Abstract
Fibroblast growth factor 23 (FGF23) is part of a previously unrecognized hormonal bone-parathyroid-kidney axis, which is modulated by 1,25(OH)2-vitamin D (1,25(OH)2D), dietary and circulating phosphate and possibly PTH. FGF23 was discovered as the humoral factor in tumors that causes hypophosphatemia and osteomalacia and through the identification of a mutant form of FGF23 that leads to autosomal dominant hypophosphatemic rickets (ADHR), a rare genetic disorder. FGF23 appears to be mainly secreted by osteocytes where its expression is up-regulated by 1,25(OH)2D and probably by increased serum phosphate levels. Its synthesis and secretion is reduced through yet unknown mechanisms that involve the phosphate-regulating gene with homologies to endopeptidases on the X chromosome (PHEX), dentin matrix protein 1 (DMP1) and ecto-nucleotide pyrophosphatase/phosphodiesterase 1 (ENPP1). Consequently, loss-of-function mutations in these genes underlie hypophosphatemic disorders that are either X-linked or autosomal recessive. Impaired O-glycosylation of FGF23 due to the lack of UDP-N-acetyl-alpha-D-galactosamine:polypeptide N-acetylgalactosaminyl-transferase 3 (GALNT3) or due to certain homozygous FGF23 mutations results in reduced secretion of intact FGF23 and leads to familial hypophosphatemic tumoral calcinosis. FGF23 acts through FGF-receptors and the coreceptor Klotho to reduce 1,25(OH)2D synthesis in the kidney and probably the synthesis of parathyroid hormone (PTH) by the parathyroid glands. It furthermore synergizes with PTH to increase renal phosphate excretion by reducing expression of the sodium-phosphate cotransporters NaPi-IIa and NaPi-IIc in the proximal tubules. Loss-of-function mutations in these two transporters lead to autosomal recessive Fanconi syndrome or to hereditary hypophosphatemic rickets with hypercalciuria, respectively.
INTRODUCTION
Identification of the genetic causes of rare familial disorders of renal phosphate handling has provided, over the past decade, important novel insights into the regulation of mammalian phosphate homeostasis. This chapter will provide an update on the current knowledge of the pathophysiology, the clinical presentation, diagnostic evaluation and therapy of FGF23-dependent and -independent disorders of phosphate homeostasis and tissue mineralization.
AUTOSOMAL DOMINANT HYPOPHOSPHATEMIC RICKETS (ADHR, OMIM 193100)
Genetics of ADHR
In 1971 Bianchine et al described a small family, in which male-to-male transmission suggested an autosomal dominant form of hypophosphatemic rickets (ADHR, for a full list of abbreviations see Table 2).1 Subsequently, Econs and McEnery identified a large family, in which numerous members are affected by ADHR.2 The authors were able to map the disorder to a locus on chromosome 12p13,3 which ultimately allowed identification of the genetic mutation leading to this rare inherited form of hypophosphatemia.4 The incidence of ADHR is unknown; thus far, only a few families have been described in which hypophosphatemia follows an autosomal dominant trait.4–6 ADHR thus appears to be much less frequent than X-linked hypophosphatemia (XLH), which affects approximately 1:20,000 births.7 The ADHR patients described to date carry a heterozygous mutation in fibroblast growth factor 23 (FGF23, chromosome 12p13.3) at amino acid positions 176 or 179, a site for cleavage by subtilisin-like proptein convertases (SPC)4–6 (Fig. 1). Although the exact mechanism is unknown, it appears that ADHR mutations enhance resistance towards SPC leading to deminished intracellular cleavage of intact FGF23. The resulting secretion of inappropriate amounts of biologically active FGF23 suggests that the identified amino acid changes are “gain-of-function” mutations, which lead to renal phosphate wasting, possibly combined with an abnormal feed-back regulation of FGF23 synthesis, as discussed below.
Table 2.
Abbreviations
| Key-Term/Acronym | Definition |
|---|---|
| GALNT3 | UDP-N-Acetyl-α-D-Galactosamine:Polypeptide N-Acetylgalactosaminyltransferease, isoform 3 appears to be important for O-glycosylation of FGF23 |
| FGF23 | Fibroblast growth Factor 23 |
| KL | Alpha Klotho |
| PHEX | Phosphate-regulating gene with Homologies to Endopeptidases on the X chromosome |
| FGF23 | Fibroblast Growth Factor 23 |
| DMP1 | Dentin Matrix Acidic Phosphoprotein 1 |
| MEPE | Matrix extracellular phosphoprotein |
| FGFR | Fibroblast Growth Factor Receptor |
| ARHP | Autosomal Recessive Hypophosphatemia |
| HHRH | Hereditary Hypophosphatemic Rickets with Hypercalciuria |
| PTHR1 | PTH/PTHrP Receptor |
| VDR | Vitamin D receptor, forms heterodimer with RXR |
| HFTC | Hyperphosphatemic Familial Tumoral Calcinosis |
| XLH | X-linked Dominant hypophosphatemia |
| ADHR | Autosomal Dominant Hypophosphatemic Rickets |
| SLC34 | Solute Carrier Family 34 (sodium-phosphate cotransporter) (also referred to as NaPi-II or NPT2), members 1 (NaPi-IIa) and 3 (NaPi-IIc) are expressed in the proximal renal tubule, member 2 (NaPi-IIb) is expressed in the intestine |
| CYP27B1 | 1-alpha-hydroyxylase, vitamin D-activating enzyme, which is expressed in the renal proximal tubule |
| TRPV | Transient receptor potential cation channel, subfamily V, members 5 and 6 are calcium-selective |
| PMCA | plasma membrane Ca2+ ATPase |
| Pit-2 | Solute Carrier Family 20 (sodium-phosphate cotransporter), member 2 |
Figure 1.
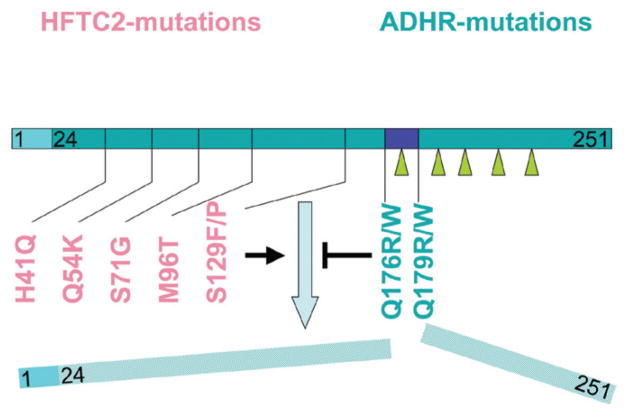
Known mutations in FGF23. Human mutations leading to hyperphosphatemic tumoral calcinosis type 2 (HFTC2, red) are located in the first half of the FGF23 molecule. These impair, directly or indirectly, O-glycosylation (solid arrows) in the second half of FGF23 and thereby prevent secretion of intact FGF23. Human FGF23 mutations leading to autosomal dominant hypophosphatemic rickets (ADHR, green) change the subtilisin-furin cleavage motif RXXR in amino acid positions 176–179 (blue box) and lead to increased secretion and stability of FGF23 that is independent of O-glycosylation.
The phosphaturic action of FGF23 requires the coreceptor alpha Klotho (KL, also see below). A genetic defect leading to a disorder resembling ADHR has recently been reported in a single sporadic case of hypophosphatemia.8 The patient, a 13 month old girl, presented with rickets due to excessive renal phosphate excretion and hyperparathyroidism and cytogenetic studies revealed a de novo chromosomal translocation with the breakpoint being located adjacent to the gene encoding KL. As a result, plasma levels of soluble KL and KL-associated beta-glucuronidase activity were increased, along with increased levels of immunoreactive FGF23. It remains uncertain, however, whether the elevated levels of FGF23 and/or PTH are solely responsible for the increased urinary excretion of phosphate, or whether the elevated levels of soluble Klotho contribute to the abnormal renal handling of this mineral.
FGF23 Synthesis and Secretion
The main sources of FGF23 are osteocytes and osteoblasts in the skeleton, but low levels of uncertain biological significance can be detected in the ventrolateral thalamic nucleus, the thymus, the small intestine and the heart.9 FGF23 synthesis is stimulated by dietary phosphate10–12 and the application of 1,25(OH)2D.13,14 Conversely, the phosphate-regulating gene with homologies to endopeptidases on the X chromosome (PHEX), dentin matrix protein 1 (DMP1) and ecto-nucleotide pyrophosphatase/phosphodiesterase 1(ENPP1) appear to have an important role in suppressing FGF23 synthesis. PHEX and DMP1 were shown to be genetically up-stream of FGF23, as the lack of either of these two proteins leads to an up-regulation of FGF23 expression in bone, most likely through indirect mechanisms.15 After cleavage of the signal sequence comprising 24 amino acids and O-glycosylation by UDP-N-acetyl-alpha-D-galactosamine:polypeptide N-acetylgalactosaminyl-transferase 3 (GALNT3), mature FGF23(25–251) is secreted into the circulation. O-glycosylation of FGF23 occurs in the 162–228 region16 and this posttranslational modification, which may involve residue 178, appears to protect FGF23 from cleavage by subtilisin-like proprotein convertases as shown with recombinant peptides in vitro.17 O-glycosylation is also essential for secretion and processing of intact FGF23 in CHO cells.17,18 Furthermore, in HEK293 cells, expression of GALNT3 is stimulated by extracellular phosphate and suppressed by extracellular calcium and 1,25(OH)2D.19 This suggests that GALNT3 may be an important component of an as-of-yet incompletely understood circuit regulating FGF23 secretion in vivo, which may be disrupted by ADHR mutations. The two FGF23 amino acid residues that are mutated in patients affected by ADHR, R176 or R179, constitute, as mentioned above, the site for cleavage by subtilisin/furin-like endopeptidases. Just like O-linked glycosylation of residue T178 in wild-type FGF23,17 mutation of either residue 176 or 179 appears to protect FGF23 against intracellular proteolytic cleavage and degradation,20 leading to the secretion of intact FGF23, although it remains unclear, how production of the ADHR mutant escapes feed-back regulation by phosphate and 1,25(OH)2D.
FGF23 Mode of Action
The phosphaturic actions of FGF23 require KL as a coreceptor. Consequently, a homozygous inactivating KL mutation was shown to lead to FGF23 resistance in a patient with familial hyperphosphatemic tumoral calcinosis type 3 (HFTC3, see below)21 and mice that are null for Kl, the murine ortholog of KL, show a corresponding phenotype.22,23 Immunoprecipitation experiments, surface plasmon resonance (SPR) spectroscopy and functional assays measuring the mitogenic response of BaF3 cells or activation of the MAPK-pathway in HEK293 cells have shown that KL, a protein with a single membrane-spanning domain, forms a ternary complex with FGF23 in conjunction with FGFR1c and other FGF receptors.24,25 Recent work using neutralizing anti-FGF23 antibodies indicates that the N-terminal portion of FGF23 interacts with FGFR1c, while the C-terminus binds to KL and both interactions appear to be important for bioactivity in vitro and in vivo.26 Low affinity binding of FGF23 to FGFR1c and other FGF receptors in the absence of KL has been shown to occur in vitro.27–29 However, this coreceptor appears to be essential for high-affinity binding of FGF23 to the receptor and its phosphate-regulating effects in vivo. The ablation of either FGFR3 or FGFR4, individually or in combination, was unable to rescue the hyp phenotype in mice suggesting that these two FGF receptors are not involved in mediating the renal effects of FGF23.30
The site of FGF23 action in the kidney is still controversial. While FGF23 decreases NPT2a and NPT2c expression31–33 and CYP27B1 activity in the proximal tubules,34–36 the coreceptor KL is expressed mainly in the distal tubules. In addition, mice injected with recombinant FGF23 show phosphorylation of MAPK and an up-regulation of the early growth-response gene 1 (Egr-1) in the distal tubules.37 These findings suggest either that FGF23 uses a noncanonical signal-transduction pathway in the proximal tubules or that it induces the secretion of an “intermediary phosphatonin” from the distal tubules, which acts in a paracrine fashion on the proximal tubules. KL may furthermore have ligand-independent actions by regulating expression of the calcium channels TRPV538 and the potassium channel ROMK in the distal tubules.39
Although the transgenic overexpression of soluble KL can prolong the life span of mice,24,40 no data on FGF23 levels or mineral ion homeostasis have been reported to date in these animals. Increased FGF23 levels in the patient described by Brownstein et al (see above) suggests that KL may interfere with the normal feed-back inhibition of FGF23 synthesis and/or secretion; however, the exact mechanism of hypophosphatemia in this patient remains unclear.
