SUMMARY
The six cortical layers have distinct anatomical and physiological properties, like different energy use and different feedforward and feedback connectivity. It is not known if and how layer-specific neural processes are reflected in the fMRI signal. To address this question we used high-resolution fMRI to measure BOLD, CBV, and CBF responses to stimuli that elicit positive and negative BOLD signals in macaque primary visual cortex. We found that regions with positive BOLD responses had parallel increases in CBV and CBF, whereas areas with negative BOLD responses showed a decrease in CBF but an increase in CBV. For positive BOLD responses, CBF and CBV increased in the center of the cortex, but for negative BOLD responses, CBF decreased superficially while CBV increased in the center. Our findings suggest different mechanisms for neurovascular coupling for BOLD increases and decreases, as well as laminar differences in neurovascular coupling.
INTRODUCTION
Despite the widespread use of functional magnetic resonance imaging (fMRI), the relative contributions of processes like feedforward, feedback, excitation, and inhibition to the blood oxygenation level-dependent (BOLD) signal remain unknown. Given the known segregation of input and output, and termination of feedforward and feedback connections in specific cortical layers (Felleman and Van Essen, 1991), high-resolution (i.e., laminar) fMRI could potentially be used to address such questions. The six cortical layers have different distributions of cell types, cell sizes, connectivity, energy use, etc., reflecting the different functions of the layers. For instance, input to a cortical area typically arrives in layer IV, while output is typically generated in layer V. In primary visual cortex (V1), the different stimulus selectivity of the layers, e.g., the magno- and parvocellular pathways, is well known (Callaway, 1998). The relative thickness of the layers also varies for different cortical areas depending on the function of the area. If these anatomical and functional differences have a counterpart in the fMRI signals, fMRI at laminar resolution might be used to elucidate such different cortical computations.
However, laminar differences under different stimulus conditions have remained elusive. There could be multiple reasons for this, for instance resolution limitations. Another possible reason is that the profile of the BOLD response as a function of cortical depth could be determined by the properties of the vasculature, with the laminar profile of the BOLD response only exhibiting amplitude differences, independent of which layers show strongest neural activity. Yet another possibility is that the point spread function (PSF) of the hemodynamic response is larger than the thickness of the layers. To address these questions and to investigate whether obvious laminar differences in the patterns of the BOLD response exist, we compared the laminar properties of positive and negative BOLD responses. We chose these stimuli because of the large differences between the responses and because the negative BOLD signal has been reported to have different properties; namely, to be more specific than the positive BOLD signal (Bressler et al., 2007) and to provide independent information about brain function (Wade and Rowland, 2010).
Negative BOLD responses have been observed in humans and animals (Allison et al., 2000; Harel et al., 2002; Huang et al., 1996; Shmuel et al., 2002, 2006; Tootell et al., 1998). In V1, negative responses can be reliably observed adjacent to positive BOLD signals (Huang et al., 1996; Shmuel et al., 2002, 2006; Tootell et al., 1998; Wade and Rowland, 2010). They were also observed upon ipsilateral inhibition in visual-, motor- and somatosensory cortex (Allison et al., 2000; Hlushchuk and Hari, 2006; Schäfer et al., 2012; Smith et al., 2004; Stefanovic et al., 2004; Whittingstall et al., 2008). Negative BOLD signals were shown to be associated with decreases in cerebral blood flow (CBF) and neural activity (Boorman et al., 2010; Devor et al., 2007; Shmuel et al., 2002, 2006). Although the above results suggest that negative BOLD signals reflect decreased neural activity and/or inhibition, it is not known whether its mechanism of neurovascular coupling is similar to the mechanism for the positive BOLD response. In fact, it is not yet fully resolved if negative BOLD signals have a purely neural origin or whether hemodynamic properties also play a role (Bianciardi et al.,2011; Harel et al., 2002), nor is the laminar profile of the negative BOLD signal known.
Here, we measured BOLD, CBF, and cerebral blood volume (CBV) in regions with positive and negative BOLD signals in anesthetized macaques and found that in regions with positive BOLD signals, CBF and CBV were also increased, while in regions where the BOLD signal was negative, CBF decreased but CBV increased. High-resolution fMRI revealed that layer-dependent differences in the BOLD, CBF, and CBV signals underlie these effects, suggesting that the mechanism of neurovascular coupling differs not only for positive and negative BOLD signals but also depending on cortical layer. Because of the laminar segregation of functionality, this may open up the possibility of using high-resolution fMRI to separately study the contributions of feedforward, feedback, excitatory, or inhibitory processes to fMRI signals.
RESULTS
High-resolution functional imaging of V1 was performed on eight anesthetized monkeys at 4.7 T (12 experiments; see Logothetis et al., 1999, and Goense et al., 2010, for technical details). BOLD, functional CBV, and CBF data were acquired while the animals were viewing rotating checkerboard stimuli and center/ring rotating checkerboard stimuli (Figure 1A) that were shown to elicit negative BOLD responses in macaques and humans (Shmuel et al., 2002, 2006). Positive BOLD responses were observed in the locations of V1 that correspond to the retinotopic representation of the fovea and the ring; negative BOLD responses were observed in the locations representing the gray area between the center spot and the ring (Figure 1B; eight-segment gradient-echo [GE] echo planar imaging [EPI], spatial resolution 0.5 × 0.375 mm2). These responses were consistent with previous results from our lab (Shmuel et al., 2006). The negative BOLD responses were weaker than the positive BOLD responses (Table 1), also in agreement with earlier observations (Shmuel et al., 2006). The functional CBV response however, showed a very different pattern from the BOLD activation pattern, with a CBV increase over the entire V1 (Figure 1D). CBV was measured in the same slices after injection of the iron-based contrast agent monocrystalline iron oxide nanocolloid (MION), using the same acquisition parameters as for the BOLD acquisition. When the CBV increases, this results in a higher MION concentration in a given voxel and causes a decrease in signal intensity (Figure 1C). Figure 1D shows the same data as Figure 1C but with an inverted color scale, reflecting the sign of the CBV changes. The CBV was increased in the areas that showed positive BOLD responses (purple in Figure 1C, yellow in Figure 1D); however, areas that showed negative BOLD responses also had an increased CBV (blue in Figure 1C, red in Figure 1D) instead of the expected decrease. Thus, in contrast to the BOLD responses, which have opposite signs in the stimulated and adjacent suppressed regions, CBV was increased in both regions, although the CBV increases in the un-stimulated regions were smaller than in the stimulated regions (Table 1). Figure 2 shows all significantly activated voxels that had both nonzero CBV and BOLD responses, indicating that positive as well as negative BOLD signals co-occurred with CBV increases (i.e., decreases in functional signal intensity after MION injection).
