Abstract
Objective
The aim of this study was to investigate the impact of pretreatment with transdermal estradiol (E2) compared to oral contraceptive pills (OCPs) on controlled ovarian stimulation (COS) response in normal responders undergoing fresh in vitro fertilization (IVF)-embryo transfer (ET) cycles.
Methods
A retrospective cohort study was performed of normal responders undergoing fresh IVF-ET cycles who received pretreatment with transdermal E2 versus OCPs prior to fresh IVF-ET. The total days of ovarian stimulation, total dosage of gonadotropins, total number of oocytes, and mature oocytes retrieved were noted. Pregnancy outcomes after ET were also recorded.
Results
A total of 2,092 patients met the inclusion criteria: 1,057 and 1,035 patients in the transdermal E2 and OCP groups, respectively. Patients in the OCP group had a longer duration of COS (10.7±1.63 days, p<0.01) than the E2 group (9.92±1.94 days). Patients in the OCP group also required higher cumulative doses of gonadotropins (2,657.3±1,187.9 IU) than those in the E2 group (2,550.1±1,270.2 IU, p=0.002). No statistically significant differences were found in the total and mature oocytes retrieved or in the rates of biochemical pregnancy, clinical pregnancy, spontaneous miscarriage, and live birth between the groups.
Conclusion
Our findings suggest that compared to OCPs, pretreatment with transdermal E2 is associated with a shorter duration of ovarian stimulation and lower gonadotropin utilization, without compromising the oocyte yield or pregnancy outcomes in normal-responder patients undergoing fresh IVF.
Keywords: Contraceptives, Oral, Combined; In vitro fertilization; Reproductive techniques, Assisted; Superovulation; Transdermal patch
Introduction
The rising utilization and success of in vitro fertilization (IVF) can be attributed to the optimization of several clinical and laboratory protocols [1]. One such example is the optimization of controlled ovarian stimulation (COS) by incorporating gonadotropin-releasing hormone antagonist (GnRH-ant)-based COS protocols [2,3]. These COS protocols have several advantages over traditional long GnRH-agonist protocols, including lower utilization of gonadotropins [2,3], a lower risk of ovarian hyperstimulation syndrome [2,3], and a lower rate of ovarian cyst formation [4]. COS with GnRH-ant based protocols can be preceded by pretreatment with oral contraceptive pills (OCPs) or estrogen, with the goal of synchronizing follicular growth [5,6]. Most of these findings have been reported in studies comparing OCPs [7,8,9,10,11,12] or estrogen [5,13,14,15,16] to no pretreatment. However, scarce data are available comparing transdermal pretreatment modalities to standard pretreatment modalities in terms of COS, embryological, or pregnancy outcomes. Thus, the primary objective of this study was to compare the impact of pretreatment with transdermal estradiol (E2) to OCPs on COS response in normal responders undergoing fresh IVF-embryo transfer (ET) cycles with GnRH-ant based protocols.
Methods
1. Inclusion and exclusion criteria
All patients undergoing fresh IVF-ET cycles between January 2008 and June 2013 at the Round O. Perelman and Claudia Cohen Center for Reproducrive Medicine were analyzed for inclusion. Patients qualified as normal responders [17] when they met the following criteria: (1) age <40 years, (2) cycle day (CD) 2/3 follicle stimulating hormone (FSH) level <12 mIU/mL, (3) CD 2/3 E2 level <75 pg/mL, and (4) anti-Müllerian hormone (AMH) level >1 ng/mL. Patients not meeting the aforementioned criteria as well as those with known history of polycystic ovarian syndrome, or poor response or cancellation in a prior IVF-ET cycle were excluded from the analysis. The institutional review board at Weill Cornell Medical College approved our retrospective study protocol.
