Abstract
In this review, I consider the varied mechanisms in cortical bone that help preserve its integrity and how they deteriorate with aging. Aging affects cortical bone in two ways: extrinsically through its effects on the individual that modify its mechanical loading experience and ‘milieu interieur'; and intrinsically through the prolonged cycle of remodelling and renewal extending to an estimated 20 years in the proximal femur. Healthy femoral cortex incorporates multiple mechanisms that help prevent fracture. These have been described at multiple length scales from the individual bone mineral crystal to the scale of the femur itself and appear to operate hierarchically. Each cortical bone fracture begins as a sub-microscopic crack that enlarges under mechanical load, for example, that imposed by a fall. In these conditions, a crack will enlarge explosively unless the cortical bone is intrinsically tough (the opposite of brittle). Toughness leads to microscopic crack deflection and bridging and may be increased by adequate regulation of both mineral crystal size and the heterogeneity of mineral and matrix phases. The role of osteocytes in optimising toughness is beginning to be worked out; but many osteocytes die in situ without triggering bone renewal over a 20-year cycle, with potential for increasing brittleness. Furthermore, the superolateral cortex of the proximal femur thins progressively during life, so increasing the risk of buckling during a fall. Besides preserving or increasing hip BMD, pharmaceutical treatments have class-specific effects on the toughness of cortical bone, although dietary and exercise-based interventions show early promise.
Introduction
Recently, we reviewed the data relevant to why the adult risk of hip fracture in economically advanced countries grows 10-fold for every 20-year increase in a person's age.1 It was argued that as has been appreciated since 1988,2 measurements of bone density made in two dimensions or three failed to account for the rapidity of this increase; and that many of the hip-weakening mechanisms revealed in the last half century of research were more closely associated with aging, either specifically of bone tissue or more generally of the body as a whole, rather than with net loss of bone tissue, that is, clinical osteoporosis. By this term, I refer to the now standard definition of loss of bone at the femoral neck measured with clinical DXA technology exceeding 2.5 SDs with reference to young normal values.
In view of the many potentially relevant fragility mechanisms, it is now interesting to explore the effects of both age and osteoporosis on the mechanical properties of cortical bone, in light of the known and growing potential of lifestyle choices and licensed pharmacological agents to impede or reverse the deterioration in its material or structural properties. Much current research focuses on the amount and distribution of bone tissue in the proximal femur without considering the effects of the passage of time on the material properties of the bone tissue. Alternatively, it does the reverse. Although this focus on individual elements of inter-connected toughening mechanisms is necessary, it is argued here that an exclusively compartmentalised approach limits our understanding of the general phenomenon of the fragility of the elderly femur, made worse by the absence of good animal models. Here I address both loss of cortical bone tissue as the human body ages and its changing mechanical nature in the context of potential remedies.
The Varied Functions of Cortical Bone
Cortical bone serves three principal mechanical functions: as a foundation for cartilage within joints; as the material basis for mechanical leverage; and as a surface for the attachment of muscles and ligaments that transfers and dissipates the mechanical loads they generate. A fourth function is to encase the bone marrow. The three mechanical functions require different specifications, but if these are compromised the likelihood of hip fracture is increased during trauma and the location of a fracture may be influenced.
The Mechanical Requirements of Cortical Bone in the Proximal Femur
Under normal conditions, cortical bone tissue deforms under a mechanical load and regains its previous anatomical form when that load is removed. In simple terms, the elasticity of cortical bone tissue is a measure of resistance to deformation that is reversible and can be quantitated by its modulus (Young's modulus).
In the case of sub-cartilaginous cortical bone, as found inside the hip joint, it is thin and supported by a network of trabecular bone. Locally thickened cortical bone is associated with osteoarthritis,3 but is of somewhat reduced density since its turnover rate is increased and the role of cortical bone in the pathogenesis of osteoarthritis remains unclear.4,5 In contrast, the femoral shaft must be relatively stiff, with a high modulus, in deference to the need to run fast and the limited ability of mature bone tissue to deform without developing an irreversible change in shape. This demands a high bending resistance to which a thick cortex contributes.
When a lower elastic modulus is required than is found in the cortical shaft of the femur, the evolutionary solution is to locate a layer of cortical bone external to and strongly connected to a foundation of trabecular bone.6 Sometimes this type of cortical bone is of comparable thickness to many of its associated trabeculae. Depending on its microscopic arrangement, as well as the thickness of the cortical component, the combined trabecular-cortical bone structure, for example, in the vertebrae where loads associated with locomotion need to be cushioned, can provide a range of elastic moduli at different distances from the inter-vertebral disc or from cartilage. In the proximal femur, to achieve varying mechanical requirements, near the hip joint the cortex is relatively thin and has a well-developed trabecular foundation; while at the mid-shaft where no reduction of modulus is desirable, the cortical bone is thicker and no trabecular bone is found. From the hip joint capsule distally, the ratio of trabecular to cortical bone declines progressively until in the sub-trochanteric region the proportion of trabecular bone becomes very small.
In this review, I examine the biological mechanisms associated with the maintenance and subsequent loss of a satisfactory mechanical specification for cortical bone in the femur.
Age-related Osteoporosis and Cortical Bone
The term cortical osteoporosis has evolved to include mechanically inappropriate cortical thinning as well as excessive porosity. In the femur, sub-periosteal bone formation continues throughout life as does endosteal resorption, leading in adulthood to a gradually expanding bony envelope.7 This age-related expansion can maintain bending resistance even as the clinical measure of osteoporosis, a bone mineral density T-score reading of <−2.5, is approached.8
Cortical Bone as a Tough Material
Toughness, the obverse of brittleness, is a property indicating high resistance to complete rupture even when a material is subjected to a mechanical load that results in permanent deformity. Almost a century ago, Griffiths pondered the brittleness of some materials like plate glass that can shatter when loads are applied that are many orders of magnitude lower than the weakest of the atomic bonds within them.9 It is now accepted that solid materials do not experience a uniform distribution of load at the molecular level: instead applied loads are highly concentrated in microscopic locations, which can then experience a microcrack through the locally applied forces being sufficient to overcome the local atomic bonds. Once a sub-microscopic crack has initiated, within brittle materials it accelerates under ongoing load to grow into a macrocrack. Conversely, tough materials like normal bone are resistant to crack growth.
Microscopic mechanisms at the nanoscale that underpin toughness depend on bone's composite nature. Bone as a material is formed of platelet-shaped crystals arranged in overlapping patterns within a proteinaceous substrate. Recently, it has been shown that citrate in bone has a critical role in anchoring mineral platelets to their collagenous matrix.10 Also, trapped between the platelets are many water molecules.10 The ratio of length to thickness in mineral platelets (their ‘aspect ratio') is critical in delivering the stiffness needed in properly functioning cortical bone. Young adult bone tissue achieves toughness both through limiting the growth of crystal size and through the behavioural properties of the proteinaceous substrate when subjected to shear forces.11 The aqueous and proteinaceous nature of the substrate protects the elongated or high-aspect-ratio mineral crystals from buckling under load. Bone also contains large amounts of lactate with no certain mechanical role. The very recent discovery of the bridging role of citrate has opened up greatly increased opportunities for understanding molecular-level toughening mechanisms and their disorders.
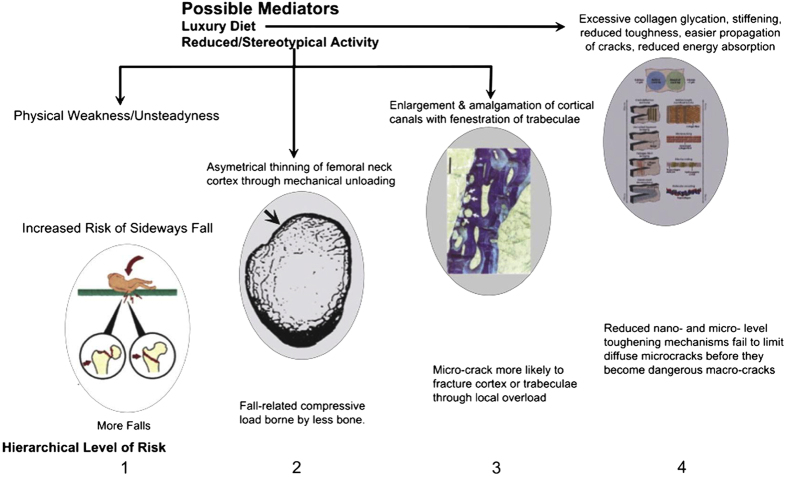
The femur has additional mechanisms at more macroscopic levels to inhibit the development of complete fracture should it be subjected to loads that overcome these molecular-level protective mechanisms. This has led to the evolutionary concept that fracture resistance has developed a hierarchical structure in which there are multiple fail-safe mechanisms operating at different scales of magnitude should a dangerous crack initiate at nanoscale (Figure 1).
Figure 1.