Figure 1. BOLD and Functional CBV Responses in a Representative Animal.
(A) Rotating checkerboard stimulus with the center covering 0°–3° and the ring covering 8°–11° of the visual field. The BOLD response to this stimulus (B) shows positive and negative activation in the stimulated and unstimulated regions, respectively. The BOLD map reflects the retinotopic representation of the stimulus in V1 and shows positive activation in the fovea and the location corresponding to the ring and a negative response at the location corresponding to the unstimulated area. The signal after MION injection is decreased (C) corresponding to an increased CBV; CBV increased in both stimulated and unstimulated regions.
(D) The same map as in (C) with inverted color scale. The CBV map shows V2 and MT activation in addition to V1 activation, because of the higher CNR of the CBV signal. Eight-segment GE-EPI: resolution, 0.5 × 0.375 × 2 mm3; TE, 20 ms; TR, 750 ms. Data were linearly detrended and spatially filtered for display purposes (Gaussian kernel, 1.5 voxels), thresholded at p < 0.05, and clustered at ≥ 10 voxels.
Table 1.
Mean Percent Change for BOLD, MION, and CBF Responses in the Stimulated and Adjacent Unstimulated Areas
| Experiment | BOLD | MION | CBF | |||
|---|---|---|---|---|---|---|
| Positive BOLD Region (%) |
Negative BOLD Region (%) |
Positive BOLD Region (%) |
Negative BOLD Region (%) |
Positive BOLD Region (%) |
Negative BOLD Region (%) |
|
| M07.2O1 | 1.6 | −0.5 | −13.6 | −2.2 | — | — |
| B09.321 | 1.2 | −0.8 | −7.2 | −1.3 | 16.8a | −4.6a |
| I09.3a1 | 1.7 | −0.4 | −6.5 | −2.8 | 19.7a | −6.2a |
| B09.3B1 | 1.1 | −0.4 | −3.8 | −2.2 | 10.2a | −4.5a |
| L07.401 | 1.0 | −0.4 | −3.6 | −1.3 | — | — |
| B09.4g1 | 1.5 | −0.4 | −5.4 | −1.4 | 13.1a | −5.5a |
| M07.6C1 | 3.0 | −0.4 | −7.6 | −0.8 | 36.5 | −10.6 |
| B09.6U1 | 2.5 | −0.9 | — | — | 22.7 | — |
| N08.7f1 | 1.8 | −0.3 | — | — | 20.7 | −9.0 |
| G11.9A1 | 1.5 | −0.2 | — | — | 16.8 | −4.1 |
| H11.b41 | 2.2 | −0.3 | — | — | 15.4 | −4.4 |
| F11.bo1 | 1.0 | −0.3 | — | — | 14.7 | −5.6 |
| Mean | 1.7 | −0.4 | −6.8 | −1.7 | 21.1 | −6.7 |
| SD | 0.6 | 0.2 | 3.4 | 0.7 | 8.1 | 2.9 |
| B09.4g1 (12.6 ms) | −2.6 | −0.8 | ||||
| M07.6C1 (12.6 ms) | −5.8 | −1.2 | ||||
| Mean | −4.2 | −1.0 | ||||
Included in the table are the mean percent change upon visual stimulation (positive BOLD region) and the changes in the adjacent unstimulated region (negative BOLD region). Note that a decrease in MION signal corresponds to an increase in CBV. The ROIs extended over the entire gray matter thickness and were defined based on the BOLD responses. Dashes, data were not acquired in a given session. In two sessions, MION data were also acquired at a shorter TE (12.6 ms) to minimize the effect of a possible BOLD contamination.
For the CBF, we used a high-resolution FAIR with a TR of 4,500 ms, except for experiments marked with this footnote, which were based on the simultaneously acquired FAIR, BOLD, and VASO data and acquired at a TR of 3,000 ms. The mean CBF was calculated based on the FAIR data only.
Figure 2. Scatterplot of All Voxels that Were Significantly Activated in the BOLD and CBV Scans in Figure 1.
Voxels that showed positive BOLD responses (red), as well as voxels that exhibited negative BOLD activation (blue), showed a decrease in signal intensity after MION injection, indicating that CBV was increased in both stimulated and unstimulated regions, although CBV increases were larger in the stimulated areas.
Figure 3 shows the time courses of the BOLD and CBV signals in the stimulated and unstimulated regions in a representative animal. The time course of the regions with positive BOLD signals showed the typical hemodynamic response, including the poststimulus undershoot after cessation of the stimulus (Figure 3A). The dynamics of the negative BOLD response also showed its characteristic pattern, a more phasic response with a faster decay than the positive BOLD signal, as observed before (Shmuel et al., 2006). The CBV response in the stimulated region had slower dynamics, i.e., the decrease of the MION-based signal intensity reaches its minimum more slowly and returns to baseline more slowly (Figure 3B), in agreement with earlier work (Leite et al., 2002; Mandeville et al., 1999a, 1999b). The MION signal also lacked an overshoot after the stimulus was turned off. Thus, CBV responses reached their plateau more slowly and returned to baseline more slowly after stimulus cessation. In contrast to the BOLD signal, the CBV signal had similar dynamics in the stimulated and unstimulated regions.
Figure 3. Time Courses of BOLD- and MION-Based Functional Activation.
(A and B) The time course of the BOLD-based (A) and MION-based (B) activation in the stimulated region is shown in red, while the time course of the activation in the adjacent unstimulated region is shown in blue. The time course of the negative BOLD response displayed a more phasic pattern than the time course of the positive BOLD response. The CBV time courses had similar dynamics in the stimulated and unstimulated regions. The stimulation period is indicated by the gray background. Error bars, SEM over repetitions.
CBF in response to the center/ring stimuli was measured by arterial spin labeling (ASL) using single-shot flow-sensitive alternating inversion recovery (FAIR) (Kim, 1995) at an in-plane spatial resolution of 0.5 × 0.5 mm2 (inversion time [TI] 1,300 ms; repetition time [TR], 4,500 ms) and showed a similar pattern to the BOLD response (Figure 4) with an increase in CBF in regions that showed a positive BOLD response and a decrease in CBF in regions that showed a negative BOLD response. Figure S1, available online, shows the difference images for the CBF responses. The CBF decreases were also smaller than the CBF increases (Table 1). These responses were similar to the responses found in humans with this type of stimuli (Pasley et al., 2007; Shmuel et al., 2002).
Figure 4. Functional CBF Is Increased in the Stimulated Areas and Decreased in the Unstimulated Areas.
