Abstract
Purpose
The purpose of the study was to study the effect of an organic light-emitting diode sleep mask on daytime alertness, wellbeing, and retinal structure/function in healthy volunteers and in diabetic macular oedema (DMO).
Patients and methods
Healthy volunteers in two groups, 18–30 yrs (A), 50–70 yrs (B) and people with DMO (C) wore masks (504 nm wavelength; 80 cd/m2 luminance; ≤8 h) nightly for 3 months followed by a 1-month recovery period. Changes from baseline were measured for (means): psychomotor vigilance task (PVT) (number of lapses (NL), response time (RT)), sleep, depression, psychological wellbeing (PW), visual acuity, contrast sensitivity, colour, electrophysiology, microperimetry, and retinal thickness on OCT.
Results
Of 60 participants, 16 (27%) withdrew, 8 (13%) before month 1, due to sleep disturbances and mask intolerance. About 36/55 (65%) who continued beyond month 1 reported ≥1 adverse event. At month 3 mean PVT worsened in Group A (RT (7.65%, P<0.001), NL (43.3%, P=0.005)) and mean PW worsened in all groups (A 28.0%, P=0.01, B 21.2%, P=0.03, C 12.8%, P<0.05). No other clinically significant safety signal was detected. Cysts reduced/resolved in the OCT subfield of maximal pathology in 67% Group C eyes. Thinning was greater at 3 and 4 months for greater baseline thickness (central subfield P<0.001, maximal P<0.05).
Conclusion
Sleep masks showed no major safety signal apart from a small impairment of daytime alertness and a moderate effect on wellbeing. Masks were acceptable apart from in some healthy participants. Preliminary data suggest a beneficial effect on retinal thickness in DMO. This novel therapeutic approach is ready for large clinical trials.
Introduction
The increased oxygen demand in the outer retina in the dark, due mostly to the 140 million rods that double their oxygen intake in the dark adapted eye during sleep, may contribute to intraretinal hypoxic drive in a range of retinal vasculopathies including diabetic retinopathy (DR) leading to upregulation of the VEGF pathway.1 If dark adaptation could be prevented or reduced, the reduction of intraretinal hypoxia may diminish the progression of DR and its complications.2
Light is a novel approach to modifying the hypoxia associated with dark adaptation.3, 4 Sleeping in an illuminated environment with light delivered through the closed lid has been tested in two small studies as a potential therapy for diabetic maculopathy.5, 6 Before light therapy can be more widely considered robust evidence on safety is required.
We developed a sleep mask containing a thin, flexible, organic light-emitting diode (OLED) to suppress dark adaptation therapeutically. We investigated if long-term nocturnal light exposure with this device could lead to sleep deprivation and effects on daytime alertness, psychological wellbeing, and retinal structure and function. We also studied safety in people with diabetic macular oedema (DMO).
Materials and methods
Healthy adult volunteers with good general health from two age groups (Group A: aged 18–30 years; Group B: aged 50–70 years) were consented and recruited into a single-centre, prospective, longitudinal, non-commercial, interventional safety study with a 3-month dosing period followed by a 1-month post dosing assessment.
Key exclusion criteria were: disease that might affect the blood–retina barrier, unstable fixation on microperimetry, history of sleep disorders, depression or psychiatric disorders, psychomotor vigilance task (PVT), number of lapses (NL) ≥17 (see below), and use of psychoactive drugs.
The study complied with the Declaration of Helsinki was approved by the National Research Ethics Committee (13/WM/0011) and the Institutional Technical Devices Committee as a device study with a CE-marked device. It complied with the Declaration of Helsinki.
People attending for monitoring of DMO were assessed (Group C). Additional inclusion criteria: best corrected visual acuity (BCVA) ≥73 ETDRS letters, retinopathy ≤ETDRS grade 47, DMO meeting definition of clinically significant macular oedema, mean subfield thickness ≥2 SD in any central five OCT subfields.
OLED sleep masks developed by Polyphotonix Medical Ltd (Figure 1) comprised a soft cushioned fabric mask containing a plastic ‘Pod' containing the light sources emitting light into the eyes through closed lids. The OLED spectrum with a peak of 504 nm was designed to closely match the scotopic response curve for selective activation of rods and to deliver a light intensity of 2 scotopic Trolands at the retina to suppress dark adaptation.6 Touching a capacitive sensor activated the mask for a maximum 8 h controlled by an internal clock with delivered dose recorded by internal sensors (hours of wear/month).
Figure 1.
Principal components of OLED sleep mask. Left panel: plastic pod containing OLED light source delivering light at 504 nm peak for a maximum of 8 h with dose delivery sensor. Right panel: soft cushioned fabric mask that houses the plastic pod.
After training, participants were instructed to wear the mask each night for 3 months and record sleep times and experiences in a diary. Participants attended monthly for 4 months and were replaced if they withdrew before completing the month 1 visit. A small honorarium and reimbursement of expenses were provided.
