Abstract
IMPORTANCE
Neuroinflammation may play a role in epilepsy. Translocator protein 18 kDa (TSPO), a biomarker of neuroinflammation, is overexpressed on activated microglia and reactive astrocytes. A preliminary positron emission tomographic (PET) imaging study using carbon 11 ([11C])–labeled PBR28 in patients with temporal lobe epilepsy (TLE) found increased TSPO ipsilateral to seizure foci. Full quantitation of TSPO in vivo is needed to detect widespread inflammation in the epileptic brain.
OBJECTIVES
To determine whether patients with TLE have widespread TSPO overexpression using [11C]PBR28 PET imaging, and to replicate relative ipsilateral TSPO increases in patients with TLE using [11C]PBR28 and another TSPO radioligand, [11C]DPA-713.
DESIGN, SETTING, AND PARTICIPANTS
In a cohort study from March 2009 through September 2013 at the Clinical Epilepsy Section of the National Institute of Neurological Disorders and Stroke, participants underwent brain PET and a subset had concurrent arterial sampling. Twenty-three patients with TLE and 11 age-matched controls were scanned with [11C]PBR28, and 8 patients and 7 controls were scanned with [11C]DPA-713. Patients with TLE had unilateral temporal seizure foci based on ictal electroencephalography and structural magnetic resonance imaging. Participants with homozygous low-affinity TSPO binding were excluded.
MAIN OUTCOMES AND MEASURES
The [11C]PBR28 distribution volume (VT) corrected for free fraction (fP) was measured in patients with TLE and controls using FreeSurfer software and T1-weighted magnetic resonance imaging for anatomical localization of bilateral temporal and extratemporal regions. Side-to-side asymmetry in patients with TLE was calculated as the ratio of ipsilateral to contralateral [11C]PBR28 and [11C]DPA-713 standardized uptake values from temporal regions.
RESULTS
The [11C]PBR28 VT to fp ratio was higher in patients with TLE than in controls for all ipsilateral temporal regions (27%–42%; P < .05) and in contralateral hippocampus, amygdala, and temporal pole (approximately 30%–32%; P < .05). Individually, 12 patients, 10 with mesial temporal sclerosis, had asymmetrically increased hippocampal [11C]PBR28 uptake exceeding the 95% confidence interval of the controls. Binding of [11C]PBR28 was increased significantly in thalamus. Relative [11C]PBR28 and [11C]DPA-713 uptakes were higher ipsilateral than contralateral to seizure foci in patients with TLE ([11C]PBR28: 2%–6%; [11C]DPA-713: 4%–9%). Asymmetry of [11C]DPA-713 was greater than that of [11C]PBR28 (F = 29.4; P = .001).
CONCLUSIONS AND RELEVANCE
Binding of TSPO is increased both ipsilateral and contralateral to seizure foci in patients with TLE, suggesting ongoing inflammation. Anti-inflammatory therapy may play a role in treating drug-resistant epilepsy.
Recent data implicate neuroinflammation as both cause and consequence of disease in patients with epilepsy. Animal studies as well as surgical specimens from patients with drug-resistant temporal lobe epilepsy (TLE) suggest that inflammation plays a key role in pathophysiological processes.1–5 Intermediates including interleukins (ILs), tumor necrosis factor α, nuclear factor–kappa B, and high-mobility group box 1 protein are expressed and toll-like receptors are upregulated in animal models of epilepsy6; they are also found in several pathological entities, including mesial temporal sclerosis (MTS), focal cortical dysplasia (FCD), and dysembroplastic neuroepithelial tumors.7–10
Activated microglia, reactive astrocytes, and inflammatory intermediates may contribute to hyperexcitability in seizure foci.11 The process may be widespread; for instance, kainic acid–induced status epilepticus resulted in immediate microglial activation in injected hippocampus and delayed IL signaling in contralateral hemisphere.12 Toll-like receptor and IL-1β antagonists suppressed seizures in animal models.5,6 Corticotropin-stimulated endogenous corticosteroid release effectively treated infantile spasms.5
Translocator protein 18 kDa (TSPO), a biomarker for neuroinflammation, has been studied in patients with epilepsy and animal models. Normally expressed at low levels in brain, TSPO is overexpressed on activated microglia and reactive astrocytes.13 In rodent brain, uptake of TSPO ligands fluorine 18 ([18F])–labeled PBR111 and [18F]DPA-714 increased in temporal regions after status epilepticus.14 Autoradiographic studies using hydrogen 3 ([3H])–labeled–(R)-PK11195, the prototypical TSPO ligand, showed increased binding in MTS surgical specimens.15 Conflicting in vivo results using carbon 11 ([11C])–labeled–(R)-PK11195 in patients likely reflect a poor signal to noise ratio.
The second-generation TSPO ligand [11C]PBR28, with higher specific binding than [11C](R)-PK11195,16 has been used in vivo to study several central nervous system disorders, including epilepsy, Alzheimer disease, and multiple sclerosis.13,16,17 Another second-generation ligand, [11C]DPA-713, had greater specificity than [11C](R)-PK11195 in discriminating healthy from lesioned brain in a rat neurodegeneration model.18 In healthy humans, [11C]DPA-713 had a much larger total distribution volume (VT) than [11C](R)-PK11195.19 The ligand [11C]DPA-713 is less sensitive than [11C]PBR28 to a TSPO polymorphism that affects binding affinity for second-generation ligands.20 To our knowledge, [11C]DPA-713 has not yet been studied in epilepsy.
