Abstract
Objectives
Babies born small for gestational age (SGA) have higher risk of neonatal morbidity and mortality as well as later life chronic disease. Objectives were to examine the extent to which pre-pregnancy body mass index (BMI) and gestational weight gain (GWG) influence risk of SGA among Japanese, and to evaluate physician response to and potential effects on GWG.
Methods
We examined SGA risk as function of maternal BMI and GWG using logistic regression with data from maternal child health handbooks obtained from women in Japan (N=383). Physicians’ written comments on weight and dietary restriction were analyzed for response to and influence on GWG.
Results
SGA babies comprised 8.6% of the sample, with 13% and 6% of the mothers being underweight and overweight respectively, and 21.7% and 19.8% of mothers gaining less and more than the recommended amounts respectively. In adjusted models, higher pre-pregnancy BMI and GWG were associated with lower risk of SGA (OR 0.71, 95% CI 0.56, 0.90; 0.75, 95% CI 0.61, 0.92 respectively) in models for girls, but not for boys. Inadequate GWG was associated with higher risk of SGA in girls (OR 6.64, 95% CI 2.18, 20.22). Physician written instructions to restrict dietary intake and weight gain followed an average weight gain of 0.69kg/week from the previous prenatal exam, and were followed by weight gains that decreased to 0.30kg/week.
Conclusions
Pre-pregnancy BMI and GWG significantly influence SGA risk in female babies. GWG may be influenced by physicians’ recommendations.
Keywords: Gestational weight gain (GWG), small for gestational age (SGA), BMI, Japan, physician role
Introduction
Babies born small for gestational age (SGA) have higher risk of poor short-term outcomes such as neonatal morbidity and mortality (Stevens-Simon and Orleans, 1999) as well as long-term health outcomes such as later life obesity (Stettler et al., 2000), diabetes (Simmons, 2005), hypertension (Law et al., 2001; Law and Shiell, 1996; Leon et al., 2005), coronary heart disease (Barker et al., 2002), and stroke (Rinaudo and Lamb, 2008). SGA is a proxy measure of intrauterine growth restriction (IUGR), which refers to less than optimal fetal growth due to numerous potential stressors such as smoking and poor nutrition. Research on Developmental Origins of Health and Disease (DOHaD) has demonstrated that the association between LBW or SGA and long-term health outcomes persists even after controlling for postnatal environment (e.g., smoking, adult obesity, social class) – the primary focus of many current public health interventions (Barker, 1995; Osmond et al., 1993). Prevalence of LBW has increased over the past few decades in Japan (Takimoto et al., 2005), leading to acute and chronic public health concerns.
Many factors affecting LBW and SGA are non-modifiable, including gestational age (except in cases of elective inductions or Cesarean sections) (Mohsin et al., 2003), maternal height (Lampl et al., 2010), primiparity, and previous history of LBW (Valero De Bernabe et al., 2004). Although the effects of maternal nutritional state at conception (with pre-pregnancy BMI as a proxy variable) and gestational weight gain (GWG) on risk of LBW and SGA are moderate compared to the above factors (Kirchengast and Hartmann, 2003; Valero De Bernabe et al., 2004), they are potentially modifiable. Ensuring adequate nutritional status at conception is challenging, but achieving adequate GWG during pregnancy is a more attainable goal. In 2006, the Japanese Ministry of Health, Labour and Welfare (MHLW) (MHLW, 2006) released new guidelines for GWG based on pre-pregnancy BMI (JSOG, 2014). Suggested weight gain according to the Japanese guidelines is considerably lower than guidelines from the Institute of Medicine (IOM) (IOM, 2009) raising questions about the appropriateness of country-specific, rather than ethnicity-specific, guidelines (Table 1). The IOM guidelines were constructed in the context of increasing maternal BMI in the US. Excessive GWG has been the focus of many studies concerned with obesity (Gillman, 2012), but few have examined low GWG and associated sequelae, especially in countries with widespread access to high quality prenatal care. In contrast to many developed countries, the prevalence of underweight among reproductive age women has been increasing in Japan (Takimoto et al., 2004; Tsukamoto et al., 2007), and many women diet during pregnancy (Takimoto et al., 2011).
TABLE 1.
Comparison of the Japanesea and US GWG guidelines based on BMI categories
| Guidelines | Body mass index (BMI) range | Recommended GWG during pregnancy to achieve appropriate birth weight |
|---|---|---|
| Japanese Ministry of Health, Labour and Welfare (MHLW), 2006 | <18.5 | 9–12 kg |
| 18.5–<25 | 7–12 kg | |
| ≥25 | 5 kgb | |
| Japanese Society of Obstetrics and Gynecology (JSOG), 1997c | <18 | 10–12 kg |
| 18–<24 | 7–10 kg | |
| ≥24 | 5–7 kg | |
| US Institute of Medicine (IOM), 2009 | <18.5 | 12.7–18.1 kg |
| 18.5–<25 | 11.3–15.9 kg | |
| 25–<30 | 6.8–11.3 kg | |
| ≥30 | 5.0–9.1 kg |
A summary of Japanese guidelines was published by the Japanese Society of Obstetrics and Gynecology (JSOG), 2014. We used the 2006 MHLW recommendations for women with BMI under 25, but the 1997 JSOG guidelines of 5–7 kg for women with BMI of 25 or greater.
