Abstract
Biocompatibility of intraocular lens (IOL) is critical to vision reconstruction after cataract surgery. Foldable hydrophobic acrylic IOL is vulnerable to the adhesion of extracellular matrix proteins and cells, leading to increased incidence of postoperative inflammation and capsule opacification. To increase IOL biocompatibility, we synthesized a hydrophilic copolymer P(MPC-MAA) and grafted the copolymer onto the surface of IOL through air plasma treatment. X-ray photoelectron spectroscopy, atomic force microscopy and static water contact angle were used to characterize chemical changes, topography and hydrophilicity of the IOL surface, respectively. Quartz crystal microbalance with dissipation (QCM-D) showed that P(MPC-MAA) modified IOLs were resistant to protein adsorption. Moreover, P(MPC-MAA) modification inhibited adhesion and proliferation of lens epithelial cells (LECs) in vitro. To analyze uveal and capsular biocompatibility in vivo, we implanted the P(MPC-MAA) modified IOLs into rabbits after phacoemulsification. P(MPC-MAA) modification significantly reduced postoperative inflammation and anterior capsule opacification (ACO), and did not affect posterior capsule opacification (PCO). Collectively, our study suggests that surface modification by P(MPC-MAA) can significantly improve uveal and capsular biocompatibility of hydrophobic acrylic IOL, which could potentially benefit patients with blood-aqueous barrier damage.
Cataract is the leading cause of vision loss worldwide, accounting for 11 million cases of blindness and 35 million cases of visual impairment per year1. So far, phacoemulsification with foldable intraocular lens (IOL) implantation is the main treatment for cataract. Foldable hydrophobic acrylic IOLs are most commonly used due to the high refractive index and relatively low posterior capsule opacification (PCO) rate2. However, extracellular matrix proteins, inflammatory cells and lens epithelial cells (LECs) can easily adhere to the hydrophobic surface, leading to a high incidence of iris posterior synechiae (IPS) and anterior capsule opacification (ACO)3,4,5,6, especially in patients with blood-aqueous barrier damage. Serious ACO may cause anterior capsule shrinkage, IOL decentration, and may hinder the examination of peripheral fundus7. These problems limit the application of hydrophobic acrylic IOL in patients with uveitis, glaucoma or diabetes.
Improvement of IOL biocompatibility, including uveal and capsular biocompatibility, is critical to reduce postoperative complications8. Uveal biocompatibility is the foreign-body reaction to the IOL implant. Disruption of the blood-aqueous barrier (BAB) in cataract surgery leads to the release of protein and inflammatory cells into the aqueous humor9. Also, the implanted IOL directly interacts with the surrounding tissue, activates the complement system, and triggers inflammation of iris, ciliary body and anterior choroid10. Capsular biocompatibility refers to the interaction between the IOL and LECs. Once the lens is removed, remnant LECs in the capsule can attach to the IOL surface, and then proliferate, migrate and differentiate into fibroblast-like cells, causing ACO and PCO8,11. Moreover, inflammatory cells such as macrophages and monocytes secrete growth factors and cytokines that may aggravate capsule opacification12.
One way to improve IOL biocompatibility is to reduce adhesion of inflammatory cells and LECs onto the IOL surface. The initial phase of cell adhesion is the interaction between cell surface adhesion molecules and extracellular matrix (ECM) proteins, such as fibronectin, laminin and collagen13. Many studies have demonstrated that hydrophilic surface can reduce protein adsorption and cell adhesion, and surface modifications of hydrophobic acrylic IOL have been attempted to increase biocompatibility without changing the bulk properties14,15,16,17. 2-methacryloyloxyethyl phosphorylcholine (MPC, Supplementary Fig. S1a) is a zwitterionic molecule and shows excellent biocompatibility since it can form a membrane-like structure and trap water molecules on the material surface, suppressing protein adsorption and cell adhesion14,18,19,20. Surface modification by MPC has been widely applied in bioimplants, tissue engineering and drug delivery systems21,22,23. Surface modification of silicone IOL by MPC can significantly reduce water contact angle, and suppress adhesion of epithelial and fibroblast-like cells14,18. However, irreversible trap of silicone oil limits the application of silicone IOL in patients with vitreoretinal diseases24. Therefore, silicone IOLs are not widely used as hydrophobic acrylic IOLs in cataract surgery. In acrylic IOL, MPC coating suppresses fibroblast and bacterial adhesion in vitro25. However, physisorbed surface coating is less stable than covalent binding. Also, uveal and capsular biocompatibility of MPC-modified hydrophobic acrylic IOL has not been evaluated in vivo.
MPC modification on hydrophobic silicone IOL did not reduce aqueous flare in a rabbit model14, suggesting that grafting MPC monomers alone is insufficient to alleviate post-operative anterior chamber inflammation. One possible explanation is that MPC does not carry enough negative charges to sufficiently reduce protein adsorption and cell adhesion as extracellular protein is usually negatively charged under physiological conditions16,17,26. Methyl acrylic acid (MAA, Supplementary Fig. S1b) is a negatively charged hydrophilic monomer due to the carboxylic acid group27, and has been applied to resist cell invasion and prevent tissue adhesions after surgery19. MAA has been widely used in the fabrication of contact lens hydrogels and showed excellent biocompatibility28,29. Here, to combine the advantages of MPC and MAA together, we used MPC and MAA to synthesize a hydrophilic copolymer P(MPC-MAA) (Supplementary Fig. S1c), and covalently grafted this copolymer onto the surface of hydrophobic acrylic IOL after ammonia plasma treatment. Then we characterized the IOL surface property and evaluated the uveal and capsular biocompatibility of P(MPC-MAA)-modified hydrophobic acrylic IOL both in vitro and in vivo.
Results
P(MPC-MAA) was synthesized and grafted onto the IOL surface
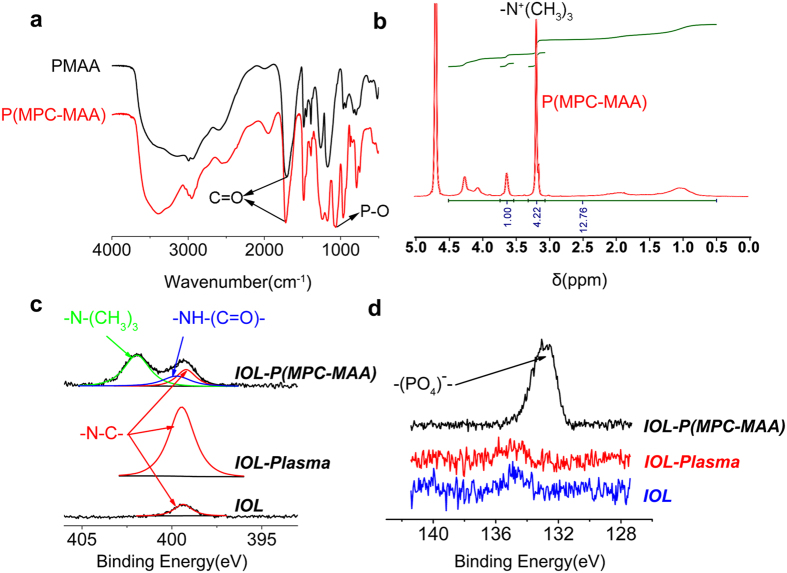
P(MPC-MAA) copolymer was synthetized via free radical polymerization. Fourier transform infrared (FT-IR) spectroscopy and proton nuclear magnetic resonance (1H NMR) spectroscopy of P(MPC-MAA) are shown in Fig. 1. A transmission absorption peak was observed at 1,720 cm−1 for all of the samples (Fig. 1a), which corresponded to the carbonyl group (C=O) in the PMAA and P(MPC-MAA). However, an absorption peak at 1,080 cm−1 was observed only in the spectra for P(MPC-MAA), which corresponded to the phosphate group (P-O) in the MPC unit30,31. The proton signals at 3.2 ppm were observed in the 1H NMR spectroscopy of P(MPC-MAA) (Fig. 1b), which was attributed to -N+(CH3)3 of the MPC units30,32,33. Collectively, these results demonstrated that the P(MPC-MAA) copolymer was successfully synthesized. The molar fractions of MPC and MAA were 5.7:4.3 calculated from 1H NMR spectroscopy. The Molecular weight (Mw) and polydispersity index (PDI, Mw/Mn) of P(MPC-MAA) copolymer were 2.3 × 105 and 3.02, respectively (Supplementary Fig. S2).
