Abstract
Nuclear spin hyperpolarization of 13C-labelled metabolites by dissolution dynamic nuclear polarization can enhance the NMR signals of metabolites by several orders of magnitude, which has enabled in vivo metabolic imaging by MRI. However, because of the short lifetime of the hyperpolarized magnetization (typically <1 min), the polarization process must be carried out close to the point of use. Here we introduce a concept that markedly extends hyperpolarization lifetimes and enables the transportation of hyperpolarized metabolites. The hyperpolarized sample can thus be removed from the polarizer and stored or transported for use at remote MRI or NMR sites. We show that hyperpolarization in alanine and glycine survives 16 h storage and transport, maintaining overall polarization enhancements of up to three orders of magnitude.
MRI imaging can be significantly enhanced by injecting highly magnetized chemical agents, but the short magnetization lifetime requires processing at the point of use. Here, the authors demonstrate a method that could extend the lifetime from seconds to hours, enabling remote preparation.
Dissolution dynamic nuclear polarization (d-DNP)1,2 is a powerful method to enhance nuclear magnetic resonance (NMR) signals by several orders of magnitude, notably in 13C-labelled metabolites. The production of 13C-hyperpolarized metabolites has opened the way to a broad range of novel experiments, such as the detection of intermediates in fast chemical reactions3 the observation of protein folding in real time4 or the detection and monitoring of cancer in humans5. In d-DNP experiments, the 13C metabolites are usually polarized at low temperatures (1.2<T<4.2 K) and moderate fields (usually 3.35<B0<6.7 T) either directly6 or indirectly7,8,9,10 by 1H→13C cross-polarization (CP)11,12. The sample formulation usually consists of a homogeneous aqueous mixture of paramagnetic polarizing agents (PAs) and metabolites sometimes containing a glass-forming agent such as glycerol. The frozen solution is then dynamically polarized by microwave irradiation. The formation of a glass upon freezing is critical for efficient DNP13,14. Alternatively, the PAs can be covalently attached to the surface of mesostructured materials that are impregnated with aqueous solutions of metabolites15, in which case one can dispense with glass-forming agents. The PAs may also be generated in situ by ultraviolet irradiation16. However, intimate contact of the nuclear spins with the PA leads to paramagnetic relaxation that is exacerbated at low fields and thus requires dissolution of the sample directly in the cryostat. Hyperpolarized solutions have lifetimes T1(13C)∼30–60 s in carboxyl groups that are sufficiently long for immediate imaging or spectroscopy, but not for transport of the sample to a distant user site.
After DNP at low temperature, the glassy mixture is rapidly melted at high field inside the DNP polarizer, and the resulting hyperpolarized solution is rapidly transferred to an NMR or magnetic resonance imaging (MRI) magnet. The hyperpolarization lifetime following dissolution is determined by the 13C longitudinal relaxation time T1(13C) in solution. The requirement of melting samples within the polarizer is one of the major limitations of d-DNP. It obviously implies that each NMR or MRI magnet needs to have a dedicated DNP polarizer located within a few metres. All attempts to remove the hyperpolarized solid sample from the polarizer, transport it and melt it remotely have so far failed. The reason for this is simply that T1(13C) becomes prohibitively short in the frozen solid samples at low magnetic fields because of the close proximity of the 13C spins to the statistically dispersed PAs17. To remove the polarized solid from the polarizer, the PAs and the substrate would need to be physically separated from each other, yet close enough for the PAs to polarize the substrate.
This can be achieved by preparing DNP samples with a suitable multi-component nano- or micro-particulate architecture. The radical-rich phase (RRP) contains the PAs and abundant spins such as protons, and the other radical-free phase (RFP) contains the molecules to be enhanced and the same abundant spins. The abundant protons are directly and rapidly polarized by DNP in the RRP, and this polarization is then slowly relayed to the protons of the RFP by proton–proton spin diffusion. The time needed for spin diffusion to propagate the proton polarization from the RRP to the RFP depends on the size of the RFP domains. Nano-crystalline suspensions have been polarized by DNP relayed by spin diffusion by van der Wel et al. in magic angle spinning studies of amyloid fibrils18,19,20,21. More recently, the concept of DNP relayed by spin diffusion has been generalized to micro-particulate samples by Emsley and co-workers, who showed that this could lead to substantial polarization in ordinary organic solids and provide information about domain structures, for example, in pharmaceutical formulations22,23,24. However, all of these studies were performed in combination with magic angle spinning DNP at temperatures ∼100 K.
In this communication, capitalizing on this concept of DNP relayed by spin diffusion, we show how micro-particles can be hyperpolarized in trapped states with high polarization levels of typically P(13C)>10%, and with long lifetimes at 4.2 K of typically T1(13C)>5 h. We also show that due to the special nature of these states, the polarized sample can be removed from the DNP polarizer while preserving much of its polarization, in contrast to 13C spins of metabolites dissolved in conventional glassy DNP samples and similar to silicon nanoparticles25,26. The polarized sample can even be transported, and finally dissolved to the liquid state in a remote location. If the RRP is formulated to be hydrophobic, the hyperpolarized metabolites (that are invariably hydrophilic) can be easily separated from the PAs and any other contaminants during the dissolution process, yielding a pure hyperpolarized solution without the need for further purification6,27,28,29,30,31.
