Abstract
The efficacy of oral phenylbutazone [PBZ; 4.4 mg/kg body weight (BW), q12h], a non-selective non-steroidal anti-inflammatory drug (NSAID), and oral meloxicam (MXM; 0.6 mg/kg BW, q24h), a COX-2 selective NSAID, were evaluated in 2 experimental pain models in horses: the adjustable heart bar shoe (HBS) model, primarily representative of mechanical pain, and the lipopolysaccharide-induced synovitis (SYN) model, primarily representative of inflammatory pain. In the HBS model, PBZ reduced multiple indicators of pain compared with the placebo and MXM. Meloxicam did not reduce indicators of pain relative to the placebo. In the SYN model, MXM and PBZ reduced increases in carpal skin temperature compared to the placebo. Meloxicam reduced lameness scores and lameness-induced changes in head movement compared to the placebo and PBZ. Phenylbutazone reduced lameness-induced change in head movement compared to the placebo. Overall, PBZ was more effective than MXM at reducing pain in the HBS model, while MXM was more effective at reducing pain in the SYN model at the oral doses used.
Résumé
Efficacité comparative du méloxicam oral et de la phénylbutazone dans deux modèles de douleur expérimentaux chez le cheval. L’efficacité de la phénylbutazone orale [PBZ; 4,4 mg/kg poids corporel (PC), q12h], d’un anti-inflammatoire non stéroïdien (AINS) non sélectif, et du méloxicam oral (MXM; 0,6 mg/kg PC, q24h), d’un AINS COX-2 sélectif, ont été évalués dans deux modèles de douleur expérimentaux chez des chevaux : le modèle du fer en cœur ajustable (HBS), qui représente surtout la douleur mécanique, et le modèle de la synovite induite par le lipopolysaccharide (SYN), qui représente principalement la douleur inflammatoire. Dans le modèle HBS, PBZ a réduit plusieurs indicateurs de douleur comparativement au placebo et au MXM. Le méloxicam n’a pas réduit les indicateurs de douleur par rapport au placebo. Dans le modèle SYN, MXM et PBZ ont réduit les hausses de la température de la peau carpienne comparativement au placebo. Le méloxicam a réduit les scores de boiterie et les changements induits par la boiterie dans le mouvement de la tête comparativement au placebo et à PBZ. La phénylbutazone a réduit le changement du mouvement de la tête induit par la boiterie comparativement au placebo. Dans l’ensemble, PBZ était plus efficace que MXM pour réduire la douleur dans le modèle HBS, tandis que MXM était plus efficace pour réduire la douleur dans le modèle SYN aux doses orales utilisées.
(Traduit par Isabelle Vallières)
Introduction
Non-steroidal anti-inflammatory drugs (NSAIDs) are commonly used to treat pain and inflammation in horses. Despite a large number of studies on individual NSAIDs, there are only a few studies directly comparing the efficacy and safety of two or more NSAIDs in horses (1–5). Choice of treatment is typically based on personal clinical experience or preference (6). In Canada, there are currently 2 oral NSAIDs approved for use in horses: phenylbutazone and aspirin. Phenylbutazone (PBZ) is more commonly used. However, oral NSAIDs approved for use in horses in other countries, such as meloxicam and firocoxib, are used extra-label in Canada.
Pain associated with musculoskeletal (MSK) injury can be either mechanical or inflammatory in origin. However, in clinical situations the 2 types of pain frequently occur together. Inflammatory pain often follows mechanical injuries and mechanical pain can occur when inflammatory processes lead to structural changes. Muscle strain is a mechanical injury which can lead to inflammation (7) and inflammatory synovitis can lead to joint distension, which can be associated with mechanical pain (8,9). Regardless of etiology, NSAIDs are often used for treatment of pain and resolution of lameness.
The primary mechanism of action of NSAIDs is inhibition of cyclooxygenase (COX) enzymes that produce prostaglandins. There are 2 forms of COX: constitutively expressed COX-1 and inducible COX-2 enzymes. Inhibition of the COX enzymes results in a reduction in the production of prostaglandins (PG) from their arachidonic acid precursor (10) and clinically results in a reduction of pain and inflammation. These NSAIDs may be classified as non-selective if they inhibit both forms at therapeutic concentrations or COX-2 selective if they primarily inhibit COX-2 forms at therapeutic concentrations. In the horse, PBZ is considered non-selective, while MXM is considered COX-2 selective (or preferential) (11).
Nociceptive pathways and responses to mechanical and inflammatory pain are different, although prostaglandins are believed to play a role in both forms of pain (12,13). The efficacy of NSAIDs in mechanical and inflammatory pain may differ for a variety of pharmacodynamic and pharmacokinetic reasons. Secondary pharmacologic targets, COX selectivity, and concentrations in serum or target tissues (inflamed tissue or the central nervous system) all influence NSAID efficacy. Comparison of clinical efficacy, therefore, requires consideration of both inflammatory and mechanical pain.
This study assessed the efficacy of oral MXM (0.6 mg/kg BW, q24h) and oral PBZ (4.4 mg/kg BW, q12h) in 2 short-term, reversible pain models in the horse: the heart bar shoe (HBS) model (14) that induces primarily mechanical pain and the LPS-induced synovitis (SYN) model that induces primarily inflammatory pain (15). It was hypothesized that MXM and PBZ would be effective in reducing pain and/or inflammation compared with the placebo and that MXM efficacy would not be significantly different from PBZ.
Materials and methods
Subjects
The study was approved by the University of Calgary’s Animal Care Committee and was conducted in accordance with the guidelines of the Canadian Council on Animal Care. Sixteen healthy horses (13.9 ± 8.2 y; 512 ± 42 kg; 8 Thoroughbreds, 3 Warmbloods, 3 Quarter Horses, and 1 Standardbred; 10 mares and 6 geldings) were included, with 8 horses assigned to each experimental model. Horses were deemed to be systemically healthy prior to study initiation on the basis of physical examination, a complete blood (cell) count (CBC), and serum bio-chemical analysis. Radiographs of the front feet (HBS-group) or carpi (SYN-group) were taken to rule out significant abnormalities. Horses did not show any evidence of foot pain when hoof testers were applied. Horses in the SYN-group were evaluated for front limb lameness at a trot in a straight line before enrolment in the study and were excluded if there was detectable front limb lameness.
All horses were housed in paddocks or pastures between experimental periods. On experimental days, horses were housed inside in individual stalls. Horses were fed grass hay ad libitum and had access to water at all times. Hay for the night was provided at the time of last assessment, which was at approximately 11 pm. Horses were treated in the morning at approximately 7:30 am and the new hay for the day was provided approximately 1 h later and throughout the day.
Overall experimental design
A randomized, blinded, 3-period, 3-way cross-over design was used. The evaluators were blinded to the treatment and the persons administering the drugs did not evaluate the horses. Horses were assigned to 1 experimental pain model and received all 3 treatments (placebo, MXM, PBZ). Lameness was induced (starting leg was randomized and alternated at each period) and treatments (order randomized) were administered at 2-week intervals. All horses had lameness induced and treatments administered on the same days. Horses were assessed (physical examination and complete assessment) the day before each experimental period to ensure no abnormalities had developed in the 2-week rest interval.
Pain was induced at t = −1 h in the HBS group and t = −2 h in the SYN group. Treatment was initiated at t = 0 h with 1 of the 3 treatments, in random order: placebo (molasses; 0.04 mL/kg BW at t = 0 and t = 12 h), meloxicam (Metacam 15 mg/mL oral suspension for horses; Boehringer Ingelheim Vetmedica, Ingelheim, Germany; 0.6 mg/kg BW at t = 0 h and placebo at t = 12 h); and phenylbutazone (Butequine oral paste; Bioniche Animal Health Canada, Belleville, Ontario; 4.4 mg/kg BW at t = 0 and t = 12 h). All treatment doses were calculated to the nearest 25 kg BW.
