Abstract
Purpose
To report surgical outcomes of microcatheter-assisted trabeculotomy following failed angle surgeries, and compare those with no previous angle surgery, in primary congenital glaucoma (PCG).
Methods
The early postoperative (12 months) results of 42 eyes of 36 patients who underwent microcatheter-assisted trabeculotomy by single surgeon for PCG were retrospectively analyzed. Group 1, 20 eyes of 16 patients, had no previous angle surgery. Group 2, 22 eyes of 20 patients, had one or two previous failed angle surgeries. Success was defined as an intraocular pressure (IOP) <21 mm Hg with at least a 30% reduction from preoperative IOP with (qualified success) or without (complete success) the use of antiglaucoma medication.
Results
Mean IOP decreased from 31.5±7.2 mm Hg on 3 (median, range: 1–5) medications in Group 1 and 34.6±7.3 mm Hg on 3 (median, range: 1–4) medications in Group 2 preoperatively to 15.6±3.1 mm Hg on 0 (median, range: 0–4) medications in Group 1 and 16.0±4.6 mm Hg on 0 (median, range: 0–2) medications in Group 2 postoperatively at 12 months (both P<0.001), respectively. The mean percentage of IOP reduction from preoperative to last postoperative visit was 46.0±20.1% in Group 1 and 45.5±25.0% in Group 2, P=0.947. Qualified and complete successes were comparable between Group 1 and Group 2 (qualified success: 90.0% vs 77.3%, P=0.294; complete success: 78.9% vs 77.3%, P=0.853). Complications were minimal.
Conclusions
Microcatheter-assisted trabeculotomy achieved significant pressure-lowering effects with a reduction in medication use in PCG, and it represents a reasonable choice of initial and repeat surgical treatment for PCG.
Introduction
Trabeculotomy ab externo is the mainstay and initial procedure of choice for the treatment of primary congenital glaucoma (PCG),1, 2, 3, 4 particularly, in children with corneal opacity that prevents adequate visualization of the angle. Rigid probe trabeculotomy has an overall success of ~60–87% after a mean follow-up of 1–3 years.5, 6, 7, 8 Additional trabeculotomies, however, are often needed in eyes with uncontrolled intraocular pressure (IOP) owing to its inability to open the full circumference of the Schlemm's canal with a single incision.9, 10 Previous angle surgeries make subsequent angle surgeries more challenging, as intact area reserved for additional surgery is limited. Beck and Lynch11 first described using 6-0 polypropylene to circumvent the entire 360° of the trabecular meshwork in patients with PCG. Nevertheless, threading a suture around the Schlemm's canal entails a risk of misdirection into the suprachoroidal space12, 13 and it may be even more difficult to advance the suture around the full circumference in eyes with previous angle surgeries.14 Recently, it has been reported that a complete or partial trabeculotomy was safely completed in eyes with childhood glaucoma with the assistance of an illuminated microcatheter, and that it provided significant IOP-lowering effects.15, 16 To date, the feasibility and effect of microcatheter-assisted trabeculotomy in eyes with previous failed angle surgeries have not been reported to our knowledge. In this study, we report surgical outcomes of microcatheter-assisted trabeculotomy in patients with PCG following failed angle surgeries, compared with those with no previous angle surgery.