Results
Overview
We show here that protons in particles of metabolites with diameters of a few micrometres are polarized at T=1.2 K and B0=6.7 T on a timescale of 30 min by DNP-assisted proton–proton spin diffusion. CP is then used to transfer the polarization from the 1H in the RFP to the 13C spins of the metabolites. After the 13C polarization has achieved a satisfactory level, the microwave irradiation is switched off and the sample temperature is increased from 1.2 to 4.2 K (ambient pressure), and the protons in both RRP and RFP phases relax towards thermal equilibrium within minutes. However, the 13C spins are effectively isolated from both protons in the RFP and PAs in the RRP, and therefore can remain hyperpolarized for prolonged periods, typically for days at 4.2 K in a field of 6.7 T. Indeed, at this point, the 13C hyperpolarization is trapped in the RFP because, first, there are no PAs acting as relaxing agents and, second, the 13C hyperpolarization cannot efficiently diffuse out of the RFP, since 13C–13C spin diffusion is intrinsically two orders of magnitude slower than 1H–1H spin diffusion in the RFP, and is ineffective in the RRP since it is not isotopically 13C enriched.
Sample formulation
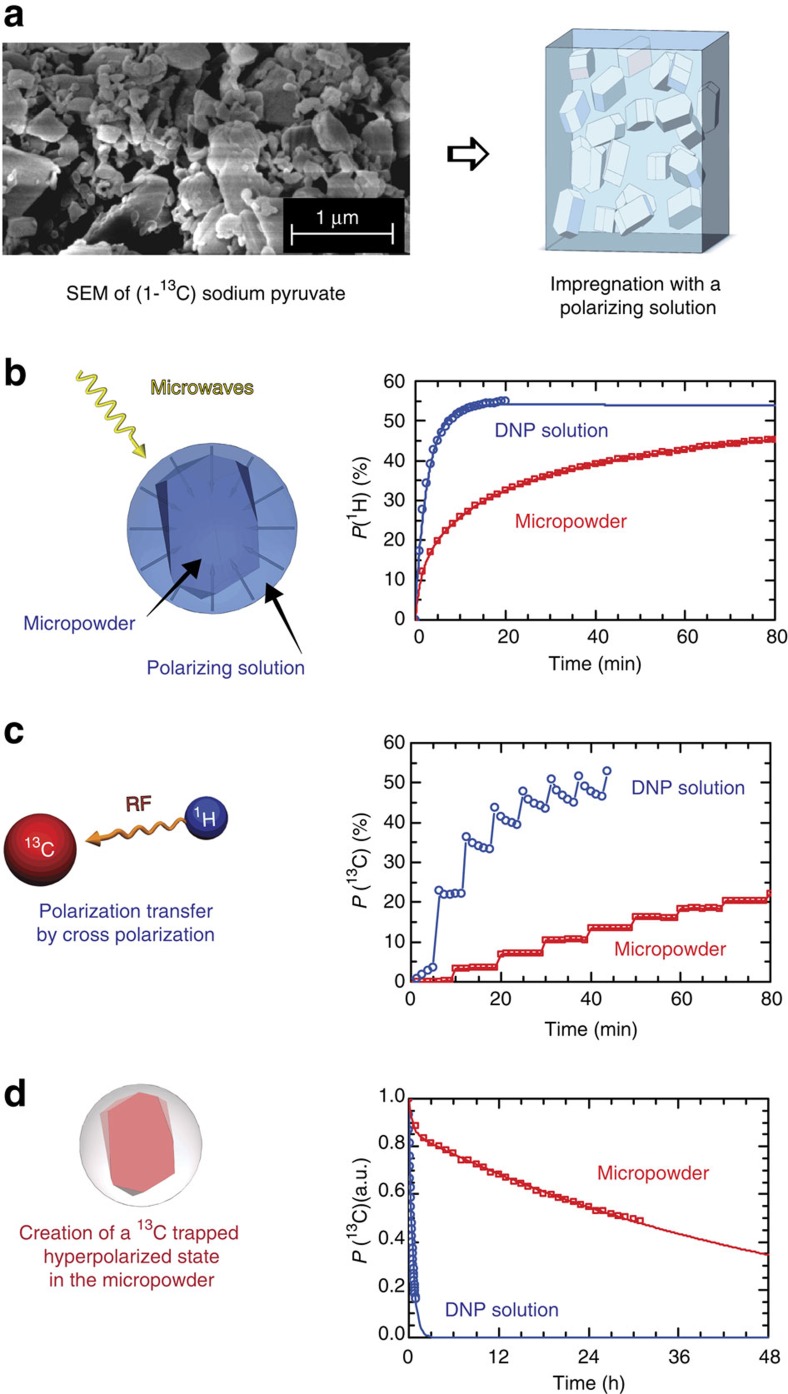
RFP of [1-13C]Glucose, [13C3, 15N]alanine, [13C2, 15N]glycine, and sodium [1-13C]pyruvate were chosen as test substances. Sodium [1-13C]pyruvate has become the metabolite of reference for in vivo clinical studies, where it provides a promising means to detect prostate tumours5. More generally, any substrate that can be prepared in the form of a micro-particulate powder is equally suitable for our method. Here powdered samples were ground by hand to an average particle diameter of 1<d<10 μm, as determined by scanning electron microscopy (SEM; Fig. 1a). Smaller particle diameters reduce the distance over which hyperpolarized 1H magnetization needs to diffuse and accelerate the build-up rates.