Using [11C]PBR28 in patients with TLE, we previously found increased TSPO ipsilateral to seizure foci relative to contralateral regions.17 Previous studies showed increased [11C](R)PK11195 binding in patients with Rasmussen encephalitis or FCD, but not TLE.21,22 Moreover, the asymmetry in our study was small, and without correcting for radioactivity in plasma we could not determine whether other brain regions were affected.
This study investigated whether TSPO is increased in regions distant from seizure foci by comparing the [11C]PBR28 distribution volume (VT) to free fraction (fp) ratio (VT/fp) from each hemisphere in patients with TLE with that of controls. We attempted to replicate previous findings of higher [11C]PBR28 uptake ipsilateral than contralateral to seizure foci in a larger patient sample, using more rigorous delineation of cortical regions. We compared [11C]PBR28 with another second-generation TSPO ligand, [11C]DPA-713, to gain preliminary experience with a ligand that might detect greater TSPO asymmetry.
Methods
Participants
Twenty-three patients with TLE (10 men, 13 women; mean [SD] age, 37 [10] years; mean [SD] weight, 82 [22] kg) were studied; 12 were included in our prior study.17 All 23 had [11C]PBR28 positron emission tomographic (PET) scans, 11 with arterial blood sampling, and 8 had [11C]DPA-713 scans (eTable 1 in the Supplement). To exclude possible physiological left-right TSPO asymmetry, we studied 11 controls with [11C]PBR28 (4 men, 7 women; mean [SD] age, 38 [11] years; mean [SD] weight, 78 [15] kg) and 7 with [11C]DPA-713 (2 men, 5 women; mean [SD] age, 33 [10] years; mean [SD] weight, 70 [15] kg). This study was approved by the National Institutes of Health Combined Neurosciences Institutional Review Board. Participants provided written informed consent before study participation.
Patients were referred to the Clinical Epilepsy Section, National Institute of Neurological Disorders and Stroke, National Institutes of Health. All were evaluated with ictal video electroencephalographic monitoring as well as 3-T Philips magnetic resonance imaging (MRI) with fluid-attenuated inversion recovery, T1-weighted (spin-echo and 3-dimensional magnetization-prepared rapid-acquisition gradient-echo), and T2-weighted sequences. Six patients subsequently had surgery. Patients were classified based on electroencephalographic findings, structural lesions on MRI, or surgical pathology (eTable 2 in the Supplement).
A recently discovered rs6971 polymorphism on the TSPO gene affects the binding affinity of second-generation TSPO PET ligands. The high (H)– and low (L)–affinity alleles are codominantly inherited, resulting in 3 genotypes: high-affinity binders (HABs; genotype HH), mixed-affinity binders (MABs; genotype HL), and low-affinity binders (genotype LL). To account for TSPO genotype, we determined each participant’s affinity status using in vitro TSPO binding assays from leukocytes,23 mathematically corrected for differences in [11C]PBR28 binding between HABs (7 patients with TLE, 5 controls) and MABs (4 patients with TLE, 6 controls). Exclusion criteria included lesions on MRI other than MTS or FCD, serious medical conditions, pregnancy, or history of substance abuse.
Radiochemistry
The ligands [11C]PBR28 and [11C]DPA-713 were synthesized according to Investigational New Drug Applications 76 441 and 116 950, respectively. Both ligands were synthesized with high radiochemical purity (>99%). At injection time, the mean (SD) specific activities were 315 (140) GBq/μmol in patients with TLE and 258 (129) GBq/μmol in controls for [11C]PBR28 and 212 (68) GBq/μmol in patients with TLE and 336 (236) GBq/μmol in controls for [11C]DPA-713.
PET Imaging
Following an 8-minute attenuation correction transmission scan, a bolus injection of [11C]PBR28 (mean [SD], patients with TLE: 678 [67] MBq, or 0.10 [0.07] nmol/kg; controls: 654 [70] MBq, or 0.09 [0.05] nmol/kg) or [11C]DPA-713 (mean [SD], patients with TLE: 696 [82] MBq, or 0.10 [0.04] nmol/kg; controls: 739 [10] MBq, or 0.10 [0.05] nmol/kg) was administered. Dynamic PET images were acquired for 90 minutes on an Advance scanner (GE Healthcare; all controls and 11 patients with TLE) or High-Resolution Research Tomograph scanner (Siemens Medical Solutions; 12 previously studied patients with TLE). The participants scanned with the High-Resolution Research Tomograph scanner did not have full quantitation and were used only to assess left-right asymmetry. We previously showed that the 2 scanners provide similar asymmetry measurements.17 Four patients with TLE had [11C]DPA-713 and [11C]PBR28 scans the same day; 4 had scans separated by 1 day to 8 weeks.