2. Clinical and laboratory protocols
Previously described protocols for COS, ovulatory trigger, oocyte retrieval, and ET were utilized [18,19]. Patients were assigned to pretreatment with E2 or OCPs based on physician preference. E2 was administered via 0.1-mg E2 patches (Vivelle-Dot estradiol transdermal system, Novartis Pharmaceuticals Co., East Hanover, NJ, USA). The active E2 component in these patches is estra-1,3,5 (10)-triene-3,17<β-diol. Patients applied 0.1-mg E2 patches 10 days after detection of the luteinizing hormone (LH) surge of the preceding menstrual cycle, and changed them every other day until the onset of menses [18]. COS with gonadotropins began on CD 2 of their menstrual cycle. Patient without menses after the application of the fourth E2 patch were underwent an assessment of their serum FSH, LH, E2, and β-human chorionic gonadotropin (β-hCG) levels as well as transvaginal pelvic ultrasonography, and if the findings were normal, proceeded with COS [8,12]. Patients undergoing pretreatment with OCPs began the pill (Ortho-Novum, Ortho-McNeil-Janssen Pharmaceuticals, Titusville, NJ, USA) on CD 1 of their preceding menstrual cycle. Following treatment with OCPs for 10 to 14 days, COS ensued approximately 2 to 3 days after discontinuation of the last pill.
The gonadotropin doses for COS were based on the patient's age, body mass index (BMI, kg/m2), antral follicle count, and serum AMH level. COS was initiated with gonadotropins (Gonal-F, EMD Serono, Rockland, MA, USA or Follistim, Merck, Kenilworth, NJ, USA; and Menopur, Ferring Pharmaceuticals, Parsippany, NJ, USA). Ovulation was suppressed with once-daily 0.25-mg Ganirelix acetate (Merck) injections, which were started when the lead follicle was >13 mm or the E2 level was 300 pg/mL [18,19]. The hCG trigger (Pregnyl, Merck or Novarel, Ferring Pharmaceuticals) was given when the two lead follicles attained a mean diameter of >17 mm. Oocyte retrieval was performed approximately 34 to 35 hours after the hCG trigger under conscious sedation. Intramuscular progesterone (50 mg daily) was started the day after retrieval. Fertilization of oocytes was achieved with conventional in vitro insemination or intracytoplasmic sperm injection [20]. All embryos were incubated in in-house culture media [18]. All ETs were performed with Wallace catheters (Smiths Medical, Norwell, MA, USA).
3. Outcome variables
Baseline demographics recorded for patients included age (years), gravidity, BMI (kg/m2), infertility diagnosis, CD 2/3 FSH level (mIU/mL), AMH level (ng/mL), and number of previous IVF attempts. COS parameters included total days of ovarian stimulation, total days of GnRH-ant administration, total dosage of gonadotropins (IU), E2 level (pg/mL) on the day of trigger, peak endometrial thickness (mm), total number of oocytes retrieved, mature oocytes retrieved, and fertilization rate (%). The number of cycles canceled as well as the number of surplus embryos cryopreserved at the blastocyst stage were also noted. Any pregnancy with positive hCG but without a gestational sac was considered a biochemical pregnancy. Clinical pregnancy was defined as the number of intrauterine gestations with fetal cardiac activity per IVF-ET cycle. Pregnancy loss after visualizing an intrauterine gestation was considered a spontaneous miscarriage. A live birth was any birth after 24 weeks of gestation.
4. Statistical analysis
Categorical variables were expressed as number of cases and percentage of occurrence and assessed using the chi-square test with the Mantel-Haenszel correction. Non-parametric variables were expressed as median (interquartile range) and were tested with the Wilcoxon rank-sum test. All continuous variables were checked for normality using the Shapiro-Wilk test and expressed as mean±standard deviation. The independent t-test was utilized for statistical comparisons of continuous variables. Statistical significance was set at p<0.05. Based on the study of Hauzman et al. [14] that showed a gonadotropin dosage difference of 65 IU between the E2 (1,692±488 IU) and OCP (1,627±565 IU) groups, a sample of size of 1,036 patients per group was estimated, assuming an α-error of 5% and a power of 80%.
