Abstract
Background:
There is limited evidence on statin risk and effectiveness for patients aged 80+. We estimated risk of recurrent myocardial infarction, muscle-related and other adverse events, and statin-related incremental costs in “real-world” older patients treated with statins versus no statins.
Methods:
We used primary care electronic medical records from the UK Clinical Practice Research Datalink. Subhazard ratios (competing risk of death) for myocardial infarction recurrence (primary end point), falls, fractures, ischemic stroke, and dementia, and hazard ratios (Cox) for all-cause mortality were used to compare older (60+) statin users and 1:1 propensity-score-matched controls (n = 12,156). Participants were followed-up for 10 years.
Results:
Mean age was 76.5±9.2 years; 45.5% were women. Statins were associated with near significant reduction in myocardial infarction recurrence (subhazard ratio = 0.84, 0.69–1.02, p = .073), with protective effect in the 60–79 age group (0.73, 0.57–0.94) but a nonsignificant result in the 80+ group (1.06, 0.78–1.44; age interaction p = .094). No significant associations were found for stroke or dementia. Data suggest an increased risk of falls (1.36, 1.17–1.60) and fractures (1.33, 1.04–1.69) in the first 2 years of treatment, particularly in the 80+ group. Treatment was associated with lower all-cause mortality. Statin use was associated with health care cost savings in the 60–79 group but higher costs in the 80+ group.
Conclusions:
Estimates of statin effectiveness for the prevention of recurrent myocardial infarction in patients aged 60–79 years were similar to trial results, but more evidence is needed in the older group. There may be an excess of falls and fractures in very old patients, which deserves further investigation.
Key Words: Statins, Myocardial infarction, Falls, Fractures, Older
Statins are cholesterol-lowering drugs commonly used to prevent myocardial infarction (MI), ischemic stroke [ST], and other cardiovascular conditions (1). Despite their widespread use in older people, evidence of efficacy and risks is limited for the very old and older people with significant comorbidities (1,2).
In the United States from 2004 to 2009, 27% of people aged 55–79 years and 24% of those aged 80 years and older received statins (3). These figures are expected to rise significantly according to recommendations from current guidelines (1,4). Although statin safety and efficacy have been consistently shown in randomized clinical trials (RCTs) of middle-aged and generally healthy younger old people (5–8), evidence in the very old and in older patients with greater burden of disease is poor. RCTs on statins enrolled a relatively low proportion of individuals aged 75 and older (1), very few patients with significant comorbidity, and no patient aged 85 and older (2). Therefore, results of available RCTs should not be extrapolated to the general older population and additional research is needed.
RCTs in real-world older patients are practically and ethically challenging, particularly for established treatments in high-risk populations; therefore, observational studies evaluating the statin risk and effectiveness might help clarify the risk-to-benefit ratio in this group. To our knowledge, only one relatively small study investigated the effect of statins in preventing MI recurrence in “real-world” patients (9). This work did not account for major confounders, explore the competing risk of death, or investigate concurrent adverse events (9).
Moreover, RCTs are often based on too small samples and too short follow-ups to provide robust evidence on adverse events. For this reason, the US Food and Drug Administration, for example, support the use of electronic medical record data to provide active surveillance of regulated medications (http://www.fda.gov/Safety/FDAsSentinelInitiative/ucm149340.htm).
The present study was aimed to investigate the effectiveness of statins for the prevention of MI recurrence in a large sample of “typical” older patients with incident MI, accounting for many potential confounders and the competing risk of death. We also explored the risk of a number of conditions leading to disability in older age (ST, severe falls, fragility fractures, and dementia) and all-cause mortality, and estimated the effect of older age (80+) and burden of diseases on the association between statins and relevant end points. Finally, we investigated the incremental costs of statins. This was accomplished using a very large database of general practitioner (GP) medical records linked to hospital records and death certificates.
Although the presence of residual confounding from unmeasured factors can never be entirely excluded in observational research, results of this study will help increase the evidence base on statin risks and effectiveness in typical older people and support future interventional studies in this section of the population.
Methods
Data Source
We used data from the UK Clinical Practice Research Datalink (CPRD), a database of anonymized electronic medical records collected by UK GPs (10). Only data from practices linked to Hospital Episode Statistics (HES; for hospital records) and Office for National Statistics (for health certificates) databases (10) were used. The CPRD has been granted Multiple Research Ethics Committee approval (05/MRE04/87) to undertake purely observational studies, and this study was approved by the Independent Scientific Advisory Committee for MHRA database research under protocol numbers 15_192R.
Study Design and Study Sample
This is a quasi-experimental study designed as a retrospective parallel-cohort study. Quasi-experiments are studies that aim to evaluate interventions but that do not use randomization (11). All participants were hospitalized for first MI between April 1, 1997 (first HES data collection) and March 31, 2014 (latest HES data collection date in the available data set), aged 60+ years at the time of, and alive 4 weeks after the acute event (CONSORT diagram with participant selection criteria in Supplementary Figure S1).
Treatment Groups
Statins (atorvastatin, fluvastatin, pravastatin, rosuvastatin, and simvastatin), regardless of strength and treatment duration, represented the exposure. Lovastatin (not commercialized in the UK), cerivastatin (withdrawn from the market), and the association simvastatin/ezetimibe were not included in the analysis. Statins were coded according to Chapter 2.12 of the British National Formulary (12) and prescriptions derived from GP records.