If close to BMI 25, about 5 kg; otherwise handles on case-by-case basis
The objective of the 1997 guidelines was to prevent preeclampsia, but because the MHLW 2006 guidelines did not have population recommendations for women with higher BMI, we used these previous obstetrical guidelines for women with higher BMI in our analyses. These JSOG 1997 guidelines were published by 中林正雄 (1999) and cited in JSOG (2014).
From the perspective of public health and individual obstetric care, it is critical to identify modifiable behavioral factors that might reduce the rate of SGA in populations and to assess whether and how physicians can intervene. During pregnancy, many women are highly motivated to make behavioral changes to improve the health of their babies. Thus obstetricians and other healthcare professionals have an opportunity to monitor and influence eating behavior and weight gain, and to intervene to reduce SGA outcomes. In this study, we examined: (1) the extent to which pre-pregnancy BMI and GWG, as well as GWG ‘adequacy’ based on BMI categories, influence risk of SGA in babies; and (2) physician response to gestational weight gain and influence of their instructions on subsequent weight gain.
Methods
As part of a longitudinal study on developmental origins of childhood obesity, data were extracted from maternal child health handbooks (MCHHs), government-issued booklets that are provided free of charge to all pregnant women in Japan and used to prospectively record data at prenatal visits, birth outcomes, and child growth and development until age 6 years. These handbooks are generally retained until adulthood as they contain all childhood vaccination records, and constitute the primary medical record for vaccinations, childhood illness, and infant-child health. The MCHH provides data on prenatal anthropometrics, blood pressure, and urine tests and also records ‘instructions’ from medical professionals. Parents of all children enrolled in four elementary and two middle schools in the Saku region of Nagano prefecture in Japan were invited to participate in this study in 2009, and asked to bring their children's MCHHs to school for copying on the day of their parent-teacher conferences. Of the 903 elementary and middle school students in the recruitment area, we received 462 MCHHs (participation rate of 51.2%). All participants provided written informed consent.
GWG was calculated as weight gain from usual self-reported weight to last measured weight. All children in the logistic regression analysis were full-term (37 weeks or older). Women were assigned to 3 BMI categories, calculated using self-reported maternal height and pre-pregnancy weight according to the 2006 Japanese MHLW categories (for BMI<25) and the 1997 Japanese Society for Obstetrics and Gynecology (JSOG) recommendations (for BMI≥25) (中林正雄, 1999), since MHLW's recommendation for BMI ≥25 was to handle case by case with no specified weight gain range. GWG was compared to the recommended weight gain ranges based on pre-conception BMI (Table 1). Women were categorized as having inadequate, appropriate, or excessive GWG based on recommended ranges.
We conducted logistic regression with SGA (defined as birthweight percentile <10%, adjusted for gestational age, sex and primiparity using Japanese standards(JSPE, 2013)) as the dependent variable, examining maternal age, pre-pregnancy BMI and GWG as predictors. Model 1 included maternal age (years), usual weight (kg) and height to calculate BMI, and ‘last measured weight – usual weight’ to calculate GWG. Model 2 used GWG categories based on Japanese guidelines (Table 1). Smoking was not included due to small sample sizes and lack of cases of SGA babies (Table 2). We verified that the association between log odds of SGA and the covariates was roughly linear using lowess curves. We assessed whether non-linear associations including quadratic terms and spline terms were necessary in the models (data not shown). Two types of sensitivity analyses were conducted (results not shown):
-
(1)
We used (last measured weight – usual self-reported weight) as an indicator of GWG. Women's last measured weight occurred with a median of 5 days (range 0-17 days, IQR=2,6) before delivery. Since we expect an increase in weight in the last weeks of pregnancy, and since usual (pre-pregnancy) weights were self-reported, we used weights estimated taking week 37 – week 17 as a sensitivity analysis to assess bias of last measured weight and self-reported measurements. We used 37 weeks as our last weight since all mothers in the analytic sample had delivery at the 37th week or later (i.e., all children were full-term). We used 17 weeks for the first weight measurement due to variability in first measured weight at prenatal exams and missing weight data for women before week 17. While the first prenatal exam with recorded weights occurred at an average gestational age of 99±19 days, shortly after the start of the second trimester, 68/383 women (17.8%) had missing measured weight data before week 17. These weights were estimated using a restricted cubic spline for each mother.
-
(2)
Usual BMI was calculated from self-reported usual (pre-pregnancy) height and weight. Since mothers with missing usual weights had significantly higher first exam weights than mothers without missing data (60.7±14.8kg vs 52.2±6.9, P<0.001) despite no differences in gestational age at first exam (P=0.24), we used BMI estimated for week 17 as a sensitivity test to assess the possibility of bias in self-reported usual weights. Pre-pregnancy weight and first exam weight are highly correlated (Pearson correlation coefficient =0.93, N=383).
TABLE 2.