Results
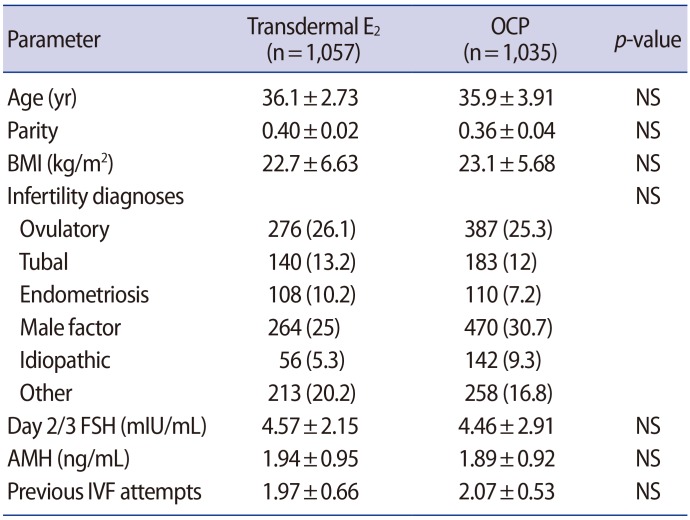
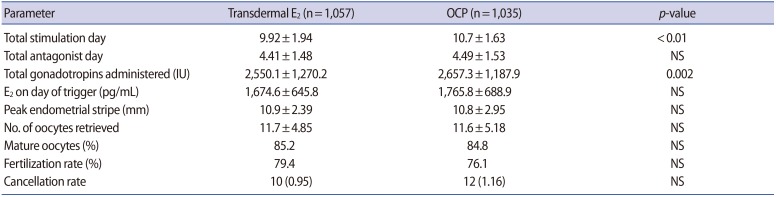
A total of 2,092 patients met the inclusion criteria: 1,057 patients in the E2 group and 1,035 patients in the OCP group. As shown in Table 1, the demographics and baseline characteristics were similar across both groups. Most patients had roughly two unsuccessful IVF-ET cycles elsewhere prior to pursuing treatment at our center. Table 2 summarizes the COS outcomes of the study cohort. Patients in the OCP group had a longer duration of COS (10.7±1.63 days) than the transdermal E2 group (9.92±1.94 days). Furthermore, patients in the OCP group required higher cumulative doses of gonadotropins (2,657.3±1,187.9 IU) than the E2 group (2,550.1±1,270.2 IU, p=0.002). Overall, no difference was noted in the total days of GnRH-ant administration, E2 level on the day of trigger, peak endometrial thickness, number of total or mature oocytes retrieved, and the fertilization rate. No difference in IVF-ET cycle cancelation was observed in the E2 and OCP pretreatment groups.
Table 1. Baseline characteristics of the study cohort (n=2,092).
Values are presented as mean±standard deviation or number (%).
E2, estradiol; OCP, oral contraceptive pill; NS, not significant; BMI, body mass index; FSH, follicle-stimulating hormone; AMH, anti-Müllerian hormone; IVF, in vitro fertilization.
Table 2. Ovarian stimulation characteristics of the study cohort (n=2,092).
Values are presented as mean±standard deviation or number (%) unless otherwise indicated.
E2, estradiol; OCP, oral contraceptive pill; NS, not significant.
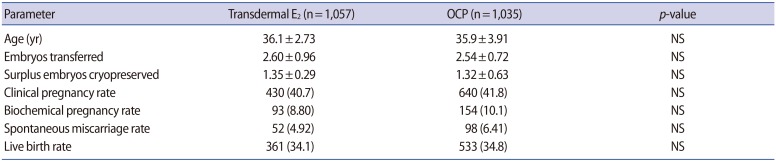
Table 3 presents the pregnancy outcomes of the study cohort. No significant difference was found in the mean age of patients or the number of embryos transferred. The number of surplus embryos cryopreserved at the blastocyst stage was also comparable among the pretreatment groups. Overall, no statistically significant differences were found in the rates of biochemical pregnancy, clinical pregnancy, spontaneous miscarriage, or live birth when comparing both groups. These findings remained unchanged even after adjusting for duration of COS, gonadotropin dose, and E2 level on the day of the trigger.
Table 3. Cycle outcomes of the study cohort (n=2,092).
Values are presented as mean±standard deviation or number (%).
E2, estradiol; OCP, oral contraceptive pill; NS, not significant.