Risk mechanisms arising from aging-related failures in biological protection against hip fracture. These are classified by length scale aligned with the protective mechanisms seen in younger bone. (Scale) Level 1: Failure to avoid sideways falls, involving personal physical decline and societal acceptance of fall risk to preserve personal freedom. Repeated falls might in principle weaken the femoral cortex through the sort of ‘delamination' mechanisms that can occur in man-made composites and making a fracture more likely at the next fall (see text). Level 2: Thinning of the superolateral half of femoral neck cortex leads to risk of its local buckling or crushing under compression in a sideways fall, progressing inevitably to complete intracapsular hip fracture. Level 3: Increased remodelling with simultaneous resorption of several adjacent canals (or trabecular surfaces) can destroy the integrity of bone's microstructure if thin bone structures are fenestrated. Here 4 osteons are arrowed: two are undergoing resorption and are about to amalgamate with two others in the resting phase near the periosteal surface of the femoral neck. The walls between osteons are hard to re-create subsequently and the resulting composite osteons have been postulated to constitute the first phase in the gradual ‘trabecularisation' of the femoral neck cortex with aging.57 Level 4: The multiple toughening mechanisms operating at nano- through microscale are shown in this cartoon from Launey et al.15 to which the reader is referred for a detailed explanation. The known or postulated effects of age-related disease and disabilities to limit the effectiveness of these mechanisms include: exposure to products that glycate collagen, so stiffening it (especially in diabetes mellitus) and, for example, fracturing crack bridges; loss of heterogeneity of mineralisation that might otherwise deflect growing cracks with energy absorption; increased crystallinity that might make some abnormally large apatite crystals vulnerable to fracture; loss with cell death or dysfunction of defined low density structures such as osteocyte canaliculi that deflect cracks; (see text). Figure previously published in Bone.1
Multiple Toughening Mechanisms Delivered at Differing Length Scales
It is evident that to be clinically significant an effect observable at a microscopic level must result in reduced mechanical performance at a more macroscopic scale—immediately or after some delay.12 The most macroscopic and obviously imperfect level of defence against fracture, here called Level 1 (Length scales are ordered from largest to smallest because of the potential need to add further scales at the more microscopic levels), is personal and societal and depends on modifying or adapting to the environment. It is outside the scope of this article. The second level of fracture resistance relates to the architectural structure of the femur as visible by the naked eye. The third level is provided by the femur's microstructure as visualised in conventional bone histomorphometry. Microscopic sculpting at both these length scales in adult life is accomplished by teams of osteoblasts and osteoclasts organised into the multicellular Basic Metabolic Units (BMUs), which create structural units (BSUs), known as osteons in cortical bone, that are ‘glued' together by a collagen-poor osteopontin-containing cement13 that appears as so-called ‘cement-lines' under the microscope. There is circumstantial evidence that at the second level, different BMUs act co-operatively.
Fourth and higher levels of organisation (up to seven have been proposed) are common to the whole skeleton and extend from the micro- through the nanoscales. The reasons normal bone tissue is resistant to crack growth at the more microscopic levels have been recently reviewed by Ritchie and colleagues in this journal14 and elsewhere.15 As a consequence of these mechanisms, human cortical bone accumulates microcracks, each of which is thought to record a damaging loading event that was aborted. Seref-Ferlengez et al.16 discussed the role of microcracks in preserving bones like the femur from fracture and showed that it was over-simplistic to assume that a high density of microcracks reflects a high propensity to fracture. Arguing that the relationship of microcracks to fracture risk is complex, they pointed out that the apparent density of microcracks depends on the rate at which a region of femoral cortex is subjected to loads high enough to initiate microscopic cracking; and inversely on the rate at which cortical bone is remodelled.
The integrity and health of cortical bone is considered critical to preventing fractures, because in a fall onto the femur the cortex generally experiences the highest mechanical loads. But, whether the microcrack whose initiation leads to a complete fracture seems to be most plausibly located within cortical bone17 or subcortical trabecular bone18 remains controversial.
This article now considers level 2 and level 3 toughening mechanisms and their age-related decline leading to increased cortical fragility. New approaches to fracture prevention are then discussed in light of their impact at different length scales.
Organisation Level 2: the Architecture of the Proximal Femur
Since Galileo's time, investigators have pondered the mechanical requirements of the mammalian femur.19 Darwin observed that the heavier wing and weaker leg bones of the wild compared with the domestic duck reflected different patterns of mechanical usage.20 Next, Wolff proposed that bones adapt their internal structure efficiently so as to minimise the effects of the mechanical loads placed upon them.21 This hypothesis requires that in regions exposed to higher than average strains, there is a net imbalance favouring bone formation over resorption.
Compression, for example, in a sideways fall, might cause fracture in other ways than through bending. Bone can disintegrate by crumbling under high compression, although thin or unsupported bony structures may sometimes buckle at rather lower strains. Buckling may explain the collapse of osteoporotic cancellous bone in the spine.22 In a fall onto the greater trochanter, the superolateral cortex, normally loaded weakly in tension, becomes heavily loaded in compression. Because of its thinness in the elderly,23,24,25,26,27 this cortex is interesting for its potential to buckle in a sideways fall.
Forces leading to fracture in bending in the absence of buckling were first estimated from DXA by Yoshikawa et al.28 It was found that the resistance to bending of the hip remains nearly constant during normal aging.29 This contrasts with the fact that, from age 40 years, there is a 10-fold increase in hip fracture risk every 20 years.30 Rapid loss of hip-bending resistance occurs only with the onset of frailty.8 Prior to that, DXA BMD (in g cm−2) typically declines somewhat faster than bending resistance. This is explained by the gradual widening of the femoral neck diameter with age,7 which for algebraic reasons reduces BMD simultaneously with increasing section modulus (a common DXA-derivable measure of bending resistance). Widening of the femoral neck is driven by periosteal bone formation associated with compensatory endosteal resorption31 and is associated with markers of sex hormone deficiency.32 In the absence of an increase in available cortical bone tissue, widening leads to cortical thinning and consequent increases in the risk of a buckle.
The contrast between the thickness of the inferomedial and the thinness of the superolateral femoral neck cortex increases with aging because the inferomedial remains largely unchanged as the superolateral grows thinner.24,25,26 This change has a small effect on bending resistance while increasing the risk of buckling or crushing superolaterally. In a sideways fall, an elderly and thinned superolateral cortex taking the full impact load might fracture through either mechanism.
Architectural changes associated with hip fracture
After matching for age, female cases of hip fracture had substantially reduced DXA-assessed bending resistance compared to controls, equivalent to 2 decades of age-related changes;8,33 but there was substantial between-group overlap. Cases also had thinner34,35 bone cortices. But re-calculated 2D DXA estimates of bending resistance (reflected in Section Modulus) were somewhat36 or little better than BMD in predicting hip fracture.37,38 Computed 3D tomography studies combined with finite element analysis (FEA) are generally superior to 2D DXA for predicting strength.39 In the Age, Gene, Environment Study, Rejkyavik (AGES Study) population-based cohort study, aging of the individual was associated with loss of FEA-assessed strength40 of the hip in both stance and falling configurations and thinning of the superior femoral neck cortex.26 This was more rapid in women than men, while in contrast hip fracture prediction was improved more in men than women. These findings were supported by two case-control studies,35,41 one suggesting that patchy as well as uniform thinning of the superolateral cortex could promote hip fragility.41
The femoral neck cortex and the lesser trochanter contains regions of highly mineralised calcified cartilage, that are anatomically associated with tendon and capsular insertions and appear to grow in relative surface area with age.42 Observationally, periosteum and the bone-cartilage tidemark have been shown to form a continuum anatomically43 and any growth in the proportion of calcified cartilage in the femoral neck with age is likely to degrade its mechanical properties through the inferior strength of calcified cartilage or even through stress-shielding of subcortical trabeculae which are only weakly compressed during slow walking. The extent of calcified cartilage in the cortex has so far not been compared between hip fracture cases and controls.
Localised cortical thinning and fracture mechanics
Cortical thinning is almost certainly relevant to hip fracture in the elderly proximal femur, as far distally as the level of the lesser trochanter. In the sub-trochanteric region, the cortex generally remains too thick for cortical thinning alone to be a primary cause of fracture. Fast cinematographic investigation of experimental (ex vivo) hip fracture has shown that the initiating crack in a basi-cervical hip fracture frequently begins in the superolateral cortex.17 It can be shown mathematically that section modulus of the femoral neck collapses suddenly once the crack becomes macroscopic; the inferomedial cortex, no longer braced by the superolateral cortex, then fractures in bending as a secondary event.24 If the initial mode of cortical failure is by buckling not crushing, supporting trabeculae which become reduced locally with age44,45,46 may be critical to cortical integrity because they have a surprisingly large effect to prevent buckling while having little capacity to prevent crushing.44,47,48 It should be noted that the so-called ‘buckling ratio' derived from DXA studies may not be linearly related to the load required to cause buckling in life, since for the two quantities to be so related requires that the femur at the point of measurement is made of homogeneous material of uniform thickness distributed in a circular cross-section.