Horses were monitored for 24 h after treatment. The assessment methods and time points varied for each group. Evaluators were blinded to treatments but were aware of the leg treated and the method used to induce lameness. A team of 4 evaluators was assigned to 2 horses — 1 in the HBS and 1 in the SYN group. A minimum of 2 evaluators from the team of 4 assessed each horse at each time point throughout the study. Any horses showing significant pain [obvious discomfort or lameness at a walk (HBS) or trot (SYN)] following the 24 h experimental period received further treatment with phenylbutazone at 4.4 mg/kg BW, PO, q12 to 24h until lameness resolved.
Heart bar shoe
As previously described (14,16,17), an adjustable heart bar shoe was placed on both front feet 1 wk before the first trial. This allowed acclimation to the new set of shoes to ensure there was no lameness associated with the shoes themselves. Pressure was placed on the frog and sole by tightening the set screw against the hinged plate. In 3 horses, the set screw stripped and the same foot was used for the 2nd and 3rd trials.
Pressure was applied at t = −1 h to produce a lameness score of 4 on the Foreman Lameness Scale (FLS) (Table 1) and an elevation in heart rate, as previously described (16). Horses were assessed immediately after pressure application and then at t = −40 min, −20 min, and 0 h to ensure that the pain score was stable prior to treatment. Pressure was adjusted as required until t = 0 h. Following a pre-treatment assessment at t = 0 h, treatment and assessments were completed at t = 1, 2, 3, 4, 6, 8, 12, 15, and 24 h post-treatment. After the t = 24 h assessment, pressure was immediately relieved from the heart bar.
Table 1.
Foreman lameness scale
| Grade 0 | Sound; no detectable lameness |
| Grade 1 | Barely detectable: no head bob and only intermittently looks lame in stall (especially when turning) and/or rare and intermittent toe pointing (e.g., 1 to 2 events/min) |
| Grade 2 | Mild lameness at a walk in the stall; mild head bob when walking or turning in stall; points toe more consistently (3 to 4 events/min) |
| Grade 3 | Moderate lameness, but not non-weight bearing; more obvious head bob at walk; toe pointing frequently (5 to 6 events/min) |
| Grade 4 | Severe lameness — non-weight bearing 50% of the time; severe head bob; toe pointing whenever not walking (e.g., almost continuous), but not always 3-legged lame at a walk |
| Grade 5 | Non-weight bearing 100% of the time |
Lameness was evaluated and scored in accordance with the FLS, as previously reported (Table 1) (16). To reduce subjectivity, individual components of the FLS were also evaluated separately and assigned a value (Table 2) to generate a Total Lameness Score (TLS) on a continuous scale. To validate the TLS, it was compared to the FLS in non-treated horses and a strong correlation was observed (Spearman’s rank correlation coefficient r = 0.96; P < 0.0001). A Global Pain Score (GPS) that incorporated additional behavioral scores (Table 3) adapted from previously published scoring systems (18,19), using only components that were demonstrated to be reproducible and were relevant to MSK pain, was determined at each time point.
Table 2.
Total lameness score
| Score | 0 | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|---|
| General lameness | None observed | Barely detectable | Mild lameness | Moderate lameness | Severe lameness | 100% non-weight-bearing |
| Head bob | None | Occasional | Mild | Moderate | Marked | 100% non-weight-bearing |
| Toe pointing | None | Rare | 1 to 2 times/min | 3 to 4 times/min | 5 to 6 times/min continuous | 100% non-weight-bearing |
| Non-weight-bearing | 0% | Occasional | Up to 25% | 50% | 75% | 100% |
Table 3.
Global pain score
| Behavior | Criteria | Score |
|---|---|---|
| Pawing the floor or pointing a foot | Quietly standing, no pawing or pointing | 0 |
| Occasional pawing/pointing (1 to 2 times/min) | 1 | |
| Frequent pawing/pointing (3 to 4 times/min) | 2 | |
| Excessive pawing/pointing (> 5 times/min) | 3 | |
| Movement | Stands relaxed or quiet movement | 0 |
| Reduced movement or mild agitation | 1 | |
| Reluctance to move (e.g., pressing rump against stall wall) or moderate agitation | 2 | |
| Refusal of movement or uncontrollable forward movement | 3 | |
| Position in stall | At stall door, looking out | 0 |
| In middle of stall, looking out | 1 | |
| Looking at side wall of stall | 2 | |
| Back to door, looking at back wall | 3 | |
| Ear position | To the front, listening (ear movement) | 0 |
| To the front, but little or no movement | 1 | |
| Ears slightly back (vertical), little movement | 2 | |
| Ears pulled back | 3 | |
| Orbital tightening | Eyes open, looking around | 0 |
| Eyes open or occasional partially closed, limited movement | 1 | |
| Eyes consistently closed up to half | 2 | |
| Eyes closed half or more, consistently | 3 |
At each assessment, behavior was first evaluated from outside the stall and the horse was video-recorded for 5 min. Heart rate and respiratory rate were evaluated in the stall with the horse at rest. The additional components of the assessment that required movement of the horse were then completed. On completion of the study, the recorded video was reviewed to ensure consistency in behavior evaluation throughout the study and a final score was assigned.
LPS-induced synovitis
Synovitis (SYN) was induced by aseptic injection of 50 ng LPS (LPS from E. coli O55:B5, Sigma Aldrich, St. Louis, Missouri, USA) in 0.5 mL lactated ringer’s solution into the intercarpal joint, as previously described (15). In a pilot study (n = 5), 50 ng LPS was found to be the lowest dose that induced a consistent, reproducible lameness at a trot in a straight line (unpublished data). A higher dose did not increase lameness and a dose of 0.5 ng per joint did not consistently induce clinical lameness.
Three horses during the last 2 experimental periods were sedated with detomidine (Dormosedan; Zoetis, New York, New York, USA), 3 mg, IV, (~6 μg/kg BW) to facilitate injection (including 3 placebo, 2 MXM, and 1 PBZ treatment period).
Baseline assessments were completed before joint injection at t = −2 h. Following a pre-treatment assessment at t = 0 h, the first treatment was administered and horses were then assessed at t = 2, 4, 6, 8, 12, 15, and 24 h. Horses were assessed for lameness and the need for rescue analgesia at t = 24 h.
Heart rate, respiratory rate, and carpal skin temperature were assessed with the horse at rest in the stall. Carpal skin temperature was assessed by infrared sensor thermometers (ThermoCheck; STEINEL Vertrieb GmbH, Herzebrock-Clarholz, Germany), with the same thermometer used for individual horses throughout the study. To ensure consistency, temperature was assessed within a 1.5 cm circle marked on the surface of the intercarpal joint, 2 cm lateral to the injection site and the extensor carpi radialis tendon. Carpal skin temperatures are expressed as change from baseline to account for any minor differences in baseline temperatures caused by changes in ambient temperatures between experimental periods.
A lameness score was assigned using the scoring system in Table 4. The difference in maximum and minimum head height (measured in mm) between the affected and non-affected leg strides was obtained using the Lameness Locator in accordance with the manufacturer’s instructions (Equinosis; Columbia, Missouri, USA), as previously described (20). Briefly, accelerometers were placed on the head and pelvis, and a gyroscope was placed on the right forelimb, allowing the proprietary computer program to calculate the differences in maximum and minimum head height between the left and right strides. Each horse was trotted in a straight line for a minimum of 25 strides to collect sufficient data points for analysis. Only front limb data were analyzed and the data are expressed as difference in maximum and minimum head height during the stride on the affected leg compared to the non-affected leg. In some horses, at the point of maximum lameness, the head height difference was not quantified by the Lameness Locator program algorithms because the minimum of 6 consistent strides in a row was not achieved. For these individual time points, following a manual inspection of the obtained data, a standard 10 mm was added to the difference in head movement observed at the flanking time points to create an imputed value that allowed analysis by 2-way repeated measures analysis of variance (ANOVA). A consistent value was added in all cases to minimize statistical bias in the results (21). This approach was validated by determining that adding 0, 10, or 20 mm to the flanking time point or deleting the affected time points did not change the interpretation of the data. All statistical analyses for head movement are reported based on a complete data set using the imputed numbers.
