Abstract
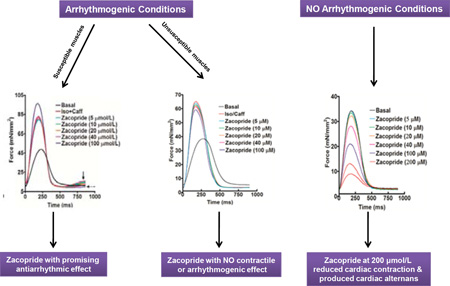
Ventricular tachycardia is the leading cause of sudden arrhythmic death in the U.S. Recently, the moderate IK1 channel activator, zacopride, was shown to suppress triggered ventricular tachycardia in rats. Nonetheless, concerns were raised about the possibility of pro-arrhythmic activity after IK1 channel stimulation based on the promising anti-arrhythmic strategy of IK1 blockade in other animal models. Therefore, the goal of the current study was to investigate the ex-vivo effects of zacopride on triggered arrhythmia and contractility in ventricular human myocardium in order to validate data that was solely obtained from animal models. Application of 100 nmol/L isoproterenol and 0.5 mmol/L caffeine led to triggered arrhythmia in isolated cardiac muscles from non-failing and end-stage failing hearts. However, the occurrence of arrhythmia in muscles of non-failing hearts was markedly higher than those of end-stage failing hearts. Interestingly, zacopride eliminated the ex-vivo triggered arrhythmia in these muscles of non-failing and failing hearts in a concentration-dependent manner, with an effective IC50 in the range of 28 to 40 µmol/L. Conversely, in the absence of isoproterenol/caffeine, zacopride led to a negative inotropic effect in a concentration-dependent manner. Reduced cardiac contraction was clearly observed at high zacopride concentration of 200 µmol/L, along with the occurrence of contractile alternans in muscles of non-failing and failing hearts. Zacopride shows promising antiarrhythmic effects against triggered arrhythmia in ventricular human myocardium. However, in the absence of Ca2+ overload/arrhythmia, zacopride, albeit at high concentrations, decreases the force of contraction and increases the likelihood of occurrence of contractile alternans, which may predispose the heart to contractile dysfunction and/or arrhythmia. Overall, our results represent a key step in translating this drug from the benchtop to the bedside in the research area.
Keywords: Zacopride, IK1 channel, Triggered Arrhythmia, Contractility, Human Heart
Graphical Abstract
Introduction
Ventricular tachycardia (VT) due to irregular ectopic beats is the leading cause of sudden arrhythmic death in the U.S [1, 2]. VT occurs in patients with structural heart disease [3] as well as in apparently healthy individuals mostly during exercise or stress-induced catecholamine flow [4]. Under different settings, abnormal diastolic Ca2+ release (DCR) through the ryanodine receptor (RyR2) on the sarcoplasmic reticulum (SR) causes this ectopic activity mainly in the form of delayed after depolarizations (DADs), resulting in triggered arrhythmias [5–8].
The cardiac inward rectifier potassium channel (IK1) is considered as the major conductance entity that regulates the resting potential (RP) and allows a considerable repolarization current during the last action potential (AP) phase. This big milieu of IK1 conductance generates very little resting membrane resistance. Yet, when the IK1 current reduction augments membrane resistance, a specified depolarizing membrane current could lead to a great extent of voltage deflection. This finding could signify a key mechanism for the occurrence of DADs-induced arrhythmias [9]. In cardiomyocytes from failing rat hearts, enhanced Ca2+ release through RyR2 has been reported to inhibit IK1, signifying a substrate for VT in heart failure (HF) [10]. Interestingly, a rise in non-adrenergic [Ca2+]i causes an increase of the IK1 current in dogs and humans, regardless of the mechanism and may provide an effective endogenous defense against cardiac arrhythmias induced by Ca2+ overload [11]. Also, during adrenergic stimulation, discrete modulatory pathways (e.g. PKA, PKC & CaMKII), through parallel IK1 targeting, may adjust it together and then develop the AP duration (APD) adaptation to varying conditions. Whereas adrenergic-induced activation of PKA and PKC reduces IK1 in both animals [12] and humans [13] and likely resulting in arrhythmia due to subsequently reduced repolarization reserve, the rise in [Ca2+]i and following activation of CaMKII leading to improved IK1 may neutralize these effects and prevent cardiac arrhythmias [11]. Hence, an anti-arrhythmic approach should augment the IK1 to get it back to a near-normal condition. As previously noted, an ideal agent for the treatment of VT may be a drug that enhances the opening of K+ channels at the RP but not at the AP peak, which means a drug that augments the IK1 current activation [9, 14]. However, the lack of IK1-specific pharmacological tools, in particular a specific agonist, was a major constraint to further investigate IK1 in the past [14].
Recently, a novel discovery of IK1 channel agonist, zacopride, has been published [15]. Zacopride was originally reported as a potent antagonist of 5-hydroxytryptamine (5-HT)3 and an agonist of 5-HT4 receptors that has been experimentally used as an anti-emetic, gastroprokinetic, and anxiolytic agent [9]. Zacopride has been reported to selectively activate the IK1 channel in rat ventricular myocytes, moderately enhance the IK1 current, hyperpolarize the RP, and decrease the APD exclusive of affecting other channels, transporters, and pumps, with subsequent suppression of triggered VT in rats [15]. Conversely, data from other studies on primates, rabbits and rats, have shown IK1 blockade as a promising anti-arrhythmic strategy [16, 17], and thus concerns were raised about the possibility of pro-arrhythmic activity after IK1 channel stimulation [17]. Indeed, humans exhibit lower repolarization reserve contributions from IK1 and IKs compared to animals, confirming species-specific determinants of repolarization and limitations of animal models for human diseases [18]. Consequently, it has been concluded that the effects of the IK1 channel agonists on heart rhythm/arrhythmias may potentially be coupled with the type of arrhythmia and species, with the need for further future investigation in humans [9, 15].
In addition to their beneficial antiarrhythmic effects, several antiarrhythmic drugs have considerable influences on myocardial contractility. Mainly, they exert negative inotropic effects that restrict their clinical use in patients with compromised cardiac function [19]. Thus, investigating the impact of antiarrhythmic drugs on the contractile profile of cardiac muscle represents an important complementary safety measure to their therapeutic efficacy. This would provide more comprehensive knowledge not only about the clinical efficacy but also about possible side effects of these drugs on the heart, which is critical for their transition towards clinical application.
Therefore, the goal of the current study was to investigate the ex-vivo effects of the moderate IK1 channel activator, zacopride, on triggered arrhythmia and contractility in ventricular myocardium of non-failing and end-stage failing human hearts.
Methods
Human Tissue Collection
All human tissues were used with approval from the Institutional Review Board (IRB) of The Ohio State University and in accordance with the Declaration of Helsinki. Informed consents were acquired from cardiac transplant patients. Non-transplantable donor hearts were acquired from Lifeline of Ohio Organ Procurement. As described previously [20], explanted hearts were obtained in the operating room, flushed immediately after removal from living donors, and thereafter transferred to the laboratory in cold cardioplegic solution containing (in mM): 110 NaCl, 16 KCl, 16 MgCl2, 10 NaHCO3, and 0.5 CaCl2. Ejection fraction of non-failing hearts, measured prior to procurement, was in the normal range with few exceptions. These exceptions did not translate to different results compared to other hearts with normal ejection fraction. None of the non-transplantable hearts had signs of coronary bypass surgery or prior myocardial infarctions. Wall thicknesses, heart weights, and heart weight-to-body weight ratios of these hearts were all in the normal range. Because it is not feasible to attain completely healthy “control” human hearts devoid of any sort of cardiovascular disease for research purposes since such hearts are almost exclusively utilized for cardiac transplantation, we opted to use the term “non-failing” as opposed to “control” in order to reflect this property as we described before [20]. End-stage failing hearts were acquired from patients undergoing cardiac transplantation at The Ohio State University Wexner Medical Center. All hearts, regardless of source, were processed with the identical protocol.