It is now understood49 that local critical stress, in which both the stresses experienced by a section of femoral neck cortex and its curvature normal to that stress are important determinants, is a measure of resistance to buckling.24,44,50 While in vivo CT-based 3D studies modelled with finite element analysis (FEA) can assess the potential for crushing51 of the thinned superolateral cortex, realistic predictions of cortical buckling, which has an absolute requirement for non-linear modelling and other refinements to avoid unrealistic stress calculations at the individual voxel level, needs to be incorporated into future FEA modelling studies of hip fracture.
Organisation Level 3: the Cortical Osteon and Regulation by its Osteocyte Network
Parfitt estimated that there are 21 × 106 cortical osteons in the human skeleton.52 As life progresses, the older osteons are progressively resorbed in whole or in part through remodelling by newly developing osteons. Collectively, partially resorbed osteons are referred to as interstitial bone. Macromechanical measures of femur strength are partly predictable from cortical porosity and much less predictable from other tests of micromechanical properties of cortical bone.53 Hence it is important to understand how the remodelling osteon achieves or fails to achieve complete refilling.
New osteons within the cortex are created by advancing teams of osteoclasts. In-filling of the spaces created within the cortex typically takes in excess of three months simply because the work rate of osteoblasts is such that removal of bone by osteoclasts is much more rapid than its osteoblastic replacement. This creates a temporary remodelling space that may become permanent if in-filling is incomplete.52 On periosteal and endosteal surfaces, erosion by osteoclasts results in rough or scalloped surfaces that are likely to generate so-called ‘stress risers' that might attract crack formation until they are subsequently smoothed over by osteoblastic bone formation. So much is now well understood; and it is commonly believed that the anti-fracture efficacy of anti-osteoporotic agents that reduce bone formation depends in part on retaining smooth bone surfaces that do not attract crack initiation.
A good deal is now known about the endocrine regulation of the balance between bone formation and resorption and how it interacts with bone cells' intrinsic regulation mechanisms; but less is known about the local detailing of bone modelling and remodelling. Recently, the contrasting effects of local geometry on bone formation in biological models of trabecular or ‘hemi-osteonal' bone formation (in Parfitt's terminology54) and in cortical osteonal bone formation were demonstrated by Bidan et al.55 They derived a plausible explanation for how the ‘trabecularisation' of the bone cortex (that is, excavation and partial resorption of cortical bone to give it a trabecular structure) as seen in growth and old age could become irreversible. The periodic tethering of formative cells to the forming surface was associated in this model with smoothing and in-filling of hemi-osteonal resorption pits of semi-circular cross-section through the generation of tension via ‘actin chords'. This reduces surface curvature. In contrast, osteonal canal surfaces develop increased curvature as their canals narrowed. Extrapolating to in vivo cortical remodelling, where large composite canals are formed in the elderly femoral neck cortex from the merging of neighbouring canals undergoing simultaneous resorption,56,57 surface curvature might be reduced rather than increased, resulting in permanently enlarged spaces much larger than a normal cortical Haversian canal. With repetition, this could create trabecular bone by sculpting it out of previously solid cortex. On the basis of computational modelling of microradiographic scan data, Fernandez et al.58 have postulated that an additional effect of osteocyte loss, which is prevalent in femoral cortical bone in the elderly, is to enlarge Haversian spaces.
The role of osteocytes in orchestrating the remodelling process is gradually becoming clearer but from our current perspective more complex. The young person's osteon or Bone Structural Unit (BSU), contains osteocytes at varying densities estimated with 3D synchrotron radiation techniques as between 20 × 103 and 90 × 103 mm−3;59,60 lining cells interposed between the matrix and the osteocytes' source of nutrition; and, only if it is actively undergoing renewal, osteoblasts and osteoclasts respectively increasing or reducing its mineralised mass. The remodelling BSU is called a BMU and is supervised by endocrine, paracrine and possibly sympathetic61 pathways.
Osteocytes are now understood also to have crucial roles in both sensing and initiating responses to mechanical loading by regulating the composition of the mineralised matrix62,63 and deploying a range of local and systemic signals, including nitric oxide,64 prostaglandins, RANK ligand65 and the osteoblast inhibitor sclerostin.66 They also regulate plasma phosphate levels, which if depressed leads to osteomalacia. However, it is principally through vitamin D deficiency that osteomalacia is implicated in a minority of hip fracture cases.67,68 Hence, osteocytes might be key regulators of cortical bone's toughness from the length scale of the osteon down to that of the mineral matrix interface.
The role of osteocytes in age-related bone loss has recently been reviewed by ourselves1 and others.69 Osteocytes are characterised by their dendrites, which interconnect with other osteocytes, surface lining cells and the cement line that defines where the formative phase began. These dendrites track through microscopic canaliculi that extend mostly radially across the thickness of the osteon. Canaliculi may also have an important permissive role in absorbing the energy associated with the microcracks that can develop under load.70
Compared with controls, in cases of hip fracture, full osteonal closure appeared delayed, explaining in part the observed increases in cortical porosity.71 As the resorption cavity is filled in, osteocytes are embedded at progressively declining densities, the rate of decline being steeper in hip fracture cases.59,72 In fracture cases, the proportion of osteocytes expressing sclerostin also increased more rapidly with maturation of the osteon73 than in controls, while also expressing nitric oxide synthases (NOS) less frequently;74,75 NOS are involved in osteocytic endocrine signalling.76 Whenever ongoing mineralisation and canal closure were observed in controls, they were associated with a higher density of recently embedded osteocytes adjacent to the osteonal canal; such densities were rarely achieved in the osteons of hip fracture cases.72
Osteocyte Aging and Death
Osteocyte death is widespread in the elderly proximal femur77,78 where, due to the femur's low rate of remodelling,79 the average newly entombed osteocyte must wait two or more decades before liberation from its surrounding bone matrix. Although osteocytes are frequently located 0.1 mm or further from their source of nutrients, osteocyte death is much less prevalent in more actively remodelling bone such as the iliac crest, suggesting that aging of the embedding tissue or of the osteocyte itself might contribute to its death. In the femoral shaft, individual osteocyte lacunae shrink in volume as the subject ages, but the numbers of lacunae in the matrix per unit volume of bone tissue does not change.60 Experimentally, Noble et al.80 showed that bone experiencing reduced loading contained higher proportions of osteocytes undergoing apoptosis. When loading exceeded normal limits, rapidly developing microdamage developed that was associated81 with greatly increased osteocyte apoptosis. This preceded the appearance of florid osteoclastic resorption and remodelling followed by in-filling.80 Parathyroid hormone (PTH), which experimentally promotes a younger osteocytic phenotype,82 and oestrogen can also promote osteocyte survival83,84 and encourage a positive remodelling balance through increasing bone formation. There has been speculation that osteocyte apoptosis is an important mechanism in the initiation of normal remodelling in femoral cortical bone. This remains unproven. Indeed, empty osteocyte lacunae accumulate with age suggesting that sometimes osteocyte death fails to trigger the remodelling of the cell's surrounding matrix. Sclerostin, a powerful inhibitor of bone formation, is normally secreted by osteocytes and chondrocytes but not by osteoblasts or lining cells66,85,86 and is likely to modify the local architecture of remodelling bone—an effect lost necessarily in bone lacking osteocytes.
Osteocytes: Additional Regulatory Roles?
Proximity to an osteocyte is associated with greater regularity in the size, orientation and satisfactory mechanical characteristics of bone crystals.87 In crystals up to 30 nm in length crystal imperfections (for example, those arising from inclusions of proteinaceous material) had little effect on tensile strength.88 To achieve adequate mechanical properties, larger crystals had an increased requirement for perfection. Crystal size grows with tissue aging89 sometimes spectacularly in hip fracture cases.90 Osteocytes may also function collectively as a syncytium:91 they are sensitive to mechanical loading, promote65 bone breakdown and regulate its growth,66 may locally regulate matrix mineralisation92 and also mobilise calcium63,93 in response to PTH signalling.94 Their functions in vertebrates may have evolved with terrestrial living.95 The concept that osteocytes function collectively has given rise to the idea that they might form part of a so-called ‘small world network',96 which is a way of efficiently delivering a complex service at low economic or biological cost in systems as diverse as parts of the brain97 and large airline networks. Osteocytes so organised might deliver diverse and complex interactions with other cells and systems such as osteoblasts, osteoclasts, phosphate and calcium homeostasis in both plasma and local bone matrix. Any small world network carries with it a potential risk of systemic breakdown in the event that a few key elements (for example, certain osteocytes or groups of osteocytes) became disabled. Conversely, the majority of less important elements may be lost with only local consequences. In summary, quiescent cortical osteons are assigned multiple endocrine and paracrine tasks in addition to their structural role. These functions depend on osteocyte survival, but it is not clear what proportion of osteocytes must survive, nor how to identify which osteocytes are especially critical to the optimum biological functioning of cortical bone in the femur.