Study procedures (all visits)
The PVT measured loss of concentration and alertness caused by sleep deprivation as increasing response time (ms) (PVT-RT) and NL (failure to respond) (PVT-NL). Using normative data we derived upper limits of normal (mean+2 SD): NL≤16; RT≤459, ≤359.7 The Karolinska Sleepiness Scale (KSS) (range 1–9 (worst))8 and the Pittsburgh Sleep Quality Index (PSQI) (range 0–21 (worst))9 questionnaires assessed level of sleepiness and sleep quality. PVT-NL, PVT-RT, KSS, and PSQI served as co-primary outcomes.
Self-reported symptoms of depression were assessed using the Centre for Epidemiologic Studies Depression scale (CESD) (abnormal ≥16)10 and psychological wellbeing using the General Health Questionnaire (GHQ12) (range 0–36, >15 evidence of distress, >20 severe distress).11
The following were also assessed: medical and ocular history, ophthalmological examination, BCVA (ETDRS letters at 1 m), Pelli Robson contrast sensitivity (CS), colour vision Cambridge colour test ((CCT); Cambridge Research Systems (Rochester, Kent, UK), normal thresholds <100 × 10−4 u'v' protan and deutan, <150 × 10−4 u'v' tritan12) 19-segment multifocal electroretinogram13 (mfERG, Roland Retiscan, Brandenburg an der Havel, Germany), electrooculogram (EOG), microperimetry (MP, Nidek MP1, Padova, Italy), mean central subfield thickness on spectral domain optical coherence tomography (SD-OCT). For Group C mean thickness was also recorded for subfield with maximal OCT pathology with a qualitative assessment of response to therapy.
Adverse events including discomfort, sleep disturbance, mood alterations, daytime wakefulness, and reasons for withdrawal were recorded and reviewed independently for relatedness (SPH, TG).
Statistical analysis
Groups of 20 were selected for the healthy cohort based on accepted guidance for paired t-tests in the lack of previously published data. Demographics and baseline variables were compared across groups using two-sided 2-sample t-test where variances were equal; if the unequal variances t-test was used, then this was reported in the results.
Analysis used STATA 13.1 (StataCorp LP, Timberlake, Richmond, UK) with log-transformations for non-normal distributions and linear regression where the dependent variable was change from baseline to 3 (primary analysis) and 4 months with hours of mask wear as a covariate (centred by average). The resultant regression intercept represented the mean change from baseline to month 3 (or 4) for the average amount of mask wear.
A 2-step method adjusted values of α for multiple comparisons of change of our four co-primary variables only: (1) standard Bonferroni for a family-wise error rate (change from baseline to month 3 in each of four co-primary outcomes) equivalent to 0.05: 0.05/4=0.013 (2) Holm–Bonferroni to compare ordered P-values for the two hypotheses in each group (Groups A and B) giving corrected significance levels of 0.013 and 0.0063. P-values are presented uncorrected and interpreted against these revised values of α.
Results
About 45 healthy volunteers were recruited, 21 Group A (18–30 years) and 24 Group B (50–70 years), and 15 participants to Group C. Patient demographics and baseline variables are presented in Supplementary Table 1. All daytime alertness, psychological wellbeing, and retinal structure/function variables were worse in Group C compared with Group B (similar mean ages), significant for CCT (2-sample t-test with unequal variances: protan and deutan P<0.01, tritan P<0.001) and PSQI (Tukey pairwise, P=0.05).
Of 60 recruited participants, 8 withdrew before month 1 and were replaced. Eight withdrew after completing month 1. Numbers completing all study visits were: A 17, B 17, and C 12. Reasons for withdrawal are listed in Table 1. Light intolerance and sleep disturbance were cited by one participant in Group A and five in Group B.
Table 1. Reasons given for withdrawal of 16 participants by study visit.