Trabeculae Isolation
Hearts were rapidly transferred from the cardioplegic solution to a cold modified Krebs–Henseleit buffer containing (in mmol/L): 120 NaCl, 5 KCl, 2 MgSO4, 1.2 NaH2PO4, 20 NaHCO3, 0.25 Ca2+, and 10 glucose, equilibrated with 95% O2-5% CO2, resulting in a pH of 7.4. Additionally, 20 mmol/L 2,3-butanedione monoxime (BDM) was added to the dissection buffer to prevent cutting injury [20]. Uniform linear trabeculae were carefully dissected from both right ventricle (RV) and left ventricle (LV), and then kept in this solution at 0–4°C until the time of the experiment (0–8 h). The dimensions of muscles were measured using a calibration reticule in the ocular of the dissection microscope (40×, resolution ~10 µm). The cross-sectional areas were calculated assuming ellipsoid cross-sectional shapes. Average dimensions (width × thickness × length) of RV and LV trabeculae from non-failing hearts were (0.33 ± 0.04 × 0.22 ± 0.03 × 3.03 ±0.53 mm) and (0.34 ±0.06 × 0.23 ± 0.04 × 3.16 ± 0.89 mm), respectively. Likewise, average dimensions of RV and LV trabeculae from end-stage failing hearts were (0.37 ± 0.04 × 0.25 ± 0.03 × 3.03 ± 0.47 mm) and (0.44 ± 0.05 × 0.30 ± 0.03 × 2.62 ± 0.22 mm), respectively.
With the use of the dissection microscope, muscles were mounted between the basket-shaped extension of a force transducer (KG7, Scientific Instruments, Heidelberg, Germany) and a hook connected to a micromanipulator as previously described [20, 21]. Muscles were superfused with the same buffer at 37°C as above (with the exception that BDM was omitted) and stimulated at close to physiologic frequency of 1 Hz. Extracellular Ca2+ concentration was raised to 2 mmol/L and muscles were allowed to stabilize for at least 30 minutes before the experimental protocol was initiated. Generally, muscles were stretched to an optimal length where a small increase in length resulted in nearly equal increases in resting tension and active developed tension. This length was selected to be comparable to the maximally attained length in-vivo at the end of diastole [22].
Arrhythmia Experiments
A total of 10 RV and 8 LV trabeculae from 10 different non-failing hearts, and 12 RV and 12 LV trabeculae from 14 different end-stage failing hearts were used in arrhythmia experiments. The characteristics of these hearts are provided in Table 1 and Table 2. These intact, twitch contracting trabeculae were allowed to develop a contractile homeostasis under near physiologic conditions (37 °C, 1 Hz at optimal length) in oxygenated Krebs-Henseleit buffer containing 2 mM Ca2+, and twitch amplitude and twitch kinetics were assessed at basal level. Once this data was collected, 100 nmol/L isoproterenol and 0.5 mmol/L caffeine were added to the perfusion solution to induce arrhythmia. Twitch contractions were monitored for 10 minutes for the development of any triggered activity. Zacopride was added at a concentration range of 5–100 µmol/L at 2 minute intervals, starting 1-to-3 minutes post arrhythmia; twitch data as well as triggered activities were recorded. Interval time and drug addition starting time were determined based on preliminary data showing that early addition of the drug is required for its antiarrhythmic efficacy (Supplementary Figure 1).
Table 1.
Characteristics of Non-failing Hearts – Arrhythmia Experiments
| Heart # | Age, yrs |
Sex | Race | Cause of Death | LVEF, % |
HR, BPM |
Heart Weight, g |
Arrhythmia After Iso/Caff |
|
|---|---|---|---|---|---|---|---|---|---|
| RV | LV | ||||||||
| 219852 | 30 | F | Caucasian | CE/RF | 55 | 135 | 299 | No | Yes |
| 331253 | 42 | F | Hispanic | CVA/ICH | 55 | 85 | 390 | Yes | Yes |
| 364587 | 19 | M | Caucasian | Blunt Injury/MVA | 25 | 131 | 300 | Yes | NA |
| 402879 | 54 | M | Caucasian | ICB/ICH | NA | NA | 474 | Yes | No |
| 415217 | 42 | M | Caucasian | ICB/ICH | NA | NA | 508 | Yes | Yes |
| 435578 | 20 | M | Caucasian | DO/Anoxia | 35 | 112 | 324 | Yes | NA |
| 514489 | 42 | F | Caucasian | Cardiac Arrest/Anoxia | 55 | 114 | 327 | Yes | Yes |
| 694855 | 46 | F | Caucasian | ICH/CVA and SAH | 60 | 94 | 356 | Yes | Yes |
| 785258 | 51 | F | Caucasian | ICH/CVA | 60 | 82 | 335 | No | No |
| 984478 | 54 | F | African American | ICB/ICH | 55 | 87 | 348 | Yes | No |
BPM, beat per minute; CE, cerebral edema; CVA, cerebral vascular accident; DO, drug overdose; F, female; HR, heart rate; ICB, intracerebral bleeding; ICH, intracerebral hemorrhage; Iso/Caff, Isoproterenol (100 nmol/L)/caffeine (0.5 mmol/L); LVEF, left ventricular ejection fraction; M, male; MVA, motor vehicle accident; NA, non applicable; RF, respiratory failure; SAH, subarachnoid hemorrhage; Yr, year.
Table 2.
Characteristics of Failing Hearts – Arrhythmia Experiments.
| Heart Heart ##Heart # |
Age, yrs | Sex | Race | Etiology | Heart Weight, g | Arrhythmia After Iso/Caff |
|
|---|---|---|---|---|---|---|---|
| RV | LV | ||||||
| 233587 | 65 | M | Caucasian | ICM | 692 | Yes | No |
| 369452 | 61 | M | African American | NICM | 540 | No | No |
| 390112 | 60 | F | Caucasian | NICM | 608 | No | No |
| 588415 | 52 | M | Caucasian | NICM | 390 | No | No |
| 599014 | 50 | M | Caucasian | NICM | 667 | No | No |
| 611422 | 68 | M | Caucasian | ICM | 576 | No | No |
| 631231 | 49 | F | Asian | CCM | 306 | No | NA |
| 645444 | 47 | M | African American | ICM | 411 | Yes | Yes |
| 728878 | 40 | M | Caucasian/African American | NICM | 747 | Yes | Yes |
| 736955 | 61 | F | Caucasian | ICM | 575 | No | No |
| 777902 | 59 | M | Caucasian | CAD | 530 | Yes | NA |
| 820447 | 68 | M | Caucasian | NICM | 567 | NA | No |
| 935541 | 51 | M | African American | NICM | 453 | NA | Yes |
| 971258 | 57 | M | Caucasian | ICM | 619 | No | No |
CAD, coronary artery disease; CCM, chemo-induced cardiomyopathy; F, female; ICM, ischemic cardiomyopathy; Iso/Caff, Isoproterenol (100 nmol/L)/caffeine (0.5 mmol/L); M, male; NA, non applicable; NICM, ischemic cardiomyopathy; yr, year.
Contractility Experiments
In another group of muscles, we studied the direct effects of zacopride on the contractile profile of ventricular myocardium. A total of 5 RV and 4 LV trabeculae from 5 different non-failing hearts, and 7 RV and 6 LV trabeculae from 6 different end-stage failing hearts were used in contractility experiments. The characteristics of these hearts are provided in Table 3 and Table 4. These intact, twitch contracting trabeculae were allowed to develop a contractile homeostasis at near physiologic conditions (37 °C, 1 Hz at optimal length) in oxygenated Krebs-Henseleit buffer containing 2 mM Ca2+; twitch amplitude and twitch kinetics were assessed at basal level. Once this data was collected, zacopride (5–200 µmol/L) was added at 3 minute intervals and twitch amplitude and twitch kinetics were assessed again.
Table 3.
Characteristics of Non-failing Hearts – Contractility Experiments
| Heart # | Age, yr | Sex | Race | Cause of Death | LVEF, % |
HR, BPM |
Heart Weight, g |
|---|---|---|---|---|---|---|---|
| 435578 | 20 | M | Caucasian | DO/Anoxia | 35 | 112 | 324 |
| 460025 | 69 | F | Caucasian | CVA/ICH | 60 | 109 | 435 |
| 925852 | 34 | F | African American | RF/Anoxia | 50 | 82 | 672 |
| 958987 | 40 | M | Caucasian | DO/Cardiac arrest | 60 | 113 | 615 |
| 987692 | 34 | F | Caucasian | MVA/Trauma | 30 | 112 | 313 |
BPM, beat per minute; CVA, cerebral vascular accident; DO, drug overdose; F, female; HR, heart rate; LVEF, left ventricular ejection fraction; M, male; MVA, motor vehicle accident; RF, respiratory failure; Yr, year.
Table 4.
Characteristics of Failing Hearts – Contractility Experiments
| Heart # | Age, yr | Sex | Race | Etiology | Heart Weight, g |
|---|---|---|---|---|---|
| 679533 | 47 | M | Caucasian | NICM | 930 |
| 777902 | 59 | M | Caucasian | CAD | 530 |
| 806554 | 39 | F | African American | NICM | 365 |
| 820447 | 68 | M | Caucasian | NICM | 567 |
| 904475 | 35 | F | Caucasian | PPCM | 262 |
| 497522 | 62 | F | Caucasian | NICM | 649 |
CAD, coronary artery disease; F, female; Iso/Caff, Isoproterenol (100 nmol/L)/caffeine (0.5 mmol/L); M, male; NICM, ischemic cardiomyopathy; PPCM, post partum cardiomyopathy; yr, year.
