Abstract
Formation of organ-specific vasculatures requires cross-talk between developing tissue and specialized endothelial cells. Here we show how developing zebrafish spinal cord neurons coordinate vessel growth through balancing of neuron-derived Vegfaa, with neuronal sFlt1 restricting Vegfaa-Kdrl mediated angiogenesis at the neurovascular interface. Neuron-specific loss of flt1 or increased neuronal vegfaa expression promotes angiogenesis and peri-neural tube vascular network formation. Combining loss of neuronal flt1 with gain of vegfaa promotes sprout invasion into the neural tube. On loss of neuronal flt1, ectopic sprouts emanate from veins involving special angiogenic cell behaviours including nuclear positioning and a molecular signature distinct from primary arterial or secondary venous sprouting. Manipulation of arteriovenous identity or Notch signalling established that ectopic sprouting in flt1 mutants requires venous endothelium. Conceptually, our data suggest that spinal cord vascularization proceeds from veins involving two-tiered regulation of neuronal sFlt1 and Vegfaa via a novel sprouting mode.
The generation of vasculature in organs is regulated by cross-talk between the developing tissue and specialized endothelial cells. Here, the authors show that vessel growth feeding the zebrafish spinal cord is coordinated by balancing neuron-derived pro-angiogenic ligand Vegfaa and its receptor, sFlt1.
The vascular network closely associates with the neuronal network throughout embryonic development, in adulthood and during tissue regeneration1,2,3. Close association of vessels and nerves allows reciprocal cross-talk involving diffusible molecules, which is important for physiological functions in both domains4,5. Arteries secrete factors that attract sympathetic axons, and adrenergic innervation of arteries allows the autonomic nervous system to control arterial tone and tissue perfusion5. The nervous system, on the other hand, requires a specialized network of blood vessels for its development and survival. Metabolically active nerves rely on blood vessels to provide oxygen necessary for sustaining neuronal activity6, and disturbances herein result in neuronal dysfunction1,7.
How nerves attract blood vessels is debated, but several studies addressing vascularization of the mouse and chicken embryonic nervous system suggest that the angiogenic cytokine VEGF-A is involved8,9,10. In the mouse peripheral nervous system axons of sensory nerves innervating the embryonic skin trigger arteriogenesis involving VEGF-A–Neuropilin-1 (NRP1) dependent signalling11,12. While these studies provide evidence for the physical proximity and cooperative patterning of the developing nerves and vasculature, relatively little is known about mechanisms controlling VEGF-A dosage at the neurovascular interface. This is of great importance considering that blood vessels are very sensitive to changes in VEGF-A protein dosage and even moderate deviations from its exquisitely controlled physiological levels result in dramatic perturbations of vascular development13,14. VEGF-A levels must therefore be well titrated, and several strategies have evolved to achieve this.
Mouse retinal neurons for example can reduce extracellular VEGF-A protein via selective endocytosis of VEGF-A–VEGF receptor-2 (KDR/FLK) complexes. Inactivation of this uptake causes non-productive angiogenesis15. In the vascular system, spatio-temporal control of VEGF-A protein dosage is thought to be achieved by soluble VEGF receptor-1 (sFLT1), an alternatively spliced, secreted isoform of the cell-surface receptor membrane-bound FLT1 (mFLT1)16,17. Soluble FLT1 binds VEGF-A with substantially higher affinity than KDR, thereby reducing VEGF-A bioavailability and attenuating KDR signalling17. While originally discovered as a vascular-specific receptor, evidence is emerging showing neuronal FLT1 expression18. To what extent endogenous neuronal Flt1 has a physiological role in titrating neuronal VEGF levels controlling angiogenesis at the neurovascular interface independent of vascular Flt1 remains to be determined.
Angiogenesis involves complex and dynamic changes in endothelial cell behaviour19. In the zebrafish embryo these events can be studied in detail at the single cell level in vivo through the use of vascular-specific reporter lines20,21. The stereotyped patterning of arteries and veins in the trunk of the zebrafish embryo prior to 48 hpf is mediated by cues derived from developing somites and the hypochord, controlling angiogenic sprout differentiation and guidance22,23. Sprouting of intersegmental arterioles (aISV) requires Vegfaa-Kdrl signalling, as loss of either kdrl or vegfaa completely abolishes ISV sprouting from the dorsal aorta (DA)24. Primary sprouting also involves a component regulated by Notch, as loss of Notch increases the endothelial propensity to occupy the tip cell position in this vessel, whereas gain of Notch restricts aISV development25. Secondary vein sprouting requires Vegfc-Flt4 signalling, as loss of either ligand or receptor blocks venous growth26,27. Developing somites are regarded as the main source for Vegfaa, while the hypochord provides Vegfc during early development22,23.
In this study we show that developing spinal cord neurons located in the trunk of the zebrafish embryo produce Vegfaa and sFlt1 affecting the angiogenic behaviour of intersegmental vessels at the neurovascular interface. We find that during early development neuronal sFlt1 restricts angiogenesis around the spinal cord. We demonstrate that on genetic ablation of neuronal sFlt1 this brake is relieved resulting in the formation of a vascular network supplying the spinal cord in a Vegfaa-Kdrl dependent manner. Using inducible neuron-specific vegfaa gain-of-function approaches and analysis of several mutants with vegfaa gain-of-function scenarios, we furthermore show that the neuronal Vegfaa dosage determines the extent of the neovasculature supplying the spinal cord, as well as sprout invasion into the spinal cord. Interestingly, loss of flt1 or augmenting neuronal vegfaa promotes sprouting from intersegmental veins involving distinctive angiogenic cell behaviours including nuclear positioning and a molecular signature not observed in primary arterial or secondary venous sprouting. Cell transplantation experiments confirm the role of neuronal flt1 in venous sprouting and furthermore show that vascular flt1 is dispensable herein. Taken together, our data suggest that spinal cord vascularization proceeds from veins and is coordinated by two-tiered regulation of neuronal sFlt1 and Vegfaa determining the onset and the extent of the vascular network that supplies the spinal cord via a novel sprouting mode.
Results
Spinal cord neurons express sflt1, mflt1 and vegf ligands
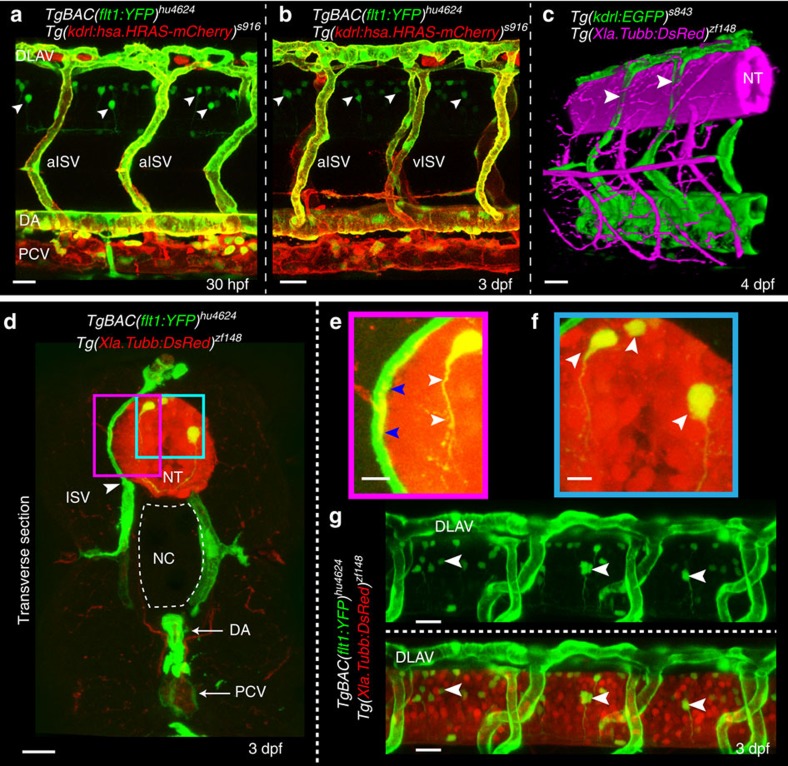
Analysis of TgBAC(flt1:YFP)hu4624;Tg(kdrl:hsa.HRAS-mCherry)s916 transgenic embryos showed flt1 expression in the aorta, arterial intersegmental vessels (aISVs), dorsal part of venous intersegmental vessels (vISVs) and spinal cord neurons located in the neural tube (Fig. 1a,b,d–g)18. Spinal cord neurons were in close proximity to blood vessels (Fig. 1c–e) and 3D-rendering of confocal z-stacks obtained from Tg(kdrl:EGFP)s843;Tg(Xla.Tubb:DsRed)zf148 double transgenic embryos showed the dorsal aspect of ISVs ‘indenting' the neural tube indicative of close contact (Fig. 1c; Supplementary Movie 1). Optical sections confirmed close contact between the outer neuronal layers of the neural tube and the dorsal part of ISVs, as well as the dorsal longitudinal anastomotic vessel (DLAV) (Fig. 1d–g). Such anatomical juxtapositioning of trunk vessels and neurons may provide a template for molecular cross-talk (Fig. 1d,e; pink box).
Figure 1. Expression of Vegf receptors and ligands at the neurovascular interface.
(a,b) Double transgenic embryos TgBAC(flt1:YFP)hu4624; Tg(kdrl:hsa.HRAS-mcherry)916 at 30 hpf and 3 dpf shows flt1 expression (green) in dorsal aorta, arterial ISV and dorsal aspect of venous ISV (3 dpf) and neurons (arrowheads). (c) 3D-rendered view of vessels (green) and nerves (purple) in Tg(kdrl:EGFP)s843;Tg(XIa.Tubb:DsRed)zf148 embryos highlighting dorsal aspect of ISVs (arrowheads) in close contact to the neural tube (NT). (d) Transverse section of the trunk of TgBAC(flt1:YFP)hu4624;Tg(XIa.Tubb:DsRed)zf148 embryos shows that ISVs (green, arrowhead) and neural tube (NT, red) are in close contact. Dorsal is up. (e) Magnified view of purple-boxed area in (d), showing direct contact of vessels with nerves at the neurovascular interface (blue arrowheads) and flt1 expressing neurons with long axonal extensions in the neural tube (white arrowheads). (f) Magnified view of blue-boxed area in (d) showing flt1 expressing neurons (arrowheads) and their axons inside neural tube (red). (g) Lateral view of TgBAC(flt1:YFP)hu4624; Tg(Xla.Tubb:DsRed)zf148 at the level of the neural tube showing flt1 expressing neurons (arrowheads) in neural tube. DA, dorsal aorta; dpf, days post fertilization; DLAV, dorsal longitudinal anastomotic vessel; hpf, hours post fertilization; ISV, intersegmental vessel; NC, notochord; NT, neural tube; PCV, posterior cardinal vein. Scale bar, 30 μm in a–d,g; 10 μm in e,f.
TaqMan analysis using FAC-sorted neuronal cells from two different neuronal reporter lines (Supplementary Fig. 1a–k) showed expression of mflt1, sflt1, kdrl, kdr, flt4 and the ligands vegfaa, vegfab, and plgf (Supplementary Fig. 1b,e)24. Flt1 was expressed in a comparable range as neuronal guidance molecules (Supplementary Fig. 1c,f). Real-time qPCR analysis for vegfaa and vegfab in the trunk of developing zebrafish embryos confirmed expression of both isoforms (Supplementary Fig. 1l,m).
Loss of flt1 induces ectopic vascular networks
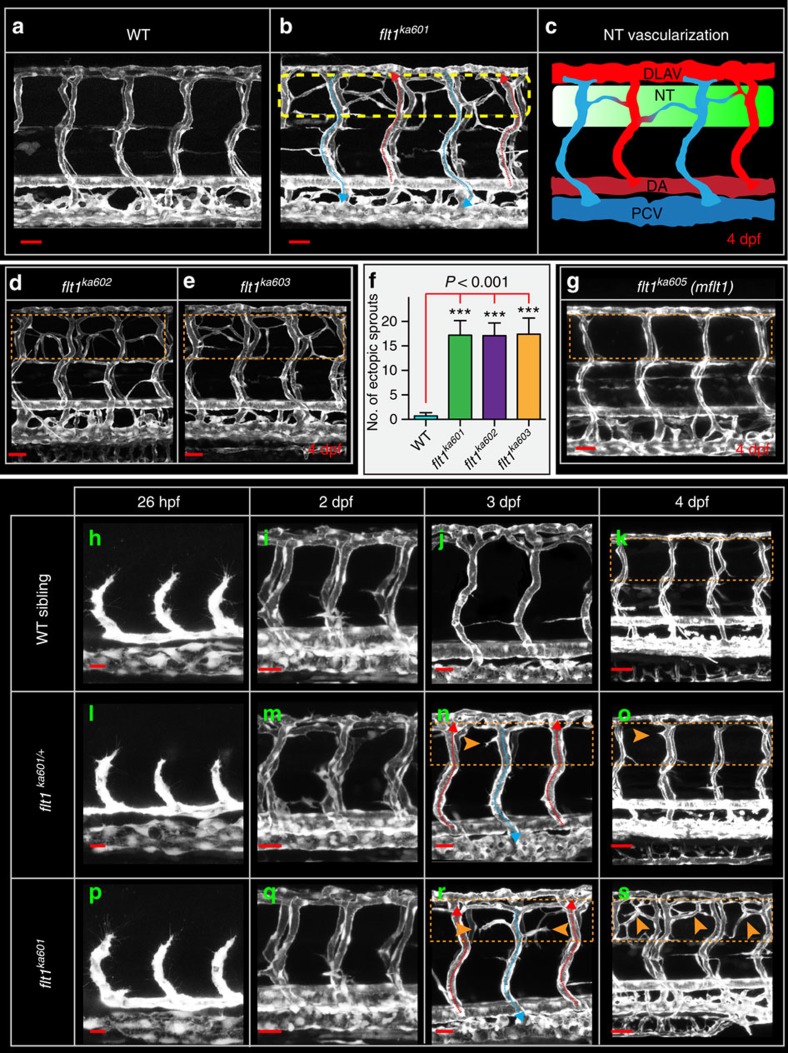
In zebrafish flt1 consists of 34 exons encoding membrane-bound mflt1 and soluble sflt1, which is formed by alternative splicing at the exon 10—intron 10 boundary (Supplementary Fig. 2a)18. To obtain loss of both mflt1 and sflt1 (flt1 full mutants) we targeted flt1-exon 3 using a CRISPR/Cas approach (Supplementary Fig. 2a–d) and analysed in detail the vascular phenotypes of three mutant alleles, flt1ka601 (−1 nt), flt1ka602 (−5 nt) and flt1ka603(+5 nt) (Fig. 2a–f; Supplementary Fig. 2a–d). To obtain mflt1-specific mutants we targeted exon 11b, the alternative exon essential for mflt1 transcription (Fig. 2g; Supplementary Fig. 2a,e)18. Both the flt1ka601 and flt1ka605 mutant showed no signs of non-sense mediated decay (Supplementary Fig. 3a,b). Zebrafish homozygous for the flt1 -1 nt allele (flt1ka601) displayed severe hyper-branching of the trunk vasculature at 3–4 dpf (Fig. 2a,b). Supernumerous amounts of branches developed in the dorsal aspect of the trunk at the level of the neural tube (Fig. 2b,c). Comparable observations were made in embryos homozygous for the flt1 −5 nt allele (flt1ka602) and the flt1 +5 nt allele (flt1ka603) (Fig. 2d,e,f). Analysis of four mflt1 mutant alleles (flt1ka605-608, Supplementary Fig. 2e) did not reveal any obvious vascular malformations or alterations in vascular branching morphogenesis (Fig. 2g). These observations are compatible with absence of angiogenic defects in mouse Flt1TK−/− embryos lacking mFlt1 signalling28,29. The vascular phenotype observed in the flt1ka601 mutants thus most likely involved soluble Flt1.