Clinical Findings in ADHR
Chronic hypophosphatemia in ADHR can lead to abnormal bone growth and mineralization, i.e., rickets in growing children and osteomalacia in adults. As in nutritional rickets, osteoid is undermineralized, leading to a blurring of the microtrabecular architecture and pseudo-fractures (Looser zones) on radiographs. The clinical consequences of rickets or osteomalacia in children or adults, respectively, are bone pain and impaired mechanical properties of the affected bones leading to deformities of the lower extremities, often leading to characteristic wind-swept deformities. Lack of chondrocyte apoptosis in the growing skeleton furthermore leads to an expansion of the epiphyses in children, giving rise to swollen wrists and rachitic rosary.41 Serum biochemical findings indicative of rickets and osteomalacia include elevated bone-specific alkaline phosphatase, osteocalcin, procollagen, pyridinoline cross-links as well as N- and C-telopeptides.42,43 Chronic hypophosphatemia also leads to muscle weakness, which when compared to the effects on the skeleton, is less well understood and may be related to the role of phosphate in intracellular signal transduction and synthesis of ATP or creatine phosphate.42,43
While rickets or osteomalacia can be observed to variable degrees in all hypophosphatemic disorders, including ADHR, subtle but important differences can guide the differential diagnostic and therapeutic decisions. When compared to other types of hypophosphatemic rickets, ADHR appears to have a quite variable clinical phenotype. The kindred described by Econs et al2 can be divided into two subgroups. Group 1 consisted of nine female patients who presented with renal phosphate-wasting during adolescence and adulthood. These individuals presented with bone pain, weakness and insufficiency fractures, but without deformities of the lower extremities. Group 2 consisted of nine male and female patients, with onset of symptoms, including bone deformities, during childhood. Two of these nine individuals lost the renal phosphate-wasting defect later in life, which is consistent with observations in the affected members of two other unrelated kindreds, in which five of eight carriers of a heterozygous, “activating” FGF23 mutation had lost the renal phosphate-wasting defect later in life.5,6 Enthesopathies, which refer to painful or indolent mineral deposits near the insertion sites of tendons usually at the spine and lower extremities, can occur in patients with XLH, ADHR, or autosomal recessive hypophosphatemia (ARHP) (see below) and are readily identified on radiographs.44 The development of these lesions may involve FGFR3 and Klotho, which are expressed in fibrocartilage cells of the entheses.45 Dental cysts or dental dysplasia, respectively, were described in patients with XLH and ADHR, but these abnormalities do not appear to be as common in ADHR as in XLH.2,5 Likewise, midfacial hypoplasia and frontal bossing may also be observed in ADHR.5 Although these findings may be related to the severity of hypophosphatemia, therapy with 1,25(OH)2D and phosphate supplements often cannot reverse or prevent these changes. Thus, local effects of FGF23 excess and activation of canonical FGF receptor signaling (Klotho-independent) or other factors up-stream of FGF23 may play a role.44,46
Diagnostic Evaluation of ADHR and Other Disorders of Phosphate Homeostasis
The clinical presentation of ADHR and other hypophosphatemic disorders includes renal phosphate wasting, leading to rickets and/or osteomalacia and abnormal vitamin D metabolism. The diagnosis therefore depends on careful evaluation of phosphate homeostasis, which can be challenging since serum phosphate concentrations are influenced by the time of day, relationship to meals and age of the subject and none of the methods for determination of tubular phosphate reabsorption are entirely satisfying. To determine the cause of abnormal serum phosphate levels in a patient, who has normal parathyroid and renal function, we generally first assess his or her tubular reabsorption for phosphate (%TRP). For this purpose the patient is asked to collect a 3-hour timed urine for measurement of phosphate and creatinine along with the corresponding serum parameters after 8 hours of fasting. %TRP is calculated according to Formula 1 and the tubular maximum of reabsorption for phosphate (TmP/GFR) is derived from a nomogram, which was devised by Walton and Bijvoet47 to correct for the nonlinear relationships of %TRP and TmP/GFR when %TRP is higher 80%.
| Formula 1 |
TmP/GFR reflects the threshold of the serum phosphate concentration above which phosphate is no longer fully reclaimed from the glomerular filtrate in the proximal tubules. While the TmP/GFR derived from the Walton and Bijvoet nomogram is generally sufficient in adults, the nomogram does not accommodate the higher normal range of serum phosphate values in newborns and toddlers.48 Thus, calculating TP/GFR provides a more accurate assessment of renal phosphate handling in the pediatric population49 (Formula 2).
| Formula 2 |
Inappropriately low %TRP in the setting of hypophosphatemia is suggestive of a proximal renal tubular defect as the underlying cause, which can be further classified by determining the patient’s vitamin D status: In patients with ADHR, %TRP and 1,25(OH)2D levels are concordantly (inappropriately) reduced, suggesting excess FGF23 activity. In contrast, appropriately elevated 1,25(OH)2D levels suggest an FGF23-independent, renal tubular defect leading to abnormal serum phosphate levels, which can for example be seen in hereditary hypophosphatemic rickets with hypercalciuria (HHRH).50 If hypophosphatemia occurs without an obvious increase in urinary phosphate excretion, nutritional deficiencies, malabsorption, or liver disease should be considered; in rare cases, a primary intestinal defect involving reduced NPT2b expression has been described in pulmonary alveolar microlithiasis (PAM)51 (see Table 1).
Table 1.
Serum biochemical findings in disorders of phosphate homeostasis
| Parameter | Hypophosphatemia | Hyperphosphatemia | ||
|---|---|---|---|---|
| FGF23-dependent | FGF23-independent | FGF23-deficient | FGF23-resistant | |
| Acquired | TIO, postrenal transplant | Post-hepatectomy | NA | NA |
| Inherited | XLH, ADHR, ARHP, OGD, OSD, FD/MAS, NF1+2 | HHRH, Fanconi with hypercalciuria | HFTC1, 2 | HFTC3 |
| S-Ca | NL | NL | NL to HIGH | NL to HIGH |
| S-PTH | NL to HIGH | NL to LOW | NL to LOW | NL to HIGH |
| S-1,25(OH)2D | NL to LOW | HIGH | HIGH | HIGH |
| U-Ca | NL to LOW | HIGH | NL to HIGH | NL to HIGH |
| S-P | LOW | LOW | HIGH | HIGH |
| S-FGF23 | NL to HIGH | LOW to NL | LOW | HIGH |
| U-P | HIGH | HIGH | LOW | LOW |
| Current Rx: | Phosphate and 1,25(OH)2D replacement | Phosphate replacement only | Phosphate binders, acetazolamide | Phosphate binders, acetazolamide |
Excess production of 1,25(OH)2D due to FGF23-independent hypophosphatemia may lead to increased absorption of calcium in the gut, resulting in hypercalciuria and some suppression of PTH production. Measuring the levels of 1,25(OH)2D and PTH, as well as serum and urinary calcium, may therefore help to distinguish FGF23-dependent from FGF23-independent forms of hypophosphatemia. It is important to keep in mind that vitamin D deficiency and secondary hyperparathyroidism may mask the findings as described for HHRH and serum and urinary studies need to be repeated after repletion of vitamin D stores.52
Measurement of FGF23 Levels
Circulating FGF23 levels can be determined from EDTA plasma or serum using several commercially available enzyme-linked immunometric assays.53,54 The intact FGF23 assay (referred to as iFGF23 assay) uses antibodies directed against N-terminal and C-terminal portions of the peptide for capture and detection, respectively (Kainos, Tokyo, Japan). In contrast, the C-terminal FGF23 assay (referred to as cFGF23 assay) (Immutopics, Inc., San Clemente, CA) uses two antibodies directed against distinct epitopes within the C-terminal region of FGF23 and thus detects intact FGF23 as well as C-terminal fragments. Both assays can help establish the diagnosis of FGF23-dependent disorders of phosphate homeostasis, when FGF23 levels are elevated above the normal range or are inappropriately normal.55,56 They can also help establishing the diagnosis of FGF23-dependent hypophosphatemic disorders such as HHRH or Fanconi syndrome, albeit with significant differences in sensitivity, which is currently best for the iFGF23 assay.54,57 The measurement of FGF23 with both assays can help establish the diagnosis of tumoral calcinosis (HFTC), since homozygous inactivating mutations in GALNT3 (HFTC1) or FGF23 (HFTC2) result in low levels of intact FGF23, yet often significant elevations of C-terminal FGF23 fragments while both intact and C-terminal FGF23 are elevated in HFTC3 (see below for details).21,58–60
Treatment of ADHR
The clinical course of ADHR is usually comparable to mild forms of XLH, which will be described in more detail below. As a result phosphate and 1,25(OH)2D supplementation are often required only during skeletal growth in childhood and adolescence. These therapeutic interventions provide symptomatic relieve and improves the bone abnormalities, but are usually unable to normalize serum phosphate levels. Treatment can be complicated by the development of secondary hyperparathyroidism, hypercalciuria and nephrocalcinosis.44,46 Thus, treatment needs to be monitored carefully for these complications and the use of calcium-sensing receptor agonists such as cinacalcet, which has been successfully used to normalize parathyroid hormone secretion and to reduce the magnitude of phosphaturia in XLH patients,61,62 may have a role in ADHR as well. We prefer potassium-containing phosphate supplements over sodium-containing phosphate supplements since the former seem to induce less sodium-related phosphaturia, although formal studies to support this practice are still missing. Thiazide diuretics may be helpful in slowing the progression of nephrocalcinosis.63 Anti-FGF23 antibodies hold promise to become a therapeutic option for humans with ADHR since treatment has been successful in hyp mice, which like patients with ADHR have high circulating FGF23 levels.26,64
OTHER DISORDERS OF RENAL PHOSPHATE EXCRETION
Acquired Hypophosphatemic Disorders
Tumor Induced Osteomalacia (TIO)
Tumor-induced osteomalacia (TIO), also referred to as oncogenic osteomalacia (OOM),65 is an acquired disorder of FGF23 excess,66 or possibly FGF7 excess.67 Tumors secreting the phosphaturic factor are usually benign mixed connective tissue tumors. Other factors such as matrix extracellular protein (MEPE)68 or secreted frizzled related protein 4 (sFRP4)69 were also isolated from TIO tumors and may contribute to the abnormal regulation of renal phosphate handling.70 Tumor-induced osteomalacia is a relatively rare condition, with only slightly more than one hundred cases described in the literature to date.65 Drezner reviewed 120 cases of tumor-induced osteomalacia and identified four distinct morphologic patterns:1 primitive-appearing, mixed connective tissue tumors;2 osteoblastoma-like tumors;3 non-ossifying fibroma-like tumors; and4 ossifying fibroma-like tumors. Hypophosphatemia was also described in patients with widespread fibrous dysplasia of bone, neurofibromatosis and linear nevus sebaceous syndrome (see further below) and concurrent with breast carcinoma, prostate carcinoma, oat cell carcinoma, small cell carcinoma, multiple myeloma and chronic lymphocytic leukemia. Proof of a causal relationship has been that removal of the tumor resulted in appropriate biochemical and radiographic improvements; however, since most cases were reported before the discovery of FGF23 (and of MEPE and sFRP-4), the phosphaturic factor secreted by these previously reported tumors has not been determined. The tumors are frequently located in the visceral scull or in the tendons of hands and feet and may only be a few millimeters large and indolent. They commonly escape detection by physical exam and computed tomography scans and may require more sensitive techniques for localization including whole-body octreotide-scans,71 PET-CT scans using [18F]-FDG,72 or [68Ga]-DOTANOC73 as tracers. Selective vein sampling74 can permit localization, even in individuals with only mildly elevated circulating FGF23 levels.75–77 Therapy consists of surgical tumor excision, once its location has been revealed, which usually results in normalization of serum phosphate levels within 24 hrs. In those patients, where localization of the tumor is impossible or if tumor resection is incomplete, symptomatic therapy, as described in more detail above, should be continued.
Other Acquired Syndromes of Renal Phosphate Wasting
Another increasingly recognized acquired syndrome of renal phosphate wasting is postrenal transplant hypophosphatemia, which often cannot be attributed to tertiary hyperparathyroidism alone.78,79 Bhan et al80 and Pande et al81 recently showed that posttransplant hypophosphatemia correlates inversely with serum FGF23 levels and coined the term “tertiary hyperphosphatoninism” due to persistent production of FGF23, which persists longer than would be expected from the half-life of the hormone.80,82 Hypophosphatemia in the setting of inappropriate renal phosphate excretion has also been recognized with severe burn-injuries83,84 and after partial hepatectomy,85 although these forms of hypophosphatemia appear to be independent of FGF23.86,87
INHERITED HYPOPHOSPHATEMIC DISORDERS OTHER THAN ADHR(SEE FIG. 2)
Figure 2.