The treatment group included all participants never treated with statins before their incident MI who had records of statin prescription within 56 days after the acute event. The control group included people never treated with statins before their first MI who did not receive a prescription of statins in the 56 days after the acute event. According to previous research (13), including “late” statin users in the control group allows both to simulate situations encountered during RCTs and avoid a biased comparison only with controls never “at risk” of being prescribed a statin. The study groups were followed up from the date of incident MI (baseline), until the occurrence of the event of interest, death, study end (ie, 10 years after baseline or March 31, 2014, whichever came first) or, only for “late statin users,” until statin prescription.
For the purpose of exploring drug persistence in people treated with statins, duration of treatment was assessed only in people alive for the entire 10-year period, as the time spanning between the first and the last prescription refill.
End Points
Primary end point was a composite of fatal MI (MI followed by death within 28 days (14)) or nonfatal MI. We used only episodes of MI leading to hospitalization and reported in HES records, to minimize misclassification given the low specificity of MI diagnosis in CPRD (15).
Secondary end points were ST, severe falls (requiring hospital admission), fragility fractures (spine, hip, wrist, humerus, pelvis, and ribs, requiring hospitalization), dementia, and all-cause mortality. These conditions were coded using ICD-10 for ST and severe falls and ICD-10 + OPCS-4 codes for fractures and derived from HES database. Dementia was coded using GP (“Read codes” adapted for CPRD) and/or HES records (ICD-10). All-cause mortality was ascertained using a combination of both Office for National Statistics and GP records. Analyses of all secondary end points were hypothesis generating and excluded people with the relevant condition at baseline.
Covariates
We used a set of 73 characteristics/conditions including enrolment period, demographics, traditional risk factors, diseases, drugs, and measures of health care utilization (those included in Table 1) as covariates to ensure an adequate control of confounding, according to previous work (16). Covariates were coded by combining GP and HES data to reduce misclassification.
Table 1.
Characteristics of the Matched Sample at Baseline by Treatment Group
| Characteristic | Controls | Statins | p Value |
|---|---|---|---|
| Number | 6,078 | 6,078 | |
| Enrolment year (%) | .797 | ||
| 1997–2001 | 34.7 | 35.5 | |
| 2002–2005 | 25.0 | 24.9 | |
| 2006–2009 | 18.2 | 17.9 | |
| 2010–2014 | 22.2 | 21.7 | |
| Demographics | |||
| Age at baseline (y, mean [SD]) | 76.4 (9.4) | 76.5 (8.9) | .555 |
| Age category (%) | .312 | ||
| 60–79 | 61.8 | 62.6 | |
| 80+ | 38.3 | 37.4 | |
| Gender (%, women) | 45.5 | 45.5 | .956 |
| Ethnicity (%) | .888 | ||
| White | 82.9 | 82.7 | |
| Nonwhite | 2.1 | 2.2 | |
| Undisclosed/unreported | 15.0 | 15.1 | |
| Index of multiple deprivations (%) | .978 | ||
| First quintile (least deprived) | 19.7 | 19.5 | |
| Second | 24.3 | 24.4 | |
| Third | 21.2 | 21.6 | |
| Fourth | 20.5 | 20.0 | |
| Fifth quintile (most deprived) | 14.1 | 14.4 | |
| Undisclosed/unreported | 0.20 | 0.20 | |
| Cardiovascular risk factors | |||
| Smoking status (%) | .845 | ||
| Never | 33.1 | 33.1 | |
| Former | 25.4 | 25.7 | |
| Current | 39.2 | 39.2 | |
| Undetermined | 2.3 | 2.0 | |
| Drinking habit (%) | .978 | ||
| Never | 13.7 | 13.9 | |
| Current, normal amount | 42.2 | 41.6 | |
| Current, unknown amount | 1.0 | 1.0 | |
| Current, heavy drinker | 8.3 | 8.4 | |
| Former | 2.4 | 2.5 | |
| Undetermined | 32.5 | 32.6 | |
| Body mass index (%) | .869 | ||
| 18.4 or less | 1.9 | 1.7 | |
| 18.5–24.9 | 20.9 | 20.9 | |
| 25–29.9 | 21.1 | 20.5 | |
| 30 or more | 9.6 | 9.9 | |
| Unmeasured | 46.5 | 47.0 | |
| Total cholesterol level ([in mmol/L], %) | .643 | ||
| Lower than 6.2 (=240mg/dL) | 27.4 | 28.2 | |
| 6.2 or higher | 8.4 | 8.2 | |
| Unmeasured | 64.1 | 63.6 | |
| Health care utilization and measures of disease burden | |||
| Flu vaccination (%) | .514 | ||
| Received (in the previous year) | 48.4 | 49.4 | |
| Not received (in the previous year) | 35.1 | 34.7 | |