During [11C]PBR28 scans of 11 patients with TLE and 11 age-matched controls, arterial input functions were generated as previously described.16 Arterial blood was sampled every 15 seconds for the first 2.5 minutes after injection, with increasing intervals of 1 to 15 minutes until the end of the scan. The [11C]PBR28 concentration was measured after separation from radiometabolites by reversed-phase chromatography. Plasma [11C]PBR28 fP was measured and normalized to a standard measurement from pooled human plasma.16
Image Analysis
Brain regions were delineated using FreeSurfer software version 5.1.1 (http://surfer.nmr.mgh.harvard.edu) as previously described.16 Briefly, T1-weighted MRIs were resliced to 1-mm isovoxel space and then corrected for inhomogeneity, processed for skull stripping, and segmented into gray and white matter according to the intensity gradient and connectivity of voxels. Misclassified voxels were corrected by minimal manual work. Cortical gray matter structures were parcellated with a probabilistic labeling algorithm by inflating the gray matter–white matter boundary and overlaying curvature information on the inflated surface. Subcortical structures were segmented and labeled by probabilistic registration technique. The probabilistic mask was applied to PET images coregistered to T1-weighted MRI resliced into isovoxel space. Decay-corrected radioactivity was measured in 42 regions (separated into left and right).
Measurement of Brain Uptake and Distribution Volume
To detect bilateral TSPO increases, we compared [11C]PBR28 VT/fp between patients with TLE and controls. Formal quantitation of [11C]PBR28 binding in brain requires correction for plasma concentration. Therefore, we calculated VT in 11 patients with TLE and 11 controls by fitting brain and plasma time-activity curves to a 2-tissue compartment model as previously described.16 Following consensus nomenclature for reversibly binding radioligands, VT is the brain to plasma radioligand concentration equilibrium ratio and is also the sum of specific (VS) and nondisplaceable (VND) uptake.24 Because only free ligand enters brain, we used the VT to fP ratio as our primary outcome measure.
Before comparing VT/fP values, we corrected for TSPO genotype. The VT values for MABs were multiplied by 1.4 because [11C]PBR28 binding is approximately 40% higher in HABs than MABs.23 We then compared genotype-corrected VT/fP in ipsilateral and contralateral regions from patients with TLE with left and right, respectively, from controls.
To determine whether TSPO was increased in seizure foci, we compared ipsilateral and contralateral brain radioligand uptake for each ligand using averaged standardized uptake values (SUVs), which correct for body weight and injected activity, from 40 to 90 minutes after injection. To compare the 2 ligands, regional asymmetry indices (AIs) were calculated from SUVs: 200% × [(ipsilateral − contralateral)/ (ipsilateral + contralateral)]. We compared left and right regional ligand uptake in controls to exclude physiological asymmetry.
Statistical Analysis
Data were normally distributed and are summarized as mean (standard deviation). The primary analysis was repeated-measures analysis of variance, with region as within-subjects factor and group as between-subjects factor, to compare VT/fP between patients with TLE and controls in ipsilateral and contralateral regions separately. This analysis tests the main hypothesis of inflammation throughout the ipsilateral and contralateral hemispheres. It gains statistical sensitivity by investigating all regions simultaneously. Significant main effects of group were followed by regional t tests without correction for multiple comparisons. Because they are only assessed after demonstrating a significant main effect of overall ipsilateral or contralateral inflammation, significant post hoc contrasts are unlikely to represent false-positive findings. Patients with TLE with and without MTS were compared using t tests. We used within-subjects paired-samples t tests to compare ipsilateral and contralateral [11C]PBR28 or [11C]DPA-713 uptake, and we used repeated-measures analysis of variance and Pearson correlations to compare AIs for [11C]PBR28 and [11C]DPA-713. Differences were considered statistically significant at P < .05.
Results
[11C]PBR28 Binding in Patients With TLE and Controls
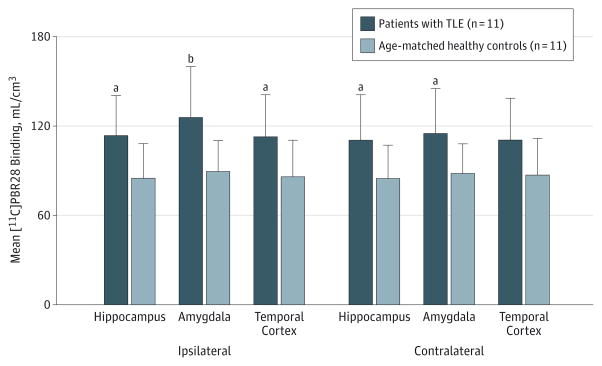
The VT/fP values were significantly higher in patients with TLE than controls in temporal and extratemporal regions ipsilateral (main effect of group: F1,20 = 5.7; P = .03) and contralateral (F1,20 = 4.4; P < .05) to seizure foci (Figure 1). In the ipsilateral hemisphere, patients with TLE had 27% to 42% higher [11C]PBR28 VT/fP values than controls in 6 temporal regions and in thalamus (all P < .05). Increases seen in cerebellum, frontal and occipital cortices, and striatum were not significant (.05 < P < .08).
Figure 1. Binding of Carbon 11 ([11C])–Labeled PBR28 in Temporal Brain Regions of Patients With Temporal Lobe Epilepsy (TLE) and Controls.
Higher [11C]PBR28 binding (measured as distribution volume [VT] to free fraction [fP] ratio, VT/fP) in patients with TLE than in controls ipsilateral and contralateral to seizure foci. Error bars indicate standard deviation.
aUncorrected P < .05.
bP < .01 for patients with TLE vs healthy controls by independent-samples t test.