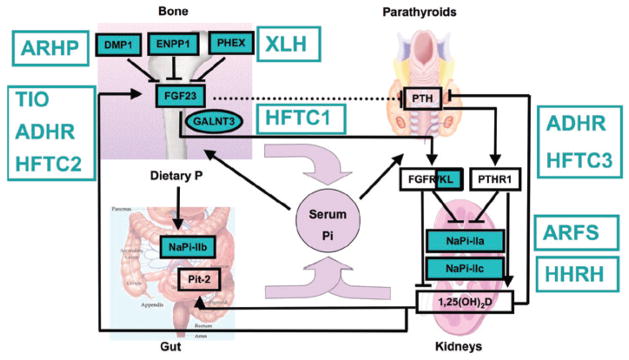
Disorders of phosphate homeostasis. FGF23 expression in bone is up-regulated by an increase in dietary phosphate intake and by 1,25(OH)2D and down-regulated, through yet unknown mechanisms, by PHEX, DMP1, ENPP1 and probably several additional proteins. FGF23 acts through one or more FGF receptors, with Klotho as coreceptor, to inhibit renal phosphate re-absorption and to decrease circulating 1,25(OH)2D levels and possibly to inhibit PTH secretion by the parathyroid glands (dashed line). The net effect of these PTH-dependent actions is a decrease in serum phosphate levels, yet an increase in serum 1,25(OH)2D levels. 1-alpha hydroxylase activity is also up-regulated by low serum phosphate levels and down-regulated by increased serum calcium and phosphate levels and by increased FGF23 levels. 1,25(OH)2D acts through VDR/RXR heterodimers to enhance the intestinal absorption of phosphate and to stimulate FGF23 synthesis and secretion by osteocytes; it furthermore inhibits PTH synthesis and secretion by the parathyroid glands. The net 1,25(OH)2D effect is an increase in serum phosphate levels. The disorders affecting phosphate homeostasis are indicated in green. Please see the text for a detailed description.
X-Linked Autosomal Hypophosphatemia (XLH, OMIM 307800)
XLH, the most common form of hypophosphatemia, was first recognized by Albright and coworkers in 1937.88 Lack of male-to-male transmission was observed by Winters in 1958,89 which suggested an X-linked mode of inheritance. Using a positional cloning approach, the genetic defect was ultimately identified in 199590 and a large number of different loss-of-function mutations in PHEX, phosphate-regulating gene with homologies to endopeptidases on the X chromosome, have since been reported.91 Deletion of the Phex gene in hyp-mice leads to increased FGF23 gene transcription in osteocytes resulting in elevated circulating levels of FGF23 and thus renal phosphate wasting,92,93 which is similar to findings in human XLH patients.53,54 It was therefore concluded that PHEX may be involved in the feed-back regulation of FGF23 secretion,94 which may explain why males and females are equally affected, although the exact mechanism remains unclear.
Although some patients have normal growth, most XLH patients show stunted growth despite treatment with phosphate and active vitamin D analogs.95 Additional clinical features include craniosynostosis, frontal bossing and mid-facial hypoplasia as described above.44,46 Owen et al recently examined female monozygotic twins with a documented PHEX mutation; one twin had overt XLH with hypophosphatemia leading to abnormal growth and rickets, while the other displayed normal renal phosphate handling, normal growth and no evidence for rickets. The authors suggested that nonrandom expression of the normal PHEX allele in critical tissues may be responsible for the discordant XLH phenotype.96
As outlined above for ADHR, treatment of XLH consists of oral phosphate supplementation and active vitamin D analogs, which provides symptomatic relieve and improves the bone abnormalities, but is usually unable to normalize serum phosphate levels. Treatment may be complicated by the development of secondary hyperparathyroidism, hypercalciuria and nephrocalcinosis44,46 and needs to be monitored carefully for these complications. The calcium-sensing receptor agonist cinacalcet has been successfully used to normalize parathyroid hormone secretion and to reduce the magnitude of phosphaturia in XLH patients, who had developed severe secondary or tertiary hyperparathyroidism.61,62 However, parathyroidectomy may ultimately be required, which results generally in improved phosphatemic control, although the postoperative course may be complicated by severe hungry bone syndrome.97 Thiazide diuretics may be helpful in slowing the progression of nephrocalcinosis.63 Replacement of the membrane-anchored PHEX with a soluble form of PHEX did not prove effective to reverse hypophosphatemia in hyp mice, while treatment with anti-FGF23 antibodies has been successful in these animals and holds promise to become a therapeutic option for humans with XLH.26,64 Growth hormone therapy was reported to improve linear growth in some patients, although it remains unclear, whether the observed improvements were in part attributable to an increase in tubular reabsorption of phosphate during growth hormone treatment.98
Autosomal Recessive Hypophosphatemia (ARHP, OMIM 241520)
ARHP is caused by homozygous, presumably loss-of-function mutations in DMP199,100 or in ecto-nucleotide pyrophosphatase/phosphodiesterase 1 (ENPP1).101,102 Intact DMP1 is cleaved into a 35 and a 57 kDa fragment, possibly by bone morphogenic protein 1 (BMP1),103 which, in turn, is activated by a complex consisting of the subtilisin propeptide convertase SPC2 and the co-activator 7B2.104 Transgenic over-expression of the C-terminal 57 kDa DMP1 fragment is both necessary and sufficient to rescue the bone phenotype (and probably the hypophosphatemia resulting from increased FGF23 secretion) of DMP1-null mice.105 This DMP1 fragment appears to have nuclear effects, which are required to prevent excess FGF23 secretion, renal phosphate wasting and hypophosphatemia.105 Thus, the C-terminal fragment may be required for suppression and/or feed back regulation of FGF23 gene transcription and/or FGF23 secretion.99 ENPP1 is a membrane-bound ecto-enzyme responsible for the generation of the mineralization inhibitor pyrophosphate (PPi).106 Loss-of function mutations in this enzyme are the cause of generalized arterial calcifications of infancy (GACI),107,108 which was associated in some patients with mild hypophosphatemia.109 Recently, two groups identified homozygous loss-of-function mutations in ENPP1 in kindreds with hypophosphatemic rickets due to FGF23-dependent renal phosphate-wasting.101,102 Further evaluation of the phenotypic expression of ENPP1 mutations indicated that members of the same kindred, carrying the same homozygous mutation can present either with arterial calcifications or with rickets. Furthermore, subtle changes, i.e., mild hypophosphatemia was observed in individuals with GACI and thickening of the cardiac valves in individuals with rickets were observed, suggesting the presence of modifiers (genetic or environmental) that underlie the variable expressivity of the disorders. The cause of FGF23 excess may be directly related to lack of PPi production, or due to accumulation of precursors, such as ATP in the extracellular space. However, presence of mild hyperphosphatemia in individuals suffering from hypophosphatasia, which is caused by loss-of-function mutations in the PPi-degrading enzyme, namely tissue nonspecific alkaline phosphatase (TNALP),110 suggests that PPi may directly or indirectly suppress FGF23 production.
Treatment of ARHP is currently symptomatic and relies, like the treatment of XLH and ADHR, on oral phosphate supplementation and repletion of 1,25(OH)2D to prevent the development of hyperparathyroidism. In the future, it may be possible to treat these groups of patients with monoclonal, inactivating antibodies against FGF23.
Hereditary Hypophosphatemic Rickets with Hypercalciuria (HHRH, OMIM 241530)
HHRH is a rare disorder with autosomal recessive inheritance that was first described in 1985 in a large consanguineous Bedouin kindred.50 Unlike patients with XLH, individuals affected by HHRH do not develop dental abscesses or craniofacial abnormalities (i.e., craniosynostosis, frontal bossing, scaphocephaly, Chiari I malformation)111,112 and different from patients affected by XLH, ADHR and ARHR, HHRH patients show suppressed or low-normal FGF23 levels.113 This reduction in FGF23, combined with the hypophosphatemia, contributes to the compensatory increase in the plasma level of 1,25(OH)2D typically observed in HHRH. This appropriate rise in the concentration of the biologically active form of vitamin D results in absorptive hypercalciuria, the cardinal feature that distinguishes HHRH from most other Mendelian hypophosphatemic disorders. Measurement of 1,25(OH)2D and urinary calcium excretion are thus essential for establishing the diagnosis of HHRH, although both may be normal, if vitamin D deficiency and/or secondary hyperparathyroidism are present.52
HHRH is caused by homozygous or compound heterozygous loss-of-function mutations in NaPi-IIc/SLC34A3.113–115 Expressivity can be quite variable and may be affected like in other hypophosphatemic forms of rickets by vitamin D status. Some heterozygous individuals may have an increased urinary calcium excretion and occasionally some of the above biochemical features of HHRH, while bone changes are generally missing. Likewise, individuals with two mutated SLC34A3 alleles can initially present with renal stones alone even in the absence of clinical symptoms associated with rickets or osteomalacia.52,116
In contrast to patients with XLH, ADHR or ARHP, who are usually treated with multiple daily doses of oral phosphate and high doses of calcitriol (i.e.,1,25(OH)2D), the effective therapy of individuals affected by HHRH consists of oral phosphate supplementation alone. The prescription of biologically active vitamin D analogs is contraindicated and may lead to hypercalcemia, hypercalciuria, nephrocalcinosis and possibly renal insufficiency.52,116
Hypophosphatemia with Osteoporosis and Nephrolithiasis Due to NaPi-IIa (OMIM 612286) or NHERF-1 Mutations (OMIM 604990)
Prie et al investigated a heterogeneous group of patients with idiopathic hypercalciuria, osteoporosis and renal stones. Using a candidate gene approach they found in 2 of 20 individuals heterozygous for nonsynonymous SNPs in NaPi-IIa/SLC34A1.117 Consistent with their findings, Lapointe et al identified additional heterozygous mutations in other patients affected by calcium nephrolithiasis and a renal phosphate leak.118 These latter NaPi-IIa mutations did not lead to functional abnormalities of sodium-dependent phosphate cotransport, when tested in vitro. In contrast, Prie et al presented experimental in vitro evidence for dominant negative effects of the NaPi-IIa/SLC34A1 alterations on proximal renal tubular phosphate reabsorption; these findings, however, were challenged by others.119 The same group more recently identified heterozygous, nonsynonymous amino acid changes in NHERF-1; three different changes were identified in 7 of 94 individuals with idiopathic hypercalciuria, osteoporosis and renal stones.120 However, two of those amino acid changes are listed in the NCBI dbSNP database as low frequency polymorphisms,121 raising the possibility that these are either not contributing to the clinical and laboratory findings in the investigated cohort or that these changes may lead more frequently to hypophosphatemia, albeit without readily appreciated clinical abnormalities. Further studies are thus required to prove that mutations in NaPi-IIa or NHERF-1 are indeed responsible for idiopathic hypercalciuria, osteoporosis and renal stones.
