Abstract
Computed tomography (CT) is able to measure the attenuation of urine in Hounsfield units (HU) on abdominal imaging studies. This study was designed to measure the correlation of urine attenuation with urine specific gravity in urine samples of 40 dogs, providing a noninvasive measure of urine concentration. The HU of urine explained 72% of the variance in measured urine specific gravity [R2 = 0.72, F(1,38) = 95.55, P < 0.001]. This noninvasive measurement can be used to estimate urine concentration in dogs undergoing abdominal CT imaging.
Résumé
Mesure de la concentration de l’urine canine par tomodensitométrie. La tomodensitométrie (TO) peut mesurer l’atténuation de l’urine en unités Hounsfield (UH) dans des études d’imagerie abdominale. Cette étude a été conçue pour mesurer la corrélation de l’atténuation de l’urine avec la gravité spécifique de l’urine dans des échantillons d’urine de 40 chiens, ce qui a fourni une mesure non invasive de la concentration de l’urine. Les UH de l’urine ont expliqué 72 % des variances dans la gravité spécifique de l’urine mesurée [R2 = 0,72, F(1,38) = 95,55, P < 0,001]. Cette mesure non invasive peut servir à estimer la concentration de l’urine des chiens subissant une imagerie abdominale réalisée par tomodensitométrie.
(Traduit par Isabelle Vallières)
Urine specific gravity is a common laboratory test performed to evaluate polyuria, hydration status, and renal function. Urine specific gravity is an estimate of osmolality, which evaluates the ability of the renal tubules to concentrate urine by active reabsorption (1). The specific gravity of dog’s urine is most commonly determined using a refractometer. Testing urine specific gravity requires a urine sample collected by free catch, catheterization, or cystocentesis.
Specific gravity is highly correlated to water content of substances (2) and has been shown to correlate with the computed tomographic (CT) measurement of urine attenuation using Hounsfield units (HU) in humans (3). Animals undergoing CT of the abdomen have quantitative imaging data collected about urine attenuation of X-ray beam during the study. This noninvasive measure of urine attenuation might be correlated to urine specific gravity in dogs and could provide quantitative information about urine concentration during imaging studies. A surrogate method of measuring urine specific gravity could facilitate studies in which urine samples were not collected, or to perform urinalysis at the same time as imaging. We hypothesized that urine attenuation measured by CT is correlated to urine specific gravity in dogs.
Urine samples collected for routine clinical use, submitted to the VMTH Clinical Diagnostic Laboratory, were used for this study, thus additional IACUC approval was not required. Urine samples were centrifuged at 335 × g for 6 min. Specific gravity of the urine supernatant was determined using a refractometer (Reichert Vet 360; Reichert Analytical Instruments, Reichert, Depew, New York, USA). All urine samples underwent sediment analysis for presence of lipid and to confirm presence of red blood cells. Other tests, performed on unspun urine, included pH and presence of protein, glucose, ketones, bilirubin, lipid, and hemoprotein (Chemstrip 10 UA; Roche Diagnostics, Indianapolis, Indiana, USA; Roche Urisys 1800 analyzer; Roche Diagnostics). The supernatant was stored in glass/plastic tubes at 4°C prior to CT imaging. Urine samples were placed in plastic racks that did not produce beam hardening during imaging.
Urine samples were imaged within 24 h of collection. The urine samples were imaged on a 16-slice clinical CT scanner (Lightspeed16; General Electric, Milwaukee, Wisconsin, USA) at 120 kV, 100 mA, and 0.625 mm collimation and were reconstructed in a high frequency algorithm. The first 20 samples were scanned a second time after inverting each tube 5 times to suspend any dependent particles. Regions of interest (ROI) were drawn on the center of the urine in each tube with an approximately standard size, avoiding partial volume artifacts from the walls of the tube (Osirix 64 bit v. 6.0; Pixmeo, Bernex, Switzerland). The mean HU of the ROI was recorded for each sample.