Subjects and methods
Study design
Our preliminary results on an interim analysis of the first 20 patients suggested PCG patients without previous surgery demonstrated a 90.0% qualified success rate after receiving the microcatheter-assisted trabeculotomy. In our clinic experiences, for those with previous sugeries, the qualified success rate of traditional rigid probe trabeculotomy was only 50%. We estimated an increase of 20% in terms of the qualified success for this novel procedure to treat PCG patients with previous surgeries. With a two-sided test using a level of 0.05 and a power of 80%, the sample size was 18 subjects per arm. On the basis of the above data, clinical data of consecutive case series of eyes undergoing trabeculotomy by single surgeon using an ophthalmic microcatheter (iTRACK 250A; iScience Interventional, Menlo Park, CA, USA) for PCG were collected from January 2014 to May 2015 at Beijing Tongren Eye Center. This retrospective case series study was approved by the Ethics Committee of Beijing Tongren Eye Center and adhered to the tenets of the Declaration of Helsinki, and it was registered under the Chinese Clinical Trials Registry (ChiCTR-OCC-15005789). Each patient's legal guardian or representative signed an informed consent. All patients had complete eye examination with detailed history and were diagnosed with PCG with the following characteristics including isolated trabeculodysgenesis without any other ocular or systemic abnormalities, elevated IOP, increased corneal diameter, corneal edema, increased axial length, and glaucomatous cupping of the optic nerve. IOP was measured at baseline and on follow-up examinations using the Icare tonometer (Icare TA01i, Icare Finland Oy, Espoo, Finland) while the subject was asleep. Patients without previous angle surgeries comprise Group 1 and those who underwent no more than two previous failed angle surgeries (trabeculotomy, trabeculotomy combined with trabeculectomy or goniotomy) comprise Group 2. Patients were further stratified into neonatal PCG (with onset after birth and <1 month), infantile PCG (with onset after 1 month and <2 years of age), and late-onset PCG (with onset after 2 years of age).17 Exclusion criteria included patients who previously had more than two angle surgeries in the candidate eye or underwent other ophthalmic surgery besides aforementioned angle surgeries. An initial examination under anesthesia was performed using an 80 MHz ultrasound biomicroscopy (iUltrasound, iScience Interventional) in all cases to determine suitability for trabeculotomy surgery. Cases with >180° of peripheral anterior synechiae were excluded. If the patients' eyes met the criteria for inclusion, both eyes were included in the analysis.
Surgical procedure and postoperative care
The details have been described previously.15, 16 Briefly, following a conjunctival peritomy, the Schlemm's canal was exposed by either scleral cut down under a superficial scleral flap or direct unroofing via a deep scleral flap. In those with previous surgeries, the site of sclera flap was located laterally to previous flap (or bleb if existed) for at least 60° to avoid the possible damage of Schlemm's canal in previous surgeries. The two ostia of the canal were dilated with high-molecular weight hyaluronic acid (Healon GV, Abbott Vision, Abbott Park, IL, USA), similarly to a viscocanalostomy. Once the Schlemm's canal was entered, the illuminated microcatheter was inserted into the canal and threaded circumferentially with the illuminated tip providing precise location of the catheter. In cases of successful 360° catheterization, the catheter tip will be retrieved from the other ostium, and both exposed ends of the catheter were grasped and pulled in opposite directions like a purse string, thus breaking through the trabecular meshwork and performing the trabeculotomy ab externo. In some cases, the microcatheter would reach an obstruction or become misdirected. In these cases, the conjunctiva was incised and a scleral cut down was performed over the illuminated catheter tip, which was retrieved at the point of obstruction. Both exposed ends of the catheter were then grasped and pulled in opposite directions like a purse string performing a partial trabeculotomy. In all cases, the scleral flap was then closed with interrupted 10-0 nylon sutures and the conjunctiva was approximated with 8-0 vicryl sutures. Gentle anterior chamber irrigation was performed via a paracentesis for rare occasions of significant hyphema. Postoperatively, tobramycin/dexamethasone (TobraDex, Alcon, Rijksweg, Belgium) 4 times per day were used initially (tapering over 2–4 weeks), along with Panoprofen (Pranopulin, Senju Pharmaceutical Co., Ltd, Osaka, Japan) 4 times daily for ~4 weeks and pilocarpine 2% (Bausch & Lomb, Rochester, NY, USA) solution 3 times daily for 1 to 3 months. We routinely use pilocarpine for at least 1 month to prevent the development of peripheral anterior synechiae after trabeculotomy regardless of the IOP and it was not considered as an IOP-lowering medication during this time. If the IOP was higher than 21 mm Hg after 1 month, the pilocarpine was continued as an IOP-lowering medication. To prevent the side effects of the long-term use of pilocarpine, the length of treatment did not exceed 3 months.