Autosomal Recessive Fanconi Syndrome (ARFS)
In 1988, Tieder et al described a consanguineous Arab kindred with childhood rickets and defective proximal tubular handing of phosphate, amino acids and glucose consistent with renal Fanconi syndrome.122 Distinct from other forms of Fanconi, their patients also had elevated 1,25(OH)2D levels and absorptive hypercalciuria. Recently, homozygosity mapping of this kindred showed linkage of the disease to chromosome 5q35.1–3 and subsequent sequence analysis of the SLC34A1/NaPi-IIa gene in the linked interval revealed a homozygous duplication g.2061_2081dup (p.I154_V160dup).123 Expression of the mutant NaPi-IIa protein in Xenopus oocytes and Opossum kidney cells showed complete loss-of-function and lack of membrane insertion, respectively. The two patients described in the 1988 report, who are now 39 and 43 years old, continue to have low TmP/GFR and their FGF23 and PTH levels were recently shown to be low-normal (despite impaired renal function), suggesting that their hypophosphatemia is FGF23- and PTH-independent. However, their previously documented absorptive hypercalciuria due to increased 1,25(OH)2D levels, had normalized in the setting of vitamin D deficiency. Although symptoms of rickets were present in childhood, both patients have been relatively asymptomatic during adulthood and discontinued phosphate supplementation. Both homozygous carriers developed CKD stage 2–3 in their 30s, which is in contrast to the other familial hypophosphatemia disorders described above. Heterozygous carriers of p.I154_V160dup had normal renal function and no evidence for renal phosphate leak or proximal tubulopathy, arguing strongly against dominant negative effects of the mutant NaPi-IIa. However, it is still possible that intracellular accumulation of the mutant transporters in homozygous carriers may be involved in the pathogenesis of their proximal tubular defect, since no comparable abnormalities were observed in mice that are null for the murine ortholog of NaPi-IIa.124, 125
Other Inherited Forms of Renal Phosphate Wasting
Osteoglophonic dysplasia (OGD, OMIM166250) is an autosomal dominant disorder caused by activating missense mutations in the gene encoding fibroblast growth factor receptor-1 (FGFR1).126,127 Affected individuals may develop hypophosphatemia and renal phosphate wasting due to an increased production of FGF23.128,129 Other clinical features include those seen in other syndromes that are caused by fibroblast growth factor receptor-1 mutations, such as craniosynostosis, midfacial hypoplasia, prognatism and rizomelic chondrodysplasia. In addition, individuals affected by OGD have symmetrical radiolucent metaphyseal defects, which may be the source of their excess FGF23 production, although direct histological evidence to support this hypothesis is to date lacking.
Opsismodysplasia (OSD, OMIM 258480)130 is an autosomal recessive skeletal dysplasia that is characterized by a delay in epiphyseal ossification, platyspondyly, metaphyseal cupping, resulting in brachydactyly with short metacarpals and phalanges. The genetic defect is unknown. Like OGD, opsismodysplasia can go along with FGF23 excess leading to renal phosphate wasting.131
Linear nevus sebaceous syndrome (LNSS)/epidermal nevus syndrome (ENS) (OMIM163200) is characterized by sebaceous nevi, often in the face, abnormalities of the central nervous system, ocular anomalies, including coloboma and skeletal defects.132–135 Most patients with LNSS or ENS carry mosaic FGFR3 mutations.136 Some patients develop hypophosphatemic rickets137,138 and recently some nevi were shown to secrete FGF23 thus providing an explanation for the underlying renal phosphate wasting.139–141 However, it is unknown whether the FGFR3 mutations alone or additional unknown somatic mutations lead to the increase in FGF23 production and thus renal phosphate-wasting.
Fibrous dysplasia (FD)/McCune Albright Syndrome (MAS) (OMIM 174800) is caused by somatic activating missense mutations in the alpha subunit of the stimulatory G-protein (encoded by GNAS).142–144 The classical triad of MAS includes polyostotic FD, café-au-lait spots and precocious puberty. However, a number of other endocrine disorders such as thyrotoxicosis, pituitary gigantism and Cushing syndrome are often present as well.144 The nonmineralizing bone lesions of FD/MAS may secrete FGF23, which can lead to hypophosphatemic rickets or osteomalacia.145–148 FGF23-mediated hypophosphatemia can also be observed in Jansen’s metaphyseal chondrodysplasia (OMIM 156400), which is caused by heterozygous activating PTH/PTHrP receptor mutations and may be, like in FD/MAS, a consequence of agonist-independent activation of the cAMP/PKA signaling pathway.149 Finally, hypophosphatemia leading to osteomalacia has been described in some individuals with neurofibromatosis 1 and 2,150,151 although the mechanism remains to be clarified.
HYPERPHOSPHATEMIC DISORDERS
Hyperphosphatemic Familial Tumoral calcinosis, HFTC (OMIM 211900)
Tumoral calcinosis is a clinically and genetically heterogeneous group of disorders first described by Giard (1898)152 and then by Duret (1899).153 Tumoral calcinosis is characterized by calcium-phosphate deposits in different tissues, but osteogenic cells and matrix formation are absent, which distinguishes this disorders from heterotopic ossification. For the purpose of this review, the hyperphosphatemic forms of familial tumoral calcinosis shall be classified as type 1–3 (HFTC1-3), which all follow an autosomal recessive mode of inheritance and all furthermore show inappropriately enhanced renal tubular absorption of phosphate leading to hyperphosphatemia as a common laboratory feature. The activity of renal 1-alpha-hydroxylase is increased resulting in elevated serum 1,25(OH)2D levels and thus increased intestinal absorption of calcium (and phosphate), suppression of parathyroid hormone production and hypercalciuria. The increased serum calcium-phosphate product results in the characteristic abnormal tissue mineralization observed in tumoral calcinosis.
Ectopic tissue mineralization in HFTC is often seen in juxtraarticular muscular and subcutaneous tissues. Patients with tumoral calcinosis often also show dental pulp stones, which may lead to a complete obliteration of the dental pulp cavities. Other clinical features, which may constitute the only clinical evidence for tumoral calcinosis, can include eye-lid calcifications, vascular calcifications and/or nephrocalcinosis. There can also be mineralization of the juxtaarticular bone marrow cavities, however, the remaining skeleton often shows low bone mineral density due to a mineralization defect, which at the moment is only poorly understood.154,155
Familial tumoral calcinosis type 1 (HFTC1) is caused by homozygous loss-of-function mutations in the gene encoding UDP-N-acetyl-alpha-D-galactosamine: polypeptide N-acetylgalactosaminyl-transferase 3 (GALNT3).58,156 GALNT3 is responsible for O-glycosylation and proper secretion of intact FGF23.17 Patients with HFTC1 characteristically have low or undetectable intact, but increased C-terminal FGF23 levels. HFTC1 is allelic with the hyperostosis-hyperphosphatemia syndrome (HSS; OMIM 610233). Patients affected by HSS show, besides the characteristic biochemical abnormalities in serum and urine, recurrent albeit transient painful swellings of the long bones and associated radiographic findings that are consistent with periosteal reaction and cortical hyperostosis.157,158 Similar or identical GALNT3 mutations thus can lead to HSS (bone abnormalities without skin involvement) or to HFTC1, but the genetic modifiers responsible for these differences in disease presentation are currently unknown.
HFTC2 is caused by mutations affecting both alleles of FGF23, which reduce the circulating levels of bioactive intact FGF23, just like in HFTC1 (Fig. 1).156,159 C-terminal FGF23 fragments are secreted, however and high levels of this fragment are often detected in the circulation.60 The mechanism by which HPTC2 mutations may lead to loss-of-function of FGF23 is unclear. Consistent with the patients’ low circulating intact FGF23 levels, we recently showed that HEK293 or COS-7 cells expressing FGF23 with proline at position 129 ([P129]FGF23) (or with other mutations identified in HFTC2 patients), secrete the intact hormone only poorly into the medium. Interestingly, total lysates of HEK293 or COS-7 cells expressing [P129]FGF23 (and other FGF23 mutants) revealed a 25 kDa form of FGF23 that was also detected in cells expressing wild-type FGF23. However, in contrast to cells expressing wild-type FGF23, a 32 kDa protein species was missing in cells expressing FGF23 mutants.
The 32 kDa protein species of FGF23 is detected with antibodies raised against N-terminal and C-terminal portions of FGF23. Thus full-length FGF23 is larger than predicted from the primary amino acid sequence of mature FGF23(25–251). This suggested that wild-type FGF23 is modified and that this modification is absent in patients carrying one of the FGF23 mutations leading to tumoral calcinosis. Enzymatic deglycosylation of wild-type FGF23 suggested that the modification consists of O-linked glycans (Fig. 3A) and that these modifications are absent in [P129]FGF23 (Fig. 3B) as well as in [G71]FGF23 and [F129]FGF23, other mutant forms of FGF23 that cause tumoral calcinosis. These findings suggest that a similar mechanism, namely lack of O-glycosylation, underlies the poor secretion observed of these FGF23 mutants. Both, HFTC1 (GALNT3 mutations) and HFTC2 (FGF23 mutations), thus appear to be disorders of abnormal O-glycosylation of FGF23.
Figure 3.
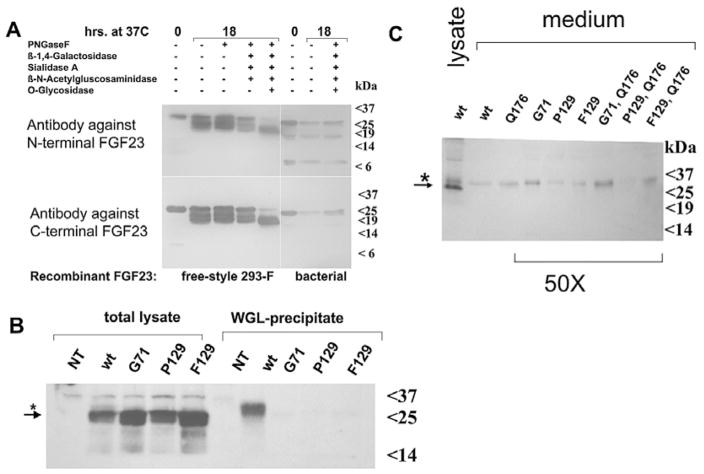
Tumoral calcinosis mutations impair O-glycosylation and secretion of FGF23. A) Enzymatic deglycosylation of recombinant FGF23: N-linked and O-linked carbohydrates were removed under denaturing conditions using approximately 1 μg of recombinant FGF23 and the E-DEGLY kit (Sigma). The molecular shift observed with O-glycosidase indicates that recombinant FGF23 is O-glycosylated. B) Total lysates from HEK293 cells expressing wild-type FGF23(1–251) (wt) or FGF23 with mutations at residues 71 or 129 (G71, P129, or F129) show equal expression of the 25 kDa protein species; the 32 kDa form of FGF23 is detected only in the glycoprotein fraction (star); NT, nontransfected. C) The 32 kDa, presumably glycosylated form of FGF23, but not the more abundant 25 kDa protein species is detected after concentrating 50-fold conditioned media from HEK293 cells expressing wild-type and mutant FGF23; this suggests that FGF23 carrying one of the mutations that cause tumoral calcinosis can undergo limited O-glycosylation thus allowing secretion (star); see text for details. Reproduced with permission from Bergwitz C et al. J Clin Endocrinol Metab 2009; 94:4267–4274,172 ©2009 The Endocrine Society.
The mechanism by which the FGF23 mutations identified in HFTC2 lead to impaired O-glycosylation of FGF23 remains unclear. However, previous reports have indicated that O-glycosylation occurs within the C-terminal portion of FGF23, i.e., the 168–228 region,16 while all HFTC2 mutations identified to date reside in the N-terminal portion of FGF23; some of these mutations furthermore do not affect potential O-glycosylation sites (H41Q,160 Q54K).161 Thus, HFTC2 mutations likely cause misfolding, which may delay or impair O-glycosylation, as previously suggested.60,159
The lack of O-glycosylation can be the consequence or the cause of poor secretion of the mutant FGF23 by HEK293 cells; the former scenario would likely result in the secretion of large quantities of an unmodified 25 kDa FGF23 into the medium of cells transfected with plasmids encoding the mutant forms of FGF23. However, even after concentrating 50-fold the conditioned medium from HEK293 expressing the mutant FGF23, the 25 kDa protein species could not be detected. Instead, small amounts of the 32 kDa protein species were observed in concentrated medium, indicating that O-glycosylation can occur, albeit inefficiently for FGF23 mutants and that these mutants can be secreted once glycosylated (Fig. 3C).