| Reported reason for withdrawal | Group A | Group B | Group C |
|---|---|---|---|
| Before month 1 | |||
| Intolerance of light and sleep disturbances | 0 | 3 | 0 |
| Unable to attend follow-up | 1 | 0 | 1 |
| Could not perform study investigations | 0 | 0 | 1 |
| No reason given | 0 | 1 | 1 |
| After month 1 | |||
| Intolerance of light and sleep disturbances | 0 | 2 | 0 |
| Unrelated medical event | 0 | 0 | 1 |
| At month 2 | |||
| Intolerance of light and sleep disturbances | 1 | 0 | 0 |
| Could not perform study investigations | 0 | 1 | 0 |
| Unable to attend follow-up | 2 | 0 | 0 |
| No reason given | 0 | 0 | 1 |
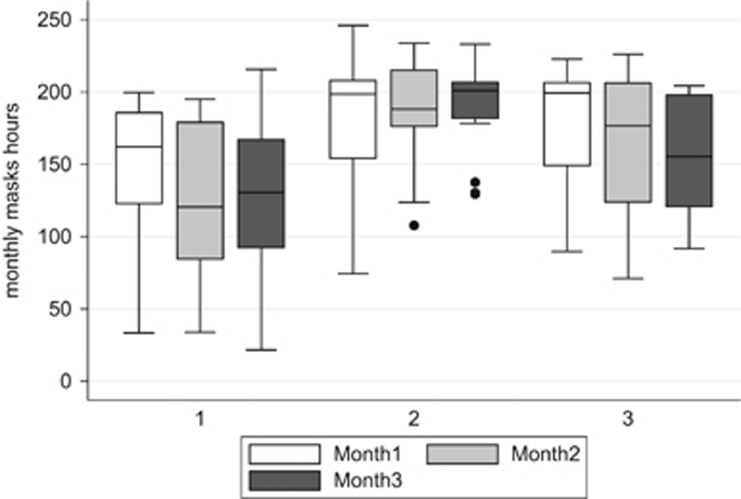
Figure 2 shows mean hours of sleep mask wear, equivalent to exposure to light therapy. Time of mask wear from baseline to month 3 (mean ±SD) was lower in Group A (410 ± 125 h) than Groups B (559±87.4, P<0.001, ANOVA) and C (495.8±139.1, P=0.002). In Group A mask wear was more variable but stayed stable, whereas in Group B it improved and became more consistent with time. On average younger healthy participants received 56% of the available light dose (with wider variability) compared with 76% in older participants (2-sample t-test with unequal variances P<0.001). Patients with DMO also had a wide variation in hours worn per month, which dropped off by month 2 (2-sample t-test with unequal variances P=0.006).
Figure 2.
Boxplots for total hours of mask wear in those participants (Group A n=17, Group B n=17, and Group c n=12) who completed 3 months of the study. Median is shown as horizontal line with upper and lower quartile as box, and range minima and maxima at ends of vertical lines; outliers with single months with reduced mask wear shown as single points (patient 29 108 h in month 2, patient 31 138 h in month 3, and patient 34 129 h in month 3).
Safety
Tables 2 and 3 show changes in study variables at months 3 and 4 compared with baseline for participants who completed 3 months of sleep mask wear (A 17, B 17, and C 12). At month 3, the PVT-RT deteriorated in both healthy groups (A 24.39 (7.25%), B 25.39 (7.65%)), statistically significant for Group A (P<0.001). PVT-NL deteriorated significantly in Group A (1.96 (43.27%), P=0.005). RT stayed depressed at month 4 whereas NL recovered. Group C had worse baseline levels of PVT than Group B but both older age groups showed no statistically significant change during the study.
Table 2. Changes in alertness, sleep quality, psychological wellbeing, and ocular function and structure at month 3 in 46 participants who completed 3 months of sleep mask wear.
|
Group A (n=17) |
Group B (n=17) |
Group C (n=12) |
||||
|---|---|---|---|---|---|---|
| Baseline (SD) range | Change at month 3 (95% CI) P-value | Baseline (SD) range | Change at month 3 (95% CI) P-value | Baseline (SD) range | Change at month 3 (95% CI) P-value | |
| PVT-NL (number) | 4.53 (4.69) 0–18 | 1.96 (0.63, 3.64) 0.005a | 4.30 (5.44) 0–24 | −0.17 (−1.15, 1.11) 0.75 | 6.08 (5.42) 1–21 | −0.8 (−1.99, 1.82) 0.93 |
| PVT-RT (ms) | 336.41 (36.62) 270.89–418.43 | 24.39 (13.35, 39.12) <0.001a | 332.10 (50.5) 276.1–456.6 | 25.39 (6.71, 42.34) 0.02 | 346.95 (13.37) 208–430 | 6.93 (−9.67, 23.53) 0.19 |