(A) The functional CBF in response to the center/ring stimulus shows a pattern similar that of the BOLD response (see Figure 1A) (p < 0.05, cluster size R 16 voxels).
(B) The functional CBF in response to a full-field rotating checkerboard. The negative CBF (A) is located more superficially than the positive CBF (B). Single-shot FAIR: spatial resolution, 0.5 × 0.5 × 3 mm3.
See Figure S1 for the difference image.
Table 1 shows the percent activation for the BOLD, CBV, and CBF signals. Functional changes were calculated in regions of interest (ROIs) corresponding to regions with positive and negative BOLD. The amplitudes of all functional signals (BOLD, CBV, and CBF) were larger in the regions with positive BOLD than in regions with negative BOLD. The MION-based CBV changes were more variable across monkeys and experiments than the BOLD signal changes due to differences in the MION-concentration in the blood, resulting from differences in weight of the animals and differences in MION clearance rates. In two animals, the CBV measurement was repeated at a shorter echo time to reduce a possible BOLD contribution to the CBV signal. This showed that, although reducing the echo time (TE) reduced the amplitude of the CBV changes, it did not alter the sign of the responses, and the CBV in stimulated and unstimulated regions was similarly affected (Table 1). Particularly in the areas displaying negative BOLD, a BOLD contribution is unlikely, because the amplitude of the negative BOLD signal (~0.5%) is below the detection threshold (~1%) of the MION scans (see Figure S2).
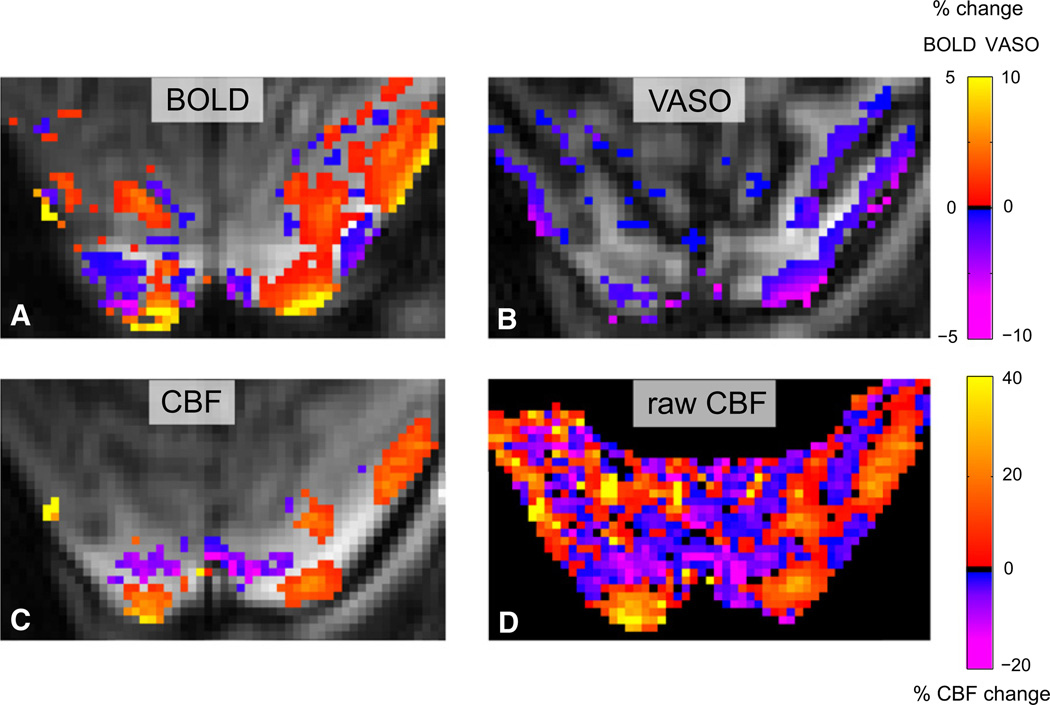
Sequential acquisition of BOLD, CBF, and CBV is unavoidable when iron-based contrast agents like MION are used. Furthermore, injection of hypertonic contrast agents can interfere with blood flow autoregulation (Grubb et al., 1974). Thus, to avoid potentially confounding effects of the MION injection and the sequential acquisition, we simultaneously acquired BOLD, CBF, and vascular space occupancy (VASO)-based CBV signals (Yang et al., 2004). The VASO signal is based on a selective nulling of the blood signal, and an increase in CBV results in a decrease of the image intensity (Lu et al., 2003). The maps showed a similar activation pattern for BOLD, CBV, and CBF compared to the separate acquisitions (Figure 5). Comparison of Figures 1 and 5 (same animal and session) also shows that the VASO- and MION-based functional CBV signals measure the same properties and shows that the results in Figure 1 are not due to an adverse effect of the MION injection.
Figure 5. Simultaneously Acquired BOLD, CBV, and CBF Signals Show the Same Behavior as Sequentially Acquired Data.
The negative BOLD signal is accompanied by a decrease in blood flow and an increase in blood volume.
BOLD response (A), VASO-based functional CBV (B), and functional CBF (C) (p < 0.05, cluster size ≥ 16 voxels). The CBF decrease in some of the unstimulated regions failed to reach significance because the negative CBF response was 5%–10%, which was smaller than the detection threshold of ~10% for this scan. The unthresholded CBF (D) shows the decreased CBF in the unstimulated regions. Spatial resolution, 1 × 0.67 × 3 mm3; TI, 925/1,300 ms; TR, 3,000 ms; TE, 8.4, 8.4, and 30.5 ms. The values of 8.4 ms refer to the echoes for CBV and CBF shown in (B), (C), and (D), and the value of 30.5 refers to the BOLD echo shown in (A).
