Abstract
Background:
To prevent onset of age-related diseases and physical and cognitive decline, interventions to slow human aging and extend health span must eventually be applied to people while they are still young and healthy. Yet most human aging research examines older adults, many with chronic disease, and little is known about aging in healthy young humans.
Method:
This article explains how this knowledge gap is a barrier to extending health span and puts forward the case that geroscience should invest in researching the pace of aging in young adults. As one illustrative example, we describe an initial effort to study the pace of aging in a young-adult birth cohort by using repeated waves of biomarkers collected across the third and fourth decades to quantify the pace of coordinated physiological deterioration across multiple organ systems (eg, pulmonary, periodontal, cardiovascular, renal, hepatic, metabolic, and immune function).
Results:
Findings provided proof of principle that it is possible to quantify individual variation in the pace of aging in young adults still free of age-related diseases.
Conclusions:
This article articulates research needs to improve longitudinal measurement of the pace of aging in young people, to pinpoint factors that slow or speed the pace of aging, to compare pace of aging against genomic clocks, to explain slow-aging young adults, and to apply pace of aging in preventive clinical trials of antiaging therapies. This article puts forward a research agenda to fill the knowledge gap concerning lifelong causes of aging.
Keywords: Longitudinal cohort study, Health span, Geroscience, Young adults
The human life span has already been extended, and medical treatments save lives, but with the undesirable result that more people are living extra years with disability and dementia today than 20 years ago (1). If human aging could be slowed by antiaging therapies, extra years of life could become extra years of health (2). Antiaging therapies show promise in animal model research, and purportedly, some therapies are near-ready for translation to humans (3,4). It has recently been shown that interventions administered to healthy organisms can slow their aging (3). Young adults are naturally the most attractive targets for therapies to extend health span because their organs are not yet damaged, making it possible in theory to prevent age-related diseases (5). Yet, most studies of human aging, including initial efforts at randomized trials of antiaging therapies (6), examine older adults. As a result, very little is known about biological aging processes in young humans, a gap in knowledge that will retard translation from animal models to human clinical application. In this article, we suggest that in addition to studying seniors, the geroscience of health-span extension should also invest in studying processes of aging in young people.
Studying Aging as a Lifelong Process: A Brief Conceptual History
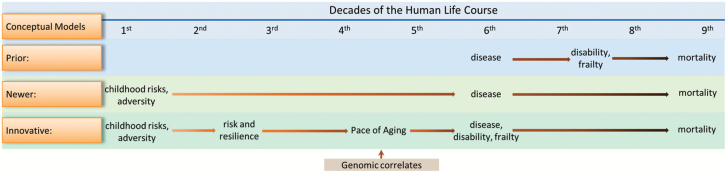
It is often remarked that “aging is a lifelong process.” The innovative nature of studying aging in the context of the whole life course (including young people) can be illustrated by tracing the recent history of evolving conceptualizations of the connection between aging and disease (Figure 1). A dominant, prior conceptual model was that diseases such as heart disease, diabetes, cancer, and dementia onset in the latter half of the life course and subsequently converted individuals who were cognitively and physically healthy into persons with disability and frailty, who died soon (top row of Figure 1). Under these assumptions, researchers recruited samples of older people, and focused on single diseases, with the aim of finding cures for them. Discoveries and treatments resulting from this research approach have demonstrably extended life expectancy, but left many patients with disability.
Figure 1.
Studying aging as a lifelong process: A brief conceptual history.
Then, a newer conceptual approach ascended, based on the recognition that age-related diseases and mortality could be predicted from very early life, even antenatally (middle row of Figure 1). As examples, intrauterine growth retardation (7), low childhood intelligence (8), and childhood adverse experiences (9) were found to be early-life antecedents of late-life disease and mortality. New technology also enabled prediction of age-related diseases by genetic endowment present at the very beginning of life (10,11). Such discoveries about the importance of early-life factors for aging persuaded scientists that aging is a lifelong process that ought to be studied in young as well as old organisms (12). Researchers began to envisage extending health span by implementing primary preventions and also to seek interventions to reverse early-life damage to health and cognition. But this conceptualization leaves an information vacuum between early-life risks and late-life disease onset.
Currently, new conceptual approaches are emerging to fill that vacuum. Now aging itself is considered a primary risk factor for nearly all age-related diseases (13,14). This idea makes it logically imperative to study aging as an antecedent to disease, and to do so requires measuring aging in healthy people in the first half of the life course before they develop age-related diseases (bottom row of Figure 1). The newer conceptual model accompanies research that measures, and manipulates, the pace of aging in young model organisms (3,4,15). But translation of this newest conceptual approach to human research is still lagging. Thus, here we advocate a conceptual approach that looks harder at the first half of the human life course, specifically by quantifying the pace of aging in young adults and by testing hypotheses about factors that bring about accelerated or slowed variation in young adults’ pace of aging (bottom row of Figure 1).