Figure 2. Flt1 mutants develop hyper-branched vascular networks at the level of the neural tube.
(a) Trunk vasculature in 4 dpf WT sibling, (b) trunk vasculature in 4 dpf flt1ka601 mutant, in Tg(kdrl:EGFP)s843 background. Perfused aISVs with red arrow, veins with blue arrow. Note the extensive amount of hyper-branching (dotted box) at the level of the neural tube. (c) Schematic representation of hyper-branching phenotype along the neural tube; ectopic vessels make anastomosis between vISV (blue) with aISVs (red). (d) Hyper-branching (dotted box) is also observed in flt1ka602 and (e) flt1ka603 mutants. (f) Quantification of hyper-branching for indicated mutant alleles. Mean±s.e.m., n=10 per group, ANOVA. (g) Membrane-bound flt1 mutant (flt1ka605) without vascular phenotype (compare dotted box in g, with control in a). (h–k) Trunk vascular network in WT embryos at indicated time points. (l–o) Trunk vasculature in flt1ka601 /+ embryos at indicated time points. (p–s) Trunk vasculature in flt1ka601 embryos at indicated time points. Arrowheads indicate ectopic branches. DA, dorsal aorta; PCV, posterior cardinal vein; DLAV, dorsal longitudinal anastomotic vessel; NT, neural tube; hpf, hours post fertilization; dpf, days post fertilization. Scale bar, 50 μm in a,b,d,e,g,i,m,q,k,o,s; 25 μm in h,l,p,j,n,r.
Since the vascular phenotypes of the flt1ka601, flt1ka602 and flt1ka603 mutant alleles (flt1 full mutants) were indistinguishable, we focused on analysing flt1ka601 embryos (Fig. 2p–s). Flt1ka601 mutants showed normal arterial-venous remodelling (Fig. 2b,p–s) and adequate perfusion of both aISVs and vISVs. No significant changes in heart frequency were noted (Supplementary Fig. 4a). The vascular phenotype of flt1ka601 mutants emerged around day 2.5 (Fig. 2q,r) with sprouts emanating exclusively from the dorsal aspect of the venous ISVs at the level of the neural tube (Supplementary Movie 2); ectopic arterial ISV sprouting was not observed (Fig. 2r). In flt1ka601/+heterozygotes (Fig. 2l–o) ectopic sprouting was rarely observed (Fig. 2n,o; Supplementary Movie 3). In wild-type (WT) embryos such endothelial cell behaviours were not observed (Fig. 2h–k, Supplementary Movie 4).
We furthermore examined whether flt1 targeting morpholino could recapitulate the flt1ka601 mutant phenotype (Supplementary Fig. 4b–h). We evaluated two dosages of a published flt1 ATG targeting morpholino (MO) and found that 1 ng flt1 MO induced hyper-branching in WT at levels comparable to flt1ka601 (Supplementary Fig. 4g,h)18,30. Injection of 1 ng MO into flt1ka601 mutant background did not induce additional sprouting defects (Supplementary Fig. 4d), suggesting that the 1ng dosage targets flt1 specifically. In contrast, 3 ng MO introduced additional branches at 2 dpf that were not observed in the flt1ka601 mutant at this stage (Supplementary Fig. 4e). Since we did not observe maternal contribution of flt1 these observations suggest that 3 ng MO introduced non-specific effects31.
Sprouts in flt1ka601 display distinctive cell behaviours
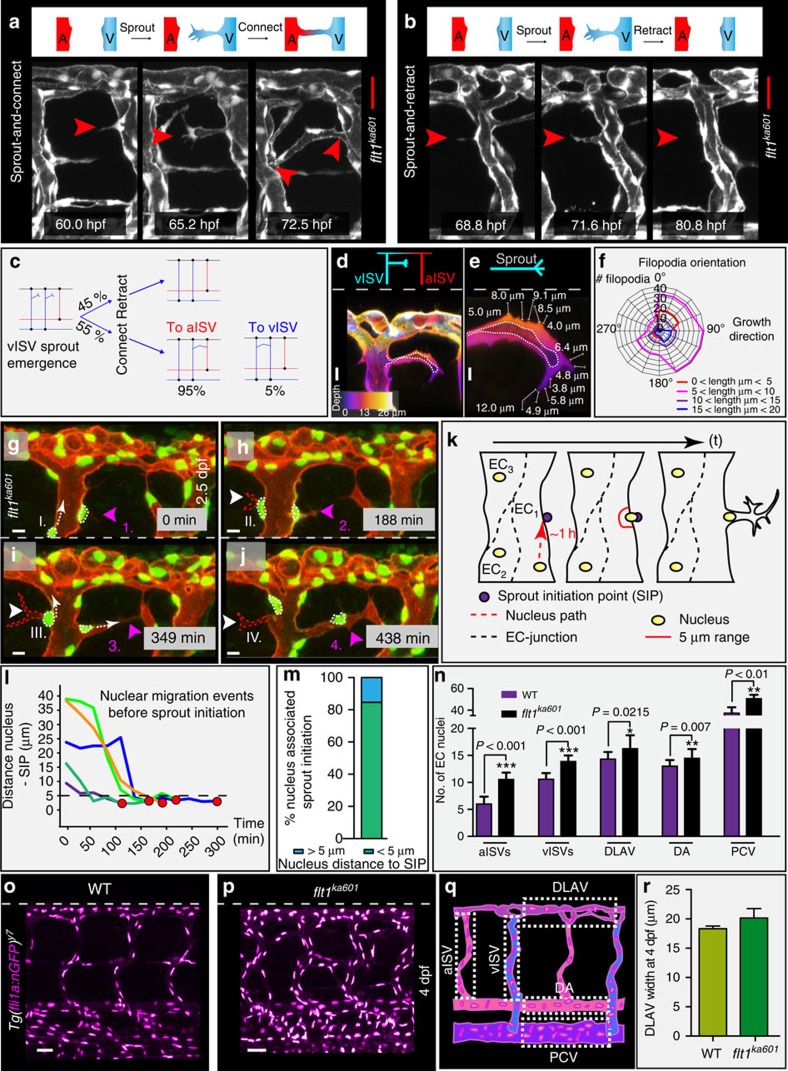
Compatible with ectopic sprouting we identified hyperactive endothelial cells extending filopodia in the dorsal aspect of vISVs of flt1ka601 mutants (Fig. 3a; Supplementary Movie 2). About 55% of hyperactive endothelial cells investigated generated a patent sprout (Fig. 3a); in the remaining 45%, filopodia and sprouts retracted (Fig. 3b). From the population of patent ectopic venous sprouts 95% formed an anastomosis with an aISV, whereas only 5% made a connection with a vISV (Fig. 3c). The preference for arterial anastomosis may be physiologically relevant as it creates a pressure gradient promoting blood flow perfusion. Sprout filopodia length ranged from 1 to 20 μm, and filopodia projected at an angle between 90 and 120° with respect to the vISV compatible with arterial anastomosis formation (Fig. 3d–f). Current models posit that Flt1 produced in angiogenic sprouts mainly prevents back-branching of nascent sprouts32. We find that in the absence of Flt1 sprouts retain their directionality and migrate away from the parent vessel. Within ISVs endothelial nuclei migrated at velocities of up to 1 μm min−1 (Fig. 3g–j,l). Careful analysis of nuclear positioning within endothelial cells revealed an association between nuclear position and sprout initiation (Fig. 3k–m, Supplementary Movie 2). Nuclei migrated actively into the direction of future sprout initiation points (SIP), and in more than 80% of the studied sprout initiations nuclear positioning was directly linked with sprout initiation (linkage was defined as nucleus-SIP distance of <5 μm at spout initiation) (Fig. 3l,m). This nuclear migration behaviour is in contrast to rearward nuclear positioning in migrating angiogenic endothelial cells in vitro33 and is not described in vivo for primary artery or secondary venous sprouting events in zebrafish. Analysis of endothelial cell numbers at 4 dpf showed increased endothelial cell numbers in aISVs, vISVs, DLAV, DA and PCV of flt1ka601 compared with WT (Fig. 3n–q); DLAV size was not statistically different (Fig. 3r). At earlier stages (17 hpf) we found no differences in endothelial cell numbers between flt1ka601 and WT (Supplementary Fig. 4i–l).
Figure 3. Imaging and quantification of sprouting kinetics in flt1ka601 mutants.
(a) Time lapse imaging of sprout initiation and anastomosis formation in flt1ka601 mutant. Sprout initiation (60.0 hpf), elongation (65.2 hpf) and connection-anastomoses (72.5 hpf) with adjacent aISV. (b) Time lapse imaging of sprout initiation and retraction in flt1ka601 mutant. Endothelial cells produce filopodia (68.8 hpf), extend a sprout (71.6 hpf), which subsequently retracts (80.8 hpf). (c) Quantification of data in (a,b) showing % of sprouts retracting (top part, 45%) or connecting (bottom part, 55%) to adjacent ISVs. In the latter scenario sprouts in 95% of cases connected to aISV (red) and in 5% of cases to vISV (blue). Angiogenic behaviour was analysed in time-lapse confocal movies, n=20 embryos. (d–f) Filopodia directionality and length in flt1ka601 mutants (n=10 embryos, n=920 filopodia). (g–j) Time lapse imaging of endothelial nuclei in Tg(fli1a:nGFP)y7; Tg(kdrl:hsa.HRAS-mcherry)s916 showing association between nuclear position and sprouting initiation point (SIP). Note that sprouts arise in close proximity to the position of the nucleus. Arrowheads indicate sprouts; nuclei at indicated time points (sprout initiation with actively migrating nucleus towards SIP I, II, III, IV and nucleus already located at SIP 1,2,3,4). (k) Schematic representation of nuclear position with respect to SIP. (l,m) Quantification of observations in g–j. Red dot indicates sprout initiation time point. Note that sprouting preferentially occurs when endothelial nuclei are within less than 5 μm from SIP (SIP below dotted line in (l)). n=5 (l) and n=13 (m). (n–q) Quantification of EC nuclei in aISV, vISVs, DLAV, DA and PCV of WT and flt1ka601 embryos at 4 dpf; mean±s.e.m., t-test, n=21 embryos per genotype. (r) Quantification of DLAV width in WT and flt1ka601 mutant, n=9 embryos per genotype. A, artery; aISV, arterial intersegmental vessel; DA, dorsal aorta; DLAV, dorsal longitudinal anastomotic vessel; EC, endothelial cell; PCV, posterior cardinal vein; SIP, sprout initiation point; V, vein; vISV, venous intersegmental vessel. Scale bar, 30 μm in a,b; 10 μm in d,e,g–j; 50 μm in o,p.
flt1ka601 display upregulation of angiogenic sprout markers
We next performed RNA sequencing of flt1ka601mutants and analysed genes implicated in sprouting angiogenesis (Supplementary Fig. 5a–c). Expression of the classical tip-stalk cell markers including notch1a, notch1b, dll4, nrarpa, nrapb, hey1, hey2, her6 and flt4 were not altered34 (Supplementary Fig. 5b). This result may not be surprising since ectopic venous sprouts emanated from venous ISVs, and Dll4-Notch signalling is absent in this domain35. Instead we found upregulated expression of other genes implied in sprouting cell behaviour. RNA-seq and qPCR of flt1 mutants showed significantly increased levels of apelin receptor-a (aplnra), angiopoietin-2a (angpt2a), and endothelial cell specific molecule-1 (esm1) (Supplementary Fig. 5b,c), genes previously shown to be enriched in angiogenic vessels36,37. In addition, we observed a significant upregulation of plgf, which encodes the Flt1-specific pro-angiogenic ligand Plgf, and lyve1, a gene expressed in veins and implied in lymphangiogenesis, in line with the venous expansion phenotype in flt1ka601 mutants (Supplementary Fig. 5c)38.