Figure 1. P(MPC-MAA) was successfully synthesized and grafted onto the IOL surface.
(a) FT-IR spectra of PMAA and P(MPC-MAA) detecting with the potassium bromide pressed-disk technique for 32 scans over the 500–4,000 cm−1 range at a resolution of 4.0 cm−1. (b) H-NMR spectra of P(MPC-MAA) determined at 400 MHz. (c,d) N1s high-resolution spectra and P1s high-resolution spectra of IOL, IOL-Plasma, and IOL-P(MPC-MAA), respectively.
To construct a protein-resistant IOL surface, P(MPC-MAA) copolymer was grafted onto the IOL surface via plasma technology. Untreated hydrophobic IOL, IOL treated by plasma alone, and P(MPC-MAA) modified IOL are abbreviated as IOL, IOL-Plasma and IOL-P(MPC-MAA), respectively. X-ray photoelectron spectroscopy (XPS) spectra of the binding energy regions of the nitrogen (N) and phosphorous (P) electrons of IOL, IOL-Plasma and IOL-P(MPC-MAA) are shown in Fig. 1c, d. Relative intensities of nitrogen element are listed in Supplementary Table S1. Compared to IOL, a strong and broad N1s peak at approximately 400 eV appeared on IOL-Plasma, indicating that plasma treatment was achieved successfully32,33,34. After P(MPC-MAA) grafting, the peaks at 401.96 and 134 eV appeared on IOL-P(MPC-MAA). These peaks corresponded to the -N-(CH3)3 and phosphate groups attributed to the MPC unit. Meanwhile, the peak at 400.04 eV corresponded to -NH-C(=O). These data indicate that P(MPC-MAA) was successfully grafted onto the IOL surface via the amidation reaction.
Surface characterization of the IOLs
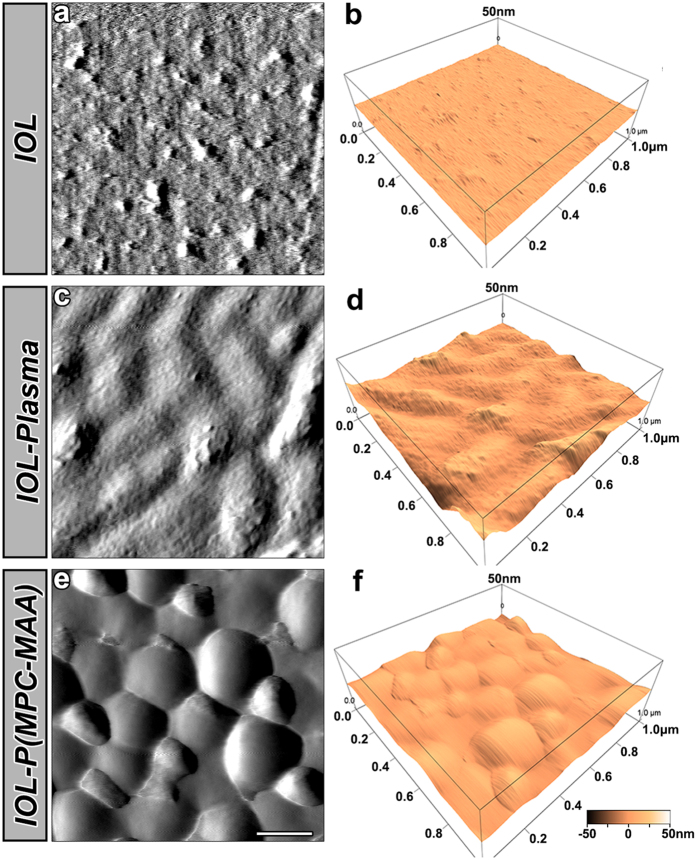
Surface topography affects protein adsorption and subsequent cell behaviors35. We first characterized the surface morphology of the samples by atomic force microscopy (AFM) (Fig. 2). IOL had a surface roughness of 0.787 nm, exhibiting a relatively even morphology with a few particles and shallow grooves (Fig. 2a,b). IOL-Plasma had many deep grooves appeared on the surface, and the roughness was increased to 4.818 nm (Fig. 2c,d). IOL-P ( MPC-MAA) exhibited many wave-like clusters of polymer chains, and the surface roughness was 3.469 nm (Fig. 2e,f).
Figure 2. Surface characterization of IOL by AFM.
Representative AFM images of the IOL, IOL-Plasma, and IOL-P(MPC-MAA) surfaces. Area size for each scan: 1.0 × 1.0 μm2. Scale bar = 200 nm.
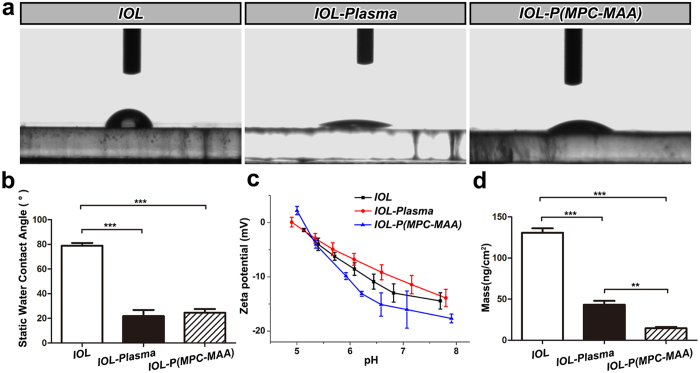
Next, we measured the water contact angles (WCAs) to characterize the hydrophilicity of the IOL surface (Fig. 3a,b). The WCA of IOL was 78.9 ± 2.2°, suggesting a hydrophobic surface property. Plasma treatment introduced amino groups onto the IOL surface, and the WCA of IOL-Plasma decreased to 21.8 ± 5.0°, indicating increased surface hydrophilicity. The WCA of IOL-P(MPC-MAA) also decreased to 24.5 ± 3.1°.
Figure 3. P(MPC-MAA) modification increases surface hydrophilicity and decreases protein adsorption.
(a) Representative images of static WCA measurement in each group at 25 °C. (b) Quantification of static WCA in each group. n = 5. **P < 0.01, ***P < 0.001. (c) Quantification of zeta potential in each group. n = 4. (d) Measurement of BSA adsorption by QCM. n = 3. **P < 0.01, ***P < 0.001.
To investigate the electrokinetic properties of the samples, we measured the zeta potential of the samples (Fig. 3c). At pH 7.2, the zeta potential of IOL was −13.6 mv. The average zeta potential of IOL-Plasma increased to −11.5 mv due to the introduction of positively charged amino groups, while the average zeta potential of IOL-P(MPC-MAA) decreased to −16.4 mv due to the introduction of the negatively charged carboxylic acid groups.
The optical characteristics of the samples, such as diopter, resolution and transmission properties, demonstrated no significant differences between IOL-P(MPC-MAA) and IOL. The haptics of all groups could endure bending and stretching 2.5 million times with a compression amplitude of +/−0.25 mm. These optical and physical properties meet the standards of State Food and Drug Administration (SFDA) in China.
IOL-P(MPC-MAA) inhibits protein adsorption
Protein adsorption is the first phenomenon observed after IOL implantation, and will affect subsequent cell interaction in the material-tissue interface in the following minutes or hours8. We used bovine serum albumin (BSA) to monitor protein adsorption on the IOL surface by quartz crystal microbalance with dissipation (QCM-D) analysis (Fig. 3d). BSA adsorption on IOL was 130.8 ± 9.9 ng/cm2, which was similar to that on other hydrophobic IOL surfaces we previously reported36. Compared to IOL, BSA adsorption on IOL-Plasma decreased to 43.1 ± 8.2 ng/cm2, and BSA adsorption on IOL-P(MPC-MAA) further decreased to 14.5 ± 3.1 ng/cm2. These data were consistent with the previous reports that increased surface hydrophilicity and introduction of negative charges onto the material surface can significantly decrease protein adsorption16,17.