Discussion
Hormonal pretreatment modalities are used to suppress a patient's endogenous gonadotropin secretion, thereby promoting the coordinated growth of early antral follicles in response to exogenous gonadotropins [5,6]. The resulting synchronization of follicles has shown to increase oocyte and embryo yield and therefore, the overall chances of pregnancy [6,7]. OCPs are perhaps the most frequently used pretreatment modality and their impact on IVF-ET cycles have been well studied in normal responders [7,8,9,10,11], poor responders [12,21] and hyper-responders [22]. Conflicting data have been reported regarding the impact of OCP pretreatment on IVF-ET outcomes in normal responders, with some studies suggesting lower oocyte yield, clinical pregnancy, and live birth rates [10,11] than observed in patients receiving no pretreatment, while other studies have not replicated these findings [7,14]. The variation in results may be attributed to the use of different OCPs with varying durations [7,14]. The large majority of studies do, however, emphasize that OCP pretreatment was associated with a longer duration of COS and higher gonadotropin utilization [9,10,11].
The use of E2 for pretreatment in normal responders was proposed as an alternative to OCPs given its shorter duration [5,14]. Administration of E2, which is generally 17-β-E2, during the luteal phase of the preceding cycle exerts negative feedback on FSH during the late luteal-early follicular phase transition, thereby suppressing follicular growth until the administration of exogenous gonadotropins [14]. In a randomized controlled trial of 100 patients comparing oral E2 to OCPs for pretreatment, Hauzman et al. [14] found no significant differences in stimulation, embryological, implantation, clinical pregnancy, spontaneous miscarriage, or live birth outcomes in fresh IVF-ET cycles. The same study also suggested that oral E2 could be used as an alternative to OCPs for scheduling or timing fresh IVF-ET cycles. It must be noted that the E2 utilized in all the aforementioned studies was E2 valerate (i.e., [17β]-3-hydroxyestra-1,3,5[10]-trien-17-yl valerate, which is the 17-pentanoyl ester of 17-β-E2).
Our center has previously described the use of another form of E2 (17-β-E2, i.e., estra-1,3,5 [10]-triene-3,17β-diol) for pretreatment in poor responder patients [23]. In order to broaden the use of this regimen, we retrospectively evaluated its utility for the pretreatment of normal responder patients. In addition to its large sample size, the current study uniquely compares transdermal E2 to OCPs. The outcomes of the OCP group are consistent with previously published findings. However, unlike the study of Hauzman et al. [14], which showed no difference in stimulation outcomes between the E2 valerate and OCP groups, our findings revealed a shorter duration of COS and lower utilization of gonadotropins in the transdermal E2 group than in the OCP group. These contrasting results may be due to the difference in sample sizes and the E2 and OCP products utilized in the two studies. For example, patients using estrane-derived and gonane-derived OCPs, with higher androgenic properties, prior to COS have been shown to have lower oocyte yield than those using anti-androgenic OCPs or those not using OCPs [24].
Despite its strengths, the current study is limited by two main shortcomings. First, the sample size calculation was based on the gonadotropin dosage difference reported by Hauzman et al. [14]. If clinical pregnancy or live birth was considered as the primary outcome of interest, then post-hoc calculations based on the same reference would suggest a sample size of at least 9,804 patients per pretreatment group. Thus, the current study is underpowered to detect a difference in clinical pregnancy or live birth rates. Second, the assignment of patients to transdermal E2 or OCP pretreatment was based on physician preference, thus introducing some selection bias to the study methodology.
In conclusion, our findings suggest that compared to OCPs, pretreatment with transdermal E2 was associated with shorter duration of COS and lower gonadotropin utilization in normal responder patients undergoing IVF-ET with GnRH-ant based protocols. The overall yield of oocytes and embryos, as well as the clinical pregnancy and live birth rates, remain unaffected by E2 or OCP pretreatment. As pretreatment with E2 begins in the preceding luteal phase, the overall length of pretreatment may also be shorter than for OCPs. While pretreatment with OCPs and oral E2 has previously been described for prospective scheduling of IVF-ET cycles, the current study presents reasonable data to suggest that OCP or E2 pretreatment may not be required in normal responders because they do not improve the overall pregnancy outcomes of fresh IVF-ET cycles. However, these suppositions need prospective validation in studies with adequate sample sizes.
Footnotes
This article was presented at the 2015 annual meeting, American Society of Reproductive Medicine, Baltimore, MD, October 17-21, 2015.