Why Is it Important to Study the Pace of Aging as a Cause of Disease?
The 2013 NIH Advances-in-Geroscience conference concluded, “The major diseases causing morbidity and mortality have one root cause in common—aging” (16). The hypothesis is that age-related chronic diseases are preceded by a gradual and interrelated loss of the body’s integrity that unfolds as a person’s chronological age increases. Consistent with this hypothesis, data from the Global Burden of Disease Project (http://www.healthdata.org/gbd, Institute for Health Metrics and Evaluation 2013) show increasing age is tightly linked to increasing morbidity from multiple chronic conditions affecting bodily systems, from the lungs to the brain (17). Data like these imply that a key to extending health span is to slow aging itself (14,18) because intervention to slow aging might be able to delay all age-related diseases simultaneously, rather than treating them one at a time (19).
Why Is it Important to Measure the Pace of Aging in Humans as well as in Animals?
Scientists have been able to quantify and manipulate the pace of aging in nonhuman model organisms in the laboratory, and announcements have been put forward that promising antiaging therapies will soon be ready for human trials (3,4). But a fundamental obstacle blocks the translational pipeline: a lack of technology to measure the pace of aging in young humans. Human life spans are long, and chronic diseases of aging onset in the later decades of life. Thus, antiaging interventions aiming to prevent disease will require decades of follow-up before outcomes of disease or mortality could be measured. Methods to measure the pace of aging in humans who have not yet developed chronic disease would make it possible to record and quantify pretreatment baseline, during-treatment change, and posttreatment outcome of participants in randomized clinical trials of rejuvenation therapies. But such measures are lacking (20).
Why Is it Important to Study Individual Variation in the Pace of Human Aging?
The experimental science of extending animal life span has tended to ignore individual variation; this work has tended to study genetically identical animals living under uniform laboratory conditions and has focused on experimentally induced variation as the variation of interest. But free-living humans are devilishly heterogeneous as compared with model organisms in the lab. A human adult’s pace of biological aging may be sped or slowed by genetic endowment, by varying early-life experience, and by individual differences in lifestyle. We think these factors must be researched and understood better because they will inevitably vex translation from preclinical health-span models to humans (21). Moreover, these factors include potential intervention targets that are uniquely human, and therefore are not easily investigated in animal research, such as perceptions and attributions, self-appraisal, purpose in life, personality traits, and mental disorders (22–25). Measures of the pace of aging in young-adult humans are needed to provide a dependent outcome variable for investigations of how individual variation in the pace of aging is affected by genetics, early-life exposures, and adult lifestyle factors.
Why Is it Important to Study the Pace of Aging in Young Humans?
The emerging field of geroscience studies mechanisms of aging and causes of age-related disease with the aim to ultimately control aging and prevent disease (26). Progress toward this aim is being constrained by research that solely examines human research participants in the last half of their lives. One reason for the focus on older adults in much geroscience research is that aging is often defined and quantified as the accumulation of age-related diseases and their complications. The result is that young people are typically excluded from aging research because they do not yet have an age-related disease. However, studying people who have age-related disease may not be the only or even the best way forward. Across all branches of medicine, primary-prevention targets have been difficult to uncover by studying patients already diagnosed with diseases, in part because diseases are typically clinically diagnosed years after their etiology is well underway. We can think of at least three advantages to conducting geroscience research in young people.
The first reason geroscience should begin to research young people is that there is now evidence that the pathogenesis of age-related diseases involves gradually accumulating damage to organ systems, beginning in the first half of the life course (27–31). Moreover, it is now known that potentially preventable risk exposures and causes of age-related disease begin in early childhood (32). An important implication of this new knowledge is that future interventions will need to be scheduled before midlife if they hope to prevent accelerated aging, pre-empt age-related disease, and improve the quality of longer lives (33). In order to design such interventions, there is a lot to be learned about exactly how damage accumulates during the years between conception, childhood, and disease onset. Studying processes of aging in younger cohorts can provide valid prospective measures of exposures and the damage they cause to organ systems (including the brain), while etiological processes are unfolding.
