Abstract
From May 2008 to December 2013, 320 cases of otitis externa were diagnosed among 2012 dogs undergoing routine physical examinations at Celtic Creatures Veterinary Clinic, Sydney River, Nova Scotia for a diagnosis frequency of 15.9% [95% confidence interval (CI): 14.3% to 17.6%]. Twenty-four percent of these dogs exhibited 1 or multiple recurrences despite initial treatment with topical antimicrobial/anti-inflammatory solutions. The frequency of diagnosis was significantly higher in breeds with pendulous ears, but was not affected by ear hairiness. There were no seasonal patterns in the frequency of diagnosis. In clinical examination of 60 dogs with otitis externa, bacteria were evident in 47% of infections. Of 10 genera cultured, Staphylococcus spp. and diptheroids were most common. In this study, analysis of clinical records provided insights into the local prevalence of otitis externa and the efficacy of treatment in routine clinical situations.
Résumé
Étude épidémiologique des chiens atteints d’une otite externe au cap Breton, en Nouvelle-Écosse. De mai 2008 à décembre 2013, 320 cas d’otite externe ont été diagnostiqués parmi 2012 chiens subissant des examens de routine à la Celtic Creatures Veterinary Clinic, pour une fréquence de diagnostic de 15,9 % (IC de 95 % : de 14,3 % à 17,6 %). Vingt-quatre pour cent des chiens ont manifesté une ou plusieurs récurrences malgré le traitement initial avec des solutions topiques antimicrobiennes/anti-inflammatoires. La fréquence du diagnostic était significativement supérieure chez les races avec des oreilles pendantes, mais elle n’était pas affectée par la présence de poils dans l’oreille. Il n’y avait aucun profil saisonnier dans la fréquence du diagnostic. Dans un examen clinique de 60 chiens avec une otite externe, les bactéries étaient évidentes dans 47 % des infections. Parmi les 10 genres pour lesquels une culture a été réalisée, Staphylococcus spp. et les dipthéroïdes étaient les plus fréquents. Dans cette étude, l’analyse des dossiers cliniques a fourni des renseignements sur la prévalence locale de l’otite externe et l’efficacité du traitement des situations cliniques de routine.
(Traduit par Isabelle Vallières)
Introduction
Otitis externa is an inflammation of the external auditory canal from the pinna to the tympanic membrane commonly observed in canine patients in small animal veterinary practice (1,2). Clinical signs include inflammation, soreness/pain, malodor, discharge, excessive scratching, and head shaking. Chronic cases are those with prolonged clinical signs or progression to closure of the ear canal, production of aural polyps, or rupture of the tympanic membrane, all of which can lead to chronic pain and deafness. Repeated infections can occur during a dog’s lifetime, with the potential for increased severity of infection and development of resistance to antimicrobial treatment when microorganisms are an associated factor (1,3).
Otitis externa has a complex etiology which complicates management of this disease (1,2,4). Primary causes of inflammation include allergies/hypersensitivities, autoimmune diseases, keratinization disorders, and parasites in the ear canal. Progression of this disease can be perpetuated by bacterial and yeast infections. Some bacteria (e.g., Staphylococcus spp., Bacillus spp.) and yeast are normally present in small numbers in ears of healthy dogs (1,4). When these become numerous or other opportunistic bacteria (e.g., Pseudomonas spp., Escherichia spp., Proteus spp., Coryneform bacteria) colonize the ear, infections may develop and prolong inflammation (1,4–8). Identification of the primary cause(s) of otitis externa is the most critical factor to address in treating otic inflammation, particularly for chronic cases, but this can be challenging (9). Elimination of secondary bacterial or yeast infections via topical antibiotic/anti-inflammatory solutions is also an important and widely practiced therapeutic approach, since elimination of the primary disease without effective management of concurrent infections typically fails to resolve clinical signs (10).
Small animal veterinary practices have valuable information on the frequency of diagnosis of diseases such as otitis externa and on the efficacy of medical treatment in routine clinical situations. Such locally relevant information, however, is often overlooked in favor of larger datasets acquired from veterinary schools [e.g., the Veterinary Medical Data Program (11)] or experimental studies which may bear less relevance to routine procedures followed in veterinary practices. In this study, records from a small animal veterinary practice in Atlantic Canada were examined to assess the seasonal frequency of diagnosis of otitis externa across dog breeds and ear types and to document evidence of recurring infections associated with routine treatment. The relative contribution of yeast and bacteria in otitis externa was also examined given that these are frequently targeted when treating this disease, and bacterial genera associated with local cases were identified.
Two complementary approaches were used in this study. First, an open cohort retrospective analysis of cases of otitis externa diagnosed in a veterinary clinic from 2008 to 2013 was undertaken to assess the frequency of diagnosis, seasonal patterns, and microbial associations with this disease. Second, a prospective analysis of ear swabs of dogs sampled at the clinic was done to verify the relative contribution of bacteria versus yeasts in ear infections and to identify those bacterial groups associated with local cases of otitis externa.