| Never received | 16.4 | 15.9 | |
| Number of drugs (%) | .256 | ||
| 0–1 | 21.1 | 20.3 | |
| 2–4 | 19.2 | 18.3 | |
| 5–9 | 26.9 | 27.8 | |
| More than 10 | 32.7 | 33.6 | |
| Charlson Index (%) | .947 | ||
| 0 | 30.5 | 30.2 | |
| 1–2 | 33.4 | 33.2 | |
| 3–4 | 14.8 | 15.1 | |
| 5 or more | 21.3 | 21.5 | |
| Nursing home visits ([previous year], %) | 0.3 | 0.3 | 1.000 |
| Residential home visits ([previous year], %) | 0.4 | 0.3 | .375 |
| More than 4 GP consultations ([previous year], %) | 51.6 | 52.2 | .502 |
| Any hospitalization ([previous year], %) | 15.8 | 16.2 | .553 |
| Any geriatrics referral ([previous year], %) | 0.9 | 1.1 | .311 |
| Any cardiology referral ([previous year], %) | 1.4 | 1.5 | .445 |
| Revascularization procedures before MI ([non-MI reasons, previous year], %) | 0.3 | 0.3 | .862 |
| Diseases at baseline | |||
| Hypertension (%) | 40.0 | 40.7 | .437 |
| Diabetes (%) | 5.3 | 5.7 | .248 |
| Stroke/transient ischemic attack (%) | 8.1 | 8.1 | .973 |
| Congestive heart failure (all stages; %) | 43.7 | 43.8 | .869 |
| Atrial fibrillation (%) | 8.5 | 8.9 | .479 |
| Heart failure (%) | 7.7 | 8.1 | .479 |
| Asthma (%) | 11.1 | 10.6 | .414 |
| Chronic obstructive pulmonary disease (%) | 9.1 | 8.9 | .704 |
| Chronic kidney diseases (stages 3–5; %) | 6.5 | 6.8 | .513 |
| Cancer (%) | 9.0 | 9.2 | .614 |
| Dementia (%) | 1.7 | 1.8 | .682 |
| Depression (%) | 14.2 | 14.7 | .470 |
| Mental health condition (%) | 1.2 | 1.3 | .742 |
| Epilepsy (%) | 1.4 | 1.4 | .938 |
| Hypothyroidism (%) | 6.6 | 6.7 | .856 |
| Incontinence (%) | 5.7 | 5.9 | .669 |
| Osteoporosis (%) | 5.3 | 5.3 | .903 |
| Osteoarthritis (%) | 26.3 | 26.7 | .608 |
| Falls (%) | 18.4 | 18.6 | .833 |
| Fractures (%) | 4.7 | 4.5 | .603 |
| Cirrhosis (%) | 0.2 | 0.3 | .563 |
| Drugs at baseline | |||
| Angiotensin-converting enzyme inhibitors (%) | 11.3 | 11.6 | .512 |
| Angiotensin receptors blockers (%) | 2.6 | 3.1 | .103 |
| Renin inhibitors (%) | 0.0 | 0.0 | .317 |
| Calcium channel blockers (%) | 6.6 | 6.9 | .406 |
| Beta-blockers | 9.6 | 10.6 | .071 |
| Alpha-adrenoceptor blocking drugs (%) | 2.1 | 2.2 | .573 |
| Centrally acting antihypertensive drugs (%) | 0.2 | 0.2 | .835 |
| Nonloop diuretics (%) | 6.9 | 7.2 | .457 |
| Potassium-sparring agents (%) | 3.0 | 2.7 | .254 |
| Loop diuretics (%) | 16.5 | 17.2 | .265 |
| Antiplatelets (%) | 22.3 | 23.5 | .142 |
| Oral anticoagulants (%) | 2.6 | 2.9 | .315 |
| Nitrates (%) | 9.3 | 10.1 | .133 |
| Digoxin (%) | 3.3 | 3.5 | .726 |
| Antiarrhythmic drugs (%) | 0.9 | 0.8 | .493 |
| Insulin (%) | 1.6 | 1.5 | .770 |
| Sulphonylureas (%) | 2.8 | 3.1 | .286 |
| Metformin (%) | 2.9 | 3.0 | .708 |
| Other antidiabetic drugs | 0.2 | 0.2 | .414 |
| Corticosteroids (including topical and inhaled; %) | 26.6 | 27.3 | .347 |
| Estrogens (%) | 0.9 | 0.9 | .923 |
| Testosterone (%) | 0.0 | 0.1 | .414 |
| Proton pump inhibitors (%) | 16.6 | 17.8 | .071 |
| H2-receptor antagonists (%) | 3.9 | 4.0 | .852 |
| First-generation antipsychotic drugs (%) | 4.8 | 5.2 | .262 |
| Second-generation antipsychotic drugs (%) | 0.5 | 0.6 | .709 |
| Tricyclic antidepressants (%) | 5.2 | 5.0 | .622 |
| Selective serotonin reuptake inhibitors (%) | 4.0 | 4.5 | .209 |
| Other antidepressants (%) | 1.1 | 1.1 | .861 |
| Anticholinesterase drugs (%) | 0.3 | 0.4 | .876 |
| Cytochrome P450 inhibiting drugs (%) | 13.2 | 13.8 | .34 |
| Anti-Parkinson’s drugs (%) | 2.0 | 2.0 | .948 |
| Drugs for incontinence (%) | 3.4 | 3.7 | .303 |
Notes: GP = general practitioner; MI = myocardial infarction.
Health care costs, including statins and other medications (17), relevant monitoring tests (4), GP visits recorded (18), outpatient (18) and inpatient (19) hospital attendances were calculated based on GP and HES recorded events. Drugs were coded using the British National Formulary (12) and prescriptions derived from GP records.
Statistical Analysis
Baseline differences of both non-matched and matched samples were reported as mean and SD or percentages and compared using analysis of variance (ANOVA) or chi-square test as appropriate.
Groups were matched 1:1 using propensity score, based on 60 of the 73 covariates initially listed (those independently associated with exposure and/or primary outcome plus a few variables included regardless their lack of association because of their potential confounding effect).