Outcome measures and statistical analysis
Clinical charts and operative reports were reviewed and the following information was recorded: demographics, duration of follow-up, age at the time of surgery, onset of the disease, preoperative diagnosis and prior surgeries, horizontal corneal diameter, the extent of successful trabeculotomy (degree), interoperative and postoperative complications, IOP, and medications at each follow-up visit. All patients were followed up at 1, 3, 6, 9, and 12 months post operation. Demographic and clinical characteristics between the groups were compared using the Student's t-test and χ2-test. Repeated-measures analysis of variance with Bonferroni adjustment was used to compare IOP and medications from preoperative baseline to 12 months between two groups. The median number of medication reduction, follow-up duration, and degree of angle with successful trabeculotomy were compared between two groups using the nonparametric Mann–Whitney U-test.
Success was defined as an IOP <21 mm Hg with at least a 30% reduction from preoperative pressure levels with (qualified success) or without (complete success) the use of antiglaucoma medication. Kaplan–Meier survival functions were constructed for complete and qualified success, and compared between groups using a robust variance estimator to account for the clustering of eyes within patients. Eyes undergoing a secondary surgical procedure were defined as failure and excluded from analysis subsequent to the intervention. The mean percentage of IOP reduction from preoperative to last postoperative visit (ie, at the last follow-up visit or when failure criteria were reached) were compared between groups using a mixed statistical model to account for the clustering of eyes within a given patient, and were compared between eyes with complete 360° trabeculotomy and partial trabeculotomy using the Mann–Whitney U-test. Correlation analysis was carried out by Spearman correlation test. A P-value of <0.05 (two-sided) was considered significant. All statistical tests were performed using the software SPSS 16.0 (SPSS, Inc., Chicago, IL, USA).
Results
Demographics and preoperative parameters
We enrolled 20 eyes of 16 patients without previous angle surgeries (Group 1) and 22 eyes of 20 patients with previous failed angle surgeries (Group 2). All 42 eyes of 36 patients were followed for a minimum of 6 months and the median follow-up duration was 12 (range: 6–12) months in both groups. No statistical differences were found with respect to gender (P=0.095), horizontal corneal diameter (P=0.907), cup to disc ratio (P=0.680), number of eyes with cloudy cornea (P=0.569), and follow-up duration (P=0.513) between the two study groups (Table 1). Disk assessment at baseline was only available on 25 eyes owing to corneal clouding. It is worth noting that the average age at the time of surgery was older in Group 2 (48.5±29.8, range: 4–108 months) than in Group 1 (33.5±32.2, range: 3–96 months), although the difference was not statistically significant (P=0.125). In Group 2, 16 eyes (72.7%) had 1 previous surgery (5 eyes with trabeculotomy, 11 eyes with trabeculotomy combined with trabeculectomy) and 6 eyes (27.3%) had 2 previous surgeries (either trabeculotomy or trabeculotomy combined with trabeculectomy). No bleb was presented in any of our patients.
Table 1. Demographic and preoperative parameters of children with childhood glaucoma having microcatheter-assisted trabeculotomy.
| Parameter | Total | Cases without previous surgery history | Cases with previous surgery history | P |
|---|---|---|---|---|
| Patients | 36 | 16 | 20 | |
| Number of eyes | 42 | 20 | 22 | 0.758a |
| Average age, months, mean±SD (range) | 41.4±31.5 (3–108) | 33.5±32.2 (3–96) | 48.5±29.8 (4–108) | 0.125b |
| Sex, number of patients | ||||
| Male | 24 | 13 | 11 | 0.095c |
| Female | 12 | 3 | 9 | |
| Diagnosis by eye, n | ||||
| Neonatal PCG (onset: birth–1 month) | 9 | 3 | 6 | 0.553a |
| Infantile PCG (onset: 1 month–2 years) | 24 | 13 | 11 | |
| Late-onset PCG (onset: >2 years) | 9 | 4 | 5 | |
| Horizontal corneal diameter, mm, mean±SD | 12.8±1.1 | 12.8±1.1 | 12.8±1.1 | 0.907b |
| Cup to disc ratio | 0.78±0.12 | 0.79±0.06 | 0.77±0.15 | 0.680b |
| Cloudy cornea, n (%) | 25 (59.5) | 11 (55.0) | 14 (63.6) | 0.569a |
| Follow-up duration, months, median (range) | 12 (6–12) | 12 (6–12) | 12 (6–12) | 0.513d |
| Average previous surgeries, mean±SD | — | — | 1.3±0.5 | — |
| 1 n(%) | 16 (72.7) | |||
| 2 n(%) | 6 (27.3) | |||
| Preoperative IOP, mean±SD | 33.1±7.4 | 31.5±7.2 | 34.6±7.3 | 0.171b |
| Preoperative number of medications, mean±SD (median, range) | 2.7±0.9 (3, 1–4) | 2.7±1.1 (3, 1–5) | 2.7±0.8 (3, 1–4) | 0.958d |
Abbreviation: n, number of eyes.