The HU of the inverted and non-inverted samples were compared with paired t-tests. Linear regression was used to evaluate the correlation of urine specific gravity and urine HU. Spearman correlation tests were used to determine whether urine solutes affected the HU measurement.
Forty consecutive canine urine samples were obtained for CT imaging. The mean age of the dogs was 8.6 y [± standard deviation (SD) 3.2 y], and mean weight was 22.6 kg (± 15.2 kg). The gender distribution was 1 female, 15 female spayed, 4 male, and 20 male neutered dogs. A variety of breeds were represented, including 31 purebreeds and 9 mixed breed dogs. Fifteen dogs were presented for signs referable to the urinary tract, and 25 dogs had medical conditions unrelated to the urinary tract.
The mean HU of the urine samples was 35.6 (± 16.3; range: 7.9 to 72.2), the mean specific gravity of the samples was 1.030 (± 0.0133; range: 1.007 to 1.057), and the mean pH of the urine was 6.75 (± 1.0; range: 5 to 9). Twenty-eight of 40 (70%) dogs had no or trace proteinuria, and 12/40 (30%) had moderate to high proteinuria. Seven of 40 (17.5%) dogs had ketonuria, and 16/40 (40%) had bilirubinuria. Twenty-eight of 40 (70%) dogs had hemoproteinuria. Twenty-three of 40 (57.5%) dogs had lipiduria. No dog had glucosuria.
Inversion of the samples did not result in significant alteration of the measured urine HU (P = 0.82). The HU of urine samples scanned the same day as collection did not differ significantly from those stored overnight (P = 0.51).
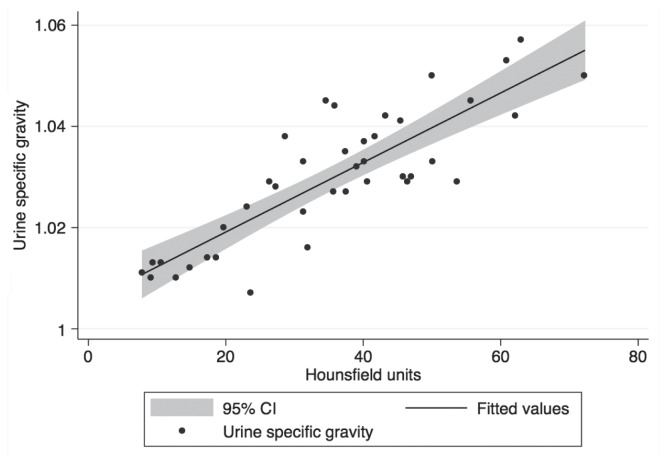
A linear regression found that the HU of urine could statistically significantly predict urine specific gravity, F(1,38) = 95.55, P < 0.001 and that HU accounted for 72% of the explained variability of specific gravity. The regression equation was: predicted specific gravity = 1.005 + 0.0007 × (HU). Spearman’s correlation found that none of the measured urine variables were significantly associated with urine HU (P > 0.05).
The HU of urine samples in this study was highly correlated to measured specific gravity. The attenuation measurements of liquids on CT are largely due to their water content and are closely related to water concentration (2). Urine is mainly comprised of water with variable concentrations of solutes. The high proportion of water in urine explains the good correlation of urine concentration to specific gravity.
Protein causes an increase in specific gravity, and also moderately increases the attenuation of pleural fluid (exudate) as measured on CT (4). Increased HU of peritoneal fluid has also been associated with colorectal perforations, hypothesized to be secondary to increased bacteria and intestinal contents within the fluid (5). We did not find protein to be a significant variable affecting urine HU measurement in dogs. There was a wide distribution of protein levels in the samples used. However, the generally low concentrations may explain the lack of attenuation changes caused by protein in urine (0.15 to 5.0 g/L) compared to an exudate (> 29 g/L).