Sample characteristics with significant differences by sex and by SGA statusa
| Total n = 383 |
Males n = 192 |
Females n = 191 |
Not SGA n = 350 |
SGA n = 33 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Mean or Freq |
SD or % |
Mean or Freq |
SD or % |
Mean or Freq |
SD or % |
Mean or Freq |
SD or % |
Mean or Freq |
SD or % |
Sex Diffb P value |
SGA Diffb P value |
| Small for gestational age | 33 | 8.6% | 13 | 6.8% | 20 | 10.5% | 0.20 | |||||
| Gestational age at birth (weeks) | 39.6 | 1.2 | 39.6 | 1.2 | 39.6 | 1.1 | 39.6 | 1.2 | 39.8 | 1.1 | 0.74 | 0.29 |
| Birth weight (g) | 3054.1 | 365.6 | 3113.7 | 348.0 | 2994.2 | 373.9 | 3110.7 | 323.3 | 2453.9 | 222.3 | <0.01 | <0.001 |
| Maternal age (years) | 30.4 | 4.0 | 30.4 | 4.2 | 30.3 | 3.7 | 30.5 | 4.0 | 29.2 | 3.4 | 0.79 | 0.07 |
| Maternal height (cm) | 157.7 | 5.5 | 157.8 | 5.3 | 157.5 | 5.7 | 158.0 | 5.4 | 154.9 | 5.8 | 0.59 | <0.01 |
| Maternal pre-pregnancy weight (kg) | 51.6 | 6.7 | 51.8 | 6.3 | 51.4 | 7.0 | 51.9 | 6.7 | 48.2 | 5.2 | 0.54 | <0.01 |
| Maternal pre-pregnancy BMI (kg/m2) | 20.7 | 2.5 | 20.8 | 2.3 | 20.7 | 2.7 | 20.8 | 2.5 | 20.1 | 2.0 | 0.77 | 0.12 |
| Parity | 0.39 | 0.07 | ||||||||||
| 0 | 188 | 49.1% | 100 | 52.1% | 88 | 46.1% | 166 | 47.4% | 22 | 66.7% | ||
| 1 | 132 | 34.5% | 60 | 31.3% | 72 | 37.7% | 123 | 35.1% | 9 | 27.3% | ||
| 2 or more children | 63 | 16.5% | 32 | 16.7% | 31 | 16.2% | 61 | 17.4% | 2 | 6.1% | ||
| Primiparity (# primparous) | 188 | 49.1% | 100 | 52.1% | 88 | 46.1% | 166 | 47.4% | 22 | 66.7% | 0.24 | 0.03 |
| Maternal Smoking (# current or past)c | 8 | 2.2% | 4 | 2.2% | 4 | 2.2% | 8 | 2.4% | 0 | 0.0% | 0.99 | 0.38 |
| GWG | 9.4 | 3.5 | 9.5 | 3.3 | 9.3 | 3.7 | 9.5 | 3.4 | 8.3 | 3.9 | 0.73 | 0.06 |
| GWG adequacyd | 0.79 | 0.07 | ||||||||||
| inadequate | 83 | 21.7% | 39 | 20.3% | 44 | 23.0% | 71 | 20.3% | 12 | 36.4% | ||
| adequate | 224 | 58.5% | 115 | 59.9% | 109 | 57.1% | 210 | 60.0% | 14 | 42.4% | ||
| excessive | 76 | 19.8% | 38 | 19.8% | 38 | 19.9% | 69 | 19.7% | 7 | 21.2% | ||
For dichotomous variables, P-values are derived from two-sided asymptotic Pearson Chi-Square tests. For continuous variables, P-values are derived from t-tests. Significant sex and and SGA differences are shown with significant P-values (P<0.05) in bold.
Welch robust test (which does not assume equality of variance) gave similar results to t-tests.
Smoking status was missing for 24 mothers. N=359 (total), 180 (male), 179 (female), 328 (not SGA), 31 (SGA).
Adequacy assessed by Japanese guidelines (see Table 1)
Abbreviations: BMI= body mass index; GWG=gestational weight gain
Significant differences in characteristics between male and female babies, and between SGA and non-SGA, were assessed using t-tests (with and without assuming equal variance) for continuous variables, and 2-sided Pearson Chi-Square tests for dichotomous variables. Variables of interest were entered into logistic regression models, and differences of P<0.05 were considered significant. We used Generalized Estimating Equations (GEE) regression with robust variance estimation to account for clustering among children of the same mother. STATA (ver. 13.1) was used for all analyses.
In addition to standard prenatal uterine height, abdominal circumference, blood pressure and urine tests results, each MCHH prenatal exam entry includes weight, doctor's instructions for patient, and name of medical institution and doctor. All doctors’ instructions and comments were reviewed, and themes related to weight, diet, eating, and intake (e.g., of salt, water, sugar, etc.) were coded into categories following guidelines for thematic analysis of qualitative data (Braun and Clarke, 2006). Data from the immediately preceding and subsequent prenatal exams were compared with data from the exam in which the comment was received to assess changes preceding and following the comment in: weight (kg), weight gain rate (kg/week), and blood pressure (mmHg).
This study is a part of the project ‘Developmental Origins of Childhood Obesity’, which was approved by Saku Central Hospital Research Ethics Review Committee on 27 May 2009 (#21-7), National Institute of Health and Nutrition Research Ethics Review Committee on 6 July 2009 (#20090706-01), and University of Delaware IRB on 10 September 2012 with continuing review approval on 9 September 2015 (#360687).
RESULTS
All MCHHs were copied and all potentially relevant data were entered into a database. Of the 462 children (236M, 226F) whose MCHHs were received, 12 were multiples, and 15 were born prematurely (< 37 weeks of gestation), resulting in 437 full-term singleton births. Because maternal height and pre-pregnancy weight were self-reported and required for calculation of BMI and GWG adequacy, missing maternal height and/or weight reduced the sample to 384. One girl was born at 43 weeks and could not have SGA calculated using available standards (JSPE, 2013), thus reducing the final sample to 383 mother-child pairs for complete-case analysis of GWG and SGA risk. Qualitative data analysis of physicians’ written comments was conducted using all 462 handbooks.