Materials and methods
Veterinary clinic
Celtic Creatures Veterinary Clinic (CCVC) is an American Animal Hospital Association accredited small animal veterinary hospital in Sydney River, Cape Breton, Nova Scotia, that first opened in 2008 with 1 licensed veterinarian. It now has 2.5 full-time equivalent veterinarians and a caseload of approximately 60% canine, 35% feline, and 5% exotic species. Physical examinations and cytological tests are carried out by licensed veterinarians and registered veterinary technicians, respectively.
Retrospective study
Records of dogs from CCVC were compiled from the time the clinic opened in May 2008 to December 2013. As part of routine physical examinations at CCVC, all dogs were initially assessed visually for redness or inflammation of the ear pinna. If any redness, malodor, or abnormal otic exudate was detected or other clinical signs of otitis externa noted or reported by owners, then an otoscopic examination and cytological analysis were performed. A positive case of microbial otitis externa was included in this study if an infectious agent (bacteria/yeast) was present on the cytological examination with at least 3 organisms per field of view (1000× magnification). Because otoscopic examinations were not performed on all dogs that entered the clinic, some cases of otitis externa may have been missed in asymptomatic dogs.
For all positive cases, the date of the infection, infectious agent (yeast, bacteria, or mixed), breed, and age of the dog were noted, we also recorded if and when there was a recurrence. To examine breed-related associations with this disease, we categorized dogs into “breed groupings” based on shared traits (e.g., spaniels, terriers; Supplementary Table 1). Supplementary tables are available on request from the corresponding author. Mongrels included those dogs registered solely as a mixed breed that could not be associated with any breed grouping.
Patterns in diagnosis of otitis externa were investigated over 6 y of records from CCVC by grouping records seasonally and determining the number of dogs with a case of otitis externa divided by the total number of dogs examined in the clinic for that season. Dogs that made repeated visits to the clinic during a season were only counted once within that season, and were scored as positive if otitis externa was diagnosed in at least 1 examination. When a dog made repeated visits to the clinic that spanned different seasons, each seasonal occurrence was entered as a new event. Recurrences were not included in this seasonal analysis: when a dog was found positive for otitis externa, subsequent visits were removed from the dataset (12). We also examined seasonal patterns in the infectious agents associated with otitis externa by determining the percentage of cases associated with bacteria, yeast, or both, within each time period analyzed.
Associations between specific phenotypic traits and the infectious agent linked to cases of otitis externa were explored by categorizing breeds according to ear form (erect versus pendulous) and extent of hair growth around the ear opening (“hairy” versus “not hairy”). Categorization based on ear form and ear hairiness followed the methodology of previous studies (12,13). If a dog was reported with otitis externa more than once, a random number generator was used to select 1 of the multiple entries for inclusion in the dataset to avoid counting repeated infections in the same dog (12).
Recurring cases of otitis externa were defined as those in which a dog that was diagnosed and treated for an otitis externa infection was returned to the clinic after a minimum period of 1 mo with the same clinical signs. To classify as a recurrence, within the period between diagnoses a dog was determined to have “clean” ears on recheck (typically at the 2- or 3-week mark) or through a telephone follow-up call with the owner. Because not all treated dogs returned to the clinic for an otoscopic examination/cytological analysis following initial treatment, it could not be determined if recurrences were associated with new events or were the result of ineffective initial treatment. The percentage of cases of otitis externa associated with recurring infections was calculated by dividing the total number of dogs with recurrences by the total number of dogs with otitis externa over the specified time period. Frequency of recurrences was determined by following the examination history of each dog and noting the number of repeat cases of this disease over the 6-year study period. To determine the timeline relative to the first diagnosed infection, we calculated the number of months between recurrences over a 2-year sliding window. The analysis did not include infections diagnosed after January 2012, since there were less than 24 mo to the end of our analysis in December 2013. To assess the tendency for recurring infections to be associated with the same or different microbial infectious agents (bacteria versus yeast), we compared diagnoses with an initial and subsequent infection in the same dog. For dogs with more than 2 infections, each new infection was compared with the diagnosis of the previous infection.
Prospective study
From May to November 2013, we undertook a prospective study of active canine otitis externa infections at CCVC to identify bacterial genera associated with these infections. Dogs exhibiting clinical signs of unilateral or bilateral otitis externa had cotton tipped swabs placed into the ear canals as deeply as possible while minimizing contact with the exterior pinna and hair. Swab samples were transferred to glass slides for cytology. Slides were stained (Dip Quick Stain, JarVet; Jorgensen Laboratories, Loveland, Colorado, USA) and were examined at 1000× magnification. The presence of yeast, bacteria, or a mixed infection was determined for each slide, and the cytological grade reported as in Zur et al (14).
Veterinarian-determined diagnosis of an otitis externa infection was made based on 2 lines of evidence: i) otoscopic examination — presence of inflammation, exudate and the appearance of the tympanic membrane; and ii) the cytology results — the presence of an infectious agent with at least 3 organisms per field of view (1000× magnification). If yeast was the sole infectious agent, this was recorded but the sample was not examined further. If bacteria were present alone or in conjunction with yeast, owners were approached to obtain permission to enroll the dog in the clinical study. All protocols received approval from Cape Breton University’s Research Ethics Board and followed the guidelines of the Canadian Council on Animal Care.