The effective atomic number of a substance determines the degree to which it absorbs X-rays, and therefore affects the CT HU. Urine has a higher effective atomic number than other body fluids (7.74 +/− 0.15), including blood, pus, and bile. High HU of urine up to 50.7 HU has been measured in dehydrated humans (6). The high attenuation of samples with higher specific gravity is likely due to the concentration of electrolytes in the urine. This may indicate that other solutes in urine have more effect on the measured HU than the presence of common solutes measured on urinalysis.
Computed tomography attenuation is affected by the k-edge of materials. Photoelectric absorption of X-rays increases abruptly at an energy just higher than that of the K shell electron. Sodium chloride has been shown to linearly increase the HU of solutions, in the range that includes renal tissue and urine, while urea had no effect (3). This is likely due to the low k-edge of urea compared to sodium chloride, and increased absorption of the X-rays by sodium chloride. Sodium in urine is regulated by tubular reabsorption, and concentration can be altered in metabolic or renal disease. Although this is not routinely measured on urinalysis, it is a possible factor affecting the variability of HU measurements in urine compared to specific gravity.
The urine samples were tested for the included variables before centrifugation. This may introduce a discrepancy between the urine tests and the CT attenuation of the sample. The remainder of the variables are soluble in water and centrifugation should not have a significant effect. No differences were found between the variables and urine HU, likely indicating any effect of centrifugation was small.
The effect of residual debris in the current study was evaluated by inverting the samples and re-scanning them. There was no significant difference in HU between these conditions indicating that the samples were free of suspended particles. When translating this technique to the clinical environment, consideration should be given to the presence of urine sediment in the live animal. The urine samples in this study were centrifuged to remove the suspended particles. Animals under anesthesia are likely to have settling of debris in the dependent portion of the bladder, making the center or superficial layer of urine the most appropriate for measurement. Debris could increase the HU of urine if included in the region of interest or suspended in the urine.
In conclusion, the measured HU of urine explained a high level of the variance in measured urine specific gravity. This noninvasive measurement can be used to estimate urine concentration in dogs undergoing abdominal CT imaging. CVJ
Figure 1.
Linear regression of urine specific gravity and Hounsfield units of canine urine samples. Predicted specific gravity = 1.005 + 0.007 × (HU).
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Watson AD. Urine specific gravity in practice. Aust Vet J. 1998;76:392–398. doi: 10.1111/j.1751-0813.1998.tb12384.x. [DOI] [PubMed] [Google Scholar]
- 2.Unger E, Littlefield J, Gado M. Water content and water structure in CT and MR signal changes: Possible influence in detection of early stroke. Am J Neuroradiol. 1988;9:687–691. [PMC free article] [PubMed] [Google Scholar]
- 3.Hsu CT, Wang ZJ, Yu ASL, et al. Physiology of renal medullary tip hyperattenuation at unenhanced CT: Urinary specific gravity and the NaCl concentration gradient. Radiology. 2008;247:147–153. doi: 10.1148/radiol.2471070585. [DOI] [PubMed] [Google Scholar]
- 4.Nandalur KR, Hardie AH, Bollampally SR, Parmar JP, Hagspiel KD. Accuracy of computed tomography attenuation values in the characterization of pleural fluid: An ROC study. Acad Radiol. 2005;12:987–991. doi: 10.1016/j.acra.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Seishima R, Okabayashi K, Hasegawa H, et al. Computed tomography attenuation values of ascites are helpful to predict perforation site. World J Gastroenterol. 2015;21:1573–1579. doi: 10.3748/wjg.v21.i5.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahnken AH, Stanzel S, Heismann B. Spectral rhoZ-projection method for characterization of body fluids in computed tomography: Ex vivo experiments. Acad Radiol. 2009;16:763–769. doi: 10.1016/j.acra.2009.01.002. [DOI] [PubMed] [Google Scholar]