Abstract
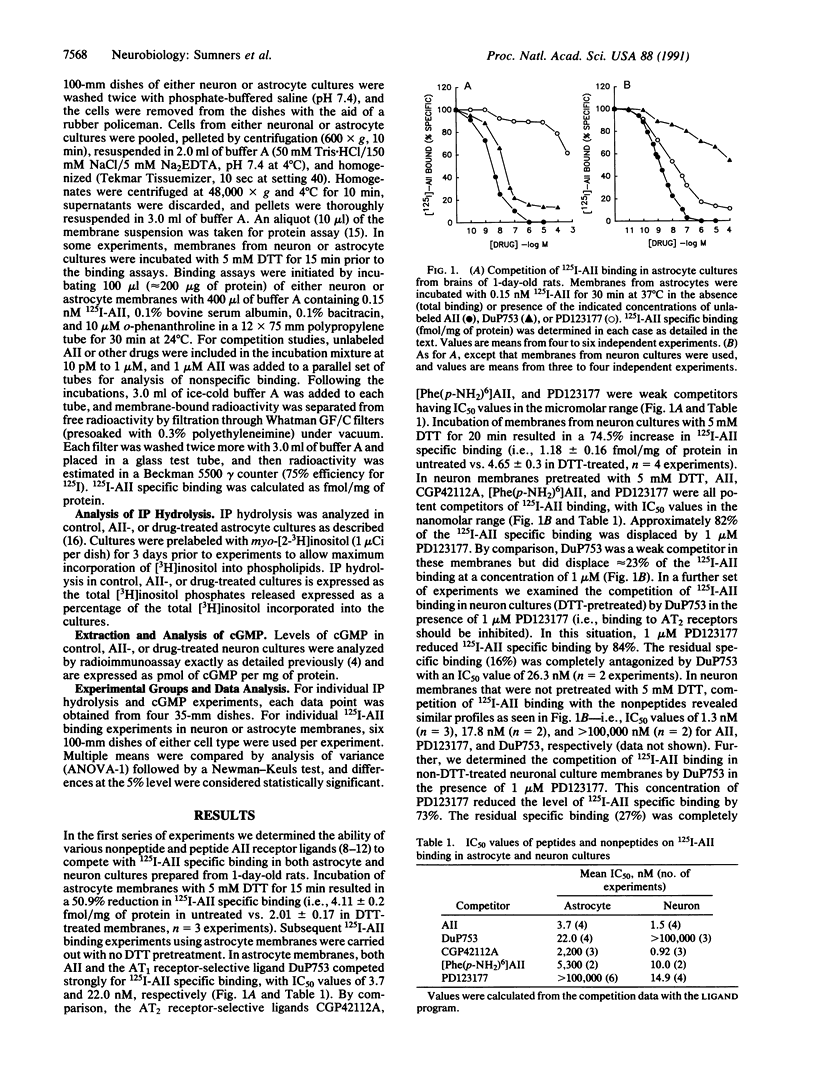
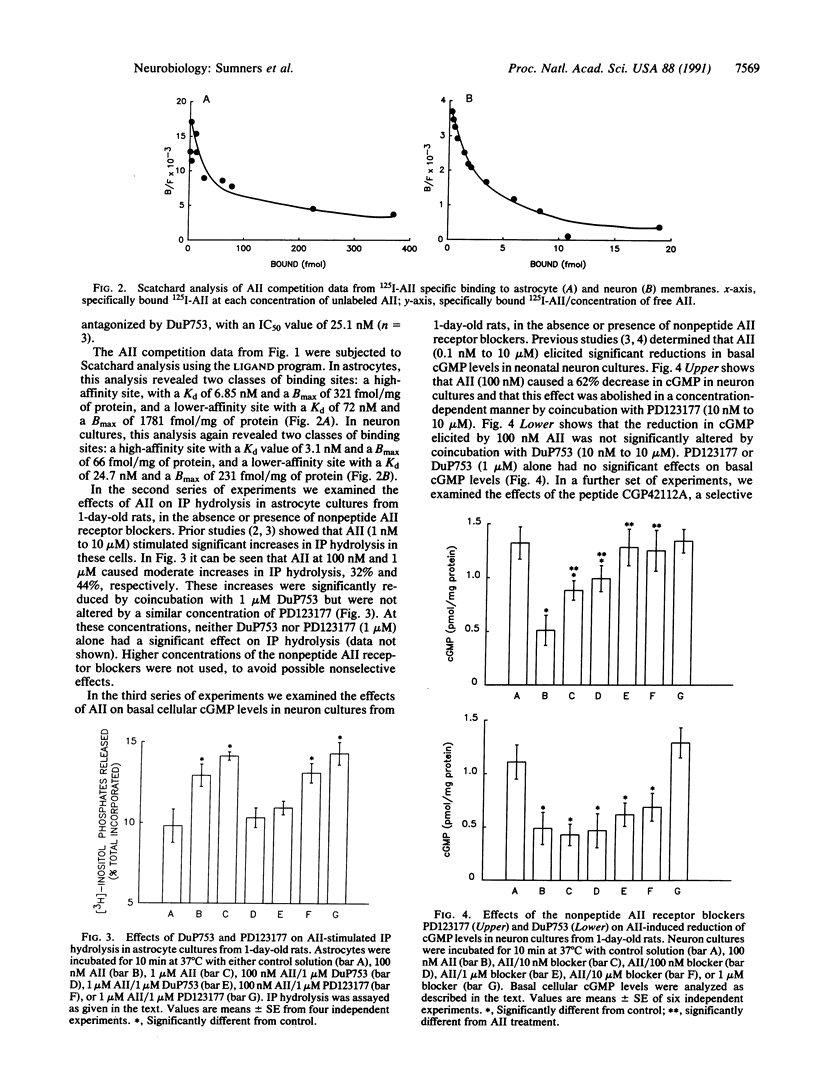
Both neurons and astrocytes contain specific receptors for angiotensin II (AII). We used selective ligands for the AT1 and AT2 types of AII receptors to investigate the expression of functional receptor subtypes in astrocyte cultures and neuron cultures from 1-day-old (neonatal) rat brain. In astrocyte cultures, competition of 125I-labeled AII (125I-AII) specific binding with AT1 (DuP753) or AT2 (PD123177, CGP42112A, [Phe(p-NH2)6]AII) selective receptor ligands revealed a potency series of AII greater than DuP753 much greater than CGP42112A greater than [Phe(p-NH2)6]AII greater than PD123177. These results suggest a predominance of the AT1 receptor subtype in neonatal astrocytes. Also, in astrocyte cultures, AII stimulated increases in inositolphospholipid hydrolysis that were significantly reduced by the AT1 receptor antagonist DuP753 but not altered by the AT2 receptor antagonist PD123177. In neonatal neuron cultures, competition of 125I-AII specific binding with the above ligands revealed a potency series of CGP42112A = AII greater than [Phe(p- NH2)6]AII greater than PD123177 much greater than DuP753. 125I-AII specific binding to neonate neuronal cultures was reduced 73-84% by 1 microM PD123177, and the residual 125I-AII specific binding was eliminated by DuP753. Also, in neuron cultures, AII induced decreases in basal cGMP that were completely blocked by PD123177 or CGP42112A but not by DuP753. Our results suggest that astrocyte cultures from neonatal rat brains contain predominantly AT1 receptors that are coupled to a stimulation of inositophospholipid hydrolysis. In contrast, neuron cultures from neonatal rat brain contain mostly AT2 receptors that are coupled to a reduction in basal cGMP levels, but a smaller population of AT1 receptors is also present in these neurons.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chang R. S., Lotti V. J. Two distinct angiotensin II receptor binding sites in rat adrenal revealed by new selective nonpeptide ligands. Mol Pharmacol. 1990 Mar;37(3):347–351. [PubMed] [Google Scholar]