In the contralateral hemisphere, patients with TLE had approximately 28% higher VT/fP values in temporal regions; results were significant in hippocampus (P = .04), amygdala (P = .02), and temporal pole (P = .04) and not significant in fusiform gyrus, temporal cortex, and entorhinal cortex/ parahippocampal gyrus (all .05 < P < .06). The VT/fP values were also increased in extratemporal regions; results were significant in the thalamus (P = .03). The [11C]PBR28 fP values had high variability but did not significantly differ between patients with TLE and controls (mean [SD], 0.04 [0.02] vs 0.05 [0.02], respectively; P = .59).
We investigated whether separate ipsilateral and contra-lateral SUV measurements (as opposed to ipsilateral vs contralateral asymmetry) could substitute for VT/fP, obviating the need for blood sampling. In contrast to VT/fP, SUVs did not significantly differ between patients with TLE who had blood sampling and controls (mean of 6 regions; mean [SD], ipsilateral: 1.0 [0.2] vs 1.0 [0.2] SUVs, respectively; contralateral: 1.0 [0.2] vs 1.0 [0.2] SUVs, respectively). Using a reference region by calculating regional to cerebellar SUV ratios revealed no significant group differences (mean for patients with TLE vs controls, ipsilateral: 1.1 vs 1.0, respectively; contralateral: 1.1 vs 1.0, respectively), except in ipsilateral temporal cortex (t = −2.8; P = .01).
Asymmetry of [11C]PBR28 and [11C]DPA-713 Uptake
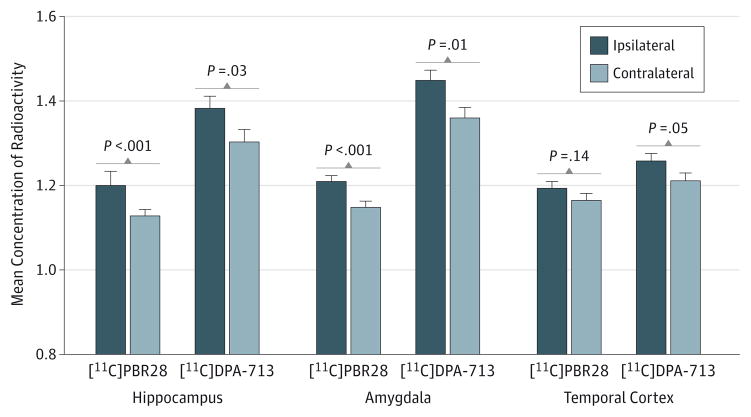
Patients with TLE had higher [11C]PBR28 and [11C]DPA-713 uptake ipsilateral than contralateral to seizure foci across temporal regions (Figure 2 and Figure 3). Ipsilateral [11C]PBR28 uptake ranged from 2% higher in fusiform gyrus and temporal cortex to 6% higher in amygdala. Mean differences were significant in hippocampus (t = 4.6; P < .001), amygdala (t = 4.2; P < .001), fusiform gyrus (t = 2.2; P = .04), and entorhinal cortex/parahippocampal gyrus (t = 3.1; P = .006) but not in temporal cortex (t = 1.5; P = .14) or pole (t = 2.0; P = .06). No significant left-right difference was observed in controls.
Figure 2. Ipsilateral vs Contralateral Carbon 11 ([11C])–Labeled PBR28 and [11C]DPA-713 Uptake.
Higher uptake of [11C]PBR28 and [11C]DPA-713 ipsilateral to seizure foci in patients with temporal lobe epilepsy. Concentration of radioactivity is expressed as the mean of standardized uptake values from 40- to 90-minute scan time. Columns indicate the means of 23 patients with temporal lobe epilepsy for [11C]PBR28 and 8 patients with temporal lobe epilepsy for [11C]DPA-713. Error bars indicate the within-subject 95% CIs. P values are from uncorrected paired-samples t tests.
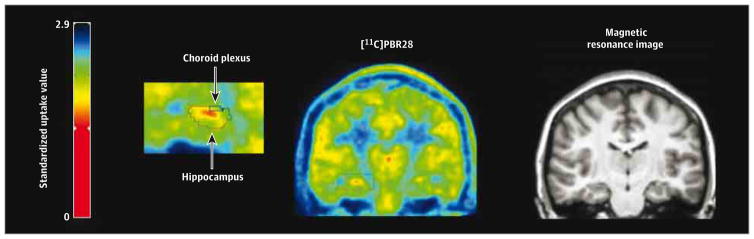
Figure 3. Increased Carbon 11 ([11C])–Labeled PBR28 Ipsilateral to Seizure Focus.
Patient with temporal lobe epilepsy with higher [11C]PBR28 uptake ipsilateral than contralateral to seizure focus. Patient with right-sided temporal lobe epilepsy and mesial temporal sclerosis on magnetic resonance imaging (right) shows increased uptake of [11C]PBR28 (left) ipsilateral to seizure focus. The left side of the image is the right hemisphere.
Twelve patients with TLE, 10 of whom had MTS, showed asymmetry in hippocampus more than 2 SDs greater than the control mean. We asked whether hippocampal atrophy might reduce radioligand asymmetry and found an inverse correlation between ipsilateral to contralateral hippocampal volume ratio and [11C]PBR28 AIs (r = −0.5; P = .02).