| KSS (score) | 3.41 (1.80) 1–7 | 0.74 (−0.33, 1.86) 0.16 | 2.41 (1.12) 1–5 | 0.47 (−0.16, 1.10) 0.13 | 2.92 (1.62) 1–7 | 0.08 (−1.41, 1.58) 0.90 |
| PSQ (score) | 2.65 (2.32) 0–8 | 0.82 (−0.16, 1.81) 0.09 | 3.60 (2.23) 1–9 | 0.06 (−1.23, 1.35) 0.92 | 4.58 (2.87) 1–11 | 0.5 (−0.57, 1.57) 0.32 |
| CESD (score) | 4.30 (3.87) 0–16 | 1.35 (−0.52, 3.23) 0.15 | 4.20 (4.50) 0–13 | −0.88 (−3.13, 1.37) 0.42 | 6.33 (6.23) 0–20 | 1.25 (−1.92, 4.42) 0.40 |
| GHQ12 (score) | 6.71 (2.02) 4–11 | 1.88 (0.51, 3.25) 0.01 | 6.94 (2.51) 3–12 | 1.47 (0.20, 2.74) 0.03 | 7.83 (2.72) 5–14 | 1.00 (0.02, 1.94) 0.046 |
| BCVA (letters) | 90.65 (3.32) 85–96 | 1.59 (−0.02, 3.19) 0.05 | 89.18 (3.28) 83–95 | 1.17 (−0.10, 2.45) 0.07 | 85.92 (5.18) 79–93 | −0.33 (−2.37, 1.70) 0.73 |
| CS (letters) | 41.29 (1.36) 37–42 | 0.35 (−0.37, 1.08) 0.32 | 41.18 (1.51) 36–42 | 0.17 (−0.64, 0.99) 0.65 | 35.0 (3.74) 30–41 | 1.0 (−0.67, 2.67) 0.11 |
| CCT protan (u'v') | 60.29 (19.47) 22–95 | −3.41 (−12.35, 5.52) 0.43 | 61.56 (14.47) 40–86 | −7.75 (−19.93, 4.43) 0.19 | 141.64 (89.35) 42–353 | −37.14 (−90.14, 15.85) 0.15 |
| CCT deutan (u'v') | 53.47 (18.57) 26–97 | 2.59 (−5.69, 10.87) 0.51 | 66.56 (19.19) 45–122 | −13.00 (−22.73, −3.27) 0.01 | 133.25 (99.47) 59–411 | −32.75 (−96.45, 30.95) 0.28 |
| CCT tritan (u'v') | 64.12 (22.51) 33–106 | −2.47 (−16.02, 11.08) 0.70 | 78.06 (36.58) 29–158 | −4.06 (−28.84, 20.71) 0.73 | 233.0 (131.55) 34–488 | −46.5 (−129.3, 36.3) 0.24 |
| mfERG amp 1 (μV) | 68.98 (16.02) 41.5–108 | 0.39 (−8.43, 9.21) 0.92 | 64.25 (12.22) 44.9–88.6 | 1.45 (−3.99, 6.91) 0.58 | 56.17 (9.76) 38.2–70.1 | 0.73 (−7.17, 8.65) 0.84 |
| mfERG lat 1 (ms) | 35.81 (2.19) 31.5–40.2 | −1.18 (−2.14, −0.22) 0.02 | 36.25 (1.72) 33.4–39.2 | −0.29 (−1.05, 0.48) 0.44 | 38.18 (2.05) 35.4–41.3 | −0.35 (−1.33, 0.62) 0.44 |
| EOG (light rise ratio) | 2.36 (0.40) 1.66–3.00 | 0.16 (−0.68, 0.99) 0.70 | 2.22 (0.42) 1.60–3.00 | 0.19 (−1.08, 1.47) 0.75 | 2.46 (0.60) 1.7–3.6 | −0.12 (−0.42, 0.18) 0.19 |
| MP1 (dB) | 19.45 (1.13) 15.6–20 | 0.25 (−0.28, 0.78) 0.33 | 19.41 (1.05) 16.6–20 | −0.06 (−0.73, 0.61) 0.86 | 13.7 (3.19) 6.6–17.8 | 1.95 (−0.39, 4.29) 0.09 |
| OCT CST (μm) | 274.53 (16.72) 238–308 | −0.53 (−2.60, 1.54) 0.60 | 286.53 (20.88) 255–319 | −2.82 (−5.62, −0.02) 0.05 | 320.50 (35.34) 274–389 | −6.25 (−20.40, 7.90) 0.35 |
| OCT maxST (μm) | 365.33 (34.69) 327–439 | −12.00 (−28.80, 4.80) 0.14 | ||||
Abbreviations: BCVA, best corrected visual acuity; CCT, Cambridge color vision test; CESD, The Centre for Epidemiologic Studies Depression scale; CS, contrast sensitivity; EOG, electrooculogram; GHQ12, General Health Questionnaire of psychological wellbeing; KSS, Karolinska Sleep Scale; mfERG, multifocal electroretinogram; MP1, microperimetry; OCT CST, optical coherence tomography mean central subfield thickness; OCT maxST, mean thickness of OCT subfield with maximal pathology; PSQI, Pittsburgh Sleep Quality Index; PVT-NL, psychomotor vigilance task (PVT) number of lapses; PVT-RT, PVT response time.
Data are presented as means with SD or 95% confidence intervals, corrected for hours of mask wear. P-values refer to paired t-tests of change in outcome (month 3 minus baseline), are uncorrected and should be interpreted against adjusted values of α (see text). Levels of P ≤ 0.05 are shown in bold.
Indicates statistically significant co-primary variable.