Laminar Differences in BOLD, CBV, and CBF
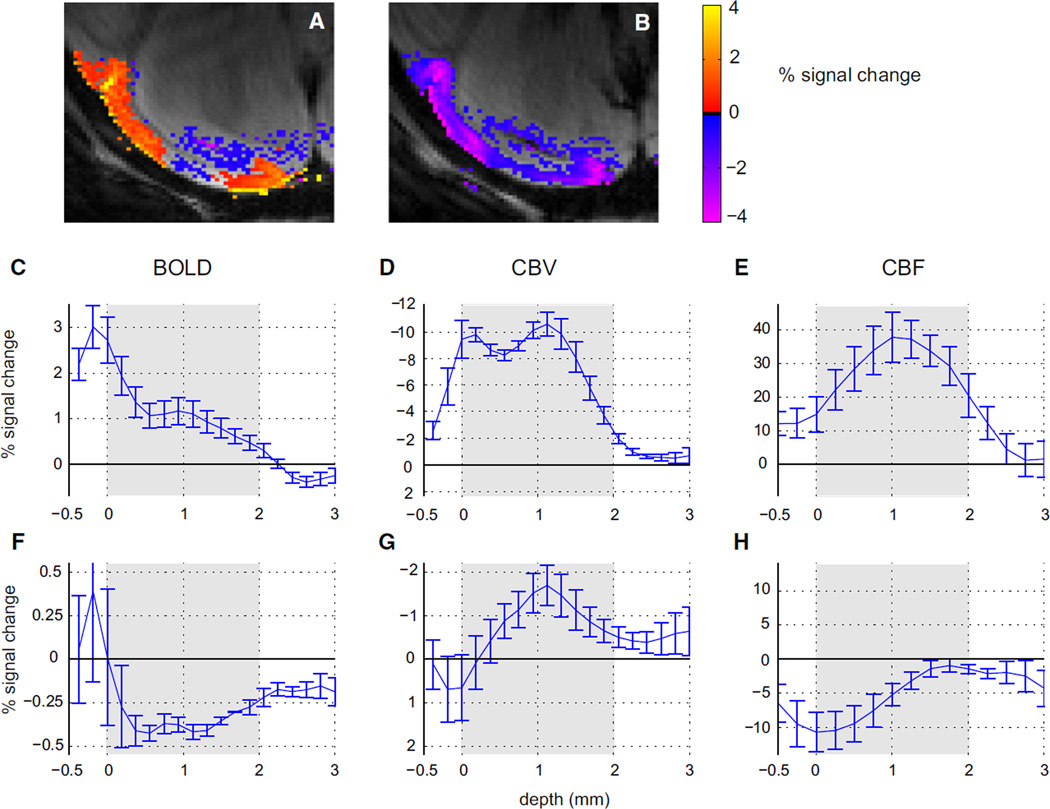
The opposite signs of the CBF and CBV suggest that the mechanism underlying the negative BOLD response is not merely the inverse of the positive BOLD response. Based on Figure 4, the negative functional CBF response seems to occur more superficially than the positive CBF response. Therefore, we used high-resolution fMRI to determine whether laminar differences in the BOLD, CBF, and CBV responses can account for our observations. Figure 6 shows the average laminar profiles calculated over the stimulated and unstimulated regions averaged over four to six experiments (see Goense and Logothetis, 2006; and Supplemental Experimental Procedures for methodological details). Figure S2 shows the profiles in a single animal. The profiles were calculated over a distance along the cortex of 6.8 ± 1.4 mm for BOLD and CBV scans and 8.3 ± 2.1 mm for CBF scans for each slice and hemisphere. The high-resolution activation maps for the BOLD- (Figure 6A) and MION-based functional CBV responses (Figure 6B) show that the positive BOLD response was maximal at the cortical surface (Figures 6A and 6C) in agreement with earlier results in monkeys, cats, and humans (Goense and Logothetis, 2006; Goense et al., 2007; Harel et al., 2006; Koopmans et al., 2011; Ress et al., 2007; Zhao et al., 2006), while the CBV response was roughly equal at the surface and in the middle layers of the cortex (Figure 6D). Note that the sign of the MION signal change was inverted in Figure 6D for easier comparison with the BOLD and CBF signals. The negative BOLD response was maximal in the center of the cortex (Figure 6A) (de Celis Alonso et al., 2008), while the negative BOLD signals at the surface sometimes failed to reach significance (Figures 6A and 6F). In the areas with negative BOLD, the functional CBV increase (i.e., MION signal decrease, the y axis is inverted again) occurred predominantly in layer IV (Figures 6B and 6G), while changes at the surface were typically not significant. The increased CBV in the regions with negative BOLD is thus due to small blood vessels or capillaries and not mediated by the large surface vessels. The peak activation for positive functional CBF occurred in layer IV (Figure 6E), similar to earlier data obtained in the macaque using continuous arterial spin labeling (CASL) (Zappe et al., 2008); the profile was similar when diffusion-weighting was added to suppress fast-flowing spins, indicating that flow in large surface vessels did not affect the CBF profiles. In contrast, the largest CBF changes in the regions displaying negative BOLD occurred at the cortical surface (Figure 6H; Figure S2).
Figure 6. Laminar Profiles of BOLD and Functional CBV and CBF Responses in Stimulated and Unstimulated Regions.
(A and B) High-resolution BOLD (A) and CBV (B) responses in a representative animal (p < 0.05, cluster size ≥ 16). In-plane resolution was 0.5 × 0.375 mm2. TE was 20 ms for the BOLD scan and 12.6 ms for the CBV scan. The maximum BOLD and CBV responses in the unstimulated region occurred in the middle of the cortex.
(C–H) Laminar profiles of the mean positive BOLD signal (C), n = 4; negative BOLD signal (F), n = 4; CBV in the stimulated region (D), TE 20 ms, n = 4; CBV in the unstimulated region (G), n = 4; CBF in the stimulated region (E), n = 6; and CBF in the unstimulated region (H), n = 5. Positive BOLD occurred mostly at the surface (C) while the functional CBV increase in excitatory regions occurred at the surface and in layer IV, shown in (D). BOLD and CBV signals in the unstimulated region, as shown in (F) and (G), are located in the middle of the cortex. CBF increases occurred in deeper layers (E), while CBF decreases occurred more superficially (H). Note that the y axis of the MION-based CBV signal has been inverted for easier comparison. Laminar profiles for BOLD and CBV were acquired at 0.5 × 0.375 mm2 resolution; CBF was acquired at 0.5 × 0.5 mm2 in-plane resolution. Error bars, SEM over experiments. The gray background in (C) through (H) indicates the approximate extent of the cortical gray matter.
See also Figures S2–S5.
The detection threshold of the acquisition is not homogeneous across the cortex and affects whether activation reaches the significance criterion (Goense et al., 2010). Due to the lower signal-to-noise ratio (SNR) at the cortical surface, detection thresholds were typically higher at the cortical surface than within gray matter (Figures S2I–S2K), and thus the same percentage change may not yield significant activation at the surface and in gray matter. Especially for the CBV measurement, detection thresholds were substantially higher in the superficial layers than in the deeper layers (Figure S2J). The high iron concentration in the large blood vessels at the surface decreases the signal intensity at the surface and thereby SNR. Thus, standard errors at the surface are typically higher, and small changes at the cortical surface may fail to reach significance.
In summary, while for stimuli that elicit positive BOLD responses, BOLD, CBV, and CBF all increased concurrently, stimuli that produce a negative BOLD response led to a decrease in CBF but an increase in CBV. These effects were layer dependent; i.e., while the decrease in CBF occurred superficially, the increase in CBV occurred in the center of the cortex. Thus, the negative BOLD response was not simply the inverse of the positive BOLD response and, most likely, produced by a different neurovascular coupling mechanism.