Culture swabs (Star Swab II; Starplex Scientific, Etobicoke, Ontario) were taken from the infected ear(s), and streaked onto Columbia agar with 5% sheep blood and onto MacConkey agar, and the plates were incubated at 37°C for 24 to 36 h. After incubation, distinct colonies were sub-cultured to Columbia agar and MacConkey agar plates to obtain pure colonies, which were Gram-stained. Diptheroids (a group of Corynebacteria) were identified based on growth appearance on Columbia agar (small 0.5 mm to 2.0 mm colonies, opaque, white, or grey in color, sometimes with a “frosted” appearance) and Gram-stain morphology. Cultures other than diptheroids were plated on trypticase soy agar (TSA), incubated at 37°C for 24 to 36 h, after which oxidase and catalase tests were performed. With information from these tests, the appropriate diagnostic API strip (API STAPH, API 20E, or API 20NE) (API; bioMérieux Canada, St. Laurent, Quebec) was inoculated and incubated following the manufacturer’s guidelines, except for API 20NE which was incubated at 37°C for 24 h. Archival samples were stored in vials containing glycerol and frozen at −80°C.
Ears of dogs not exhibiting otitis externa (control dogs) were also examined to determine the natural ear flora of healthy dogs following the procedures described. Control dogs were deemed fit for the study if they had no visible clinical signs of otitis externa and were otherwise in good health.
Statistical analyses
Lower and upper limits of the 95% confidence interval (CI) around the frequency of diagnosis of otitis externa were determined using the Wilson score interval method, with a continuity correction (15). Chi-squared tests (two-tailed, α = 0.05), were used to compare frequencies of otitis externa across breed groupings and ear types, as well as proportions of bacterial versus yeast infections across ear types and in recurring infections. Seasonal differences in the incidence of otitis externa and in the relative proportion of bacterial associated infections across the 6-year study period were assessed using a non-parametric 1-way analysis of variance (ANOVA) (Kruskal-Wallis; α = 0.05).
Results
Retrospective analysis
Over 6 y at CCVC, 320 dogs were diagnosed with at least 1 event of otitis externa infection out of 2012 dogs examined, for an overall frequency of diagnosis of 15.9% (95% CI: 14.3% to 17.6%). Cases of this disease spanned 66 breeds, with retrievers (26%), toy breeds (20%), and terriers (12.5%) representing most of the infected dogs. Frequency of diagnosis varied significantly amongst breed groupings (Chi-square, P = 0.028): bulldogs (6/20; 30.0%), schnauzers (4/15; 26.7%), spaniels (22/83; 26.5%), and hounds (14/68; 20.6%) all had disease frequencies > 20%. In contrast, doodles (4/32; 12.5%), long-hairs (18/153; 11.8%), and mixed breeds (4/52; 7.7%) had the lowest values (≤ 12.5%). With respect to individual breeds, in which sample sizes per breed were ≥ 15 dogs, springer spaniels, miniature poodles, Shetland sheepdogs, cocker spaniels, and Siberian huskies had the highest frequencies of diagnosis reaching > 20%; in contrast, collies, mixed breeds, bull terriers, golden doodles, bichon frises, pomeranians, and miniature dachshunds appeared less prone, with < 10% of dogs having infections. Miniature dachshunds were the only breed examined in which no cases of otitis externa were reported (0/15). German shepherds had a frequency of diagnosis of 14.3% over 105 dogs examined.
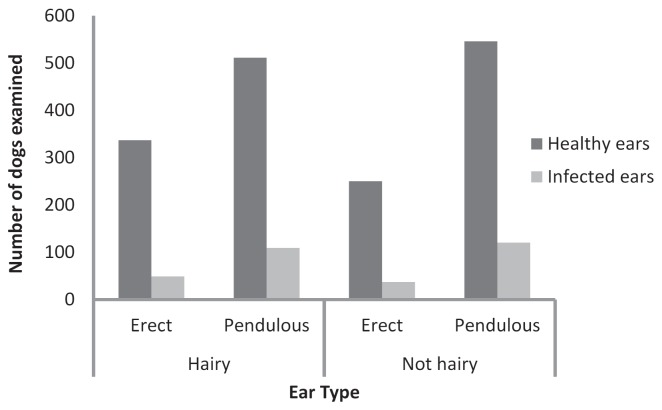
There was no significant association between ear hairiness and the frequency of otitis externa (hairy 15.7%; not hairy 16.5%; Chi-square, P = 0.64); however, dogs with pendulous ears were diagnosed significantly more frequently (17.8%) compared with those with erect ears (12.8%; Chi-square, P < 0.001; Figure 1).
Figure 1.
Distribution of dogs with healthy ears and those with otitis externa (infected ears) across different ear types as categorized on the basis of ear form (erect versus pendulous) and hairiness of the ear canal (hairy versus not hairy). Categorizations were based on the breed type recorded for each dog that entered the clinic (supplementary tables); mixed breed dogs were not included.