Table 2 shows sample characteristics with significant differences by sex and SGA. Of the 383 singletons born at full term (≥ 37 weeks and < 43 weeks) to mothers with self-reported maternal height and pre-pregnancy weight data, SGA babies comprised 8.6% (33/383) of the sample, with 13 males and 20 females. The population average pre-pregnancy weight was 51.6 kg and average BMI was 20.7. Thirteen percent (51/383) of women were classified as underweight with BMI < 18.5. Overweight or obese women comprised only 6% of the population (25/383). Significant sex differences were observed for birthweight, but none were observed for any maternal or pregnancy characteristics examined. Significant differences between SGA and non-SGA babies were observed for birthweight, maternal height, pre-pregnancy weight; and primiparity (Table 2). Compared to mothers of non-SGA babies, mothers of SGA babies were significantly shorter, and lighter in weight, and were more likely to be primiparous. Statistical trends (P<0.1) for differences between SGA and non-SGA babies were observed for maternal age, parity and GWG adequacy. The average number of prenatal visits was 11.7 (median = 12, range =6-17, IQR = 10-13).
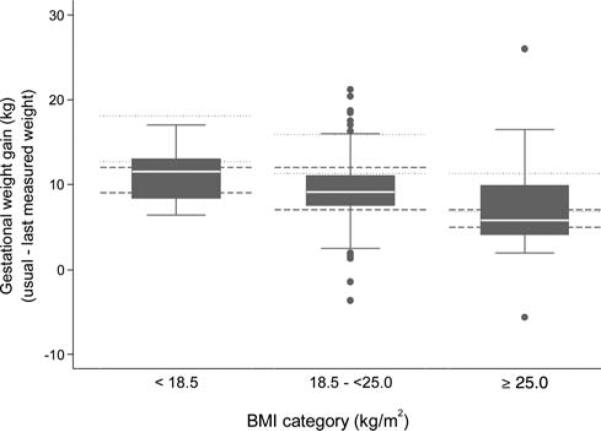
Figure 1 shows GWG by usual BMI category, with Japanese and US recommendations included for comparison. According to the 2006 MHLW recommended GWG ranges, 21.7% of this population gained less than the recommended amounts, and 19.8% gained more than the recommended amounts (Table 3).
Fig. 1.
Boxplots of gestational weight gain by BMI category (dashed lines show Japanese MHLW recommendations and dotted lines show US recommendations for upper and lower bound of recommended weight gains). BMI 5 body mass index.
TABLE 3.
Gestational weight gain assessment category by maternal usual BMI category using Japanese guidelines
| Gestational weight gain |
||||||||
|---|---|---|---|---|---|---|---|---|
| Maternal usual BMI (kg/m2) | Inadequate | Adequate | Excessive | Total | ||||
| <18.5 | 14 | (27.5%) | 17 | (33.3%) | 20 | (39.2%) | 51 | (100%) |
| 18.5–<25 | 58 | (18.8%) | 203 | (67.7%) | 48 | (15.5%) | 309 | (100%) |
| ≥25 | 11 | (47.8%) | 4 | (17.4%) | 8 | (34.8%) | 23 | (100%) |
| Total | 83 | (21.7%) | 224 | (58.5%) | 76 | (19.8%) | 383 | (100%) |
SGA Risk
Table 4 shows logistic regression results. In Model 1, higher pre-pregnancy BMI and GWG were associated with lower risk of SGA in girls (OR= 0.71, 95% CI 0.56, 0.90; and 0.75, 95% CI 0.61, 0.92, respectively). In Model 2 inadequate GWG was associated with higher risk of SGA in girls as compared to adequate GWG (OR 6.64, 95% CI 2.18, 20.22) while higher pre-pregnancy BMI was associated with a borderline significantly lower risk of SGA (OR 0.81, 95% CI 0.66, 1.00). Maternal BMI and GWG were not significantly associated with SGA risk in boys. Increasing maternal age exhibited a trend suggesting lower likelihood of SGA (P=0.05) in boys, but not girls.
TABLE 4.
Odds ratios (OR), 95% confidence intervals (CI), and significance of variables associated with small for gestational age (SGA) (≤ 10%ile) adjusted for sex, gestational age and primiparity in logistic regression by sex: (A) Males, and (B) Females.
| Model 1 (n = 192) |
Model 2 (n = 192) |
||||||
|---|---|---|---|---|---|---|---|
| Variable | OR | 95% CI | P | OR | 95% CI | P | |
| A. Males | |||||||
| Maternal age (year) | 0.88 | (0.78, 1.00) | 0.05 | 0.89 | (0.78, 1.01) | 0.07 | |
| Maternal usual BMIa (kg/m2) | 1.02 | (0.78, 1.34) | 0.86 | 1.03 | (0.79, 1.34) | 0.83 | |
| Gestational weight gain | Usual to last meas.b (kg) | 0.98 | (0.87, 1.12) | 0.81 | |||
| 3 categoriesc (ref. normal) | 0.86 | ||||||
| Inadequate | 0.78 | (0.14, 4.30) | 0.78 | ||||
| Excessive | 0.65 | (0.14, 3.12) | 0.59 | ||||
| Statistics for model check Hosmer–Lemshow test (P-value) | 0.83 | 0.40 | |||||
| Model 1 (n = 191) |
Model 2 (n = 191) |
||||||
|---|---|---|---|---|---|---|---|
| Variable | OR | 95% CI | P | OR | 95% CI | P | |
| B. Females | |||||||
| Maternal age (year) | 0.92 | (0.81, 1.05) | 0.21 | 0.97 | (0.87, 1.09) | 0.65 | |
| Maternal usual BMIa (kg/m2) | 0.71 | (0.56, 0.90) | <0.01** | 0.81 | (0.66, 1.00) | 0.05 | |
| Gestational weight gain | Usual to last meas.b (kg) | 0.75 | (0.61, 0.92) | <0.01** | |||
| 3 categoriesc (ref. normal) | <0.01** | ||||||
| Inadequate | 6.64 | (2.18, 20.22) | <0.001*** | ||||
| Excessive | 2.75 | (0.84, 8.94) | <0.09 | ||||
| Statistics for model check Hosmer–Lemshow test (P-value) | 0.67 | 0.72 | |||||
CI: confidence interval, OR odds ratio.