In all experiments, peak isometric developed force (Fdev) was determined and normalized to the cross-sectional area of the muscle. Additionally, as a force-independent parameter of force decay kinetics, time to peak force (TTP) and time from peak force to 50% relaxation (RT50) were determined.
Data Analysis and Statistics
Data are presented as mean ± SEM and were analyzed by one-way analysis of variance (ANOVA) followed by Dunnett Multiple Comparisons post-hoc test, comparing all groups to isoproterenol/caffeine group in arrhythmia experiments and to basal control group in contractility experiments. A two-tailed value of P ≤ 0.05 was considered statistically significant.
Results
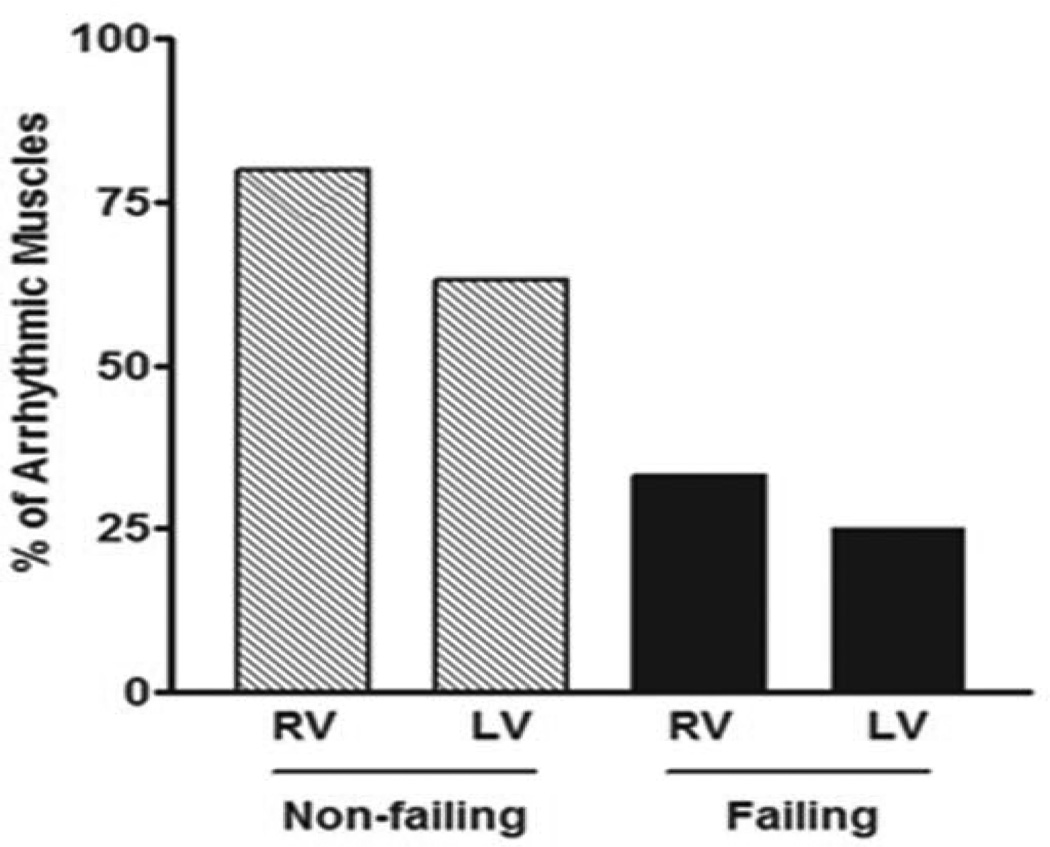
At near physiologic conditions (37 °C, 1 Hz, 2 mM Ca2+ at optimal length), our data showed that muscles from non-failing hearts were more susceptible to isoproterenol/caffeine - induced arrhythmia compared to failing hearts. Eighty percent (80 %) of RV muscles from non-failing hearts (8/10) showed triggered activity compared to ≈ 33% (4/12) of their failing counterparts. Similarly, ≈ 63 % of LV muscles from non-failing hearts (5/8) exhibited arrhythmic behavior compared to 25 % (3/12) of those from failing hearts (Figure 1).
Figure 1.
Percentage of arrhythmia occurrence in ventricular trabeculae isolated from non-failing [RV: right ventricle (8 out of 10) & LV: left ventricle (5 out of 8)] and failing [RV (4 out of 12) & LV (3 out of 12)] human hearts.
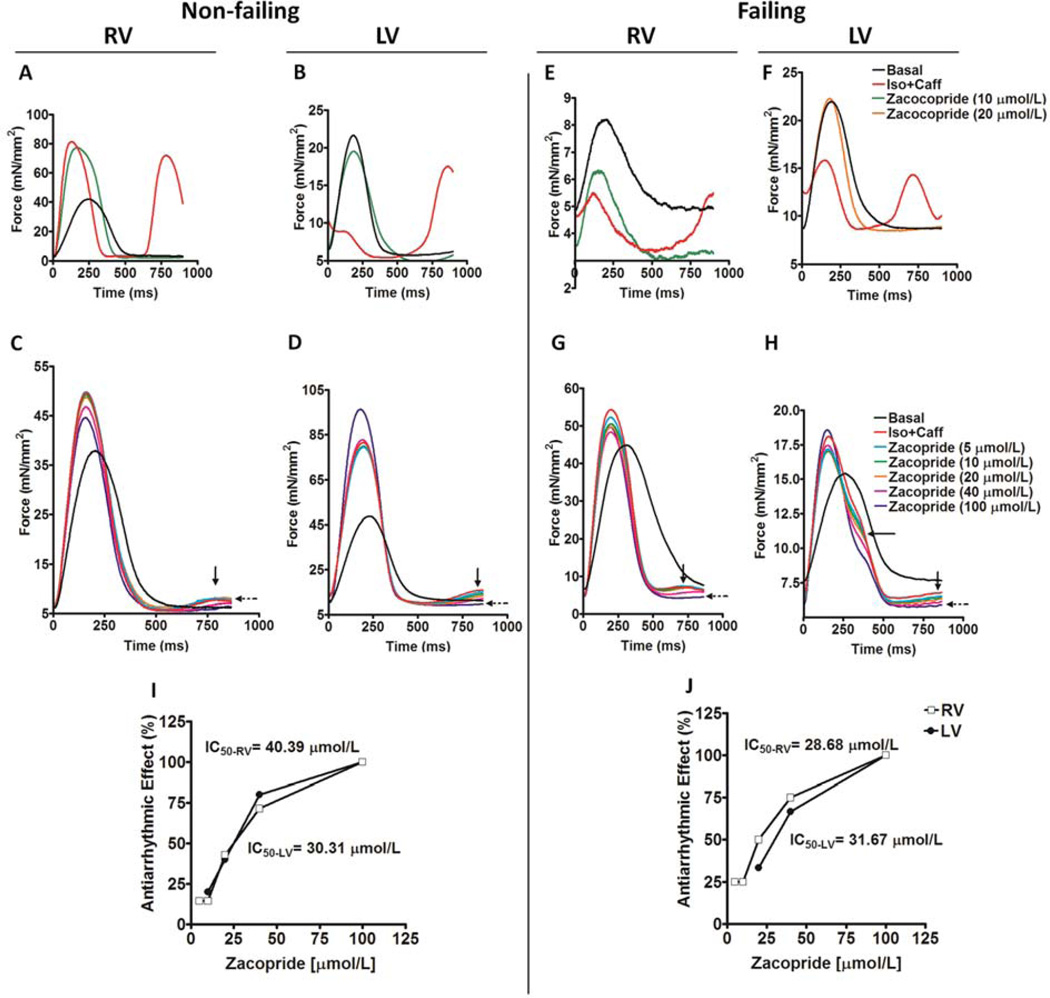
In the presence of isoproterenol/caffeine, arrhythmias in the form of extra systolic contractions with either high (Figure 2A and 2B) or low (Figure 2C and 2D) amplitudes were triggered in trabeculae isolated from both RV and LV of non-failing hearts, respectively. Similarly, isoproterenol/caffeine treatment resulted in triggered arrhythmia with either high (Figure 2E and 2F) or low (Figure 2G and 2H) amplitudes in trabeculae isolated from both RV and LV of failing hearts, respectively. Zacopride, added within 1 to 3 minutes after development of arrhythmia, was able to suppress these triggered activities of variant amplitudes in all susceptible muscles. Zacopride showed these effects in a concentration-dependent manner as is shown in Figure 2C, D, G and H. Calculated median inhibitory concentrations (IC50s) of zacopride in the RV and LV from non-failing hearts are 40.4 and 30.3 µmol/L, respectively (Figure 2I). However, calculated IC50s of zacopride in the RV and LV from failing hearts are 28.7 and 31.7 µmol/L, respectively (Figure 2J).