The[11C]PBR28 AIs in hippocampus were significantly higher in patients with MTS than in patients without MTS (8% vs 2%, respectively; P = .02) but not in other regions. In contrast, VT/fP did not differ based on the presence of MTS. There were no significant effects of seizure frequency (generalized tonic-clonic: F2,20 = 0.24; P = .80; complex partial: F3,19 = 0.87; P = .47) or epilepsy duration (F4,18 = 1.3; P = .43) on [11C]PBR28 uptake.
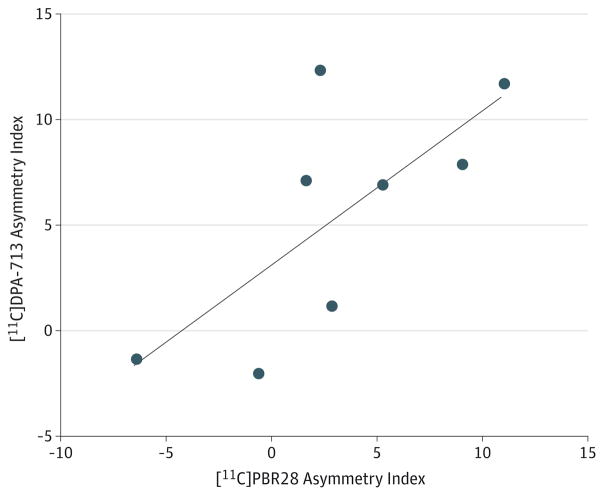
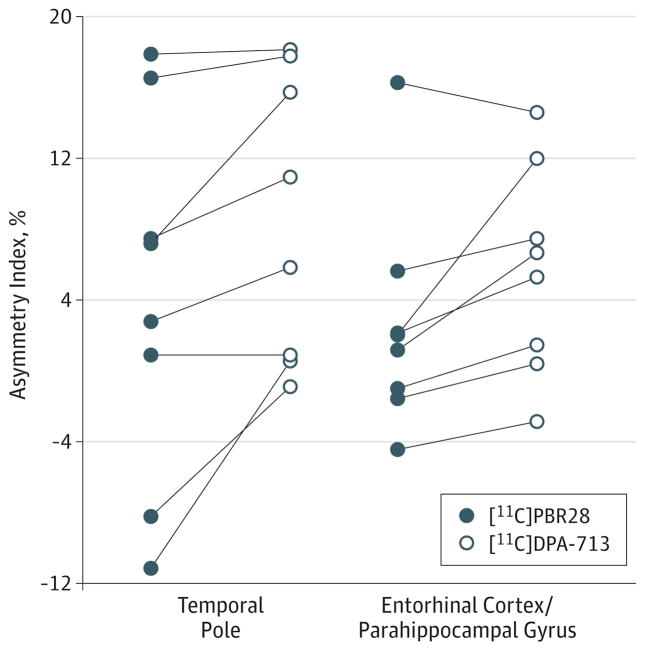
Consistent with [11C]PBR28 results, ipsilateral [11C]DPA-713 uptake ranged from 4% higher in fusiform gyrus and temporal cortex to 9% higher in temporal pole (Figure 2). Mean differences were significant in hippocampus (t = 2.6; P = .03), amygdala (t = 3.4; P = .01), fusiform gyrus (t = 2.5; P = .04), entorhinal cortex/parahippocampal gyrus (t = 2.8; P = .03), and temporal pole (t = 3.1; P = .02) but not in temporal cortex (t = 2.4; P = .05). Controls had a small but significant difference in [11C]DPA-713 uptake between the left and right fusiform gyrus (t = −3.8; P = .01). In 8 patients with TLE studied with both radioligands, AIs were well correlated (r = 0.7; P = .04) (Figure 4) but significantly higher for [11C]DPA-713 than [11C]PBR28 (F = 29.4; P = .001) (Figure 5).
Figure 4. Correlation of Carbon 11 ([11C])–Labeled DPA-713 and [11C]PBR28 Asymmetry Indices.
Each circle indicates the asymmetry index of 1 patient with temporal lobe epilepsy in hippocampus as a representative region. The asymmetry indices were calculated as 200% × [(ipsilateral - contralateral)/(ipsilateral + contralateral)]. Pearson r = 0.72 (P = .04). The line indicates the best-fit linear regression line, with a slope of 0.70 (95% CI, 0.02–1.39).
Figure 5. Comparison of Asymmetry Indices of Carbon 11 ([11C])–Labeled DPA-713 and [11C]PBR28.
Higher asymmetry indices of [11C]DPA-713 compared with [11C]PBR28. Regions shown had significant differences in asymmetry indices by repeated-measures analysis of variance and pairwise t tests. Each circle indicates the asymmetry index of a single patient with temporal lobe epilepsy. The asymmetry indices were calculated as 200% × [(ipsilateral - contralateral)/ (ipsilateral + contralateral)].
Discussion
We found increased TSPO in patients with TLE, extending beyond the seizure focus and involving bilateral regions. By using an automated brain segmentation procedure, we obviated observer bias inherent in the manual segmentation used in our prior study and reduced potential spillover from the choroid plexus.17 We detected relative TSPO binding increases with [11C]DPA-713, a pyrazolopyrimidineacetamide ligand, in addition to [11C]PBR28, an aryloxyanilide ligand. We replicated our prior finding of relatively increased ipsilateral [11C]PBR28 brain uptake in a larger patient sample and found even greater increases with [11C]DPA-713. Taken together, the data show that TSPO asymmetry in patients with TLE is a robust finding, independent of segmentation procedure and radioligand chemical class.