Figure 1. Illustration of the steps leading to a locked hyperpolarized state.
(a) Preparation of an impregnated powder. The radical-free phase (RFP), here a micro-powder of hydrophilic [1-13C] sodium pyruvate ground by hand, as shown on the SEM image, is impregnated with the radical-rich phase (RRP), here a hydrophobic glass-forming solution. (b) Spin diffusion relayed dynamic nuclear polarization (DNP). The resulting micro-formulation is placed in the polarizer and irradiated with microwaves. Initially, DNP enhances the proton polarization within the RRP and proton–proton spin diffusion transports the polarization to the RFP. The graph shows the 1H DNP build-up at 6.7 T and 1.2 K in (blue circles, DNP solution) for a homogeneous frozen glassy mixture of sodium [1-13C]pyruvate in H2O:D2O:glycerol-d8 (2:3:5) and doped with 40 mM TEMPOL without RFP, and (red squares, micro-powder) the build-up in micro-particles of pure sodium [1-13C]pyruvate (the RFP) that were impregnated with a solution of toluene-d6:THF-d8:THF (8:1:1) doped with 40 mM TEMPOL-benzoate as RRP. The curves show single- and bi-exponential fits to the data, as described in the text. (c) 1H→13C cross-polarization (CP). Several CP pulse sequences (see methods for details on the pulse sequence) are applied to transfer the proton polarization in the RFP to carbon-13 of the metabolites. The graph shows the 13C CP-DNP build-up curves of the same glassy RRP (blue circles) and micro-particulate RFP (red squares). (d) 13C-trapped state at 4.2 K. As the carbon-13 nuclei in the RFP are not in close contact with the paramagnetic radicals of the RRP, the hyperpolarized 13C magnetization has a long lifetime T1(13C) at 4.2 K. The graph shows the decay of the 13C nuclear spin polarization at 4.2 K and 6.7 T in the same glassy RRP (blue circles) and micro-particulate RFP (red squares).
The RRP consists of a glass-forming solution of PA chosen such that the RFP is not soluble in the RRP, such as 80 mM TEMPOL-benzoate in toluene/THF (v:v=8:2; see Methods for details on wet impregnation of the RFP with the RRP, including procedures to homogenize the distribution, and to fix the sample by freezing). We have not yet investigated bio-compatible solvents that could replace toluene. This DNP medium was chosen because, first, a proton polarization P(1H)>50% can be achieved in <5 min at 1.2 K and 6.7 T (Fig. 1b), second, none of the metabolites that we have tested are soluble in this mixture and, third, since the mixture is not soluble in water, it separates after dissolution. Other combinations of frozen glass-forming solvents and PA's are possible, and may turn out to be more effective.
Spin diffusion relayed DNP
The blue curve in Fig. 1b shows the 1H DNP build-up at 1.2 K and 6.7 T for a typical RRP, a homogeneous frozen glassy solution made by dissolving sodium [1-13C]pyruvate in H2O:D2O:glycerol-d8 (2:3:5) doped with 40 mM TEMPOL, without any micro-particles containing metabolites (that is, without any RFP). The polarization reaches P(1H)>50% with a mono-exponential build-up with a characteristic time constant τDNP(1H)=150 s. The red curve in Fig. 1b shows the 1H DNP build-up obtained under the same conditions by wet impregnation of 20 mg of micro-crystalline sodium [1-13C]pyruvate RFP powder (proton density of 34 mmol cm−3) with 60 μl of toluene-d6:THF-d8:THF (8:1:1) doped with 40 mM TEMPOL-benzoate as RRP (proton density of 9.4 mmol cm−3). The 1H DNP build-up curve is now bi-exponential with a fast component τDNP fast(1H)=155 s arising from the direct polarization within the RRP, and a new slow component τDNP slow(1H)=1900, s corresponding to polarization of the RFP that builds up through proton–proton spin diffusion across the phase barrier between the RRP and the RFP. This clearly shows that the polarization enhanced by DNP propagates, albeit slowly, from the RRP to the RFP.
The DNP enhanced proton polarization can then be transferred to the 13C spins in the RFP by 1H→13C CP at low temperatures7,8,11,12,32. Although our micro-particulate DNP sample formulation is drastically different from the usual glassy solutions, 1H to 13C by CP-DNP was performed in essentially the same manner. Figure 1c shows the stepwise build-up of the 13C magnetization of sodium [1-13C]pyruvate obtained by multiple-contact CP from 1H to 13C at 1.2 K, compared with a similar 1H→13C CP build-up measured on the same amount of sodium [1-13C]pyruvate, but dissolved in the conventional glassy matrix H2O:D2O:glycerol-d8 (2:3:5) doped with 40 mM TEMPOL33. Though these two 1H→13C CP build-up curves look similar, it is worth highlighting two major differences, first, the time between two consecutive CP contacts needs to be extended to allow proton–proton spin diffusion to carry the polarization into the RFP particles between CP steps (ΔtCP=20 min in the two-component sample, instead of 5 min in the conventional glassy matrix) and, second, 13C relaxation between CP contacts, which usually limits the 13C build-up, is essentially absent in the micro-particulate RFP because of the absence of contact with the PAs. These two new features imply that the 13C polarization is slower to build-up, but can in principle achieve polarization levels as high as P(13C)=P(1H).