χ2-test.
Independent-samples t-test.
Fisher's exact test.
Mann–Whitney U-test.
Degree of trabeculotomy with either microcatheter or trabeculotome
Schlemm's canal was cannulated with the catheter, either completely or partially, in all cases. More eyes in Group 1 (15 eyes, 75.0%) than in Group 2 (4 eyes, 18.2%) had complete 360° trabeculotomy. More eyes in Group 2 (18 eyes, 81.8%) than in Group 1 (5 eyes, 25.0%) had partial trabeculotomy with microcatheter. Among the five eyes in Group 1, three were neonatal, one was infantile, and one was late-onset PCG. Among the 18 eyes in Group 2, six were neonatal PCG and three of them had two previous surgeries, 10 were infantile PCG, and three of them had two previous surgeries, and two were late-onset PCG with one previous surgery. The median degree of successful trabeculotomy was significantly higher in Group 1 (360, range: 120–360) than in Group 2 (195, range: 120–360, P=0.007).
Change in IOP and antiglaucoma medication usage
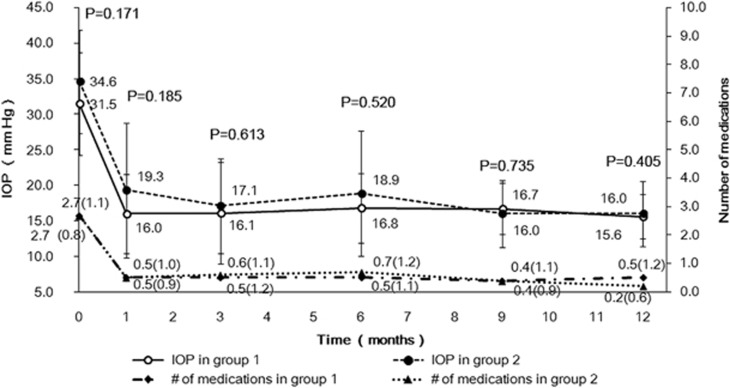
Mean IOP decreased from 31.5±7.2 mm Hg in Group 1 and 34.6±7.3 mm Hg in Group 2 preoperatively to 15.6±3.1 mm Hg (P<0.001) and 16.0±4.6 mm Hg (P<0.001) postoperatively at 12 months, respectively. Median number of medications decreased from 3 (range: 1–5) in Group 1 and 3 (range: 1–4) in Group 2 preoperatively to 0 (range: 0–4; P=0.002) in Group 1 and 0 (range: 0–2; P<0.001) in Group 2 postoperatively at 12 months. In both groups, IOP and medication use were significantly reduced from baseline at all postoperative visits (both P<0.001, repeated-measures analysis). Multivariable repeated-measures analysis of variance controlling for age (P=0.571), age of onset (P=0.963), horizontal corneal diameter (P=0.258), and preoperative IOP (P=0.530) was performed to adjust for possible confounding factors or imbalances, as well as the influences of these 4 covariates on IOP. This revealed no significant differences in IOP and the mean number of medications between the two groups at any postoperative time point (P>0.05, Figure 1). The mean percentage of IOP reduction from preoperative to last postoperative visit was similar between both groups (46.0±20.1% in Group 1 and 45.5±25.0% in Group 2; P=0.947). The median number of medications decreased from 3 (range: 1–5) in Group 1 and 3 (1–4) in Group 2 preoperatively to 0 (range: 0–4) at last postoperative visit in both groups with no difference between the two groups (P=0.792).