DISCUSSION
Using ring-shaped rotating checkerboard stimuli, we reliably evoked negative BOLD responses in V1, which were accompanied by decreases in CBF, as in humans (Pasley et al., 2007; Shmuel et al., 2002, 2006; Wade and Rowland, 2010). CBV however, was increased in the regions with negative BOLD. Using high-resolution fMRI, we found that the laminar profiles of the BOLD, CBF, and CBV responses in the unstimulated regions are not merely inverted versions of the responses in the stimulated regions but that their laminar distribution is markedly different. This indicates that neurovascular coupling differs in the stimulated and unstimulated regions and also that neurovascular coupling differs depending on cortical depth.
Hemodynamic Properties
The fMRI methods used in this work are sensitive to different aspects of the hemodynamic response, i.e., the BOLD signal originates from water protons in and near capillaries, venules, and veins, contrast-agent-based CBV signals reflect water in and near arteries, veins, and capillaries save for large vessels; and the ASL signal arises predominantly from water protons in arterioles and capillaries and their exchange with tissue water (He et al., 2012; Kennan et al., 1994; Weisskoff et al., 1994). It has been shown that hemodynamic regulation is heterogeneous and that functionally induced microvascular changes can occur at small spatial scales, i.e., at the level of columns and layers (Chaigneau et al., 2003; Erinjeri and Woolsey, 2002). Laminar differences in blood volume and flow have been observed in baseline conditions as well as after stimulation, showing that blood flow regulation differs between layers and between superficial vessels and parenchyma (Choi et al., 2010; Moskalenko et al., 1998; Zaharchuk et al., 1999). Baseline blood flow and vascularization are highest in the center of the cortex (Duvernoy et al., 1981; Gerrits et al., 2000; Moskalenko et al., 1998; Weber et al., 2008). Upon stimulation, blood flow increases throughout the cortex, with the highest CBF increases in the middle layers (Moskalenko et al., 1998; Norup Nielsen and Lauritzen, 2001; Takano et al., 2006). The BOLD, CBF, and CBV signals are a combination of the changes in the hemodynamic response and the signal characteristics of the fMRI methods: the BOLD signal is maximal at the cortical surface with a secondary peak in layer IV, reflecting the increased flow and oxygenation in the superficial veins and the middle layers; CBF and CBV peak in layer IV, reflecting the higher CBF and CBV in the center of the cortex and the sensitivity of these methods to microvessels, while the peak at the surface for CBV may reflect the increased CBV in superficial arteries and arterioles (Duong et al., 2000; Harel et al., 2006; Silva et al., 2000; Yu et al., 2012; Zappe et al., 2008; Zhao et al., 2006).
We found that the properties of the negative BOLD response are not the inverse of the positive BOLD signal. The decrease in CBF at the cortical surface and in the superficial layers and the increase in CBV in the middle of the cortex indicate that blood flow at the surface and in the upper layers is reduced while the middle layers are hyperemic. Negative BOLD signals arise because of an excess of deoxyhemoglobin (dHb), which occurs when the net inflow of fresh blood is insufficient relative to the O2 consumption. At the cortical surface, the negative BOLD signal seems to be driven by the decrease in CBF. The CBV at the surface also showed a small decrease, but this failed to reach significance because detection thresholds at the surface are elevated due to the high iron concentration in the large surface vessels (Figure S2). Similarly, the negative BOLD signal at the surface (~0.5%) did not reach significance in all animals (Figure S2). The behavior of the hemodynamic response at the cortical surface agrees well with results obtained by optical imaging, which showed arteriolar vasoconstriction and decreased CBV, CBF, and oxygenation in the inhibitory surrounding areas in rat somatosensory cortex (Boorman et al., 2010; Devor et al., 2007). Given that the responses measured with optical imaging arise mostly from the upper cortical layers, the negative BOLD responses and decreased CBF observed in the upper layers correspond well with optical imaging data.
In the deeper layers, there was a lack of inflow of fresh blood and an unexpected increase in CBV. Because the ASL signal reflects the inflow of fresh blood and is primarily sensitive to arterioles and capillaries and exchanging water, the CBV increase might occur due to dilation of capillaries or small vessels in the middle of the cortex. This could be an autoregulatory or redistribution phenomenon. If autoregulation occurs at the microscopic level as well as at the global level, the decrease in perfusion pressure in the center of the cortex due to the reduction of flow in the superficial vessels and upper layers might lead to an increase in CBV in the center. A capillary dilation with arterial constriction would require backpressure from the venous side to lead to a larger CBV. Given the relative lack of a CBF response in the deeper layers, the negative BOLD signal in the deeper layers may arise as a result of local vasodilation and increase in dHb due to an insufficient inflow of fresh blood. However, the CBV increase in the middle layers could also be mediated by local dilation of capillaries induced by inhibitory neural activity (Cauli et al., 2004; Fergus and Lee, 1997; Fernández-Klett et al., 2010). Hyperemia by increasing the capillary volume might increase the availability of oxygen by increasing the overall blood volume. The signals we observed in the deeper layers are not expected to have a direct counterpart in optical imaging, because of the limited depth resolution of optical imaging. High-resolution fMRI can thus provide a unique window into such responses.
Hyperemia of the brain parenchyma with arterial constriction has been observed with optical imaging during the poststimulus undershoot (Chen et al., 2011), and such a mechanism could potentially also lead to the hyperemia in the deeper layers observed during the negative BOLD response. Chen et al. (2011) hypothesized that there may be a dilation of capillaries mediated by pericytes. The laminar pattern of the negative BOLD response we observed shows similarities with the poststimulus undershoot measured with fMRI. The BOLD undershoot in cat visual cortex occurred in both tissue and surface vessels. The CBV in gray matter, however, remained elevated after stimulus cessation, while CBV at the surface decayed rapidly to baseline (Yacoub et al., 2006; Zhao et al., 2007); this was also observed in the macaque (data not shown). The above observations suggest the possibility that the poststimulus undershoot and the negative BOLD response may share a similar mechanism, resulting in a decrease of blood flow in the large vessels at the surface, while the parenchyma (the deeper layers) stays hyperemic. However, the temporal overlap makes the individual contributions to the poststimulus undershoot difficult to disentangle, and vascular compliance effects can also explain the time course of the CBV (Buxton et al., 1998, 2004; Leite et al., 2002; Mandeville et al., 1999a, 1999b). Acquiring the time course of the CBF at laminar resolution could resolve the potential similarities between the negative BOLD and the poststimulus undershoot.