Origin of endothelial cells in ectopic venous sprouts
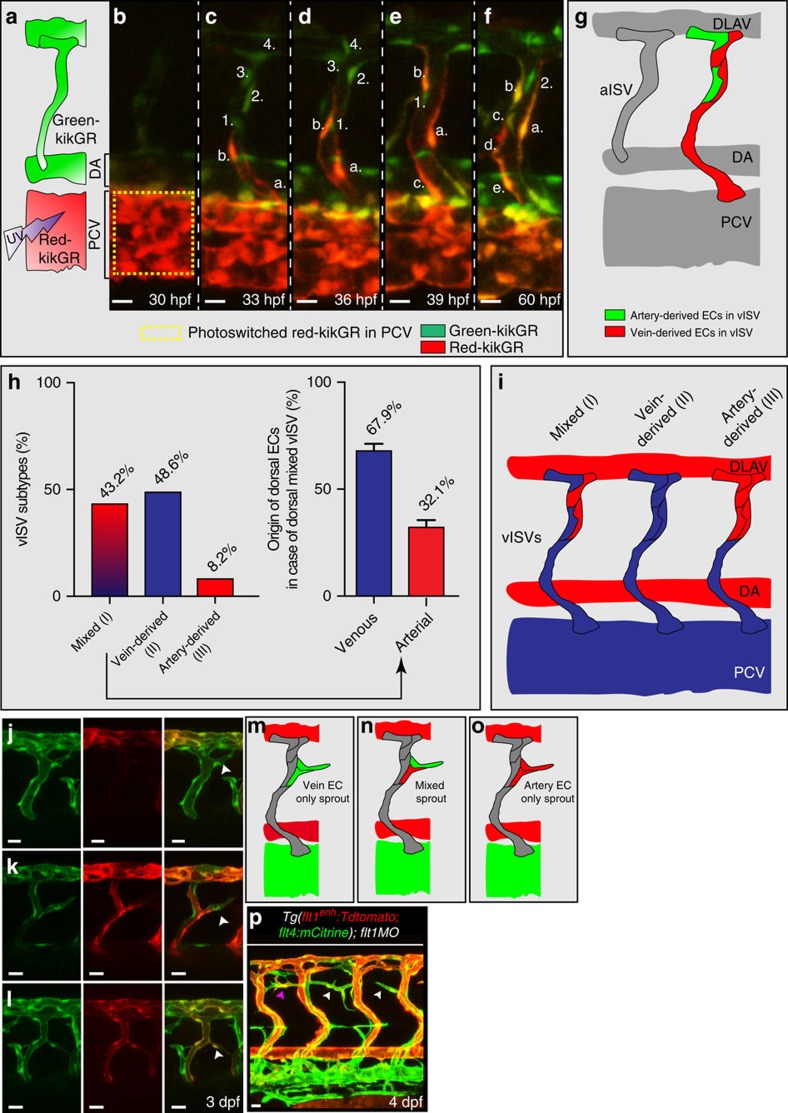
It is established that artery-derived ECs, on arteriovenous (AV) remodelling, contribute to the dorsal aspect of vISVs (ref. 39). Besides these remodelled artery-derived cells, another source may be PCV-derived venous endothelial cells as they can migrate over long distances40. However, a specific contribution of these venous ECs in populating the dorsal aspect of vISVs has not been shown thus far. To determine whether PCV-derived venous cells can colonize the dorsal aspect of vISVs, we performed cell tracking experiments using the Tg(kdrl:nlskikGR)hsc7 transgenic line (Fig. 4a–i). A small part of the PCV was photo-converted at 30 hpf and individual venous endothelial cells were tracked in the period 30–60 hpf by time-lapse imaging (Fig. 4a–f, Supplementary Movie 5). We observed three scenarios (Fig. 4i). In scenario (I): PCV-derived venous endothelial cells migrated into the vISV and reached the most dorsal aspect of the vISV (Fig. 4c–e). In the dorsal aspect of vISVs, PCV-derived endothelial cells were observed together with the remodelled artery-derived endothelial cells (Fig. 4f,g; artery-derived cells in green). Scenario (I), which we refer to as ‘mixed' (both artery and vein-derived EC), accounted for 43.2% of cases (Fig. 4h,i). Of the mixed population 67.9% of endothelial cells were of venous origin and 32.1% of arterial origin (Fig. 4h, right panel). In scenario (II), the dorsal part of vISV only contained PCV-derived venous endothelial cells; artery-derived endothelial cells were absent. Scenario (II) accounted for 48.6% of cases (Fig. 4h,i). In scenario (III) we find that the dorsal part of vISV only contained artery-derived ECs; in this scenario the dorsal aspect of vISVs was not colonized by migrating PCV-derived venous endothelial cells (Fig. 4h,i). This scenario was observed in 8.2% of cases.
Figure 4. Arterial and venous-derived endothelial cells populate the dorsal part of venous ISV and contribute to sprouting upon loss of flt1.
(a) Endothelial cell tracking in Tg(kdrl:nlsKikGR)hsc7 embryos. Photo-converted PCV-derived venous endothelial cells express red-kikGR. (b–f) Endothelial cell tracing during 30–60 hpf, showed that PCV-derived endothelium, indicated in red & labelled a,b,c,d,e, migrated along the ISV, from ventral to dorsal up to the most dorsal part of vISVs (cell labelled b). Pre-existing arterial endothelial cells in ISV, in green and labelled 1,2,3,4, shows artery-derived ECs in the dorsal part of vISVs (cell labelled 2) adjacent of PCV-derived EC (cell labelled b,a). (g) Schematic representation of the scenario imaged in b–f. (h) Left panel: Identity analysis of endothelial cells in the dorsal part of vISV revealed three different scenarios: (I) mixed, both arterial and venous-derived endothelium were present, (II) only vein-derived endothelium, (III) only artery-derived endothelium. (n=10 experiments & 6ISVs/embryo). Right panel: % of artery and vein-derived endothelium in the mixed population scenario. (i) Schematic representation of the three identity scenarios in dorsal part of vISV. Arterial derived EC in red, venous-derived EC in blue. (j–l) Ectopic sprouting scenarios in flt1 morphants in Tg(flt1enh:Tdtomato; flt4:mCitrine), n=6 embryos. (j) Ectopic venous sprout devoid of flt1enh expressing artery-derived EC (arrowhead). (k) Ectopic venous sprout (arrowhead) containing both arterial and venous-derived ECs; the flt1enh expressing artery-derived EC is juxtaposed to the venous-derived cell at the tip (arrowhead). (l) Ectopic venous sprout only containing flt1enh expressing artery-derived ECs; flt1enh (red) and flt4 (green) were expressed by the same cell which appears in yellow (arrowhead). (m–o) Schematic representation of the three ectopic venous sprouting scenarios. (p) Ectopic sprouting upon loss of flt1 in Tg(flt1enh:Tdtomato; flt4:mCitrine) (representative of 5 embryos). Flt1enh positive sprouts (pink arrowhead) and sprouts devoid of flt1enh (white arrowhead). MO, flt1 morpholino, 1ng. aISV, arterial intersegmental vessel; DA, dorsal aorta; DLAV, dorsal longitudinal anastomotic vessel; PCV, posterior cardinal vein; vISV, venous intersegmental vessel. Scale bar, 20 μm in j–l, 10 μm in b–f,p.
The flt1enh promoter marks ISV-ECs of arterial origin39. Loss of flt1 in Tg(flt1enh:Tdtomato; flt4:mCitrine) showed ectopic venous sprouts containing flt1enh-expressing ECs (Fig. 4k,l,n–p). In the same embryo, we furthermore noted ectopic venous sprouts devoid of flt1enh expressing ECs (Fig. 4j,m,p), suggesting that these sprouts were only made of vein-derived ECs (Fig. 4m). To confirm a contribution of PCV-derived venous endothelium we performed cell tracking experiments in Tg(kdrl:nlskikGR)hsc7 on loss of flt1 and indeed we found that PCV-derived venous ECs were capable of contributing to ectopic sprouting (Supplementary Fig. 6a). Interestingly, besides sprouts exclusively containing artery-derived, or venous-derived endothelium (Fig. 4m,o), we observed composite sprouts with artery and venous-derived endothelial cells juxtapositioned (Fig. 4k,n).
Vegfaa gain-of-function promotes venous sprouting
Before 48 hpf trunk arterial sprouting is driven by Vegfaa and venous sprouting by Vegfc (refs 24, 26, 27). Since loss of flt1 mimics vegfaa gain-of-function, we expected changes in arterial branching in flt1ka601. Rather surprisingly, we observed ectopic venous sprouting after 2.5 dpf (Fig. 3a,b; Fig. 5a,b,e). Primary artery development was not affected in flt1 mutants (Supplementary Fig. 6b,c,f,g), although primary arterial sprouts developed in close proximity to the neural tube (Supplementary Fig.6h–k).
Figure 5. flt1ka601 mutants and vegfaa gain-of-function scenarios promote ectopic venous sprouting.
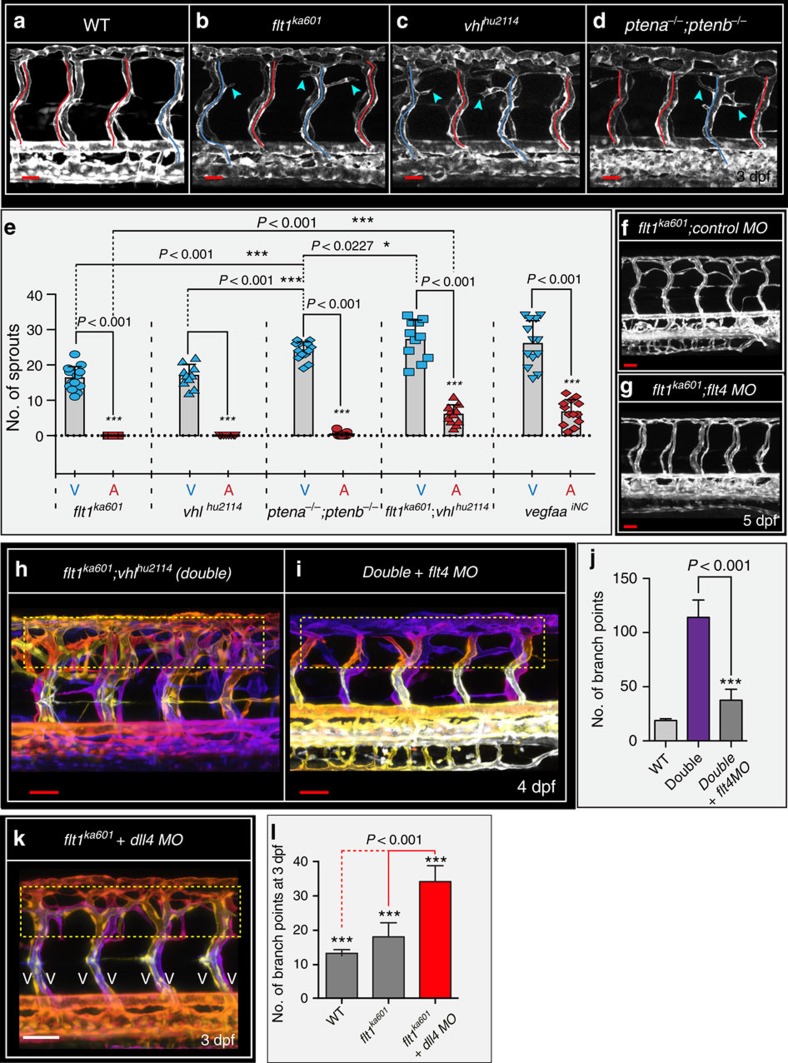
(a–d). Trunk vasculature at 3 dpf in WT (a), flt1ka601 (b), vhlhu2114 (c) and ptena−/−;ptenb−/− double mutants (d) in Tg(kdrl:EGFP)s843 background. Note ectopic sprouts originate from vISVs (blue arrowheads) in mutants. aISVs indicated in red, vISVs in blue. (e) Quantification of ectopic sprouting in indicated mutants and inducible neuronal-specific vegfaa gain-of-function. In all models ectopic sprouting preferentially occurs in veins, mean±s.e.m., n=10-13/per group, t-test. (f,g) flt1ka601 mutants show hyper-branching and knockdown of flt4 in flt1ka601 mutant rescues hyper-branching; n=21 embryos per group. (h,i) Knockdown of flt4 in flt1ka601; vhlhu2114 double mutants (double) rescues hyper-branching; compare yellow dotted box in h,i. The position of vessels is colour-coded. Note: on loss of flt4 the trunk vasculature consists almost exclusively of aISV. (j) Quantification of h,i. Mean±s.e.m., n=12 embryos per group, t-test. (k,l) Loss of dll4 in flt1ka601 mutants augments ectopic branching compared with untreated flt1ka601 mutants. Note: on loss of dll4 the trunk vasculature consists almost exclusively of vISV; n=11 embryos per group, t-test. aISV, intersegmental artery; MO, morpholino; vISV, intersegmental vein. Scale bar, 30 μm in a–d,f; 50 μm in f–i,k.
Ectopic venous sprouting was conserved in several other vegfaa gain-of-function scenarios, including vhlhu2114 mutants and ptena−/−;ptenb−/− double mutants (Fig. 5c–e). Von Hippel-Lindau protein (pVHL) is essential for the proteolytic degradation of Hif-1α, an evolutionary conserved transcription factor important for regulating vegfaa transcription41,42. Loss of vhl prevents Hif-1α degradation and augments vegfaa expression41,42. Accordingly, vhlhu2114 mutants developed ectopic sprouts emanating from vISVs but not from aISVs (Fig. 5c,e). Changes in primary aISV sprouting were not observed (Supplementary Fig. 6d,f,g).
PTEN is a tumour suppressor gene acting as a PI3K/Akt signalling attenuator and linked to the progression of many tumours involving VEGF-A (refs 43, 44). In zebrafish, two orthologues of pten exist, and ptena−/−;ptenb−/− double mutant zebrafish show increased vegfaa levels44. Detailed analysis of ptena−/−;ptenb−/− double mutants identified pronounced ectopic venous sprouting at the level of the neural tube (Fig. 5d,e). In pten double mutants ectopic venous sprout numbers were higher when compared with flt1ka601 single mutant or vhlhu2114 single mutant (Fig. 5e). In addition, in a small percentage of ISVs, ptena−/−;ptenb−/− double mutants displayed very few ectopic arterial sprouts (Fig. 5e).
Mechanistically, loss of vhl and flt1 augments Vegfaa function at different levels, through increased vegfaa transcription and higher Vegfaa protein bioavailability, respectively. We reasoned that combining both mutants should increase Vegfaa and activate downstream Kdrl signalling even further. Indeed, flt1ka601;vhlhu2114 double mutants showed more severe hyper-branching of the trunk vasculature when compared with single mutants (Fig. 5e,h). Accordingly, flt1ka601;vhlhu2114 double mutants developed more ectopic venous sprouts when compared with either single flt1ka601 or single vhlhu2114 mutants (Fig. 5e). The flt1ka601;vhlhu2114 double mutants also developed a small number of ectopic arterial sprouts after 2.5 dpf (Fig. 5e). However, venous sprout numbers were three times higher (P<0.001) than arterial sprout numbers at this stage (Fig. 5e). Changes in primary aISV (24 hpf) sprouting were not observed (Supplementary Fig. 6e,f,g). Endoxifen-induced neuronal-specific overexpression of vegfaa165 at 52 hpf in WT embryos also promoted ectopic venous sprouting (Fig. 5e; Supplementary Fig. 8e,f). In addition, a smaller number of ectopic arterial sprouts was noted, similar to flt1ka601;vhlhu2114 double mutants (Fig. 5e). Taken together, ectopic venous sprouting was conserved in five vegfaa gain-of-function scenarios.