- Chiu A. T., Herblin W. F., McCall D. E., Ardecky R. J., Carini D. J., Duncia J. V., Pease L. J., Wong P. C., Wexler R. R., Johnson A. L. Identification of angiotensin II receptor subtypes. Biochem Biophys Res Commun. 1989 Nov 30;165(1):196–203. doi: 10.1016/0006-291x(89)91054-1. [DOI] [PubMed] [Google Scholar]
- Chiu A. T., Herblin W. F., McCall D. E., Ardecky R. J., Carini D. J., Duncia J. V., Pease L. J., Wong P. C., Wexler R. R., Johnson A. L. Identification of angiotensin II receptor subtypes. Biochem Biophys Res Commun. 1989 Nov 30;165(1):196–203. doi: 10.1016/0006-291x(89)91054-1. [DOI] [PubMed] [Google Scholar]
- Chiu A. T., McCall D. E., Nguyen T. T., Carini D. J., Duncia J. V., Herblin W. F., Uyeda R. T., Wong P. C., Wexler R. R., Johnson A. L. Discrimination of angiotensin II receptor subtypes by dithiothreitol. Eur J Pharmacol. 1989 Oct 24;170(1-2):117–118. doi: 10.1016/0014-2999(89)90145-3. [DOI] [PubMed] [Google Scholar]
- Dudley D. T., Panek R. L., Major T. C., Lu G. H., Bruns R. F., Klinkefus B. A., Hodges J. C., Weishaar R. E. Subclasses of angiotensin II binding sites and their functional significance. Mol Pharmacol. 1990 Sep;38(3):370–377. [PubMed] [Google Scholar]
- Gehlert D. R., Gackenheimer S. L., Reel J. K., Lin H. S., Steinberg M. I. Non-peptide angiotensin II receptor antagonists discriminate subtypes of 125I-angiotensin II binding sites in the rat brain. Eur J Pharmacol. 1990 Oct 2;187(1):123–126. doi: 10.1016/0014-2999(90)90348-a. [DOI] [PubMed] [Google Scholar]
- Koepke J. P., Bovy P. R., McMahon E. G., Olins G. M., Reitz D. B., Salles K. S., Schuh J. R., Trapani A. J., Blaine E. H. Central and peripheral actions of a nonpeptidic angiotensin II receptor antagonist. Hypertension. 1990 Jun;15(6 Pt 2):841–847. doi: 10.1161/01.hyp.15.6.841. [DOI] [PubMed] [Google Scholar]
- MacLean M. R., Raizada M. K., Sumners C. The influence of angiotensin II on catecholamine synthesis in neuronal cultures from rat brain. Biochem Biophys Res Commun. 1990 Mar 16;167(2):492–497. doi: 10.1016/0006-291x(90)92050-a. [DOI] [PubMed] [Google Scholar]