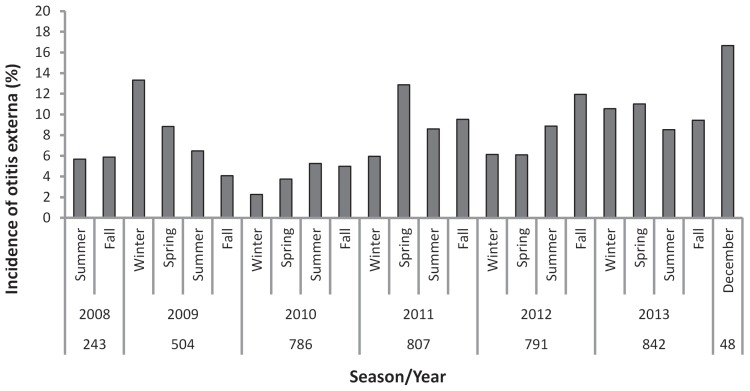
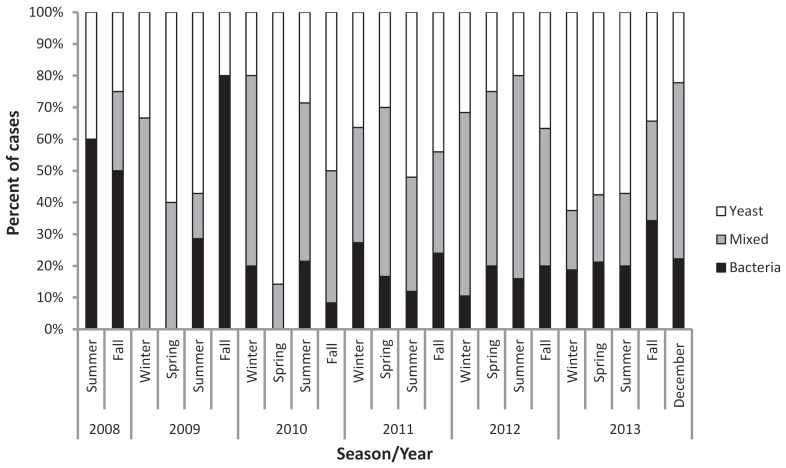
The annual incidence of otitis externa increased over our sampling period (Figure 2), reaching a high of 16.7% in December 2013 — the last period sampled. No significant differences were found in incidence levels across the 4 seasons (Kruskal-Wallis, P = 0.92); the average seasonal incidence was 7.7% (95% CI: 6.4% to 9.0%). Infectious agents were recorded in 89% (284/320) of the 320 clinical records at the time of diagnosis; in the remaining 36 cases, otitis externa was diagnosed, but the microbial association (bacterial, yeast, or mixed) was not specified in the clinical record. Based on complete records, yeasts were associated with the majority of infections (82.0%), either as sole or mixed infections. The relative frequency of occurrence of bacteria and yeast in otitis externa cases varied dramatically across sampling periods, but no consistent seasonal trends were apparent (Figure 3; Kruskal-Wallis, P = 0.61). Bacteria-associated infections (either bacteria or mixed) accounted for more than 37.5% of otitis externa cases in every season except spring 2010, and in 6 separate seasons, bacteria were associated with ≥ 75% of infections.
Figure 2.
Incidence of otitis externa diagnosed in dogs undergoing routine physical examinations at CCVC from 2008 to 2013 categorized by season. Dogs may be represented more than once across seasons; however, once a dog was diagnosed with otitis externa it was not included further in the dataset (i.e., recurrences are not included). The sample size refers to the number of dogs that were examined in the clinic during each time period.
Figure 3.
The percentage of cases of otitis externa diagnosed each season (from 2008 to 2013) that were associated with bacterial, yeast, or mixed (bacterial and yeast) infections. Values are expressed as percentages due to the varying number of cases each year.
The proportion of yeast, bacteria, or mixed infections did not differ significantly between dog breeds based on ear form, with bacteria associated with 54.5% and 59.1% of pendulous and erect eared dogs, respectively (Chi-square, P = 0.75). Likewise, no significant association was found based on ear hairiness (52.1% versus 59.6% for hairy/not hairy ears, respectively; Chi-square, P = 0.21).