Ectopic sprouting in flt1ka601 mutants requires veins
To prove that in flt1ka601 mutant sprouts indeed emanated from veins, we interfered with early arterial-venous remodelling by blocking flt4 (ref. 26) (Fig. 5f,g; Supplementary Fig. 6l). Loss of flt4 in flt1ka601 mutants interfered with arterial-venous remodelling; as a consequence almost all trunk ISVs remained arterial26 (Supplementary Fig. 6l). In line with the requirement for veins, the flt1ka601 hyper-branching phenotype was rescued (Fig. 5f,g). Furthermore, flt4 loss-of-function in the flt1ka601;vhlhu2114 double mutants (denoted as double in Fig. 5h,i) also significantly reduced branching complexity (Fig. 5h-j; method quantification of branch points in Supplementary Fig. 1n). As ectopic sprouting requires venous endothelium, we next reasoned that promoting vISV formation in flt1ka601 mutants should augment branching. Vessel identity and Notch signalling are linked. In zebrafish, it is established that loss of the Notch ligand Dll4 promotes venous cell fate and dll4 loss-of-function embryos display a trunk vasculature consisting almost exclusively of vISVs45. Accordingly, loss of dll4 in flt1ka601 mutants significantly augmented ectopic branching when compared with control flt1ka601 mutants (Fig. 5k,l).
Notch, pericytes and ectopic venous sprouting in flt1ka601
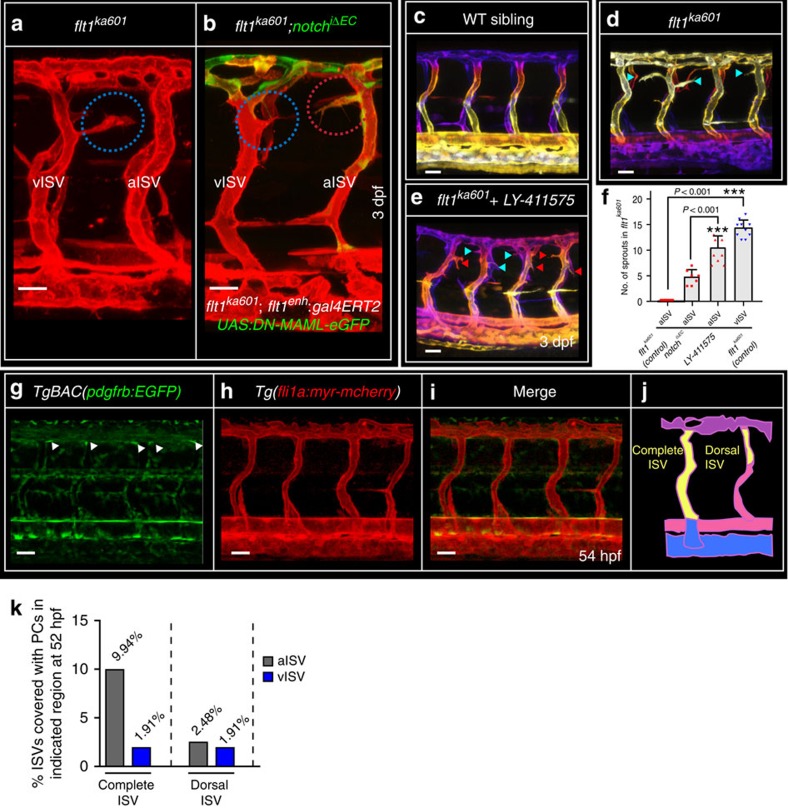
One explanation for the low arterial responsiveness in vegfaa gain-of-function scenarios may involve high arterial Notch activity since Notch acts as a repressor of sprouting in arteries, downstream of Vegfaa signalling25,35,46. To inhibit endothelial Notch signalling in arterial ISVs of flt1ka601 mutants, we expressed a dominant negative form of the Notch co-activator MAML (DN-MAML-EGFP) in an endoxifen inducible manner (Fig. 6a,b)47. We used the flt1enh promoter construct which is mainly active in aISVs (ref. 39) to drive gal4-ERT2;UAS:DN-MAML-eGFP (notchiΔEC) in flt1ka601 mutants. Transgene expression was initiated at 52 hpf by adding endoxifen. Endothelial-specific DN-MAML gain-of-function in flt1ka601 mutants induced ectopic aISV sprouting at the level of the neural tube (Fig. 6a,b,f). Even more pronounced ectopic arterial sprouting was observed with the γ-secretase inhibitor LY-411575 that blocks Notch activation; adding LY-411575 at 2 dpf activated ectopic arterial sprouting in flt1ka601 mutants (Fig. 6c–f). Venous sprout numbers were not significantly changed upon DN-MAML (16.1±3.45 versus 17.1±2.88) or LY-411575 treatment (15.9±2.89 versus 14.2±1.69). Addition of LY-411575 to WT at 2 dpf had no effect. To explain differential AV responsiveness, we also considered differences in pericyte cell coverage (Fig. 6g–j). Overall, pericytes were scarce with 88% of all ISVs investigated not being covered by pericytes. In the remaining 12% of cases, pericytes were found in both aISVs (9.94%) and vISVs (1.91%) along the ISV ventral-dorsal axis. In the most dorsal aspect of aISV and vISV, the region where ectopic sprouting occurs in flt1ka601, pericytes were comparable between aISV and vISV (2.48% and 1.91% respectively, Fig. 6k).
Figure 6. Notch inhibits ectopic arterial sprouting in flt1ka601 mutants.
(a), flt1ka601 mutants show ectopic venous sprouts (blue circles), but no arterial sprouts. (b) Inhibiting arterial Notch by endoxifen-induced arterial ISV-specific expression of dominant negative MAML-eGFP (Notch iΔEC) at 52 hpf under control of the flt1enh promoter in flt1ka601 mutant results in the emergence of ectopic arterial sprouts (red circles); representative image from 7 experiments. (c–e) Trunk vasculature of WT (c), flt1ka601(d) and flt1ka601 treated with Notch inhibitor LY-411575 (e). LY-411575 was added at 2 dpf. Note the emergence of ectopic arterial sprouts upon LY-411575 treatment. (red arrowhead: arterial sprout; blue arrowhead: venous sprout). (f) Quantification of experiments in (a–e), mean±s.e.m., n=7 for NotchiΔEC, n=10 for LY-41157 treatment, n=10 for flt1ka601; t-test. (g–i) Imaging of pericytes in TgBAC(pdgfrb:EGFP);Tg(fli1a:myr-mcherry) double transgenic at 54 hpf. (j) Schematic representation of pericyte number counting in ISVs as performed in (k). (k) Quantification of pericyte recruitment in aISVs and vISVs at 54 hpf. (n=246 ISVs from 14 embryos). ISV, intersegmental artery; Notch iΔEC, inducible ISV-specific loss of Notch; vISV, intersegmental veina. Scale bar, 25 μm in a–e,g–i.
Vegf and Flt1 determine extent of spinal cord vascularization
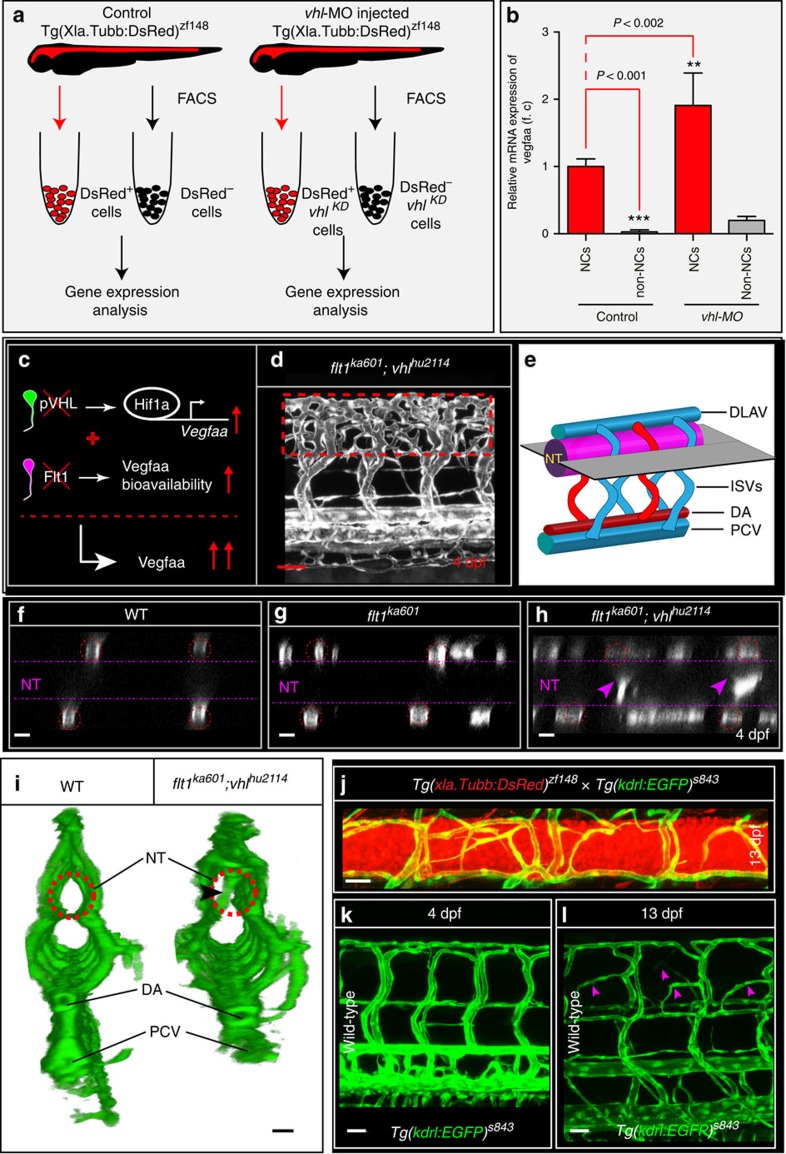
Neurons expressed vegfaa (Supplementary Fig. 1), and neuronal cells of both 3 dpf WT and vhl loss-of-function embryos had significantly higher vegfaa levels than non-neuronal cells (Fig. 7a,b; FACS settings in Supplementary Fig. 7a–d). Furthermore, neuronal vegfaa expression was significantly increased in vhl loss-of-function when compared with WT (Fig. 7a,b). Thus, at this stage of development neurons are the major source of vegfaa, and not other tissues like developing muscle48. We next examined whether neurons can direct sprouts into the neural tube (Fig. 7c–i). We compared the flt1ka601;vhlhu2114 double mutant (Fig. 7c,d) with flt1ka601 mutant and WT and found striking changes in optical sections of the neurovascular interface (Fig. 7e–h). In flt1ka601, sprouts occasionally projected into the neural tube (Fig. 7g), whereas in flt1ka601;vhlhu2114 double mutants many branches invaded the neural tube (Fig. 7h,i).
Figure 7. Neurons are a major source of Vegfaa and attract sprouting vessels.
(a) FACS procedure for obtaining neuronal cells in control and vhl morphants using Tg(Xla.Tubb:DsRed)zf148 neuronal reporter embryos. (b) Quantification of vegfaa expression using real-time qPCR in FAC-sorted cell populations at 3 dpf. Note that neuronal cells expressed significantly more vegfaa than non-neuronal cells. Loss of vhl promoted neuronal vegfaa expression. Mean±s.e.m., n=3 separate experiments in triplicate (two-way ANOVA). (c) Schematic representation: loss of vhl augments vegfaa transcription, loss of flt1 augments Vegfaa bioavailability; combining both mutants augments Vegfaa bioavailability above single mutant level. (d) Trunk vasculature in flt1ka601;vhlhu2114 double mutants at 4 dpf. Note the severe hyper-branching at the level of the neural tube, red-dotted box. (e) Schematic representation of optical section (shown in f–h) through the neural tube and associated trunk vasculature. (f–h) Dorsal view on optical section through WT (f), flt1ka601 (g) and flt1ka601;vhlhu2114 double mutants (h). Note invasion of sprouts into the neural tube in double mutants (arrowheads in h). Red circle indicates position of ISVs, dotted line neural tube boundary. (i) Transverse 3D-rendered view of vasculature (green) through the trunk in WT (left panel) and flt1ka601;vhlhu2114 double mutants (right panel); note vessels penetrating the neural tube in mutant (compare vessel in dotted circle right panel, arrowhead; such vessels are absent in WT left panel; representative image from 3 separate experiments). (j) Representative image of spinal cord vascular network in Tg(xIa.Tubb:DsRed)zf148; Tg(kdrl:EGFP)s843 double transgenic at 13 dpf. (k,l) Comparison of trunk vasculature in WT at 4 dpf (k) and at 13 dpf (l); note the emergence of ectopic branches (pink arrowheads) at level of the spinal cord. DA, dorsal aorta; f.c. fold change; KD, knockdown; NT, neural tube; NC, neuronal cell; PCV, posterior cardinal vein. Mutants are in Tg(kdrl:EGFP)s843 background. Scale bar, 50 μm in d; 25 μm in f–l.
In the mutants with vegfaa gain-of-function, the spinal cord becomes vascularized relatively early, between 3 and 4 dpf. In WT, the spinal cord is vascularized much later in development starting in the period between 12 and 14 dpf (Fig. 7j–l). In those older WT embryos, sprouts preferentially emanated from venous ISVs, displayed nuclear positioning as described for the flt1 mutant (Supplementary Fig. 7e,f) and the onset of vascularization of the WT spinal cord coincides with decreased sflt1 expression during this stage of development (Supplementary Fig. 7g).