Elevated absolute [11C]PBR28 binding in regions distant from seizure foci suggests that inflammation in TLE is widespread, which may help explain low side-to-side binding asymmetry. Despite approximately 30% increases in VT/fP, SUVs did not significantly differ between patients with TLE and controls. Therefore, full quantitation with an arterial input function is likely required to detect widespread TSPO changes in vivo.
Hippocampal [11C]PBR28 uptake asymmetry was significantly greater in patients with MTS than in those without MTS. Expression of TSPO may be greater in MTS than other TLE pathological substrates. Moreover, increasing ipsilateral hippocampal volume loss predicted greater relative increases in ipsilateral [11C]PBR28 uptake. This finding is particularly interesting, as hippocampal volume loss might have been predicted to reduce binding. However, volume loss reflects focal gliosis and neuronal loss. The ligand [11C]PBR28 is a glial rather than neuronal marker; thus, greater relative increases in ipsilateral binding may reflect increasing MTS pathological severity. The association between ipsilateral hippocampal atrophy and increased [11C]PBR28 binding is consistent with an inflammatory component of MTS.
In contrast to [11C]PBR28 uptake asymmetry, absolute [11C]PBR28 binding did not differ significantly based on presence of MTS. It is possible that [11C]PBR28 lacks sensitivity to detect group differences in VT/fP, which contains noise from plasma data, and our cohort was small.
The VT corrected for fP is theoretically more accurate than VT alone because only free drug enters brain. Because it is a separate measurement, fP adds noise but may be important in epilepsy studies if antiepileptic drugs alter radioligand fP by plasma protein displacement.25 We therefore chose VT/fP as our primary outcome measure. No patients were taking antiepileptic drugs with known anti-inflammatory effects.
Increased in vivo [11C](R)-PK11195 uptake in the affected hemisphere has been reported in a few patients with encephalitis and 1 patient with FCD.21,22 No changes in [11C](R)-PK11195 uptake were observed in hippocampal sclerosis, likely owing to a low specific to nonspecific binding ratio.22 Here and in our prior study, [11C]PBR28 and [11C]DPA-713 detected increased activity in ipsilateral hippocampus despite atrophy. In addition to visible atrophy in some patients, ipsilateral hippocampal volume identified by FreeSurfer software was 13% smaller than contralateral (mean [SD], 3.9 [0.7] vs 4.5 [0.6] cm3, respectively). Greater asymmetry of [11C]DPA-713 compared with [11C]PBR28 likely reflects higher specific binding, which could be directly assessed using a blocking agent.26
Inflammation may lead to recurrent seizures in several ways. Altering glutamatergic and γ-aminobutyric acid–ergic neurotransmission may increase neuronal excitability. Proinflammatory cytokines are increased in various animal models of epilepsy, and their activation vs inactivation decreases and increases seizure threshold, respectively.9,27 These cytokines promote cell damage and excitotoxic effects, inhibit glutamate uptake, stimulate glutamatergic N-methyl-D-aspartate receptors, and reduce γ-aminobutyric acid receptor function.27
Seizure-related inflammation might contribute to cell loss and synaptic reorganization. In human mesial TLE and in epilepsy associated with FCD, IL-1β and IL receptor type 1 were expressed by astrocytes, microglia, and neurons.4 In human MTS, astroglial, microglial, and neuronal complement component C1q, C3c, and C3d expression occurred in association with neuronal loss.28
Inflammation may be linked to blood-brain barrier (BBB) alterations that can trigger seizures and lead to drug resistance. Disruption of the BBB in epilepsy models is associated with albumin deposition and IL-1β receptor activation.2 Transforming growth factor β activation may play an important role in epileptogenesis after BBB compromise.29 Neuroinflammation may lead to antiepileptic drug resistance by upregulating efflux transporters at the BBB.30 In addition, bilateral inflammation might help explain the failure of temporal lobectomy to achieve seizure freedom in more than 50% to 60% of patients, despite apparent well-localized seizure onset.31
One limitation of our AI measurements is that TSPO affinity status was not determined in patients with TLE from the prior study, which was completed before the polymorphism was discovered. To estimate the effects of TSPO genotype on [11C]PBR28 AIs, we considered VT and VND values from healthy humans.26 A 10% increase in specific binding (SUVS, or SUVT - SUVND) translates to a higher AI in HABs than in MABs (5% vs 3%, respectively). The [11C]DPA-713 SUVs were not corrected for TSPO genotype because VND was not known.
Disruption of the BBB near seizure foci could allow greater radiometabolite brain entry and inflate observed TSPO asymmetry. However, in vitro autoradiography studies demonstrate TSPO overexpression in surgical tissue from patients with TLE.15 Also, we found absolute TSPO increases contralateral to seizure foci, where the BBB should be intact.
Spillover from the choroid plexus might contaminate signal in adjacent regions. We minimized the effect by separating the choroid plexus with FreeSurfer software and found almost symmetric [11C]PBR28 uptake (mean AI, −1%), in contrast to significant asymmetry in hippocampus. Moreover, some areas were too distant (up to 4 cm) to be affected by choroid plexus signal (point spread function, GE camera: 0.7 cm). We found increased absolute TSPO binding in extratemporal regions in patients with TLE compared with controls.