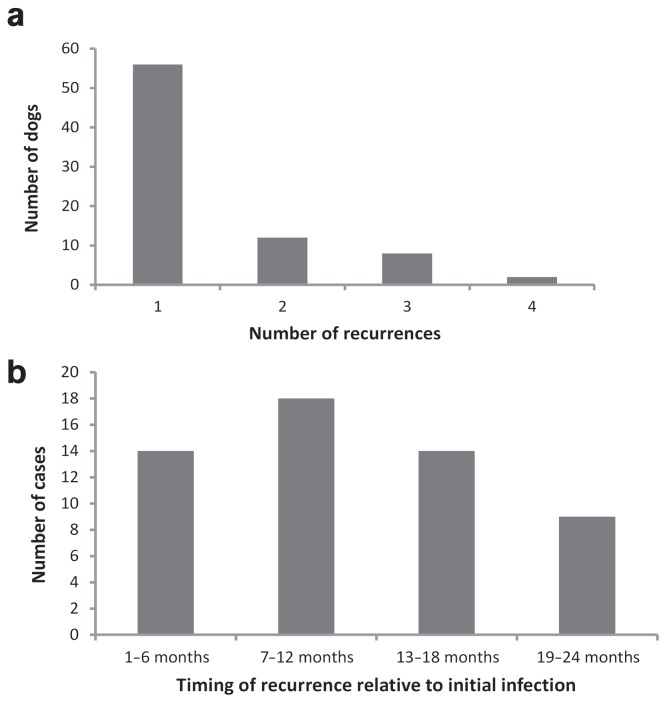
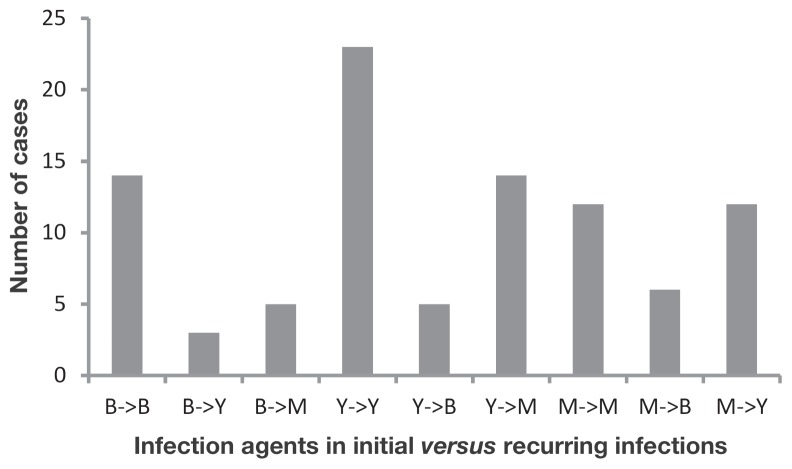
Seventy-eight dogs (24% of infected dogs) returned to the clinic with otitis externa once or multiple times during the course of the study despite initial treatment with a variety of topical antimicrobial/anti-inflammatory solutions. Most of these cases represented a single event, although as many as 4 recurring infections were documented in 2 dogs (Figure 4a). Return visits occurred most often in cases associated with yeast infections (25.6%), but were also high in mixed (21.4%) and bacterial infections (20.0%). The incidence of recurring infections was highest in dogs sampled during the first 2 y of the study (39% and 42%, respectively), reflecting the longer period over which recurrences could occur in these dogs relative to those examined during the latter part of the study. When analyzed over a 2-year sliding window, recurrences were more likely to occur within the 7- to 12-month time frame following initial diagnosis, with declining incidence after 1 y (4b). Recurring infections were significantly more likely to be associated with the same infectious agent as the initial infection for both bacterial (Chi-square, P = 0.009) and yeast-based cases (Figure 5; Chi-square, P = 0.003) of otitis externa, but not for mixed cases (Chi-square, P = 0.30). Neither ear type nor ear hairiness was associated with an increased frequency of recurring infections over the 6-year study period (ear type: Chi-square, P = 0.94; ear hairiness: Chi-square, P = 0.46).
Figure 4.
a — The number of dogs experiencing 1, 2, 3, or 4 recurrences among the 78 individuals that had multiple infections of otitis externa over the 6-year study period; b — The number of recurrences within 2 y of initial infection based on dogs first diagnosed with otitis externa from June 2008 to December 2011.
Figure 5.
Number of cases in which the infectious agent remained the same or was different between subsequent infections of otitis externa. Data are based on 94 cases examined from 2008 to 2013 in which the infectious agent was determined for both the initial and recurring infection of otitis externa. (B — bacteria; Y — yeast; M — mixed)
Prospective study
Sixty dogs with otitis externa spanning 21 breeds entered our prospective study from May to November 2013. Based on culture and cytology, 28 (47%) of these were positive for bacterial or mixed infections, whereas 32 had yeast alone. In total, 48 distinct types of bacterial colony representing 10 genera were isolated from the 28 dogs with bacteria/mixed infections. Staphylococcus was the most common bacterial genus (36% of cases), followed by the diptheroid group (23%). Klebsiella spp. and Proteus spp. were both isolated from 8% of the cultures, and Pseudomonas sp. was found in 6%. Other bacteria were grouped together as other Gram-positive bacteria, including Micrococcus spp. and Kocuria spp. and other Gram-negative species, consisting of Pantoea spp., Escherichia spp. and Weeksella spp. which made up 8% and 11% of the isolated cultures, respectively. The diptheroids were never isolated on their own. They were always found in conjunction with other bacteria.
Cytological analyses of the ears of the 10 control dogs revealed that 6 were positive for yeast, 3 were positive for bacteria, and 1 did not have any bacteria or yeast detected. Three bacterial genera were isolated from healthy ears: Staphylococcus spp., Bacillus spp., and a Streptococcus spp.