Neuronal sFlt1 and Vegfaa regulate sprouting from veins
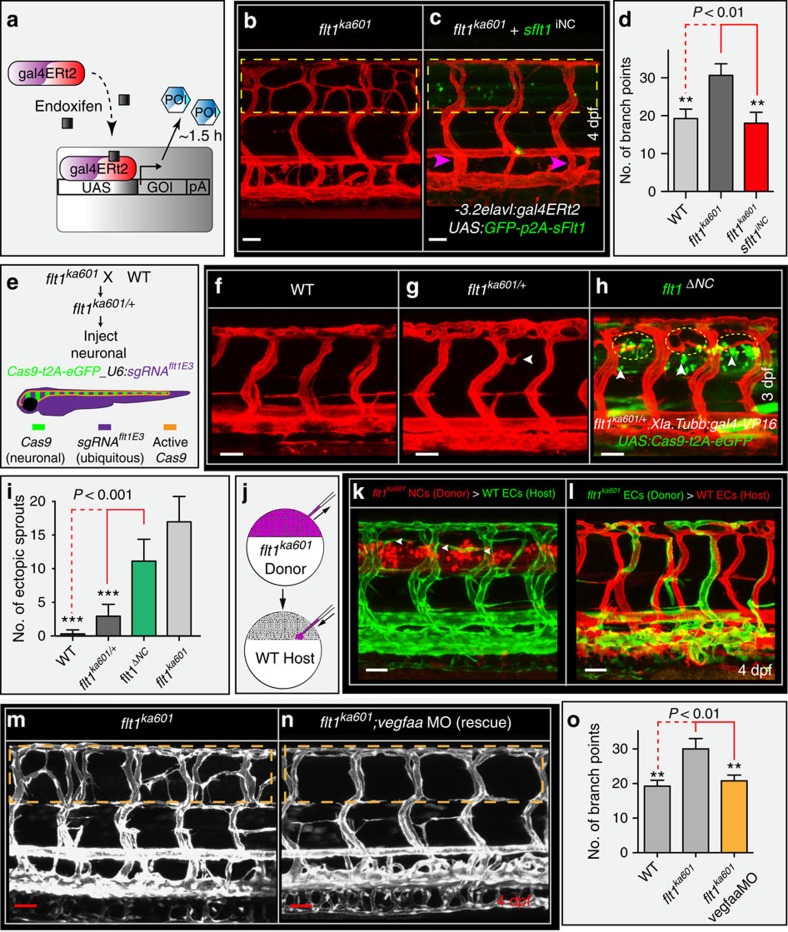
We next generated tissue-specific and inducible flt1 and vegfaa gain-of-function models. Loss of neuronal sFlt1 in flt1ka601 mutants may augment neuron-derived Vegfaa availability and promote ISV sprouting. Hence, restoring neuronal sFlt1 in flt1ka601 mutants should provide a rescue, whereas neuronal-specific flt1 loss-of-function should induce hypersprouting. To test the first scenario we expressed -3.2elavl3:gal4-ERT2;UAS:GFP-p2A-sflt1 (sflt1iNC) in flt1ka601 mutants (Fig. 8a–d, branch quantification method in Supplementary Fig. 1n). This construct allows precise time-controlled expression of sflt1 specifically in neurons. We found that transgene activation in neurons at 52 hpf, just before the emergence of the ectopic sprouts in flt1ka601 mutants, rescued the vascular hyper-branching phenotype (Fig. 8b–d).
Figure 8. Neuronal Flt1 regulates vascular branching by titrating neuronal Vegfaa.
(a) Schematic representation of endoxifen inducible gain-of-function approach in zebrafish. In the present situation Gal4 is under the control of neuron-specific promoters elavl or XIa.Tubb. Expression can be observed within 1.5 h upon endoxifen application. (b) Hyper-branching in flt1ka601 mutants (dotted box). (c) Endoxifen inducible neuron-specific sflt1 gain-of-function rescues hyper-branching in flt1ka601 mutants; compare dotted box in c and b. Purple arrowheads indicate vISVs; endoxifen was applied at 52 hpf. (d) Quantification of rescue in (b,c), mean±s.e.m, n=15-19 embryos per group. (e) Approach for generating a neuron-specific flt1 mutant. Cas9 was expressed under control of neuronal promoter Xla.Tubb; sgRNA was expressed ubiquitously, resulting in Cas9 activity in neuronal cells only (domain marked by orange border). Heterzygous flt1ka601/+ were used to facilitate biallelic knockout. (f–h) Neuron-specific loss of flt1 (flt1ΔNC) induces ectopic sprouting (h), sprouts in yellow dotted ellipse, arrowheads indicate neuronal cells with Cas9 expression. (i) Quantification of ectopic sprouting for indicated genotypes. Note that neuron-specific loss of flt1 significantly augments ectopic sprouting (green bar) mean±s.e.m, n=16 embryos per group, t-test. (j–l) Transplantation of flt1 mutant neuronal cells (k) and endothelial cells (l) into WT. Note: transplantation of flt1 mutant neuronal cells induced sprouting (k, arrowheads); 9 out of 12 neuronal cell transplantations resulted in sprout formation. In all 10 endothelial cell transplantations, sprouts were absent (l). (m,n) Low dose morpholino-mediated reduction of vegfaa expression in flt1ka601 mutants rescues sprouting defects; compare dotted box in (m,n). (o) Quantification of rescue in (m,n), mean±s.e.m., n>5 per group, t-test. DA, dorsal aorta; PCV, posterior cardinal vein; DLAV, dorsal longitudinal anastomotic vessel; NT, neural tube. GOI, gene of interest; POI, protein of interest; iNC, inducible, neuronal cell specific gain-of-function; ΔNC, neuron-specific loss of flt1; MO, morpholino. Scale bar, 50 μm in b–h,m,n; 25 μm in k,l.
We next explored whether neuron-specific loss of flt1 is sufficient to induce ISV hyper-branching (Fig. 8e–i). To accomplish neuron-specific loss of flt1 we expressed the flt1 targeting sgRNAflt1E3 (U6:sgRNAflt1E3, the same sgRNA as used to generate flt1ka601 mutants; expressed in all cells) together with the Cas9 construct employing the Gal4-UAS system under the control of the pan-neuronal promoter Xla.Tubb (-3.8Xla.Tubb:gal4-VP16/UAS:Cas9-t2A-eGFP (flt1ΔNC); (Fig. 8e))49. To optimize the biallelic knockout efficacy, we injected the construct into embryos heterozygous for the flt1 −1 nt allele (flt1ka601/+). GFP signal was detected in spinal cord neurons indicating efficient Xla.Tubb-driven neuron-specific expression of Cas9 (Fig. 8h). Neuronal loss of flt1 significantly induced ectopic venous sprout formation when compared with WT and flt1ka601/+heterozygous mutants (Fig. 8f–i). In contrast, sprouting was not observed when Cas9 was expressed under a vascular promoter (Supplementary Fig. 8a) or in embryos only carrying the sgRNA without Cas9.
To substantiate the contribution of neuronal sflt1 we next employed multiplexed custom designed miRNAs directed against sflt1 3′UTR arranged with a common miR-155 backbone50 (Supplementary Fig. 8b). The constructs were expressed under control of vascular (flt1enh) and neuronal (Xla.Tubb) specific promoters. Targeting neuronal sflt1 resulted in ectopic sprouting (Supplementary Fig. 8c), but targeting vascular sflt1 failed to induce sprouts (Supplementary Fig. 8d).
Next we performed cell transplantation experiments, which demonstrated that neuronal flt1 and not vascular flt1 is the physiologically relevant mediator of sprouting at the level of the neural tube (Fig. 8j–l). Transplantation of flt1 mutant neurons into WT hosts induced ectopic sprouting (Fig. 8k). In contrast, transplantation of flt1 mutant endothelial cells into WT hosts failed to induce sprouting (Fig. 8l).
To prove that neuron-derived Vegfaa promotes hyper-branching, we generated neuronal tissue-specific and inducible vegfaa165 gain-of-function zebrafish (Supplementary Fig. 8e,f; quantification in Fig. 5e). Transgenic expression was initiated by adding endoxifen after completion of AV remodelling at 52 hpf. In this scenario hyper-branched neovascular networks formed at the level of the neural tube, similar to flt1ka601 mutants (Supplementary Fig. 8e,f). Neuronal vegfaa121 was also capable of inducing sprouting (Supplementary Fig. 8g). In contrast, neuron-specific and inducible vegfc gain-of-function, induced at 54 hpf, did not induce ectopic sprouts (Supplementary Fig. 8h). Timing of transgene expression was relevant as inducible neuron-specific vegfaa165 overexpression prior to completion of AV remodelling resulted in thickened abnormal vascular structures (Supplementary Fig. 8i,j). In the same line, neuron-specific constitutive overexpression of sflt1 completely annihilated ISV formation (Supplementary Fig. 8k).
To confirm that the flt1ka601 phenotype involved gain of Vegfaa, we titrated vegfaa levels using a low dose vegfaa targeting morpholino51. Reducing vegfaa in flt1ka601 mutants rescued the hyper-branching phenotype (Fig. 8m–o). Vegfaa signals via Kdrl and application of ki8751, an established Kdrl tyrosine kinase inhibitor in zebrafish52 to flt1ka601 mutants at 2.5 dpf annihilated the formation of the ectopic neovascular networks (Supplementary Fig. 8l,m,o). In contrast, the Flt4-specific tyrosine kinase inhibitor MAZ51 (ref. 52) did not rescue hyper-branching in flt1ka601 (Supplementary Fig. 8l,n,o). Vegfaa-driven primary artery sprouting can occur in the absence of blood flow perfusion. To test if Vegfaa-driven ectopic venous sprouting in flt1ka601 mutants is affected by blood flow, we modulated cardiac activity and flow with BDM or tricaine. We found that loss of flow completely rescued ectopic hyper-sprouting in flt1ka601 mutants (Supplementary Fig. 9a-e). Inhibition of PI3 kinase with wortmannin also significantly reduced ectopic sprouting (Supplementary Fig. 9f).
Discussion
Intimate cross-talk between vessels and the nervous system is important for tissue homeostasis. During embryonic development, neuronal stem cells differentiate into mature neurons, a process that associates with a change in cellular metabolism53. Concomitantly with developmental neurogenesis, changes occur in the vascular network feeding the spinal cord. We show in the zebrafish embryo that neurons in the developing spinal cord express the pro-angiogenic ligand Vegfaa and anti-angiogenic soluble Vegf receptor-1, sFlt1, which acts as a Vegfaa scavenger (Fig. 9). Spinal cord neurons are in close contact to the developing trunk vasculature, and we show that these vessels are responsive to changes in neuronal sFlt1 and Vegfaa. Using a combination of global and tissue-specific loss of flt1 mutants, and further substantiated by vegfaa loss- and gain-of-function experiments as well as cell transplantations, we demonstrate that neuronal sFlt1 restricts neuronal Vegfaa and vessel branching morphogenesis at the neurovascular interface. Differential regulation of vegfaa and sflt1 allows orchestration of the onset and extent of spinal cord vascularization (Fig. 9). We propose that neurons may use sFlt1-Vegfaa to adjust vascularization according to their developmental needs.
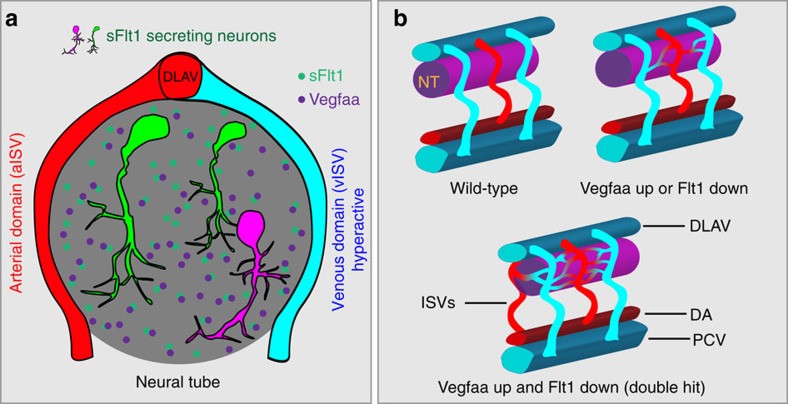
Figure 9. Schematic representation of neurovascular communication involving neuronal sFlt1-Vegfaa and sprouting from intersegmental veins.
(a) Spinal cord neurons produce both sFlt1 and Vegfaa in close proximity to the dorsal aspect of intersegmental arteries and veins. (b) Schematic representation of vascularization around the neural tube in WT (top left), flt1ka601 single mutant or vhlhu2114 single mutant (top right), and flt1ka601;vhlhu2114 double mutant (bottom). Loss of flt1 or vhl induces the formation of a peri-neural tube network, and combining both mutants in addition promotes sprouting into the neural tube. NT, neural tube; ISV, intersegmental vessel (a-arterial, v-venous); DA, dorsal aorta; PCV, posterior cardinal vein; DLAV, dorsal longitudinal anastomotic vessel; hpf, hours post fertilization.
Flt1ka601 mutants develop ectopic sprouts emanating from venous ISVs around embryonic day 2.5. Neuron-specific targeting of flt1 or sflt1 using CRISPR/Cas9- and miRNA-based approaches respectively, result in flt1ka601 comparable phenotypes. Transplantation of flt1 mutant neurons into WT hosts induces ectopic sprouting which is not observed after transplantation of flt1 mutant endothelial cells, suggesting that neuronal flt1 is the physiologically relevant mediator in our mutant. Neuronal-specific gain of sflt1, reducing vegfaa levels, or inhibition of Kdrl signalling provide a rescue suggesting that ectopic venous sprouting is mediated by the Vegfaa-Kdrl signalling axis. Accordingly, ectopic venous sprouting from the dorsal aspect of vISVs is conserved in five independent vegfaa gain-of-function scenarios.
Previous studies have shown that during AV remodelling, aISV-derived endothelial cells remain integrated in the dorsal aspect of vISVs (ref. 39). We confirm that remodelled artery-derived endothelial cells indeed contribute to this domain although they are not the sole or most important endothelial source. Using in vivo cell tracking we find posterior cardinal vein-derived endothelial cells migrating against the direction of blood flow to populate venous ISVs including the dorsal aspect where sprouts are formed. Here, venous-derived endothelium can co-exist with the artery-derived endothelium. With respect to the endothelial cells populating the dorsal part of vISVs, our data now reveal three different scenarios. The dorsal aspect can contain a mix of both artery and venous-derived endothelium (43%), only vein-derived endothelium (48%) or only artery-derived endothelium (8%). On loss of flt1, both artery- and vein-derived endothelium give rise to ectopic vISV sprouts. This prompts toward the concept that integration into the local venous ISV environment constitutes a permissive factor for sprouting, regardless of the endothelial origin.