Table 3. Changes in alertness, sleep quality, psychological wellbeing, and ocular function and structure 1 month after discontinuing sleep mask wear in 46 participants who completed 3 months of wear.
|
Group A (n=17) |
Group B (n=17) |
Group C (n=12) |
||||
|---|---|---|---|---|---|---|
| Baseline (SD) range | Change at month 4 (95% CI) P-value | Baseline (SD) range | Change at month 4 (95% CI) P-value | Baseline (SD) range | Change at month 4 (95% CI) P-value | |
| PVT-NL (number) | 4.53 (4.69) 0–18 | 0.59 (−0.26, 11.28) 0.07 | 4.30 (5.44) 0–24 | 1.39 (−0.95, 5.27) 0.27 | 6.08 (5.42) 1–21 | 1.25 (−7.93, 4.43) 0.41 |
| PVT-RT (ms) | 336.41 (36.62) 270.89–418.43 | 28.02 (10.24, 46.70) 0.005 | 332.10 (50.5) 276.1–456.6 | 27.66 (10.11, 46.10) 0.01 | 346.95 (13.37) 208–430 | 10.03 (−5.70, 25.77) 0.09 |
| KSS (score) | 3.41 (1.80) 1–7 | 0.41 (−0.68, 1.50) 0.44 | 2.41 (1.12) 1–5 | 0.29 (−0.33, 0.92) 0.33 | 2.92 (1.62) 1–7 | −0.58 (−1.81, 0.64) 0.32 |
| PSQ (score) | 2.65 (2.32) 0–8 | 0.12 (−0.73, 0.97) 0.77 | 3.60 (2.23) 1–9 | 0.71 (−0.63, 2.04) 0.28 | 4.58 (2.87) 1–11 | 0.92 (−0.82, 2.66) 0.27 |
| CESD (score) | 4.30 (3.87) 0–16 | 1.38 (−1.05, 3.80) 0.24 | 4.20 (4.50) 0–13 | 0.19 (−2.65, 3.02) 0.88 | 6.33 (6.23) 0–20 | 3.67 (−1.19, 8.52) 0.12 |
| GHQ12 (score) | 6.71 (2.02) 4–11 | 0.94 (−0.39, 2.27) 0.15 | 6.94 (2.51) 3–12 | 1.06 (−0.79, 2.91) 0.24 | 7.83 (2.72) 5–14 | 2.08 (−0.19, 4.36) 0.07 |
| BCVA (letters) | 90.65 (3.32) 85–96 | 2.44 (0.68, 4.20) 0.001 | 89.18 (3.28) 83–95 | 1.59 (0.23, 2.95) 0.025 | 85.92 (5.18) 79–93 | 0.00 (−2.56, 2.56) 1.00 |
| CS (letters) | 41.29 (1.36) 37–42 | 0.56 (−0.26, 1.39) 0.17 | 41.18 (1.51) 36–42 | 0.06 (−0.47, 0.59) 0.82 | 35.0 (3.74) 30–41 | 2.57 (0.84, 4.33) 0.01 |
| CCT protan (u'v') | 60.29 (19.47) 22–95 | −8.88 (−9.78, 2.01) 0.10 | 61.56 (14.47) 40–86 | −2.19 (−14.14, 9.76) 0.70 | 141.64 (89.35) 42–353 | −37.56 (−95.04, 19.92) 0.18 |
| CCT deutan (u'v') | 53.47 (18.57) 26–97 | −1.29 (−11.68, 9.10) 0.80 | 66.56 (19.19) 45–122 | −10.00 (−19.14, 2.55) 0.11 | 133.25 (99.47) 59–411 | −46.17 (−114.1, 21.77) 0.16 |
| CCT tritan (u'v') | 64.12 (22.51) 33–106 | −3.47 (−22.11, 15.16) 0.70 | 78.06 (36.58) 29–158 | 0.19 (−18.07, 18.44) 0.98 | 233.0 (131.55) 34–488 | −54.83 (−125.5, 15.8) 0.12 |
| mfERG amp 1 (μV) | 19.45 (1.13) 15.6–20 | 0.10 (−0.28, 0.48) 0.59 | 19.41 (1.05) 16.6–20 | 0.30 (−0.14, 1.14) 0.30 | 55.73 (9.42) 38.2–70.1 | −2.71 (−10.75, 5.33) 0.47 |
| mfERG lat 1 (ms) | 68.98 (16.02) 41.5–108 | 1.70 (−7.35, 10.75) 0.70 | 64.25 (12.22) 44.9–88.6 | 7.81 (0.88, 14.74) 0.03 | 38.20 (1.95) 35.4–41.3 | −0.72 (−1.94, 0.49) 0.21 |
| MP1 (dB) | 35.81 (2.19) 31.5–40.2 | −1.22 (−2.14, −0.30) 0.01 | 36.25 (1.72) 33.4–39.2 | 0.08 (0.84, 1.01) 0.85 | 13.7 (3.19) 6.6–17.8 | 2.29 (0.18, 4.41) 0.04 |
| OCT CST (μm) | 274.53 (16.72) 238–308 | 0.24 (−1.41, 1.88) 0.77 | 286.53 (20.88) 255–319 | 1.24 (−3.18, 5.65) 0.56 | 320.50 (35.34) 274–389 | −7.58 (−22.08, 6.91) 0.27 |
| OCT maxST (μm) | 365.33 (34.69) 327–439 | −15.58 (−32.93, 1.76) 0.07 | ||||
Abbreviations: BCVA, best corrected visual acuity; CCT, Cambridge color vision test; CESD, The Centre for Epidemiologic Studies Depression scale; CS, contrast sensitivity; EOG, electrooculogram; GHQ12, General Health Questionnaire of psychological wellbeing; KSS, Karolinska Sleep Scale; mfERG, multifocal electroretinogram; MP1, microperimetry; OCT CST, optical coherence tomography mean central subfield thickness; OCT maxST, mean thickness of OCT subfield with maximal pathology; PSQI, Pittsburgh Sleep Quality Index; PVT-NL, psychomotor vigilance task (PVT) number of lapses; PVT-RT, PVT response time.