Table 4.
Synovitis lameness score
| 0 | No lameness at a walk or trot |
| 1 | Intermittent lameness at a trot in a straight line |
| 2 | Consistent lameness, mild head bob at a trot in a straight line |
| 3 | Consistent obvious lameness, with moderate to severe head bob at a trot in a straight line |
| 4 | Lame with head bob at a walk |
| 5 | Non-weight-bearing lame |
Statistical analysis
All data are reported as mean ± standard error (SE), unless indicated otherwise. Statistical comparisons were achieved by using a 2-way repeated measures ANOVA to determine residual error and then carrying out a post-hoc analysis using Tukey’s multiple comparison method to identify significant differences at each time point between placebo, MXM, and PBZ, using commercially available software (Graph Pad Software, La Jolla, California, USA). Differences were considered significant when P < 0.05. For ease of presentation, only significant differences at individual time points are reported. In addition, t-tests were used to determine if there was any effect of the use of detomidine on the pre-treatment lameness scores in the synovitis group.
Group size was determined by comparison to previously published studies and using a simplified power analysis. Power analysis of 2-way repeated measure ANOVAs is complex and is based on a large number of assumptions. Therefore, predicted sample size was estimated based on comparisons with multiple paired t-tests. A power calculation using a β = 0.8 and α = 0.05 indicated a sample size of 8 horses per group would be sufficient to detect a 25% difference.
Results
Heart bar shoe model
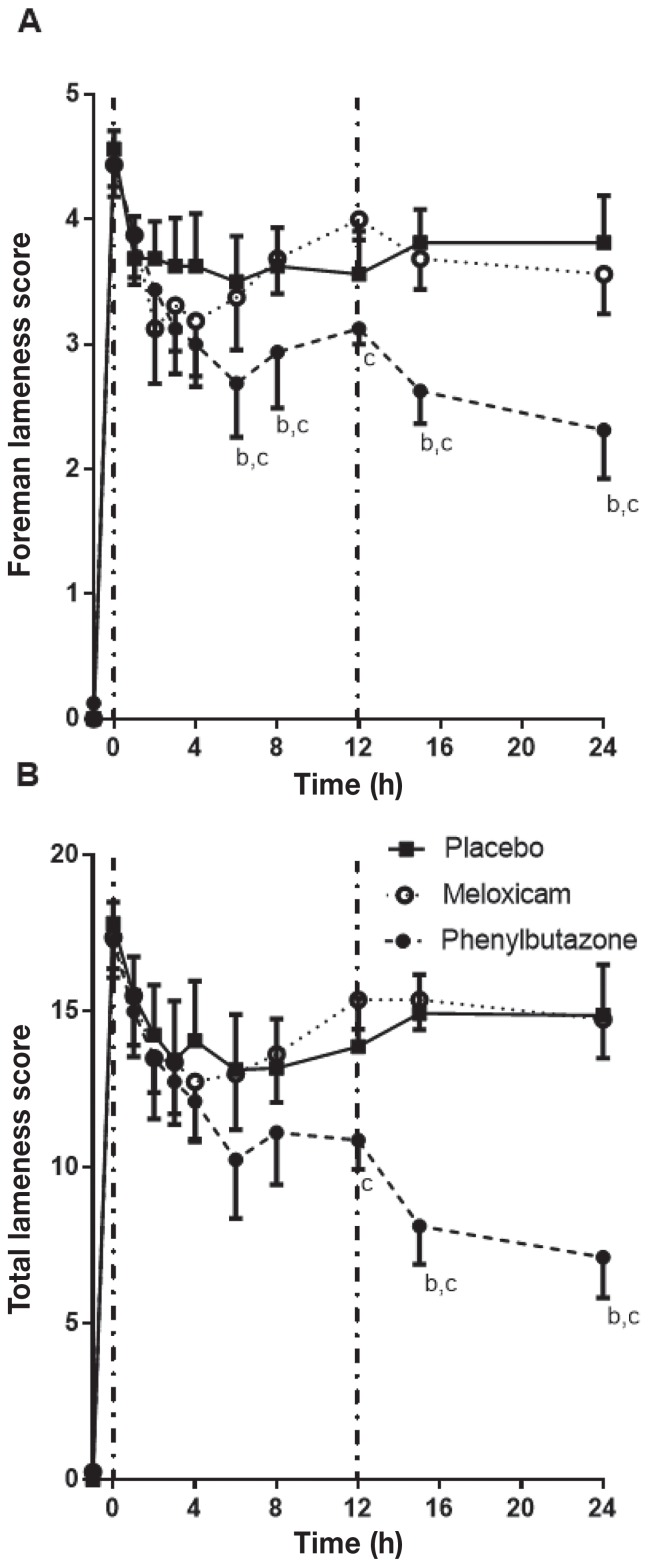
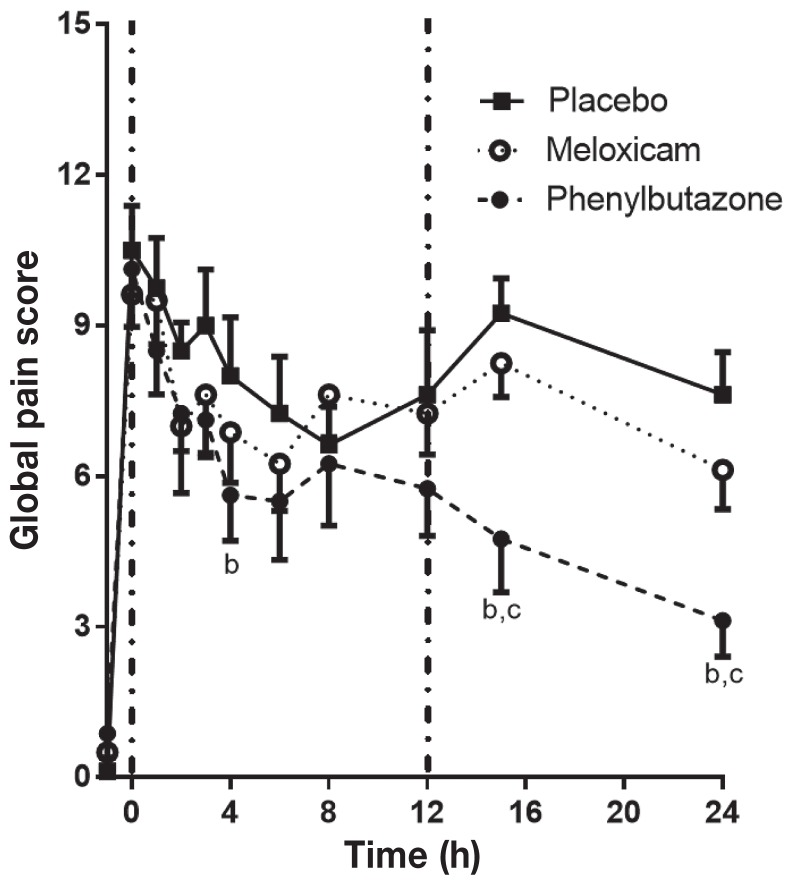
Application of pressure with the HBS increased the score on the FLS from a baseline of 0 to an average of 4.5 (Figure 1). With placebo treatment, the FLS stabilized by t = 1 h and remained between 3.5 and 3.8 for the duration of the experimental period, similar to previous reports (14,16,17). Phenylbutazone significantly reduced the FLS and TLS scores compared to the placebo and MXM at multiple time points (Figure 1), with the greatest effect at t = 15 to 24 h. Meloxicam did not significantly change the FLS or the TLS compared to the placebo at any time point. The Global Pain Score (GPS; Figure 2) followed a similar pattern, with PBZ resulting in a significant reduction compared to the placebo at t = 4, 15, and 24 h and to MXM at t = 15 and 24 h. Meloxicam did not significantly change the GPS compared to the placebo at any time point.