Remote hyperpolarization
Extended hyperpolarization lifetimes. The primary novelty associated with this new sample formulation resides in the markedly different 13C spin-lattice relaxation times T1(13C). The 13C spins of metabolites dissolved in glassy matrices are inevitably in contact through electron–nuclear dipolar interactions with the PAs that act as paramagnetic relaxation centres. In the micro-particle formulation on the other hand, the 13C spins of the RFP are physically separated from the PAs on a micrometre length scale that is much larger than that of the electron–nuclear dipolar interaction. Figure 1d shows how the spin-lattice relaxation time T1(13C) of sodium [1-13C]pyruvate can be markedly extended at 4.2 K and 6.7 T by switching from a glassy frozen solution, where T1(13C)=20 min, to the micro-particulate sample, where T1(13C) is extended to 37 h. We measured relaxation times exceeding 20 h in [1-13C]glucose, and 5 h in [13C3, 15N]alanine and [13C2, 15N]glycine.
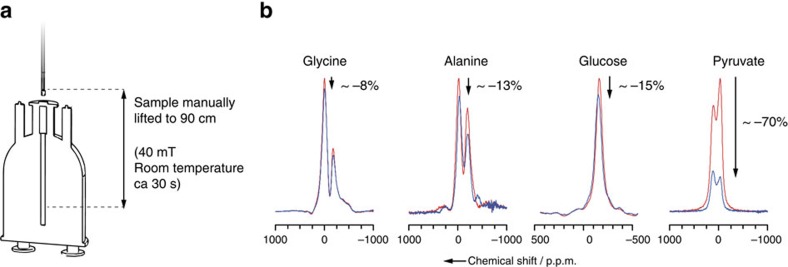
Sample transfer and storage. The paramagnetic-free environment in the RFP enables a marked extension of the nuclear spin-lattice relaxation time T1(13C). Transport to remote locations becomes possible provided that one uses a simple cryogenic transport device maintaining a small static magnetic field. This was demonstrated recently in the context of brute force hyperpolarization where neat [1-13C]pyruvic acid was thermally polarized without PAs by cooling to 2 K in a field of 14 T (refs 34, 35). Special care needs to be taken with regard to the magnetic field for sample shuttling out of the polarizer, so as to avoid excessive relaxation losses and low-field nuclear thermal mixing36,37 as recently illustrated by Peat et al.26 To test the feasibility of our strategy towards transporting hyperpolarization, Fig. 2 shows how lifting the sample manually above the polarizer for 30 s in a 40 mT magnetic field nicely preserves the hyperpolarization for [1-13C]glucose, [13C3, 15N]alanine or [13C2, 15N]glycine and to a lesser extent for sodium [1-13C]pyruvate. For pyruvate, the manual transfer strategy is not satisfactory, and a faster automated, cold, higher field transfer would help34,35 overcoming the fast relaxation arising from the presence of methyl groups and quadrupolar sodium nuclear spins in the vicinity of 13C.
Figure 2. Preserving hyperpolarization out of the polarizer.
(a) Procedure for lifting the sample above the polarizer and putting it back. In practice, the sample is lifted manually by 2 cm into a coil that provides a supplementary field of 40 mT, parallel to the B0 field of the polarizer. In 5 s, the sample and the supplementary coil are then lifted together 90 cm above the centre of the main B0 field. The sample is kept at room temperature for ∼30 s and is put back into the polarizer in ∼5 s. (b) 13C NMR spectra before and after lifting the solid powder out of the polarizer. 1H decoupled 13C NMR of [13C2, 15N]glycine, [13C3, 15N]alanine, [1-13C]glucose and [1-13C]pyruvate measured with 0.5° nutation angle pulses after 10 min 1H DNP followed by a single 1H–13C cross-polarization contact, before (red line) and after (blue line) lifting the sample according to the procedure described in a, leading to polarization losses of ca. –8%, –13%, –15% and –70%, respectively, illustrated by the arrows.
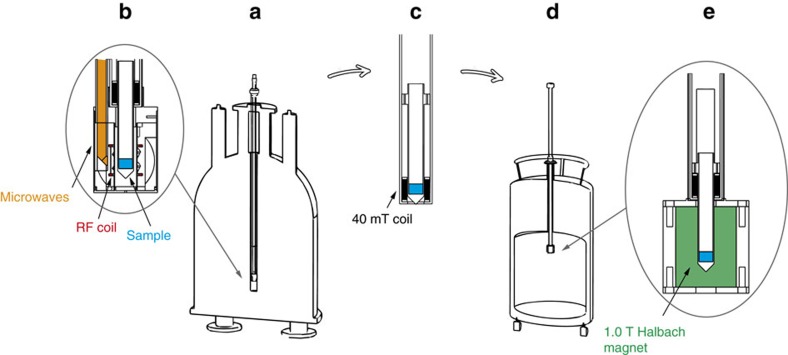
The feasibility of transfer and storage is demonstrated for a mixture of [13C3, 15N]alanine and [13C2, 15N]glycine powders impregnated with the RRP. For that purpose, we have upgraded our existing state-of-the-art DNP polarizer with a transfer, storage and transport system, as shown in Fig. 3. The sample was polarized at 1.2 K in our polarizer (Fig. 1a,b) for 60 min, while three CP sequences were applied at 20, 40 and 60 minutes, leading to a polarization level P(13C)∼15±5%. The sample was manually transferred out of the 6.7 T polarizer in a 40 mT magnetic field (Fig. 3c) and inserted into the magnetic insert of the cryogenic Dewar (1.0 T and 4.2 K; Fig. 3d,e) and stored for 16 h. No attempts were made so far to optimize the 13C lifetimes during the waiting time of 16 h. Higher fields, lower temperatures, sample purification, oxygen removal and annealing of the RFP are expected to improve the lifetimes, as shown by Kempf et al., who reported T1(13C)>24 h at 6 K and 4 T in neat pyruvic acid after annealing34,35. In this case, we used the sample without purification for this proof-of-concept experiment.