Figure 1.
Comparison of mean IOP and number of medications between eyes without previous angle surgery (Group 1) and eyes with previous angle surgeries (Group 2) after microcatheter-assisted trabeculotomy for pediatric glaucoma over 12-month follow-up. No significant differences were found between the two groups at any time point. Number of medications: mean (SD).
The mean percentage of IOP reduction was not significantly different between eyes with complete 360° trabeculotomy (46.0±20.1%, mean±SD) and partial trabeculotomy with microcatheter (45.5±25.0%, mean±SD, P=0.947), and it had a positive correlation with the extent of trabeculotomy, but this was not statistically significant (r=0.179, P=0.256; Spearman rank correlation).
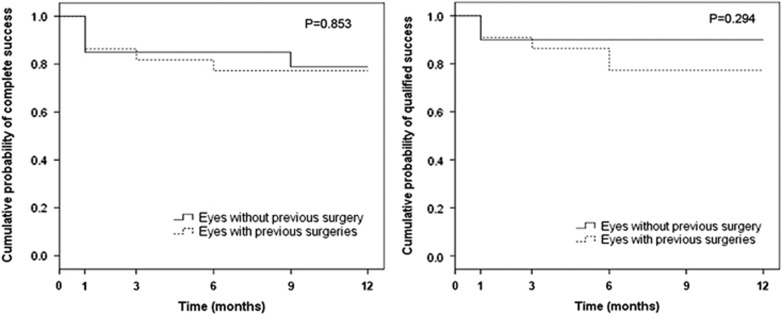
Success rate
Table 2 shows the cumulative success rate at various postoperative time points and Figure 2 shows the Kaplan–Meier survival plots. At 12-month follow-up, Group 1 achieved a 78.9% complete and 90.0% qualified success rate, whereas Group 2 achieved 77.3% and 77.3%, respectively. There was no statistical difference between the two groups (complete success: P=0.853, Figure 2, left; qualified success: P=0.294, Figure 2, right). In Group 2, one eye underwent transscleral diode laser cyclophotocoagulation at 9-month follow-up, hence was considered a failure and censored from analysis subsequent to the intervention. Even in those eyes with partial trabeculotomy with microcatheter, 4 out of 5 eyes in Group 1 and 13 out of 18 eyes in Group 2 had successful control of IOP (<21 mm Hg with or without medications) with mean IOP reduction of 45.6±20.1% in Group 1 and 55.1±12.1% in Group 2 at last follow-up.
Table 2. The cumulative success rate of microcatheter-assisted trabeculotomy in childhood glaucoma.
| Follow-up |
Complete success (%) |
Qualified success (%) |
N(eyes) |
|||
|---|---|---|---|---|---|---|
| Group 1 | Group 2 | Group 1 | Group 2 | Group 1 | Group 2 | |
| 1 month | 85.0 | 86.4 | 90.0 | 84.2 | 20 | 22 |
| 3 months | 85.0 | 81.8 | 90.0 | 90.9 | 20 | 22 |
| 6 months | 85.0 | 77.3 | 90.0 | 86.4 | 20 | 22 |
| 9 months | 78.9 | 77.3 | 90.0 | 77.3 | 16 | 21 |
| 12 months | 78.9 | 77.3 | 90.0 | 77.3 | 14 | 17 |
Group 1: eyes without previous angle surgery; Group 2: eyes with previous angle surgeries. Success was defined as an intraocular pressure (IOP) <21 mm Hg with at least a 30% reduction from preoperative pressure levels with (qualified success) or without (complete success) the use of antiglaucoma medication. Eyes undergoing a secondary surgical procedure were classified as failures and censored from analysis subsequent to the intervention.
Figure 2.