Flt1ka601 mutants display ectopic sprouting in vISVs but not in aISVs, indicating that AV vessel identity or compartment-specific cues may be involved in the novel sprouting type described here. Notch is tightly linked to both AV vessel specification and sprouting, as Notch programs arterial identity and Notch signalling represses sprouting of arteries25,45. Lack of Notch associates with venous identity and Notch signalling is absent in venous ISVs (ref. 35). Interference with AV remodelling or Notch indeed affects sprouting numbers in flt1ka601. Inhibiting the remodelling of arterial ISVs into venous ISVs in flt1ka601, and thus creating a trunk vasculature that almost exclusively consists of aISVs, rescues ectopic hypersprouting. Conversely, promoting venous cell fate and creating a trunk that consists of vISVs by knock-down of dll4 augments vascular branching in flt1ka601. Inhibiting Notch by endothelial expression of DN-MAML or pharmacological treatment with LY-411575 stimulate ectopic arterial sprouting in flt1ka601, without affecting venous sprout numbers. Thus active Notch signalling in arteries most likely accounts for the observed AV sprouting differences. We propose that the artery-derived endothelial cells that become incorporated in vISVs on AV remodelling are relieved from Notch and adopt a venous fate, enabling them to respond to changes in local Vegfaa.
Our data show that vISVs contribute to the vascularization of the spinal cord with Vegfaa-Kdrl signalling mediating vISV branching events. Interestingly, in other domains of the zebrafish trunk venous vasculature, endothelial cells are responsive to alternative signals. The Vegfc-Flt4 signalling pathway drives secondary venous sprouting from the PCV contributing to the formation of the lymphatic vasculature. BMP-Smad signalling has been shown to promote venous sprouting in the caudal vein plexus54. Furthermore, PCV-derived endothelial cells contribute to the formation of the gut vasculature55. Regeneration of the tail fin vasculature upon injury also starts from the venous side56. The concept emerging from these observations is that initiation of organ vascularization is initiated or proceeds from the venous vasculature. The mouse embryo coronary vasculature is vein-derived, and coronary arteries form by developmental reprogramming of venous endothelium57. Context-dependent heterogeneity in signalling mechanisms establishing venous branching, may allow versatile control of tissue vascularization in a spatio-temporal manner.
Using sFlt1 as a rheostat to control Vegfaa bioavailability, constitutes a means to regulate Vegfaa independent of vegfaa promoter activity, vegfaa mRNA or protein stability. We propose that this enables neurons to dynamically fine-tune the extent and onset of peri-neural vascular network formation and sprouting into the spinal cord. While the peri-neural network may serve to sustain growth of the developing nervous system, vessel sprouting into the spinal cord and relief of hypoxia has been associated with changes in neuronal stem cell metabolism, triggering differentiation events53. Therefore, untimely or excessive vascularization of the spinal cord is potentially harmful as it may promote premature stem cell differentiation and disrupt the carefully orchestrated neuronal specification process. We propose a two-tiered checkpoint mechanism involving sFlt1 and Vegfaa, requiring two decisions to guide vascularization, namely Vegfaa up- and sFlt1 downregulation, to protect neurons from harmful angiogenesis and oxygen variations during early stages on the one hand, and on the other hand to enable more mature neurons to attract sufficient vessels into the spinal cord after stem cell differentiation has been completed.
Methods
Ethics statement
Zebrafish husbandry and experimental procedures were performed in accordance with the German animal protection standards and were approved by the government of Baden-Württemberg, Regierungspräsidium Karlsruhe, Germany (Akz.: 35-9185.81/G-93/15).
Transgenic lines
Tg(fli1a:EGFP)y1, Tg(kdrl:hsa.-HRAS-mcherry)s916, TgBAC(flt1:YFP)hu4624, Tg(fli1a:nGFP)y7, Tg(Xla.Tubb:DsRed)zf148, Tg(kdrl:EGFP)s843, Tg(HuC:EGFP)as8, Tg(mnx1:GFP)ml2, Tg(flt1enh:tdTomato)hu5333, Tg(flt4:mCitrine)hu7135, Tg(kdrl:nlskikGR)hsc7, Tg(fli1a:myr-mcherry), TgBAC(pdgfrb:EGFP) as well as vhlhu2114 and ptena−/−;ptenb−/+mutants were used as published39,44,58,59,60,61.
Morpholino injections
Morpholino antisense oligomers (MOs; Gene Tools) were prepared at a stock concentration of 1 mM according to the manufacturer. MOs were injected into the yolk of one-cell stage embryos. We used the flt4 ATG MO, 5′-CTCTTCATTTCCAGGTTTCAAGTCC-3′ (4 ng), the flt1 ATG MO, 5′-ATATCGAACATTCTCTTGGTCTTGC-3′ (1 ng or 3 ng), the vhl e1i1 splice MO 5′-GCATAATTTCACGAACCCACAAAAG-3′ (6 ng), the vegfaa ATG MO 5′-GTATCAAATAAACAACCAAGTTCAT-3′ (0.3 ng), the dll4 MO 5′-TAGGGTTTAGTCTTACCTTGGTCAC-3′ (6 ng), and a control MO 5′-CTCTTACCTCAGTTACAATTTATA-3′ (10 ng) (refs 18, 26, 45, 51, 62).
mRNA injection and generation of transgenic and mutant lines
For the generation of mutants 1 nl of a mixture containing 600 ng μl−1 capped and polyadenylated Cas9-nls mRNA and 50 ng μl−1 sgRNA was injected into one-cell stage embryos63. Cas9 mRNA was produced by in vitro transcription from the MLM3613 plasmid using the mMessage mMachine T7 Ultra Kit (Ambion). The MLM3613 plasmid was a gift from Keith Joung (Addgene plasmid #42251). For the generation of transgenic lines 1 nl of a mixture of 12.5 ng μl−1 transposase mRNA and 25 ng μl−1 plasmid DNA was injected into one-cell stage embryos.
Cell transplantations
Cell transplantations were performed using 3.5 hpf donor and host blastula-stage embryos. Approximately 50–100 cells were taken from the donor's animal pole and transferred close to the host's lateral marginal zone (for ECs) or slightly above for neuronal cells. Donors and hosts carried distinct neuronal and endothelial-specific reporters to identify the source of ECs and neurons within chimeras.
Generating flt1 mutants
The zebrafish flt1 gene consists of 34 exons encoding membrane-bound flt1 (mflt1) and a shorter soluble flt1 (sflt1) form. Soluble flt1 is generated through alternative splicing of flt1 mRNA at the exon 10 - Intron 10 boundary (Supplementary Fig. 2a). To annihilate the production of both mflt1 and sflt1 and obtain flt1 mutants, we targeted exon 3, using a CRISPR/Cas approach. We designed five sgRNAs targeting exon 3, encoding the extracellular Ig1 domain relevant for Vegfaa binding. Oligonucleotides containing the GG-N18 targeting sequence and overhangs were purchased from Eurofins (Ebersberg, Germany). The annealed oligos were ligated into DR274 which was a gift from Keith Joung (Addgene plasmid # 42250)63. The corresponding genomic region (surrounding exon 3) was amplified by PCR using primer pair Flt1_E3_gDNA_r and Flt1_E3_gDNA_f and indels were quantified with T7EI assay or direct Sanger sequencing of the PCR product as described (for primer sequences see Supplementary Table 6)63. The T7EI cleavage products of 211 and 249 bp were quantified using ImageJ. The sgRNAflt1E3 (Supplementary Table 4) with the highest cleavage rate (∼70%) was used to generate the flt1 mutants. WT embryos were coinjected with sgRNAflt1E3 plus capped and polyadenylated Cas9 mRNA. Four independent lines with frame shift mutations were investigated in more detail. The flt1ka601 (exon 3 -1nt allele), flt1ka602(exon 3 -5nt allele), flt1ka603(exon 3 +5nt allele) and flt1ka604(exon 3 -14nt allele) have a premature termination codon (PTC) resulting in a truncated protein devoid of a functional extracellular Vegfaa binding domain. Embryos carrying the mutation were raised and outcrossed to vascular and neuronal reporter lines (Tg(kdrl:eGFP)s843, Tg(fli1a:eGFP)y1, Tg(fli1a:nGFP)y7, Tg(kdrl:hsa.HRAS-mcherry)s916, and Tg(Xla.Tubb:DsRed)zf148).
Generation of mflt1-specific mutants
To generate mflt1 mutants we used a CRISPR/Cas approach and designed an sgRNA targeting E11b, the first specific mflt1 exon18. In this scenario splicing of intron 10 and exon 11a relevant for generating sflt1 mRNA remains unaffected. Oligos Flt1E11_O1_A_15 and Flt1E11_O2_A_15 were annealed and cloned into DR274 as described for flt1 mutants. Founders were identified by PCR and subsequent Sanger sequencing, using primers Flt1E11A2386576F and Flt1E11A2386151R. We identified four frame shift mutants harbouring a PTC in exon 11b. Flt1ka605(exon 11b +28nt), flt1ka606(exon 11b +20nt), flt1ka607(exon 11b −1nt) and flt1ka608 (exon 11b −1nt and one mutation) mflt1 mutants were outcrossed to Tg(kdrl:EGFP)s843 and Tg(Xla.Tubb:DsRed)zf148. All four mflt1 mutants were phenotypically comparable and in this manuscript only the mflt1 mutant flt1ka605 is shown. All sgRNA sequences and oligos used for annealed oligo cloning into DR274 are listed in Supplementary Table 4,5.
Generation of p5E entry clones
The NBT_tauGFP plasmid was a kind gift by Enrique Amaya. The 3.8 kb regulatory element derived from neural specific beta tubulin was removed from the NBT_tauGFP using SalI and HindIII and subcloned into SalI and HindIII digested and dephosphorylated p5E_MCS (ref. 64). The 1 kb flt1 enhancer/promoter fragment from the pMiniTol2_flt1_ECR5a_pro_181_YFP (ref. 39) construct was subcloned into p5E_MCS using KpnI and HindIII. The resulting plasmids were named p5E_ Xla.Tubb-3.8 and p5E_flt1enh.
Generation of a universal p2A-GFP middle entry clone
To easily detect transgenic cells the pME_eGFP (#455) from the Tol2kit (ref. 64) was modified by site-directed mutagenesis PCR. The p2A sequence was added before the stop codon of GFP using pME_eGFP specific primer with 5′end extension coding for the p2A peptide and a SmaI restriction site just downstream of p2A for convenient subcloning (pME_eGFP_p2A_fw and pME_eGFP_p2A_rev primer).
pME entry clones used for gain-of-function experiments
pME_eGFP-p2A_SmaI was digested with SmaI and XhoI. The inserts vegfaa165, vegfC and sflt1 were amplified from zebrafish cDNA using primers vegfaa_p2A_fw/rev, vegfc_p2A_fw/rev and sflt1_p2A_fw/rev. The PCR products were digested with XhoI and gel purified. Vector and inserts were ligated following the manufactures instructions (NEB T4 DNA Ligase). The resulting plasmids were named pME_eGFP-p2A_vegfaa165, pME_eGFP-p2A_vegfc and pME_eGFP-p2A_sflt1.
gal4ERt2 middle entry clone generation
To spatially and temporally regulate transgene expression an inducible gal4-ERT2 fusion protein was constructed. The Gal4 DNA binding domain was fused at its C-terminus with a mutant oestrogen ligand-binding domain ERT2 that carries a VP16-derived non-deleterious transactivation domain TA4 (ta4, 39 aa) at its C-terminus65. Among all possible sequential orders of domains, this arrangement was inferred to have a low background with a high induction rate66. A middle entry clone pENTR/D-creERT2 was modified by replacing Cre recombinase domain (1,053 bp, flanked by NotI and XhoI sites at its 5′ and 3′ termini, respectively) with a PCR product encoding Gal4 DNA binding domain (1–146 aa) with Kozak sequence in the 5′ vicinity of the start codon. To replace the stop codon at the 3′ terminus of ERT2 domain with TA4 domain, a C-terminal half of a ERT2 domain (115–316 aa, flanked by in-frame NcoI at its 5′ terminus) was replaced with a PCR product encoding the C-terminal half of the ERT2 domain without a stop codon (115–315 aa) with in-frame AgeI site at its 3′ terminus. The AgeI site, and 3′ downstream EcoRI site were utilized to insert two synthetic double-stranded oligonucleotides encoding the TA4 domain and the stop codon.
Generation of gateway expression clones
pME_DN-MAML-eGFP was kindly provided by Caroline Burns47. p5E_flt1enh, pME_DN-MAML-eGFP and p3E_polyA were recombined into pDestTol2CG2 according to the manufacturer's instructions (Thermo Fisher, LR Clonase II plus). The resulting plasmid was named pCG2_flt1_ DN-MAML-eGFP. p5E_Xla.Tubb-3.8, pME_eGFP-p2A_sflt1 and p3E_polyA were recombined into pDestTol2CG2 (pCG2_Xla.Tubb-3.8_eGFP-p2A-sflt1). p5E_Xla.Tubb-3.8, pME_eGFP-p2A_sflt1 and p3E_polyA were recombined into pDestTol2CG2 (pCG2_Xla.Tubb-3.8_eGFP-p2A-sflt1). p5E_elavl-3.2 (R.W., unpublished observations), pME_gal4ERT2 and p3E_polyA were recombined into pDestTol2CG2 (pCG2_elavl-3.2_gal4-ERT2). p5E_flt1enh, pME_gal4ERT2 and p3E_polyA were recombined into pDestTol2CG2 (pCG2_ flt1enh_gal4-ERT2).
Generation of tissue-specific KO constructs
pME-Cas9-T2A-GFP and pDestTol2pA2-U6:gRNA were a gift from Leonard Zon (Addgene plasmid # 63156 and # 63155)49. pDestTol2pA2-U6:gRNAflt1E3 was generated by annealed oligo cloning. Oligos U6_flt1E3_1 and U6_flt1E3_2 were cloned into pDestTol2pA2-U6:gRNA following BseRI restriction digest. To drive Cas9 expression specifically in neurons, the Gal4 driver construct pCG2_Xla:Tubb-3.8_gal4ERT2 was generated by recombining p5E_Xla.Tubb-3.8, pME_gal4ERT2, p3E_polyA and pDestTol2CG2. To drive Cas9 expression specifically in endothelial cells, the Gal4 driver construct pCG2_flt1enh_gal4ERT2 was generated by recombining p5E_ flt1enh, pME_gal4ERT2, p3E_polyA and pDestTol2CG2. For the Gal4 effector construct, p5E_UAS, pME_cas9-t2a-eGFP and p3E_polyA were recombined into pDestTol2pA2-U6:sgRNAflt1E3 (pCG2_UAS_Cas9-t2A-eGFP_U6_gRNAflt1E3).