EOG was not recorded at this visit. Data are presented as means with SD or 95% confidence intervals, corrected for hours of mask wear. Data on the effect of mask wear are also presented as effect of 100 h wear. P-values are uncorrected and shown in bold if ≤ 0.05.
Interpreting our secondary outcomes requires some caution due to multiple comparisons. Psychological wellbeing (GHQ12) worsened in all the groups at month 3 (A 28.0%, P=0.01; B 21.2%, P=0.03; C 12.7%, P<0.05) with a small but statistically significant greater effect with increasing mask wear.
In older participants there was a consistent improvement in CCT thresholds at month 3, statistically significant for deutan (13.00 (19.5%) P=0.01). There were small increases in BCVA at month 4: Group A +2.44 letters (P<0.001), Group B +1.59 letters (P=0.025). No change was detected in Group C at month 3 in BCVA, CS, mfERG, EOG, or for any other variables in any group. We detected no clinically important effect on primary or secondary variables associated with duration of recorded mask wear apart from GHQ12.
About 75% participants in Groups A and B and 40% in Group C who wore the sleep mask for ≥1 month reported ≥1 adverse event (Supplementary Table 2). Events were mostly attributed to the fabric mask housing the ‘Pod'. One SAE deemed unlikely to be related to the sleep mask occurred in Group C.
About 40/45 (95%) healthy participants and 13/15 (87%) DMO patients completed sleep diaries during the dosing period (61% for all 3 months). Thirty-three reported mask discomfort and slippage. Three participants (none in Group C) reported the light as an issue and then only at month 1; all three completed the study.
DMO
For Group C all measures of retinal function and structure showed no statistically significant improvement or change with increasing hours of mask wear (Tables 2 and 3); however there were trends for improvement across all measures. There was a near significant OCT effect for the field of maximal pathology at month 4 (P=0.07). About 10/15 (67%) showed a reduction/clearance of cysts in the maximum pathology subfield.
Statistically significant greater changes in mean thicknesses per 1 μm change in baseline thickness were seen for CST and maxCT at 3 and 4 months: month 3: CST: −0.77 (−1.03,−0.53), P<0.001; month 4: CST: −0.79 (−1.05,−0.53), P<0.001; maxST: month 3: −0.44 (−0.87,−0.02), P=0.05, month 4: −0.62 (−0.96,−0.28), P<0.01. For the subfield of maximal pathology, for each 10 μm increase in baseline thickness, there was a corresponding greater reduction of thickness of 4–6 μm.
Discussion
This is the first safety study, to our knowledge, of a sleep mask containing an OLED device designed as a potential therapy for retinal diseases.
We designed a stimulus aimed at evenly reducing the rod dark current without stimulating cones. Previous work has used LED light, which has a narrow spectral bandwidth and is directional.5, 6 Emission from our OLED was close to Lambertian and homogenous across the plane of the OLED, ensuring even illumination of the retina and minimising the effect of eye movement during sleep. This allowed the brain to adapt to the presence of the light through Troxler's fading.14 After attenuation of the output spectrum by the lid and ocular media, mesopic retinal illumination drops from 80 candelas/m2 to a level of around 2 candelas/m2.15 The effect of this wavelength on the melanopsin containing retinal ganglion cells responsible for circadian synthesis could suppress and shorten melatonin onset and duration, and potentially impact sleep, thermoregulation, blood pressure, and glucose homeostasis.16, 17
Sleep disturbance and intolerance to light appeared to be the principal reasons given for withdrawal, especially in older volunteers; in younger people withdrawal was associated with lack of compliance with mask wear and/or attendance. The level of light intolerance of 5–10% appears acceptable as light therapy moves into efficacy trials. Further dose calibration experiments changing intensity or with shorter duration may be justified depending on future efficacy trials.
The significant withdrawal rate of 24% (11% in the first month of usage) and the self-reported adverse event rate of ~75% all indicate that further modifications of mask design are needed for home compliance to become optimal. Patients with DME reported less adverse events possibly due to higher motivation in participants with treatable disease.