Mineralised Bone Tissue Heterogeneity
The lamellar structure of mature osteonal bone with its alternating densities may assist in the deflection of cracks.98 In hip fracture cases naive to anti-osteoporosis treatment, there were decreases in matrix mineralisation in the cases99,100 together with reduced heterogeneity of both the mineral:matrix and carbonate:phosphate ratios and increased crystallinity.101 In contrast, cases and controls had similar indentation modulus and hardness. This suggests compensation for reduced mineralisation by a stiffer organic phase in hip fracture cases, making the bone tissue potentially less tough,99,100 consistent with the finding of Norman et al.102 that reduced mineralisation is positively associated with diffuse damage and microcrack density.
Cortical osteons in young people have a steeper gradient of mineralisation from the canal to the cement line than in older subjects, with the highest mineralisation levels being adjacent to the canal,103 where the volumetric density of osteocytic nuclei is lowest.59,72 This mineralisation gradient may have been the first demonstration of an important source of heterogeneity. It also raises the question whether reduced heterogeneity and increased stiffness of the organic phase in hip fracture cases might be related to the ages of the osteons studied.
Remodelling and Fracture Risk
Increased osteonal age as a potential contributor to hip fracture risk appears at first sight paradoxical in view of the well-known increase in remodelling post-menopause contributing to osteoporosis. The paradox might be resolved if this type of remodelling caused net bone loss or created new bone with sub-optimal mechanical properties such as reduced heterogeneity. Remodelling osteons are clustered anatomically.56,57 A mechanism whereby remodelling osteons, which amalgamate to form giant composite osteons, might promote the trabecularisation of the cortex received some support from Bidan et al.'s investiogations already referred to.55 Subcortical femoral trabeculae, in contrast to most iliac trabeculae, when viewed under polarised light show that their internal lamellae are usually not parallel to either trabecular surface, suggesting they might have been sculpted out of pre-existing compact cortical bone by osteoclasts.104
New imaging techniques now make it possible to investigate the detailed 3D structure of individual osteons in the femoral cortex and the anatomical relationships between them.105,106 These offer new avenues for understanding how conversion of the cortex to trabecular bone, leading to cortical thinning,104,107 develops and to how osteonal structures might help preserve the toughness of bone once a crack threatens to expand beyond the osteon's own territory.108
Another cause of increased remodelling is the secondary hyperparathyroidism associated with reduced vitamin D levels, commonly seen in the elderly. Before it develops into frank osteomalacia with demineralisation of bone tissue, this can result in accelerated net bone loss, cortical thinning and trabecular fenestration as reviewed by Chavassieux et al.109
Finally, in view of its location at the potentially highly stressed cortical surface of the femur, sub-periosteal bone remodelling deserves attention. Power et al. found that the extent of sub-periostal alkaline phosphatase in the femoral neck (reflecting new bone formation) was associated with the proportion of crenellated intracortical canals apparently undergoing osteoclastic resorption.110
Organisation Level 4: Sub-microscopic Toughening Mechanisms
These have been extensively reviewed recently and the reader is referred to excellent summaries in this journal and elsewhere.14,15,111,112 The prevalence of mechanical stress-concentrators such as notches and flaws must inevitably be increased in the cortex by increased remodelling, typically seen after menopause. Normal mature human femoral bone subjected to cyclic loading develops microcracks that grow then rapidly decelerate,113 reflecting multiple toughening mechanisms absorbing the energy associated with cracking without excessive sacrifice of strength.108,114 Their length scales range from the molecular (for example, re-formable bonding of collagen115 and cross-links116 through that of the potentially crack-deflecting osteocyte canaliculus (∼250 nm diameter).70
The Behaviour of Cracks
Crack energy is directional: deflection absorbs a crack's energy. When the matrix of bone is not homogeneous, a developing crack will experience deflections.117 So heterogeneity of its material properties is desirable and intrinsic to normal lamellar bone.112,118
On a larger length scale, osteons also absorb or deflect cracks.119 Repair of bone sufficiently damaged by the development of an enlarging crack may necessitate remodelling. Because femoral cortical bone is more liable to crack longitudinally, cracks that start transversely are usually diverted longitudinally so that an undetectable tendency to cortical splitting, akin to the delamination of plywood, might be the first adverse consequence of a heavy fall onto the greater trochanter. However, the superolateral cortex of the femoral neck can become as thin as 0.5–1.0 mm in old age,44,120 so because of their size few osteons capable of crack capture can remain within it. This makes thin cortices (like trabeculae) dependent on the more microscopic crack-capturing mechanisms.
Un-remodelled cracks accumulate with aging in the femur.121 Zioupos and Currey122 and Diab et al.123 showed ex vivo that older bone formed linear microcracks in preference to diffuse damage with an exponential decrease in fatigue life with age.123 By analogy with man-made composites, longitudinal splitting short of generating a clinical fracture, in which cracking occurs parallel to the periosteal surface,17 must increase the tendency of the superolateral cortex to buckle under load in a future fall.124
At the nanoscale level, collagen molecules undergo increased glycation with ageing, which is likely to make bone harder and reduce toughness.14,125 Femoral cortical bone tissue turnover has only rarely been studied, Wand et al.79 estimating its half life as approaching two decades, which gives plenty of time for exposure to circulating glycating sugars to increase collagen cross links.126
Hazards Associated with the Type 2 Diabetes Epidemic
The recent increase in consumption of thermally processed foods resulting in high levels of pro-oxidant advanced glycation end-products (AGEs) likely increases glycation rates. These molecules also promote inflammation (which when local can lead to bone loss) and appetite, so are of mutual interest in both the study of hip fracture and type 2 diabetes.127 Diabetics provide about 10% of hip fracture cases and have a 1.6 to 1.7-fold increased risk after adjusting for BMD.128,129
Reducing Hip Fracture Risk: Current Approaches and Future Prospects
Because healthy bone strength is organised so that it can prevent cracks propagating both while they are still at the nanoscale or at any larger scale up to that of the whole femur, effective anti-fracture interventions might reduce fracture risk by enhancing protection at any length scale. Moreover, the comparative lack of correlation of measurements related to bone strength at the nano- and microscales with those at the level of the whole femur53 should be viewed positively, as it offers the additive potential for simultaneously intervening at multiple length scales.
Lifestyle and Dietary Interventions
In men, in their seventh decade, substantial local increases in superolateral cortical thickness were achieved in a clinical trial with targeted mechanical loading, through regular hopping on one leg 50 times daily.130 This level of physical activity, quite easily achieved in healthy 60-year-old men, is likely unachievable in people beginning exercise from their eighth decade after lifelong sedentary behaviour; but, it reinforces observational studies suggesting that commitment to a physically active lifestyle in older persons postpones the age-related increase in hip fracture risk.
The need to trial the effects of reducing consumption of AGEs on risk of type 2 diabetes is self-evident. There is a similarly strong case for including older subjects at risk of hip fracture to measure the intervention's effect on femoral strength and integrity.
Pharmaceutical Interventions
Broadly speaking, currently prescribed bisphosphonates and other anti-resorptive agents such as denosumab reduce the risk of all types of hip fracture131 other than the so-called atypical sub-trochanteric fracture. Bisphosphonates have an effect that is several-fold larger in reducing trochanteric and intra-capsular fractures than can be explained by their effect to increase bone density. Two ideas have been put forward to explain this dissociation:132 because they reduce remodelling, anti-resorptive agents must also reduce the number of mechanical stress-concentrating resorption pits where a dangerous crack might initiate; and because they might have a beneficial effect on osteocyte function. These ideas are extensively discussed by Geissler et al.133 in light of the adverse effects of bisphosphonates and probably other anti-resorptive agents134 on sub-trochanteric fracture in long-term therapy.
There might be several factors contributing to this increased risk: the reduced birth rate of osteocytes in bisphosphonate-treated cortical bone, coupled with their longer potential dwell-time in the matrix (and hence risk of death in situ), that results from reduced remodelling; enhanced accumulation of AGEs due to the effect of bisphosphonates to prolong the life of bone tissue; and possibly a specific bisphosphonate-effect to enhance the long-term accumulation of deleterious cross-links.135,136
In vitro mechanical testing suggested that bone anabolic agents do one of two contrasting things besides increasing bone formation. They either increase the heterogeneity of cortical bone in the case of PTH receptor type 1 agonists; or in the case of anti-sclerostin antibodies they appeared to leave heterogeneity unchanged.137,138 The potentially toughening effect of teriparatide and abaloparatide is predicted from their effects to reduce the overall bone tissue age in treated patients and increase tissue heterogeneity. Anti-sclerostin antibodies might only reduce bone tissue age.138,139 Close attention will now be paid to contrasting the effects of the PTH1 receptor binders and the anti-sclerostin antibodies in their long-term effects on those fractures associated with the brittleness of ageing cortical bone.