Recently, a 13-year old girl with a disorder resembling HFTC1 and HFTC2, was reported, who had extremely high circulating levels of intact and C-terminal FGF23.21 Radiographs of the patient showed osteopenia, patchy sclerosis in the hands, feet, long bones and calvaria, intracranial calcifications and calcifications of the dura and carotid arteries. Interestingly and distinct from the first two forms of HFTC, she had elevated PTH levels due to four-gland parathyroid hyperplasia. Very high circulating levels of intact FGF23 had also been observed in mice that are null for klotho (KL), the coreceptor for FGF23 (see above). The authors therefore decided to analyze the gene encoding KL, which led to the discovery of a homozygous missense mutation in the second putative beta-glycosidase domain that is presumably inactivating, leading to end-organ resistance to FGF23.21
Like humans with HFTC, mice that are null for FGF23,162,163 GALNT3,164 KL22 suffer from hyperphosphatemia and increased 1,25(OH)2D levels due to the loss of biologically active FGF23 or due to end-organ resistance to FGF23, respectively. Consistent with an increased intestinal absorption of calcium from the gut, mice with these genetic modifications also display mild hypercalcemia, suppressed PTH levels and hypercalciuria. The resulting increase in the calcium-phosphorus product in each of these disorders is thought to cause the characteristic tissue calcifications. The homozygous ablation of Npt2a,154 the VDR,163 or CYP27B1165 rescues the serum-biochemical abnormalities of FGF23-null mice and the homozygous ablation of CYP27B1 was also shown to rescue the klotho-null mice.166 Likewise, diets low in phosphate or low in vitamin D167 can normalize the changes in mineral ion homeostasis of FGF23- and klotho-null animals, although mineralization defects in the skeleton may persist.154,162,168
Heterozygous carriers of the genetic mutations that cause HFTC1-3 may have mildly abnormal blood chemistries,156 but do not develop significant calcified lesions and they do not require specific treatment. Treatment of homozygous individuals currently relies on minimizing the intestinal absorption of phosphate through appropriate binders such as aluminum hydroxide or sevelamer60 and on inhibiting renal phosphate reabsorption with acetazolamide;169 in one study treatment with PTH was temporarily able to induce phosphaturia.170 In the future, patients affected by HFTC may be treated with calcilytic agents that reduce the activity of the calcium-sensing receptor and thus stimulate endogenous PTH secretion171 or with recombinant FGF23, if this becomes available for use in humans.
CONCLUSION
FGF23 was identified initially as the hypophosphatemic factor that is abundantly expressed in TIO tumors and that is mutated in individuals affected by ADHR. This phosphaturic hormone was subsequently found to be readily detectable in the circulation of healthy individuals and research over the past years strongly suggests that it is an important regulator of normal phosphate homeostasis. Dysregulation of FGF23 occurs in more than 13 acquired and inherited disorders of phosphate homeostasis and it is emerging as a predictor of disease progression in chronic kidney disease and of mortality of incident and long-term dialysis patients. Further investigations are required to understand the regulation of FGF23 expression through changes in dietary intake and/or serum phosphate levels and through PTH and 1,25(OH)2D. It will also be important to understand which FGF receptors mediate the actions of FGF23 in parathyroids and kidney and how PHEX, DMP1, ENPP1 and most likely several other proteins contribute to the regulation of FGF23 synthesis and secretion.
References
- 1.Bianchine JW, Stambler AA, Harrison HE. Familial hypophosphatemic rickets showing autosomal dominant inheritance. Birth Defects Orig Artic Ser. 1971;7:287–295. [PubMed] [Google Scholar]
- 2.Econs MJ, McEnery PT. Autosomal dominant hypophosphatemic rickets/osteomalacia: clinical characterization of a novel renal phosphate-wasting disorder. J Clin Endocrinol Metab. 1997;82:674–681. doi: 10.1210/jcem.82.2.3765. [DOI] [PubMed] [Google Scholar]
- 3.Econs M, McEnery P, Lennon F, et al. Autosomal dominant hypophosphatemic rickets is linked to chromosome 12p13. J Clin Invest. 1997;100:2653–2657. doi: 10.1172/JCI119809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.ADHR Consortium T. White KE, Evans WE, et al. Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat Genet. 2000;26:345–348. doi: 10.1038/81664. [DOI] [PubMed] [Google Scholar]
- 5.Gribaa M, Younes M, Bouyacoub Y, et al. An autosomal dominant hypophosphatemic rickets phenotype in a Tunisian family caused by a new FGF23 missense mutation. J Bone Miner Metab. 2009;28:111–115. doi: 10.1007/s00774-009-0111-5. [DOI] [PubMed] [Google Scholar]
- 6.Kruse K, Woelfel D, Strom TM. Loss of renal phosphate wasting in a child with autosomal dominant hypophosphatemic rickets caused by a FGF23 mutation. Horm Res. 2001;55:305–308. doi: 10.1159/000050018. [DOI] [PubMed] [Google Scholar]
- 7.Burnett CH, Dent CE, Harper C, et al. Vitamin D-Resistant Rickets. Analysis of Twenty-Four Pedigrees with Hereditary and Sporadic Cases. Am J Med. 1964;36:222–232. doi: 10.1016/0002-9343(64)90085-3. [DOI] [PubMed] [Google Scholar]
- 8.Brownstein CA, Adler F, Nelson-Williams C, et al. A translocation causing increased alpha-klotho level results in hypophosphatemic rickets and hyperparathyroidism. Proc Natl Acad Sci USA. 2008;105:3455–3460. doi: 10.1073/pnas.0712361105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamashita T, Yoshioka M, Itoh N. Identification of a novel fibroblast growth factor, FGF-23, preferentially expressed in the ventrolateral thalamic nucleus of the brain. Biochem Biophys Res Commun. 2000;277:494–498. doi: 10.1006/bbrc.2000.3696. [DOI] [PubMed] [Google Scholar]
- 10.Burnett SM, Gunawardene SC, Bringhurst FR, et al. Regulation of C-terminal and intact FGF-23 by dietary phosphate in men and women. J Bone Miner Res. 2006;21:1187–1196. doi: 10.1359/jbmr.060507. [DOI] [PubMed] [Google Scholar]
- 11.Perwad F, Zhang MY, Tenenhouse HS, et al. Fibroblast growth factor 23 impairs phosphorus and vitamin D metabolism in vivo and suppresses 25-hydroxyvitamin D-1alpha-hydroxylase expression in vitro. Am J Physiol Renal Physiol. 2007;293:F1577–F1583. doi: 10.1152/ajprenal.00463.2006. [DOI] [PubMed] [Google Scholar]
- 12.Nagano N, Miyata S, Abe M, et al. Effect of manipulating serum phosphorus with phosphate binder on circulating PTH and FGF23 in renal failure rats. Kidney Int. 2006;69:531–537. doi: 10.1038/sj.ki.5000020. [DOI] [PubMed] [Google Scholar]
- 13.Nishi H, Nii-Kono T, Nakanishi S, et al. Intravenous calcitriol therapy increases serum concentrations of fibroblast growth factor-23 in dialysis patients with secondary hyperparathyroidism. Nephron Clin Pract. 2005;101:c94–c99. doi: 10.1159/000086347. [DOI] [PubMed] [Google Scholar]
- 14.Kolek OI, Hines ER, Jones MD, et al. 1alpha,25-Dihydroxyvitamin D3 upregulates FGF23 gene expression in bone: the final link in a renal-gastrointestinal-skeletal axis that controls phosphate transport. Am J Physiol Gastrointest Liver Physiol. 2005;289:G1036–G1042. doi: 10.1152/ajpgi.00243.2005. [DOI] [PubMed] [Google Scholar]
- 15.Quarles LD. Endocrine functions of bone in mineral metabolism regulation. J Clin Invest. 2008;118:3820–3828. doi: 10.1172/JCI36479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shimada T, Muto T, Urakawa I, et al. Mutant FGF-23 responsible for autosomal dominant hypophosphatemic rickets is resistant to proteolytic cleavage and causes hypophosphatemia in vivo. Endocrinology. 2002;143:3179–3182. doi: 10.1210/endo.143.8.8795. [DOI] [PubMed] [Google Scholar]
- 17.Kato K, Jeanneau C, Tarp MA, et al. Polypeptide GalNAc-transferase T3 and familial tumoral calcinosis. Secretion of fibroblast growth factor 23 requires O-glycosylation. J Biol Chem. 2006;281:18370–18377. doi: 10.1074/jbc.M602469200. [DOI] [PubMed] [Google Scholar]
- 18.Frishberg Y, Ito N, Rinat C, et al. Hyperostosis-hyperphosphatemia syndrome: a congenital disorder of O-glycosylation associated with augmented processing of fibroblast growth factor 23. J Bone Miner Res. 2007;22:235–242. doi: 10.1359/jbmr.061105. [DOI] [PubMed] [Google Scholar]
- 19.Chefetz I, Kohno K, Izumi H, et al. GALNT3, a gene associated with hyperphosphatemic familial tumoral calcinosis, is transcriptionally regulated by extracellular phosphate and modulates matrix metalloproteinase activity. Biochim Biophys Acta. 2009;1792:61–67. doi: 10.1016/j.bbadis.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.White KE, Carn G, Lorenz-Depiereux B, et al. Autosomal-dominant hypophosphatemic rickets (ADHR) mutations stabilize FGF-23. Kidney Int. 2001;60:2079–2086. doi: 10.1046/j.1523-1755.2001.00064.x. [DOI] [PubMed] [Google Scholar]
- 21.Ichikawa S, Imel EA, Kreiter ML, et al. A homozygous missense mutation in human KLOTHO causes severe tumoral calcinosis. J Clin Invest. 2007;117:2684–2691. doi: 10.1172/JCI31330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuro-o M, Matsumura Y, Aizawa H, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 23.Bai X, Dinghong Q, Miao D, et al. Klotho ablation converts the biochemical and skeletal alterations in FGF23 (R176Q) transgenic mice to a Klotho-deficient phenotype. Am J Physiol Endocrinol Metab. 2009;296:E79–E88. doi: 10.1152/ajpendo.90539.2008. [DOI] [PubMed] [Google Scholar]
- 24.Kurosu H, Yamamoto M, Clark JD, et al. Suppression of aging in mice by the hormone Klotho. Science. 2005;309:1829–1833. doi: 10.1126/science.1112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Urakawa I, Yamazaki Y, Shimada T, et al. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444:770–774. doi: 10.1038/nature05315. [DOI] [PubMed] [Google Scholar]
- 26.Yamazaki Y, Tamada T, Kasai N, et al. Anti-FGF23 neutralizing antibodies show the physiological role and structural features of FGF23. J Bone Miner Res. 2008;23:1509–1518. doi: 10.1359/jbmr.080417. [DOI] [PubMed] [Google Scholar]
- 27.Zhang X, Ibrahimi OA, Olsen SK, et al. Receptor specificity of the fibroblast growth factor family. The complete mammalian FGF family. J Biol Chem. 2006;281:15694–15700. doi: 10.1074/jbc.M601252200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu X, White KE. Fibroblast growth factor 23 and its receptors. Ther Apher Dial. 2005;9:308–312. doi: 10.1111/j.1744-9987.2005.00287.x. [DOI] [PubMed] [Google Scholar]
- 29.Yamashita T, Konishi M, Miyake A, et al. Fibroblast growth factor (FGF)-23 inhibits renal phosphate reabsorption by activation of the mitogen-activated protein kinase pathway. J Biol Chem. 2002;277:28265–28270. doi: 10.1074/jbc.M202527200. [DOI] [PubMed] [Google Scholar]
- 30.Liu S, Zhou J, Tang W, et al. Pathogenic role of Fgf23 in Dmp1-null mice. Am J Physiol Endocrinol Metab. 2008;295:E254–E261. doi: 10.1152/ajpendo.90201.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Segawa H, Yamanaka S, Ohno Y, et al. Correlation between hyperphosphatemia and type II Na-Pi cotransporter activity in klotho mice. Am J Physiol Renal Physiol. 2007;292:F769–F779. doi: 10.1152/ajprenal.00248.2006. [DOI] [PubMed] [Google Scholar]