Figure 2.
Original representative recordings of twitches (normalized to cross-sectional area) in non-failing human myocardium at basal status, in presence of Iso + Caff [isoproterenol (100 nmol/L)/caffeine (0.5 mmol/L)] and zacopride effective antiarrhythmic concentrations when arrhythmias in the form of extra systolic contractions with either high (A & B) or low (C & D) amplitudes are triggered in trabeculae isolated from both RV, right ventricle (heart # 415217 & 514489) and LV, left ventricle (heart # 219852 & 514489) of non-failing hearts, respectively. Original representative recordings of twitches (normalized to cross-sectional area) in failing human myocardium at basal status, in presence of Iso + Caff and zacopride effective antiarrhythmic concentrations when arrhythmias in the form of extra systolic contractions with either high (E & F) or low (G & H) amplitudes are triggered in trabeculae isolated from both RV (heart # 233587 & 645444) and LV (heart # 728878 & 645444) of failing hearts, respectively. IC50: median inhibitory concentrations of zacopride in the RV and LV of non-failing (I) and failing (J) human hearts.
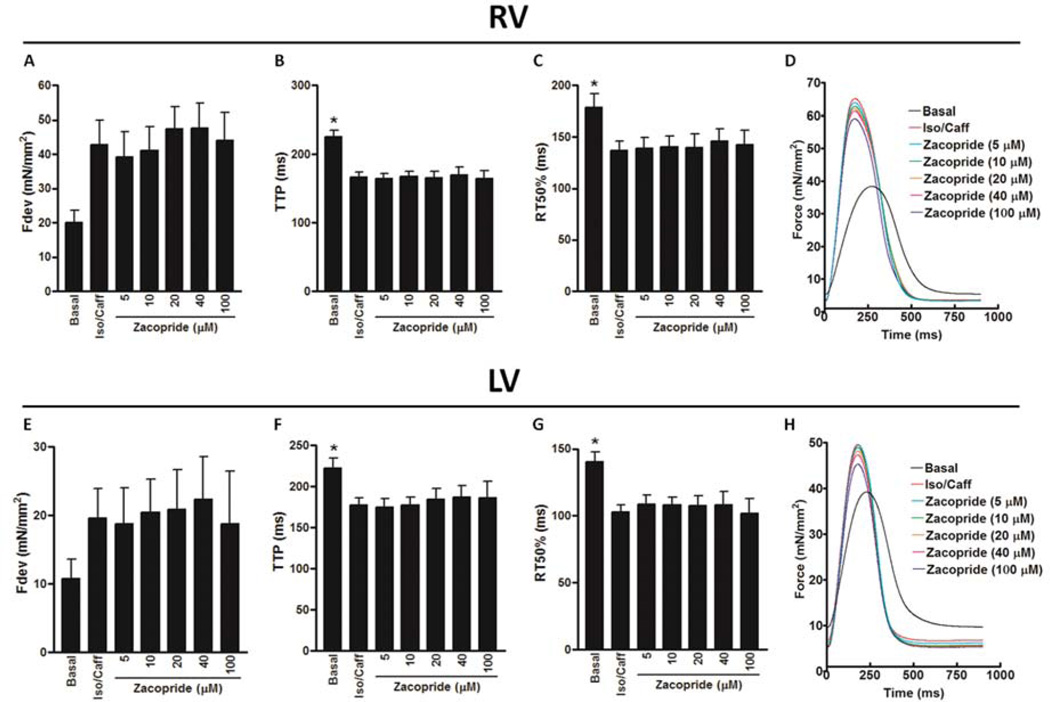
Isoproterenol/caffeine treatment in cardiac trabeculae from RV of failing hearts that were unsusceptible to its arrhythmogenic effects caused an increase in the Fdev (42.8 ± 7.1 mN/mm2), which was not quite significant (P = 0.0596) compared to basal Fdev (20.0 ± 3.6 mN/mm2) (Figure 3A). However, it resulted in significant decreases in contractile kinetic parameters, including TTP (166 ± 7 ms; P < 0.01) and RT50 (137 ± 9 ms; P < 0.05) compared to their values at basal conditions (TTP: 225± 10 ms and RT50:178± 14 ms) (Figure 3B & C), respectively. Zacopride in a concentration range of 5 to 100 µmol/L did not significantly change the Fdev, contractile kinetic parameters or induce any kind of arrhythmia in the presence of isoproterenol/caffeine (Figure 3A – D). Equally, isoproterenol/caffeine treatment in cardiac trabeculae from LV of failing hearts that were unsusceptible to its arrhythmogenic effects caused an insignificant increase in the Fdev (19.6 ± 4.3 mN/mm2) compared to its basal value (10.7 ± 2.8 mN/mm2) (P = 0.6610) (Figure 3E). Nonetheless, it resulted in significant decreases in contractile kinetic parameters, including TTP (177± 9 ms; P < 0.05) and RT50 (103± 5 ms; P < 0.01) compared to their values at basal conditions (TTP; 222± 12 ms and RT50; 140± 8 ms) (Figure 3F & G), respectively. Again, zacopride in the same concentration range did not significantly change the Fdev, contractile kinetic parameters or induce any kind of arrhythmia in the presence of isoproterenol/caffeine (Figure 3E – H). Increasing zacopride concentration up to 200 µmol/L did not change the contractile profile or induce any kind of arrhythmia in a trabecula from the LV of a failing heart under the same conditions (Supplementary Figure 2).
Figure 3.
The effect of zacopride on Iso/Caff [isoproterenol (100 nmol/L)/caffeine (0.5 mmol/L)]-induced changes in contractility of ventricular trabeculae isolated from the RV, right ventricle of failing human hearts, which are not susceptible to Iso/Caff-provoked triggered arrhythmia including: Fdev, peak isometric developed force (A); TTP, time to peak force (B); and RT50, time from peak force to 50% relaxation (C). Original representative recordings of twitches (normalized to cross-sectional area) in the RV of failing human myocardium (heart # 611422) that is not susceptible to Iso/Caff-provoked triggered arrhythmia at basal status, in presence of Iso/Caff and zacopride concentrations (5 – 100 µmol/L) (D). The effect of zacopride on Iso/Caff-induced changes in contractility of ventricular trabeculae isolated from the LV, left ventricle of failing human hearts, which are not susceptible to Iso/Caff-provoked triggered arrhythmia including: Fdev (E); TTP, time to peak force (F); and RT50 (G). Original representative recordings of twitches (normalized to cross-sectional area) in the LV of failing human myocardium (heart # 611422) that are not susceptible to Iso/Caff-provoked triggered arrhythmia at basal status, in presence of Iso/Caff and zacopride concentrations (5 – 100 µmol/L) (H). *A significant change compared to Iso/Caff, P ≤ 0.05. n = 4 – 9 based on time-dependent increase in zacopride concentration.