Conflict of interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Sunderam S, Kissin DM, Crawford SB, Folger SG, Jamieson DJ, Warner L, et al. Assisted reproductive technology surveillance–United States, 2013. MMWR Surveill Summ. 2015;64:1–25. doi: 10.15585/mmwr.ss6411a1. [DOI] [PubMed] [Google Scholar]
- 2.Gilliam ML. Gonadotrophin-releasing hormone antagonists for assisted reproductive technology. Obstet Gynecol. 2011;118:706–707. doi: 10.1097/AOG.0b013e31822bbbb2. [DOI] [PubMed] [Google Scholar]
- 3.Al-Inany HG, Youssef MA, Ayeleke RO, Brown J, Lam WS, Broekmans FJ. Gonadotrophin-releasing hormone antagonists for assisted reproductive technology. Cochrane Database Syst Rev. 2016;4:CD001750. doi: 10.1002/14651858.CD001750.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pereira N, Amrane S, Hobeika E, Lekovich JP, Chung PH, Rosenwaks Z. Cyst aspiration or GnRH antagonist administration for ovarian cysts detected at the start of fresh in vitro fertilization cycles. Gynecol Endocrinol. 2016;32:562–565. doi: 10.3109/09513590.2016.1139565. [DOI] [PubMed] [Google Scholar]
- 5.Fanchin R, Salomon L, Castelo-Branco A, Olivennes F, Frydman N, Frydman R. Luteal estradiol pre-treatment coordinates follicular growth during controlled ovarian hyperstimulation with GnRH antagonists. Hum Reprod. 2003;18:2698–2703. doi: 10.1093/humrep/deg516. [DOI] [PubMed] [Google Scholar]
- 6.de Ziegler D, Jaaskelainen AS, Brioschi PA, Fanchin R, Bulletti C. Synchronization of endogenous and exogenous FSH stimuli in controlled ovarian hyperstimulation (COH) Hum Reprod. 1998;13:561–564. doi: 10.1093/humrep/13.3.561. [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Velasco JA, Bermejo A, Ruiz F, Martinez-Salazar J, Requena A, Pellicer A. Cycle scheduling with oral contraceptive pills in the GnRH antagonist protocol vs the long protocol: a randomized, controlled trial. Fertil Steril. 2011;96:590–593. doi: 10.1016/j.fertnstert.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 8.Rombauts L, Healy D, Norman RJ; A comparative randomized trial to assess the impact of oral contraceptive pretreatment on follicular growth and hormone profiles in GnRH antagonist-treated patients. Hum Reprod. 2006;21:95–103. doi: 10.1093/humrep/dei302. [DOI] [PubMed] [Google Scholar]
- 9.Kolibianakis EM, Papanikolaou EG, Camus M, Tournaye H, Van Steirteghem AC, Devroey P. Effect of oral contraceptive pill pretreatment on ongoing pregnancy rates in patients stimulated with GnRH antagonists and recombinant FSH for IVF: a randomized controlled trial. Hum Reprod. 2006;21:352–357. doi: 10.1093/humrep/dei348. [DOI] [PubMed] [Google Scholar]
- 10.Griesinger G, Venetis CA, Marx T, Diedrich K, Tarlatzis BC, Kolibianakis EM. Oral contraceptive pill pretreatment in ovarian stimulation with GnRH antagonists for IVF: a systematic review and meta-analysis. Fertil Steril. 2008;90:1055–1063. doi: 10.1016/j.fertnstert.2007.07.1354. [DOI] [PubMed] [Google Scholar]
- 11.Griesinger G, Kolibianakis EM, Venetis C, Diedrich K, Tarlatzis B. Oral contraceptive pretreatment significantly reduces ongoing pregnancy likelihood in gonadotropin-releasing hormone antagonist cycles: an updated meta-analysis. Fertil Steril. 2010;94:2382–2384. doi: 10.1016/j.fertnstert.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 12.Kovacs P, Barg PE, Witt BR. Hypothalamic-pituitary suppression with oral contraceptive pills does not improve outcome in poor responder patients undergoing in vitro fertilization-embryo transfer cycles. J Assist Reprod Genet. 