* <P < 0.05.
P < 0.01.
P < 0.001.
Based on reported usual height and weight.
Calculated as a difference between the weight measured at the last prenatal visit and the reported usual weight.
According to Japanese recommended gestational weight gain during pregnancy (Ministry of Health, Labour and Welfare, Japan, 2006).
Sensitivity Analyses
Sensitivity analyses performed by conducting logistic regression using GWG from 17-37 weeks instead of usual-last measured exam, and maternal BMI at 17 weeks (and GWG from first to last exam) gave similar results in terms of significant variables and odds ratios, although with increased significance for most variables as sample sizes were higher (data not shown).
Physician Roles
Physician instructions notated in handbooks were coded into the following categories: (1) restrict weight gain and/or dietary intake (including: (a) caution about weight or weight increase, (b) guidance about meals, (c) restrict calories, (d) restrict snacking); (2) restrict water; (3) restrict salt; (4) restrict water and/or salt; and (5) lose weight. Fifty comments related to diet and/or weight gain were recorded for 23 pregnancies. Comments on weight gain and/or restricting eating appeared in 30 exams, and on water- or salt-restriction in 23 exams, as early as 16 weeks gestation. Physician comments about weight gain or water and salt restriction followed an average weight gain rate of 0.6-0.7kg/week and were followed by weight gain rates that decreased by more than half to 0.3kg/week.
No comments advising women to increase weight gain were recorded in any of the 462 handbooks in spite of the fact that 22% had inadequate weight gain according to Japanese guidelines. One woman (pre-pregnancy weight of 49kg and BMI of 20.4) was instructed to not gain more than 10kg during pregnancy, a common recommendation in Japan. The absence of comments about inadequate weight gain was striking in several cases. One woman reported her pre-pregnancy weight as 54kg (BMI 24.7), but her first prenatal exam weight was recorded as 48kg (BMI 22). Her last recorded weight during pregnancy was only 50.4kg. She gave birth to a girl at 38.1wks weighing 2340g after a total weight gain of 2.4kg from her first exam, or a total GWG of −3.6kg from her pre-pregnancy weight. Yet no comments about weight were ever recorded. Later in the MCHH, a physician recorded concerns about the baby's delayed movement and weight gain. According to the reproductive history record in the MCHH, she had a previous LBW birth, further increasing the risk of SGA for her daughter.
DISCUSSION
WHO does not currently make GWG recommendations, although determinants of LBW were reported over 25 years ago in the Bulletin of the WHO, and the top 3 for developed countries were smoking, poor gestational nutrition and low pre-pregnancy weight (Kramer, 1987). In contrast, both the US IOM and Japan have clear GWG guidelines that vary depending on maternal pre-conception BMI, yet do not vary by sex of the baby, nor by ethnicity in the case of IOM despite significant differences between US and Japanese guidelines (Table 1). The current results suggest that GWG recommendations may be particularly critical for women carrying female fetuses, and that optimal GWG may vary by ethnicity and population.
Girls’ increased sensitivity to maternal nutritional status during gestation (as measured by GWG) relative to boys may be related to apparent sex differences in the effects of a fetus on mother's glucose metabolism. A recent study found that women carrying a male fetus had higher odds (OR 1.39) of developing gestational diabetes (Retnakaran et al., 2015). While the paper focused on the implications for the mother's health, it is interesting to consider the implications for the baby. That is, boys may be more able than girls to obtain the glucose needed for growth by manipulating the mother's metabolism. Fetal sex differences in growth trajectories in response to maternal size have been documented (Lampl et al., 2010), and need further exploration in diverse populations.
Optimal GWG ranges may vary by population and ethnicity. Interestingly, when this Japanese population is analyzed according to the 2009 US IOM guidelines, most (>75%) gained less than and only 3% gained more than the recommended amounts during pregnancy (data not shown), compared to 22% and 20% respectively by the Japanese guidelines. Yet only 8.6% of the births were SGA, much lower than expected if >75% had inadequate GWG. These results are consistent with results from other studies of Japanese GWG and SGA (Harita et al., 2012), although those studies generally combine males and females in their analyses (Tanaka et al., 2014). The lower-than-expected prevalence of SGA based on IOM-defined guidelines for this population raises questions about the appropriateness of IOM guidelines for Japanese-Americans, and possibly other subpopulations with Asian ancestry (Ee et al., 2014).