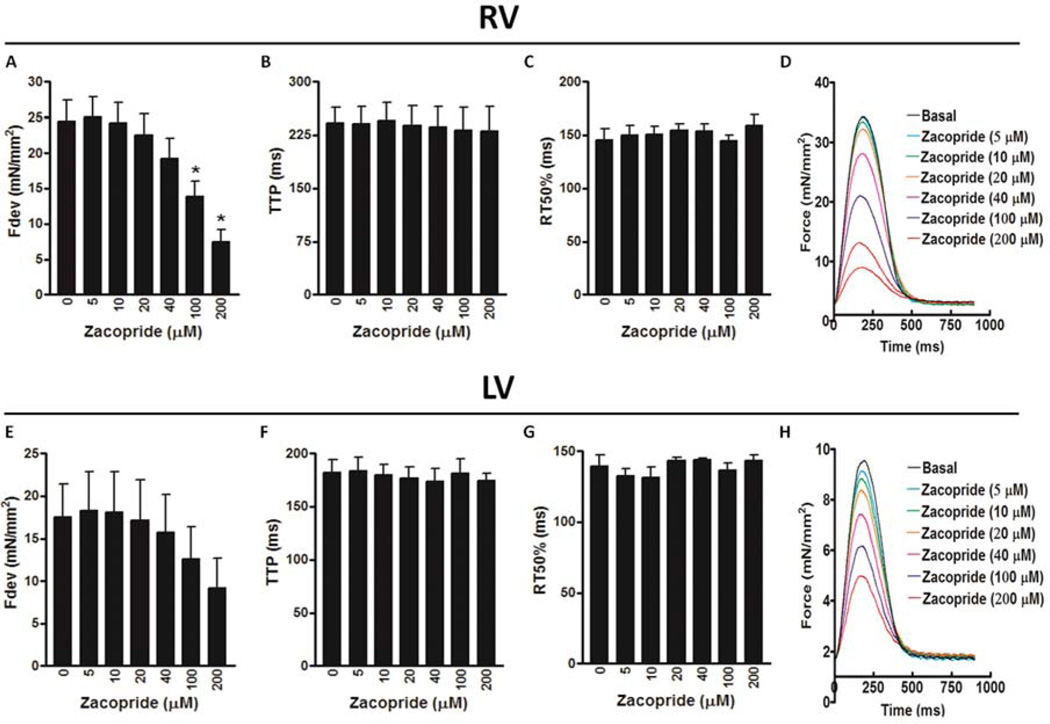
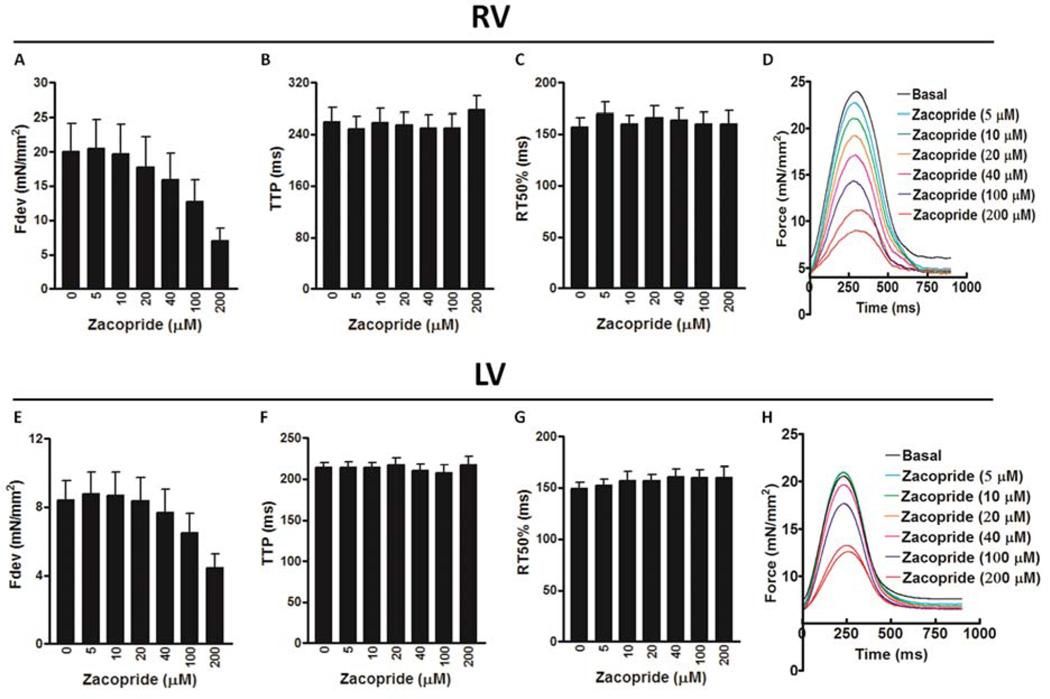
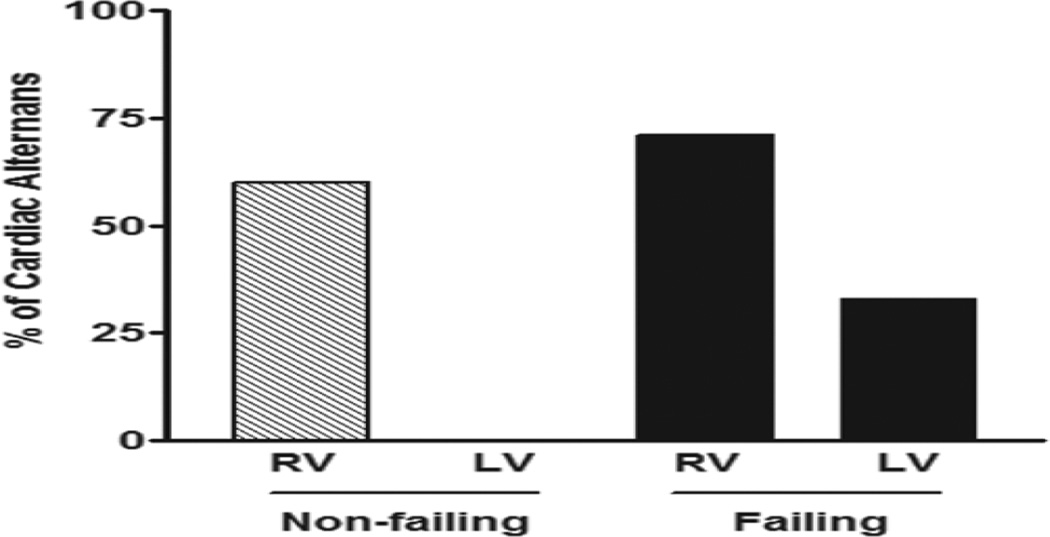
Further, we investigated the direct effects of zacopride (5 – 200 µmol/L) on contractile profiles of trabeculae isolated from both non-failing and failing human hearts. In cardiac trabeculae from RV of non-failing hearts, zacopride resulted in a concentration-dependent decrease in the Fdev, which reached significance at 100 µmol/L (13.9 ± 2.2 mN/mm2; P < 0.05) and 200 µmol/L (7.4 ± 1.7 N/mm2; P < 0.01) compared to basal Fdev (24.4 ± 3.0 mN/mm2) (Figure 4A). However, there were no significant differences in the TTP or RT50 at all investigated concentrations of zacopride compared to their values at basal conditions (Figure 4B & C, respectively). Surprisingly, zacopride at a concentration of 200 µmol/L only resulted in contractile alternans as demonstrated in Figure 4D. Likewise, cardiac trabeculae from LV of non-failing hearts exhibited a concentration-dependent decrease in the Fdev but it did not reach significance (P = 0.7719) (likely due to low n, n = 4) (Figure 4E). Also, there were no significant differences in all contractile kinetic parameters, including TTP (Figure 4F) and RT50 (Figure 4G) at all investigated concentrations of zacopride compared to their values at basal conditions. Besides, we did not notice any contractile alternans in these muscles at high zacopride concentration of 200 µmol/L as revealed in Figure 4H. On the other hand, cardiac trabeculae from the RV of failing hearts demonstrated a concentration-dependent decrease in the Fdev but it did not reach significance (P = 0.1972) (Figure 5A). Also, there were no significant differences in all contractile kinetic parameters, including TTP (Figure 5B) and RT50 (Figure 5C) at all investigated concentrations of zacopride compared to their values at basal conditions. Again, zacopride caused contractile alternans in 5 out of the 7 investigated RV trabeculae; 4 as expected at 200 µmol/L (Figure 5D) and one appeared earlier at 10 µM. Likewise, cardiac trabeculae from the LV of failing hearts exhibited a concentration-dependent decrease in the Fdev but it did not reach significance (P = 0.2200) (Figure 5E). Also, there were no significant differences in all contractile kinetic parameters, including TTP (Figure 5F) and RT50 (Figure 5G) at all investigated concentrations of zacopride compared to their values at basal conditions. In contrast to trabeculae from the LV of non-failing hearts that did not show any contractile alternans, 2 out of 6 trabeculae from the LV of failing hearts exhibited contractile alternans at 200 µmol/L (Figure 5H). In summary, 60 % of RV muscles from non-failing hearts (3/5) showed contractile alternans compared to ≈ 71 % (5/7) of their failing counterparts at 200 µmol/L of zacopride. Yet, none of the LV muscles (0/4) from non-failing hearts exhibited contractile alternans compared to 33 % (2/6) of those from failing hearts (Figure 6).
Figure 4.
The effect of zacopride on contractile parameters of ventricular trabeculae isolated from the RV, right ventricle of non-failing human hearts, including: Fdev, peak isometric developed force (A); TTP, time to peak force (B); and RT50, time from peak force to 50% relaxation (C). Superimposed original representative recordings of twitches (normalized to cross-sectional area) in the RV of non-failing human myocardium (heart # 958987) in the presence of zacopride (5 – 200 µmol/L) showing a concentration-dependent decrease in the Fdev and occurrence of contractile alternans at the highest applied concentration of 200 µmol/L (D). The effect of zacopride on contractile parameters of ventricular trabeculae isolated from the LV, left ventricle of non-failing human hearts, including, Fdev (E), TTP (F) and RT50 (G). Superimposed original representative recordings of twitches (normalized to cross-sectional area) in the LV of non-failing human myocardium (heart # 958987) in the presence of zacopride (5 – 200 µmol/L) showing a concentration-dependent decrease in the Fdev (H). *A significant change compared to basal (zacopride concentration: 0 µmol/L), P ≤ 0.05 and n = 4 – 5.