Tissue-specific miR155-flt1-1-2-3 knockdown constructs
sflt1 3′UTR-specific miRNAs were designed using the BLOCK-IT RNAi Designer website (https://rnaidesigner.thermofisher.com/rnaiexpress/). To enhance miRNA effectiveness three sflt1 3′UTR-specific target sites with miRNA155 backbone were cloned in series. A fragment containing the three multiplexed miRNAs were synthesized by Eurofins Genomics and cloned into 641-pMER-GFP-miR155empty and 641-pMER-DsRED-miR155empty using restriction enzymes BamHI and XhoI50. The target sites are listed in Supplementary Table 7. The expression construct with Xla.Tubb or flt1enh promoter was cloned using gateway cloning. p5E_Xla.Tubb-3.8, 641-pMER-GFP-miR155-sflt1-1-2-3 and p3E_polyA were recombined into pDestTol2CG2 (pCG2_Xla.Tubb_ GFP-miR155-sflt1-1-2-3). p5E_flt1enh,641-pMER-DsRed-miR155-sflt1-1-2-3 and p3E_polyA were recombined into pDestTol2CG2 (pCG2_flt1enh_DsRed-miR155-sflt1-1-2-3).
FACS
Approximately 500 embryos Tg(mnx1:GFP)ml2, Tg(HuC:EGFP)as8, Tg(Xla.Tubb:DsRed)zf148 or vhl MO injected Tg(Xla.Tubb:DsRed)zf148 embryos were dechorionated at 24 hpf using pronase (0.5 mg/ml). Cells were dissociated using FACSMax as recommended by the manufacturer. Tg(mnx1:GFP)ml2, Tg(HuC:EGFP)as8 embryos were dissociated and sorted at 24 hpf, control and vhl MO injected Tg(Xla.Tubb:DsRed)zf148 embryos were dissociated and sorted at 3 dpf. Dissociated cells were FACS sorted using BD-FACS-Aria I and Aria II. The sorted cells (∼0.5 × 106 cells per experiment) were spun down at 310 g for 5 min and resuspended in lysis buffer contained in the RNeasy mini kit (Qiagen). RNA was extracted as described in the manual. Because of limited amounts of RNA the QuantiTect Whole Transcriptome Kit (Qiagen) was used to preamplify and reverse transcribe the RNA to make cDNA. cDNA was diluted 1:250 for real-time qPCR.
Gene expression analysis by real-time qPCR and TaqMan
Total RNA of zebrafish embryos was isolated with TRIzol, purified with RNeasy mini kit (Qiagen) and quantity and quality were measured using an Agilent 2,100 Bioanalyzer (Agilent Technologies) according to the manufacturer's instructions. We performed DNase on-column digestion using RNase-free DNase Set (Qiagen) according to the manufacturer, followed by cDNA synthesis using the Thermoscript First-Strand Synthesis System (Thermo Fisher Scientific). Primer probe sets (FAM and TAMRA labels) were obtained from Thermo Fisher Scientific. Amplification was carried out using an ABI Prism 7,000 thermocycler (Applied Biosystems). qPCR was conducted with SYBR Green PCR Master Mix (Thermo Scientific) in a StepOnePlus real-time qPCR system (Applied Biosystems). Primers for real-time qPCR were ordered from Eurofins Genomics. Gene expression data were normalized against zebrafish elongation factor 1-alpha. Primers and probes are listed in Supplementary Table 1–3.
RNA-seq library preparation and sequencing
Zebrafish RNA was isolated and purified from 4 dpf zebrafish larvae using TRIzol and RNeasy mini kit (Qiagen) as recommended by the manufacturers. A cDNA library was generated using the TruSeq Ilumina RNA sample prepv2 kit according to the manufacturer's protocol. The cDNA library was sequenced on a HiSeq2000 according to the manufacturer's protocols (Illumina).
Identification of differentially expressed genes
Raw sequencing reads were mapped to the transcriptome and the zebrafish reference genome (GRCz10 danRer10) using Bowtie2.0 and TopHat 2.0 (ref. 67). On average 44,490,573 reads (81,6% of total reads) were assigned to genes with Cufflinks and HTSeq software package. Differentially expressed genes (control vs. mutant) were identified using DESeq and Cuffdiff67,68. Genes were defined as differentially expressed if ≥2 fold significantly regulated (P<0.05) with two independent methods (DEseq and Cuffdiff).
Zebrafish histological sectioning
Dechorionated larvae were fixed in 4%PFA for 2 h and subsequently transferred to 20% DMSO/ 80% Methanol and incubated overnight at −20 °C. Larvae were then washed in 100 mM NaCl, 100mMTris-HCl, pH7.4 for 30 min at room temperature. Washed larvae were embedded in gelatin from cold water fish skin/sucrose (Sigma). Larvae were sectioned (20 μm) in a cryomicrotome.
Inhibitor treatments
All stock solutions were prepared in DMSO. Embryos were dechorionated at 24 hpf using Pronase (Roche, Basel, Switzerland). For Notch signalling inhibition embryos were incubated from 2 dpf with 10 μM of LY-411575 (Sigma, St Louis, MO, USA) and imaged at 3 dpf. For VEGFR2 and VEGFR3 inhibition embryos were treated with 25 μM MAZ51 (Merck Millipore, Billerica, Massachusetts, USA) from 2.5 dpf or from 3 dpf with 0.125 μM ki8751 (Sigma, St Louis, MO, USA) and imaged at 4 dpf. To inhibit PI3K/Akt signalling embryos were incubated with 1.25 μM wortmannin from 3 dpf and imaged at analysed at 4 dpf. Heartbeat was blocked using 15 mM 2,3-Butanedione 2-monoxime (BDM) dissolved in E3 media. Control embryos were mock treated with DMSO (Sigma, St Louis, MO, USA). Embryos were randomly assigned to experimental groups. Investigators were blinded to inhibitor treatment.
Photoconversion of kikGR and migration tracking
Dechorionated embryos were embedded in 0.7% low-melting agarose at 30 hpf and a small part of the posterior cardinal vein of Tg(kdrl:nlskikGR)hsc7 transgenics was converted for several seconds using UV-light with the smallest available field diaphragm of the Leica Sp8 confocal microscope. Subsequently embryos removed from the agarose and allowed to develop in E3 medium until imaging or were immediately used for time-lapse imaging.
Gal4ERT2 endoxifen activation
Endoxifen (Sigma) was solved in DMSO. Zebrafish embryos expressing Gal4ERT2 were incubated from 52 hpf onwards in 0.5 μM endoxifen in E3 medium in the dark. GFP positive cells could be observed approximately 1.5 h after induction.
Vascular network analysis
To assess sprout number and length, we developed a semi-automated analysis of the DLAV-ISV vessel network using ImageJ (Supplementary Fig. 1n). Image-stacks of ISVs were acquired using the Leica SP8 confocal microscope. Stack projections of one side of the trunk were generated. Dorsal region of the ISVs was used for analysis. Using ImageJ a Gaussian blur filter was applied followed by a black/white threshold and subsequent skeletonization to generate a skeleton of the vasculature. Segment number, branch point number and total branch length were calculated using the ‘analyse skeleton' plugin. The semi-automated pipeline was applied for analysis of 4 dpf vascular networks, while sprout numbers in 2–3 dpf zebrafish embryos were counted manually.
Imaging
Zebrafish larvae were embedded in 0.7% low-melting agarose with 0.112 mg ml−1 Tricaine (E10521, Sigma) and 0.003% PTU (P7629, Sigma) in glass bottom dishes (MatTek, P35G-0.170-14-C). Images presented in this study were acquired using a Leica SP8 confocal microscope with × 20 multi-immersion and × 40 water immersion objectives and LAS X software. Images were processed using ImageJ. Vascular branching was quantified using a semi-automated ImageJ pipeline (Supplementary Fig. 1n). Animal numbers used are indicated in figure legends. For zebrafish mutants more than 100 embryos per genotype were analysed. In morpholino experiments morphologically malformed embryos were excluded from analysis.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 6. Each dataset was tested for normal distribution (D'Agostino and Pearson test). Parametric method (unpaired Students t-test) was only applied if the data were normally distributed. For non-normal distributed data sets, a non-parametric test (Mann Whitney U test) was applied. When appropriate in case of multiple comparisons, ANOVA plus Bonferroni correction was applied. P values <0.05 were considered significant. Data are represented as mean±s.e.m., unless otherwise indicated. *P<0.05, **P<0.01 and ***P<0.001.
Data availability
The authors declare that all data supporting the findings of this study are available within the article and its Supplementary Information files or from the corresponding author on reasonable request. The RNA-seq data generated in this study has been deposited into the Gene Expression Omnibus database with the accession code http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE89350.
Additional information
How to cite this article: Wild, R. et al. Neuronal sFlt1 and Vegfaa determine venous sprouting and spinal cord vascularization. Nat. Commun. 8, 13991 doi: 10.1038/ncomms13991 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Supplementary Figures, Supplementary Tables.
3D-rendering of vessels and nerves. 3D-rendered view of blood vessels (green) and nerves (blue) in Tg(kdrl:EGFP)s843; Tg(XIa.Tubb:DsRed)zf148 embryo at 4dpf.
Angiogenic cell behaviors in flt1ka601. Time-lapse imaging of ectopic vessel sprouting in flt1ka601 full mutant in Tg(fli1a:nEGFP)y7; Tg(kdrl:has.HRASmCherry)s916 background. Vessels in red, endothelial cell nuclei in green. White arrowhead indicates developing sprout. Pink arrowhead indicates the corresponding endothelial cell nucleus. Sprout emanates from the dorsal aspect of the intersegmental vein at the level of the neural tube, and anastomoses with adjacent intersegmental vessel. Developmental stage and scale bar are indicated (bottom left and right corner respectively). Scale bar represents 20μm.
Angiogenic cell behaviors in flt1ka601/+. Time-lapse imaging of angiogenic cell behavior in flt1ka601/+ heterozygous mutant in Tg(fli1a:nEGFP)y7; Tg(kdrl:has.HRAS-mCherry)s916 background. Vessels in red, endothelial cell nuclei in green. White and pink arrowheads indicate hyperactive endothelial cells and filopodia extensions. Developmental stage and scale bar are indicated (bottom left and right corner respectively). Scale bar represents 20μm.
Angiogenic cell behaviors in WT. Time-lapse imaging of endothelial cell behavior in WT sibling in Tg(fli1a:nEGFP)y7; Tg(kdrl:has.HRASmCherry)s916 background. Vessels in red, endothelial cell nuclei in green. Developmental stage and scale bar are indicated (bottom left and right corner respectively). Scale bar represents 20μm.
Origin of vISV endothelial cells. Endothelial cell tracking in Tg(kdrl:nlsKikGR)hsc7 embryo upon photo-switching at 30hpf. At 30hpf, a small part of the posterior cardinal vein (PCV) was photo-converted by exposure to UV light. Photo-converted PCV derived cells express red-kikGR; all endothelial cells express green-kikGR. Arrowheads indicate PCV derived endothelial cells migrating into intersegmental vessels up until the dorsal lateral anastomotic vessel. Developmental stage indicated in bottom right corner. Scale bar represents 20μm.
Acknowledgments
We thank the colleagues of the KIT–European Zebrafish Resource Center (EZRC) for handling and maintenance of the zebrafish lines. We are very grateful to Dr Caroline Burns, Cardiovascular Research Center, Charlestown for sharing the pME_DN-MAML-GFP entry clone. We thank Dr Thomas Becker and Dr Jean Giacomotto, Brain and Mind Research Institute, University of Sydney for the 641-pMER-GFP/DsRed-miR155empty plasmids. We thank Leonard I. Zon M.D., Boston Children's Hospital and Dana Farber Cancer Institute, Boston for the pDestTol2CG2-U6:gRNA and the pME-Cas9-T2A-GFP constructs and Dr Keith Joung, Massachusetts General Hospital, Charlestown for DR274 and MLM3613 plasmids. We are also very grateful to Dr Jeroen den Hertog, Hubrecht Institute, Utrecht for sharing the zebrafish ptena−/−;ptenb−/− double mutants. S.S.-M, U.S. and F.L.N. are members of the EuFishBioMed zebrafish initiative. S.S.-M. and F.l.N. are supported by grants from the Deutsche Forschungsgemeinschaft (DFG)–FOR2325 ‘Interactions at the Neurovascular Interface'. Y.H. is supported by an individual grant from the Danish Council for Independent Research. We acknowledge support by Deutsche Forschungsgemeinschaft and Open Access Publishing Fund of Karlsruhe Institute of Technology.
Footnotes
Author contributions R.W. designed and performed experiments and interpreted experimental data. A.K. and R.W. performed and analysed FACS experiments. K.A. participated in manuscript preparation. L.P. performed and analysed inhibitor experiments. J.K. performed and analysed Taqman experiments. M.T. & U.S. contributed the Gal4ERT2 construct, Y.H. performed FACS analyses, K.A. & N.M. performed the analysis on pericyte contribution. A.van I. and S.S.-M. contributed transgenic fish, constructs, interpreted data and discussed the conceptual framework. F.l.N. conceived and designed the project, analysed the data and supervised the overall project. F.l.N. and R.W. wrote the manuscript.