Neurobehavioural function as a measure of sleep disturbance was reduced at month 3 in PVT-RT for both age groups and PVT-NL for younger participants. The delays in RT of 24.39 (younger) and 25.39 (older) were within the SD of typical response times for healthy non-sleep-deprived participants (40–100 ms) in our and other published data.9, 18 PVT-NL showed a larger 43% mean change in younger participants but changed little in older participants; impaired PVT-NL has been reported in sleep-deprived young men but not older men.19, 20 The worsening in NL returned to normal by month 4.
Although our results are generally reassuring we believe that PVT is an important safety measurement and should be included in dark adaptation blocking therapy trials. Eight of our participants developed loss of performance during the study (Supplementary Tables 3 and 4). NL rose as high as 40 in one participant and in 3 there was concordance between worsening NL and RT. Worsening reaction times and NL have been observed after extended periods of wakefulness21 and associated with poor driving performance.22 We developed age-specific upper criteria, extrapolated from published normative data,7 useful in future clinical trials (<52 years: NL=17; 52–64 years: NL=26; >64 years: NL=31).
Some secondary outcomes (BCVA, OCT, mfERG latency, and CCT deutan) showed statistically significant changes at month 3 but none were in an unsafe direction.
The GHQ12 evidence of worsening wellbeing is of some concern. GHQ12 was a secondary outcome; so the findings should be interpreted with caution but the worsening was significant in all the groups (28% reduction for younger healthy controls) and some participants moved from the no distress to the some distress or severe distress categories. There were no significant changes in depressive symptoms on CESD.
We extended our safety study to a cohort of people with DMO primarily to study safety but also to investigate an effect on macular oedema. These participants performed worse across all study variables including daytime alertness, psychological wellbeing, and retinal structure/function when compared with healthy volunteers with similar ages (Group B). This is not unexpected for retinal structure and function, especially colour dysfunction23 but less familiar to ophthalmology for poor wellbeing and alertness. Visually impaired people have reported lowered alertness and performance parameters24 and people with diabetes suppressed neuropsychological test scores compared with age-matched controls.25
All study parameters that measured retinal function and structure improved by month 3 apart from EOG. Improvements persisted into month 4 but only reached statistical significance for CS and MP1. The depression scores improved but psychological wellbeing worsened at month 3 as in the healthy cohort.
We detected a small beneficial effect of mask wear on DMO as measured by OCT. This effect was larger for those with more severe thickness, may be clinically relevant, was statistically significant and comprised a 10–20% reduction in thickness. Arden et al6 reported 6-month OCT and BCVA changes in an uncontrolled patient population with bilateral DMO where one eye was treated with a light mask with four light-emitting diodes with similar parameters as in our OLED. The authors reported a reduction in number of retinal cysts and detected no adverse events, claiming the therapy to be safe and acceptable. Their study had a number of limitations in the post hoc statistical analysis and no safety data were collected. However our results are consistent with their findings. Faced with a global epidemic of diabetes a readily available, inexpensive, non-invasive home therapy that can be used at the earliest stages of disease development, would be a major therapeutic asset.
Our study detected no major safety signal and allows a move to phase 3 clinical trials of light therapy during sleep for retinal diseases. Data from our DMO cohort provide useful additional pilot evidence of a potential beneficial effect. We believe that the therapy will be acceptable to most potential patients including older people and people with diabetes. However it will be challenging for some individuals, with a small but acceptable proportion likely to be intolerant beyond a few days. Including behavioural and psychological investigations of the effect on sleep disturbance in future clinical trials will be important including carefully designed monitoring and exit criteria. Usefulness will depend on improvements in mask design for compliance, tolerability, and acceptability.

Acknowledgments
The study was supported by Small Business Research Initiative, Health Enterprise East, NHS Midlands and East on behalf of the Technology Strategy Board, United Kingdom.
Footnotes
Supplementary Information accompanies this paper on Eye website (http://www.nature.com/eye)
IG is the Medical and Scientific Adviser to Polyphotonix Medical Ltd. MNH is the Operations Director at Polyphotonix Medical Ltd. The remaining authors declare no conflict of interest.