Conclusions
This study demonstrates that patients with TLE have increased TSPO in both temporal lobes and extratemporal regions and confirms that the effect is most pronounced in temporal regions ipsilateral to seizure foci and in patients with MTS. These findings suggest a role for neuroinflammation in TLE and support development of anti-inflammatory approaches to treating drug-resistant epilepsy.
Acknowledgments
Funding/Support: This work was supported by the Intramural Research Programs of the National Institute of Mental Health and National Institute of Neurological Disorders and Stroke, National Institutes of Health.
Footnotes
Conflict of Interest Disclosures: None reported.
Role of the Funder/Sponsor: All authors except Ms Gershen were employees of the National Institutes of Health when this research was performed, and they directed the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, and approval of the manuscript; and decision to submit the manuscript for publication.
Supplemental content at jamaneurology.com
Author Contributions: Ms Gershen and Dr Zanotti-Fregonara had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Ms Gershen and Dr Zanotti-Fregonara are co–first authors and contributed equally to the study. Study concept and design: Zanotti-Fregonara, Kreisl, Innis, Theodore.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Gershen, Zanotti-Fregonara, Hirvonen, Theodore.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Gershen, Hirvonen, Hong, Theodore.
Obtained funding: Innis, Theodore.
Administrative, technical, or material support: Dustin, Kreisl, Brouwer, Zoghbi, Innis, Theodore. Study supervision: Zanotti-Fregonara, Hirvonen, Pike, Innis, Theodore.
Additional Contributions: Desiree Ferraris Araneta, CRNP, and Denise Rallis-Frutos, DNP, APRN/PMH, MsED, MSN, National Institutes of Health, Bethesda, Maryland, provided clinical coverage for positron emission tomographic scanning. Ioline Henter, MA, National Institutes of Health, provided editorial support. Daniel Goldenholz, MD, PhD, National Institutes of Health, participated in clinical patient evaluation. Alicia Woock, BA, National Institutes of Health, assisted in radiometabolite analysis. All performed the work as part of their official US government duties and received no compensation for their efforts.
References
- 1.Aronica E, Ravizza T, Zurolo E, Vezzani A. Astrocyte immune responses in epilepsy. Glia. 2012;60(8):1258–1268. doi: 10.1002/glia.22312. [DOI] [PubMed] [Google Scholar]
- 2.Librizzi L, Noè F, Vezzani A, de Curtis M, Ravizza T. Seizure-induced brain-borne inflammation sustains seizure recurrence and blood-brain barrier damage. Ann Neurol. 2012;72(1):82–90. doi: 10.1002/ana.23567. [DOI] [PubMed] [Google Scholar]
- 3.Ravizza T, Vezzani A. Status epilepticus induces time-dependent neuronal and astrocytic expression of interleukin-1 receptor type I in the rat limbic system. Neuroscience. 2006;137(1):301–308. doi: 10.1016/j.neuroscience.2005.07.063. [DOI] [PubMed] [Google Scholar]
- 4.Ravizza T, Gagliardi B, Noé F, Boer K, Aronica E, Vezzani A. Innate and adaptive immunity during epileptogenesis and spontaneous seizures: evidence from experimental models and human temporal lobe epilepsy. Neurobiol Dis. 2008;29(1):142–160. doi: 10.1016/j.nbd.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 5.Vezzani A, French J, Bartfai T, Baram TZ. The role of inflammation in epilepsy. Nat Rev Neurol. 2011;7(1):31–40. doi: 10.1038/nrneurol.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maroso M, Balosso S, Ravizza T, et al. Toll-like receptor 4 and high-mobility group box-1 are involved in ictogenesis and can be targeted to reduce seizures. Nat Med. 2010;16(4):413–419. doi: 10.1038/nm.2127. [DOI] [PubMed] [Google Scholar]
- 7.Crespel A, Coubes P, Rousset M-C, et al. Inflammatory reactions in human medial temporal lobe epilepsy with hippocampal sclerosis. Brain Res. 2002;952(2):159–169. doi: 10.1016/s0006-8993(02)03050-0. [DOI] [PubMed] [Google Scholar]
- 8.Iyer A, Zurolo E, Spliet WGM, et al. Evaluation of the innate and adaptive immunity in type I and type II focal cortical dysplasias. Epilepsia. 2010;51(9):1763–1773. doi: 10.1111/j.1528-1167.2010.02547.x. [DOI] [PubMed] [Google Scholar]
- 9.Ravizza T, Boer K, Redeker S, et al. The IL-1β system in epilepsy-associated malformations of cortical development. Neurobiol Dis. 2006;24(1):128–143. doi: 10.1016/j.nbd.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 10.Zurolo E, Iyer A, Maroso M, et al. Activation of Toll-like receptor, RAGE and HMGB1 signalling in malformations of cortical development. Brain. 2011;134(pt 4):1015–1032. doi: 10.1093/brain/awr032. [DOI] [PubMed] [Google Scholar]
- 11.Ravizza T, Noé F, Zardoni D, Vaghi V, Sifringer M, Vezzani A. Interleukin converting enzyme inhibition impairs kindling epileptogenesis in rats by blocking astrocytic IL-1β production. Neurobiol Dis. 2008;31(3):327–333. doi: 10.1016/j.nbd.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 12.Pernot F, Heinrich C, Barbier L, et al. Inflammatory changes during epileptogenesis and spontaneous seizures in a mouse model of mesiotemporal lobe epilepsy. Epilepsia. 2011;52(12):2315–2325. doi: 10.1111/j.1528-1167.2011.03273.x. [DOI] [PubMed] [Google Scholar]