Among those categorized as having inadequate GWG by IOM guidelines (Fig. 1), weight gains averaged 5.5kg less than the recommended range midpoint, much more than the weight of a healthy baby (2.5-4kg). Since GWG includes 3 components (the products of conception (fetus and placenta), maternal tissues, and maternal fat reserves) (CIPWMCH, 2007), the low rates of SGA relative to IOM GWG adequacy suggest that fetal growth may be prioritized over maternal tissues and fat reserves, and optimal GWG influenced by maternal characteristics may exhibit population differences. Given that Asian BMI cutoffs have been set differently for chronic disease risk (e.g. less than 18·5 kg/m2 underweight; 18·5–23 kg/m2 increasing but acceptable risk; 23–27·5 kg/m2 increased risk; and 27·5 kg/m2 or higher high risk) from those for Europeans and their descendants (Shiwaku et al., 2004), perhaps Asian populations require less maternal tissues or fat reserves during pregnancy to achieve adequate fetal growth. Results of this study suggest that further research is required to assess whether GWG recommendations should be modified by ethnicity and population to optimize birth outcomes. The IOM guidelines may not be biologically universal, particularly problematic given the diversity of the US population.
The analysis of physician instructions and their effect on weight gain suggests that patients do follow physician instructions with respect to diet and weight gain, and thus physicians may have an important role to play in modifying dietary behavior to ensure adequate GWG to improve outcomes related to SGA. Advised and target weight gains have been strongly associated with actual weight gains (Cogswell et al., 1999), underscoring the need for public health guidelines and prenatal monitoring of appropriate target weight gains. Indeed, all restrictive diet- and weight-related comments (e.g., restrict weight gain, snacking, water intake, salt intake) appeared to have effects on weight gain rate, approximately halving weight gain rate to the subsequent exam, suggesting an ability of physician instructions to influence behavior related to weight gain. While verbal instructions may have been given more often than written comments, the nature of the latter (e.g., recommending food and weight gain restriction; absence of comments about inadequate weight gain) are probably indicative of the types of or lack of instructions women received during their prenatal checkups.
The lack of comments about inadequate weight gain in this population, although 22% were categorized as inadequate compared to 20% categorized as excessive, may reflect a legacy of chisaku umare, ookiku sodatte (birth small, raise large). A majority of Japanese women believe that smaller babies will translate to an easier delivery (Takimoto et al., 2011), and this widespread belief might lead obstetricians to focus more on efforts to limit rather than encourage weight gain, despite increasing rates of SGA babies and high proportions of women with low pre-pregnancy BMI (Takimoto et al., 2005). If the Developmental Origins of Health and Disease paradigm is correct, increases in SGA babies are likely to lead to increases in later life chronic disease in this population (Barker, 2004; Martin-Gronert and Ozanne, 2012).
This study's strengths lie in the coupling of detailed clinical prenatal visits (average of 11.7 per mother) with birth records in a population where almost all women receive regular prenatal care. Additionally, written records of physician instructions during pregnancy were available for qualitative analysis. In contrast to many developed countries, Japan faces a high proportion of underweight women of reproductive age and thus provides an excellent population in which to examine the Developmental Origins of Health and Disease paradigm. Low maternal pre-pregnancy BMI and inadequate GWG appear to place girls at particular risk of SGA, possibly due to differences in metabolism and growth strategies (Lampl et al., 2010). Conclusions of this study about the effects of GWG on SGA may be limited by small sample sizes, particularly for boys, which constrain interpretation of logistic regression results. It may be that girls are more sensitive to maternal BMI and GWG than boys, or that our sample was underpowered to detect such relationships among boys. Some children who were born SGA and had associated health problems or disabilities may not have been enrolled in local schools, thus potentially biasing the sample and limiting our analyses only to those children who were healthy enough to enroll in local public schools. Possible selection-bias may have occurred if parents who remembered to bring their handbooks to parent-teacher conferences were different from other parents. Analysis of physicians’ comments can be considered exploratory due to small sample sizes. Further qualitative research is needed to understand women's and providers’ knowledge and beliefs about healthy GWG.
CONCLUSIONS
Many Japanese women (13%) not only have low BMI at conception, but many also have inadequate GWG according to Japanese recommendations (21.7%), with much higher proportions of women categorized as having inadequate GWG according to the IOM guidelines. Maternal pre-pregnancy BMI and GWG inadequacy are significant predictors of SGA in girls, with lower BMI and lower GWG associated with higher risk of SGA. GWG appears to be a modifiable behavioral variable, potentially amenable to influence from physicians’ instructions.
This study's results suggest that education of health care providers and women is needed to decrease the rates of SGA, by recommending adequate target weight gains based on BMI categories and making changes to medical record forms (such as MCHHs in Japan) and prenatal exams to facilitate monitoring and interventions to ensure appropriate weight gain, particularly for women carrying female babies. Maternal height and weight should be measured at the earliest prenatal exam to estimate pre-pregnancy BMI; optimal GWG range should be explained; and weight gain rate between exams and adequacy should be recorded at each prenatal exam. Similar to sex-specific infant and child growth charts, perhaps sex-specific GWG charts could be created, with optimal ranges identified for each BMI category, possibly modified by ethnicity and population.
ACKNOWLEDGMENTS
We thank the women and children who generously shared their maternal child health handbooks and made this research possible. We gratefully acknowledge the cooperation and assistance of staff from the National Institute of Health and Nutrition; Saku Public Health Center and Saku Central Hospital; the Boards of Education in Yachiho and Koumi; and Yachiho and Koumi elementary and middle schools. We thank M. Kaihoku for research coordination and data entry, K. Maizuru for data entry, M. Griffin for extensive data clean-up and preliminary analyses, Y. Ito for consultation about the use of Japanese anthropometric indicators and S. Noazin for data cleaning.