Figure 5.
The effect of zacopride on contractile parameters of ventricular trabeculae isolated from the RV, right ventricle of failing human hearts, including: Fdev, peak isometric developed force (A); TTP, time to peak force (B); and RT50, time from peak force to 50% relaxation (C). Superimposed original representative recordings of twitches (normalized to cross-sectional area) in the RV of failing human myocardium (heart # 904475) in the presence of zacopride (5 – 200 µmol/L) showing a concentration-dependent decrease in the Fdev and occurrence of contractile alternans at the highest applied concentration of 200 µmol/L (D). The effect of zacopride on contractile parameters of ventricular trabeculae isolated from the LV, left ventricle of failing human hearts, including, Fdev (E), TTP (F) and RT50 (G). Superimposed original representative recordings of twitches (normalized to cross-sectional area) in the LV of non-failing human myocardium (heart # 820447) in the presence of zacopride (5 – 200 µmol/L) showing a concentration-dependent decrease in the Fdev and occurrence of contractile alternans at the highest applied concentration of 200 µmol/L (H). n = 5 – 7.
Figure 6.
Percentage of the occurrence of contractile alternans in ventricular trabeculae isolated from non-filing [RV, right ventricle (3 out of 5) & LV, left ventricle (0 out of 4)] and failing [RV (5 out of 7) & LV (2 out of 6)] human hearts.
Discussion
Generally, antiarrhythmic drugs have had restricted success and can occasionally trigger arrhythmia [23]. Even with the recent success of implantable defibrillators and the promising future of gene therapy, there is still an imperative need for novel antiarrhythmic drugs. A better understanding of cardiac ion channels and new ways to target selection and compound screening will grant innovative prospects for future drug discovery [23]. Recently, the moderate IK1 channel activator zacopride was shown to suppress triggered VT in rats [15]. Nonetheless, concerns were raised about the possibility of pro-arrhythmic activity after IK1 channel stimulation based on a promising anti-arrhythmic strategy of IK1 blockade in other animal models [16, 17]. Translation of results obtained in animal models to human pathophysiology is often hampered by species-dependent differences. For instance, humans exhibit lower repolarization reserve contributions from IK1 and IKs compared to dogs [18]. Also, the rat ventricle lacks functional IKr and IKs [24]. In addition, the extreme dissimilarity of electrical substrate in mouse/rat hearts, including the lack of any appreciable AP plateau phase, very rapid heart rate and potentially unstable RP, may cause a negligible role for IK1 compared to larger hearts with slower heart rates, and stable AP plateaus, as humans possess [25]. Therefore, the goal of the current study was to investigate the ex-vivo effects of zacopride on triggered arrhythmia and contractility in ventricular human myocardium in order to validate data that was solely obtained from animal models.
Triggered arrhythmias originate from DADs that are most frequently observed under conditions of SR Ca2+ overload, where the high SR Ca2+ content can cause spontaneous Ca2+ release after the termination of the AP due to the activation of Na+/Ca2+ exchange (NCX), and the decline of IK1 in both animals [26, 27] and humans [13, 28, 29]. In this regard, caffeine allows for increases in the open probability of the RyR2 without depleting the SR, and it decreases the threshold for SR Ca2+ release [30, 31]. On the other hand, isoproterenol increases the SR Ca2+ content to a high enough level to reach the threshold for SR Ca2+ release [32]. Our recent data showed a synchronization mechanism that exists for the generation of triggered activity in multicellular cardiac preparations of genetically mutated murine myocardium [33] and wild-type rat hearts under conditions of Ca2+ dysregulation induced either by isoproterenol (100 nmol/L) alone [33], or a combination of isoproterenol (100 nmol/L) and caffeine (0.5 mmol/L) (unpublished). In the current study, the same concentrations of isoproterenol/caffeine combination led to triggered arrhythmia in isolated cardiac muscles from both non-failing and end-stage failing hearts. However, the occurrence of arrhythmia in the RV and LV muscles of non-failing hearts was markedly higher than those of end-stage failing hearts. Consistent with these results, it has been reported that myocardial catecholamines’ diminution and myocardial β-adrenergic receptors’ down-regulation may elucidate tolerance of patients with severe congestive HF to VT as well as intolerance to conventional antiarrhythmic drugs [34]. Similarly, previous data indicate that myocardium from failing human heart is less sensitive to stimulation by isoproterenol than normal myocardium [35]. Furthermore, isoproterenol-induced inhibition of IK1 has been shown to be significantly reduced in human ventricular myocytes from the failing heart compared to the non-failing heart [13]. In contrast, it has been revealed that residual β-adrenergic responsiveness conspires to significantly augment the susceptibility to triggered arrhythmias in HF through IK1 down-regulation, NCX upregulation and SR Ca2+ overload-induced spontaneous SR Ca2+ release and after contractions in rabbits [26, 27]. Nonetheless, the authors of these studies stated that preserved β-adrenergic responsiveness that may take place only in HF that is not end-stage [36] is an essential contributor to arrhythmogenicity in HF [27]. In end-stage human HF, there are fewer sudden arrhythmic deaths likely due to the entire loss of β-adrenergic responsiveness [34, 35]. During this stage, susceptibility for arrhythmia may be lower, as pump failure continues, due to the inability of SR Ca2+ load to reach a level that is high enough for spontaneous SR Ca2+ release even though increased NCX and decreased IK1 may persist [26]. This clearly confirms the lower incidence rate of triggered arrhythmia in muscles of end-stage failing hearts compared to those of non-failing hearts in this study.
Zacopride is a moderate IK1 channel stimulator that selectively amplifies the IK1 current up to 40%, through the activation of the Kir2.1 homomeric channel via a PKA-dependent pathway [15, 37]. This modest enhancement in IK1 is different from that induced by the IK1 channel gene-transfer strategy, where the IK1 is increased by more than 100%, which makes zacopride a promising antiarrhythmic candidate [9, 15]. Importantly, zacopride has been established to suppress both ex-vivo and in-vivo induced triggered arrhythmia in rats [15]. Consistently, our current data demonstrate the ability of zacopride to eliminate the ex-vivo triggered arrhythmia in muscles from both non-failing and failing human hearts in a concentration-dependent manner, with an effective IC50 in the range of 28 to 40 µmol/L. In line with these effective high zacopride concentrations, a previous study showed that mandatory zacopride concentration to attain a maximal efficacy for the IK1 activation is greatly elevated in human embryonic kidney-293 cells (100 µmol/L) [37] compared to rat ventricular myocytes (1 µmol/L) [15]. Moreover, zacopride (at the highest applicable concentration, 200 µmol/L) did not affect isoproterenol-induced cardiac contractile changes, including Fdev, TTP, RT50 and RT90 in the majority of failing heart muscles, which were not susceptible to the arrhythmogenic effect of isoproterenol/caffeine combination. In addition, zacopride did not induce any arrhythmia in the presence of isoproterenol/caffeine on these muscles. Similarly, application of K+ channel openers inhibited isoproterenol-induced after contractions in both guinea-pig and human myocytes. Also, these K+ channel openers returned the contraction to a point close to or higher than those with isoproterenol alone, but did not change the isoproterenol-induced alterations in TTP or RT50 and did not repeat the appearance of after contractions [38]. From this study the authors concluded that the isoproterenol inotropic and lusitropic effects are separable from its arrhythmogenic effects. Additionally, the inability of K+ channel openers to antagonize these isoproterenol-induced contractile changes may imply their independency of PKA-dependent pathways [38]. Thus, they proposed different mechanisms regarding these drug effects, which include: 1) AP shortening could diminish the possibility of L-type Ca2+ channel reactivation, which might be an underlying mechanism in the development of arrhythmia, 2) a shorter plateau region may possibly cause less Ca2+ influx due to faster calcium current deactivation, as a result free [Ca2+]i and following SR loading might be minimized and 3) in the presence of short AP plateau NCX Ca2+ efflux may be more favored compared to NCX Ca2+ influx, which mainly occurs with a longer AP [38]. This may explain the similar effects of zacopride on isoproterenol/caffeine-induced arrhythmia and contractile changes; nevertheless, it requires further investigation, particularly, due to other reports which show that IK1 in human ventricular myocytes can be inhibited by isoproterenol-induced PKA-mediated phosphorylation [13].