Discussion
With a diagnosis frequency of 15.9% among 2012 dogs examined over 6 y at CCVC, the results fall within the commonly cited 5% to 20% prevalence range for otitis externa (1,3,4,8,14,16). These high values underscore the importance of otitis externa in dogs (1). The increase in incidence of otitis externa over the 6-year study period at CCVC may be due to increased vigilance associated with 2 new veterinarians who joined the practice in Spring, 2010 and Fall, 2013.
Otitis externa is not evenly distributed across all canine breeds. Factors that predispose dogs to developing otitis externa include shapes of the ear canal and pinna, excessive moisture in the ear canal, and trauma to the ear due to excessive cleaning (12,14,17,18). Higher incidence of otitis externa is often reported in specific breeds of dogs (e.g., poodles, cocker spaniels, Brittany spaniels, Labrador retrievers, shar-peis, and German shepherds) that tend to have abnormalities of the ear, allergies, keratinization disorders, and hyperactive ceruminous glands (3,14,16,18,19). Our results documented the highest frequencies of diagnosis in springer and cocker spaniels, miniature poodles, Shetland sheepdogs, and Siberian huskies, a result not entirely consistent with other studies. Particularly unexpected was the relatively low frequency of otitis externa in German shepherds, since this breed is well-known for hyperactivity of its cerumen-producing glands (1,18).
Breeds with pendulous ears were associated with a higher frequency of otitis externa relative to breeds with erect ears in our study, although ear hairiness was not. These results corroborate, at least in part, the findings of Hayes et al (12) who first documented a significantly higher risk of otitis externa in dogs with hairy, pendulous ears. These ear traits may increase disease risk by affecting air flow, heat radiation, and convection from the ear canal relative to erect, hair-free ears, providing conditions ideal for microorganism growth. Contrary to expectation, however, Huang and Huang (13) did not find elevated external ear temperatures in dogs with hairy or pendulous ears. Higher frequencies of otitis externa in hairier ears have been linked to matted hair in the ear canal (5), entrapment of grass awns causing irritation and inflammation (19), and increase in ceruminous and sebaceous gland tissue (20).
Few studies have examined seasonal changes in the incidence of otitis externa, yet ambient temperature and humidity can result in subtle but important changes in the microenvironment of the ear (12,21,22). Using records from veterinary medical teaching hospitals in the US, Hayes et al (12) documented higher rates of diagnosis in the late summer and fall, and found that air temperature, rainfall, and relative humidity (with a 1- to 2-month lag) explained a substantial amount of variation in the hospital prevalence of first diagnosed cases at each hospital location. While our qualitative analysis noted changes in the incidence of otitis externa and type of microorganisms associated with otitic ears across seasons and years, there was no consistent pattern or significant seasonal effect. A more detailed analysis of canine lifestyles and activity patterns across seasons in Cape Breton (19) in the context of seasonal variation in weather may help to understand these results more fully.
Approximately 1 in 4 dogs diagnosed with otitis externa in our study experienced recurring clinical signs following initial treatment. The potential for recurrence is likely even higher than this, given that ~40% of dogs that entered our study in the first 2 y returned with otitis externa over the subsequent 4 to 5 y of records. Recurrences could reflect a number of factors, including the use of ineffective medications, the presence of microbe strains resistant to topical antibiotics/antifungals, or poor owner compliance. Failure to treat the primary cause(s) of otitis externa, which is critical to resolving ongoing inflammation in the ear canal, could contribute to the potential for this disease to recur (2,9). Determination of primary causes is often bypassed in routine treatment of otitis externa in an effort to quickly alleviate clinical signs and suffering that arise with microbial infections. The high frequency of recurrences documented herein, however, suggests that increased emphasis should be placed on addressing primary causes, particularly for chronic cases of this disease. For an affected individual, this will involve a thorough history and assessment of lifestyle and diet, a physical and dermatological examination, and an understanding of specific breed-related predispositions to this disease (9,14,19).
Treatment of microbial infections remains an important short-term therapeutic objective in alleviating clinical signs of otitis externa. Both bacteria and yeast were associated with otitis externa in our study, with both pure and mixed infections of each. Bacteria cultured from dogs with otitis externa at CCVC were similar to genera found in past investigations; however, differences were evident in the prevailing genera. The common presence of Staphylococcus spp. was consistent with the literature (4,6,8,14,18); however, we reported a higher incidence of diptheroids (23% of cases) compared with most other studies (4,18; 1.2 %, and 10.3% of dogs, respectively). In addition, previous studies documented higher incidences of Pseudomonas spp. (7% to 35.5%; 4,6,7,23) compared with 6% herein. Regarding control dogs, the 3 genera of bacteria and yeast we documented have also been reported in controls of another study (4).
Identification of bacterial cultures was restricted to the genus level, given that API strips do not accurately identify organisms from veterinary origin to species level (24–26). Identifying bacterial cultures to species and testing their resistance to antibiotics commonly used to treat otitis externa would provide additional insights in local cases. Post-treatment evaluation of the otic inflammation is the most certain way to determine success of a treatment rather than in vitro analysis of bacterial isolates and susceptibility patterns (2,9). However, in vitro studies remain beneficial for selecting the optimal medication for treating the infection, especially in cases in which empirical treatment is unsuccessful (4,6,8,9,18,27).