Conclusions
The 1992 NIA Bone quality workshop140 stimulated many research studies reviewed here aimed at understanding what, apart from BMD, makes the femur fracture-resistant. Hopefully we shall soon understand why physically active and generally osteoporotic elderly osteoporotic women in rural equatorial Africa have tough if osteoporotic bones and rarely suffer hip fractures,141 whereas in the affluent world more sedentary elderly women, even those without osteoporosis, are at much greater fracture risk. What seems certain is that our improving understanding of the role of cortical bone in the femur will continue to promote improvements in the clinical management of hip fracture in the years ahead.
Footnotes
The author declares that in the last two years he has received compensation for providing written advice to Eli Lilly on the history of publicly funded research on the use of hPTH(1-34), that is, teriparatide, for treating osteoporosis. Prior to 2012 the author received compensation for providing scientific advice to Procter and Gamble, Novartis, Lilly and Merck.
References
- Reeve J, Loveridge N. The Fragile elderly hip: mechanisms associated with age-related loss of strength and toughness. Bone 2014; 61: 138–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui SL, Slemenda CW, Johnston CC Jr. Age and bone mass as predictors of fracture in a prospective study. J Clin Invest 1988; 81: 1804–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turmezei TD, Treece GM, Gee AH, Fotiadou AF, Poole KES. Quantitative 3D analysis of bone in hip osteoarthritis using clinical computed tomography. Eur Radiol 2016; 26: 2047–2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burr DB. Anatomy and physiology of the mineralized tissues: role in the pathogenesis of osteoarthrosis. Osteoarthritis Cartilage 2004; 12(Suppl A): S20–S30. [DOI] [PubMed] [Google Scholar]
- Dequeker J, Mokassa L, Aerssens J. Bone density and osteoarthritis. J Rheumatol 1995; 43(Suppl): 98–100. [PubMed] [Google Scholar]
- Flynn MJ, Cody DD. The assessment of vertebral bone macroarchitecture with X-ray computed tomography. Calcif Tissue Int 1993; 53: S170–S175. [DOI] [PubMed] [Google Scholar]
- Kaptoge S, Dalzell N, Loveridge N, Beck TJ, Khaw KT, Reeve J. Effects of gender, anthropometric variables and aging on the evolution of hip strength in men and women aged over 65. Bone 2003; 32: 561–570. [DOI] [PubMed] [Google Scholar]
- Beck TJ, Oreskovic TL, Stone KL, Ruff CB, Ensrud K, Nevitt MC et al. Structural adaptation to changing skeletal load in the progression towards hip fragility: the Study of Osteoporotic Fractures. J Bone Miner Res 2001; 16: 1108–1119. [DOI] [PubMed] [Google Scholar]
- Griffiths AA. The phenomena of rupture and flow in solids. Philos Trans R Soc Lond A 1921; 221: 163–198. [Google Scholar]
- Duer M. The contribution of solid-state NMR spectroscopy to understanding biomineralization: atomic and molecular structure of bone. J Magn Reson 2015; 253: 98–110. [DOI] [PubMed] [Google Scholar]
- Gao HJ. Application of fracture mechanics concepts to hierarchical biomechanics of bone and bone-like materials. Int J Fract 2006; 138: 101–137. [Google Scholar]
- Hernandez CJ, Keaveny TM. A biomechanical perspective on bone quality. Bone 2006; 39: 1173–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee MD, Nanci A. Osteopontin at mineralized tissue interfaces in bone, teeth, and osseointegrated implants: ultrastructural distribution and implications for mineralized tissue formation, turnover, and repair. Microsc Res Tech 1996; 33: 141–164. [DOI] [PubMed] [Google Scholar]
- Zimmermann EA, Busse B, Ritchie RO. The fracture mechanics of human bone: influence of disease and treatment. Bonekey Rep 2015; 4: 743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launey ME, Buehler MJ, Ritchie RO. On the mechanistic origins of toughness in bone. Annu Rev Mater Res 2010; 40: 25–53. [Google Scholar]
- Seref-Ferlengez Z, Kennedy OD, Schaffler MB. Bone microdamage, remodeling and bone fragility: how much damage is too much damage? Bonekey Rep 2015; 4: 644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bakker PM, Manske SL, Ebacher V, Oxland TR, Cripton PA, Guy P. During sideways falls proximal femur fractures initiate in the superolateral cortex: evidence from high-speed video of ex vivo fractures. J Biomech 2009; 42: 1917–1925. [DOI] [PubMed] [Google Scholar]
- Zysset PK. Where does hip fracture initiate? Bonekey Rep 2014; 3: 601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galilei Linceo, G. Discorsi e Dimostrazioni Matematiche (tr. H Crew and A de Salvio: ‘dialogues concerning two new sciences' MacMillan 1914, reprinted Dover edition, page 131). Elsevier: Leiden, The Netherlands, 1638.
- Darwin C. On the Origin of Species by Means of Natural Selection John Murray: London, UK, 1859. [Google Scholar]
- Wolff J. Das Gesetz der Transformation der Knochen A Hirschwald: Berlin, Germany, 1892. [Google Scholar]
- Bell GH, Dunbar O, Beck JS, Gibb A. Variations in strength of vertebrae with age and their relation to osteoporosis. Calcif Tissue Res 1967; 1: 75–86. [DOI] [PubMed] [Google Scholar]
- Crabtree N, Lunt M, Holt G, Kröger H, Burger H, Grazio S et al. Hip geometry, bone mineral distribution and bone strength in European men and women: the EPOS study. Bone 2000; 27: 151–160. [DOI] [PubMed] [Google Scholar]
- Mayhew PM, Thomas CDL, Clement JG, Loveridge N, Beck TJ, Bonfield W et al. Relation between age, femoral neck cortical stability, and hip fracture risk. Lancet 2005; 366: 129–135. [DOI] [PubMed] [Google Scholar]
- Poole KES, Mayhew PM, Rose CM, Brown JK, Bearcroft PJ, Loveridge N et al. Changing structure of the femoral neck across the adult female lifespan. J Bone Miner Res 2010; 25: 482–491. [DOI] [PubMed] [Google Scholar]
- Johannesdottir F, Aspelund T, Reeve J, Poole KES, Sigurdsson S, Harris TB et al. Age-related regional losses of cortical and trabecular bone in femoral neck in elderly women and men: The AGES-Reykjavik Longitudinal Study. J Bone Miner Res 2013; 28: 2165–2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treece GM, Poole KES, Gee AH. Imaging the femoral cortex: thickness, density and mass from clinical CT. Med Image Anal 2012; 16: 952–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa T, Turner CH, Peacock M, Slemenda CW, Weaver CM, Teegarden D et al. Geometric structure of the femoral neck measured using dual-energy X-ray absorptiometry. J Bone Miner Res 1994; 9: 1053–1064. [DOI] [PubMed] [Google Scholar]
- Kaptoge S, Dalzell N, Jakes R, Wareham N, Khaw KT, Beck TJ et al. Hip section modulus, a measure of bending resistance, is more strongly related to physical activity than BMD. Osteoporos Int 2003; 14: 941–949. [DOI] [PubMed] [Google Scholar]
- Melton LJ III. A gompertzian view of osteoporosis. Calcif Tissue Int 1990; 46: 285–286. [DOI] [PubMed] [Google Scholar]
- Power J, Loveridge N, Rushton N, Parker M, Reeve J. Intracapsular bone formation on the external 'periosteal' surface of the femoral neck: an investigation in cases of hip fracture and controls. Osteoporos Int 2003; 14: 146–151. [DOI] [PubMed] [Google Scholar]
- Kaptoge S, Dalzell N, Folkerd E, Doody D, Khaw KT, Beck TJ et al. Sex hormone status may modulate rate of expansion of proximal femur diameter in older women alongside other skeletal regulators. J Clin Endocrinol Metab 2007; 92: 304–313. [DOI] [PubMed] [Google Scholar]
- Crabtree NJ, Kroger H, Martin A, Pols HAP, Lorenc R, Nijs J et al. Improving risk assessment: hip geometry, bone mineral distribution and bone strength in hip fracture cases and controls. The EPOS Study. Osteoporos Int 2002; 13: 48–54. [DOI] [PubMed] [Google Scholar]
- Bell K, Loveridge N, Power J, Garrahan N, Stanton M, Lunt M et al. Structure of the femoral neck in hip fracture: cortical bone loss in the inferoanterior to superoposterior axis. Journal of Bone and Mineral Research 1999; 14: 112–120. [DOI] [PubMed] [Google Scholar]