Although IK1 enhancement to stabilize RP is beneficial, especially in the treatment of triggered arrhythmia, it could also be harmful because of the reduction in excitability, propagation rate, and AP duration [26]. In the absence of isoproterenol/caffeine, zacopride led to a negative inotropic effect in a concentration-dependent manner. Reduced cardiac contraction was observed only at high zacopride concentration of 200 µmol/L, along with the occurrence of cardiac alternans in muscles from both non-failing (RV) and failing (RV & LV) human hearts. Likewise, K+ channel openers that returned the contraction to a point close to or higher than those with isoproterenol alone [38] resulted in significant shortening of cardiac APD and reduction of the twitch amplitude in the absence of isoproterenol [39]. Furthermore, it has been reported that AP shortening is responsible for the negative inotropic effect of nifedipine in neonatal cardiomyocyte due to reduction in activator Ca2+ influx through NCX [40]. On the other hand, alternans is a risk factor for cardiac arrhythmia, and the main mechanism underlying alternans resides in a disturbance of Ca2+ signaling [41] as well as in recirculation of Ca2+ between heart beats [42]. However, the actual mechanism for these zacopride-induced contractile defects and alternans needs to be assessed.
Of note, in this study there are some differences in the responses of muscles obtained from the RV and LV of both non-failing and failing hearts. Mostly, the RV muscles demonstrated higher responses to isoproterenol/caffeine and/or zacopride compared to LV muscles. The RV and LV encompass diverse embryologic, structural, metabolic, and electrophysiologic features, which could explain the difference between RV and LV in these experimental responses [43]. For example, repolarizing K+ currents ITO1 and IKs, but not IK1 are larger in RV than LV of canine myocardium [44]. Also, RV of canine myocardium exhibited enhanced β-adrenergic responsiveness compared to the LV [43].
Conclusions
Under arrhythmogenic conditions, zacopride shows promising antiarrhythmic effects against triggered arrhythmia induced by isoproterenol/caffeine in ventricular human myocardium. Interestingly, this occurred with zacopride IC50 concentration range, most likely, devoid of contractile side effects, which gives this drug a higher chance to be effectively used in the treatment of human ventricular tachyarrhythmia, yet, applicable dosage should be carefully determined. In the absence of Ca2+ overload/arrhythmia induced by isoproterenol/caffeine, zacopride, albeit at high concentrations, decreases the force of contraction and increases likelihood of occurrence of contractile alternans, which may predispose the heart to contractile dysfunction and/or arrhythmia, especially in those who are using the drug at high doses as an anti-emetic, gastroprokinetic or anxiolytic agent. Overall, our results represent a key step in translating zacopride from the benchtop to the bedside in the research area to truly investigate the potential in-vivo effects of this drug on both arrhythmia and contractility in human hearts.
Study Limitations
The inability of zacopride to affect the force of contraction or induce arrhythmia in the presence of Ca2+ overload could signify a differential mechanism of action for this drug in the heart, yet, this needs to be elucidated. In addition, the ability of this drug to directly induce contractile dysfunction and cardiac alternans requires further assessment. Due to time-constraints in using live human tissue and the limited number of experimental protocols that can be completed on such intact muscles, this study focused on the contractile aspects, and contractile read-outs of arrhythmic behavior. The effects of zacopride on AP as well as on Ca2+ transients in susceptible human heart muscles are important factors that remain to be investigated to further elucidate the antiarrhythmic mechanism of this drug.
Supplementary Material
Acknowledgments
This work is supported by NIH R01HL113084 (PMLJ) and AHA GRA-16POST27760155 (ME).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts-of-interest
None.
References
- 1.Kong MH, Fonarow GC, Peterson ED, Curtis AB, Hernandez AF, Sanders GD, et al. Systematic review of the incidence of sudden cardiac death in the United States. J Am Coll Cardiol. 2011;57:794–801. doi: 10.1016/j.jacc.2010.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bers DM. Excitation–Contraction Coupling and Cardiac Contractile Force. 2nd. Dordrecht: Springer; 2001. [Google Scholar]
- 3.Pogwizd SM, McKenzie JP, Cain ME. Mechanisms underlying spontaneous and induced ventricular arrhythmias in patients with idiopathic dilated cardiomyopathy. Circulation. 1998;98:2404–2414. doi: 10.1161/01.cir.98.22.2404. [DOI] [PubMed] [Google Scholar]
- 4.Leenhardt A, Lucet V, Denjoy I, Grau F, Ngoc DD, Coumel P. Catecholaminergic polymorphic ventricular tachycardia in children. A 7-year follow-up of 21 patients. Circulation. 1995;91:1512–1519. doi: 10.1161/01.cir.91.5.1512. [DOI] [PubMed] [Google Scholar]
- 5.Ter Keurs HEDJ, Boyden PA. Calcium and arrhythmogenesis. Physiol Rev. 2007;87:457–506. doi: 10.1152/physrev.00011.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laurita KR, Rosenbaum DS. Mechanisms and potential therapeutic targets for ventricular arrhythmias associated with impaired cardiac calcium cycling. J Mol Cell Cardiol. 2008;44:31–43. doi: 10.1016/j.yjmcc.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Györke S, Carnes C. Dysregulated sarcoplasmic reticulum calcium release: Potential pharmacological target in cardiac disease. Pharmacol Ther. 2008;119:340–354. doi: 10.1016/j.pharmthera.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Radwanski PB, Poelzing S. NCX is an important determinant for premature ventricular activity in a drug-induced model of Andersen-Tawil syndrome. Cardiovasc Res. 2011;92:57–66. doi: 10.1093/cvr/cvr180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu BW, Cao JM. On the risk concerns of zacopride, a moderate IK1 channel agonist with cardiac protective action. J Cardiovasc Pharmacol. 2014;64:357–359. doi: 10.1097/FJC.0000000000000148. [DOI] [PubMed] [Google Scholar]
- 10.Fauconnier J, Lacampagne A, Rauzier JM, Vassort G, Richard S. Ca2+-dependent reduction of IK1 in rat ventricular cells: a novel paradigm for arrhythmia in heart failure? Cardiovasc Res. 2005;68:204–212. doi: 10.1016/j.cardiores.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 11.Nagy N, Acsai K, Kormos A, Sebők Z, Farkas AS, Jost N, et al. [Ca2+]i-induced augmentation of the inward rectifier potassium current (IK1) in canine and human ventricular myocardium. Pflugers Arch. 2013;465:1621–1635. doi: 10.1007/s00424-013-1309-x. [DOI] [PubMed] [Google Scholar]
- 12.Koumi S, Wasserstrom JA, Ten Eick RE. Beta-adrenergic and cholinergic modulation of inward rectifier K+ channel function and phosphorylation in guinea-pig ventricle. J Physiol. 1995;486(Pt 3):661–678. doi: 10.1113/jphysiol.1995.sp020842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koumi S, Backer CL, Arentzen CE, Sato R. Beta-Adrenergic modulation of the inwardly rectifying potassium channel in isolated human ventricular myocytes. Alteration in channel response to beta-adrenergic stimulation in failing human hearts. J Clin Invest. 1995;96(6):2870–2881. doi: 10.1172/JCI118358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lopatin AN, Nichols CG. Inward rectifiers in the heart: an update on IK1. J Mol Cell Cardiol. 2001;33:625–638. doi: 10.1006/jmcc.2001.1344. [DOI] [PubMed] [Google Scholar]
- 15.Liu QH, Li XL, Xu YW, Lin YY, Cao JM, Wu BW. A novel discovery of IK1 channel agonist: zacopride selectively enhances IK1 current and suppresses triggered arrhythmias in the rat. J Cardiovasc Pharmacol. 2012;59:37–48. doi: 10.1097/FJC.0b013e3182350bcc. [DOI] [PubMed] [Google Scholar]