Supplementary Material
References
- Linsenmeier RA, Braun RD, McRipley MA, Padnick LB, Ahmed J, Hatchell DL et al. Retinal hypoxia in long-term diabetic cats. Invest Ophthalmol Vis Sci 1998; 39: 1647–1657. [PubMed] [Google Scholar]
- Arden GB, Wolf JE, Tsang Y. Does dark adaptation exacerbate diabetic retinopathy? Evidence and a linking hypothesis. Vision Res 1998; 38: 1723–1729. [DOI] [PubMed] [Google Scholar]
- de Gooyer TE, Stevenson KA, Humphries P, Simpson DA, Gardiner TA, Stitt AW. Retinopathy is reduced during experimental diabetes in a mouse model of outer retinal degeneration. Invest Ophthalmol Vis Sci 2006; 47: 5561–5568. [DOI] [PubMed] [Google Scholar]
- Arden GB. The absence of diabetic retinopathy in patients with retinitis pigmentosa: implications for pathophysiology and possible treatment. Br J Ophthalmol 2001; 85: 366–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arden GB, Gunduz MK, Kurtenbach A, Volker M, Zrenner E, Gunduz SB et al. Preliminary trial to determine whether prevention of dark adaptation affects the course of early diabetic retinopathy. Eye 2010; 24: 1149–1155. [DOI] [PubMed] [Google Scholar]
- Arden GB, Jyothi S, Hogg CH, Lee YF, Sivaprasad S. Regression of early diabetic macular oedema is associated with prevention of dark adaptation. Eye 2011; 25: 1546–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaida K, Takahashi M, Akertstedt T, Nakata A, Otsuka Y, Haratani T et al. Validation of the Karolinska sleepiness scale against performance and EEG variables. Clin Neurophysiol 2006; 117: 1574–1581. [DOI] [PubMed] [Google Scholar]
- Akerstedt T, Gillberg M. Subjective and objective sleepiness in the active individual. Int J Neurosci 1990; 52: 29–37. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 1989; 28: 193–213. [DOI] [PubMed] [Google Scholar]
- Radloff LS, Lock BZ The community mental health assessment survey and the CES-D scale In: Weissman M, Myers J, Ross C eds. Community Surveys. Rutgers University Press: New Brunswick, NJ, 1986. [Google Scholar]
- Goldberg DP, Gater R, Sartorius N, Ustun TB, Piccinelli M, Gureje O et al. The validity of two versions of the GHQ in the WHO study of mental illness in general health care. Psychol Med 1997; 27(1): 191–197. [DOI] [PubMed] [Google Scholar]
- Wuerger S. Colour constancy across the life span: evidence for compensatory mechanisms. PLoS One 2013; 8: e63921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan RP, Fisher AC, Brown MC. Examination of short binary sequences for mfERG recording. Doc Ophthalmol 2006; 113: 21–27. [DOI] [PubMed] [Google Scholar]
- Martinez-Conde S, Macknik SL, Hubel D. The role of fixational eye movements in visual perception. Nat Rev Neurosci 2004; 5: 229–240. [DOI] [PubMed] [Google Scholar]
- Robinson J, Bayliss S, Fielder AR. Transmission of light across the adult and neonatal eyelid in vivo. Vis Res 1991; 31: 1837–1840. [DOI] [PubMed] [Google Scholar]
- Boudreau P, Dumont GA, Boivin DB. Circadian adaption to night shift work influences sleep performance, mood and the autonomic modulation of the heart. PLoS One 2013; 8: e70813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooley JJ, Chamberlain K, Smith KA, Khalsa SB, Rajaratnam SM, Van Reen E et al. Exposure to room light before bedtime suppresses melatonin onset and shortens melatonin duration in humans. J Clin Endocrinol Metab 2011; 96: E463–E472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip P, Taillard J, Sagaspe P, Valtat C, Sanchez-Ortuno M, Moore N et al. Age, performance and sleep deprivation. J Sleep Res 2004; 13: 105–110. [DOI] [PubMed] [Google Scholar]
- Lamond N, Dorrian J, Roach GD, McCulloch K, Holmes AL, Burgess HJ et al. The impact of a week of simulated night work on sleep, circadian phase and performance. Occup Environ Med 2003; 60: e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam M, Rêtey JV, Khatami R, Landolt HP. Age-related changes in the time course of vigilant attention during 40 hours without sleep in men. Sleep 2006; 29: 55–57. [DOI] [PubMed] [Google Scholar]
- Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep 2003; 26: 117–126. [DOI] [PubMed] [Google Scholar]
- Baulk SD, Biggs SN, Reid KJ, van den Heuvel CJ, Dawson D. Chasing the silver bullet: measuring driver fatigue using simple and complex tasks. Accid Anal Prev 2008; 40: 396–402. [DOI] [PubMed] [Google Scholar]
- Gualtieri M, Feitosa-Santana C, Lago M, Nishi M, Ventura DF. Early visual changes in diabetic patients with no retinopathy measured by color discrimination and electroretinography. Psychol Neurosci 2013; 6: 227–234. [Google Scholar]
- Lockley SW, Arendt J, Skene DJ. Visual impairment and circadian rhythm disorders. Dialogues Clin Neurosci 2007; 9: 301–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palta P, Schneider ALC, Biessels GJ, Touradji P, Hill-Briggs F. Magnitude of cognitive dysfunction in adults with type 2 diabetes: a meta-analysis of six cognitive domains and the most frequently reported neuropsychological tests within domains. J Int Neuropsychol Soc 2014; 20: 278–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.