The treatment of diseases with complex etiologies such as otitis externa may benefit from the careful examination of clinical records from veterinary practices. Disease prevalence can vary temporally and geographically, as can the prevalence of bacteria and yeast species associated with otitis externa and their susceptibility to antimicrobial/antifungal agents. Bacterial strains can also be expected to change through time in response to selective pressure through use of topical antibiotics and fungicides (7,8,23,28). In Canada, there have been relatively few recent surveys of the diversity and susceptibility of bacterial species associated with otitis externa (6), and while there is general consistency in the common bacteria present (5,6, our study), there are differences in prevalence as well as in resistance to antibiotics. Knowledge of the microbial species present locally and their susceptibility to specific antibiotics should facilitate more effective treatment and reduce the likelihood of chronic cases or the emergence of antibiotic-resistant bacteria.
Working with datasets from small animal veterinary practices can have substantial challenges and limitations that should be considered in future studies undertaking similar approaches. In small start-up practices such as CCVC, there can be inconsistencies in record-keeping until standard methodological approaches have been developed. Such issues are not unique to small animal practices, however, and can also be a problem in larger retrospective datasets (11). In addition, datasets sampled from veterinary practices will be subject to different standards and limitations compared to those collected from more circumscribed experimental studies. For instance, routine clinical practices do not typically subject every canine patient to an otoscopic examination; consequently, asymptomatic dogs with otitis externa may be missed, leading to underestimates of the true prevalence of this disease. For practicing veterinarians, however, an understanding of what is diagnosed in a clinic is important, as is the recurrence of clinical signs (either through direct observation or reports from owners). Likewise, large differences between the results of experimental studies and actual outcomes in veterinary practices can be informative and can reflect the need for changing medical interventions and procedures.
Acknowledgments
We thank Lillian Kelly for her assistance and invaluable experience in the laboratory, the staff of Celtic Creatures Vet Clinic, Dr. David McCorquodale and Dr. Robert Bailey at Cape Breton University for their support of this project, and Matt Saab and Dr. Anne Muckle for their comments and suggestions on the manuscript. We also notably appreciate the comments of the two anonymous reviewers who helped to improve the manuscript considerably. We acknowledge IDEXX Laboratories for donating clinical supplies and NSERC for supporting LP through an Industrial USRA. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
This study was funded by the Industrial NSERC Undergraduate Student Research Award granted to Laura Perry.
References
- 1.August JR. Otitis externa: A disease of multifactorial etiology. Vet Clin North Am Small Anim Pract. 1988;18:731–742. doi: 10.1016/s0195-5616(88)50076-1. [DOI] [PubMed] [Google Scholar]
- 2.Murphy KM. A review of techniques for the investigation of otitis externa and otitis media. Clin Tech Small Anim Pract. 2001;16:236–241. doi: 10.1053/svms.2001.27601. [DOI] [PubMed] [Google Scholar]
- 3.Angus JC, Lichtensteiger C, Campbell KL, Schaeffer DJ. Breed variations in histopathologic features of chronic severe otitis externa in dogs: 80 cases (1995–2001) J Am Vet Med Assoc. 2002;221:1000–1006. doi: 10.2460/javma.2002.221.1000. [DOI] [PubMed] [Google Scholar]
- 4.Lyskova P, Vydrzalova M, Mazurova J. Identification and antimicrobial susceptibility of bacteria and yeasts isolated from healthy dogs and dogs with otitis externa. J Vet Med Ser A. 2007;54:559–563. doi: 10.1111/j.1439-0442.2007.00996.x. [DOI] [PubMed] [Google Scholar]
- 5.Rycroft AK, Saben HS. A clinical study of otitis externa in the dog. Can Vet J. 1977;18:64–70. [PMC free article] [PubMed] [Google Scholar]
- 6.Hariharan H, Coles M, Poole D, Lund L, Page R. Update on antimicrobial susceptibilities of bacterial isolates from canine and feline otitis externa. Can Vet J. 2006;47:253–255. [PMC free article] [PubMed] [Google Scholar]
- 7.Aalbæk B, Bemis DA, Schjærff M, Kania SA, Frank LA, Guardabassi L. Coryneform bacteria associated with canine otitis externa. Vet Microbiol. 2010;145:292–298. doi: 10.1016/j.vetmic.2010.03.032. [DOI] [PubMed] [Google Scholar]