IOL-P(MPC-MAA) inhibits the adhesion and proliferation of lens epithelial cells in vitro
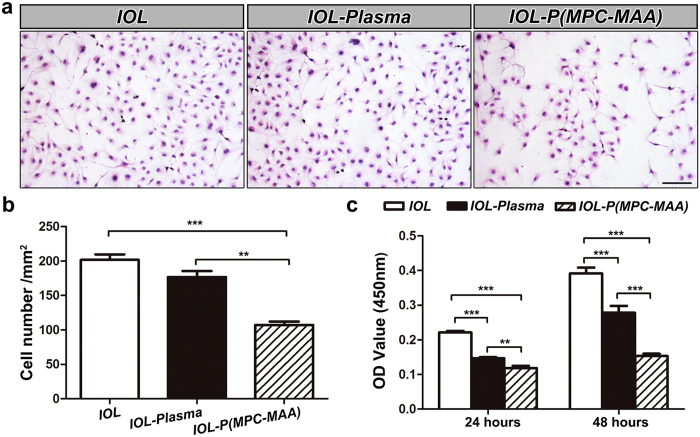
Cell interaction in the material-tissue interface include an initial phase of cell adhesion followed by subsequent cell proliferation and migration12. We used human lens epithelial cell (LEC) line SRA01/04 to evaluate cell behaviors on modified IOLs in vitro. Adhesion of LECs on IOL-P(MPC-MAA) (107.1 ± 5.1/mm2) was significantly decreased compared to that on IOL (201.7 ± 8.1/mm2) and IOL-Plasma (176.7 ± 8.9/mm2) (Fig. 4a,b). However, there was no significant difference between cell adhesion on IOL and IOL-Plasma ( P = 0.174). In order to characterize cell proliferation on the IOL surfaces, we incubated LECs on the IOL for 24 and 48 hours and performed a cell viability assay. Compared to IOL and IOL-Plasma, IOL-P(MPC-MAA) significantly decreased cell proliferation after 24 and 48 hours of incubation (Fig. 4c). Collectively, these results demonstrate that P(MPC-MAA) modification significantly increases cell repellency of the IOL surface.
Figure 4. IOL-P(MPC-MAA) inhibits the adhesion and proliferation of LECs in vitro.
(a) Representative inverted phase contrast microscope images of LECs attached to the surface of IOL, IOL-Plasma, and IOL-P(MPC-MAA), respectively. Scale bar = 100 μm. (b) Quantification of the number of cells attached to the surfaces of IOLs in (a). In each group, 5 IOLs were chosen, and in each IOL, five fields were selected with one in the central and four in peripheral quadrants at random. (c) Cell viability assay shows the proliferation of LECs on the surfaces of IOLs after incubation for 24 and 48 hours, respectively. n = 3. **P < 0.01, ***P < 0.001.
IOL-P(MPC-MAA) reduces postoperative inflammation
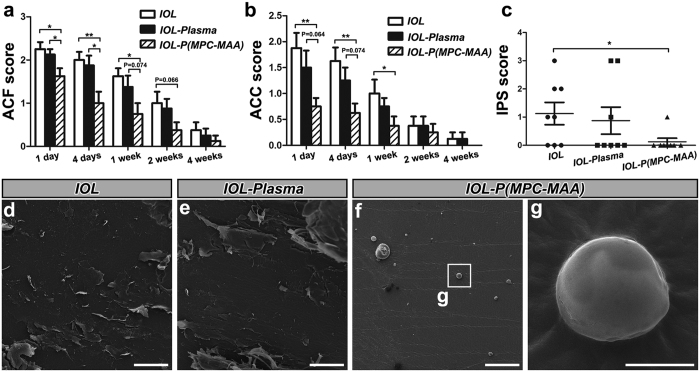
Uveal biocompatibility of the IOL can be assessed by the severity of postoperative inflammation8. Breakdown of the blood-aqueous barrier and the foreign body reaction to the IOL implant results in release of protein and cells into the anterior chamber, which can be manifested as anterior chamber flare (ACF) and anterior chamber cell (ACC) respectively37. Therefore, we first evaluated ACF and ACC scores as indicators of inflammation (Fig. 5a,b). Similar to the inflammatory responses in human patients after IOL implantation4,38, both ACF and ACC scores peaked 1 day postoperatively, and then decreased to the baseline after 4 weeks. Rabbit eyes with implantation of IOL-P(MPC-MAA) had significantly lower ACF and ACC scores 1 day, 4 days, and 1 week after surgery. Persistent inflammation may cause IPS, which refers to the adhesion of the iris to the anterior surface of the IOL or lens capsule. Eight weeks after surgery, slit lamp examination showed that IOL-P(MPC-MAA) implantation group had a significantly lower IPS score than IOL implantation group (Fig. 5c, Supplementary Fig. S3a). We also noticed other postoperative complications occurred in IOL, IOL-Plasma groups, including pupil capture (1 eye in IOL group and 1 eye in IOL-Plasma group), IOL displacement (1 eye in IOL group and 1 eye in IOL-Plasma group) and severe cortical proliferation (1 eye in IOL group) (Supplementary Fig. S3b). However, no obvious postoperative complication due to inflammation was found in IOL-P(MPC-MAA) implantation group. Intraocular pressure (IOP) values were within normal range in all the groups (Supplementary Fig. S4).
Figure 5. IOL-P(MPC-MAA) reduces postoperative inflammation after cataract surgery.
(a,b) Quantification of ACF and ACC scores in each group 1 day, 4 days, 1 week, 2 weeks, and 4 weeks after surgery, respectively. n = 8. *P < 0.05, **P < 0.01. (c) Quantification of IPS scores in each group 8 weeks after surgery. n = 8. *P < 0.05, **P < 0.01. (d–g) Representative SEM images of IOL, IOL-Plasma, and IOL-P(MPC-MAA) surfaces 8 weeks after surgery. (d–f) Scale bar = 100 μm. (g) Scale bar = 5 μm.
To further characterize the cellular response to the IOL implants, we extracted the IOLs 8 weeks after surgery and performed scanning electron microscopy (SEM). A large number of amorphous debris and polygonal cells were found adhered to the surfaces of IOL (Fig. 5d,e). However, only a few debris and small round cells were found on the surfaces of IOL-P(MPC-MAA) (Fig. 5f,g). Collectively, these results indicate that P(MPC-MAA) modification greatly improves uveal biocompatibility of hydrophobic acrylic IOLs in vivo.