Grant sponsorship:
Wenner-Gren Foundation for Anthropological Research (#7902); University of Delaware General University Research grant (#12A00454); intramural funding from the National Institute of Health and Nutrition (Tokyo, Japan).
Footnotes
Author Contributions: MM conceived and planned the study, collected the data, performed preliminary analyses, drafted and revised the manuscript. GY conducted data cleaning and final statistical analyses. PS consulted on final analyses and revised the manuscript. All authors edited the manuscript for intellectual content and provided critical comments on the manuscript.
LITERATURE CITED
- Barker DJ. Fetal origins of coronary heart disease. Bmj. 1995;311(6998):171–174. doi: 10.1136/bmj.311.6998.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ. The developmental origins of adult disease. J Am Coll Nutr. 2004;23(6 Suppl):588S–595S. doi: 10.1080/07315724.2004.10719428. [DOI] [PubMed] [Google Scholar]
- Barker DJ, Eriksson JG, Forsen T, Osmond C. Fetal origins of adult disease: strength of effects and biological basis. Int J Epidemiol. 2002;31(6):1235–1239. doi: 10.1093/ije/31.6.1235. [DOI] [PubMed] [Google Scholar]
- Braun V, Clarke V. Using thematic analysis in psychology. Qualitative Research in Psychology. 2006;3(2):77–101. [Google Scholar]
- CIPWMCH (Committee on the Impact of Pregnancy Weight on Maternal and Child Health) Influence of Pregnancy Weight on Maternal and Child Health: Workshop Report. Institute of Medicine and National Research Council of the National Academies, editor: The National Academies Press; 2007. p. 116. [Google Scholar]
- Cogswell ME, Scanlon KS, Fein SB, Schieve LA. Medically advised, mother's personal target, and actual weight gain during pregnancy. Obstet Gynecol. 1999;94(4):616–622. doi: 10.1016/s0029-7844(99)00375-0. [DOI] [PubMed] [Google Scholar]
- Ee TX, Allen JC, Jr., Malhotra R, Koh H, Ostbye T, Tan TC. Determining optimal gestational weight gain in a multiethnic Asian population. J Obstet Gynaecol Res. 2014;40(4):1002–1008. doi: 10.1111/jog.12307. [DOI] [PubMed] [Google Scholar]
- Gillman MW. Gestational weight gain: now and the future. Circulation. 2012;125(11):1339–1340. doi: 10.1161/CIRCULATIONAHA.112.091751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harita N, Kariya M, Hayashi T, Sato KK, Aoki T, Nakamura K, Endo G, Narimoto K. Gestational bodyweight gain among underweight Japanese women related to small-for-gestational-age birth. J Obstet Gynaecol Res. 2012;38(9):1137–1144. doi: 10.1111/j.1447-0756.2012.01848.x. [DOI] [PubMed] [Google Scholar]
- IOM. Weight gain during pregnancy: Reexamining the guidelines. Institute of Medicine and National Research Council of the National Academies; Washington, D.C.: 2009. [PubMed] [Google Scholar]
- JSOG (Japanese Society of Obstetrics and Gynecology) In: Japanese Society of Obstetrics and Gynecology, editor. Tokyo, Japan: 2014. [1 Feb 2016]. 助産雑誌 2014 年 11 月号 特 集 『産婦人科診療ガイドライン-産礁編 2014』の改ポイント説 (Guideline for Obstetrical Practice in Japan 2014). 医学孢院. http://www.jsog.or.jp/activity/pdf/gl_sanka_2014.pdf. [Google Scholar]
- JSPE (Japanese Society for Pediatric Endocrinology) Japanese Society for Pediatric Endocrinology, editor. [1 Feb 2016];2013 体格指数計ファイル[Calculation file for anthropometric indices]. http://jspe.umin.jp/medical/files/taikakubirthlongcrossv1.xlsx.