Figure 3. Illustration of the remote hyperpolarization set-up.
(a) The DNP polarizer consists of a 6.7-T wide bore magnet and a 1.2-K cryostat equipped with (b) a DNP probe where the sample is inserted and irradiated with microwaves for 1H DNP and radio-frequency fields for 1H–13C cross-polarization. The sample can then be manually removed from the polarizer in (c) a transfer stick comprising a coil sustaining a magnetic field of ca. 40 mT (100 turns, current of 4 A) and subsequently inserted in (d) a conventional liquid helium transport Dewar with (e) a magnetic insert providing a 1.0 T static magnetic field for storage or transport.
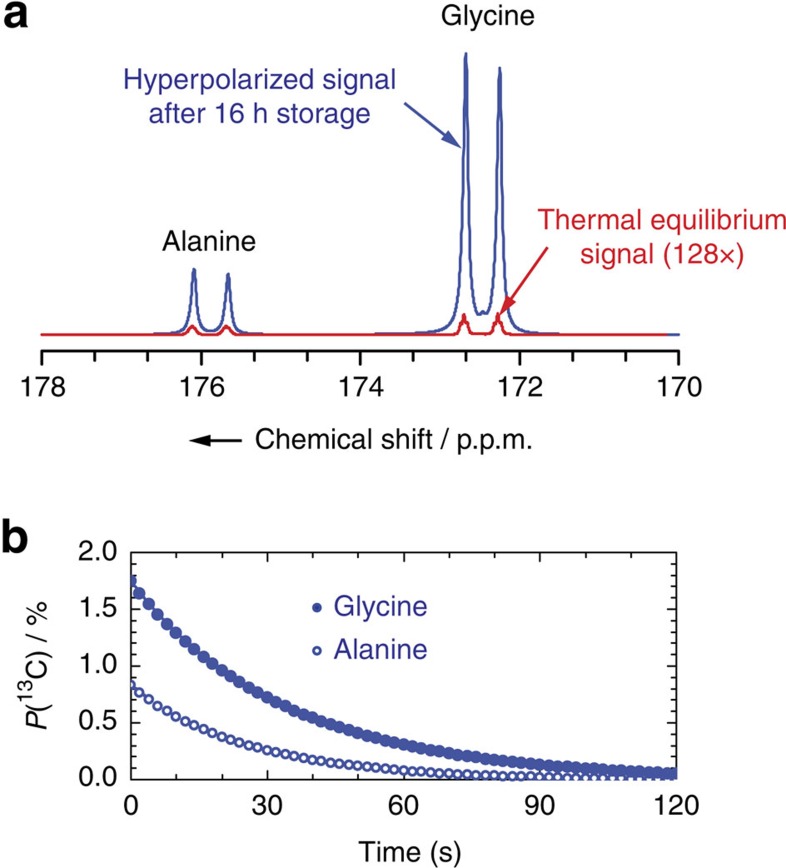
Dissolution. Following a 16-h hold, the sample was then dissolved directly from the transport Dewar following a previously described procedure9 with 5 ml of pressurized heated water (420 K and 1 MPa). The resulting hyperpolarized solution was pushed through a magnetic tunnel38 and injected into a 5-mm sample tube in a 500-MHz liquid-state NMR spectrometer. Figure 4a shows the hyperpolarized signal compared with thermal equilibrium, featuring enhancement factors ɛDNP=821 and 1728, corresponding to polarizations P(13C)=0.84% and 1.76% for alanine and glycine, respectively. In the dissolution product, we measured 13C spin-lattice relaxation times T1(13C)=26.3 and 34.9 s for alanine and glycine, respectively, which are typical for radical-free solutions (Fig. 4b). This is explained by the fact that the PA used (TEMPOL-benzoate) is insoluble in water and therefore remains in the hydrophobic organic RRP phase that separates28 from the aqueous phase, while the alanine and glycine readily dissolve in the aqueous phase. With our micro- particulate sample formulation, the thermodynamics of dissolution is advantageous compared with water-based glassy solutions since, first, the heat capacity CP and latent heat of fusion (LV) of organic solvents are significantly smaller than those of water and, second, the enthalpy of solution (ΔHsoln) of organic crystals is generally negative, so that dissolution is exothermic. Dissolution can therefore be performed with the existing dissolution devices6,9,39,40, and possibly with a smaller dilution factor.
Figure 4. Hyperpolarization preserved during a 16-h storage.