References
- Zacchigna S., Lambrechts D. & Carmeliet P. Neurovascular signalling defects in neurodegeneration. Nat. Rev. Neurosci. 9, 169–181 (2008). [DOI] [PubMed] [Google Scholar]
- White I. A., Gordon J., Balkan W. & Hare J. M. Sympathetic reinnervation is required for mammalian cardiac regeneration. Circ. Res. 117, 990–994 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam J. et al. Coronary veins determine the pattern of sympathetic innervation in the developing heart. Development 140, 1475–1485 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichmann A., Le Noble F., Autiero M. & Carmeliet P. Guidance of vascular and neural network formation. Curr. Opin. Neurobiol. 15, 108–115 (2005). [DOI] [PubMed] [Google Scholar]
- Larrivee B., Freitas C., Suchting S., Brunet I. & Eichmann A. Guidance of vascular development: Lessons from the nervous system. Circ. Res. 104, 428–441 (2009). [DOI] [PubMed] [Google Scholar]
- James J. M. & Mukouyama Y. Neuronal action on the developing blood vessel pattern. Semin. Cell Dev. Biol. 22, 1019–1027 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schratzberger P. et al. Reversal of experimental diabetic neuropathy by VEGF gene transfer. J. Clin. Invest. 107, 1083–1092 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan K. A., Ambler C. A., Chapman D. L. & Bautch V. L. The neural tube patterns vessels developmentally using the VEGF signaling pathway. Development 131, 1503–1513 (2004). [DOI] [PubMed] [Google Scholar]
- James J. M., Gewolb C. & Bautch V. L. Neurovascular development uses VEGF-A signaling to regulate blood vessel ingression into the neural tube. Development 136, 833–841 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raab S. et al. Impaired brain angiogenesis and neuronal apoptosis induced by conditional homozygous inactivation of vascular endothelial growth factor. Thromb. Haemost. 91, 595–605 (2004). [DOI] [PubMed] [Google Scholar]
- Mukouyama Y. S., Shin D., Britsch S., Taniguchi M. & Anderson D. J. Sensory nerves determine the pattern of arterial differentiation and blood vessel branching in the skin. Cell 109, 693–705 (2002). [DOI] [PubMed] [Google Scholar]
- Mukouyama Y.-S., Gerber H.-P., Ferrara N., Gu C. & Anderson D. J. Peripheral nerve-derived VEGF promotes arterial differentiation via neuropilin 1-mediated positive feedback. Development 132, 941–952 (2005). [DOI] [PubMed] [Google Scholar]
- Carmeliet P. et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature 380, 435–439 (1996). [DOI] [PubMed] [Google Scholar]
- Miquerol L., Langille B. L. & Nagy A. Embryonic development is disrupted by modest increases in vascular endothelial growth factor gene expression. Development 127, 3941–3946 (2000). [DOI] [PubMed] [Google Scholar]
- Okabe K. et al. Neurons limit angiogenesis by titrating VEGF in retina. Cell 159, 584–596 (2014). [DOI] [PubMed] [Google Scholar]
- Olsson A.-K., Dimberg A., Kreuger J. & Claesson-Welsh L. VEGF receptor signalling in control of vascular function. Nat. Rev. Mol. Cell Biol. 7, 359–371 (2006). [DOI] [PubMed] [Google Scholar]
- Shibuya M. Vascular endothelial growth factor (VEGF) and its receptor (VEGFR) signaling in angiogenesis: a crucial target for anti- and pro-angiogenic therapies. Genes Cancer 2, 1097–1105 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger J. et al. Flt1 acts as a negative regulator of tip cell formation and branching morphogenesis in the zebrafish embryo. Development 138, 2111–2120 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsson L. et al. Endothelial cells dynamically compete for the tip cell position during angiogenic sprouting. Nat. Cell Biol. 12, 943–953 (2010). [DOI] [PubMed] [Google Scholar]
- Isogai S., Lawson N. D., Torrealday S., Horiguchi M. & Weinstein B. M. Angiogenic network formation in the developing vertebrate trunk. Development 130, 5281–5290 (2003). [DOI] [PubMed] [Google Scholar]
- Jin S.-W., Beis D., Mitchell T., Chen J.-N. & Stainier D. Y. R. Cellular and molecular analyses of vascular tube and lumen formation in zebrafish. Development 132, 5199–5209 (2005). [DOI] [PubMed] [Google Scholar]
- Covassin L. D., Villefranc J. A., Kacergis M. C., Weinstein B. M. & Lawson N. D. Distinct genetic interactions between multiple Vegf receptors are required for development of different blood vessel types in zebrafish. Proc. Natl Acad. Sci. USA 103, 6554–6559 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang D. et al. The role of vascular endothelial growth factor (VEGF) in vasculogenesis, angiogenesis, and hematopoiesis in zebrafish development. Mech. Dev. 108, 29–43 (2001). [DOI] [PubMed] [Google Scholar]
- Covassin L. D. et al. A genetic screen for vascular mutants in zebrafish reveals dynamic roles for Vegf/Plcg1 signaling during artery development. Dev. Biol. 329, 212–226 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siekmann A. F. & Lawson N. D. Notch signalling limits angiogenic cell behaviour in developing zebrafish arteries. Nature 445, 781–784 (2007). [DOI] [PubMed] [Google Scholar]
- Hogan B. M. et al. Vegfc/Flt4 signalling is suppressed by Dll4 in developing zebrafish intersegmental arteries. Development 136, 4001–4009 (2009). [DOI] [PubMed] [Google Scholar]
- Villefranc J. A. et al. A truncation allele in vascular endothelial growth factor c reveals distinct modes of signaling during lymphatic and vascular development. Development 140, 1497–1506 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiratsuka S., Minowa O., Kuno J., Noda T. & Shibuya M. Flt-1 lacking the tyrosine kinase domain is sufficient for normal development and angiogenesis in mice. Proc. Natl Acad. Sci. USA 95, 9349–9354 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiratsuka S. et al. Membrane fixation of vascular endothelial growth factor receptor 1 ligand-binding domain is important for vasculogenesis and angiogenesis in mice. Mol. Cell. Biol. 25, 346–354 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avraham-Davidi I. et al. ApoB-containing lipoproteins regulate angiogenesis by modulating expression of VEGF receptor 1. Nat. Med. 18, 967–973 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi A. et al. Genetic compensation induced by deleterious mutations but not gene knockdowns. Nature 524, 230–233 (2015). [DOI] [PubMed] [Google Scholar]
- Chappell J. C., Taylor S. M., Ferrara N. & Bautch V. L. Local guidance of emerging vessel sprouts requires soluble Flt-1. Dev. Cell 17, 377–386 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen G. G. & Worman H. J. Nuclear positioning. Cell 152, 1376–1389 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert S. P. & Stainier D. Y. R. Molecular control of endothelial cell behaviour during blood vessel morphogenesis. Nat. Rev. Mol. Cell Biol. 12, 551–564 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quillien A. et al. Distinct Notch signaling outputs pattern the developing arterial system. Development 141, 1544–1552 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Toro R. et al. Identification and functional analysis of endothelial tip cell-enriched genes. Blood 116, 4025–4033 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser G. A., Kaminker J. S. & Tessier-Lavigne M. Microarray analysis of retinal endothelial tip cells identifies CXCR4 as a mediator of tip cell morphology and branching. Blood 115, 5102–5110 (2010). [DOI] [PubMed] [Google Scholar]
- Okuda K. S. et al. lyve1 expression reveals novel lymphatic vessels and new mechanisms for lymphatic vessel development in zebrafish. Development 139, 2381–2391 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussmann J. et al. Arteries provide essential guidance cues for lymphatic endothelial cells in the zebrafish trunk. Development 137, 2653–2657 (2010). [DOI] [PubMed] [Google Scholar]
- Nicenboim J. et al. Lymphatic vessels arise from specialized angioblasts within a venous niche. Nature 522, 56–61 (2015). [DOI] [PubMed] [Google Scholar]
- Carmeliet P. et al. Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature 394, 485–490 (1998). [DOI] [PubMed] [Google Scholar]
- van Rooijen E. et al. von Hippel-Lindau tumor suppressor mutants faithfully model pathological hypoxia-driven angiogenesis and vascular retinopathies in zebrafish. Dis. Model. Mech. 3, 343–353 (2010). [DOI] [PubMed] [Google Scholar]
- Gomez-Manzano C. et al. Mechanisms underlying PTEN regulation of vascular endothelial growth factor and angiogenesis. Ann. Neurol. 53, 109–117 (2003). [DOI] [PubMed] [Google Scholar]
- Choorapoikayil S., Weijts B., Kers R., de Bruin A. & den Hertog J. Loss of Pten promotes angiogenesis and enhanced vegfaa expression in zebrafish. Dis. Model. Mech. 6, 1159–1166 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie J. D. et al. Endothelial signalling by the Notch ligand Delta-like 4 restricts angiogenesis. Development 134, 839–844 (2007). [DOI] [PubMed] [Google Scholar]
- Suchting S. et al. The Notch ligand Delta-like 4 negatively regulates endothelial tip cell formation and vessel branching. Proc. Natl Acad. Sci. USA 104, 3225–3230 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L. et al. Notch signaling regulates cardiomyocyte proliferation during zebrafish heart regeneration. Proc. Natl Acad. Sci. USA 111, 1403–1408 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahlhut C., Suárez Y., Lu J., Mishima Y. & Giraldez A. J. miR-1 and miR-206 regulate angiogenesis by modulating VegfA expression in zebrafish. Development 139, 4356–4364 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ablain J., Durand E. M., Yang S., Zhou Y. & Zon L. I. A CRISPR/Cas9 vector system for tissue-specific gene disruption in zebrafish. Dev. Cell 32, 756–764 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacomotto J., Rinkwitz S. & Becker T. S. Effective heritable gene knockdown in zebrafish using synthetic microRNAs. Nat. Commun. 6, 7378 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs S., Chen J.-N., Garrity D. M. & Fishman M. C. Patterning of angiogenesis in the zebrafish embryo. Development 129, 973–982 (2002). [DOI] [PubMed] [Google Scholar]
- Kwon H.-B. H.-B. et al. The parallel growth of motoneuron axons with the dorsal aorta depends on Vegfc/Vegfr3 signaling in zebrafish. Development 140, 4081–4090 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange C. et al. Relief of hypoxia by angiogenesis promotes neural stem cell differentiation by targeting glycolysis. EMBO J. 35, 924–941 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley D. M. et al. Distinct signalling pathways regulate sprouting angiogenesis from the dorsal aorta and the axial vein. Nat. Cell Biol. 13, 686–692 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hen G. et al. Venous-derived angioblasts generate organ-specific vessels during zebrafish embryonic development. Development 142, 4266–4278 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C. et al. Arteries are formed by vein-derived endothelial tip cells. Nat. Commun. 5, 5758 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Red-Horse K., Ueno H., Weissman I. L. & Krasnow M. A. Coronary arteries form by developmental reprogramming of venous cells. Nature 464, 549–553 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan B. et al. ccbe1 is required for embryonic lymphangiogenesis and venous sprouting. Nat. Genet. 41, 396–398 (2009). [DOI] [PubMed] [Google Scholar]
- Peri F. & Nüsslein-Volhard C. Live imaging of neuronal degradation by microglia reveals a role for v0-ATPase a1 in phagosomal fusion in vivo. Cell 133, 916–927 (2008). [DOI] [PubMed] [Google Scholar]
- van Impel A. et al. Divergence of zebrafish and mouse lymphatic cell fate specification pathways. Development 141, 1228–1238 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando K. et al. Clarification of mural cell coverage of vascular endothelial cells by live imaging of zebrafish. Development 143, 1328–1339 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhakumar K. et al. A zebrafish model to study and therapeutically manipulate hypoxia signaling in tumorigenesis. Cancer Res. 72, 4017–4027 (2012). [DOI] [PubMed] [Google Scholar]
- Hwang W. Y. et al. Efficient genome editing in zebrafish using a CRISPR-Cas system. Nat. Biotechnol. 31, 227–229 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan K. M. et al. The Tol2kit: a multisite gateway-based construction kit for Tol2 transposon transgenesis constructs. Dev. Dyn. 236, 3088–3099 (2007). [DOI] [PubMed] [Google Scholar]
- Distel M., Wullimann M. F. & Köster R. W. Optimized Gal4 genetics for permanent gene expression mapping in zebrafish. Proc. Natl Acad. Sci. USA 106, 13365–13370 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louvion J. F., Havaux-Copf B. & Picard D. Fusion of GAL4-VP16 to a steroid-binding domain provides a tool for gratuitous induction of galactose-responsive genes in yeast. Gene 131, 129–134 (1993). [DOI] [PubMed] [Google Scholar]
- Trapnell C. et al. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 7, 562–578 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders S. & Huber W. Differential expression analysis for sequence count data. Genome Biol. 11, R106 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures, Supplementary Tables.
3D-rendering of vessels and nerves. 3D-rendered view of blood vessels (green) and nerves (blue) in Tg(kdrl:EGFP)s843; Tg(XIa.Tubb:DsRed)zf148 embryo at 4dpf.
Angiogenic cell behaviors in flt1ka601. Time-lapse imaging of ectopic vessel sprouting in flt1ka601 full mutant in Tg(fli1a:nEGFP)y7; Tg(kdrl:has.HRASmCherry)s916 background. Vessels in red, endothelial cell nuclei in green. White arrowhead indicates developing sprout. Pink arrowhead indicates the corresponding endothelial cell nucleus. Sprout emanates from the dorsal aspect of the intersegmental vein at the level of the neural tube, and anastomoses with adjacent intersegmental vessel. Developmental stage and scale bar are indicated (bottom left and right corner respectively). Scale bar represents 20μm.
Angiogenic cell behaviors in flt1ka601/+. Time-lapse imaging of angiogenic cell behavior in flt1ka601/+ heterozygous mutant in Tg(fli1a:nEGFP)y7; Tg(kdrl:has.HRAS-mCherry)s916 background. Vessels in red, endothelial cell nuclei in green. White and pink arrowheads indicate hyperactive endothelial cells and filopodia extensions. Developmental stage and scale bar are indicated (bottom left and right corner respectively). Scale bar represents 20μm.
Angiogenic cell behaviors in WT. Time-lapse imaging of endothelial cell behavior in WT sibling in Tg(fli1a:nEGFP)y7; Tg(kdrl:has.HRASmCherry)s916 background. Vessels in red, endothelial cell nuclei in green. Developmental stage and scale bar are indicated (bottom left and right corner respectively). Scale bar represents 20μm.
Origin of vISV endothelial cells. Endothelial cell tracking in Tg(kdrl:nlsKikGR)hsc7 embryo upon photo-switching at 30hpf. At 30hpf, a small part of the posterior cardinal vein (PCV) was photo-converted by exposure to UV light. Photo-converted PCV derived cells express red-kikGR; all endothelial cells express green-kikGR. Arrowheads indicate PCV derived endothelial cells migrating into intersegmental vessels up until the dorsal lateral anastomotic vessel. Developmental stage indicated in bottom right corner. Scale bar represents 20μm.
Data Availability Statement
The authors declare that all data supporting the findings of this study are available within the article and its Supplementary Information files or from the corresponding author on reasonable request. The RNA-seq data generated in this study has been deposited into the Gene Expression Omnibus database with the accession code http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE89350.