IOL-P(MPC-MAA) inhibits anterior capsule opacification
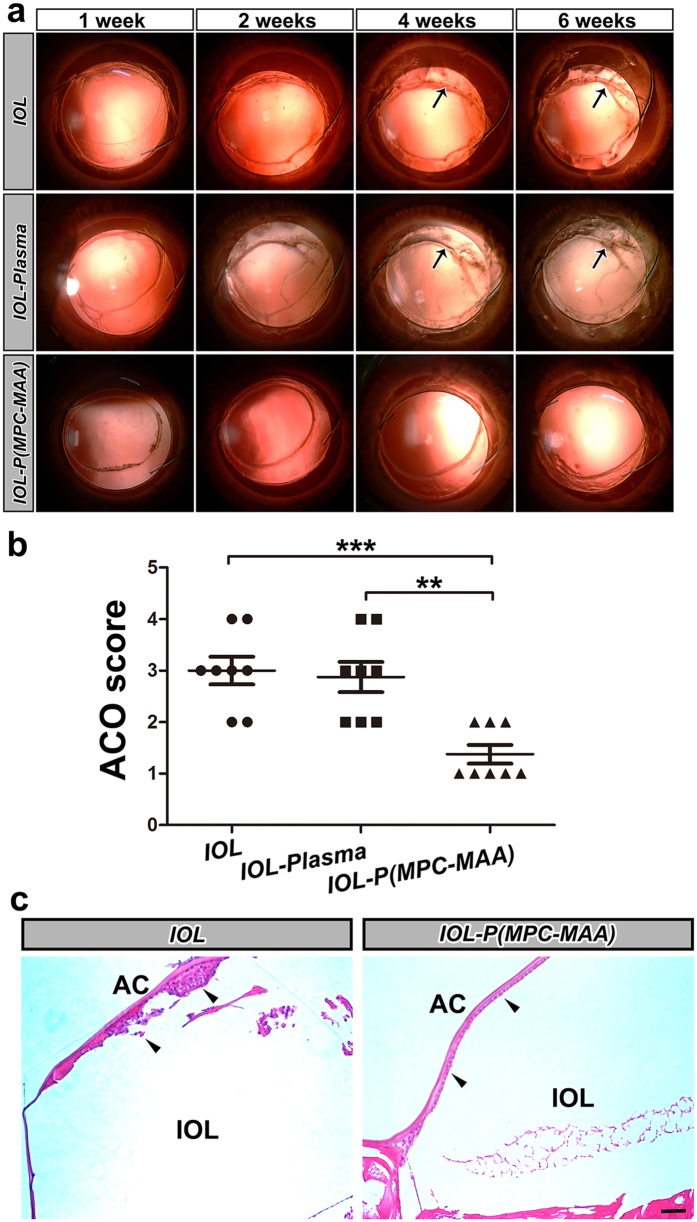
ACO is caused by proliferation and epithelial-mesenchymal transition (EMT) of the remnant LECs between the inner surface of the anterior capsule and IOL implant39. In our study, IOL and IOL-plasma implantation groups developed ACO 2 weeks after surgery (Fig. 6a). After 4 weeks, severe fibrosis occurred on the anterior capsule covering IOL and IOL-plasma optics, leading to anterior capsule shrinkage (black arrows). However, in IOL-P(MPC-MAA) implantation group, ACO developed slowly, and the anterior capsule was relatively transparent 6 weeks after surgery. The ACO score of IOL-P(MPC-MAA) group was significantly lower than that of the IOL and IOL-plasma groups 6 weeks postoperatively (Fig. 6b). Also, histopathological examination showed that multilayer LECs presented underneath the anterior capsule in IOL implantation group, while LECs were arranged regularly in a single layer in IOL-P(MPC-MAA) group (Fig. 6c, black arrowheads). The expression levels of EMT markers fibronectin (Fn) and α-smooth muscle actin (α-SMA) were lower in IOL-P(MPC-MAA) group compared to those in IOL group (Supplementary Fig. S5). TEM showed that in IOL group, LECs underneath the anterior capsule presented an elongated fibroblast-like appearance with massive ECM deposition (Supplementary Fig. S6a). However, in IOL-P(MPC-MAA) group, LECs maintained epithelial morphology with a few ECM depositions (Supplementary Fig. S6b). These results indicate that IOL-P(MPC-MAA) significantly suppressed LECs proliferation and EMT under the anterior capsule and thus inhibited ACO formation.
Figure 6. IOL-P(MPC-MAA) suppresses ACO formation after cataract surgery.
(a) Representative retroillumination slit lamp photos of anterior capsule opacification in each group 1 week, 2 weeks, 4 weeks, and 6 weeks after surgery, respectively. Black arrows indicate anterior capsule fibrosis and shrinkage. (b) Quantification of ACO scores in each group 6 weeks after surgery. n = 8. **P < 0.01, ***P < 0.001. (c) Representative HE staining images of anterior capsule in each group 8 weeks after surgery. Black arrowheads indicate LECs under anterior capsule. AC: anterior capsule. Scale bar = 50 μm.
IOL-P(MPC-MAA) does not affect posterior capsule opacification
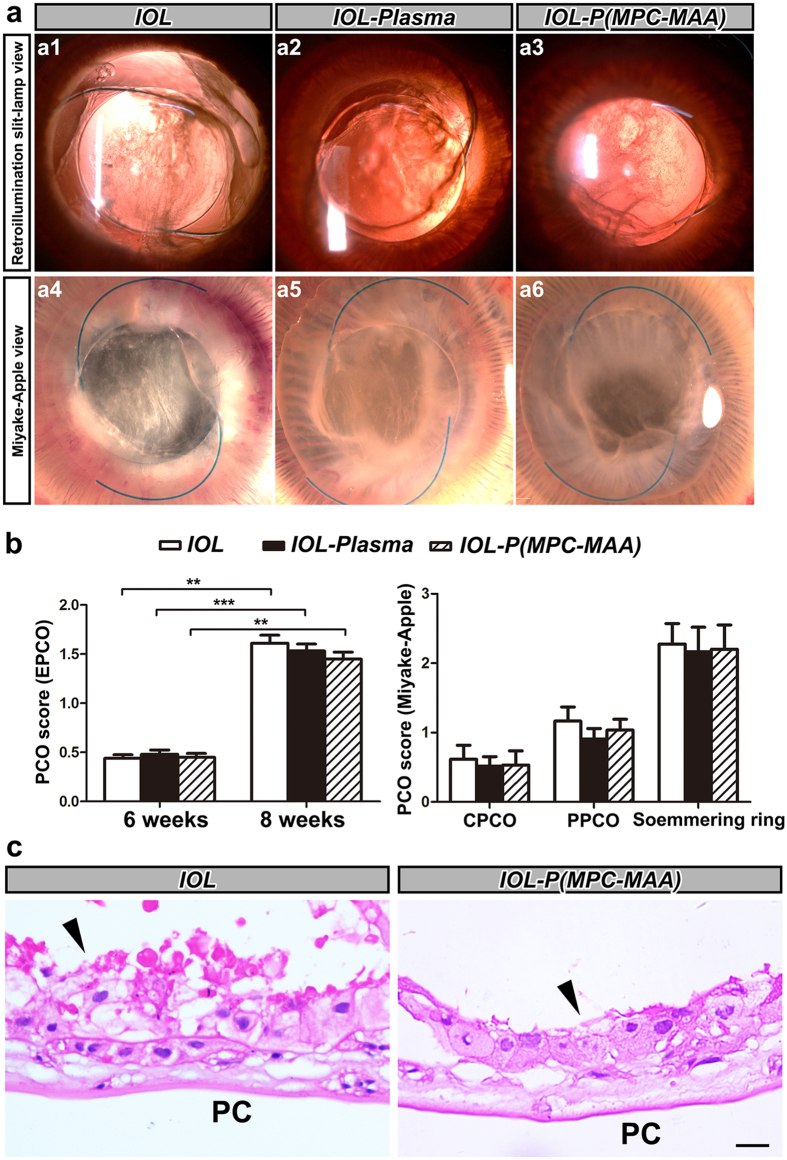
PCO is caused by migration of remnant LECs to the posterior capsule39. In our study, slit lamp examination showed that all three groups developed moderate PCO 8 weeks after surgery (Fig. 7a). Fundus examination showed that the optic disk, retinal vessels and choroid vessels could not be clearly seen 8 weeks postoperatively (Supplementary Fig. S7). EPCO 2000 analysis showed no significant difference in all the groups, and Miyake-Apple view analysis showed no difference of CPCO, PPCO and Soemmering’s area in all the groups (Fig. 7b). In both IOL and IOL-P(MPC-MAA) groups, LECs exhibited fibroblast-like morphology and were arranged irregularly underneath the posterior capsule (Fig. 7c, black arrowheads) with massive ECM surrounded (Supplementary Fig. S6c,d). These results suggest that MPC-MAA surface grafting does not affect PCO formation.
Figure 7. IOL-P(MPC-MAA) does not affect PCO formation after cataract surgery.
(a) Representative photos of posterior capsule opacification in each group 8 weeks after surgery. a1–a3: retroillumination slit-lamp view, a4–a6: Miyake-Apple view. (b) Quantification of PCO scores in each group by EPCO 2000 software or Miyake-Apple view analysis, respectively. n = 8. **P < 0.01, ***P < 0.001. CPCO: central posterior capsule opacification, PPCO: periphery posterior capsule opacification. (c) Representative HE staining images of posterior capsule in each group 8 weeks after surgery. Black arrowheads indicate migration and proliferation of LECs on the posterior capsule. PC: posterior capsule. Scale bar = 50 μm.