Figure 1.
Foreman (A) and total (B) lameness scores in horses (n = 8) with heart bar shoes following treatment with a placebo (black square), meloxicam (MXM; open circle), or phenylbutazone (PBZ; black circle). Pressure was applied at t = −1 h and treatments were administered at t = 0 h (vertical dashed line) and t = 12 h (placebo or PBZ; second vertical dashed line). Significant differences at each time point were determined by a post-hoc Tukey’s analysis following a 2-way repeated measures ANOVA. Each point represents the mean ± standard error and significant differences are shown as follows: a = P < 0.05 placebo versus MXM; b = P < 0.05 placebo versus PBZ; c = P < 0.05 MXM versus PBZ.
Figure 2.
Global pain scores in horses with heart bar shoes following treatment with placebo, meloxicam, or phenylbutazone. See Figure 1 for full explanation of figure legends.
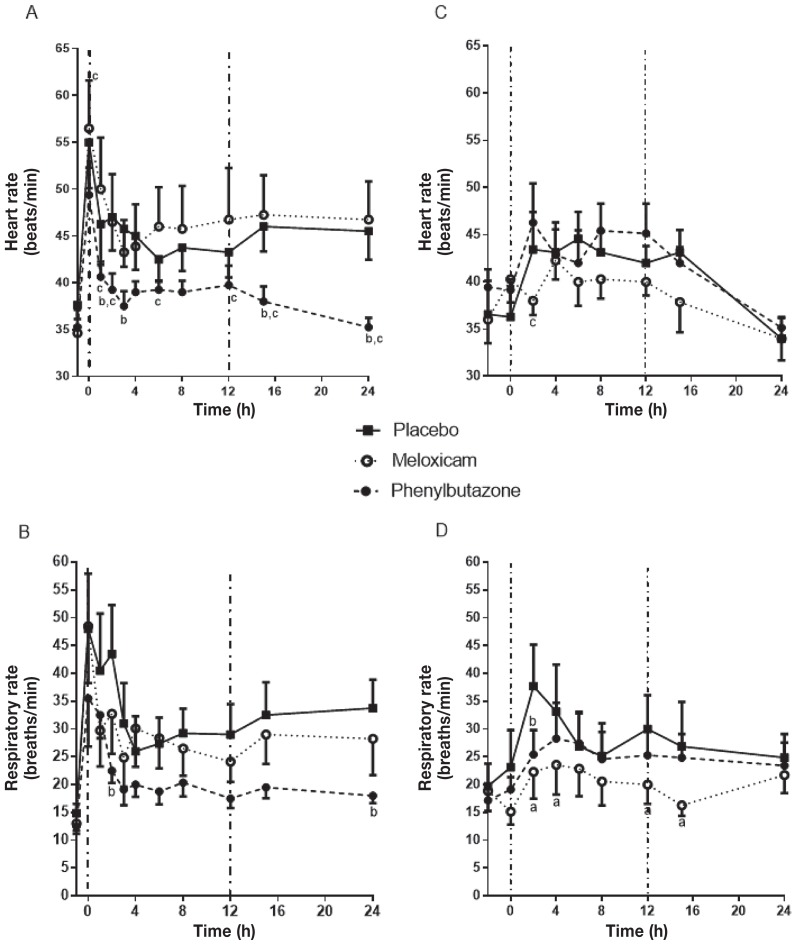
Heart rate increased from approximately 35 at baseline to 55 beats/min (bpm) at t = 0 h and then stabilized at approximately 45 bpm by t = 1 h in placebo-treated horses (Figure 3A). Meloxicam did not significantly change heart rate compared to the placebo at any time point. Heart rate in PBZ-treated horses was significantly lower than that of the placebo at t = 2, 3, 15, and 24 h and significantly lower than that in horses treated with MXM at t = 0, 1, 2, 6, 8, 12, 15, and 24 h. Phenylbutazone returned the heart rate to baseline by t = 2 h. Similarly, respiratory rate initially increased 3-fold in placebo-treated horses, and then stabilized at a rate approximately twice baseline (Figure 3B). Meloxicam did not significantly change respiratory rate at any time point, but PBZ resulted in significantly lower respiratory rates at t = 2 and t = 24 h.
Figure 3.
Heart rate (A) and respiratory rate (B) with heart bar shoes and heart rate (C) and respiratory rate (D) in horses with LPS-induced synovitis following treatment with a placebo, meloxicam, or phenylbutazone. For the heart bar shoe, pressure was applied at t = −1 h. Synovitis was induced at t = −2 h by aseptic injection of 50 ng LPS into the intercarpal joint. See Figure 1 for full explanation of figure legends.
On completion of the treatment period, 5 placebo-treated horses, 3 MXM-treated horses, and no PBZ-treated horses required rescue analgesia. This was a significant difference between groups in the number of horses requiring rescue analgesia (Chi-square; P < 0.05).
Synovitis model
Lipopolysaccharide (LPS) injection induced synovitis accompanied by signs of pain and inflammation in all horses. One of the horses in the SYN group developed a bilateral front limb lameness characterized by sensitivity to hoof testers across the sole between the first and second treatment period. This horse was therefore removed from the study. Thus, the SYN group had 7 horses that completed the trial and only those results are included.
To determine if the horses that received detomidine could be included in the analysis, pre-treatment values at t = 0 h (2 h post-synovitis induction, but pre-NSAID treatment) for all parameters were compared between those treatment periods where horses received detomidine and those where they did not. Further, the pre-treatment values for all treatment periods in which horses received detomidine were compared to all other treatment periods. No significant differences in any parameter were observed (data not shown), confirming that detomidine did not influence the degree of synovitis induced and was not providing detectable analgesia at t = 0 h. Therefore, all horses were included in the analysis.
Induction of synovitis was associated with lameness (Synovitis Lameness Score over 2; Table 4), a rise in carpal skin temperature, and variable degrees of joint effusion by t = 0 h (2 h after joint injection). Synovitis was associated with an increase in the heart rate and respiratory rate (Figures 3C and 3D). In the placebo group, heart rate peaked between t = 2 and t = 6 h (4 to 8 h post-joint injection) and returned to baseline by t = 24 h. Neither MXM nor PBZ reduced heart rate compared to the placebo. The respiratory rate peaked at t = 2 h and then returned towards baseline by t = 24 h. Meloxicam significantly reduced the respiratory rate compared to the placebo at t = 2, 4, 12, and 15 h. Phenylbutazone also significantly reduced the respiratory rate compared to the placebo at t = 2 h, but MXM and PBZ were not significantly different at any time point.