A second reason to take advantage of young cohorts is that scientists cannot assume that causes and correlates of problematic aging uncovered in studies of individuals older than 50 years will also explain individual differences in the pace of aging in people younger than 50 years. For example, genetic correlates in extreme-longevity samples have been found to differ from genetic correlates in older-adult samples (10). Thus, studying the pace of aging in younger cohorts can answer the question of whether factors that influence aging in younger adults and older adults are the same or different.
A third reason geroscientists should embrace young cohorts is to improve study designs and to reduce bias and noise in their data (11,34). Studying aging in the already-aged introduces selection bias. Many individuals with a fast pace of aging may not survive into their sixth or seventh decades of life, yielding missing data for the cases of greatest interest. Studying aging in individuals who already have chronic disease also introduces “noise” from disease symptoms, medication regimens, and treatment responses that interfere with the “signal” of basic processes of aging. Studying the pace of aging in younger cohorts can avoid these ubiquitous sources of bias and noise.
Cross-sectional Approaches to Measuring Individual Differences in Biological Age
Measuring biological aging is a recent enterprise (13), and there is no consensus yet about the best methods (35–37). Candidate biomarkers of aging are numerous (eg, telomere length and methylation profiles), but findings are mixed (20,38–41). (A biomarker is anything that can be used as a measureable indicator of a disease state or other physiological state of an organism.) As an alternative to single-marker aging indicators, multibiomarker algorithms have been recommended as a way to achieve more reliable measurement (42–46). As such, research groups are using data to identify multibiomarker aggregates that correlate with participants’ chronological age and/or predict participants’ mortality better than does their chronological age.
Most multibiomarker composite measures proposed so far are designed for cross-sectional data. Such one-wave cross-sections have inherent limitations which render them unable to track aging as change within lives of individuals. First, they cannot disentangle aging-related change from other factors that influence biomarker levels, such as pre-existing poor health sustained since childhood, or an acute spell of illness around the time of data collection. Second, in samples where the participants vary in age but the biomarkers are all surveyed at one time point, age differences in biomarker levels are not independent of cohort effects (eg, historical improvements in health-related living conditions can make younger participants look better than older participants on biomarkers.) Third, even if a cross-sectional algorithm predicts mortality well in a sample with age variation, its utility for quantification of aging in younger persons remains uncertain because most deaths observed during follow-up occur to a sample’s oldest participants. Finally, cross-sectional measures are static whereas aging is a dynamic process sometimes referred to as “damage accrual”; one-wave indicators are unable to truly track whether a young person’s pace of age-related biological decline is actually accelerating or slowing down (47).
A Longitudinal Approach to Measuring the Pace of Biological Aging
A novel approach is to study aging longitudinally, while tracking a cohort of young adults who are approaching the middle of the life course, a developmental period when individuals start to diverge in their aging trajectories, but before most diseases onset. We are undertaking one such study, the Dunedin Study, which has followed a representative birth cohort of 1037 individuals since their birth in 1972–1973 and is now entering its fifth decade with 95% retention (48). The cohort is primarily white, and it is population representative; a lifetime of research participation has not improved Study members’ health as compared with the New Zealand National Health & Nutrition Survey (eg, body mass index, smoking, and GP visits) (48). Cohort members are largely still healthy; by age 40, only 11 study members (1%) have been diagnosed with an age-related condition: heart attack, stroke, or type 2 diabetes. New Zealand is a useful laboratory because it suffers the same types of income inequalities found in the United States and the United Kingdom (GINI coefficient = 0.34 for New Zealand vs 0.38 for the United States, 0.35 for the United Kingdom). Like the United Kingdom, New Zealand spends less on health care than the United States (New Zealand = 8% vs United States = 17% of gross domestic product), but has a longer life expectancy (New Zealand = 80.7 vs United States = 78.5 years) and has a national health care system.
Taking advantage of this longitudinal cohort study, we developed a measure of the pace of aging. We designed the measure in accordance with contemporary geroscience theory about human aging as a gradual and interrelated loss of integrity in every organ system, beginning in the first half of the life course (13,14,16,19). In the Dunedin Study, at three measurement occasions spanning 12 years, we repeatedly measured blood pressure, cardiorespiratory fitness, pulmonary function, periodontal disease, anthropometric indices, lipid parameters, glycated hemoglobin, indicators of kidney and liver function, and systemic inflammation, as well as telomere length. Of course, every biomarker has its own unique sources of individual variation at any specific point in time. Temporary sickness can spike a biomarker, and other sources of measurement error insert abnormal values into assay data. Our approach was designed to look beyond individual measurements to capture correlated trends among biomarkers as they changed with advancing chronological age. Our hypothesis was that if, for example, declining cardiorespiratory fitness taps aging, then a research participant who showed declining cardiorespiratory fitness on testing from Time 1 to Time 2 to Time 3 should likewise show correlated decline in, for example, their kidney, lung, and immune function. Accordingly, we measured the pace of aging by assessing decline in a panel of biomarkers repeatedly collected. Biomarkers were assessed at ages 26, 32, and 38 years (and will be repeated at age 45 years in 2017). The repeated-measures approach separates sources of variation. One source is baseline individual differences in physiology, which in part reflect poor health from earlier in life, and thus cannot unambiguously represent aging. Another source is acute change in a biomarker that recovers; again, not aging. The last source is physiological change over time in the direction of decline, as shown in many biomarkers from multiple organ systems simultaneously tracked over the same time period. According to geroscience theory, only the latter represents the pace of aging.