Discussion
Optimization of IOL biocompatibility is critical for vision reconstruction after cataract surgery. Uveal biocompatibility is typically important for hydrophobic acrylic IOL because many studies have shown that hydrophobic IOL will cause more inflammatory responses than hydrophilic IOL after implantation3,40,41. Both the cataract surgery and the IOL implant trigger the release and adhesion of inflammatory cells, including macrophages and giant cells, onto the IOL surface8, leading to a high incidence of IPS and ACO, especially in patients with blood-aqueous barrier damage. MPC has excellent biocompatibility since the phosphorylcholine group on MPC mimics the neutral phospholipids of the cell membrane14. Previous studies have shown that grafting MPC onto silicone IOL surface can reduce adhesion of macrophages20. However, grafting MPC monomers does not reduce aqueous flare in vivo, possibly due to inadequate negative charges on the material surface14. In this study, we synthesized the copolymer P(MPC-MAA) and covalently grafted this copolymer onto the surface of hydrophobic acrylic IOLs. Compared to MPC monomer, P(MPC-MAA) has two advantages. First, P(MPC-MAA) is heavily negatively charged (Fig. 3c). The introduction of negative charges by MAA resulted in a significant reduction of protein adsorption (Fig. 3d) and cell adhesion (Fig. 4). Second, the intermolecular repulsion between MAA in the copolymer could make more MPC diffuse into the aqueous humor, so that the IOL surface was more inert to the surrounding biological system. Therefore, P(MPC-MAA) modification significantly reduced post-operative inflammation after IOL implantation (Fig. 5a–c) and showed excellent biocompatibility in vivo.
The remaining anterior LECs (A cells) following cataract surgery have the potential to form fibrous tissue and cause capsular opacification around the capsulorhexis margin, resulting in ACO. Formation of ACO includes two stages: an early stage of LEC proliferation and a late stage involving EMT and ECM production. The LEC proliferation process is regulated by various cytokines and growth factors, such as IL-1, IL-6, transforming growth factor (TGF) and fibroblast growth factor (FGF), which are secreted by residual LECs and inflammatory cells42,43,44. Hydrophobic IOL has a higher incidence rate of ACO than hydrophilic IOL, because hydrophobic surfaces tend to attract more remnant LECs and inflammatory cells to adhere and proliferate5,6,45. Our results showed that surface modification by P(MPC-MAA) significantly suppressed ACO formation, which could be a direct consequence of decreased LECs adhesion and proliferation. Also suppression LECs and inflammatory cells adhesion may lead to less secretion of cytokines, contributing to a relatively mild inflammatory response in IOL-P(MPC-MAA) group than IOL and IOL-Plasma groups. This is consistent with other studies that hydrophilic surface modifications such as HSM coating38 or PEG-grafting15,46 could significantly reduce LECs adhesion and postoperative foreign-body reaction of hydrophobic IOL.
Posterior capsule opacification (PCO), also known as secondary cataract, results from proliferation, migration and EMT of residual LECs across the posterior capsule. In clinical application, hydrophobic IOL has a relatively low PCO rate compared to hydrophilic IOL, as the rapid adhesion of IOL to the posterior capsule can effectively inhibit the migration of LECs12,47. In this study, we did not observe a difference in PCO severity between IOL, IOL plasma and IOL-P(MPC-MAA) groups. Similarly, Xiaodan et al.14 also did not find a change in PCO incidence after grafting MPC on silicone IOL. It is possible that the surface property of IOL may be not as important as the optic configuration in the prevention of PCO. Many studies have shown that a sharp optic edge is the key factor for preventing LECs migration from anterior to the posterior capsule48,49,50. Although all the IOLs we used in this study had sharp optic edges, we still observed PCO formation in all the groups 8 weeks after surgery, possibly because rabbit LECs have higher proliferation and migration capacity than human LECs.
Interestingly, we noticed that introduction of amino groups by ammonia plasma treatment alone could also increase surface hydrophilicity, decrease protein adsorption and cell proliferation. However, our previous study showed that the increased hydrophilicity of IOL after plasma treatment can only last for 14 days51. On the contrary, covalent immobilization of hydrophilic molecules onto the material surface can greatly weaken the hydrophobic recovery process20,46. Here, we showed that although the hydrophilicities of IOL-Plasma and IOL-P(MPC-MAA) were comparable after modification, IOL-P(MPC-MAA) exhibited more protein resistance. Moreover, only IOL-P(MPC-MAA) showed decreased postoperative inflammation and ACO formation in vivo. Therefore, modification by plasma treatment alone is insufficient for the improvement of IOL biocompatibility.
In conclusion, we synthesized a new copolymer P(MPC-MAA) and successfully grafted the copolymer onto the surface of hydrophobic acrylic IOL by plasma technology. IOL-P(MPC-MAA) showed increased surface hydrophilicity and reduced protein adsorption while maintaining the bulk optical and physical properties. IOL-P(MPC-MAA) significantly inhibited LECs adhesion and proliferation in vitro. and suppressed postoperative inflammation and ACO formation in vivo. Overall, these results suggest that P(MPC-MAA) modification improved uveal and capsular biocompatibility of hydrophilic acrylic IOLs. More studies need to be carried out to assess the long-term biocompatibility of IOL-P(MPC-MAA).
Methods
Synthesis and purification of P(MPC-MAA)
0.01 mol MPC (Nanjing Institute of Natural Science and Technology Development, Nanjing, China) and 0.01 mol MAA (Kemiou Chemical Reagent Co., Ltd, Tianjing, China) were dissolved in 20 g of ultrapure water (monomer concentration: 1 mol/L). After argon was introduced for 30 minutes, a sodium sulfite/ammonium sulfate initiator system (ammonium persulfate, sodium sulfite = 1:1.5) was added at a concentration of 0.03 mol/L. The reaction was carried out at 37 °C for 24 hours and was stopped with liquid nitrogen. After introducing anhydrous ethanol, sedimentation was carried out and collected by a suction filter. The sediment was again dissolved in water and dialyzed for 3 days. Then, the sample was freeze-dried for 2 days, and P(MPC-MAA) was obtained. FT-IR spectra was obtained using an FT-IR analyzer (VECTOR-22, Bruker, Germany) with the potassium bromide pressed-disk technique for 32 scans over the 500–4,000 cm−1 range at a resolution of 4.0 cm−1. The composition of the polymers was determined by 1H NMR (AVANCE 300, Bruker, Germany) spectral measurements at 400 MHz.
P(MPC-MAA) grafting
Hydrophobic acrylic IOLs (EYEGOOD Medicals Co., Ltd, Zhuhai, China) were treated with ammonia plasma in a DL-01 model plasma generator (Omega Machinery Electronic Technology Co. Ltd., Suzhou, China) with power at 80 W for 15 minutes. P(MPC-MAA) solution was obtained containing EDC and HOBt (1-hydroxy-benzotriazole) at the ratio of 1:1.5:2 (-COOH:EDC:HOBt). IOLs were immersed in the 2.5% P(MPC-MAA) solution overnight, and dried under nitrogen flow. IOLs for cell assays and in vivo implantation were autoclaved with ethylene oxide, sealed in a sterilized package and stored at room temperature.
X-ray photoelectron spectroscopy(XPS)
XPS was measured by a photoelectron spectrometer (AXIS ULTR DLD, Kratos, England) using an Al Kα (1486.4 eV) monochromatic X-ray source at a pressure of 2 × 10−9 Torr and a scan area of 0.7 × 0.3 mm2. The high resolution scanning of elemental spectra was performed at 40 eV pass energy.
Atomic force microscopy(AFM)
Surface topography was tested with an atomic force microscope (MFP-3D-S, Asylum Research, USA). AFM imaging was performed under dry conditions at room temperature (24 ± 2 °C). Samples were analyzed over a 1.0 × 1.0 μm region at a resolution of 512 × 512 pixels. Root mean square roughness values were determined from height retrace images for each sample.