- 32.Baum M, Schiavi S, Dwarakanath V, et al. Effect of fibroblast growth factor-23 on phosphate transport in proximal tubules. Kidney Int. 2005;68:1148–1153. doi: 10.1111/j.1523-1755.2005.00506.x. [DOI] [PubMed] [Google Scholar]
- 33.Segawa H, Onitsuka A, Aranami F, et al. RENAL WEEK 2006. San Francisco: 2007. Npt2a and Npt2c in Mice Play Distinct and Synergistic Roles in Inorganic Phosphate Metabolism and Skeletal Development. abstract SA-FC101. [DOI] [PubMed] [Google Scholar]
- 34.Strom TM, Jüppner H. PHEX, FGF23, DMP1 and beyond. Curr Opin Nephrol Hypertens. 2008;17:357–362. doi: 10.1097/MNH.0b013e3282fd6e5b. [DOI] [PubMed] [Google Scholar]
- 35.Liu S, Gupta A, Quarles LD. Emerging role of fibroblast growth factor 23 in a bone-kidney axis regulating systemic phosphate homeostasis and extracellular matrix mineralization. Curr Opin Nephrol Hypertens. 2007;16:329–335. doi: 10.1097/MNH.0b013e3281ca6ffd. [DOI] [PubMed] [Google Scholar]
- 36.Fukumoto S, Yamashita T. FGF23 is a hormone-regulating phosphate metabolism—unique biological characteristics of FGF23. Bone. 2007;40:1190–1195. doi: 10.1016/j.bone.2006.12.062. [DOI] [PubMed] [Google Scholar]
- 37.Farrow EG, Davis SI, Summers LJ, et al. Initial FGF23-mediated signaling occurs in the distal convoluted tubule. J Am Soc Nephrol. 2009;20:955–960. doi: 10.1681/ASN.2008070783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang Q, Hoefs S, van der Kemp AW, et al. The beta-glucuronidase klotho hydrolyzes and activates the TRPV5 channel. Science. 2005;310:490–493. doi: 10.1126/science.1114245. [DOI] [PubMed] [Google Scholar]
- 39.Cha SK, Hu MC, Kurosu H, et al. Regulation of renal outer medullary potassium channel and renal K(+) excretion by Klotho. Mol Pharmacol. 2009;76:38–46. doi: 10.1124/mol.109.055780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Masuda H, Chikuda H, Suga T, et al. Regulation of multiple ageing-like phenotypes by inducible klotho gene expression in klotho mutant mice. Mech Ageing Dev. 2005;126:1274–12783. doi: 10.1016/j.mad.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 41.Donohue MM, Demay MB. Rickets in VDR null mice is secondary to decreased apoptosis of hypertrophic chondrocytes. Endocrinology. 2002;143:3691–3694. doi: 10.1210/en.2002-220454. [DOI] [PubMed] [Google Scholar]
- 42.Narchi H, El Jamil M, Kulaylat N. Symptomatic rickets in adolescence. Arch Dis Child. 2001;84:501–503. doi: 10.1136/adc.84.6.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Francis RM, Selby PL. Osteomalacia. Baillieres Clin Endocrinol Metab. 1997;11:145–163. doi: 10.1016/s0950-351x(97)80569-1. [DOI] [PubMed] [Google Scholar]
- 44.Econs MJ, Samsa GP, Monger M, et al. X-Linked hypophosphatemic rickets: a disease often unknown to affected patients. Bone Miner. 1994;24:17–24. doi: 10.1016/s0169-6009(08)80127-4. [DOI] [PubMed] [Google Scholar]
- 45.Liang G, Katz LD, Insogna KL, et al. Survey of the enthesopathy of X-linked hypophosphatemia and its characterization in Hyp mice. Calcif Tissue Int. 2009;85:235–246. doi: 10.1007/s00223-009-9270-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.DiMeglio LA, Econs MJ. Hypophosphatemic rickets. Rev Endocr Metab Disord. 2001;2:165–173. doi: 10.1023/a:1010054727323. [DOI] [PubMed] [Google Scholar]
- 47.Walton RJ, Bijvoet OL. Nomogram for derivation of renal threshold phosphate concentration. Lancet. 1975;2:309–310. doi: 10.1016/s0140-6736(75)92736-1. [DOI] [PubMed] [Google Scholar]
- 48.Brodehl J, Gellissen K, Weber HP. Postnatal development of tubular phosphate reabsorption. Clin Nephrol. 1982;17:163–171. [PubMed] [Google Scholar]
- 49.Alon U, Hellerstein S. Assessment and interpretation of the tubular threshold for phosphate in infants and children. Pediatr Nephrol. 1994;8:250–251. doi: 10.1007/BF00865491. [DOI] [PubMed] [Google Scholar]
- 50.Tieder M, Modai D, Samuel R, et al. Hereditary hypophosphatemic rickets with hypercalciuria. N Engl J Med. 1985;312:611–617. doi: 10.1056/NEJM198503073121003. [DOI] [PubMed] [Google Scholar]
- 51.Corut A, Senyigit A, Ugur SA, et al. Mutations in SLC34A2 cause pulmonary alveolar microlithiasis and are possibly associated with testicular microlithiasis. Am J Hum Genet. 2006;79:650–656. doi: 10.1086/508263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kremke B, Bergwitz C, Ahrens W, et al. Hypophosphatemic rickets with hypercalciuria due to mutation in SLC34A3/NaPi-IIc can be masked by vitamin D deficiency and can be associated with renal calcifications. Exp Clin Endocrinol Diabetes. 2009;117:49–56. doi: 10.1055/s-2008-1076716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yamazaki Y, Okazaki R, Shibata M, et al. Increased circulatory level of biologically active full-length FGF-23 in patients with hypophosphatemic rickets/osteomalacia. J Clin Endocrinol Metab. 2002;87:4957–4960. doi: 10.1210/jc.2002-021105. [DOI] [PubMed] [Google Scholar]
- 54.Jonsson KB, Zahradnik R, Larsson T, et al. Fibroblast growth factor 23 in oncogenic osteomalacia and X-linked hypophosphatemia. N Engl J Med. 2003;348:1656–1663. doi: 10.1056/NEJMoa020881. [DOI] [PubMed] [Google Scholar]
- 55.Endo I, Fukumoto S, Ozono K, et al. Clinical usefulness of measurement of fibroblast growth factor 23 (FGF23) in hypophosphatemic patients: proposal of diagnostic criteria using FGF23 measurement. Bone. 2008;42:1235–1239. doi: 10.1016/j.bone.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 56.Imel EA, Peacock M, Pitukcheewanont P, et al. Sensitivity of fibroblast growth factor 23 measurements in tumor-induced osteomalacia. J Clin Endocrinol Metab. 2006;91:2055–2061. doi: 10.1210/jc.2005-2105. [DOI] [PubMed] [Google Scholar]
- 57.Imel EA, Hui SL, Econs MJ. FGF23 concentrations vary with disease status in autosomal dominant hypophosphatemic rickets. J Bone Miner Res. 2007;22:520–526. doi: 10.1359/jbmr.070107. [DOI] [PubMed] [Google Scholar]
- 58.Topaz O, Shurman DL, Bergman R, et al. Mutations in GALNT3, encoding a protein involved in O-linked glycosylation, cause familial tumoral calcinosis. Nat Genet. 2004;36:579–581. doi: 10.1038/ng1358. [DOI] [PubMed] [Google Scholar]
- 59.Ichikawa S, Guigonis V, Imel EA, et al. Novel GALNT3 mutations causing hyperostosis-hyperphosphatemia syndrome result in low intact fibroblast growth factor 23 concentrations. J Clin Endocrinol Metab. 2007;92:1943–1947. doi: 10.1210/jc.2006-1825. [DOI] [PubMed] [Google Scholar]
- 60.Larsson T, Yu X, Davis SI, et al. A novel recessive mutation in fibroblast growth factor-23 causes familial tumoral calcinosis. J Clin Endocrinol Metab. 2005;90:2424–2427. doi: 10.1210/jc.2004-2238. [DOI] [PubMed] [Google Scholar]
- 61.Geller JL, Khosravi A, Kelly MH, et al. Cinacalcet in the management of tumor-induced osteomalacia. J Bone Miner Res. 2007;22:931–937. doi: 10.1359/jbmr.070304. [DOI] [PubMed] [Google Scholar]
- 62.Alon US, Levy-Olomucki R, Moore WV, et al. Calcimimetics as an adjuvant treatment for familial hypophosphatemic rickets. Clin J Am Soc Nephrol. 2008;3:658–664. doi: 10.2215/CJN.04981107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Seikaly MG, Baum M. Thiazide diuretics arrest the progression of nephrocalcinosis in children with X-linked hypophosphatemia. Pediatrics. 2001;108:E6. doi: 10.1542/peds.108.1.e6. [DOI] [PubMed] [Google Scholar]
- 64.Aono Y, Yamazaki Y, Yasutake J, et al. Therapeutic effects of anti-FGF23 antibodies in hypophosphatemic rickets/osteomalacia. J Bone Miner Res. 2009;24:1879–1888. doi: 10.1359/jbmr.090509. [DOI] [PubMed] [Google Scholar]
- 65.Drezner MK. Tumor-induced osteomalacia. Rev Endocr Metab Disord. 2001;2:175–186. doi: 10.1023/a:1010006811394. [DOI] [PubMed] [Google Scholar]
- 66.Shimada T, Mizutani S, Muto T, et al. Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc Natl Acad Sci USA. 2001;98:6500–6505. doi: 10.1073/pnas.101545198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Carpenter TO, Ellis BK, Insogna KL, et al. Fibroblast growth factor 7: an inhibitor of phosphate transport derived from oncogenic osteomalacia-causing tumors. J Clin Endocrinol Metab. 2005;90:1012–1020. doi: 10.1210/jc.2004-0357. [DOI] [PubMed] [Google Scholar]
- 68.Rowe PS, de Zoysa PA, Dong R, et al. MEPE, a new gene expressed in bone marrow and tumors causing osteomalacia. Genomics. 2000;67:54–68. doi: 10.1006/geno.2000.6235. [DOI] [PubMed] [Google Scholar]
- 69.Jan De Beur S, Finnegan R, Vassiliadis J, et al. Tumors associated with oncogenic osteomalacia express genes important in bone and mineral metabolism. J Bone Miner Res. 2002;17:1102–1110. doi: 10.1359/jbmr.2002.17.6.1102. [DOI] [PubMed] [Google Scholar]
- 70.Berndt T, Craig T, Bowe A, et al. Secreted frizzled-related protein 4 is a potent tumor-derived phosphaturic agent. J Clin Invest. 2003;112:785–794. doi: 10.1172/JCI18563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nguyen BD, Wang EA. Indium-111 pentetreotide scintigraphy of mesenchymal tumor with oncogenic osteomalacia. Clin Nucl Med. 1999;24:130–131. doi: 10.1097/00003072-199902000-00016. [DOI] [PubMed] [Google Scholar]
- 72.Dupond JL, Magy N, Mahammedi M, et al. Oncogenic osteomalacia: the role of the phosphatonins. Diagnostic usefulness of the Fibroblast Growth Factor 23 measurement in one patient. Rev Med Interne. 2005;26:238–241. doi: 10.1016/j.revmed.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 73.Hesse E, Moessinger E, Rosenthal H, et al. Oncogenic osteomalacia: exact tumor localization by coregistration of positron emission and computed tomography. J Bone Miner Res. 2007;22:158–162. doi: 10.1359/jbmr.060909. [DOI] [PubMed] [Google Scholar]
- 74.Takeuchi Y, Suzuki H, Ogura S, et al. Venous sampling for fibroblast growth factor-23 confirms preoperative diagnosis of tumor-induced osteomalacia. J Clin Endocrinol Metab. 2004;89:3979–3982. doi: 10.1210/jc.2004-0406. [DOI] [PubMed] [Google Scholar]
- 75.Westerberg PA, Olauson H, Toss G, et al. Preoperative tumor localization by means of venous sampling for fibroblast growth factor-23 in a patient with tumor-induced osteomalacia. Endocr Pract. 2008;14:362–367. doi: 10.4158/EP.14.3.362. [DOI] [PubMed] [Google Scholar]
- 76.Nasu T, Kurisu S, Matsuno S, et al. Tumor-induced hypophosphatemic osteomalacia diagnosed by the combinatory procedures of magnetic resonance imaging and venous sampling for FGF23. Intern Med. 2008;47:957–961. doi: 10.2169/internalmedicine.47.0745. [DOI] [PubMed] [Google Scholar]
- 77.van Boekel G, Ruinemans-Koerts J, Joosten F, et al. Tumor producing fibroblast growth factor 23 localized by two-staged venous sampling. Eur J Endocrinol. 2008;158:431–437. doi: 10.1530/EJE-07-0779. [DOI] [PubMed] [Google Scholar]
- 78.Parfitt AM, Kleerekoper M, Cruz C. Reduced phosphate reabsorption unrelated to parathyroid hormone after renal transplantation: implications for the pathogenesis of hyperparathyroidism in chronic renal failure. Miner Electrolyte Metab. 1986;12:356–362. [PubMed] [Google Scholar]