Kaplan–Meier survival plot of the cumulative probability of success for eyes without previous angle surgery (Group 1) and eyes with previous angle surgeries (Group 2) after microcatheter-assisted trabeculotomy for pediatric glaucoma over 12 months. Success was defined as an IOP <21 mm Hg with at least a 30% reduction from preoperative pressure levels with (qualified success) or without (complete success) the use of antiglaucoma medication. Eyes undergoing a secondary surgical procedure were classified as failures and censored from analysis subsequent to the intervention. The differences between groups were analyzed by the log-rank test.
Complications and secondary procedures
Blood reflux was noted intraoperatively and minimal to small hyphema was seen on the first postoperative day in all subjects. Two subjects in Group 2 had small amounts of residual blood in the anterior chamber at their 1-week postoperative assessment. At the 1-month follow-up examination, no patient in either group had any evident hyphema. No cases of iris tear, Descemet's tears, choroidal detachment, or persistent hypotony were noted.
Discussion
Despite the reported high success rate (80–90%) of trabeculotomy in PCG, Ikeda et al9 reported that to achieve successful control of IOP, 59.8% eyes required 1 trabeculotomy, 18.8% required 2 trabeculotomies, and 7.1% required 3 trabeculotomies, and some even required trabeculectomy (2.7%) or cyclocryotherapy (0.9%) in addition to the trabeculotomies. Given the relatively high success rate and low incidence of intra and postoperative complications of trabeculotomy, as compared with mitomycin C-augmented trabeculectomy, aqueous shunts, and cyclodestructive surgery,8, 10 two or three trabeculotomies may be performed in eyes with uncontrolled IOPs in our practice, similar to what was reported by others.9, 18, 19, 20 Although these cases are typically considered to have poor prognosis, the previous angle surgeries may make these cases even more challenging, owing to the limited area intact for additional surgery and difficulty accessing the inferior region with the trabeculotome. The main advantage of microcatheter-assisted trabeculotomy is the ability to treat the entire angle to the maximum degree with a single incision, especially the inferior angle for eyes with previous angle surgeries, thereby maximizing IOP-lowering benefit. In this retrospective study, a significant reduction of IOP and medication use was detected in PCG with previous failed angle surgeries treated by microcatheter-assisted trabeculotomy. Although the degree of Schlemm's canal canalized by the microcatheter was less in these eyes than that in eyes undergoing this procedure as an initial intervention owing to previous surgical destruction of Schlemm's canal, the IOP-lowing effect was comparable between these two groups (77.3% vs 90.0% qualified success rate, 77.3% vs 78.9% complete success rate for eye with vs without previous angle surgery). The mean percentage of IOP reduction was not significantly different between eyes with complete 360° trabeculotomy (46.0±20.1%, mean±SD) and partial trabeculotomy with microcatheter (45.5±25.0%, mean±SD, P=0.947). Segmental outflow has been reported in human eyes, at any given time, only a fraction of the outflow pathways are actively involved in aqueous humor drainage21, 22, 23 and only two aqueous veins could account for all of aqueous outflow in normal human eye.24 We then can speculate that the circumferential angle has no need to be opened to 360° to maintain the normal IOP; a partial of 120° or more may be enough for outflow drainage, as approved by the successfully IOP control of the traditional 120° trabeculotomy when the surgery correctly cut open 120° angle. However, limited by our small sample size, this warrants further investigation.
The available surgical repertoire for PCG has remained relatively unchanged for many years with most progress owing to modifications of the existing surgeries. A major advance has been the concept of incising the whole angle (circumferential trabeculotomy) with either suture11, 12, 13, 14 or microcatheter.15, 16 With surgical destruction of the Schlemm's canal and potential severe angle maldevelopment involving Schlemm's canal,19, 25, 26, 27 the microcatheter could not pass 360°. Therefore, it would be encountered in eyes with previous angle surgeries frequently and even in eyes without previous angle surgeries. In cases without previous angle surgeries in our study, complete catheterization was achieved in 15 of 20 (75.0%) PCG eyes with the assistance of the illuminated microcatheter. It is consistent with the success rate reported in literature (67 to 75%).15, 16 Circumferential trabeculotomy however may be difficult, if not impossible, in cases with previous angle surgeries owing to surgical destruction of the Schlemm's canal. In the 22 eyes with previous failed angle surgeries, complete catheterization with the microcatheter was achieved in four eyes, indicative of an intact Schlemm's canal despite previous surgical injuries. This may explain why previous traditional trabeculotomy failed, as the trabeculotome may have been inadvertently or inaccurately placed or advanced.28 The illuminated tip facilitates the placement and advancement of the microcatheter in Schlemm's canal and improves accuracy, making partial trabeculotomy more effective than traditional rigid probe trabeculotomy.29
In this study, the microcatheter-assisted trabeculotomy resulted in no complications other than transient hyphema requiring no intervention in all our patients. With the illuminated tip continuously verifying the catheter location within the Schlemm's canal, the microcatheter improves the safety of 360° trabeculotomy by preventing inadvertent misdirection and tissue disruption.