Each cohort member’s pace of aging was calculated from longitudinal analysis of the 18 biomarkers (for details, see (17)). Briefly, we used mixed-effects growth models to calculate each study member’s personal slope for each of the 18 biomarkers one by one and then computed each study member’s pace of aging as the sum of 18 slopes. Our analyses revealed four findings. First, growth-curve models showed declining function in the biomarkers from ages 26 to 38 years (eg, higher triglycerides, lower maximal aerobic capacity, and higher blood pressure). Second, addressing the critical theoretical prediction, the “slopes” of change in the biomarkers were themselves positively inter-correlated, showing correlated age-related decline across multiple organ systems. Third, the pace of this correlated biomarker decline varied among individuals; some cohort members declined faster than others. Fourth, sensitivity analyses showed that contrary to some expectations, the pace of aging did not depend on change in any particular lifestyle-influenced biomarker, such as rising body mass index (49).
The Dunedin cohort, having reached only the midpoint of the life span, lacked data to test whether the pace of aging measure predicts mortality. However, we were able to validate this measure in two ways. The first validation showed that study members who had been experiencing a more rapid biological pace of aging over the preceding 12 years had also reached an older estimated biological age in 2011–2012, when all cohort members were 38 years old (17). For this analysis, we calculated for each study member a previously published 10-biomarker cross-sectional indicator of biological age (44,50). This biological age indicator at age 38 years was a good validation benchmark because in NHANES participants aged 30 to 75 years, this cross-sectional indicator predicted mortality 20 years later, did so better than chronological age, and accounted for the association between chronological age and mortality (50). As hypothesized, the correlation between Study members’ pace of aging over the past 12 years and their attained biological age at the last follow-up was significant and moderate (Pearson correlation = .38).
The second set of validation studies showed that individual differences in the biological pace of aging were significantly correlated, already by age 38, with diminished functional capacity, as would be expected in a gerontological study of older adults (17). Study members with a more rapid pace of aging were less physically able: They had more difficulty than age peers with the Unipedal Stance Test of balance, the Grooved Pegboard Test of fine-motor coordination, and the Grip Strength test. Study members with a more rapid pace of aging showed a decline in cognitive performance, net of their childhood baseline level on the same neuropsychological tests, and this was particularly true for tests of fluid intellectual abilities such as processing speed. Study members with a faster pace of biomarker aging also looked older according to an independent panel of raters who evaluated facial photographs of our Study members at age 38. These initial findings provide proof of principle that variation in the pace of aging can be quantified in young adults.
What Are the Pressing Research Needs?
We call for research on the pace of aging in other young cohorts to inform geroscientists about processes of aging in young humans, who are the eventual market for antiaging therapies. The leap from healthy young lab animal to healthy young human may not be so simple. Humans’ aging is under more multifactorial influence than lab animals’ aging, and this heterogeneity is likely to complicate and even compromise clinical trial outcomes; but if we knew more about aging in young humans, trials could be planned to maximize chances of success. Moreover, at present there is no outcome measure that can be used to show if a treatment has worked in young adults. Measuring aging in free-living humans early in their life spans brings interesting new scientific opportunities and research needs, as noted below in this section.