(a) Hyperpolarized 1-13C signal in [13C3, 15N]alanine or [13C2, 15N]glycine. Before storage at 4.2 K and 1.0 T, 5.5 mg [13C3, 15N]alanine and 9 mg [13C2, 15N]glycine were impregnated with 45 μl of toluene:THF (8:2) doped with 80 mM TEMPOL-benzoate (RRP) and polarized at 6.7 T and 1.2 K. Following a 16-h hold, the sample was dissolved in the storage Dewar in 700 ms with superheated water (5 ml at 420 K) and pushed through a magnetic tunnel38 towards the 500-MHz spectrometer in 4.5 s, and injected in a 5-mm NMR tube in 2 s. This is to be compared with a thermal equilibrium signal (red line, 128 × ) measured after complete relaxation of the 13C magnetization, with 16 pulses with 90° angles applied every 300 s. (b) Hyperpolarization decay after dissolution to room temperature. The complete 1-13C signal relaxation curve was measured with 5° nutation angle pulses applied every 2 s for [13C3, 15N]alanine (blue circle) and [13C2, 15N]glycine (blue filled circle).
Discussion
This proof-of-concept experiment is not yet optimized, and the levels of polarization obtained after transfer, storage and dissolution are still about one order of magnitude below what can be achieved in conventional ‘on-site' d-DNP in frozen glassy samples. The reasons for the lower polarization are multiple.
First, DNP would be more efficient, typically by a factor two, if the particles were tailored to the right dimensions. Indeed, small particle diameters are critical to reduce the distance over which hyperpolarized 1H magnetization needs to diffuse. Zirconia ball milling or other preparation methods could be envisaged, such as precipitation/crystallization from a saturated solution, co-crystallization, spray-drying, re-crystallization by solvent evaporation and so on.
Second, polarization losses above 10% are currently observed upon transfer of the sample to the cryogenic storage Dewar. This could be minimized if the transfer were done more rapidly, at a lower temperature and in a higher magnetic field.
Third, losses during storage and transport could possibly be minimized if the storage magnetic field were increased. As shown in Fig. 1d, polarization losses in sodium [1-13C]pyruvate at 4.2 K and 6.7 T are only ∼40% over 16 h.
Finally, sample formulation could be further improved by a series of measures among which removal of traces of paramagnetic ions with suitable ligands, purification by re-crystallization, annealing, and deoxygenation to promote longer hyperpolarization lifetimes.
In conclusion, we have introduced a novel approach to dissolution DNP that uses samples with a micro-particulate architecture where substrates such as metabolites are physically separated from the PAs, the former in an RFP, the latter in an RRP. This enables the creation of ‘trapped' hyperpolarized states by a combination of proton DNP in the RRP, proton–proton spin diffusion across the phase separation between the RRP and the RFP, and CP from 1H to 13C within the RFP. The lifetime T1(13C) of the hyperpolarization P(13C) in the micro-particulate samples studied here ranges between 5 and 37 h. This approach enables the removal of a solid hyperpolarized by DNP from the polarizer, while preserving its polarization. This is reminiscent of samples that have been hyperpolarized by so-called brute force methods (that is, at much lower temperatures without radicals32,33), although DNP can be much faster and can achieve much higher polarization levels. We have demonstrated that one can store and transport hyperpolarized molecules to remote locations using a simple cryogenic transport device. Finally, we have shown how the micro-particulate samples can be dissolved in hot water so that the aqueous RFP is physically separated from the organic RRP. The resulting pure hyperpolarized solution of metabolites can in principle be used in any existing hyperpolarized in vivo or in vitro magnetic resonance experiment. With this new approach, the production of hyperpolarized molecules could in principle be scaled up, using dedicated high-throughput multiple-sample25 remote polarizers.
Methods
Sample preparation
[1-13C]Glucose (Sigma Aldrich), [13C3, 15N]alanine, [13C2, 15N]glycine and sodium [1-13C]pyruvate (Cambridge Isotopes) were ground by hand to obtain the RFP. The RRPs were prepared by dissolving 4-hydroxy-TEMPO-benzoate (Sigma Aldrich) in mixtures of toluene-d6 (Armar Chemicals), THF-d8 (Armar Chemicals), toluene and THF (Sigma Aldrich). The finely ground RFP were then impregnated with the RRP solution directly in the DNP sample holder with 3 μl of the RRP added per milligram of RFP. The impregnated mixture in the DNP sample holder was then sonicated for 10 s before insertion in the DNP polarizer that resulted in rapid freezing of the sample in liquid helium.
Remote DNP apparatus
The system builds on our previously described DNP apparatus9 that consists of a 6.7-T DNP polarizer equipped with a CP-DNP probe (Fig. 3a,b) in which the sample changer tube (Fig. 3c) has been modified to accommodate a solenoidal coil (100 turns) so as to sustain a ca. 40 mT magnetic field during sample transfer (with 4 A current). After DNP was completed, the sample was manually transferred in ca. 10 s from the 6.7-T polarizer to a 1.0-T Halbach magnet in a liquid helium cryogenic transport Dewar (Fig. 3d,e). The polarized [13C3, 15N]alanine and [13C2, 15N]glycine powders were left in the storage Dewar for 16 h. Dissolution then took place directly in the storage Dewar.
DNP experiments
DNP was performed at B0=6.7 T and T=1.2 K by frequency modulated microwave irradiation (PμW=87.5 mW and fμW=188.3 GHz) and combined with CP from 1H to 13C in the RFP, using CP contacts of 1–10 ms and B1=20 kHz at intervals of 20 min to allow the proton polarization to migrate by spin diffusion from the RRP to the RFP. Further details are available in Fig. 5a of ref. 41.