Another stimulus paradigm that reliably yields negative BOLD responses is ipsilateral inhibition, and it is likely that this paradigm would result in similar laminar profiles to the ones found here. Although negative BOLD responses have also been shown in cases of physiological challenge, like seizures or low blood pressure (Nagaoka et al., 2006; Schridde et al., 2008), its mechanism and laminar profiles might very well differ from the stimulus-driven negative BOLD response. However, this requires further study.
Energy Use and Neural Mechanism
Decreases in the cerebral metabolic rate of oxygen consumption (CMRO2) were seen in areas with negative BOLD using MRI-based methods (Shmuel et al., 2002; Stefanovic et al., 2004). Although it is likely that the reduced neural activity (Shmuel et al., 2006) leads to a reduced energy use, this cannot automatically be inferred, and CMRO2 changes could also be layer dependent. Layer-dependent CMRO2 is suggested by observations that glucose and O2 consumption are highest in layer IV (Carroll and Wong-Riley, 1984; Li and Freeman, 2011, 2012; Tootell et al., 1988b), while 2-deoxyglucose autoradiography in V1 showed that for areas adjacent to stimulated areas, glucose use depended on stimulus properties and retinotopic location (Tootell et al., 1988b).
The increase in CBV in the deeper layers might be driven by a cortical-layer-dependent increase in energy use, which could be the result of layer-dependent or neuron-type-dependent increases in neural activity. A possible driver of the microvascular dilation is an increase in the activity of inhibitory interneurons; these are often missed with standard microelectrodes, but they can have high firing rates. Inhibitory activity has been shown to cost energy (McCasland and Hibbard, 1997; Nudo and Masterton, 1986) and might lead to a vascular response also. Thus, although it is likely that energy use is reduced in the case of negative BOLD, its laminar profile and the exact driving mechanism of the vascular response still need to be determined.
The neural mechanism for the negative BOLD response is also still unknown (Pasley et al., 2007; Shmuel et al., 2002, 2006; Wade and Rowland, 2010). Inhibition via horizontal connections may play a role, although the spatial extent of the negative BOLD response suggests longer range interactions. Other possible neural mechanisms that could account for the decreases in the fMRI responses are a reduced input from the lateral geniculate nucleus, reduced or inhibitory feedback from higher cortical areas like V2 or MT (Angelucci and Bressloff, 2006; Angelucci and Bullier, 2003), or a reduction in inhibitory as well as excitatory activity, which can be explained by an inhibition-stabilized network (Ozeki et al., 2009; Tsodyks et al., 1997). Further study is needed to resolve the neural mechanism, for instance, by using different stimuli, elimination of feedback by pharmacological inactivation, or by combining high-resolution fMRI with multi-site electrophysiological recording.
Measurement of Functional CBV
Our results indicate that CBV-based fMRI measures different properties than BOLD-based fMRI (Smirnakis et al., 2007). This indicates that CBV-based fMRI signals cannot be assumed to always reflect the same underlying processes as BOLD-based fMRI. On one hand, this can complicate the interpretation of comparative fMRI studies or of VASO- and BOLD-based responses. On the other hand, these differences can potentially be exploited to better understand fMRI signals or to disentangle different neural processes.
The results presented here have implications for comparative fMRI studies between macaques and humans. In the majority of the macaque fMRI studies, iron-based contrast agents are used to boost the contrast-to-noise ratio (CNR) of the functional signal (Vanduffel et al., 2001). Although comparative studies allow direct comparison between monkeys and humans under the same stimulus or task (Nasr et al., 2011; Tsao et al., 2003, 2008; Vanduffel et al., 2002), when iron-based contrast agents are used, the results may not always be exactly comparable. Our results indicate that BOLD and functional CBV responses are not fully equivalent, and CBV-based methods may be unable to unambiguously discriminate between processes that result in positive and negative BOLD signals, for instance, excitation versus inhibition.
The similarity of the results obtained with VASO- and MION-based CBV suggests that the VASO- and MION-based CBV methods measure similar properties (Jin and Kim, 2008). The VASO- and MION-based CBV signals both suffer from the drawback that they cannot unambiguously distinguish processes leading to positive and negative BOLD responses. However, this may also be advantageous, because if the VASO and BOLD responses always reflect the same processes, VASO would just be a low-SNR version of BOLD. The different behavior of the VASO signal provides the option to study CBV responses in humans to better understand the fMRI mechanism and improve comparisons between human and animal CBV studies.
The CBV measurement can potentially be improved by using spin-echo (SE)-based methods, which are less susceptible to signal dropout near large vessels and can improve the detectability of CBV changes at the cortical surface (Zhao et al., 2006). However, SNR and CNR for SE-CBV methods are lower, which negated the aforementioned advantage in our setup. Further improvement of SNR by improving radio frequency technology may allow applicability of SE-EPI as well as increasing spatial resolution.
Summary
While excitatory activity increases CBF and CBV in superficial as well as in deeper layers, we found that stimuli that cause negative BOLD responses reduced the blood flow at the cortical surface but increased the blood volume in the deeper layers. Our results imply that the mechanism of neurovascular coupling differs for the two types of stimuli. Furthermore, the different behaviors seen in both deep and superficial layers for these stimuli suggest that neurovascular coupling mechanisms are layer dependent. The laminar differences in neural responses; energy consumption; and the segregation of input, output, and feedback connections in V1 have all been well characterized (Felleman and Van Essen, 1991; Johnson et al., 2001; Ringach et al., 2002; Tootell et al., 1988a). Our results suggest such processes might be reflected as laminar differences in the fMRI responses. Other processes, like perception or attention, may also leave a laminar signature in the fMRI profiles, as they do in the neural signals (Mehta et al., 2000). This could potentially allow the use of high-resolution fMRI to study cortical processing at the level of the microcircuits as well as allow us to separate the individual contributions of feedforward, feedback, excitatory, or inhibitory processes to fMRI signals.
EXPERIMENTAL PROCEDURES
Subjects and Anesthesia Protocol
Experiments (n = 12) were performed on eight healthy monkeys (Macaca mulatta; six females, two males; 4–9 kg, 3–12 years). All experiments were approved by the local authorities (Regierungspräsidium Baden-Württemberg, Tübingen, Germany) and were in full compliance with the guidelines of the European Community (EUVD 86/609/EEC) for the care and use of laboratory animals. Experiments were performed on a Bruker Avance-II 4.7T vertical scanner running ParaVision 5.0/5.1 (Bruker Biospec 47/40v, Bruker Biospin GmbH, Ettlingen, Germany). A custom-built four-channel receive array was used in combination with a detunable partial volume transmit coil (Goense et al., 2010).