- Millan M. A., Carvallo P., Izumi S., Zemel S., Catt K. J., Aguilera G. Novel sites of expression of functional angiotensin II receptors in the late gestation fetus. Science. 1989 Jun 16;244(4910):1340–1342. doi: 10.1126/science.2734613. [DOI] [PubMed] [Google Scholar]
- Myers L. M., Raizada M. K., Sumners C. Effects of phorbol esters and a calcium ionophore on angiotensin II binding in rat brain synaptosomes. Neurochem Res. 1989 Jan;14(1):25–30. doi: 10.1007/BF00969753. [DOI] [PubMed] [Google Scholar]
- Myers L. M., Sumners C. Regulation of angiotensin II binding sites in neuronal cultures by catecholamines. Am J Physiol. 1989 Oct;257(4 Pt 1):C706–C713. doi: 10.1152/ajpcell.1989.257.4.C706. [DOI] [PubMed] [Google Scholar]
- Olson J. A., Jr, Shiverick K. T., Ogilvie S., Buhi W. C., Raizada M. K. Angiotensin II induces secretion of plasminogen activator inhibitor 1 and a tissue metalloprotease inhibitor-related protein from rat brain astrocytes. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1928–1932. doi: 10.1073/pnas.88.5.1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeilschifter J. Angiotensin II B-type receptor mediates phosphoinositide hydrolysis in mesangial cells. Eur J Pharmacol. 1990 Aug 2;184(1):201–202. doi: 10.1016/0014-2999(90)90684-x. [DOI] [PubMed] [Google Scholar]
- Raizada M. K., Muther T. F., Sumners C. Increased angiotensin II receptors in neuronal cultures from hypertensive rat brain. Am J Physiol. 1984 Nov;247(5 Pt 1):C364–C372. doi: 10.1152/ajpcell.1984.247.5.C364. [DOI] [PubMed] [Google Scholar]
- Raizada M. K., Phillips M. I., Crews F. T., Sumners C. Distinct angiotensin II receptor in primary cultures of glial cells from rat brain. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4655–4659. doi: 10.1073/pnas.84.13.4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe B. P., Grove K. L., Saylor D. L., Speth R. C. Angiotensin II receptor subtypes in the rat brain. Eur J Pharmacol. 1990 Sep 21;186(2-3):339–342. doi: 10.1016/0014-2999(90)90457-h. [DOI] [PubMed] [Google Scholar]