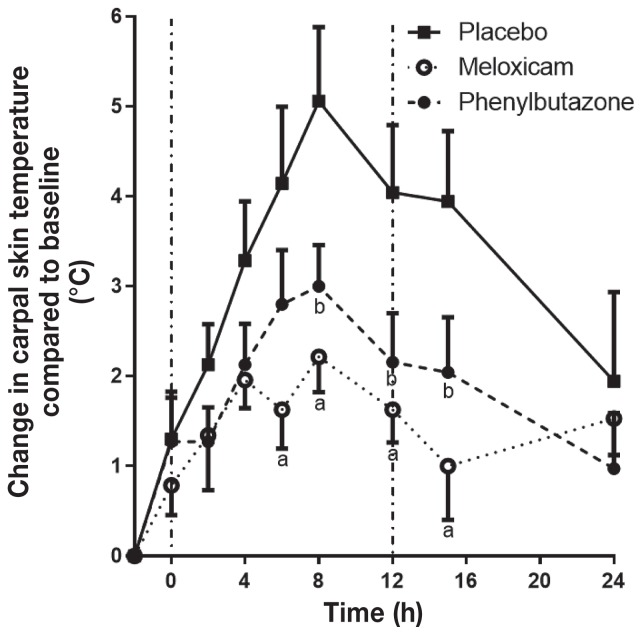
The carpal skin temperature peaked at t = 8 h in the placebo group, with an average increase of 5.1 ± 0.8°C above baseline, before starting to decline (Figure 4). Meloxicam significantly reduced the carpal skin temperature increase compared with the placebo from t = 6 to t = 15 h and PBZ reduced the temperature increase from t = 8 to t = 15 h. Phenylbutazone and MXM were not significantly different from each other at any time point.
Figure 4.
Change in carpal skin temperature of horses relative to baseline (n = 7) with LPS-induced synovitis following treatment with placebo, meloxicam, or phenylbutazone. See Figure 1 for full explanation of figure legends.
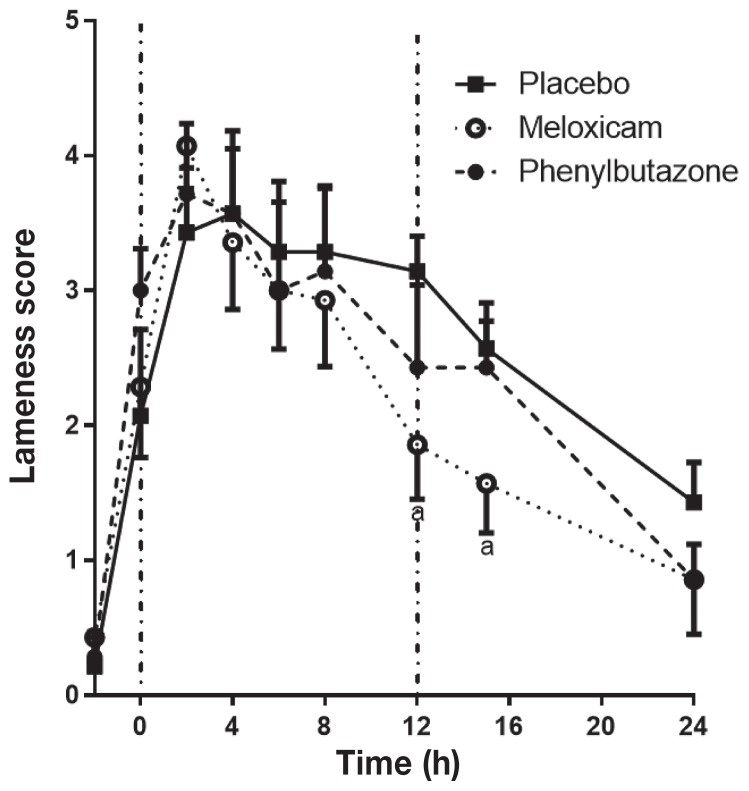
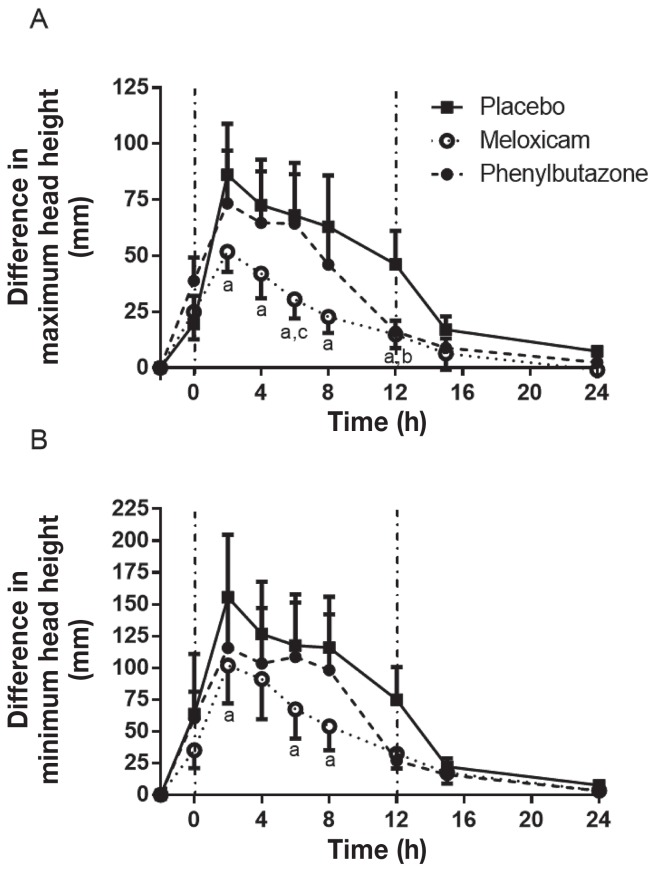
Lameness scores peaked at t = 4 h with the placebo and then began to decrease at t = 12 h so that lameness scores were close to baseline by t = 24 h (Figure 5). Phenylbutazone did not reduce the lameness score at any time point compared to the placebo, while MXM significantly reduced the lameness score at t = 12 and t = 15 h. Meloxicam and PBZ were not significantly different at any time point, although the difference did approach significance at t = 15 h (P = 0.10). An objective assessment of lameness on a continuous scale was achieved using the Lameness Locator® to assess changes in maximum and minimum head height difference during the stride. The maximum head height difference (head height during the impact phase, affected versus non-affected side) increased markedly following induction of synovitis, reaching a peak at t = 2 h (Figure 6A). Meloxicam resulted in a significant reduction in the peak increase in maximum head height difference at t = 2 h and resulted in a more rapid return towards baseline, with the change in head motion being significantly less than placebo at t = 4, 6, 8, and 12 h, and significantly less than PBZ at 6 h. Phenylbutazone only reduced maximum head height compared to placebo at t = 12 h. The minimum head height difference (head height during the stance phase) also increased following induction of synovitis, reaching a peak at t = 2 h (Figure 6B). Meloxicam significantly reduced the peak change in minimum head height difference at t = 2, 6, and 8 h compared to the placebo. Phenylbutazone did not significantly alter the minimum head height difference. No horses required rescue analgesia upon completion of any SYN period.
Figure 5.
Synovitis lameness scores in horses with LPS-induced synovitis following treatment with placebo, meloxicam, or phenylbutazone. See Figure 1 for full explanation of figure legend.
Figure 6.
Differences in maximum head height (A) and minimum head height (B) during the stride on the affected leg compared to the unaffected leg in horses with LPS-induced synovitis following treatment with placebo, meloxicam, or phenylbutazone. See Figure 1 for full explanation of figure legend.