- 8.Zamankhan Malayeri H, Jamshidi S, Zahraei Salehi T. Identification and antimicrobial susceptibility patterns of bacteria causing otitis externa in dogs. Vet Res Commun. 2010;34:435–444. doi: 10.1007/s11259-010-9417-y. [DOI] [PubMed] [Google Scholar]
- 9.Jacobson LS. Diagnosis and medical treatment of otitis externa in the dog and cat. J S Afr Vet Assoc. 2002;73:162–170. doi: 10.4102/jsava.v73i4.581. [DOI] [PubMed] [Google Scholar]
- 10.Angus JC. Otic cytology in health and disease. Vet Clin Small Anim Pract. 2004;34:411–424. doi: 10.1016/j.cvsm.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Hayes HM, Jr, Wilson GP, Moraff H. Proceedings of the International Symposium on Animal Health and Disease Data Banks. US Department of Agriculture; Washington, DC: 1979. The Veterinary Medical Data Program (VMDP): Past, present, and future; pp. 127–132. Miscellaneous publication (United States. Dept. of Agriculture) [Google Scholar]
- 12.Hayes HM, Jr, Williams Pickle LW, Wilson GP. Effects of ear type and weather on the hospital prevalence of canine otitis externa. Res Vet Sci. 1987;42:294–298. [PubMed] [Google Scholar]
- 13.Huang H-P, Huang H-M. Effects of ear type, sex, age, body weight, and climate on temperatures in the external acoustic meatus of dogs. Am J Vet Res. 1999;60:1173–1176. [PubMed] [Google Scholar]
- 14.Zur G, Lifshitz B, Bdolah-Abram T. The association between the signalment, common causes of canine otitis externa and pathogens. J Small Anim Pract. 2011;52:254–258. doi: 10.1111/j.1748-5827.2011.01058.x. [DOI] [PubMed] [Google Scholar]
- 15.Newcombe RG. Two-sided confidence intervals for the single proportion: Comparison of seven methods. Stat Med. 1998;17:857–872. doi: 10.1002/(sici)1097-0258(19980430)17:8<857::aid-sim777>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 16.Fernández G, Barboza G, Villalobos A, Parra O, Finol G, Ramírez R. Isolation and identification of microorganisms present in 53 dogs suffering otitis externa. Rev Científica. 2006;16:23–30. [Google Scholar]
- 17.Webster FL, Whyard BH, Brandt RW, Jones WG. Treatment of otitis externa in the dog with Gentocin otic. Can Vet J. 1974;15:176–177. [PMC free article] [PubMed] [Google Scholar]
- 18.Kiss G, Radványi S, Szigeti G. New combination for the therapy of canine otitis externa. I. Microbiology of otitis externa. J Small Anim Pract. 1997;38:51–56. doi: 10.1111/j.1748-5827.1997.tb02987.x. [DOI] [PubMed] [Google Scholar]
- 19.Saridomichelakis MN, Farmaki R, Leontides LS, Koutinas AF. Aetiology of canine otitis externa: A retrospective study of 100 cases. Vet Dermatol. 2007;18:341–347. doi: 10.1111/j.1365-3164.2007.00619.x. [DOI] [PubMed] [Google Scholar]
- 20.Huang H-P, Little CJL, McNeil PE. Histological changes in the external ear canal of dogs with otitis externa. Vet Dermatol. 2009;20:422–428. doi: 10.1111/j.1365-3164.2009.00853.x. [DOI] [PubMed] [Google Scholar]
- 21.Grono LR. Studies of the microclimate of the external auditory canal in the dog. I. Aural temperature. Res Vet Sci. 1970;11:307–311. [PubMed] [Google Scholar]
- 22.Grono LR. Studies of the microclimate of the external auditory canal in the dog. III. Relative humidity within the external auditory meatus. Res Vet Sci. 1970;11:316–319. [PubMed] [Google Scholar]
- 23.Bugden DL. Identification and antibiotic susceptibility of bacterial isolates from dogs with otitis externa in Australia. Aust Vet J. 2013;91:43–46. doi: 10.1111/avj.12007. [DOI] [PubMed] [Google Scholar]
- 24.Koop G, De Visscher A, Collar CA, et al. Identification of coagulase-negative Staphylococcus species from goat milk with the API Staph identification test and with transfer RNA-intergenic spacer PCR combined with capillary electrophoresis. J Dairy Sci. 2012;95:7200–7205. doi: 10.3168/jds.2012-5747. [DOI] [PubMed] [Google Scholar]
- 25.Swanson EC, Collins MT. Use of the API 20E system to identify veterinary Enterobacteriaceae. J Clin Microbiol. 1980;12:10–14. doi: 10.1128/jcm.12.1.10-14.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watts JL, Owens WE, Nickerson SC. Identification of staphylococci from bovine udders: Evaluation of the API 20GP system. Can J Microbiol. 1986;32:359–361. doi: 10.1139/m86-069. [DOI] [PubMed] [Google Scholar]
- 27.Lilenbaum W, Veras M, Blum E, Souza GN. Antimicrobial susceptibility of staphylococci isolated from otitis externa in dogs. Lett Appl Microbiol. 2000;31:42–45. doi: 10.1046/j.1472-765x.2000.00759.x. [DOI] [PubMed] [Google Scholar]
- 28.Blue JL, Wooley RE. Antibacterial sensitivity patterns of bacteria isolated from dogs with otitis externa. J Am Vet Med Assoc. 1977;171:362–363. [PubMed] [Google Scholar]