- Speth R. C., Kim K. H. Discrimination of two angiotensin II receptor subtypes with a selective agonist analogue of angiotensin II, p-aminophenylalanine6 angiotensin II. Biochem Biophys Res Commun. 1990 Jun 29;169(3):997–1006. doi: 10.1016/0006-291x(90)91993-3. [DOI] [PubMed] [Google Scholar]
- Sumners C., Myers L. M. Angiotensin II decreases cGMP levels in neuronal cultures from rat brain. Am J Physiol. 1991 Jan;260(1 Pt 1):C79–C87. doi: 10.1152/ajpcell.1991.260.1.C79. [DOI] [PubMed] [Google Scholar]
- Sumners C., Myers L. M., Kalberg C. J., Raizada M. K. Physiological and pharmacological comparisons of angiotensin II receptors in neuronal and astrocyte glial cultures. Prog Neurobiol. 1990;34(5):355–385. doi: 10.1016/0301-0082(90)90032-c. [DOI] [PubMed] [Google Scholar]
- Sumners C., Shalit S. L., Kalberg C. J., Raizada M. K. Norepinephrine metabolism in neuronal cultures is increased by angiotensin II. Am J Physiol. 1987 Jun;252(6 Pt 1):C650–C656. doi: 10.1152/ajpcell.1987.252.6.C650. [DOI] [PubMed] [Google Scholar]
- Tsutsumi K., Saavedra J. M. Increased dithiothreitol-insensitive, type 2 angiotensin II receptors in selected brain areas of young rats. Cell Mol Neurobiol. 1991 Apr;11(2):295–299. doi: 10.1007/BF00769042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsumi K., Saavedra J. M. Quantitative autoradiography reveals different angiotensin II receptor subtypes in selected rat brain nuclei. J Neurochem. 1991 Jan;56(1):348–351. doi: 10.1111/j.1471-4159.1991.tb02602.x. [DOI] [PubMed] [Google Scholar]
- Wamsley J. K., Herblin W. F., Alburges M. E., Hunt M. Evidence for the presence of angiotensin II-type 1 receptors in brain. Brain Res Bull. 1990 Sep;25(3):397–400. doi: 10.1016/0361-9230(90)90226-p. [DOI] [PubMed] [Google Scholar]
- Wong P. C., Hart S. D., Zaspel A. M., Chiu A. T., Ardecky R. J., Smith R. D., Timmermans P. B. Functional studies of nonpeptide angiotensin II receptor subtype-specific ligands: DuP 753 (AII-1) and PD123177 (AII-2). J Pharmacol Exp Ther. 1990 Nov;255(2):584–592. [PubMed] [Google Scholar]