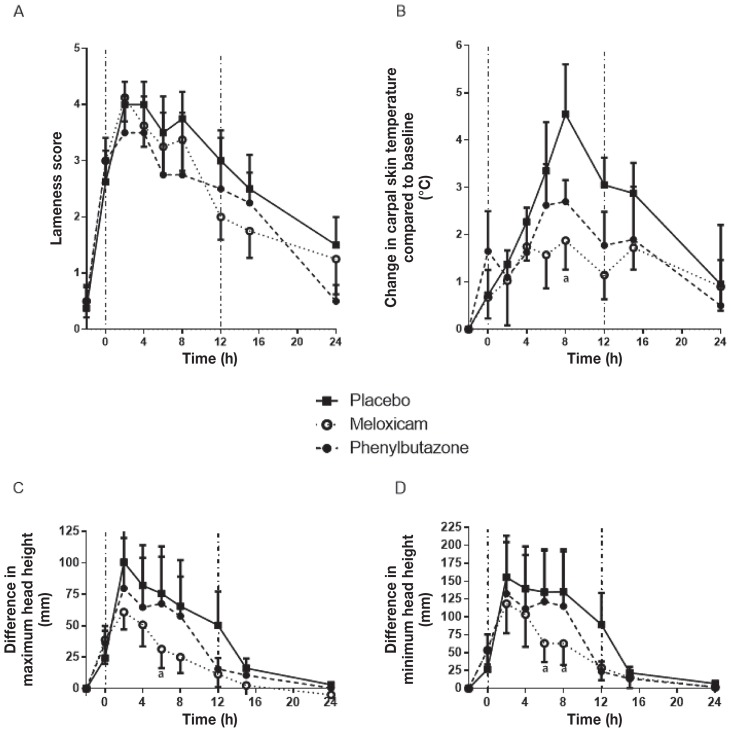
To further confirm that detomidine administration did not affect the results, the complete analysis was repeated excluding the horses that received detomidine. The data trends aligned with the full data set as reported; however, because of the loss of power associated with the decreased number of subjects, only the peak effects of MXM at 6 or 8 h were significant for joint temperature and changes in head height. No significant difference in Lameness Scores was observed at any time point (Figure 7).
Figure 7.
Re-analysis of results, excluding horses receiving detomidine, of assessment of LPS-induced synovitis following treatment with placebo, meloxicam, or phenylbutazone: Synovitis lameness scores (A), change in carpal skin temperature compared to baseline (B), difference in maximum head height (C), and difference in minimum head height (D). See Figure 1 for full explanation of figure legend.
Discussion
Given the differences in the pathophysiology of mechanical and inflammatory pain and the pharmacodynamic and pharmacokinetic differences between NSAIDs, 2 experimental pain models were used to compare the efficacy of oral MXM and PBZ at the recommended oral doses. Previous studies employing the HBS model typically compared medications over ≤ 13 h (16,17) and used IV administration. As MXM is typically dosed q24h, these studies were carried out over a 24-hour period. Meloxicam has not previously been assessed in the HBS model. A variety of agents (Freund’s adjuvant, LPS, carrageenan), doses and dose intervals have been used to induce synovitis in horses and there has not been an accepted standard for comparison of NSAID efficacy. It was therefore important to demonstrate efficacy compared to placebo in the pain models as well as the comparative efficacy.
Heart rate and the FLS score are responsive to NSAID treatment in the HBS model (16,17). A TLS using a scoring system based on the descriptors in the FLS was used to ensure a continuous linear scale and to minimize subjective variability between raters by assigning individual scores to each component instead of relying on a single impression score by the evaluator that incorporated all components at once. Furthermore, a behavioral scale (the Global Pain Score) was applied to provide a more global assessment of pain that did not rely solely on lameness. In the HBS model, the increases in heart rate and FLS score in this study were similar to those previously reported (16,17). Oral PBZ ameliorated the increase in HR, RR, FLS, TLS, and GPS at multiple time points compared to the placebo and MXM. Oral MXM did not significantly alter any of the parameters assessed compared to the placebo. Heart rate decreased significantly at t = 2 h after PBZ treatment, compared with the placebo. There was a significant difference between MXM and PBZ at time zero in the HR, before drug treatment, but neither was different from placebo. The FLS score and GPS were not different at t = 0 h, so this likely reflects a type 1 error at this one time point and does not reflect a lower pain induction in the PBZ treatment period. The reduction in GPS and TLS did not become significant until t = 4 and t = 12 h, respectively, following treatment and differences were greatest at t = 15 and t = 24 h. The difference in onset time between statistically significant changes in HR, GPS, FLS, and TLS may reflect differences in expression of pain indicators (i.e., reduction in pain may lead to changes in heart rate prior to changes in lameness). Alternatively, it may just reflect differences in reaching statistical significance because the overall response patterns were similar across the different assessment parameters. The need for pain relief at the end of the experimental pain period was most frequent in the placebo and MXM groups. This may be because of reduced pain and inflammation during the experimental period or because of prolonged action of PBZ. Thus, PBZ was more effective than MXM in this model of pain, regardless of the assessment parameter considered.
The maximum observable response occurred after the second dose of PBZ. This may reflect a lag time to effect with PBZ, the delay in absorption with oral administration, or a higher plasma concentration achieved after the second dose. Previous studies in the HBS model have used IV administration of PBZ and demonstrated an earlier and greater effect than observed herein (1,16), suggesting that slower time to peak concentrations with oral dosing or the higher plasma concentrations achieved after the second dose are likely more important than a pharmacologic lag time to effect. It would appear prudent to assume a delay in clinical response of at least 4 h following administration of oral PBZ, with the maximum response not occurring until after the second dose at 15 to 24 h.
Over a period of several weeks following completion of the experimental portion of the study, all horses in the HBS group developed evidence of solar necrosis in one or both feet. This has previously been reported (1), but appears to have occurred with a greater frequency in our study. This may be related to the duration of pressure (24 h versus 12 to 13 h more commonly used) or perhaps to a more cranial pressure point of the heart bar shoe. Horses were assessed before each experimental period and solar necrosis or increased sole pain (application of hoof testers) was not observed during the trial. Furthermore, the pressure required to induce pain (as assessed by the number of turns of the screw required to induce pain) was consistent between weeks, even in the instances that the same foot was used for consecutive treatment periods. In other studies, the same foot has been used at more frequent (weekly) intervals (1,16,17). Given that the order of treatment was randomized, alternate feet were used (with a few exceptions) and sole necrosis was not observed during the trial, the occurrence of sole necrosis at a later date does not appear to invalidate the results. It does, however, highlight a limitation of this model.
Overall, these results demonstrate a superior efficacy of oral PBZ compared to oral MXM in a model that primarily induces mechanical pain. The results do not discount the possibility that MXM would be effective for mechanical pain after intravenous injection, at a higher oral dose, or if the pain was less severe.
Intra-articular LPS injection reliably induces a transient joint inflammation and pain that starts within 2 h post-injection and resolves within 24 to 48 h, similar to what was observed in our study (Figures 4–6) (15,22–24). Carpal skin temperature, stride length and lameness scores have been shown to reflect pain and inflammation, and to be responsive to NSAID treatment (25) in SYN models. Instead of stride length, which was found to be variable and difficult to measure in a pilot study, the commercially available Lameness Locator was used to objectively measure changes in head position associated with lameness (26). Joint circumference and joint flexion were also measured, but similar to previous studies (22,25) the results were highly variable and unreliable so they are not reported here.
The time to peak lameness in this study was between t = 2 to t = 4 h (4 to 6 h after injection). The changes in head movement following induction of synovitis were greatest at t = 2 h (4 h after induction), while the peak increase in carpal skin temperature was later at t = 8 h. These differences to peak times suggest that the measured parameters are reflecting different combinations of pain and inflammation.
Heart and respiratory rate were monitored for untoward systemic responses to LPS. Heart and respiratory rate did increase after induction of synovitis (P < 0.001 and P < 0.02, respectively), but there was no other evidence of endotoxemia (e.g., hyperemic mucous membranes, elevated temperature). Heart rate and respiratory rate were not reliable indicators of pain in this model as the horses had to be trotted to evaluate lameness and movement of 1 horse disturbed all horses in the barn (note that the HBS model horses were kept in a separate wing).