Dissolution experiments
Dissolution was performed as described elsewhere39 by injecting 5 ml of D2O preheated to T=420 K at 1.0 MPa onto the powdered impregnated materials in 700 ms. The dissolved sample was then pushed in 4.5 s by helium gas with a pressure of 0.6 MPa through a 1.5-mm inner diameter polytetrafluoroethylene tube protected by a 0.9-T magnetic tunnel38 to a home-built injector just above a 5-mm sample tube in a 11.7 T magnet (500 MHz for protons). The sample was then injected in ca. 2 s. The complete sequence of dissolution, transfer and injection takes ∼7.2 s. A series of 5° pulses was then applied to the 13C nuclei at regular intervals (2–5 s).
Scanning electron microscopy
SEM was performed with a FEI XLF-30 SEM instrument at 10 kV accelerating voltage. An amount of 10 mg of finely ground RFP was placed on a film of conducting polymer attached to the sample holder. The sample was coated with 25 nm gold to ensure surface conductivity.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Additional information
How to cite this article: Ji, X. et al. Transportable hyperpolarized metabolites. Nat. Commun. 8, 13975 doi: 10.1038/ncomms13975 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
We thank Dr Pascal Miéville for valuable assistance. S.J. thanks James Kempf for invaluable discussions and Roger Mottier from the mechanical workshop. SEM pictures were recorded by Grégoire Baroz and Julien Michellod. This work was supported by the Swiss National Science Foundation (SNF), the Ecole Polytechnique Fédérale de Lausanne, Bruker BioSpin Switzerland AG, the Centre National de la Recherche Scientifique, Equipements d'Excellence (EQUIPEX) Contract ANR-10-EQPX-47-01, and the European Research Council (ERC) Advanced Grants 320860 and 339754.
Footnotes
Author contributions J.M. and S.J. built the apparatus. A.B., X.J., B.V., D.G., A.J.R. and S.J carried out the experiments. L.E., G.B., A.B. and S.J. conceived the ideas, analysed the data and wrote the paper.
References
- Abragam A. & Goldman M. Principles of dynamic nuclear-polarization. Rep. Prog. Phys. 41, 395–467 (1978). [Google Scholar]
- Ardenkjaer-Larsen J. H. et al. Increase in signal-to-noise ratio of>10,000 times in liquid-state NMR. Proc. Natl Acad. Sci. USA 100, 10158–10163 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y., Heo G. S., Zeng H. F., Wooley K. L. & Hilty C. Detection of living anionic species in polymerization reactions using hyperpolarized NMR. J. Am. Chem. Soc. 135, 4636–4639 (2013). [DOI] [PubMed] [Google Scholar]
- Chen H. Y., Ragavan M. & Hilty C. Protein folding studied by dissolution dynamic nuclear polarization. Angew. Chem. Int. Ed. 52, 9192–9195 (2013). [DOI] [PubMed] [Google Scholar]
- Nelson S. J. et al. Metabolic imaging of patients with prostate cancer using hyperpolarized [1-C-13]pyruvate. Sci. Transl. Med. 5, 198ra108 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardenkjaer-Larsen J. H. et al. Dynamic nuclear polarization polarizer for sterile use intent. NMR Biomed. 24, 927–932 (2011). [DOI] [PubMed] [Google Scholar]
- Jannin S., Bornet A., Colombo S. & Bodenhausen G. Low-temperature cross polarization in view of enhancing dissolution dynamic nuclear polarization in NMR. Chem. Phys. Lett. 517, 234–236 (2011). [Google Scholar]
- Bornet A., Melzi R., Jannin S. & Bodenhausen G. Cross polarization for dissolution dynamic nuclear polarization experiments at readily accessible temperatures 1.2<T<4.2K. Appl. Magn. Reson. 43, 107–117 (2012). [Google Scholar]
- Bornet A. et al. Boosting dissolution dynamic nuclear polarization by cross polarization. J. Phys. Chem. Lett. 4, 111–114 (2013). [DOI] [PubMed] [Google Scholar]
- Batel M. et al. Dissolution dynamic nuclear polarization efficiency enhanced by Hartmann-Hahn cross polarization. Chem. Phys. Lett. 554, 72–76 (2012). [Google Scholar]
- Hartmann S. R. & Hahn E. L. Nuclear double resonance in the rotating frame. Phys. Rev 128, 2042 (1962). [Google Scholar]
- Pines A., Waugh J. S. & Gibby M. G. Proton-enhanced nuclear induction spectroscopy—method for high-resolution NMR of dilute spins in solids. J. Chem. Phys. 56, 1776 (1972). [Google Scholar]
- Elisei E. et al. Dynamic nuclear polarization of a glassy matrix prepared by solid state mechanochemical amorphization of crystalline substances. Chem. Commun. 51, 2080–2083 (2015). [DOI] [PubMed] [Google Scholar]
- Flori A., Liserani M., Bowen S., Ardenkjaer-Larsen J. H. & Menichetti L. Dissolution dynamic nuclear polarization of non-self-glassing agents: spectroscopy and relaxation of hyperpolarized [1-13C]acetate. J. Phys. Chem. A 119, 1885–1893 (2015). [DOI] [PubMed] [Google Scholar]