First, there is a need for research to develop better, more reliable, valid, and practical measurement technology to quantify the pace of aging in young adults. One obvious need is for more waves of biomarker data; quantifying the pace of aging will be enhanced with four or more biomarker data points (51). In longitudinal panel studies, every additional wave of data increases the reliability and precision of measuring change and adds power. Moreover, four waves enhance capacity to test alternative functional forms of change. Another need is to know how far apart the waves must be to detect aging sensitively. Our waves were about 5 years apart, but shorter intervals should be tested (52). Furthermore, research needs to evaluate the relative performance of biomarker subsets with the aim of identifying the most efficient “short-form” of the pace of aging that is most feasible. We used all 18 biomarkers that were repeatedly measured in the Dunedin Study, and our analyses showed that associations between pace of aging and validating measures of physical and cognitive functioning did not depend on any one biomarker (17). However, other biomarkers that we did not have may be able to improve measurement, including repeated measures of physical function. Also, we weighted all biomarkers equally to transparently avoid assumptions, and to avoid a finding that was too cohort specific. Nonetheless, another research need is to refine weightings of biomarker contributions to pace-of-aging measurement. In fact, tests are needed of geroscience theory’s central claim that aging unites organ systems; are there systems that do not age in concert with others? The ultimate need is to develop pace-of-aging measures that are good enough that they can be used to identify individuals who are most in need of intervention before clinical symptoms onset; that is, young individuals who are already aging more rapidly than their age peers.
Second, there is a need to find out how the pace of aging tracks over the entire life course. Studies tracking pace of aging earlier, in children, adolescents, and young adults, are needed to uncover when and how aging trajectories begin to diverge. For example, does rapid pace of aging bear any relation to early pubertal development? The ideal data resource would comprise repeatable biomarkers that cover the entire life course validly, as has been reported for blood pressure (53). Studies tracking pace of aging in midlife and older adults are also needed (54). Do individuals who age more rapidly in their 20s and 30s continue to age more rapidly onward through midlife and into late life? Preliminary evidence suggests they do; our pace-of-aging measure correlated positively with a cross-sectional indicator of biological age (50) which has been reported to predict mortality better than does chronological age. However, cohort studies with late-life follow-up are needed to evaluate how well pace of aging in young adults forecasts their health span and life span.
Third, there is a need to build an evidence base about what factors correlate with the pace of aging in young adults. Human aging has multifactorial origins. Therefore, to speed the development of novel intervention strategies, it will be necessary to know what factors are creating the variation in young humans’ pace of aging. For example, cohort studies having repeated measures can test if within-individual change in the longitudinal pace of aging tracks temporally alongside change in behavioral correlates (eg, if depression remits, or physical activity improves, does the pace of aging slow?).
Fourth, there is a need to test longitudinal repeated measures of the pace of aging against cross-sectional genomic measures now being put forward, often referred to as genomic clocks (55–58). Are these various measurements strongly correlated with each other and do they tap the same aging process? If so, genomic clocks ought to predict: (a) each other, (b) chronological age, (c) mortality, (d) cognitive and physical function, and (e) deterioration in multiple organ systems over meaningful time spans: that is, the measured pace of aging. The genomic research agenda could also include studies that interrogate the pace of aging as a phenotype. Genomic studies can search for profiles of gene methylation and expression of the pace of aging, not just chronological age, and genome-wide association studies can study the pace of aging, in addition to extreme longevity.
Fifth, because delayed aging is not necessarily the flipside of accelerated aging, researchers should additionally ask: What genomes and lifestyles characterize young adults who stay biologically young while their peers age? With good measurement of the pace of aging, such unusually resilient individuals can be identified for research while they are still young. Study of such individuals may reveal molecular and behavioral pathways to rejuvenation.
Sixth, there is a need to develop measures of the pace of aging that are feasible for use as baseline and outcome criteria in future randomized clinical trials that will try to slow aging in humans. Pace of aging measures need to be good surrogates for late-life disease and mortality, but feasible for use with trial participants for whom disease and death are far in the future. Practical measures of how fast a young clinical trial participant is aging are needed to show which treatments work, and which do not, and for whom. For example, participants already aging slowly may have little room to improve in a therapeutic trial, whereas those aging most rapidly might be treatment resistant.
Why Should Young People’s Pace of Aging Be on Everyone’s Mind?
Currently, Western people live relatively healthy lives until their 60s, when morbidity starts to accumulate until death (59). A nightmare scenario for population health is that death will be postponed to older and older ages, but morbidity will not (21). The full nightmare will probably not happen, because there is evidence that health span has been extending (60). Despite this evidence for compression of morbidity into older age groups, already the Global Burden of Disease Project found that compared with people in 1990 those in 2010 were living more years with disability from age-related conditions such as heart disease, type 2 diabetes, stroke, pulmonary disease, and dementia (1). Treating these unprevented diseases in late life has proven costly and largely ineffective, and consequently, effective strategies are needed to prevent age-related diseases before they onset. The goal, therefore, is not only to increase life expectancy but to ensure that added years at the end of life are healthy years of living and to improve the quality of longer lives (2). To achieve this goal, morbidity needs to be postponed closer to death, that is, aging research must enable still-healthy young people to age more slowly and stay young longer. It is now accepted that factors in early life lead to age-related disease in later life but there is still a knowledge gap about the process of aging in between, and filling it will inform primary prevention. Studying aging as an antecedent cause of disease will become possible if researchers can quantify differences among healthy individuals in their pace of aging. Moreover, the only way intervention researchers will know if trials have succeeded (or failed) to slow aging in young people is if they can accurately measure each young person’s pace of aging. Yet, most human geroscience omits young people. We look forward to a geroscience of the young.