- Yang L, Udall WJ, McCloskey EV, Eastell R. Distribution of bone density and cortical thickness in the proximal femur and their association with hip fracture in postmenopausal women: a quantitative computed tomography study. Osteoporos Int 2014; 25: 251–263. [DOI] [PubMed] [Google Scholar]
- Naylor KE, McCloskey EV, Eastell R, Yang L. Use of DXA-based finite element analysis of the proximal femur in a longitudinal study of hip fracture. J Bone Miner Res 2013; 28: 1014–1021. [DOI] [PubMed] [Google Scholar]
- Kaptoge S, Beck TJ, Reeve J, Stone KL, Hillier TA, Cauley JA et al. Prediction of incident hip fracture risk by femur geometry variables measured by hip structural analysis in the Study of Osteoporotic Fractures. J Bone Miner Res 2008; 23: 1892–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaCroix AZ, Beck TJ, Cauley JA, Lewis CE, Bassford T, Jackson R et al. Hip structural geometry and incidence of hip fracture in postmenopausal women: what does it add to conventional bone mineral density? Osteoporos Int 2010; 21: 919–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zysset PK, Dall'ara E, Varga P, Pahr DH. Finite element analysis for prediction of bone strength. Bonekey Rep 2013; 2: 386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang TF, Sigurdsson S, Karlsdottir G, Oskarsdottir D, Sigmarsdottir A, Chengshi J et al. Age-related loss of proximal femoral strength in elderly men and women: the Age Gene/Environment Susceptibility Study--Reykjavik. Bone 2012; 50: 743–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole KES, Treece GM, Mayhew PM, Vaculík J, Dungl P, Horák M et al. Cortical thickness mapping to identify focal osteoporosis in patients with hip fracture. PLoS ONE 2012; 7: e38466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea JE, Vajda EG, Bloebaum RD. Evidence of a hypermineralised calcified fibrocartilage on the human femoral neck and lesser trochanter. J Anat 2001; 198: 153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havelka S, Horn V. Joint cartilage tidemark and periosteum: Two components of one envelope. Acta Univ Carol [Med] (Praha) 1986; 32: 311–318. [PubMed] [Google Scholar]
- Thomas CDL, Mayhew PM, Power J, Poole KES, Loveridge N, Clement JG et al. Femoral neck trabecular bone: loss with ageing and role in preventing fracture. J Bone and Miner Res 2009; 24: 1808–1818. [DOI] [PubMed] [Google Scholar]
- Tsangari H, Findlay DM, Fazzalari NL. Structural and remodeling indices in the cancellous bone of the proximal femur across adulthood. Bone 2007; 40: 211–217. [DOI] [PubMed] [Google Scholar]
- Yang L, Udall WJ, McCloskey EV, Eastell R. Distribution of bone density and cortical thickness in the proximal femur and their association with hip fracture in postmenopausal women: a quantitative computed tomography study. Osteoporos Int 2014; 25: 251–263. [DOI] [PubMed] [Google Scholar]
- Hirsch C, Brodetti A. The weight-bearing capacity of structural elements in femoral necks. Acta Orthop Scand 1956; 26: 15–24. [PubMed] [Google Scholar]
- Manske SL, Liu-Ambrose T, Cooper DML, Kontulainen S, Guy P, Forster BB et al. Cortical and trabecular bone in the femoral neck both contribute to proximal femur failure load prediction. Osteoporos Int 2009; 20: 445–453. [DOI] [PubMed] [Google Scholar]
- Timoshenko SP, Gere JM. Theory of Elastic Stability McGraw Hill: New York, NY, USA, 1979. [Google Scholar]
- Hetenyi M. Beams on Elastic Foundation: Theory with Applications in the Fields of Civil and Mechanical Engineering University of Michigan Press: Detroit, MI, USA, 1946. [Google Scholar]
- Carpenter RD, Beaupré GS, Lang TF, Orwoll ES, Carter DR. MrOS Study Group. Osteoporotic Fractures in Men (MrOS) Study Group New QCT analysis approach shows the importance of fall orientation on femoral neck strength. J Bone Miner Res 2005; 20: 1533–1542. [DOI] [PubMed] [Google Scholar]
- Parfitt AM. The physiological and clinical significance of bone histomorphometric data. In: Recker R (ed.).Bone Histomorphometry, Techniques and Interpretation CRC Press: Boca Raton, FL, USA, 1983; pp. 143–224. [Google Scholar]
- Mirzaali MJ, Schwiedrzik JJ, Thaiwichai S, Best JP, Michler J, Zysset PK et al. Mechanical properties of cortical bone and their relationships with age, gender, composition and microindentation properties in the elderly. Bone 2016; 93: 196–211. [DOI] [PubMed] [Google Scholar]
- Parfitt AM. Osteonal and hemi-osteonal remodelling: the spatial and temporal framework for signal traffic in adult human bone. J Cell Biochem 1994; 55: 273–286. [DOI] [PubMed] [Google Scholar]
- Bidan CM, Kommareddy KP, Rumpler M, Kollmannsberger P, Bréchet YJ, Fratzl P et al. How linear tension converts to curvature: geometric control of bone tissue growth. PLoS ONE 2012; 7: e36336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan G, Loveridge N, Bell KL, Power J, Rushton N, Reeve J. Spatial clustering of osteonal remodelling: a cause of focal weakness in the femoral neck cortex in hip fracture. Bone 2000; 26: 305–313. [DOI] [PubMed] [Google Scholar]
- Bell KL, Loveridge N, Jordan GR, Power J, Constant CR, Reeve J. A novel mechanism for the induction of the increased cortical porosity in cases of intra-capsular hip fracture. Bone 2000; 27: 297–304. [DOI] [PubMed] [Google Scholar]
- Fernandez JW, Das R, Cleary PW, Hunter PJ, Thomas CD, Clement JG. Using smooth particle hydrodynamics to investigate femoral cortical bone remodelling at the Haversian level. Int J Numer Method Biomed Eng 2013; 29: 129–143. [DOI] [PubMed] [Google Scholar]
- Hannah KM, Thomas CDL, Clement JG, De Carlo F, Peele AG. Bimodal distribution of osteocyte lacunar size in the human femoral cortex as revealed by micro-CT. Bone 2010; 47: 866–871. [DOI] [PubMed] [Google Scholar]
- Carter Y, Thomas CD, Clement JG, Cooper DML. Femoral osteocyte lacunar density, volume and morphology in women across the lifespan. J Struct Biol 2013; 183: 519–526. [DOI] [PubMed] [Google Scholar]
- Takeda S, Elefteriou F, Levasseur R, Liu X, Zhao L, Parker KL et al. Leptin regulates bone formation via the sympathetic nervous system. Cell 2002; 111: 305–317. [DOI] [PubMed] [Google Scholar]
- Dallas SL, Prideaux M, Bonewald LF. The Osteocyte: an endocrine cell and more. Endocr Rev 2013; 34: 658–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysolmerski JJ. Osteocytes remove and replace perilacunar mineral during reproductive cycles. Bone 2013; 54: 230–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaman G, Pitsillides AA, Rawlinson SCF, Suswillo RLF, Mosley JR, Cheng MZ et al. Mechanical strain stimulates nitric oxide production by rapid activation of endothelial nitric oxide synthase in osteocytes. J Bone Miner Res 1999; 14: 1123–1131. [DOI] [PubMed] [Google Scholar]
- Nakashima T, Hayashi M, Fukunaga T, Kurata K, Oh-Hora M, Feng JQ et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med 2011; 17: 1231–1234. [DOI] [PubMed] [Google Scholar]
- Poole KE, van Bezooijen RL, Loveridge N, Hamersma H, Papapoulos SE, Lowik C et al. Sclerostin is a delayed secretion product of osteocytes that inhibits bone formation. FASEB J 2005; 19: 1842–1844. [DOI] [PubMed] [Google Scholar]
- Hordon LD, Peacock M. Osteomalacia and osteoporosis in femoral neck fracture. Bone Miner 1990; 11: 247–259. [DOI] [PubMed] [Google Scholar]
- Compston JE, Vedi S, Croucher PI. Low prevalence of osteomalacia in elderly patients with hip fracture. Age Ageing 1991; 20: 132–134. [DOI] [PubMed] [Google Scholar]
- Jilka RL, O'Brien CA. The role of osteocytes in age-related bone loss. Curr Osteoporos Rep 2016; 14: 16–25. [DOI] [PubMed] [Google Scholar]
- Ebacher V, Guy P, Oxland TR, Wang RZ. Sub-lamellar microcracking and roles of canaliculi in human cortical bone. Acta Biomater 2012; 8: 1093–1100. [DOI] [PubMed] [Google Scholar]
- Bell KL, Loveridge N, Power J, Stanton M, Meggitt BF, Reeve J. Regional differences in cortical porosity in the fractured femoral neck. Bone 1999; 24: 57–64. [DOI] [PubMed] [Google Scholar]