Static water contact angle (WCA) measurement
WCA was characterized with a contact angle goniometer (OCA15, Dataphysics, Germany) at 25 °C using distilled water as a reference liquid. A total of 1.00 μL of reference liquid was pumped onto the surface through a stainless steel needle at a rate of 1.0 μL/s. The results are mean values calculated from five independent measurements on different points of the films.
Zeta potential measurement
Zeta potential was obtained using an electrokinetic analyzer (SurPASS, Anton Paar Surpass, Austria). For the determination of zeta potential, streaming current measurements were performed using an Adjustable Gap Cell (SurPASS, Anton Paar Surpass, Austria). 1.0 mM potassium chloride (KCl) solution was used as the background electrolyte, and 0.1 M potassium hydroxide as well as 0.1 M hydrochloric acid solutions were used to adjust the pH value to 7.2.
Optical and physical characteristics
Diopter, resolution, transmission properties and anti-fatigue resistance of the IOL haptics were assessed according to the standards of State Food and Drug Administration (SFDA) by Medical Equipment Quality Supervision and Inspection Center of Zhejiang Province in China.
BSA adsorption assay
BSA adsorption of the surface was measured by quartz crystal microbalance with dissipation (QCM-D, E4, Q-Sense, Sweden). Briefly, the BSA solution (dissolved in PBS buffer at a concentration of 50 μg/mL) was introduced onto the samples. After balancing, PBS was introduced again to wash off the non-adsorbed protein. Then, BSA adsorption was obtained from Q-Tools.
Cell adhesion assay
IOLs were placed into a 48-well plate. 300 μL SRA01/04 cell (human lens epithelial cell) suspension at a concentration of 1 × 104/mL was loaded onto the IOL surface. After incubation for 12 hours, the IOLs were stained with hematoxylin and eosin (HE) and examined with an inverted phase contrast microscope (CKX41, Olympus, Japan). Five fields were selected with one in the central and four in peripheral quadrants at random. Image-Pro Plus 6.0 was used to quantify the number of LECs in each field. At least 5 IOLs in each group were tested.
Cell viability assay
The seeding procedure was the same as described above. After incubation for 24 or 48 hours, 200 μL Dulbecco’s Modified Eagle Medium (DMEM) with 10% fetal bovine serum (FBS) and 20 μL Cell Counting Kit-8 (CCK-8) reagent were added to IOLs. Wells without IOLs were used as controls. After incubation for 1 hour, the OD values at 450 nm were measured with a microplate reader. The assay was repeated 3 times.
Phacoemulsification and IOL implantation
Twenty-four 1.5 kg male New Zealand albino rabbits were divided into three groups at random. Phacoemulsification was performed on the left eye using the Legacy 20000 System (Alcon Laboratories, Fort Worth, TX, USA). Briefly, a 3.2-mm corneal limbus tunnel incision was made at the 12 o’clock position, followed by a central continuous curvilinear capsulorhexis with 5.5 mm in diameter. Then, the lens materials were extracted and IOL was implanted into the capsule. The tunnel incision was closed with interrupted 10-0 nylon sutures. All surgeries were performed by one surgeon (M.X.W.), who was blind to the group assignment. All experiments were conducted in accordance with the ethical guidelines set forth by the Laboratory Animal Care and Use Committee of the Association for Research in Vision and Ophthalmology (ARVO). The study protocol was reviewed and approved by the Animal Ethics Committee of Zhongshan Ophthalmic Center, Sun Yat-sen University, China.
Follow-up ophthalmic examinations
Digital slit lamp photos were taken by the SL-D7 anterior eye segment analysis system (Topcon Medical Systems, Inc., Tokyo, Japan) at indicated times postoperatively. Intraocular pressure (IOP) was measured by a Tono-Pen tonometer (Reichert Inc., Seefeld, Germany). Fundus images were acquired by a fundus camera (Topcon Medical Systems, Inc., Tokyo, Japan). All examinations were conducted by two researchers who were blind to the group assignment. Serious PCO usually occurred at 8 weeks due to the strong proliferative ability of rabbit LECs, so we defined 8 weeks postoperatively as the endpoint of the ophthalmic examinations.
Inflammation evaluation
Anterior chamber flare (ACF), anterior chamber cell (ACC), iris posterior synechiae (IPS) were scored to evaluate uveal biocompatibility of the IOL as previously described52. The grading is summarized in Supplementary Table S2. Postoperative complications, such as corneal edema, glaucoma, IOL displacement, pupil capture, and cortical proliferation, were also recorded.
ACO scoring
Six weeks after surgery, ACO was scored from grade 0 to IV based on the severity of the anterior capsule opacity and the contraction of the anterior capsulorhexis opening: Grade 0: clear (transparent) anterior capsule; Grade I: opacification localized at the edge of the capsulorhexis; Grade II: moderate and diffuse opacification, in some cases with areas of capsular folding; Grade III: intense opacification, with areas of capsular folding; Grade IV: constriction (phimosis) of the capsulorhexis opening45.
PCO scoring
PCO was quantified by Evaluation of Posterior Capsule Opacification (EPCO) 2000 software or Miyake-Apple view analysis. Standard retroillumination pictures were taken 6 and 8 weeks postoperatively, imported into EPCO 2000 and processed as previously described53.
Eight weeks after surgery, the rabbits were euthanatized and the eye balls were enucleated for Miyake-Apple view analysis. Eye balls were sectioned at the equator and gross examinations were performed from the posterior aspect. Miyake-Apple view analysis of PCO was conducted as previously described54.
Hematoxylin-eosin (HE) staining
Three capsules with IOLs in each group were fixed in 10% formalin for 2 hours, dehydrated and embedded in paraffin. Sections were cut on a microtome (RM2235, Leica, Germany) at 4 μm and stained with HE.
Immunofluorescent staining
Three capsules without IOLs in each group were embedded and stored at −80 °C. Sections (8 μm) were cut using a cryostat, fixed in cold acetone for 10 min, incubated with 0.2% Triton X-100 for 10 min, and blocked with 1% BSA. Sections were then incubated with primary antibodies at 4 °C overnight, and incubated with secondary antibody for 1 hour at room temperature. The sources and dilutions of antibodies are: mouse anti-fibronectin (1:100, ab6328, Abcam, MA, USA), mouse anti-α-SMA (1:100, ab7817, Abcam), Alexa Fluor 488-conjugated anti-mouse IgG (1:1000, #4408, Cell Signaling Technology, MA, USA) and Alexa Fluor 555-conjugated anti-mouse IgG (1:1000, #4409, Cell Signaling Technology).
Scanning electron microscopy(SEM)
Five IOLs in each group were extracted, fixed in 2.5% glutaraldehyde, and post-fixed in 1% osmium tetroxide. Dehydration was carried out through a graded ethanol series at room temperature. After critical drying point, the IOLs were sputter-coated with gold and examined using a scanning electron microscope (Quanta 200, FEI, Hillsboro, Oregon, USA).
Transmission electron microscopy (TEM)
Two capsules without IOLs were fixed in 2.5% glutaraldehyde, post-fixed in 1% osmium tetroxide (w/v 0.1 M phosphate buffer), dehydrated through a graded ethanol series and embedded in resin. About 80 nm ultrathin sections were obtained and stained with uranyl acetate and lead citrate, and examined by a transmission electronic microscope (Tecnai G2 Spirit Twin, FEI, Hillsboro, Oregon, USA).
Statistical analysis
SPSS 17.0 software package (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. All data are represented as means ± S.D. Kruskal-Wallis test was used to analyze the ACC, ACF, IPS, ACO and PCO scores. One-way analysis of variance (ANOVA) followed by Bonferroni’s post hoc test was used to compare means of the other results. All statistical tests were two tailed. P < 0.05 was considered statistically significant.