- 13.Chen MK, Guilarte TR. Translocator protein 18 kDa (TSPO): molecular sensor of brain injury and repair. Pharmacol Ther. 2008;118(1):1–17. doi: 10.1016/j.pharmthera.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dedeurwaerdere S, Callaghan PD, Pham T, et al. PET imaging of brain inflammation during early epileptogenesis in a rat model of temporal lobe epilepsy. EJNMMI Res. 2012;2(1):60. doi: 10.1186/2191-219X-2-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson EW, de Lanerolle NC, Kim JH, et al. “Central” and “peripheral” benzodiazepine receptors: opposite changes in human epileptogenic tissue. Neurology. 1992;42(4):811–815. doi: 10.1212/wnl.42.4.811. [DOI] [PubMed] [Google Scholar]
- 16.Kreisl WC, Lyoo CH, McGwier M, et al. Biomarkers Consortium PET Radioligand Project Team. In vivo radioligand binding to translocator protein correlates with severity of Alzheimer’s disease. Brain. 2013;136(pt 7):2228–2238. doi: 10.1093/brain/awt145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirvonen J, Kreisl WC, Fujita M, et al. Increased in vivo expression of an inflammatory marker in temporal lobe epilepsy. J Nucl Med. 2012;53(2):234–240. doi: 10.2967/jnumed.111.091694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boutin H, Chauveau F, Thominiaux C, et al. 11C-DPA-713: a novel peripheral benzodiazepine receptor PET ligand for in vivo imaging of neuroinflammation. J Nucl Med. 2007;48(4):573–581. doi: 10.2967/jnumed.106.036764. [DOI] [PubMed] [Google Scholar]
- 19.Endres CJ, Pomper MG, James M, et al. Initial evaluation of 11C-DPA-713, a novel TSPO PET ligand, in humans. J Nucl Med. 2009;50(8):1276–1282. doi: 10.2967/jnumed.109.062265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Owen DRJ, Gunn RN, Rabiner EA, et al. Mixed-affinity binding in humans with 18-kDa translocator protein ligands. J Nucl Med. 2011;52(1):24–32. doi: 10.2967/jnumed.110.079459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Butler T, Ichise M, Teich AF, et al. Imaging inflammation in a patient with epilepsy due to focal cortical dysplasia. J Neuroimaging. 2013;23(1):129–131. doi: 10.1111/j.1552-6569.2010.00572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Banati RB, Goerres GW, Myers R, et al. [11C](R)-PK11195 positron emission tomography imaging of activated microglia in vivo in Rasmussen’s encephalitis. Neurology. 1999;53(9):2199–2203. doi: 10.1212/wnl.53.9.2199. [DOI] [PubMed] [Google Scholar]
- 23.Kreisl WC, Jenko KJ, Hines CS, et al. Biomarkers Consortium PET Radioligand Project Team. A genetic polymorphism for translocator protein 18 kDa affects both in vitro and in vivo radioligand binding in human brain to this putative biomarker of neuroinflammation. J Cereb Blood Flow Metab. 2013;33(1):53–58. doi: 10.1038/jcbfm.2012.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Innis RB, Cunningham VJ, Delforge J, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27(9):1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- 25.Theodore WH, Giovacchini G, Bonwetsch R, et al. The effect of antiepileptic drugs on 5-HT-receptor binding measured by positron emission tomography. Epilepsia. 2006;47(3):499–503. doi: 10.1111/j.1528-1167.2006.00458.x. [DOI] [PubMed] [Google Scholar]
- 26.Owen DR, Guo Q, Kalk NJ, et al. Determination of [(11)C]PBR28 binding potential in vivo: a first human TSPO blocking study. J Cereb Blood Flow Metab. 2014;34(6):989–994. doi: 10.1038/jcbfm.2014.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vezzani A, Balosso S, Ravizza T. The role of cytokines in the pathophysiology of epilepsy. Brain Behav Immun. 2008;22(6):797–803. doi: 10.1016/j.bbi.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 28.Aronica E, Boer K, van Vliet EA, et al. Complement activation in experimental and human temporal lobe epilepsy. Neurobiol Dis. 2007;26(3):497–511. doi: 10.1016/j.nbd.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 29.Kim SY, Buckwalter M, Soreq H, Vezzani A, Kaufer D. Blood-brain barrier dysfunction-induced inflammatory signaling in brain pathology and epileptogenesis. Epilepsia. 2012;53(suppl 6):37–44. doi: 10.1111/j.1528-1167.2012.03701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bauer B, Hartz AMS, Pekcec A, Toellner K, Miller DS, Potschka H. Seizure-induced up-regulation of P-glycoprotein at the blood-brain barrier through glutamate and cyclooxygenase-2 signaling. Mol Pharmacol. 2008;73(5):1444–1453. doi: 10.1124/mol.107.041210. [DOI] [PubMed] [Google Scholar]
- 31.Wiebe S, Blume WT, Girvin JP, Eliasziw M Effectiveness and Efficiency of Surgery for Temporal Lobe Epilepsy Study Group. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001;345(5):311–318. doi: 10.1056/NEJM200108023450501. [DOI] [PubMed] [Google Scholar]