- Power J, Doube M, van Bezooijen R, Loveridge N, Reeve J. Osteocyte recruitment declines as the osteon fills in: interacting effects of osteocytic sclerostin and previous hip fracture on the size of cortical canals in the femoral neck. Bone 2012; 50: 1107–1114. [DOI] [PubMed] [Google Scholar]
- Power J, Poole KE, van Bezooijen R, Doube M, Caballero-Alías AM, Lowik C et al. Sclerostin and the regulation of bone formation: Effects in hip osteoarthritis and femoral neck fracture. J Bone Miner Res 2010; 25: 1867–1876. [DOI] [PubMed] [Google Scholar]
- Caballero A, Loveridge N, Pitsillides A, Parker M, Kaptoge S, Lyon A et al. Osteocytic expression of constitutive NO synthase isoforms in the femoral neck cortex: a case-control study of intra-capsular hip fracture. J Bone Miner Res 2005; 20: 268–273. [DOI] [PubMed] [Google Scholar]
- Loveridge N, Fletcher S, Power J, Caballero AM, Das-Gupta V, Rushton N et al. Patterns of osteocytic endothelial nitric oxide synthase expression in the femoral neck cortex: differences between cases of intracapsular hip fracture and controls. Bone 2002; 30: 866–871. [DOI] [PubMed] [Google Scholar]
- Sunters A, Armstrong VJ, Zaman G, Kypta RM, Kawano Y, Lanyon LE et al. Mechano-transduction in osteoblastic cells involves strain-regulated estrogen receptor alpha-mediated control of insulin-like growth factor (IGF) receptor sensitivity to anbient IGF, leading to phosphoinositol 3-kinase/AKT-dependent Wnt/LRP5 receptor-independent activation of beta-catenin signaling. J Biol Chem 2010; 285: 8743–8758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunstan CR, Evans RA, Hills E, Wong SY, Higgs RJ. Bone death in hip fracture in the elderly. Calcif Tissue Int 1990; 47: 270–275. [DOI] [PubMed] [Google Scholar]
- Power J, Noble BS, Loveridge N, Bell KL, Rushton N, Reeve J. Osteocyte viability in the femoral neck cortex: an association with cortical remodelling in cases of hip fracture and controls. Calcif Tissue Int 2001; 69: 13–19. [DOI] [PubMed] [Google Scholar]
- Wand JS, Smith T, Green JR, Hesp R, Bradbeer JN, Reeve J. Whole body and site-specific bone remodelling in patients with previous femoral fractures. Relationships between reduced physical activity, reduced bone mass and increased bone resorption. Clin Sci 1992; 83: 665–675. [DOI] [PubMed] [Google Scholar]
- Noble B, Peet N, Stevens H, Brabbs A, Mosley J, Reilly G et al. Mechanical loading: biphasic osteocyte survival and targeting of osteoclasts for bone destruction in rat cortical bone. Am J Physiol Cell Physiol 2003; 284: 934c–944c. [DOI] [PubMed] [Google Scholar]
- Verborgt O, Gibson GJ, Schaffler MB. Loss of osteocyte integrity in association with microdamage and bone remodelling after fatigue in vivo. J Bone Miner Res 2000; 15: 60–67. [DOI] [PubMed] [Google Scholar]
- Prideaux M, Dallas SL, Zhao N, Johnsrud ED, Veno PA, Guo D et al. Parathyroid hormone induces bone cell motility and loss of mature osteocyte phenotype through L-calcium channel dependent and independent mechanisms. PLoS ONE 2015; 10: e0125731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomkinson A, Reeve J, Shaw RW, Noble BS. The death of osteocytes via apoptosis accompanies estrogen withdrawal in human bone. J Clin Endocrinol Metab 1997; 82: 3128–3135. [DOI] [PubMed] [Google Scholar]
- Jilka RL, Weinstein RS, Bellido T, Robertson P, Parfitt AM, Manolagas SC. Increased bone formation by prevention of osteoblast apoptosis with parathyroid hormone. J Clin Invest 1999; 104: 439–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bezooijen RL, Roelen BA, Visser A, van der Wee-Pals L, de Wilt E, Karperien M et al. Sclerostin is an osteocyte-expressed negative regulator of bone formation, but not a classical BMP antagonist. J Exp Med 2004; 199: 805–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins GJ, Rowe PS, Lim HP, Welldon KJ, Ormsby R, Wijenayaka AR et al. Sclerostin is a locally acting regulator of late-osteoblast/preosteocyte differentiation and regulates mineralisation through a MEPE-ASARM-dependent mechanism. J Bone Miner Res 2011; 26: 1425–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerschnitzki M, Kollmannsberger P, Burghammer M, Duda GN, Weinkamer R, Wagermaier W et al. Architecture of the osteocyte network correlates with bone material quality. J Bone Miner Res 2013; 28: 1837–1845. [DOI] [PubMed] [Google Scholar]
- Gao HJ, Ji B, Jager IL, Arzt E, Fratzl P. Materials become insensitive to flaws at nanoscale: Lessons from nature. Proc Natl Acad Sci USA 2003; 100: 5597–5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterji SK, Wall JC, Jeffery JW. Age-related changes in the orientation and particle size of the mineral phase in human femoral cortical bone. Calcif Tissue Int 1981; 33: 567–574. [DOI] [PubMed] [Google Scholar]
- Kent GN, Dodds RA, Klenerman L, Watts RW, Bitensky L, Chayen J. Changes in crystal size and orientation of acidic glycosaminoglycans at the fracture site in fractured necks of femur. J Bone Joint Surg Br 1983; 65: 189–194. [DOI] [PubMed] [Google Scholar]
- Palumbo C, Ferretti M, Marotti G. Osteocyte dendrogenesis in static and dynamic bone formation: an ultrastructural study. Anat Rec 2004; 278A: 474–480. [DOI] [PubMed] [Google Scholar]
- Atkins GJ, Findlay DM. Osteocyte regulation of bone mineral: a little give and take. Osteoporos Int 2012; 23: 2067–2079. [DOI] [PubMed] [Google Scholar]
- Talmage DW, Talmage RV. Calcium homeostasis: How bone solubility relates to all aspects of bone physiology. J Musculoskelet Neuronal Interact 2007; 7: 108–112. [PubMed] [Google Scholar]
- Saini V, Marengi DA, Barry KJ, Fulzele KS, Heiden E, Liu X et al. Parathyroid hormone (PTH)/PTH-related peptide type 1 receptor (PPR) signaling in osteocytes regulates anabolic and catabolic skeletal responses to PTH. J Biol Chem 2013; 288: 20122–20134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witten PE, Huysseune A. A comparative view on mechanisms and functions of skeletal remodelling in teleost fish, with special emphasis on osteoclasts and their function. Biol Rev 2009; 84: 315–346. [DOI] [PubMed] [Google Scholar]
- Watts DJ, Strogatz SH. Collective dynamics of 'small-world' networks. Nature 1998; 393: 440–442. [DOI] [PubMed] [Google Scholar]
- Bullmore E, Sporns O. The economy of brain network organization. Nat Rev Neurosci 2012; 13: 336–349. [DOI] [PubMed] [Google Scholar]
- Ciarelli TE, Tjhia CK, Rao DS, Qiu S, Parfitt AM, Fyhrie DP. Trabecular packet-level lamellar density patterns differ by fracture status and bone formation rate in white females. Bone 2009; 45: 903–908. [DOI] [PubMed] [Google Scholar]
- Loveridge N, Power J, Reeve J, Boyde A. Bone mineralization density and femoral neck fragility. Bone 2004; 35: 929–941. [DOI] [PubMed] [Google Scholar]
- Fratzl-Zelman N, Roschger P, Gourrier A, Weber M, Misof BM, Loveridge N et al. Combination of nanoindentation and quantitative backscattered electron imaging revealed altered bone material properties associated with femoral neck fragility. Calcif Tissue Int 2009; 85: 335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourion-Arsiquaud S, Lukashova L, Power J, Loveridge N, Reeve J, Boskey AL. Fourier transformed infra-red imaging of femoral neck bone: reduced heterogeneity of mineral:matrix and carbonate:phosphate and more variable crystallinity in treatment-naïve fracture cases compared to fracture-free controls. J Bone Miner Res 2013; 2013: 150–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman TL, Little TM, Yeni YN. Age-related changes in porosity and mineralisation and in-service damage accumulation. J BiomechJournal of Biomechanics 2008; 41: 2868–2873. [DOI] [PubMed] [Google Scholar]
- Ortner DJ. Aging effects on osteon remodeling. Calcif Tissue Res 1975; 18: 27–36. [DOI] [PubMed] [Google Scholar]
- Zebaze RM, Ghasem-Zadeh A, Bohte A, Iuliano-Burns S, Mirams M, Price RI et al. Intracortical remodelling and porosity in the distal radius and post-mortem femurs of women: a cross-sectional study. Lancet 2010; 375: 1729–1736. [DOI] [PubMed] [Google Scholar]