Additional Information
How to cite this article: Tan, X. et al. Improvement of Uveal and Capsular Biocompatibility of Hydrophobic Acrylic Intraocular Lens by Surface Grafting with 2-Methacryloyloxyethyl Phosphorylcholine-Methacrylic Acid Copolymer. Sci. Rep. 7, 40462; doi: 10.1038/srep40462 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
This work was supported by the 973 Program (No. 2015CB964600), the Major International Joint Research Project (No. 81320108008), the Industry-University-Research Cooperation Project of the Ministry of Education of Guangdong Province (No. 2010B090400416), the Science and Technology Program of Guangdong Province (No. 2013B051000029), the National Natural Science Funds for Young Scholar (No. 81600707), the Science and Technology Program of Guangzhou City (No. 11A22060326), the National Key Technologies R&D Program (No. 2012BAI17B02), the Guangzhou Science and Technology Program (No. 201508020123), and the National Natural Science Foundation of China (No. 50803018). We thank EYEGOOD Medicals Co., Ltd for providing the foldable hydrophobic acrylic intraocular lens.
Footnotes
Author Contributions X.H.T., J.Z.Z., J.C., Y.Z., M.X.W., Y.Z.L. and L.R. conceived and designed the study. X.H.T., Y.Z., Z.Z.L., Y.Y.Q. and M.X.W. conducted the animal experiments. J.Z.Z., J.C., L.W., S.L., Y.J.W. and L.R. developed the IOL. X.H.T., J.Z.Z., Y.Z. wrote the manuscript. M.X.W., Y.Z.L., and L.R. reviewed and approved the manuscript.
References
- Khairallah M. et al. Number of People Blind or Visually Impaired by Cataract Worldwide and in World Regions, 1990 to 2010. Invest Ophthalmol Vis Sci 56, 6762–6769, doi: 10.1167/iovs.15-17201 (2015). [DOI] [PubMed] [Google Scholar]
- Wang G. et al. In vivo implantation of hydrophobic acrylic intraocular lenses with surface modification. Eye Sci 28, 176–179 (2013). [PubMed] [Google Scholar]
- Abela-Formanek C., Amon M., Kahraman G., Schauersberger J. & Dunavoelgyi R. Biocompatibility of hydrophilic acrylic, hydrophobic acrylic, and silicone intraocular lenses in eyes with uveitis having cataract surgery: Long-term follow-up. J Cataract Refract Surg 37, 104–112, doi: 10.1016/j.jcrs.2010.07.038 (2011). [DOI] [PubMed] [Google Scholar]
- Richter-Mueksch S. et al. Uveal and capsular biocompatibility after implantation of sharp-edged hydrophilic acrylic, hydrophobic acrylic, and silicone intraocular lenses in eyes with pseudoexfoliation syndrome. J Cataract Refract Surg 33, 1414–1418, doi: 10.1016/j.jcrs.2007.05.009 (2007). [DOI] [PubMed] [Google Scholar]
- Saika S., Miyamoto T. & Ohnishi Y. Histology of anterior capsule opacification with a polyHEMA/HOHEXMA hydrophilic hydrogel intraocular lens compared to poly(methyl methacrylate), silicone, and acrylic lenses. J Cataract Refract Surg 29, 1198–1203 (2003). [DOI] [PubMed] [Google Scholar]
- Miyake K., Ota I., Miyake S. & Maekubo K. Correlation between intraocular lens hydrophilicity and anterior capsule opacification and aqueous flare. J Cataract Refract Surg 22 Suppl 1, 764–769 (1996). [DOI] [PubMed] [Google Scholar]
- Macky T. A. et al. Anterior capsule opacification. Int Ophthalmol Clin 41, 17–31 (2001). [DOI] [PubMed] [Google Scholar]
- Werner L. Biocompatibility of intraocular lens materials. Curr Opin Ophthalmol 19, 41–49, doi: 10.1097/ICU.0b013e3282f20132 (2008). [DOI] [PubMed] [Google Scholar]
- Liu Y., Luo L., He M. & Liu X. Disorders of the blood-aqueous barrier after phacoemulsification in diabetic patients. Eye ( Lond) 18, 900–904, doi: 10.1038/sj.eye.6701349 (2004). [DOI] [PubMed] [Google Scholar]
- Amon M. & Menapace R. In vivo documentation of cellular reactions on lens surfaces for assessing the biocompatibility of different intraocular implants. Eye ( Lond) 8(Pt 6), 649–656, doi: 10.1038/eye.1994.161 (1994). [DOI] [PubMed] [Google Scholar]
- Mamalis N. Intraocular lens biocompatibility. J Cataract Refract Surg 28, 1–2 (2002). [DOI] [PubMed] [Google Scholar]
- Saika S. Relationship between posterior capsule opacification and intraocular lens biocompatibility. Prog Retin Eye Res 23, 283–305 (2004). [DOI] [PubMed] [Google Scholar]
- Parsons J. T., Horwitz A. R. & Schwartz M. A. Cell adhesion: integrating cytoskeletal dynamics and cellular tension. Nat Rev Mol Cell Biol 11, 633–643, doi: 10.1038/nrm2957 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X. D., Yao K., Zhang Z., Zhang Y. & Wang Y. Uveal and capsular biocompatibility of an intraocular lens with a hydrophilic anterior surface and a hydrophobic posterior surface. J Cataract Refract Surg 36, 290–298, doi: 10.1016/j.jcrs.2009.09.027 (2010). [DOI] [PubMed] [Google Scholar]
- Kim M. K. et al. Effect of poly(ethylene glycol) graft polymerization of poly(methyl methacrylate) on cell adhesion. In vitro and in vivo study. J Cataract Refract Surg 27, 766–774 (2001). [DOI] [PubMed] [Google Scholar]
- Yamada K. et al. High-Density and Highly Surface Selective Adsorption of Protein–Nanoparticle Complexes by Controlling Electrostatic Interaction. Japanese Journal of Applied Physics 45, 4259–4264 (2006). [Google Scholar]
- Patil S., Sandberg A., Heckert E., Self W. & Seal S. Protein adsorption and cellular uptake of cerium oxide nanoparticles as a function of zeta potential. Biomaterials 28, 4600–4607, doi: 10.1016/j.biomaterials.2007.07.029 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X. D., Li H. Y., Lin L. & Yao K. Reduced silicone oil adherence to silicone intraocular lens by surface modification with 2-methacryloyloxyethyl phosphoryl-choline. Curr Eye Res 38, 91–96, doi: 10.3109/02713683.2012.704477 (2013). [DOI] [PubMed] [Google Scholar]
- Ishiyama N. et al. The prevention of peritendinous adhesions by a phospholipid polymer hydrogel formed in situ by spontaneous intermolecular interactions. Biomaterials 31, 4009–4016, doi: 10.1016/j.biomaterials.2010.01.100 (2010). [DOI] [PubMed] [Google Scholar]
- Yao K., Huang X. D., Huang X. J. & Xu Z. K. Improvement of the surface biocompatibility of silicone intraocular lens by the plasma-induced tethering of phospholipid moieties. J Biomed Mater Res A 78, 684–692, doi: 10.1002/jbm.a.30741 (2006). [DOI] [PubMed] [Google Scholar]
- Kyomoto M. & Ishihara K. Self-Initiated Surface Graft Polymerization of 2-Methacryloyloxyethyl Phosphorylcholine on Poly(ether ether ketone) by Photoirradiation. Acs Appl Mater Inter 1, 537–542 (2009). [DOI] [PubMed] [Google Scholar]
- Monge S., Canniccioni B., Graillot A. & Robin J. J. Phosphorus-containing polymers: a great opportunity for the biomedical field. Biomacromolecules 12, 1973–1982, doi: 10.1021/bm2004803 (2011). [DOI] [PubMed] [Google Scholar]
- Zheng Z. W., Ren L., Zhai Z. C., Wang Y. J. & Hang F. Surface modification on polyethylene terephthalate films with 2-methacryloyloxyethyl phosphorylcholine. Mat Sci Eng C-Mater 33, 3041–3046 (2013). [DOI] [PubMed] [Google Scholar]
- Khawly J. A., Lambert R. J. & Jaffe G. J. Intraocular lens changes after short- and long-term exposure to intraocular silicone oil. An in vivo study. Ophthalmology 105, 1227–1233, doi: 10.1016/S0161-6420(98)97025-7 (1998). [DOI] [PubMed] [Google Scholar]