- 16.Rees SA, Curtis MJ. Specific IK1 blockade: a new antiarrhythmic mechanism? Effect of RP58866 on ventricular arrhythmias in rat, rabbit, and primate. Circulation. 1993;87:1979–1989. doi: 10.1161/01.cir.87.6.1979. [DOI] [PubMed] [Google Scholar]
- 17.Curtis MJ. Activation of IK1 by zacopride: amelioration of left ventricular remodeling, but at what risk? J Cardiovasc Pharmacol. 2014;64:343–344. doi: 10.1097/FJC.0000000000000140. [DOI] [PubMed] [Google Scholar]
- 18.Jost N, Virág L, Comtois P, Ordög B, Szuts V, Seprényi G, et al. Ionic mechanisms limiting cardiac repolarization reserve in humans compared to dogs. J Physiol. 2013;591:4189–4206. doi: 10.1113/jphysiol.2013.261198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peralta AO, John RM, Gaasch WH, Taggart PI, Martin DT, Venditti FJ. The class III antiarrhythmic effect of sotalol exerts a reverse use-dependent positive inotropic effect in the intact canine heart. J Am Coll Cardiol. 2000;36(4):1404–1410. doi: 10.1016/s0735-1097(00)00833-0. [DOI] [PubMed] [Google Scholar]
- 20.Milani-Nejad N, Canan BD, Elnakish MT, Davis JP, Chung JH, Fedorov VV, et al. The Frank-Starling mechanism involves deceleration of cross-bridge kinetics and is preserved in failing human right ventricular myocardium. Am J Physiol Heart Circ Physiol. 2015;309(12):H2077–H2086. doi: 10.1152/ajpheart.00685.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elnakish MT, Moldovan L, Khan M, Hassanain HH, Janssen PM. Myocardial Rac1 exhibits partial involvement in thyroxin-induced cardiomyocyte hypertrophy and its inhibition is not sufficient to improve cardiac dysfunction or contractile abnormalities in mouse papillary muscles. J Cardiovasc Pharmacol. 2013;61(6):536–544. doi: 10.1097/FJC.0b013e31828d4b9d. [DOI] [PubMed] [Google Scholar]
- 22.Rodriguez EK, Hunter WC, Royce MJ, Leppo MK, Douglas AS, Weisman HF. A method to reconstruct myocardial sarcomere lengths and orientations at transmural sites in beating canine hearts. Am J Physiol Heart Circ Physiol. 1992;263(1):H293–H306. doi: 10.1152/ajpheart.1992.263.1.H293. [DOI] [PubMed] [Google Scholar]
- 23.Sanguinetti MC, Bennett PB. Antiarrhythmic drug target choices and screening. Circ Res. 2003;93(6):491–499. doi: 10.1161/01.RES.0000091829.63501.A8. [DOI] [PubMed] [Google Scholar]
- 24.Pogwizd SM. Nonreentrant mechanism underlying spontaneous ventricular arrhythmias in a model of nonischemic heart failure in rabbits. Circulation. 1995;92:1034–1048. doi: 10.1161/01.cir.92.4.1034. [DOI] [PubMed] [Google Scholar]
- 25.Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signaling pathways. Nat Rev Mol Cell Biol. 2006;7:589–600. doi: 10.1038/nrm1983. [DOI] [PubMed] [Google Scholar]
- 26.Pogwizd SM, Schlotthauer K, Li L, Yuan W, Bers DM. Arrhythmogenesis and contractile dysfunction in heart failure: roles of sodium–calcium exchange, inward rectifier potassium current, residual beta-adrenergic responsiveness. Circ Res. 2001;8:1159–1167. doi: 10.1161/hh1101.091193. [DOI] [PubMed] [Google Scholar]
- 27.Desantiago J, Ai X, Islam M, Acuna G, Ziolo MT, Bers DM, et al. Arrhythmogenic effects of beta2-adrenergic stimulation in the failing heart are attributable to enhanced sarcoplasmic reticulum Ca load. Circ Res. 2008;102(11):1389–1397. doi: 10.1161/CIRCRESAHA.107.169011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schlotthauer K, Schattmann J, Bers DM, Maier LS, Schutt U, Minami K, et al. Frequency-dependent changes in contribution of SR Ca2+ to Ca2+ transients in failing human myocardium assessed with ryanodine. J Mol Cell Cardiol. 1998;30:1285–1294. doi: 10.1006/jmcc.1998.0690. [DOI] [PubMed] [Google Scholar]
- 29.Verkerk AO, Veldkamp MW, Baartscheer A, Schumacher CA, Klöpping C, van Ginneken AC, et al. Ionic mechanism of delayed after depolarizations in ventricular cells isolated from hum an end-stage failing hearts. Circulation. 2001;104(22):2728–2733. doi: 10.1161/hc4701.099577. [DOI] [PubMed] [Google Scholar]
- 30.Nieman CJ, Eisner DA. Effects of caffeine, tetracaine, and ryanodine on calcium-dependent oscillations in sheep cardiac Purkinje fibers. J Gen Physiol. 1985;86:877–889. doi: 10.1085/jgp.86.6.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trafford AW, Sibbring GC, Diaz ME, Eisner DA. The effects of low concentrations of caffeine on spontaneous Ca release in isolated rat ventricular myocytes. Cell Calcium. 2000;28:269–276. doi: 10.1054/ceca.2000.0156. [DOI] [PubMed] [Google Scholar]
- 32.Venetucci LA, Trafford AW, Eisner DA. Increasing ryanodine receptor open probability alone does not produce arrhythmogenic calcium waves: threshold sarcoplasmic reticulum calcium content is required. Circ Res. 2007;100:105–111. doi: 10.1161/01.RES.0000252828.17939.00. [DOI] [PubMed] [Google Scholar]
- 33.Brunello L, Slabaugh JL, Radwanski PB, Ho HT, Belevych AE, Lou Q, et al. Decreased RyR2 refractoriness determines myocardial synchronization of aberrant Ca2+ release in a genetic model of arrhythmia. Proc Natl Acad Sci U S A. 2013;110:10312–10317. doi: 10.1073/pnas.1300052110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kjekshus J. Arrhythmias and mortality in congestive heart failure. Am J Cardiol. 1990;65(19):42I–48I. doi: 10.1016/0002-9149(90)90125-k. [DOI] [PubMed] [Google Scholar]
- 35.Ginsburg R, Bristow MR, Billingham ME, Stinson EB, Schroeder JS, Harrison DC. Study of the normal and failing isolated human heart: decreased response of failing heart to isoproterenol. Am Heart J. 1983;106(3):535–540. doi: 10.1016/0002-8703(83)90698-1. [DOI] [PubMed] [Google Scholar]
- 36.Bristow MR, Ginsburg R, Umans V, Fowler M, Minobe W, Rasmussen R, et al. β1- and β2-adrenergicreceptor subpopulations in nonfailing and failing human ventricular myocardium: coupling of both receptor subtypes to muscle contraction and selective β1-receptor down-regulation in heart failure. Circ Res. 1986;59:297–309. doi: 10.1161/01.res.59.3.297. [DOI] [PubMed] [Google Scholar]
- 37.Zhang L, Liu Q, Liu C, Zhai X, Feng Q, Xu R, et al. Zacopride selectively activates the Kir2.1 channel via a PKA signaling pathway in rat cardiomyocytes. Sci China Life Sci. 2013;56(9):788–796. doi: 10.1007/s11427-013-4531-z. [DOI] [PubMed] [Google Scholar]
- 38.Tweedie D, O'Gara P, Harding SE, MacLeod KT. The effect of alterations to action potential duration on beta-adrenoceptor-mediated after contractions inhuman and guinea-pig ventricular myocytes. J Mol Cell Cardiol. 1997;29(5):1457–1467. doi: 10.1006/jmcc.1997.0385. [DOI] [PubMed] [Google Scholar]
- 39.Nichols CG, Ripoll C, Lederer WJ. ATP-sensitive potassium channel modulation of the guinea pig ventricular action potential and contraction. Circ Res. 1991;68(1):280–287. doi: 10.1161/01.res.68.1.280. [DOI] [PubMed] [Google Scholar]
- 40.Go A, Srivastava S, Collis L, Coetzee WA, Artman M. Negative inotropic effect of nifedipine in the immature rabbit heart is due to shortening of the action potential. Pediatr Res. 2005;57(3):399–403. doi: 10.1203/01.PDR.0000150798.83920.5A. [DOI] [PubMed] [Google Scholar]
- 41.Kanaporis G, Blatter LA. The mechanisms of calcium cycling and action potential dynamics in cardiac alternans. Circ Res. 2015;116(5):846–856. doi: 10.1161/CIRCRESAHA.116.305404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wohlfart B. Analysis of mechanical alternans in rabbit papillary muscle. Acta Physiol Scand. 1982;115(4):405–414. doi: 10.1111/j.1748-1716.1982.tb07098.x. [DOI] [PubMed] [Google Scholar]
- 43.Molina CE, Johnson DM, Mehel H, Spätjens RL, Mika D, Algalarrondo V, et al. Interventricular differences in β-adrenergic responses in the canine heart: role of phosphodiesterases. J Am Heart Assoc. 2014;3(3):e000858. doi: 10.1161/JAHA.114.000858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Volders PG, Sipido KR, Carmeliet E, Spätjens RL, Wellens HJ, Vos MA. Repolarizing K+ currents ITO1 and IKs are larger in right than left canine ventricular midmyocardium. Circulation. 1999;99(2):206–210. doi: 10.1161/01.cir.99.2.206. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.