The experimental setup and procedures were described in detail in Logothetis et al. (1999). Monkeys were scanned in an upright position in a specially designed primate chair. Experiments were performed under general anesthesia; after preanesthesia with glycopyrrolate (0.01 mg · kg−1) and ketamine (15 mg · kg−1), and induction with fentanyl (3 µg · kg−1), thiopental (5 mg · kg−1) and succinylcholine chloride (3 mg · kg−1), animals were intubated and ventilated using a Servo Ventilator 900C (Siemens, Germany) maintaining an end-tidal CO2 of 33–35 mm Hg and oxygen saturation above 95%. Anesthesia was maintained with remifentanil (0.5–1.5 µg · kg−1 min) and mivacurium chloride (4–6 mg · kg−1 · h−1) to ensure paralysis of the eye muscles. Body temperature was maintained between 38.5–39.5°C. Jonosteril (Fresenius Kabi, Germany) with 2.5% glucose was infused at a rate of 10 ml · kg−1 · hr−1. Intravascular volume and blood pressure were maintained by administering hydroxyethyl starch as needed (Volulyte, Fresenius Kabi, Germany). One to two drops of 1% cyclopentolate hydrochloride were administered to each eye to achieve mydriasis, and the animal was fitted with hard contact lenses to bring the eyes to focus on the stimulus plane (hard PMMA lenses, Wöhlk, Kiel, Germany). Visual stimuli were presented using a custom-built projector and SVGA fiber-optic system with a resolution of 640 × 480 pixels. We used full-field black-and-white rotating checkerboards and rotating center/ring stimuli, analogous to the stimuli used by Shmuel et al. (Shmuel et al., 2002, 2006). The stimuli (Figure 1A) consisted of a foveal stimulus of 1°–3° in the center and a ring which typically extended from 8°–12°. The sizes of the center and ring were adjusted up to 2° such that the region showing a negative BOLD was located in the middle of the operculum; this ensured that the negative BOLD response occurred in the region of the cortex where the SNR was highest and where the cortical thickness was most uniform, to allow for the most accurate determination of laminar profiles. Full-field rotating checkerboard stimuli were used as control. A block design was used in which 64 images were acquired in four blocks of eight images each on and off for BOLD and CBV experiments, and 128 images in four blocks of 16 images each on and off for CBF experiments.
Data Acquisition
For BOLD and CBV scans, the same eight-segment GE-EPI sequence was used. Thirteen to fifteen slices were acquired oriented perpendicular to the cortical surface of V1. The matrix was 128 × 128; field of view (FOV), 64 × 48mm2 (in-plane resolution, 0.5 × 0.375 mm2); slice thickness, 2 mm; receiver bandwidth (BW), 100 kHz; EPI acquisition window, 20.5 ms; flip angle, 40°; TE, 20 ms; and TR, 750 ms. For functional CBV experiments (n = 7), MION, obtained from the Center for Molecular Imaging Research, Massachusetts General Hospital (Boston, MA, USA), was injected. Citrate-buffered MION (7–8 mg/kg [iron]) was injected intravenously, which decreased the image intensity to about half the preinjection level. BOLD scans were acquired right before the MION injection, and all CBV data were acquired within 1½ hr after MION injection to avoid washout effects. In two experiments (in two animals) CBV data were also acquired at a TE of 12.6 ms to reduce any possible BOLD contamination and to reduce signal dropout at the cortical surface.
Functional CBF (n = 10) was measured using FAIR (Kim, 1995) and/or with the sequence by Yang et al. (2004) that simultaneously acquires FAIR, BOLD, and VASO signals. The EPI module was the same for the FAIR and simultaneous BOLD/CBF/VASO sequence and was a single-shot EPI with a BW of 132 kHz and a slice thickness of 3 mm. For the simultaneous BOLD/CBF/VASO sequence, data were typically acquired at a resolution of 0.75 × 0.75 mm2, with a FOV of 64 × 32 mm2 and a matrix size of 86 × 43. The TI was 925 ms for the VASO-echo and 1,300 ms for the FAIR-echo at a TR of 3,000 ms. The TI for the VASO-echo was determined based on the inversion of blood in veins in the calcarine sulcus. The echo times were 8.4, 8.4, and 30.5 ms for the VASO-echo, FAIR-echo, and BOLD-echo, respectively. Hyperbolic secant pulses were used for inversion. For high-resolution FAIR (n = 6), a single slice was acquired oriented perpendicular to the cortical surface with a FOV of 64 × 24 mm2 or 64 × 16 mm2, a matrix of 128 × 48 or 128 × 32 (resolution, 0.5 × 0.5 × 3 mm3 ), a TI of 1,400 ms, and a TR of 4,500 ms. The shortest possible TE was used ranging from 8.6 to 11.6 ms depending on the matrix and FOV. To determine whether flow in large vessels affects the CBF profiles, a diffusion-weighted SE FAIR-EPI was used as a control. Its sequence parameters were the same as for the GE-based high-resolution FAIR, except that the TE was 26.4 ms and the b factor was 20.
Data Analysis
Data were analyzed using custom-written routines in MatLab (The Math-Works). Activation maps were generated using t tests. No smoothing was applied in the analyses (the exception is Figure 1, where data were smoothed for display purposes). For measuring the VASO-CBV signal, only the nonselective inversion was used, reducing the number of images per scan to 64. To determine the mean percent functional signal change in the regions with positive and negative BOLD, ROIs for the positive and negative BOLD were drawn in the operculum of V1, based on the high-resolution raw (i.e., not thresholded for significant activation) BOLD percentage change activation maps. The same ROIs were used to calculate functional CBV changes. For calculation of functional CBF, ROIs were drawn based on the unthresholded CBF percentage change maps after verifying the locations of the ROIs in the BOLD scans.