- Shigeta M., Tanaka T., Koike N., Yamakawa N. & Usui M. Suppression of fibroblast and bacterial adhesion by MPC coating on acrylic intraocular lenses. J Cataract Refr Surg 32, 859–866 (2006). [DOI] [PubMed] [Google Scholar]
- Bondar O. V., Saifullina D., Shakhmaeva I., Mavlyutova I. & Abdullin T. Monitoring of the zeta potential of human cells upon reduction in their viability and interaction with polymers. Acta naturae 4, 78 (2012). [PMC free article] [PubMed] [Google Scholar]
- Nam K., Watanabe J. & Ishihara K. The characteristics of spontaneously forming physically cross-linked hydrogels composed of two water-soluble phospholipid polymers for oral drug delivery carrier I: hydrogel dissolution and insulin release under neutral pH condition. European journal of pharmaceutical sciences: official journal of the European Federation for Pharmaceutical Sciences 23, 261–270, doi: 10.1016/j.ejps.2004.07.012 (2004). [DOI] [PubMed] [Google Scholar]
- Tranoudis I. & Efron N. Parameter stability of soft contact lenses made from different materials. Contact Lens and Anterior Eye 27, 115–131 (2004). [DOI] [PubMed] [Google Scholar]
- Tranoudis I. & Efron N. In-eye performance of soft contact lenses made from different materials. Contact Lens and Anterior Eye 27, 133–148 (2004). [DOI] [PubMed] [Google Scholar]
- Kyomoto M. et al. Self-initiated surface grafting with poly (2-methacryloyloxyethyl phosphorylcholine) on poly (ether-ether-ketone). Biomaterials 31, 1017–1024 (2010). [DOI] [PubMed] [Google Scholar]
- Kyomoto M. et al. Poly (2-methacryloyloxyethyl phosphorylcholine) grafting and vitamin E blending for high wear resistance and oxidative stability of orthopedic bearings. Biomaterials 35, 6677–6686 (2014). [DOI] [PubMed] [Google Scholar]
- Gong M. et al. Cell membrane mimetic films immobilized by synergistic grafting and crosslinking. Soft Matter 9, 4501–4508 (2013). [Google Scholar]
- Lu C.-Y. et al. Surface grafting density analysis of high anti-clotting PU-Si-g-P(MPC) films. Applied Surface Science 258, 3920–3926, doi: 10.1016/j.apsusc.2011.12.064 (2012). [DOI] [Google Scholar]
- He F., Li J. & Ye J. Improvement of cell response of the poly(lactic-co-glycolic acid)/calcium phosphate cement composite scaffold with unidirectional pore structure by the surface immobilization of collagen via plasma treatment. Colloids and Surfaces B: Biointerfaces 103, 209–216, doi: 10.1016/j.colsurfb.2012.10.018 (2013). [DOI] [PubMed] [Google Scholar]
- Tanaka T., Shigeta M., Yamakawa N. & Usui M. Cell adhesion to acrylic intraocular lens associated with lens surface properties. J Cataract Refract Surg 31, 1648–1651, doi: 10.1016/j.jcrs.2004.11.050 (2005). [DOI] [PubMed] [Google Scholar]
- Zheng Z., Jiao Y., Ren L. & Wang Y. Biological protein-resistance layer construction of recombinant hirudin on polymethyl methacrylate IOL surface. J Biomed Mater Res A 103, 878–886, doi: 10.1002/jbm.a.35226 (2015). [DOI] [PubMed] [Google Scholar]
- Freddo T. F. A contemporary concept of the blood-aqueous barrier. Prog Retin Eye Res 32, 181–195 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krall E. M. et al. Intraindividual aqueous flare comparison after implantation of hydrophobic intraocular lenses with or without a heparin-coated surface. J Cataract Refract Surg 40, 1363–1370, doi: 10.1016/j.jcrs.2013.11.043 (2014). [DOI] [PubMed] [Google Scholar]
- Raj S. M., Vasavada A. R., Johar S. R., Vasavada V. A. & Vasavada V. A. Post-operative capsular opacification: a review. Int J Biomed Sci 3, 237–250 (2007). [PMC free article] [PubMed] [Google Scholar]
- Abela-Formanek C. et al. Uveal and capsular biocompatibility of hydrophilic acrylic, hydrophobic acrylic, and silicone intraocular lenses. Journal of Cataract & Refractive Surgery 28, 50–61 (2002). [DOI] [PubMed] [Google Scholar]
- Mullner-Eidenbock A. et al. Cellular reaction on the anterior surface of 4 types of intraocular lenses. J Cataract Refract Surg 27, 734–740 (2001). [DOI] [PubMed] [Google Scholar]
- Nishi O., Nishi K., Fujiwara T., Shirasawa E. & Ohmoto Y. Effects of the cytokines on the proliferation of and collagen synthesis by human cataract lens epithelial cells. The British journal of ophthalmology 80, 63–68 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi O., Nishi K. & Ohmoto Y. Synthesis of interleukin-1, interleukin-6, and basic fibroblast growth factor by human cataract lens epithelial cells. J Cataract Refract Surg 22 Suppl 1, 852–858 (1996). [DOI] [PubMed] [Google Scholar]
- Tan X. et al. Sprouty2 Suppresses Epithelial-Mesenchymal Transition of Human Lens Epithelial Cells through Blockade of Smad2 and ERK1/2 Pathways. PLoS One 11, e0159275, doi: 10.1371/journal.pone.0159275 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner L. et al. Anterior capsule opacification: correlation of pathologic findings with clinical sequelae. Ophthalmology 108, 1675–1681 (2001). [DOI] [PubMed] [Google Scholar]
- Lin L., Wang Y., Huang X. D., Xu Z. K. & Yao K. Modification of hydrophobic acrylic intraocular lens with poly(ethylene glycol) by atmospheric pressure glow discharge: A facile approach. Applied Surface Science 256, 7354–7364 (2010). [Google Scholar]
- Zaugg B., Auffarth G. U., Borkenstein A., Apple D. J. & Kleinmann G. New concepts on “no space – no cells“. New information derived from almost 57 years of observation of implanted Ridley lens. Klinische Monatsblätter Für Augenheilkunde (2009). [Google Scholar]
- Mencucci R., Favuzza E., Boccalini C., Gicquel J. J. & Raimondi L. Square-edge intraocular lenses and epithelial lens cell proliferation: implications on posterior capsule opacification in an in vitro model. Bmc Ophthalmology 15, 1–5 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi O. & Nishi K. Preventing posterior capsule opacification by creating a discontinuous sharp bend in the capsule. Journal of Cataract & Refractive Surgery 25, 521–526 (1999). [DOI] [PubMed] [Google Scholar]
- Auffarth G. U., Golescu A., Becker K. A. & Völcker H. E. Quantification of posterior capsule opacification with round and sharp edge intraocular lenses. Ophthalmology 110, 772–780 (2003). [DOI] [PubMed] [Google Scholar]
- Zheng Z. W., Deng K. M., Ren L. & Wang Y. J. Surface Modification of Hydrophobic Acrylic Intraocular Lens by Ammonia Low-temperature Plasma. Chem J Chinese U 33, 1350–1354 (2012). [Google Scholar]
- Walters T. R., Goldberg D. F., Peace J. H. & Gow J. A. Bromfenac ophthalmic solution 0.07% dosed once daily for cataract surgery: results of 2 randomized controlled trials. Ophthalmology 121, 25–33, doi: 10.1016/j.ophtha.2013.07.006 (2014). [DOI] [PubMed] [Google Scholar]
- Zemaitiene R., Jasinskas V., Barzdziukas V. & Auffarth G. U. Prevention of posterior capsule opacification using different intraocular lenses (results of one-year clinical study). Medicina ( Kaunas) 40, 721–730 (2004). [PubMed] [Google Scholar]
- Nishi O., Nishi K. & Osakabe Y. Effect of intraocular lenses on preventing posterior capsule opacification: design versus material. J Cataract Refract Surg 30, 2170–2176, doi: 10.1016/j.jcrs.2004.05.022 (2004). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.