- 79.Levi M. Post-transplant hypophosphatemia. Kidney Int. 59:2377–2387. doi: 10.1046/j.1523-1755.2001.00755.x. [DOI] [PubMed] [Google Scholar]
- 80.Bhan I, Shah A, Holmes J, et al. Post-transplant hypophosphatemia: Tertiary ‘Hyper-Phosphatoninism’? Kidney Int. 2006;70:1486–1494. doi: 10.1038/sj.ki.5001788. [DOI] [PubMed] [Google Scholar]
- 81.Pande S, Ritter CS, Rothstein M, et al. FGF-23 and sFRP-4 in chronic kidney disease and postrenal transplantation. Nephron Physiol. 2006;104:p23–p32. doi: 10.1159/000093277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Khosravi A, Cutler CM, Kelly MH, et al. Determination of the elimination half-life of fibroblast growth factor-23. J Clin Endocrinol Metab. 2007;92:2374–2377. doi: 10.1210/jc.2006-2865. [DOI] [PubMed] [Google Scholar]
- 83.Nordstrom H, Lennquist S, Lindell B, et al. Hypophosphataemia in severe burns. Acta Chir Scand. 1977;143:395–399. [PubMed] [Google Scholar]
- 84.Dickerson RN, Gervasio JM, Sherman JJ, et al. A comparison of renal phosphorus regulation in thermally injured and multiple trauma patients receiving specialized nutrition support. JPEN J Parenter Enteral Nutr. 2001;25:152–159. doi: 10.1177/0148607101025003152. [DOI] [PubMed] [Google Scholar]
- 85.Nafidi O, Lepage R, Lapointe RW, et al. Hepatic resection-related hypophosphatemia is of renal origin as manifested by isolated hyperphosphaturia. Ann Surg. 2007;245:1000–1002. doi: 10.1097/SLA.0b013e31805d0882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nafidi O, Lapointe RW, Lepage R, et al. Mechanisms of renal phosphate loss in liver resection-associated hypophosphatemia. Ann Surg. 2009;249:824–827. doi: 10.1097/SLA.0b013e3181a3e562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Salem RR, Tray K. Hepatic resection-related hypophosphatemia is of renal origin as manifested by isolated hyperphosphaturia. Ann Surg. 2005;241:343–348. doi: 10.1097/01.sla.0000152093.43468.c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Albright F, Butler AM, Bloomberg E. Rickets resistant to vitamin D therapy. Am J Dis Child. 1937;54:529–547. [Google Scholar]
- 89.Winters RW, Graham JB, Williams TF, et al. A genetic study of familial hypophosphatemia and vitamin D resistant rickets with a review of the literature. Medicine (Baltimore) 1958;37:97–142. doi: 10.1097/00005792-195805000-00001. [DOI] [PubMed] [Google Scholar]
- 90.HYP-Consortium. A gene (PEX) with homologies to endopeptidases is mutated in patients with X-linked hypophosphatemic rickets. Nat Genet. 1995;11:130–136. doi: 10.1038/ng1095-130. [DOI] [PubMed] [Google Scholar]
- 91.Sabbagh Y, Jones AO, Tenenhouse HS. PHEXdb, a locus-specific database for mutations causing X-linked hypophosphatemia. Hum Mutat. 2000;16:1–6. doi: 10.1002/1098-1004(200007)16:1<1::AID-HUMU1>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 92.Liu S, Guo R, Simpson LG, et al. Regulation of fibroblastic growth factor 23 expression but not degradation by PHEX. J Biol Chem. 2003;278:37419–37426. doi: 10.1074/jbc.M304544200. [DOI] [PubMed] [Google Scholar]
- 93.Sitara D, Razzaque MS, Hesse M, et al. Homozygous ablation of fibroblast growth factor-23 results in hyperphosphatemia and impaired skeletogenesis and reverses hypophosphatemia in Phex-deficient mice. Matrix Biol. 2004;23:421–432. doi: 10.1016/j.matbio.2004.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu S, Quarles LD. How fibroblast growth factor 23 works. J Am Soc Nephrol. 2007;18:1637–1647. doi: 10.1681/ASN.2007010068. [DOI] [PubMed] [Google Scholar]
- 95.Makras P, Hamdy NA, Kant SG, et al. Normal growth and muscle dysfunction in X-linked hypophosphatemic rickets associated with a novel mutation in the PHEX gene. J Clin Endocrinol Metab. 2008;93:1386–1389. doi: 10.1210/jc.2007-1296. [DOI] [PubMed] [Google Scholar]
- 96.Owen CJ, Habeb A, Pearce SH, et al. Discordance for X-linked hypophosphataemic rickets in identical twin girls. Horm Res. 2009;71:237–244. doi: 10.1159/000201113. [DOI] [PubMed] [Google Scholar]
- 97.Rivkees SA, el-Hajj-Fuleihan G, Brown EM, et al. Tertiary hyperparathyroidism during high phosphate therapy of familial hypophosphatemic rickets. J Clin Endocrinol Metab. 1992;75:1514–1518. doi: 10.1210/jcem.75.6.1464657. [DOI] [PubMed] [Google Scholar]
- 98.Seikaly MG, Brown R, Baum M. The effect of recombinant human growth hormone in children with X-linked hypophosphatemia. Pediatrics. 1997;100:879–884. doi: 10.1542/peds.100.5.879. [DOI] [PubMed] [Google Scholar]
- 99.Lorenz-Depiereux B, Bastepe M, Benet-Pages A, et al. DMP1 mutations in autosomal recessive hypophosphatemia implicate a bone matrix protein in the regulation of phosphate homeostasis. Nat Genet. 2006;38:1248–1250. doi: 10.1038/ng1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Feng JQ, Ward LM, Liu S, et al. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet. 2006;38:1310–1315. doi: 10.1038/ng1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Levy-Litan V, Hershkovitz E, Avizov L, et al. Autosomal-recessive hypophosphatemic rickets is associated with an inactivation mutation in the ENPP1 gene. Am J Hum Genet. 86:273–278. doi: 10.1016/j.ajhg.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lorenz-Depiereux B, Schnabel D, Tiosano D, et al. Loss-of-function ENPP1 mutations cause both generalized arterial calcification of infancy and autosomal-recessive hypophosphatemic rickets. Am J Hum Genet. 86:267–272. doi: 10.1016/j.ajhg.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.von Marschall Z, Fisher LW. Dentin matrix protein-1 isoforms promote differential cell attachment and migration. J Biol Chem. 2008;283:32730–32740. doi: 10.1074/jbc.M804283200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yuan B, Meudt J, Feng JO, et al. 7B2 protein mediated inhibition of DMP1 cleavage in osteoblasts enhances FGF-23 production in hyp-mice. JBMR. 2008;23:s16. (abstract 1053) [Google Scholar]
- 105.Lu Y, Qin C, Xie Y, et al. Studies of the DMP1 57-kDa functional domain both in vivo and in vitro. Cells Tissues Organs. 2009;189:175–185. doi: 10.1159/000151727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Terkeltaub R. Physiologic and pathologic functions of the NPP nucleotide pyrophosphatase/phosphodiesterase family focusing on NPP1 in calcification. Purinergic Signal. 2006;2:371–377. doi: 10.1007/s11302-005-5304-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rutsch F, Vaingankar S, Johnson K, et al. PC-1 nucleoside triphosphate pyrophosphohydrolase deficiency in idiopathic infantile arterial calcification. Am J Pathol. 2001;158:543–554. doi: 10.1016/S0002-9440(10)63996-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rutsch F, Ruf N, Vaingankar S, et al. Mutations in ENPP1 are associated with ‘idiopathic’ infantile arterial calcification. Nat Genet. 2003;34:379–381. doi: 10.1038/ng1221. [DOI] [PubMed] [Google Scholar]
- 109.Ramjan KA, Roscioli T, Rutsch F, et al. Generalized arterial calcification of infancy: treatment with bisphosphonates. Nat Clin Pract Endocrinol Metab. 2009;5:167–172. doi: 10.1038/ncpendmet1067. [DOI] [PubMed] [Google Scholar]
- 110.Chodirker BN, Evans JA, Seargeant LE, et al. Hyperphosphatemia in infantile hypophosphatasia: implications for carrier diagnosis and screening. Am J Hum Genet. 1990;46:280–285. [PMC free article] [PubMed] [Google Scholar]
- 111.Jones A, Tzenova J, Frappier D, et al. Hereditary hypophosphatemic rickets with hypercalciuria is not caused by mutations in the Na/Pi cotransporter NPT2 gene. J Am Soc Nephrol. 2001;12:507–514. doi: 10.1681/ASN.V123507. [DOI] [PubMed] [Google Scholar]
- 112.Tenenhouse HS, Econs MJ. Mendelian Hypophosphatemias. In: Scriver CR, Beaudet AL, Valle D, Sly WS, Vogelstein B, Childs B, Kinzler KW, editors. The Metabolic and Molecular Bases of Inherited Diseases. 8. McGraw-Hill; New York: 2001. pp. 5039–5067. [Google Scholar]
- 113.Lorenz-Depiereux B, Benet-Pages A, Eckstein G, et al. Hereditary Hypophosphatemic Rickets with Hypercalciuria Is Caused by Mutations in the Sodium-Phosphate Cotransporter Gene SLC34A3. Am J Hum Genet. 2006;78:193–201. doi: 10.1086/499410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bergwitz C, Roslin NM, Tieder M, et al. SLC34A3 Mutations in Patients with Hereditary Hypophosphatemic Rickets with Hypercalciuria Predict a Key Role for the Sodium-Phosphate Cotransporter NaPi-IIc in Maintaining Phosphate Homeostasis. Am J Hum Genet. 2006;78:179–192. doi: 10.1086/499409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ichikawa S, Sorenson AH, Imel EA, et al. Intronic Deletions in the SLC34A3 Gene Cause Hereditary Hypophosphatemic Rickets with Hypercalciuria. J Clin Endocrinol Metab. 2006;91:4022–4027. doi: 10.1210/jc.2005-2840. [DOI] [PubMed] [Google Scholar]
- 116.Jaureguiberry G, Carpenter TO, Forman S, et al. A novel missense mutation in SLC34A3 that causes hereditary hypophosphatemic rickets with hypercalciuria in humans identifies threonine 137 as an important determinant of sodium-phosphate cotransport in NaPi-IIc. Am J Physiol Renal Physiol. 2008;295:F371–F379. doi: 10.1152/ajprenal.00090.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Prie D, Huart V, Bakouh N, et al. Nephrolithiasis and osteoporosis associated with hypophosphatemia caused by mutations in the type 2a sodium-phosphate cotransporter. N Engl J Med. 2002;347:983–991. doi: 10.1056/NEJMoa020028. [DOI] [PubMed] [Google Scholar]
- 118.Lapointe JY, Tessier J, Paquette Y, et al. NPT2a gene variation in calcium nephrolithiasis with renal phosphate leak. Kidney Int. 2006;69:2261–2267. doi: 10.1038/sj.ki.5000437. [DOI] [PubMed] [Google Scholar]
- 119.Virkki LV, Forster IC, Hernando N, et al. Functional characterization of two naturally occurring mutations in the human sodium-phosphate cotransporter type IIa. J Bone Miner Res. 2003;18:2135–2141. doi: 10.1359/jbmr.2003.18.12.2135. [DOI] [PubMed] [Google Scholar]
- 120.Karim Z, Gerard B, Bakouh N, et al. NHERF1 mutations and responsiveness of renal parathyroid hormone. N Engl J Med. 2008;359:1128–1135. doi: 10.1056/NEJMoa0802836. [DOI] [PubMed] [Google Scholar]
- 121.Bergwitz C, Bastepe M. NHERF1 Mutations and Responsiveness of Renal Parathyroid Hormone. NEJM. 2008;359:2615–2617. doi: 10.1056/NEJMc086284. [DOI] [PubMed] [Google Scholar]
- 122.Tieder M, Arie R, Modai D, et al. Elevated serum 1,25-dihydroxyvitamin D concentrations in siblings with primary Fanconi’s syndrome. N Engl J Med. 1988;319:845–849. doi: 10.1056/NEJM198809293191307. [DOI] [PubMed] [Google Scholar]
- 123.Magen D, Berger L, Coady MJ, et al. A loss-of-function mutation in NaPi-IIa and renal Fanconi’s syndrome. N Engl J Med. 2010;362:1102–1109. doi: 10.1056/NEJMoa0905647. [DOI] [PubMed] [Google Scholar]
- 124.Beck L, Karaplis AC, Amizuka N, et al. Targeted inactivation of Npt2 in mice leads to severe renal phosphate wasting, hypercalciuria and skeletal abnormalities. Proc Natl Acad Sci USA. 1998;95:5372–5377. doi: 10.1073/pnas.95.9.5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Iwaki T, Sandoval-Cooper MJ, Tenenhouse HS, et al. A missense mutation in the sodium phosphate cotransporter Slc34a1 impairs phosphate homeostasis. J Am Soc Nephrol. 2008;19:1753–1762. doi: 10.1681/ASN.2007121360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Beighton P, Cremin BJ, Kozlowski K. Osteoglophonic dwarfism. Pediatr Radiol. 1980;10:46–50. doi: 10.1007/BF01644343. [DOI] [PubMed] [Google Scholar]