The ability of PBZ and MXM to ameliorate pain and inflammation in SYN models is consistent with previous reports (23,24). However, no previous studies have directly compared PBZ and MXM in the horse using induced synovitis models. Phenylbutazone and MXM significantly reduced the increase in carpal skin temperature throughout the assessment period compared to the placebo, but were not significantly different from each other (Figure 4). Only MXM significantly reduced the lameness score compared to the placebo, producing significantly lower pain scores at t = 12 and t = 15 h (Figure 5). By t = 24 h, lameness in the placebo group had returned towards baseline so identifying significant changes at that time point was not possible. Oral PBZ did not significantly reduce lameness scores compared to the placebo or MXM at any time point. Thus, the subjective lameness scores alone were not able to clearly document differences between PBZ and MXM in the SYN model.
As shown in Figure 6, MXM was more effective than PBZ at reducing SYN associated changes in head movement. There are 2 possible interpretations for the differences between MXM and PBZ in the SYN model. One is that MXM was absorbed more rapidly and achieved effective concentrations in blood and in the joint more quickly than PBZ. An argument against this pharmacokinetic explanation is that MXM and PBZ both maximally reduced carpal skin temperature by t = 8 h and with similar time courses. However, in the HBS model the greatest effects of PBZ on lameness were not observed until t = 15 and t = 24 h, despite reducing heart rate within 1 to 2 h. An alternative and more likely interpretation is that MXM was more effective than PBZ at the doses used and was able to mitigate inflammatory pain earlier in the experimental model, when it was more severe. Regardless of the underlying explanation, MXM had an earlier onset of action in the SYN model compared to PBZ and had the same relative efficacy at t = 12 h. Unfortunately, it is not possible to draw conclusions about relative efficacy after t = 12 h due to waning clinical signs of pain and inflammation.
One concern in this study was the use of detomidine to permit joint injections in 3 horses. The stress of joint injection made these horses difficult to handle, and it was considered an animal welfare and human safety issue to attempt joint injection without sedation. The antinociceptive effects of 20 μg/kg BW detomidine are lost in less than 2 h (27), so the lower dose used in this study (~6 μg/kg BW) was not be expected to have any effect at the time the NSAIDs were administered (2 h after detomidine). There was no significant effect of detomidine on the pre-treatment values for any of the measured parameters. Furthermore, removal of the horses receiving detomidine from the analysis did not change the overall patterns of response, although statistical power was lost because of the reduced numbers and only MXM produced any significant reductions in the assessed parameters (Figure 7). Therefore, all horses were included in the final analysis.
The results of our study are consistent with the important role of COX-2 in inflammatory pain. Meloxicam and PBZ are both able to inhibit COX-2 and thus reduce peripheral inflammation. Accordingly, both NSAIDs had significant effects in the synovitis model. In contrast, only PBZ was effective in the HBS model. The ability to ameliorate mechanical pain may be related to COX-1 inhibition and concentrations achieved in the spinal cord (28). Both COX-1 and COX-2 enzymes are expressed at different levels and times in the spinal cord and surrounding tissues. Intrathecal injection (thus eliminating peripheral effects) of a COX-1 inhibitor in a murine model of neuropathic pain significantly minimized mechanical allodynia (29) and inhibition of central COX-1 is important in ameliorating surgical pain (12,30). It is reasonable to assume similar processes occur in the equid spinal cord. This may explain the ability of PBZ, a non-selective COX-inhibitor, to ameliorate pain in the HBS model and a lack of effect of MXM, a COX-2 selective inhibitor.
The purpose of this study was to determine and compare the efficacy of MXM and PBZ in 2 models of equine pain. The transient induced lameness models used herein decreased variability compared to naturally occurring disease and allowed some distinction between mechanical and inflammatory pain. The use of experimental models allowed a placebo-controlled, cross-over study.
Phenylbutazone was more effective than MXM in controlling the primarily mechanical pain associated with the HBS model, whereas MXM was more effective in the SYN model, which is primarily associated with inflammatory pain at the oral doses used. Our results suggest that oral MXM may be more effective and have a more rapid onset in clinical conditions with a significant inflammatory component, whereas PBZ may be more effective when a significant source of pain is mechanical in origin when using the oral dosage regimen. Naturally occurring MSK conditions typically have a combination of mechanical and inflammatory pain; therefore, clinical response to treatment in individual horses may vary with the relative contributions of mechanical and inflammatory pain. Additional studies in naturally occurring clinical disease could further support the translation of these findings to clinical conditions.
In addition to efficacy, another factor to consider when selecting a NSAID is relative safety. As MXM is COX-2 selective in the horse, it would be anticipated to have a superior gastrointestinal safety profile. Experimental studies suggest that meloxicam may have fewer adverse GI effects than phenylbutazone (31,32).
Meloxicam is not currently approved for use in horses in Canada; however, since PBZ and aspirin are the only oral approved oral NSAIDs for horses in Canada, practitioners are using other NSAID products in an extra-label manner. Use of drugs in an extra-label manner is only appropriate if an approved drug is not suitable for use in a given patient because of administration or dosing challenges, efficacy issues, or safety issues. The results of this study suggest that preference for MXM or PBZ may depend on the specific clinical situation. Meloxicam may be a preferred choice in select clinical cases based on efficacy and safety considerations. It has previously been shown that generic human meloxicam tablets administered in molasses are bioequivalent to the product used in this study (33) and would therefore be expected to produce a similar response.
Acknowledgments
The authors thank Drs. Emma Read and Jerry Bailey for assistance with the intercarpal joint injections. Dr. Jonathan Foreman provided a heart bar shoe prototype so we were able to manufacture our own shoes and provided valuable advice on using the heart bar shoe model. We thank Lisa Colangeli, Kate Armstrong, Wendy Hawes, Danita Phelan, and Sandra Pinkham for technical assistance. CVJ
Footnotes
This work was supported by the University of Calgary Faculty of Veterinary Medicine.