- Kirchengast S, Hartmann B. Impact of maternal age and maternal somatic characteristics on newborn size. Am J Hum Biol. 2003;15(2):220–228. doi: 10.1002/ajhb.10139. [DOI] [PubMed] [Google Scholar]
- Kramer MS. Determinants of low birth weight: methodological assessment and meta-analysis. Bull World Health Organ. 1987;65(5):663–737. [PMC free article] [PubMed] [Google Scholar]
- Lampl M, Gotsch F, Kusanovic JP, Gomez R, Nien JK, Frongillo EA, Romero R. Sex differences in fetal growth responses to maternal height and weight. Am J Hum Biol. 2010;22(4):431–443. doi: 10.1002/ajhb.21014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law CM, Egger P, Dada O, Delgado H, Kylberg E, Lavin P, Tang GH, von Hertzen H, Shiell AW, Barker DJ. Body size at birth and blood pressure among children in developing countries. Int J Epidemiol. 2001;30(1):52–57. doi: 10.1093/ije/30.1.52. [DOI] [PubMed] [Google Scholar]
- Law CM, Shiell AW. Is blood pressure inversely related to birth weight? The strength of evidence from a systematic review of the literature. J Hypertens. 1996;14(8):935–941. [PubMed] [Google Scholar]
- Leon DA, Koupil I, Mann V, Tuvemo T, Lindmark G, Mohsen R, Byberg L, Lithell H. Fetal, developmental, and parental influences on childhood systolic blood pressure in 600 sib pairs: the Uppsala Family study. Circulation. 2005;112(22):3478–3485. doi: 10.1161/CIRCULATIONAHA.104.497610. [DOI] [PubMed] [Google Scholar]
- Martin-Gronert MS, Ozanne SE. Mechanisms underlying the developmental origins of disease. Rev Endocr Metab Disord. 2012;13(2):85–92. doi: 10.1007/s11154-012-9210-z. [DOI] [PubMed] [Google Scholar]
- MHLW. In: Department of Children and Family. Mother and Child Health Preservation Section), editor. Tokyo: 2006. [26 Jan 2016]. pp. 61–74. 「妊産婦のための食生指針」の策なについて (About the Plan of Eating/Dietary Guidelines for Expectant and Nursing Mothers). In: 厚生労働厚生労 働等・児童家庭局 母子保健課 (Ministry of Health, Labour and Welfare, and Employment Equality. 厚生働省発衫働 (Ministry of Health, Labour and Welfare Publisher) http://www.mhlw.go.jp/houdou/2006/02/dl/h0201-3a4.pdf. [Google Scholar]
- Mohsin M, Wong F, Bauman A, Bai J. Maternal and neonatal factors influencing premature birth and low birth weight in Australia. J Biosoc Sci. 2003;35(2):161–174. doi: 10.1017/s0021932003001615. [DOI] [PubMed] [Google Scholar]
- Osmond C, Barker DJ, Winter PD, Fall CH, Simmonds SJ. Early growth and death from cardiovascular disease in women. BMJ. 1993;307(6918):1519–1524. doi: 10.1136/bmj.307.6918.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retnakaran R, Kramer CK, Ye C, Kew S, Hanley AJ, Connelly PW, Sermer M, Zinman B. Fetal sex and maternal risk of gestational diabetes mellitus: the impact of having a boy. Diabetes Care. 2015;38(5):844–851. doi: 10.2337/dc14-2551. [DOI] [PubMed] [Google Scholar]
- Rinaudo PF, Lamb J. Fetal origins of perinatal morbidity and/or adult disease. Semin Reprod Med. 2008;26(5):436–445. doi: 10.1055/s-0028-1087109. [DOI] [PubMed] [Google Scholar]
- Shiwaku K, Anuurad E, Enkhmaa B, Kitajima K, Yamane Y. Appropriate BMI for Asian populations. Lancet. 2004;363(9414):1077. doi: 10.1016/S0140-6736(04)15856-X. [DOI] [PubMed] [Google Scholar]
- Simmons R. Developmental origins of adult metabolic disease: concepts and controversies. Trends Endocrinol Metab. 2005;16(8):390–394. doi: 10.1016/j.tem.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Stettler N, Tershakovec AM, Zemel BS, Leonard MB, Boston RC, Katz SH, Stallings VA. Early risk factors for increased adiposity: a cohort study of African American subjects followed from birth to young adulthood. Am J Clin Nutr. 2000;72(2):378–383. doi: 10.1093/ajcn/72.2.378. [DOI] [PubMed] [Google Scholar]
- Stevens-Simon C, Orleans M. Low-birthweight prevention programs: the enigma of failure. Birth. 1999;26(3):184–191. doi: 10.1046/j.1523-536x.1999.00184.x. [DOI] [PubMed] [Google Scholar]
- Takimoto H, Mitsuishi C, Kato N. Attitudes toward pregnancy related changes and self-judged dieting behavior. Asia Pac J Clin Nutr. 2011;20(2):212–219. [PubMed] [Google Scholar]
- Takimoto H, Yokoyama T, Yoshiike N, Fukuoka H. Increase in low-birth-weight infants in Japan and associated risk factors, 1980-2000. J Obstet Gynaecol Res. 2005;31(4):314–322. doi: 10.1111/j.1447-0756.2005.00294.x. [DOI] [PubMed] [Google Scholar]
- Takimoto H, Yoshiike N, Kaneda F, Yoshita K. Thinness among young Japanese women. Am J Public Health. 2004;94(9):1592–1595. doi: 10.2105/ajph.94.9.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Ashihara K, Nakamura M, Kanda T, Fujita D, Yamashita Y, Terai Y, Kamegai H, Ohmichi M. Associations between the pre-pregnancy body mass index and gestational weight gain with pregnancy outcomes in Japanese women. J Obstet Gynaecol Res. 2014;40(5):1296–1303. doi: 10.1111/jog.12353. [DOI] [PubMed] [Google Scholar]
- Tsukamoto H, Fukuoka H, Inoue K, Koyasu M, Nagai Y, Takimoto H. Restricting weight gain during pregnancy in Japan: a controversial factor in reducing perinatal complications. Eur J Obstet Gynecol Reprod Biol. 2007;133(1):53–59. doi: 10.1016/j.ejogrb.2006.07.031. [DOI] [PubMed] [Google Scholar]
- Valero De Bernabe J, Soriano T, Albaladejo R, Juarranz M, Calle ME, Martinez D, Dominguez-Rojas V. Risk factors for low birth weight: a review. Eur J Obstet Gynecol Reprod Biol. 2004;116(1):3–15. doi: 10.1016/j.ejogrb.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 1999;51(12):507–510. 中 林 正 雄 . 妊娭中毒病の栄養管理指針 (Nutritional management guidelines for pregnancy toxemia). 白産婦誌 (Acta Obstetrica et Gynaecologica Japonica) [Google Scholar]