End point analyses used survival analysis with competing risk models (20), to account for the high frequency of death within this age group, and results were reported as subhazard ratio (SHR) and 95% confidence intervals (95% CI), according to Fine and Gray (21). Cox proportional hazard models (using practice ID as strata) were used to analyze all-cause mortality and results were displayed as HR and 95% CIs.
Data were analyzed by censoring follow-up time of “late users” (control group only) when statin prescription was issued according to previous research (13). Alternate results obtained without this censoring were also presented as Supplementary Material.
Analyses on MI, ST, dementia, and all-cause mortality excluded events occurring the first 2 years of follow-up. Exclusion of the first 2 years of follow-up was based on exploratory analyses (data not shown) and meant to (i) reduce “reverse causation” issues (people more likely to die in the short period were less likely to be treated and patients more likely to have immediate MI recurrence were more likely to receive statins), (ii) reduce the confounding effect of early nonatherosclerotic coronary events (ie, restenosis or late stent thrombosis), and (iii) account for the timing of statin effect on cardiovascular outcomes that is likely to be apparent many years after treatment initiation (5). The main model for falls and fractures included the first 2 years of follow-up based on considerations regarding the shorter timing of statins effects on skeletal muscle. Results from alternate models including first 2 years of follow-up for MI, ST, dementia, and all-cause mortality, and excluding the first 2 years of follow-up for falls and fractures were also presented as supplementary material.
To investigate the effect of age and burden of disease on outcomes, using interactions terms, participants were divided into age (60–79 and 80+) and disease burden groups. The Charlson Comorbidity Index was used to assess disease burden because this tool was adapted and validated in the CPRD (22). Patients divided into two disease burden groups (Charlson Index: <5, first three quartiles, and ≥5, last quartile (22)). Age and disease burden analyses were not data driven but prespecified in the approved protocol, as one of the main objects of the present research.
A similar analysis investigated the effect of post-MI revascularization procedures (percutaneous transluminal coronary angioplasty or coronary artery by-pass graft) on the association between statins and recurrent MI.
Numbers needed to treat were calculated using a published formula (23).
An alpha level of 0.05 was chosen as the threshold for statistical significance for the primary end point and a 0.10 level for interaction terms. All secondary end point analyses were considered exploratory.
Data were analyzed using the Stata 13 (StataCorp. 2013, Stata Statistical Software: Release 13. College Station, TX: StataCorp LP).
Results
Sample Characteristics
After propensity score matching, the study sample included 12,156 people (6,078 per group). Mean age at baseline was 76.5 (SD: 9.2), ranging from 60 to 105.4 years, and women comprised 45.5% of the sample.
In the treatment group, 78.1% of patients received one statin, 18.9% two, and the remaining three or four. Of those who received one statin, 65% were treated with simvastatin, 28.9% with atorvastatin, and the remaining with pravastatin, fluvastatin, or rosuvastatin. In the control group, 42.6% of the patients received statin treatment ≥57 days after the acute event. Of these “late” statin users, 29.8% received statins within the first 3 months, 34.5% within the first year, and the remaining 36.1% from 1 to 10 years after the first MI.
Eighty percent of participants aged 60–79 years and 58% of those aged 80+ who were alive for the entire 10-year follow-up were still on statins 2 years after the incident MI; these proportions decreased to 67.6% and 35.2% at year 4 and to 58.6% and 20.9%, respectively, at year 6.
After matching, study groups did not differ for any of the 73 measured baseline characteristics (Table 1).
Primary End Point
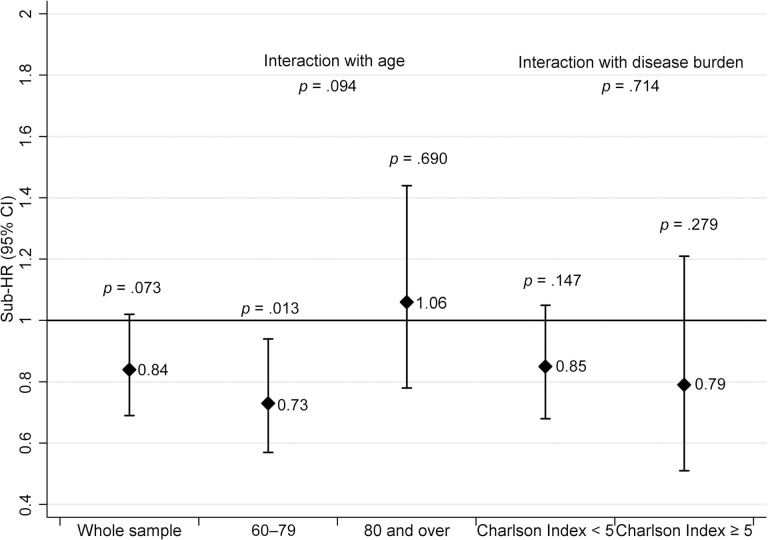
Figure 1 shows SHRs and 95% CI for recurrent MI for the whole study sample and by age and disease burden group. Patients were followed-up for 43,314 person-years. The rate of MI recurrence was 19.2 per 1,000 person-years (831 MIs). People treated with statins were less likely to have MI recurrence, although the association was only marginally significant in the whole sample. Statins showed a significant benefit in the 60–79 but not in the 80+ group. Disease burden did not affect the estimates.
Figure 1.
Effectiveness of statins for prevention of recurrence of myocardial infarction in the whole sample (60+) and by age and disease burden group (competing risk of death, excluding first 2 years’ events).