Funding
This work was supported by the U.S. National Institute on Aging (NIA; grants AG032282, AG048895, and AG049789); UK Medical Research Council (grant MR/K00381X); and ESRC (grant ES/M010309/1). Additional support was provided by the Jacobs Foundation. D.W.B. received support from the NIA through P30 AG028716. R.P. was supported by the NZ Health Research Council and New Zealand Ministry of Business, Innovation and Employment (MBIE).
Conflict of Interest
The authors report no conflict of interest.
References
- 1. Murray CJ, Atkinson C, Bhalla K, et al. The state of US health, 1990–2010: burden of diseases, injuries, and risk factors. JAMA. 2013;310:591–608. doi:10.1001/jama.2013.13805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Burch JB, Augustine AD, Frieden LA, et al. Advances in geroscience: impact on healthspan and chronic disease. J Gerontol A Biol Sci Med Sci. 2014;69(suppl 1):S1–S3. doi:10.1093/gerona/glu041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. de Cabo R, Carmona-Gutierrez D, Bernier M, Hall MN, Madeo F. The search for antiaging interventions: from elixirs to fasting regimens. Cell. 2014;157:1515–1526. doi:10.1016/j.cell.2014.05.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Longo VD, Antebi A, Bartke A, et al. Interventions to slow aging in humans: Are we ready? Aging Cell. 2015;14:497–510. doi:10.1111/acel.12338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fontana L, Kennedy BK, Longo VD, Seals D, Melov S. Medical research: treat ageing. Nature. 2014;511:405–407. doi:10.1038/511405a [DOI] [PubMed] [Google Scholar]
- 6. Hayden E. Anti-ageing pill pushed as bona fide drug. Nature. 2015;522:265–266. doi:10.1038/522265a [DOI] [PubMed] [Google Scholar]
- 7. Barker DJ, Winter PD, Osmond C, Margetts B, Simmonds SJ. Weight in infancy and death from ischaemic heart disease. Lancet. 1989;2:577–580. [DOI] [PubMed] [Google Scholar]
- 8. Calvin CM, Deary IJ, Fenton C, et al. Intelligence in youth and all-cause-mortality: systematic review with meta-analysis. Int J Epidemiol. 2011;40:626–644. doi:10.1093/ije/dyq190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Felitti VJ, Anda RF, Nordenberg D, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998;14:245–258. [DOI] [PubMed] [Google Scholar]
- 10. Brooks-Wilson AR. Genetics of healthy aging and longevity. Hum Genet. 2013;132:1323–1338. doi:10.1007/s00439-013-1342-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Barzilai N, Guarente L, Kirkwood TBL, et al. The place of genetics in ageing research. Nat Rev Genet. 2012;8:589–594. doi:10.1038/nrg3290 [DOI] [PubMed] [Google Scholar]
- 12. Kuh D, Cooper R, Richards M, Ben-Shlomo Y, eds. A Life-course Approach to Healthy Ageing. Oxford: Oxford University Press; 2014. [Google Scholar]
- 13. Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi:10.1016/j.cell.2013.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kaeberlein M. Longevity and aging. F1000 Prime Reports. 2013;5:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mirzaei H, Suarez JA, Longo VD. Protein and amino acid restriction, aging and disease: from yeast to humans. Trends Endocrinol Metab. 2014;25:558–566. doi:10.1016/j.tem.2014.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kennedy BK. NIH Conference on Advances in Geroscience: Impact on Healthspan and Chronic Disease; October 21, 2013; National Institutes of Health, Bethesda, MD. [Google Scholar]
- 17. Belsky DW, Caspi A, Houts R, et al. Quantification of biological aging in young adults. Proc Natl Acad Sci USA. 2015;112:E4104–E4110. doi:10.1073/pnas.1506264112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hayflick L. The future of ageing. Nature. 2000;408:267–269. [DOI] [PubMed] [Google Scholar]
- 19. Kirkland JL. Translating advances from the basic biology of aging into clinical application. Exp Gerontol. 2013;48:1–5. doi:10.1016/j.exger.2012.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pitt JN, Kaeberlein M. Why is aging conserved and what can we do about it? PLoS Biol. 2015;13:e1002131. doi:10.1371/journal.pbio.1002131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guarente L. Aging research—where to we stand and where are we going? Cell. 2014;159:15–19. doi:10.1016/j.cell.2014.08.041 [DOI] [PubMed] [Google Scholar]