Our study is limited by the small sample size and relatively short follow-up duration. And the retrospective nature of this study may need further prospective randomized controlled trials to illustrate this issue better.
In conclusion, a flexible microcatheter with an illuminated, atraumatic tip can be successfully used to catheterize Schlemm's canal, and perform partial and full circumferential trabeculotomy for the treatment of PCG. It seems to provide excellent pressure-lowering effect with minimal complications in the early postoperative course (12 months) in PCG with previous angle surgeries, and the effect was comparable to that in eyes that underwent this procedure as an initial intervention. Further research is needed to determine the long-term success of this procedure.
Acknowledgments
We thank Dr Debbie S Kuo of Duke University for her input during the manuscript revision.
The authors declare no conflict of interest.
References
- McPherson SD Jr. Results of external trabeculotomy. Trans Am Ophthalmol Soc 1973; 71: 163–167. [PMC free article] [PubMed] [Google Scholar]
- Quigley HA. Childhood glaucoma: results with trabeculotomy and study of reversible cupping. Ophthalmology 1982; 89(3): 219–226. [DOI] [PubMed] [Google Scholar]
- Luntz MH. The advantages of trabeculotomy over goniotomy. J Pediatr Ophthalmol Strabismus 1984; 21(4): 150–153. [PubMed] [Google Scholar]
- Anderson DR. Trabeculotomy compared to goniotomy for glaucoma in children. Ophthalmology 1983; 90(7): 805–806. [DOI] [PubMed] [Google Scholar]
- Akimoto M, Tanihara H, Negi A, Nagata M. Surgical results of trabeculotomy ab externo for developmental glaucoma. Arch Ophthalmol 1994; 112(12): 1540–1544. [DOI] [PubMed] [Google Scholar]
- Gregersen E, Kessing SV. Congenital glaucoma before and after the introduction of microsurgery. Results of ‘macrosurgery' 1943-1963 and of microsurgery (trabeculotomy/ectomy) 1970-1974. Acta Ophthalmol (Copenh) 1977; 55(3): 422–430. [DOI] [PubMed] [Google Scholar]
- Rohen JW, Harms H, Barany E. Discussion on new methods of glaucoma surgery. Bibl Ophthalmol 1970; 81: 154–160. [PubMed] [Google Scholar]
- Chang TC, Cavuoto KM. Surgical management in primary congenital glaucoma: four debates. J Ophthalmol 2013; 2013: 612708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda H, Ishigooka H, Muto T, Tanihara H, Nagata M. Long-term outcome of trabeculotomy for the treatment of developmental glaucoma. Arch Ophthalmol 2004; 122(8): 1122–1128. [DOI] [PubMed] [Google Scholar]
- Terraciano AJ, Sidoti PA. Management of refractory glaucoma in childhood. Curr Opin Ophthalmol 2002; 13(2): 97–102. [DOI] [PubMed] [Google Scholar]
- Beck AD, Lynch MG. 360 degrees trabeculotomy for primary congenital glaucoma. Arch Ophthalmol 1995; 113(9): 1200–1202. [DOI] [PubMed] [Google Scholar]
- Neely DE. False passage: a complication of 360 degrees suture trabeculotomy. J AAPOS 2005; 9(4): 396–397. [DOI] [PubMed] [Google Scholar]
- Verner-Cole EA, Ortiz S, Bell NP, Bell NP, Feldman RM. Subretinal suture misdirection during 360 degrees suture trabeculotomy. Am J Ophthalmol 2006; 141(2): 391–392. [DOI] [PubMed] [Google Scholar]