Number needed to treat for MI recurrence was 154.6 (104.5–248.2); undergoing revascularization was associated with better statin effectiveness (revascularization: SHR = 0.41, 95% CI = 0.27–0.51, p < .001; no revascularization: 0.95, 0.76–1.19, p = .685; p for interaction = .003).
When the first 2 years of follow-up were included in the analysis (Supplementary Table S1), statins were associated with a greater MI risk, particularly in older people (60–79: 0.99, 0.81–1.21, p = .976; 80+: 1.46, 1.18–1.81, p < .001; p for interaction = .025).
Secondary End Points
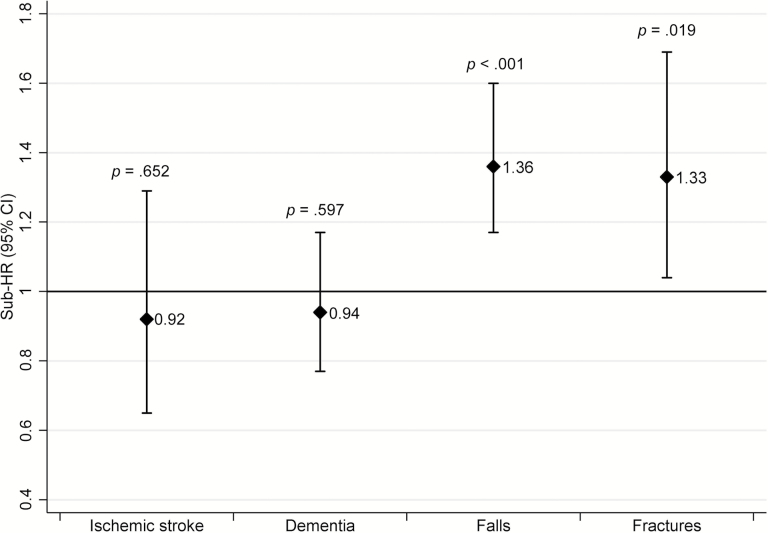
The incidence rate for ST was 7.1 per 1,000 person-years (n = 196 cases), for dementia was 16.7 (n = 446), for severe falls was 24.8 (n = 1,026 episodes), for fragility fractures was 7.6 (n = 322), and all-cause mortality rate was 115.1 (n = 5,165) per 1,000 person-years.
Figure 2 shows SHR and 95% CI for ST, dementia, falls, and fractures. No association was found between ST and dementia. People treated with statins were at greater risk of severe falls and fragility fractures.
Figure 2.
Risk of disabling conditions of older age in the whole sample (60+, competing risk of death, excluding first 2 years’ events for ischemic stroke and dementia).
The risk of falls (60–79: 1.13, 0.91–1.40, p = .260; 80+: 1.82, 1.45–2.30, p < .001; p for interaction = .012) and fractures (60–79: 1.00, 0.70–1.41, p = .993; 80+: 1.91, 1.36–2.67, p < .001; p for interaction = .019) was greater in people aged 80+ then in their younger counterparts.
Although the estimates were not significant, SHRs for ST were lower in the 60–79 than in the 80+ group (60–79: 0.73, 0.42–1.14, p = .168; 80+: 1.37, 0.81–2.33; p for interaction = .098).
No interaction with age was found for dementia. Burden of diseases did not affect the estimates of ST, dementia, falls, and fractures (data not shown).
When events occurring during the first 2 years of follow-up were excluded from the analysis, people in the treatment group were less likely to fall than those in the control group (0.73, 0.64–0.85, p < .001; Supplementary Table S2).
Participants in the statin group had lower risk for all-cause mortality (HR = 0.62, 0.57–0.68, p < .001). Increasing age (60–79: 0.62, 0.55–0.72, p < .001; 80+: 0.77, 0.67–0.89, p < .001; p for interaction = .010), but not burden of disease, affected the association between statins and all-cause mortality. When follow-up of statin-treated controls was not censored at the time of statin initiation, the benefit of statins on all-cause mortality was substantially attenuated (HR = 0.83, 0.78–0.90; p < .001).
Over 10 years, mean total cost per patient in the statin group was £24,011 (~$36,000, at exchange rate of $1.50 per £1). For the control group, the mean total cost was £23,094 (~$34,700). The mean cost difference between the groups was £917 (−3,930 to 5,630) (~$1,400) per patient. In the 60–79 age group, statins resulted in cost savings of −£13,234 (−35,122 to 2,287) (~$20,000) but increased costs in the 80+ group £6,729 (5,099–8,265) (~$10,000).
Same associations but lower estimates were found when follow-up of people taking statins in the control group was not censored at the time of statin prescription. Overall mean cost difference was −£176 (−2,299 to 1,789) reflecting a cross-over use of statins by 42% (2,564/6,078) of participants in the control group after the start of the observation period. The mean cost difference between the groups was £92 per patient. In the 60–79 age group, statins resulted in cost savings of −£3,962 (−8,012 to −175) but increased costs in the 80+ group £3,377 (1,319 to 5,077).
Discussion
To our knowledge, this is the first study investigating statin effectiveness for the prevention of MI recurrence in a large sample of “real-world” older people with incident MI, accounting for a large number of covariates and exploring the competing risk of death. Results showed that statins were effective in younger old people in reducing recurrent MI, with similar effect sizes to those from RCTs. In testing for interactions with advanced age, we found evidence of more modest protective effects in the older group, but CIs were wide and more evidence will be needed to clarify the effect sizes. Burden of disease did not affect the estimates. Undergoing post-MI revascularization was associated with greater statin benefit.