- Gajan D. et al. Hybrid polarizing solids for pure hyperpolarized liquids through dissolution dynamic nuclear polarization. Proc. Natl Acad. Sci. USA 111, 14693–14697 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichhorn T. R. et al. Hyperpolarization without persistent radicals for in vivo real-time metabolic imaging. Proc. Natl Acad. Sci. USA 110, 18064–18069 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg W. E. Nuclear spin-lattice relaxation caused by paramagnetic Impurities. Phys. Rev. 119, 79 (1960). [Google Scholar]
- van der Wel P. C. A., Hu K. N., Lewandowski J. & Griffin R. G. Dynamic nuclear polarization of amyloidogenic peptide nanocrystals: GNNQQNY, a core segment of the yeast prion protein Sup35p. J. Am. Chem. Soc. 128, 10840–10846 (2006). [DOI] [PubMed] [Google Scholar]
- Debelouchina G. T. et al. Dynamic nuclear polarization-enhanced solid-state NMR spectroscopy of GNNQQNY nanocrystals and amyloid fibrils. Phys. Chem. Chem. Phys. 12, 5911–5919 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debelouchina G. T. et al. Higher order amyloid fibril structure by MAS NMR and DNP spectroscopy. J. Am. Chem. Soc. 135, 19237–19247 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayro M. J. et al. Intermolecular structure determination of amyloid fibrils with magic-angle spinning and dynamic nuclear polarization NMR. J. Am. Chem. Soc. 133, 13967–13974 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini A. J. et al. Dynamic nuclear polarization enhanced NMR spectroscopy for pharmaceutical formulations. J. Am. Chem. Soc. 136, 2324–2334 (2014). [DOI] [PubMed] [Google Scholar]
- Rossini A. J. et al. Dynamic nuclear polarization NMR spectroscopy of microcrystalline solids. J. Am. Chem. Soc. 134, 16899–16908 (2012). [DOI] [PubMed] [Google Scholar]
- Pinon A. C., Rossini A. J., Widdifield C. M., Gajan D. & Emsley L. Polymorphs of Theophylline characterized by DNP enhanced solid-state NMR. Mol. Pharm. 12, 4146–4153 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batel M. et al. A multi-sample 94 GHz dissolution dynamic-nuclear-polarization system. J. Magn. Reson. 214, 166–174 (2012). [DOI] [PubMed] [Google Scholar]
- Peat D. T. et al. Low-field thermal mixing in [1-C-13] pyruvic acid for brute-force hyperpolarization. Phys. Chem. Chem. Phys. 18, 19173–19182 (2016). [DOI] [PubMed] [Google Scholar]
- Miéville P. et al. Scavenging free radicals to preserve enhancement and extend relaxation times in NMR using dynamic nuclear polarization. Angew. Chem. Intl. Ed. 49, 6182–6185 (2010). [DOI] [PubMed] [Google Scholar]
- Harris T., Bretschneider C. & Frydman L. Dissolution DNP NMR with solvent mixtures: Substrate concentration and radical extraction. J. Magn. Reson. 211, 96–100 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach A. M., Miller P., Telfeyan E. & Whitt D. B. Method and apparatus for the dissolution and filtration of a hyperpolarized agent with a neutral dissolution media. US2009263325-A1 (2009).
- Lumata L. et al. BDPA: an efficient polarizing agent for fast dissolution dynamic nuclear polarization NMR spectroscopy. Chem. Eur. J. 17, 10825–10827 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumata L. et al. The efficiency of DPPH as a polarising agent for DNP-NMR spectroscopy. RSC Adv. 2, 12812–12817 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jannin S., Bornet A., Melzi R. & Bodenhausen G. High field dynamic nuclear polarization at 6.7 T: Carbon-13 polarization above 70% within 20 min. Chem. Phys. Lett. 549, 99–102 (2012). [Google Scholar]
- Can T. V. et al. Overhauser effects in insulating solids. J. Chem. Phys. 141, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch M. L., Kalechofsky N., Belzer A., Rosay M. & Kempf J. G. Brute-Force Hyperpolarization for NMR and MRI. J. Am. Chem. Soc. 137, 8428–8434 (2015). [DOI] [PubMed] [Google Scholar]
- Hirsch M. L. et al. Transport and imaging of brute-force C-13 hyperpolarization. J. Magn. Reson. 261, 87–94 (2015). [DOI] [PubMed] [Google Scholar]
- Abragam A. & Proctor W. G. Experiments on spin temperature. Phys. Rev 106, 160–161 (1957). [Google Scholar]
- Abragam A. & Proctor W. G. Spin temperature. Phys. Rev. 109, 1441–1458 (1958). [Google Scholar]
- Milani J. et al. A magnetic tunnel to shelter hyperpolarized fluids. Rev. Sci. Instrum. 86, 024101 (2015). [DOI] [PubMed] [Google Scholar]
- Comment A. et al. Design and performance of a DNP prepolarizer coupled to a rodent MRI scanner. Concepts Magn. Reson. B 31B, 255–269 (2007). [Google Scholar]
- Comment A. et al. Principles of operation of a DNP prepolarizer coupled to a rodent MRI scanner. Appl. Magn. Reson. 34, 313–319 (2008). [Google Scholar]
- Bornet A. et al. Microwave-gated dynamic nuclear polarization. Phys. Chem. Chem. Phys. 18, 30530–30535 (2016). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.