- 22. Hill PL, Turiano NA, Spiro A, 3rd, Mroczek DK. Understanding inter-individual variability in purpose: longitudinal findings from the VA Normative Aging Study. Psychol Aging. 2015;30:529–533. doi:10.1037/pag0000020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kwan CM, Love GD, Ryff CD, Essex MJ. The role of self-enhancing evaluations in a successful life transition. Psychol Aging. 2003;18:3–12. [DOI] [PubMed] [Google Scholar]
- 24. Munoz E, Sliwinski MJ, Scott SB, Hofer S. Global perceived stress predicts cognitive change among older adults. Psychol Aging. 2015;30:487–499. doi:10.1037/pag0000036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ziegler M, Cengia A, Mussel P, Gerstorf D. Openness as a buffer against cognitive decline: the Openness-Fluid-Crystallized-Intelligence (OFCI) model applied to late adulthood. Psychol Aging. 2015;30:573–588. doi:10.1037/a0039493 [DOI] [PubMed] [Google Scholar]
- 26. Kennedy BK, Berger SL, Brunet A, et al. Geroscience: linking aging to chronic disease. Cell. 2014;159:709–713. doi:10.1016/j.cell.2014.10.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Finch CE, Crimmins EM. Inflammatory exposure and historical changes in human life-spans. Science. 2004;305:1736–1739. doi:10.1126/ science.1092556 [DOI] [PubMed] [Google Scholar]
- 28. Gavrilov LA, Gavrilova NS. Early-life programming of aging and longevity: the idea of high initial damage load (the HIDL hypothesis). Ann NY Acad Sci. 2004;1019:496–501. doi:10.1196/annals.1297.091 [DOI] [PubMed] [Google Scholar]
- 29. Gillman MW. Developmental origins of health and disease. New Engl J Med. 2005;353:1848–1850. doi:10.1056/NEJMe058187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kirkwood TBL, Austad SN. Why do we age? Nature. 2000;408:233–238. doi:10.1038/35041682 [DOI] [PubMed] [Google Scholar]
- 31. Sayer AA, Cooper C, Evans JR, et al. Are rates of ageing determined in utero? Age Ageing. 1998;27:579–583. [DOI] [PubMed] [Google Scholar]
- 32. Power C, Kuh D, Morton S. From developmental origins of adult disease to life course research on adult disease and aging: insights from birth cohort studies. Annu Rev Public Health. 2013;34:7–28. doi:10.1146/annurev-publhealth-031912-114423 [DOI] [PubMed] [Google Scholar]
- 33. Brayne C. The elephant in the room - healthy brains in later life, epidemiology and public health. Nat Rev Neurosci. 2007;8:233–239. doi:10.1038/nrn2091 [DOI] [PubMed] [Google Scholar]
- 34. Heyn H, Li N, Ferreira HJ, et al. Distinct DNA methylomes of newborns and centenarians. Proc Natl Acad Sci USA. 2012;109:10522–10527. doi:10.1073/pnas.1120658109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jackson SHD, Weale MR, Weale RA. Biological age—what is it and can it be measured? Arch Gerontol Geriatr. 2003;36:103–115. [DOI] [PubMed] [Google Scholar]
- 36. Levine ME. Response to Dr. Mitnitski’s and Dr. Rockwood’s Letter to the Editor: Biological age revisited. J Gerontol A Biol Sci Med Sci. 2014;69:297–298. doi:10.1093/gerona/glt138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mitnitski A, Howlett SA, Rockwood K. Heterogeneity of human aging and its assessment. J Gerontol A Biol Sci Med Sci. 2016. doi:10.1093/gerona/glw089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Johnson TE. Recent results: biomarkers of aging. Exp Gerontol. 2006;41:1243–1246. [DOI] [PubMed] [Google Scholar]
- 39. Mather KA, Jorm AF, Parslow RA, Christensen H. Is telomere length a biomarker of aging? A review. J Gerontol A Biol Sci Med Sci. 2011;66:202–213. doi:10.1093/gerona/glq180 [DOI] [PubMed] [Google Scholar]
- 40. Simm A, Nass N, Bartling B, et al. Potential biomarkers of ageing. Biol Chem. 2008;389:257–265. doi:10.1515/BC.2008.034 [DOI] [PubMed] [Google Scholar]
- 41. Sprott RL. Biomarkers of aging and disease: introduction and definitions. Exp Gerontol. 2010;45:2–4. doi:10.1016/j.exger.2009.07.008 [DOI] [PubMed] [Google Scholar]