Risk of falls and fractures might be higher, particularly in the very old during the first years of treatment. No association with ST or dementia was found. Finally, people treated with statins were at lower risk of all-cause mortality, and yet the benefit was lower in the very old. Statin treatment was associated with cost-savings in the 60–79 but higher costs in the 80+ group.
Data on older people have been provided in RCTs on statins including both primary and secondary prevention patients (24–26), and results are not easy to directly compare. Overall, our estimates for the primary end point (MI) are similar to those of RCTs on statins in the age group (60–79) usually enrolled in RCTs (5). To the best of our knowledge, only one observational study has evaluated the effectiveness of statins on the prevention of MI recurrence in “real-world” older patients (9). In this study, conducted in a relatively small sample (n = 1,410) of older patients, the authors found that, after adjusting for age, smoking habit, hypertension, diabetes, and low- and high-density lipoprotein cholesterol levels, people in the statin group were less likely to develop fatal and nonfatal MI than people in the control group (relative risk = 0.49, 0.43–0.57) (9). Given the limited number of potential confounders included in the analysis, the risk of residual confounding, particularly “reverse causation,” cannot be excluded in this study.
As expected, statin treatment immediately after MI was probably driven by a combination of better short-term prognosis (approximately fourfold greater first-year mortality rates in controls, data not shown) and greater risk of imminent MI recurrence (increased MI risk associated with statins when events occurred in the first years were included).
The fact that statin benefit might decrease in older age has been considered biologically plausible and previously reported in studies on statins and mortality (27). Of note, age also markedly affects the association between cholesterol levels and mortality for ischemic heart disease (28). However, we cannot exclude that the observed reduced benefit in people 80+ might result from poor treatment persistence.
All secondary end point analyses of this study should be considered exploratory.
Our results showed that statin was not associated with dementia risk. This is in line with a recent Cochrane review (29). The lack of association with ST requires consideration. Although not significant, estimates obtained in the 60–79 group are similar to those of RCTs and our study was not powered to capture such an effect with small number of events.
We found increased risk in falls and fractures, especially in people 80+ during the first treatment years. Statins can cause myopathy, from subclinical to life-threatening forms, and age and comorbidity are important risk factors (30). Its consequences in vulnerable older people can potentially be more dangerous than in younger/healthier older patients. Although statin use has been associated with lower energy and greater muscle exertion (31), longitudinal decrease in muscle performance, and increased risk of falls in small studies of older people (32), other authors found no association (33) or even benefit on skeletal muscle (34). The fact that statins are protective from falls after the first 2 years, when follow-up time in statin-prescribed controls was not censored, is not easy to explain. This might result from a combination of fall rate reduction in the treatment group after the second year of follow-up (discontinuation of treatment in case of adverse reactions and/or timing of muscle damaging effect) and concurrent fall rate increase among “late statin users” (once they start receiving treatment) in the control group that could not be captured when “late statin users” follow-up time was censured. Alternatively, when taken for longer periods, statins might reduce the fall risk by slowing the decline of cardiovascular function.
A number of studies have investigated the association between statin use and all-cause mortality (27) in older people with previous cardiovascular disease. Our estimates were remarkably consistent with those of most published reports (27). The fact that estimates of statin benefit for all-cause mortality is greater than that for MI recurrence might be explained by long-term noncardiovascular beneficial statin effects such as those on cancer (35); however, we cannot exclude the presence of residual confounding.
Previous modeling studies (36) found that statin therapy for secondary prevention is associated with increased costs to the health care system. In contrast, this analysis presents evidence from 10-year observational follow-up that statins may result in cost-savings in people aged 60–79 years but increased costs in 80+. These results warrant further cost-effectiveness analysis that accounts for the accrual of health care costs and quantity and quality of life benefits to patients.
There are inevitably limitations in the analysis presented. Although statin prescription and the main conditions studied are likely to be accurately ascertained in the combination of primary care and hospital inpatient records used, there may be some underdiagnosis of dementia and underrecording of falls, but there is no apparent reason why these limitations would be associated with statin receipt after MI. The propensity scoring approach models effects only in those cases and controls that have overlapping scores, reducing the sample size analyzed, although the patients included in analyses are those for whom clinical decisions about adding in statins varied after MI in apparently similar cases.
Observational analyses like the one presented here are always limited in not being able to definitively exclude the existence of residual confounding that might have resulted from unmeasured factors, although the very high number of variables used in our propensity scoring should have minimized such biases. Given that the statin-treated group enjoyed lower mortality rates during the up to 10-year follow-up, the observed associations with injurious falls and fractures are unlikely to have been driven by a general excess morbidity in the statins group, but appear to be a specific effect that is difficult to explain by residual negative health difference between the statin-treated patients and their controls, after matching. The main analysis for the primary end point (MI recurrence) excluded the first 2 years of follow-up and therefore the results obtained cannot be generalizable to the early period of treatment. However, because the beneficial effect of statins for cardiovascular prevention occurs 1–3 years after treatment start (5), we are confident that this exclusion, although helpful to address reverse causation, did not significantly bias the primary end point estimates. Finally, a number of unmeasured factors might have contributed to the high “noise-to-signal” ratio reflected by the large variability and CIs in our real-world older people. Although an “a priori” sample size calculation was performed based on the point estimates obtained by Baigent and colleagues (5), given the greater proportion of older patients in our sample and higher heterogeneity seen in this group, we cannot exclude that the overall primary end point analysis was slightly underpowered.