- Beck AD, Lynn MJ, Crandall J, Mobin-Uddin O. Surgical outcomes with 360-degree suture trabeculotomy in poor-prognosis primary congenital glaucoma and glaucoma associated with congenital anomalies or cataract surgery. J AAPOS 2011; 15(1): 54–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girkin CA, Rhodes L, McGwin G, Marchase N, Cogen MS. Goniotomy versus circumferential trabeculotomy with an illuminated microcatheter in congenital glaucoma. J AAPOS 2012; 16(5): 424–427. [DOI] [PubMed] [Google Scholar]
- Sarkisian SR Jr. An illuminated microcatheter for 360-degree trabeculotomy [corrected] in congenital glaucoma: a retrospective case series. J AAPOS 2010; 14(5): 412–416. [DOI] [PubMed] [Google Scholar]
- Beck A, Chang TCP, Freedman S. Definition, classification and differential diagnosis in childhood glaucoma. In: Weinreb RN, Grajewski A, Papadopoulos M, Grigg J, Freedman S (eds). WGA Consensus Series – 9. Kugler Publications: Amsterdam, 2013, pp 3–10..
- Shaffer RN. Prognosis of goniotomy in primary infantile glaucoma (trabeculodysgenesis). Trans Am Ophthalmol Soc 1982; 80: 321–325. [PMC free article] [PubMed] [Google Scholar]
- Walton DS, Katsavounidou G. Newborn primary congenital glaucoma: 2005 update. J Pediatr Ophthalmol Strabismus 2005; 42(6): 333–341. [DOI] [PubMed] [Google Scholar]
- Papadopoulos M, Edmunds B, Fenerty C, Khaw PT. Childhood glaucoma surgery in the 21st century. Eye 2014; 28(8): 931–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller KE, Bradley JM, Vranka JA, Acott TS. Segmental versican expression in the trabecular meshwork and involvement in outflow facility. Invest Ophthalmol Vis Sci 2011; 52: 5049–5057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CY, Liu Y, Gong H. Effects of Y27632 on aqueous humor outflow facility with changes in hydrodynamic pattern and morphology in human eyes. Invest Ophthalmol Vis Sci 2013; 54: 5859–5870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hann CR, Fautsch MP. Preferential fluid flow in the human trabecular meshwork near collector channels. Invest Ophthalmol Vis Sci 2009; 50: 1692–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone M, Martin E, Jamil A. Pulsatile flow into the aqueous veins: manifestations in normal and glaucomatous eyes. Exp Eye Res 2011; 92: 318–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry LP, Jakobiec FA, Zakka FR, Walton DS. Newborn primary congenital glaucoma: histopathologic features of the anterior chamber filtration angle. J AAPOS 2012; 16: 565–568. [DOI] [PubMed] [Google Scholar]
- Tawara A, Inomata H. Developmental immaturity of the trabecular meshwork in congenital glaucoma. Am J Ophthalmol 1981; 92: 508–525. [DOI] [PubMed] [Google Scholar]
- Hollander DA, Sarfarazi M, Stoilov I, Wood IS, Fredrick DR, Alvarado JA. Genotype and phenotype correlations in congenital glaucoma: CYP1B1 mutations, goniodysgenesis, and clinical characteristics. Am J Ophthalmol 2006; 142: 993–1004. [DOI] [PubMed] [Google Scholar]
- McPherson Jr SD, McFarland D. External trabeculotomy for developmental glaucoma. Ophthalmology 1980; 87(4): 302–305. [DOI] [PubMed] [Google Scholar]
- Shi Y, Wang H, Yin J, Li M, Zhang X, Xin C et al. Microcatheter-assisted trabeculotomy versus rigid probe trabeculotomy in childhood glaucoma. Br J Ophthalmol 2015; 100(9): 1257–1262. [DOI] [PubMed] [Google Scholar]