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Foreman J, Ruemmler R. Phenylbutazone and flunixin meglumine used singly or in combination in experimental lameness in horses. Equine Vet J. 2011;43:12–17. doi: 10.1111/j.2042-3306.2011.00485.x. [DOI] [PubMed] [Google Scholar]
- 2.Owens JG, Kamerling SG, Stanton SR, Keowen ML. Effects of ketoprofen and phenylbutazone on chronic hoof pain and lameness in the horse. Equine Vet J. 1995;27:296–300. doi: 10.1111/j.2042-3306.1995.tb03080.x. [DOI] [PubMed] [Google Scholar]
- 3.Erkert RS, MacAllister CG, Payton ME, Clarke CR. Use of force plate analysis to compare the analgesic effects of intravenous administration of phenylbutazone and flunixin meglumine in horses with navicular syndrome. Am J Vet Res. 2005;66:284–288. doi: 10.2460/ajvr.2005.66.284. [DOI] [PubMed] [Google Scholar]
- 4.Naylor RF, Taylor AH, Knowles EJ, et al. Comparison of flunixin meglumine and meloxicam for post operative management of strangulating small intestinal lesions. Equine Vet J. 2014;46:427–434. doi: 10.1111/evj.12224. [DOI] [PubMed] [Google Scholar]
- 5.Doucet MY, Bertone AL, Hendrickson D, et al. Comparison of efficacy and safety of paste formulations of firocoxib and phenylbutazone in horses with naturally occurring osteoarthritis. J Am Vet Med Assoc. 2008;232:91–97. doi: 10.2460/javma.232.1.91. [DOI] [PubMed] [Google Scholar]
- 6.Sanchez L, Robertson S. Pain control in horses: What do we really know? Equine Vet J. 2014;46:517–523. doi: 10.1111/evj.12265. [DOI] [PubMed] [Google Scholar]
- 7.Nikolaou PK, Macdonald BL, Glisson RR, Seaber AV, Garrett WE. Biomechanical and histological evaluation of muscle after controlled strain injury. Am J Sports Med. 1987;15:9–14. doi: 10.1177/036354658701500102. [DOI] [PubMed] [Google Scholar]
- 8.Jayson M, St. Dixon A. Intra-articular pressure in rheumatoid arthritis of the knee. Pressure changes during passive joint distension. Ann Rheum Dis. 1970;29:261. doi: 10.1136/ard.29.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goddard N, Gosling P. Intra-articular fluid pressure and pain in osteoarthritis of the hip. J Bone Joint Surg [Br] 1988;70:52–55. doi: 10.1302/0301-620X.70B1.3339061. [DOI] [PubMed] [Google Scholar]
- 10.Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nature New Biol. 1971:232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- 11.Beretta C, Garavaglia G, Cavalli M. COX-1 and COX-2 inhibition in horse blood by phenylbutazone, flunixin, carprofen and meloxicam: An in vitro analysis. Pharmacol Res. 2005;52:302–306. doi: 10.1016/j.phrs.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 12.Zhu X, Conklin DR, Eisenach JC. Preoperative inhibition of cyclooxygenase-1 in the spinal cord reduces postoperative pain. Anesth Analg. 2005;100:1390–1393. doi: 10.1213/01.ANE.0000148127.53832.8E. [DOI] [PubMed] [Google Scholar]
- 13.Prochazkova M, Dolezal T, Sliva J, Krsiak M. Different patterns of spinal cyclooxygenase-1 and cyclooxygenase-2 mrna expression in inflammatory and postoperative pain. Basic Clin Pharmacol Toxicol. 2006;99:173–177. doi: 10.1111/j.1742-7843.2006.pto_457.x. [DOI] [PubMed] [Google Scholar]
- 14.Foreman JH, Lawrence LM. Lameness and heart rate elevation in the exercising horse. J Equine Vet Sci. 1991;11:353–356. [Google Scholar]
- 15.Palmer JL, Bertone AL. Experimentally-induced synovitis as a model for acute synovitis in the horse. Equine Vet J. 1994;26:492–495. doi: 10.1111/j.2042-3306.1994.tb04056.x. [DOI] [PubMed] [Google Scholar]
- 16.Foreman J, Barange A, Lawrence L, Hungerford L. Effects of single-dose intravenous phenylbutazone on experimentally induced, reversible lameness in the horse. J Vet Pharmacol Ther. 2008;31:39–44. doi: 10.1111/j.1365-2885.2007.00925.x. [DOI] [PubMed] [Google Scholar]
- 17.Foreman J, Bergstro B, Golden K, et al. Dose titration of the clinical efficacy of intravenously administered flunixin meglumine in a reversible model of equine foot lameness. Equine Vet J. 2012;44:17–20. doi: 10.1111/j.2042-3306.2012.00655.x. [DOI] [PubMed] [Google Scholar]
- 18.Dalla Costa E, Minero M, Lebelt D, Stucke D, Canali E, Leach MC. Development of the Horse Grimace Scale (HGS) as a pain assessment tool in horses undergoing routine castration. PloS One. 2014;9:e92281. doi: 10.1371/journal.pone.0092281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pritchett LC, Ulibarri C, Roberts MC, Schneider RK, Sellon DC. Identification of potential physiological and behavioral indicators of postoperative pain in horses after exploratory celiotomy for colic. Appl Anim Behav Sci. 2003;80:31–43. [Google Scholar]
- 20.Keegan KG, Kramer J, Yonezawa Y, et al. Assessment of repeatability of a wireless, inertial sensor–based lameness evaluation system for horses. Am J Vet Res. 2011;72:1156–1163. doi: 10.2460/ajvr.72.9.1156. [DOI] [PubMed] [Google Scholar]
- 21.Gallo P, Chuang-Stein C. A note on missing data in noninferiority trials. Drug Inf J. 2009;43:469–474. [Google Scholar]
- 22.Lucia J, Coverdale J, Arnold C, Winsco K. Influence of an intra-articular lipopolysaccharide challenge on markers of inflammation and cartilage metabolism in young horses. J Anim Sci. 2013;91:2693–2699. doi: 10.2527/jas.2012-5981. [DOI] [PubMed] [Google Scholar]
- 23.de Grauw J, Lest C, Brama P, Rambags B, van Weeren P. In vivo effects of meloxicam on inflammatory mediators, MMP activity and cartilage biomarkers in equine joints with acute synovitis. Equine Vet J. 2009;41:693–699. doi: 10.2746/042516409x436286. [DOI] [PubMed] [Google Scholar]
- 24.de Grauw J, van Loon J, van de Lest C, Brunott A, van Weeren P. In vivo effects of phenylbutazone on inflammation and cartilage-derived bio-markers in equine joints with acute synovitis. Vet J. 2014;201:51–56. doi: 10.1016/j.tvjl.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 25.Toutain P-L, Cester CC. Pharmacokinetic-pharmacodynamic relationships and dose response to meloxicam in horses with induced arthritis in the right carpal joint. Am J Vet Res. 2004;65:1533–1541. doi: 10.2460/ajvr.2004.65.1533. [DOI] [PubMed] [Google Scholar]
- 26.McCracken M, Kramer J, Keegan K, et al. Comparison of an inertial sensor system of lameness quantification with subjective lameness evaluation. Equine Vet J. 2012;44:652–656. doi: 10.1111/j.2042-3306.2012.00571.x. [DOI] [PubMed] [Google Scholar]
- 27.Rohrback H, Korpivaara T, Schatzmann U. Comparison of the effects of the alpha-2 agonists detomidine, romifidine and xylazine on nociceptive withdrawal reflex and temporal summation in horses. Vet Anaesth Analg. 2009;36:384–395. doi: 10.1111/j.1467-2995.2009.00466.x. [DOI] [PubMed] [Google Scholar]
- 28.Bannwarth B, Netter P, Pourel J, Royer RJ, Gaucher A. Clinical pharmacokinetics of onsteroidal anti-inflammatory drugs in the cerebrospinal fluid. Biomed Pharmacother. 1989;43:121–126. doi: 10.1016/0753-3322(89)90140-6. [DOI] [PubMed] [Google Scholar]
- 29.Kanda H, Kobayashi K, Yamanaka H, Noguchi K. COX-1-dependent prostaglandin D2 in microglia contributes to neuropathic pain via DP2 receptor in spinal neurons. Glia. 2013;61:943–956. doi: 10.1002/glia.22487. [DOI] [PubMed] [Google Scholar]
- 30.Zhu X, Conklin D, Eisenach JC. Cyclooxygenase-1 in the spinal cord plays an important role in postoperative pain. Pain. 2003;104:15–23. doi: 10.1016/s0304-3959(02)00465-7. [DOI] [PubMed] [Google Scholar]
- 31.Noble G, Edwards S, Lievaart J, Pippia J, Boston R, Raidal S. Pharmacokinetics and safety of single and multiple oral doses of meloxicam in adult horses. J Vet Intern Med. 2012;26:1192–1201. doi: 10.1111/j.1939-1676.2012.00976.x. [DOI] [PubMed] [Google Scholar]
- 32.D’Arcy-Moskwa E, Noble G, Weston L, Boston R, Raidal S. Effects of meloxicam and phenylbutazone on equine gastric mucosal permeability. J Vet Intern Med. 2012;26:1494–1499. doi: 10.1111/j.1939-1676.2012.01004.x. [DOI] [PubMed] [Google Scholar]
- 33.Vivancos M, Barker J, Engbers S, Fischer C, et al. Pharmacokinetics and bioequivalence of 2 meloxicam oral dosage formulations in healthy adult horses. Can Vet J. 2015;56:730–736. [PMC free article] [PubMed] [Google Scholar]