- Arhatari BD, Cooper DML, Thomas CDL, Clement JG, Peele AG. Imaging the 3D structure of secondary osteons in human cortical bone using phase-retrieval tomography. Phys Med Biol 2011; 56: 5265–5274. [DOI] [PubMed] [Google Scholar]
- Cooper DML, Erickson B, Peele AG, Hannah K, Thomas CD, Clement JG. Visualization of 3D osteon morphology by synchrotron radiation micro-CT. J Anat 2011; 219: 481–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersh ME, Pandy MG, Bui QM, Jones AC, Arns CH, Knackstedt MA et al. The heterogeneity in femoral neck structure and strength. J Bone Miner Res 2013; 28: 1022–1028. [DOI] [PubMed] [Google Scholar]
- Zimmermann EA, Schaible E, Bale H, Barth HD, Tang SY, Reichert P et al. Age-related changes in the plasticity and toughness of human cortical bone at multiple length scales. Proc Natl Acad Sci USA 2011; 108: 14416–14421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavassieux P, Seeman E, Delmas PD. Insights into material and structural basis of bone fragility from diseases associated with fractures: how determinants of the biomechanical properties of bone are compromised by disease. Endocr Rev 2007; 28: 151–164. [DOI] [PubMed] [Google Scholar]
- Power J, Loveridge N, Lyon A, Rushton N, Parker M, Reeve J. Osteoclastic cortical erosion as a determinant of sub-periosteal osteoblastic bone formation in the femoral neck's response to BMU imbalance. Effects of stance-related loading and hip fracture. Osteoporos Int 2005; 6: 1049–1056. [DOI] [PubMed] [Google Scholar]
- Ruppel ME, Miller LM, Burr DB. The effect of the microscopic and nanoscale structure on bone fragility. Osteoporos Int 2008; 19: 1251–1265. [DOI] [PubMed] [Google Scholar]
- Fratzl P. When the cracks begin to show. Nat Mater 2008; 7: 610–612. [DOI] [PubMed] [Google Scholar]
- Akkus O, Rimnac CM. Cortical bone tissue resists fatigue fracture by deceleration and arrest of microcrack growth. J Biomech 2001; 34: 757–764. [DOI] [PubMed] [Google Scholar]
- Peterlik H, Roschger P, Klaushofer K, Fratzl P. From brittle to ductile fracture of bone. Nat Mater 2006; 5: 52–55. [DOI] [PubMed] [Google Scholar]
- Thompson JB, Kindt JH, Drake B, Hansma HG, Morse DE, Hansma PK. Bone indentation recovery time correlates with bond reforming time. Nature 2001; 414: 773–776. [DOI] [PubMed] [Google Scholar]
- Paschalis EP, Tatakis DN, Robins S, Fratzl P, Manjubala I, Zoehrer R et al. Lathyrism-induced alterations in collagen cross-links influence the mechanical properties of bone material without affecting the mineral. Bone 2011; 49: 1232–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalla RK, Stolken JS, Kinney JH, Ritchie RO. Fracture in human cortical bone: local fracture criteria and toughening mechanisms. J Biomech 2005; 38: 1517–1525. [DOI] [PubMed] [Google Scholar]
- Fratzl P, Gupta HS, Fischer FD, Kolednik O. Hindered crack propagation in materials with periodically varying Young's modulus—lessons from biological materials. Adv Mater 2007; 19: 2657–2661. [Google Scholar]
- Yeni YN, Norman TL. Fracture toughness of human femoral neck: effect of microstructure, composition, and age. Bone 2000; 26: 499–504. [DOI] [PubMed] [Google Scholar]
- Johannesdottir F, Poole KES, Reeve J, Siggeirsdottir K, Aspelund T, Mogensen B et al. Distribution of cortical bone in the femoral neck and hip fracture: A prospective case-control analysis of 143 incident hip fractures; the AGES-REYKJAVIK Study. Bone 2011; 48: 1268–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffler MB, Boyce TM, Lundin-Cannon KD, Milgrom C, Fyhrie DP. Age-related architectural changes and microdamage accumulation in the human femoral neck cortex. Trans Orthop Res Soc 1995; 20: 549. [Google Scholar]
- Zioupos P, Currey JD. Changes in the stiffness, strength and toughness of human cortical bone with age. Bone 1998; 22: 57–66. [DOI] [PubMed] [Google Scholar]
- Diab T, Sit S, Rho J, Vashishth D. Age-dependent fatigue behaviour of human cortical bone. Eur J Morphol 2005; 42: 53–59. [DOI] [PubMed] [Google Scholar]
- Nilsson K-F, Asp LE, Alpman JE, Nystedt L. Delamination buckling and growth for delaminations at different depths in a slender composite panel. Int J Solids Struct 2001; 38: 3039–3071. [Google Scholar]
- Wang X, Shen X, Li X, Mauli Agrawal C. Age-related changes in the collagen network and toughness of bone. Bone 2002; 31: 1–7. [DOI] [PubMed] [Google Scholar]
- Banse X, Sims TJ, Bailey AJ. Mechanical properties of adult vertebral cancellous bone: correlation with collagen intermolecular cross-links. J Bone Miner Res 2002; 17: 1621–1628. [DOI] [PubMed] [Google Scholar]
- Vlassara H, Striker GE. AGE restriction in diabetes mellitus: a paradigm shift. Nat Rev Endocrinol 2013; 7: 526–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris R, Parker M. Diabetes mellitus and hip fracture: a study of 5966 cases. Injury 2011; 42: 1313–1316. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Sugimoto T. Bone metabolism and fracture risk in type 2 diabetes mellitus [Review]. Endocr J 2011; 58: 613–624. [DOI] [PubMed] [Google Scholar]
- Allison SJ, Poole KES, Treece GM, Gee AH, Tonkin C, Rennie WJ et al. The influence of high-impact exercise on cortical and trabecular bone mineral content and 3D distribution across the proximal femur in older men: a randomized controlled unilateral intervention. J Bone Miner Res 2015; 30: 1709–1716. [DOI] [PubMed] [Google Scholar]
- Mackey DC, Black DM, Bauer DC, McCloskey EV, Eastell R, Mesenbrink P et al. Effects of antiresorptive treatment on nonvertebral fracture outcomes. J Bone Miner Res 2011; 26: 2411–2418. [DOI] [PubMed] [Google Scholar]
- Watts NB, Cooper C, Lindsay R, Eastell R, Manhart MD, Barton IP et al. Relationship between changes in bone mineral density and vertebral fracture risk associated with risedronate: greater increases in bone mineral density do not relate to greater decreases in fracture risk. J Clin Densitom 2004; 7: 255–261. [DOI] [PubMed] [Google Scholar]
- Geissler JR, Bajaj D, Fritton JC. Cortical bone tissue mechanical quality and biological mechanisms possibly underlying atypical fractures. J Biomech 2015; 48: 883–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papapoulos S, Lippuner K, Roux C, Lin CJ, Kendler DL, Lewiecki EM et al. The effect of 8 or 5 years of denosumab treatment in postmenopausal women with osteoporosis: results from the FREEDOM Extension study. Osteoporos Int 2015; 26: 2773–2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burr DB, Liu Z, Allen MR. Duration-dependent effects of clinically relevant oral alendronate doses on cortical bone toughness in beagle dogs. Bone 2015; 71: 58–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acevedo C, Bale H, Gludovatz B, Wat A, Tang SY, Wang M et al. Alendronate treatment alters bone tissues at multiple structural levels in healthy canine cortical bone. Bone 2015; 81: 352–363. [DOI] [PubMed] [Google Scholar]
- Boskey AL, Donnelly E, Boskey E, Spevak L, Ma Y, Zhang W et al. Examining the relationships between bone tissue composition, compositional heterogeneity and fragility fracture: a Matched Case Controlled FTIRI Study. J Bone Miner Res 2016; 31: 1070–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross RD, Edwards LH, Acerbo AS, Ominsky MS, Virdi AS, Sena K et al. Bone matrix quality after sclerostin antibody treatment. J Bone Miner Res 2014; 29: 1597–1607. [DOI] [PubMed] [Google Scholar]
- Dempster DW, Roschger P, Misof BM, Zhou H, Paschalis EP, Alam J et al. Differential effects of teriparatide and zoledronic acid on bone mineralization density distribution at 6 and 24 months in the SHOTZ Study. J Bone Miner Res 2016; 31: 1527–1535. [DOI] [PubMed] [Google Scholar]
- Sherman S, Hadley EC. Aging and bone quality: an underexplored frontier. Calcif Tissue Int 1993; 53: S1. [DOI] [PubMed] [Google Scholar]
- Aspray TJ, Prentice A, Cole T, Sawo Y, Reeve J, Francis RM. Low bone mineral content is common but osteoporotic fractures are rare in elderly rural gambian women. J Bone Miner Res 1996; 11: 1019–1025. [DOI] [PubMed] [Google Scholar]