- 127.Sklower Brooks S, Kassner G, Qazi Q, et al. Osteoglophonic dysplasia: review and further delineation of the syndrome. Am J Med Genet. 1996;66:154–162. doi: 10.1002/(SICI)1096-8628(19961211)66:2<154::AID-AJMG6>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 128.White KE, Cabral JM, Davis SI, et al. Mutations that cause osteoglophonic dysplasia define novel roles for FGFR1 in bone elongation. Am J Hum Genet. 2005;76:361–367. doi: 10.1086/427956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Farrow EG, Davis SI, Mooney SD, et al. Extended mutational analyses of FGFR1 in osteoglophonic dysplasia. Am J Med Genet A. 2006;140:537–539. doi: 10.1002/ajmg.a.31106. [DOI] [PubMed] [Google Scholar]
- 130.Maroteaux P, Stanescu V, Stanescu R, et al. Opsismodysplasia: a new type of chondrodysplasia with predominant involvement of the bones of the hand and the vertebrae. Am J Med Genet. 1984;19:171–182. doi: 10.1002/ajmg.1320190117. [DOI] [PubMed] [Google Scholar]
- 131.Zeger MD, Adkins D, Fordham LA, et al. Hypophosphatemic rickets in opsismodysplasia. J Pediatr Endocrinol Metab. 2007;20:79–86. doi: 10.1515/jpem.2007.20.1.79. [DOI] [PubMed] [Google Scholar]
- 132.Solomon LM, Fretzin DF, Dewald RL. The epidermal nevus syndrome. Arch Dermatol. 1968;97:273–285. [PubMed] [Google Scholar]
- 133.Gellis SS, Feingold M. Linear nevus sebaceous syndrome. Am J Dis Child. 1970;120:139–140. [PubMed] [Google Scholar]
- 134.Rogers M. Epidermal nevi and the epidermal nevus syndromes: a review of 233 cases. Pediatr Dermatol. 1992;9:342–344. doi: 10.1111/j.1525-1470.1992.tb00623.x. [DOI] [PubMed] [Google Scholar]
- 135.Menascu S, Donner EJ. Linear nevus sebaceous syndrome: case reports and review of the literature. Pediatr Neurol. 2008;38:207–210. doi: 10.1016/j.pediatrneurol.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 136.Hafner C, van Oers JM, Vogt T, et al. Mosaicism of activating FGFR3 mutations in human skin causes epidermal nevi. J Clin Invest. 2006;116:2201–2207. doi: 10.1172/JCI28163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Skovby F, Svejgaard E, Moller J. Hypophosphatemic rickets in linear sebaceous nevus sequence. J Pediatr. 1987;111:855–857. doi: 10.1016/s0022-3476(87)80204-4. [DOI] [PubMed] [Google Scholar]
- 138.Zutt M, Strutz F, Happle R, et al. Schimmelpenning-Feuerstein-Mims syndrome with hypophosphatemic rickets. Dermatology. 2003;207:72–76. doi: 10.1159/000070948. [DOI] [PubMed] [Google Scholar]
- 139.Hoffman WH, Jüppner HW, Deyoung BR, et al. Elevated fibroblast growth factor-23 in hypophosphatemic linear nevus sebaceous syndrome. Am J Med Genet A. 2005;134:233–236. doi: 10.1002/ajmg.a.30599. [DOI] [PubMed] [Google Scholar]
- 140.John M, Shah NS. Hypophosphatemic rickets with epidermal nevus syndrome. Indian Pediatr. 2005;42:611–612. [PubMed] [Google Scholar]
- 141.Hoffman WH, Jain A, Chen H, et al. Matrix extracellular phosphoglycoprotein (MEPE) correlates with serum phosphorus prior to and during octreotide treatment and following excisional surgery in hypophosphatemic linear sebaceous nevus syndrome. Am J Med Genet A. 2008;146A:2164–2168. doi: 10.1002/ajmg.a.32395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Schwindinger WF, Francomano CA, Levine MA. Identification of a mutation in the gene encoding the alpha subunit of the stimulatory G protein of adenylyl cyclase in McCune-Albright syndrome. Proc Natl Acad Sci USA. 1992;89:5152–5156. doi: 10.1073/pnas.89.11.5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Weinstein LS, Shenker A, Gejman PV, et al. Activating mutations of the stimulatory G protein in the McCune-Albright syndrome. New Engl J Med. 1991;325:1688–1695. doi: 10.1056/NEJM199112123252403. [DOI] [PubMed] [Google Scholar]
- 144.Weinstein L, Yu S, Warner D, et al. Endocrine Manifestations of Stimulatory G Protein alpha-Subunit Mutations and the Role of Genomic Imprinting. Endocr Rev. 2001;22:675–705. doi: 10.1210/edrv.22.5.0439. [DOI] [PubMed] [Google Scholar]
- 145.Yamamoto T, Miyamoto KI, Ozono K, et al. Hypophosphatemic rickets accompanying McCune-Albright syndrome: evidence that a humoral factor causes hypophosphatemia. J Bone Miner Metab. 2001;19:287–295. doi: 10.1007/s007740170012. [DOI] [PubMed] [Google Scholar]
- 146.Riminucci M, Collins MT, Fedarko NS, et al. FGF-23 in fibrous dysplasia of bone and its relationship to renal phosphate wasting. J Clin Invest. 2003;112:683–692. doi: 10.1172/JCI18399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Collins MT, Chebli C, Jones J, et al. Renal phosphate wasting in fibrous dysplasia of bone is part of a generalized renal tubular dysfunction similar to that seen in tumor-induced osteomalacia. J Bone Miner Res. 2001;16:806–813. doi: 10.1359/jbmr.2001.16.5.806. [DOI] [PubMed] [Google Scholar]
- 148.Kobayashi K, Imanishi Y, Koshiyama H, et al. Expression of FGF23 is correlated with serum phosphate level in isolated fibrous dysplasia. Life Sci. 2006;78:2295–2301. doi: 10.1016/j.lfs.2005.09.052. [DOI] [PubMed] [Google Scholar]
- 149.Brown WW, Jüppner H, Langman CB, et al. Hypophosphatemia with elevations in serum fibroblast growth factor 23 in a child with Jansen’s metaphyseal chondrodysplasia. J Clin Endocrinol Metab. 2009;94:17–20. doi: 10.1210/jc.2008-0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Haviv YS, Silver J. Late onset oncogenic osteomalacia-associated with neurofibromatosis type II. Clin Nephrol. 2000;54:429–430. [PubMed] [Google Scholar]
- 151.Konishi K, Nakamura M, Yamakawa H, et al. Hypophosphatemic osteomalacia in von Recklinghausen neurofibromatosis. Am J Med Sci. 1991;301:322–328. doi: 10.1097/00000441-199105000-00006. [DOI] [PubMed] [Google Scholar]
- 152.Giard JM. Sur la calcifcation hibernale. Compes Rend Seanes Soc Biol So. 1998;34:1013–1015. [Google Scholar]
- 153.Duret MH. Tumeurs multiples et singulieres des bourses sereuses. Bull Mem Soc Ant Paris. 1899;74:725–731. [Google Scholar]
- 154.Sitara D, Kim S, Razzaque MS, et al. Genetic evidence of serum phosphate-independent functions of FGF-23 on bone. PLoS Genet. 2008;4:e1000154. doi: 10.1371/journal.pgen.1000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Martin A, David V, Laurence JS, et al. Degradation of MEPE, DMP1 and release of SIBLING ASARM-peptides (minhibins): ASARM-peptide(s) are directly responsible for defective mineralization in HYP. Endocrinology. 2008;149:1757–1772. doi: 10.1210/en.2007-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ichikawa S, Lyles KW, Econs MJ. A novel GALNT3 mutation in a pseudoautosomal dominant form of tumoral calcinosis: evidence that the disorder is autosomal recessive. J Clin Endocrinol Metab. 2005;90:2420–2423. doi: 10.1210/jc.2004-2302. [DOI] [PubMed] [Google Scholar]
- 157.Frishberg Y, Topaz O, Bergman R, et al. Identification of a recurrent mutation in GALNT3 demonstrates that hyperostosis-hyperphosphatemia syndrome and familial tumoral calcinosis are allelic disorders. J Mol Med. 2005;83:33–38. doi: 10.1007/s00109-004-0610-8. [DOI] [PubMed] [Google Scholar]
- 158.Frishberg Y, Araya K, Rinat C, Sprecher E, et al. Hyperostosis-hyperphosphatemia syndrome caused by mutations in GALNT3 and associated with augmented processing of FGF-23. American Society of Nephrology; Philadelphia: 2004. p. F-P0937. [Google Scholar]
- 159.Benet-Pages A, Orlik P, Strom TM, et al. An FGF23 missense mutation causes familial tumoral calcinosis with hyperphosphatemia. Hum Mol Genet. 2005;14:385–390. doi: 10.1093/hmg/ddi034. [DOI] [PubMed] [Google Scholar]
- 160.Masi L, Gozzini A, Carbonell S, et al. A novel recessive mutation in fibroblast growth factor-23 (FGF23) causes a tumoral calcinosis. J Bone Miner Res. 2005:S128. [Google Scholar]
- 161.Garringer HJ, Malekpour M, Esteghamat F, et al. Molecular genetic and biochemical analyses of FGF23 mutations in familial tumoral calcinosis. Am J Physiol Endocrinol Metab. 2008;295:E929–E937. doi: 10.1152/ajpendo.90456.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Shimada T, Kakitani M, Yamazaki Y, et al. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest. 2004;113:561–568. doi: 10.1172/JCI19081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hesse M, Fröhlich LF, Zeitz U, et al. Ablation of vitamin D signaling rescues bone, mineral and glucose homeostasis in Fgf-23 deficient mice. Matrix Biol. 2007;26:75–84. doi: 10.1016/j.matbio.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 164.Ichikawa S, Sorenson AH, Austin AM, et al. Ablation of the Galnt3 gene leads to low-circulating intact fibroblast growth factor 23 (Fgf23) concentrations and hyperphosphatemia despite increased Fgf23 expression. Endocrinology. 2009;150:2543–2550. doi: 10.1210/en.2008-0877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Razzaque MS, Sitara D, Taguchi T, et al. Premature aging-like phenotype in fibroblast growth factor 23 null mice is a vitamin D-mediated process. Faseb J. 2006;20:720–722. doi: 10.1096/fj.05-5432fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ohnishi M, Nakatani T, Lanske B, et al. Reversal of mineral ion homeostasis and soft-tissue calcification of klotho knockout mice by deletion of vitamin D 1alpha-hydroxylase. Kidney Int. 2009;75:1166–1172. doi: 10.1038/ki.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Stubbs JR, Liu S, Tang W, et al. Role of hyperphosphatemia and 1,25-dihydroxyvitamin d in vascular calcification and mortality in fibroblastic growth factor 23 null mice. J Am Soc Nephrol. 2007;18:2116–2124. doi: 10.1681/ASN.2006121385. [DOI] [PubMed] [Google Scholar]
- 168.Liu S, Zhou J, Tang W, et al. Pathogenic role of Fgf23 in Hyp mice. Am J Physiol Endocrinol Metab. 2006;291:E38–E49. doi: 10.1152/ajpendo.00008.2006. [DOI] [PubMed] [Google Scholar]
- 169.Yamaguchi T, Sugimoto T, Imai Y, et al. Successful treatment of hyperphosphatemic tumoral calcinosis with long-term acetazolamide. Bone. 1995;16(4 Suppl):247S–250S. doi: 10.1016/8756-3282(95)00019-a. [DOI] [PubMed] [Google Scholar]
- 170.Lufkin EG, Wilson DM, Smith LH, et al. Phosphorus excretion in tumoral calcinosis: response to parathyroid hormone and acetazolamide. J Clin Endocrinol Metab. 1980;50:648–653. doi: 10.1210/jcem-50-4-648. [DOI] [PubMed] [Google Scholar]
- 171.Gowen M, Stroup GB, Dodds RA, et al. Antagonizing the parathyroid calcium receptor stimulates parathyroid hormone secretion and bone formation in osteopenic rats. J Clin Invest. 2000;105:1595–1604. doi: 10.1172/JCI9038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Bergwitz C, Banerjee S, Abu-Zahra H, et al. Defective O-glycosylation due to a novel homozygous S129P mutation is associated with lack of fibroblast growth factor 23 secretion and tumoral calcinosis. J Clin Endocrinol Metab. 2009;94:4267–4274. doi: 10.1210/jc.2009-0961. [DOI] [PMC free article] [PubMed] [Google Scholar]