- 42. Cohen AA, Milot E, Li Q, et al. Detection of a novel, integrative aging process suggests complex physiological integration. PLoS One. 2015;10: e0116489. doi:10.1371/journal.pone.0116489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gruenewald TL, Seeman TE, Ryff CD, Karlamangla AS, Singer BH. Combinations of biomarkers predictive of later life mortality. Proc Natl Acad Sci USA. 2006;103:14158–14163. doi:10.1073/pnas.0606215103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Klemera P, Doubal S. A new approach to the concept and computation of biological age. Mech Ageing Dev. 2006;127:240–248. doi:10.1016/j.mad.2005.10.004 [DOI] [PubMed] [Google Scholar]
- 45. Lara J, Cooper R, Nissan J, et al. A proposed panel of biomarkers of healthy ageing. BMC Med. 2015;13:222. doi:10.1186/s12916-015-0470-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rosso AL, Sanders JL, Arnold AM, et al. Multisystem physiologic impairments and changes in gait speed of older adults. J Gerontol A Biol Sci Med Sci. 2015;70:319–324. doi:10.1093/gerona/glu176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhang WB, Pincus Z. Predicting all-cause mortality from basic physiology in the Framingham Heart Study. Aging Cell. 2016;15:39–48. doi:10.1111/acel.12408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Poulton R, Moffitt TE, Silva PA. The Dunedin Multidisciplinary Health and Development Study: overview of the first 40 years, with an eye to the future. Soc Psychiatry Psychiatr Epidemiol. 2015;50:679–693. doi:10.1007/s00127-015-1048-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Belsky DW. Reply to Newman: quantification of biological aging in young adults is not the same thing as the onset of obesity. Proc Natl Acad Sci USA. 2015;112:E7164–E7165. doi:10.1073/pnas.1518878112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Levine ME. Modeling the rate of senescence: can estimated biological age predict mortality more accurately than chronological age? J Gerontol A Biol Sci Med Sci. 2013;68:667–674. doi:10.1093/gerona/gls233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Willett JB, Singer JD, Martin NC. The design and analysis of longitudinal studies of development and psychopathology in context: statistical models and methodological recommendations. Dev Psychopathol. 1998;10:395–426. [DOI] [PubMed] [Google Scholar]
- 52. Glei DA, Goldman N, Rodriguez G, Weinstein M. Beyond self-reports: changes in biomarkers as predictors of mortality. Popul Dev Rev. 2014;40:331–360. doi:10.1111/j.1728-4457.2014.00676.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wills AK, Lawlor DA, Matthews FE, et al. Life course trajectories of systolic blood pressure using longitudinal data from eight UK cohorts. PLoS Med. 2011;8:e1000440. doi:10.1371/journal.pmed.1000440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lixie E, Edgeworth J, Shamir L. Comprehensive analysis of large sets of age-related physiological indicators reveals rapid aging around the age of 55 years. Gerontology. 2015;61:526–533. doi:10.1159/000381584 [DOI] [PubMed] [Google Scholar]
- 55. Horvath S. DNA methylation age of human tissues and cell types. Genome Biol. 2013;14:R115. doi:10.1186/gb-2013-14-10-r115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hannum G, Guinney J, Zhao L, et al. Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol Cell. 2013;49:359–367. doi:10.1016/j.molcel.2012.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Peters MJ, Joehanes R, Pilling LC, et al. The transcriptional landscape of age in human peripheral blood. Nat Commun. 2015;6:8570. doi:10.1038/ncomms9570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sood S, Gallagher IJ, Lunnon K, et al. A novel multi-tissue RNA diagnostic of healthy ageing relates to cognitive health status. Genome Biol. 2015;16:185. doi:10.1186/s13059-015-0750-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mitnitski A, Rockwood K. The rate of aging: the rate of deficit accumulation does not change over the adult life span. Biogerontology. 2016;17:199–204. doi:10.1007/s10522-015-9583-y [DOI] [PubMed] [Google Scholar]
- 60. Crimmins EM. Trends in the health of the elderly. Annu Rev Public Health. 2004;25:79–98. doi:10.1146/annurev.publhealth.25.102802.124401 [DOI] [PubMed] [Google Scholar]