Along with the limitations, it is worth noting that the analysis includes all eligible patients in the data set (ie, the equivalent of a 100% response rate) and likely negligible loss to follow-up in hospital and death certificate data during our up to 10-year analysis of outcomes. The estimates produced are therefore likely to represent “real-world” outcomes in typical clinical practice during the period studied. Also worth noting is that we have not excluded frail or dependent groups including those in nursing and residential homes.
Further work, including RCTs, is needed to replicate these findings in independent populations and to clarify the mechanisms of the excess falls and fractures, establishing whether these are driven by the well-known effects of statins on muscle or through other mechanisms in older people.
In conclusion, our quasi-experimental analysis of effectiveness of statins for secondary prevention of MI produced estimates in line with results of RCTs for patients aged 60–79 years, but more evidence is needed in the older groups. We found evidence of excess falls and fractures in very old patients, which deserve further investigation. If these results are confirmed, higher falls and fracture rates need to be considered in judgments about the appropriateness of statin use in older patients. Very old patients in our analysis were less likely to stay on treatment for a period long enough to provide benefit but long enough to risk serious adverse reactions.
Supplementary Material
Supplementary material can be found at: http://biomedgerontology.oxfordjournals.org/
Funding
This research is funded by the National Institute for Health Research (NIHR) School for Public Health Research (SPHR). Grant number: IS-SPH-0211-10100 - SPHR-SWP-AWP-PR2. SPHR is a partnership between the Universities of Sheffield, Bristol, Cambridge, Exeter; UCL; the London School for Hygiene and Tropical Medicine; the LiLaC collaboration between the Universities of Liverpool and Lancaster and Fuse (the Centre for Translational Research in Public Health, a collaboration between Newcastle, Durham, Northumbria, Sunderland, and Teesside Universities).
Supplementary Material
Acknowledgments
A.B. is a former employee of Pfizer (until November 2012). P.M.H., J.D., J.A.M., K.B., J.Z.-S., R.E.M.M., W.E.H., and D.M. have no conflict of interest.
The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the UK Department of Health.
References
- 1. Stone NJ, Robinson JG, Lichtenstein AH, et al. ; American College of Cardiology/American Heart Association Task Force on Practice Guidelines 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 Pt B):2889–2934. doi:10.1016/j.jacc.2013.11.002 [DOI] [PubMed] [Google Scholar]
- 2. Fleg JL, Forman DE, Berra K, et al. ; American Heart Association Committees on Older Populations and Exercise Cardiac Rehabilitation and Prevention of the Council on Clinical Cardiology, Council on Cardiovascular and Stroke Nursing, Council on Lifestyle and Cardiometabolic Health Secondary prevention of atherosclerotic cardiovascular disease in older adults: a scientific statement from the American Heart Association. Circulation. 2013;128:2422–2446. doi:10.1161/01.cir.0000436752.99896.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chokshi NP, Messerli FH, Sutin D, Supariwala AA, Shah NR. Appropriateness of statins in patients aged >/=80 years and comparison to other age groups. Am J Cardiol. 2012;110:1477–1481. doi:10.1016/j.amjcard.2012.06.058 [DOI] [PubMed] [Google Scholar]
- 4. NICE. Lipid Modification: Cardiovascular Risk Assessment and the Modification of Blood Lipids for the Primary and Secondary Prevention of Cardiovascular Disease. NICE Guidelines [CG181]; 2014. https://www.nice.org.uk/guidance/cg181. Accessed December 2, 2015. [Google Scholar]
- 5. Baigent C, Keech A, Kearney PM, et al. ; Cholesterol Treatment Trialists’ (CTT) Collaborators Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–1278. doi:10.1016/S0140-6736(05)67394-1 [DOI] [PubMed] [Google Scholar]
- 6. Mihaylova B, Emberson J, Blackwell L, et al. ; Cholesterol Treatment Trialists’ (CTT) Collaborators The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. 2012;380:581–590. doi:10.1016/S0140-6736(12)60367-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Taylor F, Ward K, Moore TH, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2011;1:CD004816 doi:10.1002/14651858.CD004816.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Savarese G, Gotto AM, Jr, Paolillo S, et al. Benefits of statins in elderly subjects without established cardiovascular disease: a meta-analysis. J Am Coll Cardiol. 2013;62:2090–2099. doi:10.1016/j.jacc.2013.07.069 [DOI] [PubMed] [Google Scholar]
- 9. Aronow WS, Ahn C. Incidence of new coronary events in older persons with prior myocardial infarction and serum low-density lipoprotein cholesterol > or = 125mg/dl treated with statins versus no lipid-lowering drug. Am J Cardiol. 2002;89:67–69. [DOI] [PubMed] [Google Scholar]
- 10. Herrett E, Gallagher AM, Bhaskaran K, et al. Data resource profile: Clinical Practice Research Datalink (CPRD). Int J Epidemiol. 2015;44:827–836. doi:10.1093/ije/dyv098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Harris AD, McGregor JC, Perencevich EN, et al. The use and interpretation of quasi-experimental studies in medical informatics. J Am Med Inform Assoc. 2006;13:16–23. doi:10.1197/jamia.M1749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ah-See KW. British National Formulary. 65th ed. London, UK: London BMJ Group and Pharmaceutical Press; 2013. [Google Scholar]
- 13. Weiner MG, Xie D, Tannen RL. Replication of the Scandinavian Simvastatin Survival Study using a primary care medical record database prompted exploration of a new method to address unmeasured confounding. Pharmacoepidemiol Drug Saf. 2008;17:661–670. doi:10.1002/pds.1585 [DOI] [PubMed] [Google Scholar]
- 14. Tunstall-Pedoe H, Morrison C. Coronary heart disease in women. Women may be more ill when they reach hospital. BMJ. 1994;309:1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bhattarai N, Charlton J, Rudisill C, Gulliford MC. Coding, recording and incidence of different forms of coronary heart disease in primary care. PLoS One. 2012;7:e29776 doi:10.1371/journal.pone.0029776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Smeeth L, Douglas I, Hall AJ, Hubbard R, Evans S. Effect of statins on a wide range of health outcomes: a cohort study validated by comparison with randomized trials. Br J Clin Pharmacol. 2009;67:99–109. doi:10.1111/j.1365-2125.2008.03308.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. HSCIC. HSCIC prescription cost analysis 2014 http://www.hscic.gov.uk/catalogue/PUB17274/pres-cost-anal-eng-2014-rep.pdf Accessed December 2, 2015.