2001;18:391–394. doi: 10.1023/A:1016626607387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blockeel C, Engels S, De Vos M, Haentjens P, Polyzos NP, Stoop D, et al. Oestradiol valerate pretreatment in GnRH-antagonist cycles: a randomized controlled trial. Reprod Biomed Online. 2012;24:272–280. doi: 10.1016/j.rbmo.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 14.Hauzman EE, Zapata A, Bermejo A, Iglesias C, Pellicer A, Garcia-Velasco JA. Cycle scheduling for in vitro fertilization with oral contraceptive pills versus oral estradiol valerate: a randomized, controlled trial. Reprod Biol Endocrinol. 2013;11:96. doi: 10.1186/1477-7827-11-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smulders B, van Oirschot SM, Farquhar C, Rombauts L, Kremer JA. Oral contraceptive pill, progestogen or estrogen pre-treatment for ovarian stimulation protocols for women undergoing assisted reproductive techniques. Cochrane Database Syst Rev. 2010;(1):CD006109. doi: 10.1002/14651858.CD006109.pub2. [DOI] [PubMed] [Google Scholar]
- 16.Cedrin-Durnerin I, Bstandig B, Parneix I, Bied-Damon V, Avril C, Decanter C, et al. Effects of oral contraceptive, synthetic progestogen or natural estrogen pre-treatments on the hormonal profile and the antral follicle cohort before GnRH antagonist protocol. Hum Reprod. 2007;22:109–116. doi: 10.1093/humrep/del340. [DOI] [PubMed] [Google Scholar]
- 17.Kligman I, Rosenwaks Z. Differentiating clinical profiles: predicting good responders, poor responders, and hyperresponders. Fertil Steril. 2001;76:1185–1190. doi: 10.1016/s0015-0282(01)02893-x. [DOI] [PubMed] [Google Scholar]
- 18.Huang JY, Rosenwaks Z. Assisted reproductive techniques. Methods Mol Biol. 2014;1154:171–231. doi: 10.1007/978-1-4939-0659-8_8. [DOI] [PubMed] [Google Scholar]
- 19.Pereira N, Reichman DE, Goldschlag DE, Lekovich JP, Rosenwaks Z. Impact of elevated peak serum estradiol levels during controlled ovarian hyperstimulation on the birth weight of term singletons from fresh IVF-ET cycles. J Assist Reprod Genet. 2015;32:527–532. doi: 10.1007/s10815-015-0434-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palermo GD, Neri QV, Rosenwaks Z. To ICSI or Not to ICSI. Semin Reprod Med. 2015;33:92–102. doi: 10.1055/s-0035-1546825. [DOI] [PubMed] [Google Scholar]
- 21.Shastri SM, Barbieri E, Kligman I, Schoyer KD, Davis OK, Rosenwaks Z. Stimulation of the young poor responder: comparison of the luteal estradiol/gonadotropin-releasing hormone antagonist priming protocol versus oral contraceptive microdose leuprolide. Fertil Steril. 2011;95:592–595. doi: 10.1016/j.fertnstert.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 22.Damario MA, Barmat L, Liu HC, Davis OK, Rosenwaks Z. Dual suppression with oral contraceptives and gonadotrophin releasing-hormone agonists improves in-vitro fertilization outcome in high responder patients. Hum Reprod. 1997;12:2359–2365. doi: 10.1093/humrep/12.11.2359. [DOI] [PubMed] [Google Scholar]
- 23.Dragisic KG, Davis OK, Fasouliotis SJ, Rosenwaks Z. Use of a luteal estradiol patch and a gonadotropin-releasing hormone antagonist suppression protocol before gonadotropin stimulation for in vitro fertilization in poor responders. Fertil Steril. 2005;84:1023–1026. doi: 10.1016/j.fertnstert.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 24.Barad DH, Kim A, Kubba H, Weghofer A, Gleicher N. Does hormonal contraception prior to in vitro fertilization (IVF) negatively affect oocyte yields? A pilot study. Reprod Biol Endocrinol. 2013;11:28. doi: 10.1186/1477-7827-11-28. [DOI] [PMC free article] [PubMed] [Google Scholar]