- 18. Curtis L. PSSRU unit costs of health and social care 2014: University of Kent, 2014 http://www.pssru.ac.uk/project-pages/unit-costs/2014/ Accessed December 2, 2015.
- 19. Health Do. NHS reference costs 2015 https://www.gov.uk/government/collections/nhs-reference-costs Accessed December 2, 2015.
- 20. Berry SD, Ngo L, Samelson EJ, Kiel DP. Competing risk of death: an important consideration in studies of older adults. J Am Geriatr Soc. 2010;58:783–787. doi:10.1111/j.1532-5415.2010.02767.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 22. Khan NF, Perera R, Harper S, Rose PW. Adaptation and validation of the Charlson Index for Read/OXMIS coded databases. BMC Fam Pract. 2010;11:1 doi:10.1186/1471-2296-11-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stang A, Poole C, Bender R. Common problems related to the use of number needed to treat. J Clin Epidemiol. 2010;63:820–825. doi:10.1016/j.jclinepi.2009.08.006 [DOI] [PubMed] [Google Scholar]
- 24. Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7–22. doi:10.1016/S0140-6736(02)09327-3 12114036 [Google Scholar]
- 25. Lloyd SM, Stott DJ, de Craen AJ, et al. Long-term effects of statin treatment in elderly people: extended follow-up of the PROspective Study of Pravastatin in the Elderly at Risk (PROSPER). PLoS One. 2013;8:e72642 doi:10.1371/journal.pone.0072642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Roberts CG, Guallar E, Rodriguez A. Efficacy and safety of statin monotherapy in older adults: a meta-analysis. J Gerontol A Biol Sci Med Sci. 2007;62:879–887. [DOI] [PubMed] [Google Scholar]
- 27. Strandberg TE, Kolehmainen L, Vuorio A. Evaluation and treatment of older patients with hypercholesterolemia: a clinical review. JAMA. 2014;312:1136–1144. doi:10.1001/jama.2014.10924 [DOI] [PubMed] [Google Scholar]
- 28. Lewington S, Whitlock G, Clarke R, et al. ; Prospective Studies Collaboration Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet. 2007;370:1829–1839. doi:10.1016/S0140-6736(07)61778-4 [DOI] [PubMed] [Google Scholar]
- 29. McGuinness B, Craig D, Bullock R, Passmore P. Statins for the prevention of dementia. Cochrane Database Syst Rev. 2009;2:CD003160 doi:10.1002/14651858.CD003160.pub2 [DOI] [PubMed] [Google Scholar]
- 30. Abd TT, Jacobson TA. Statin-induced myopathy: a review and update. Expert Opin Drug Saf. 2011;10:373–387. doi:10.1517/14740338.2011.540568 [DOI] [PubMed] [Google Scholar]
- 31. Golomb BA, Evans MA, Dimsdale JE, White HL. Effects of statins on energy and fatigue with exertion: results from a randomized controlled trial. Arch Intern Med. 2012;172:1180–1182. doi:10.1001/archinternmed.2012.2171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Scott D, Blizzard L, Fell J, Jones G. Statin therapy, muscle function and falls risk in community-dwelling older adults. QJM. 2009;102:625–633. doi:10.1093/qjmed/hcp093 [DOI] [PubMed] [Google Scholar]
- 33. Swiger KJ, Martin SS, Tang F, et al. Cognitive and physical function by statin exposure in elderly individuals following acute myocardial infarction. Clin Cardiol. 2015;38:455–461. doi:10.1002/clc.22423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Riechman SE, Andrews RD, Maclean DA, Sheather S. Statins and dietary and serum cholesterol are associated with increased lean mass following resistance training. J Gerontol A Biol Sci Med Sci. 2007;62:1164–1171. [DOI] [PubMed] [Google Scholar]
- 35. Cai H, Zhang G, Wang Z, Luo Z, Zhou X. Relationship between the use of statins and patient survival in colorectal cancer: a systematic review and meta-analysis. PLoS One. 2015;10:e0126944 doi:10.1371/journal.pone.0126944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ward S, Lloyd Jones M, Pandor A, et al. A systematic review and economic evaluation of statins for the prevention of coronary events. Health Technol Assess. 2007;11:1–160, iii. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.