Functional activation as a function of cortical depth was analyzed by calculating the profiles perpendicular to the cortex (see the Supplemental Experimental Procedures for a detailed description of the analysis procedures and the factors affecting the laminar resolution). The areas over which the profiles were calculated were defined based on the extent of the negative BOLD activation, which amounted to a distance of 7–8 mm along the cortex for each slice and hemisphere. Two to three slices were used for BOLD and CBV profiles. The same coordinates were used to calculate BOLD and functional CBV profiles. For the laminar profiles for the positive BOLD responses, profiles were calculated from activation maps in response to a full-field checkerboard, allowing the use of the same coordinates (i.e., using the same stretch of cortex) for the positive and negative BOLD responses. We verified that using activation maps derived from a full-field checkerboard yielded the same laminar profiles and percent signal changes as using the regions with positive BOLD from the ring stimuli. To allow for the most accurate calculation of signal changes at the cortical surface, only experiments for which there was no scanner drift within and between scans were included in the laminar analysis. Scanner drift can potentially lead to a misalignment of up to one voxel in the phase-encoding direction (anterior-posterior). Due to the sensitivity profile of the receive array and the strong activation at the cortical surface in GE-BOLD and GE-CBV scans, image registration software is often not able to accurately realign such data; hence, any data with scanner drift were excluded from the analysis.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Dr. Daniel Gembris and Dr. Franek Hennel from Bruker BioSpin GmbH for the sequence to simultaneously measure the BOLD, CBF, and VASO signals and Dr. Mark Augath for technical support. Dr. Kevin Whittingstall provided comments on earlier versions of the manuscript. We also thank the reviewers for their suggestions. The research was supported by the Max-Planck Society and in part by the Intramural Program of the National Institutes of Health, National Institute of Neurological Disorders and Stroke, Bethesda, MD, USA (to H.M.).
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information includes five figures and Supplemental Experimental Procedures and can be found with this article online at http://dx.doi.org/10.1016/j.neuron.2012.09.019.
REFERENCES
- Allison JD, Meador KJ, Loring DW, Figueroa RE, Wright JC. Functional MRI cerebral activation and deactivation during finger movement. Neurology. 2000;54:135–142. doi: 10.1212/wnl.54.1.135. [DOI] [PubMed] [Google Scholar]
- Angelucci A, Bullier J. Reaching beyond the classical receptive field of V1 neurons: horizontal or feedback axons? J Physiol Paris. 2003;97:141–154. doi: 10.1016/j.jphysparis.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Angelucci A, Bressloff PC. Contribution of feedforward, lateral and feedback connections to the classical receptive field center and extraclassical receptive field surround of primate V1 neurons. Prog Brain Res. 2006;154:93–120. doi: 10.1016/S0079-6123(06)54005-1. [DOI] [PubMed] [Google Scholar]
- Bianciardi M, Fukunaga M, van Gelderen P, de Zwart JA, Duyn JH. Negative BOLD-fMRI signals in large cerebral veins. J Cereb Blood Flow Metab. 2011;31:401–412. doi: 10.1038/jcbfm.2010.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boorman L, Kennerley AJ, Johnston D, Jones M, Zheng Y, Redgrave P, Berwick J. Negative blood oxygen level dependence in the rat: a model for investigating the role of suppression in neurovascular coupling. J Neurosci. 2010;30:4285–4294. doi: 10.1523/JNEUROSCI.6063-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressler D, Spotswood N, Whitney D. Negative BOLD fMRI response in the visual cortex carries precise stimulus-specific information. PLoS ONE. 2007;2:e410. doi: 10.1371/journal.pone.0000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton RB, Wong EC, Frank LR. Dynamics of blood flow and oxygenation changes during brain activation: the balloon model. Magn Reson Med. 1998;39:855–864. doi: 10.1002/mrm.1910390602. [DOI] [PubMed] [Google Scholar]
- Buxton RB, Uludağ K, Dubowitz DJ, Liu TT. Modeling the hemodynamic response to brain activation. Neuroimage. 2004;23(Suppl 1):S220–S233. doi: 10.1016/j.neuroimage.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Callaway EM. Local circuits in primary visual cortex of the macaque monkey. Annu. Rev. Neurosci. 1998;21:47–74. doi: 10.1146/annurev.neuro.21.1.47. [DOI] [PubMed] [Google Scholar]
- Carroll EW, Wong-Riley MT. Quantitative light and electron microscopic analysis of cytochrome oxidase-rich zones in the striate cortex of the squirrel monkey. J. Comp, Neurol. 1984;222:1–17. doi: 10.1002/cne.902220102. [DOI] [PubMed] [Google Scholar]
- Cauli B, Tong XK, Rancillac A, Serluca N, Lambolez B, Rossier J, Hamel E. Cortical GABA interneurons in neurovascular coupling: relays for subcortical vasoactive pathways. J Neurosci. 2004;24:8940–8949. doi: 10.1523/JNEUROSCI.3065-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaigneau E, Oheim M, Audinat E, Charpak S. Two-photon imaging of capillary blood flow in olfactory bulb glomeruli. Proc. Natl. Acad.Sci. USA. 2003;100:13081–13086. doi: 10.1073/pnas.2133652100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BR, Bouchard MB, McCaslin AF, Burgess SA, Hillman EM. High-speed vascular dynamics of the hemodynamic response. Neuroimage. 2011;54:1021–1030. doi: 10.1016/j.neuroimage.2010.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JK, Mandeville JB, Chen YI, Grundt P, Sarkar SK, Newman AH, Jenkins BG. Imaging brain regional and cortical laminar effects of selective D3 agonists and antagonists. Psychopharmacology (Berl.) 2010;212:59–72. doi: 10.1007/s00213-010-1924-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Celis Alonso BC, Lowe AS, Dear JP, Lee KC, Williams SC, Finnerty GT. Sensory inputs from whisking movements modify cortical whisker maps visualized with functional magnetic resonance imaging. Cereb. Cortex. 2008;18:1314–1325. doi: 10.1093/cercor/bhm163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devor A, Tian P, Nishimura N, Teng IC, Hillman EM, Narayanan SN, Ulbert I, Boas DA, Kleinfeld D, Dale AM. Suppressed neuronal activity and concurrent arteriolar vasoconstriction may explain negative blood oxygenation level-dependent signal. J Neurosci. 2007;27:4452–4459. doi: 10.1523/JNEUROSCI.0134-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duong TQ, Silva AC, Lee SP, Kim SG. Functional MRI of calcium-dependent synaptic activity: cross correlation with CBF and BOLD measurements. Magn. Reson. Med. 2000;43:383–392. doi: 10.1002/(sici)1522-2594(200003)43:3<383::aid-mrm10>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Duvernoy HM, Delon S, Vannson JL. Cortical blood vessels of the human brain. Brain Res Bull. 1981;7:519–579. doi: 10.1016/0361-9230(81)90007-1. [DOI] [PubMed] [Google Scholar]
- Erinjeri JP, Woolsey TA. Spatial integration of vascular changes with neural activity in mouse cortexJCereb. Blood Flow Metab. 2002;22:353–360. doi: 10.1097/00004647-200203000-00013. [DOI] [PubMed] [Google Scholar]
- Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cereb. Cortex. 1991;1:1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- Fergus A, Lee KS. GABAergic regulation of cerebral microvascular tone in the rat. J. Cereb. Blood Flow Metab. 1997;17:992–1003. doi: 10.1097/00004647-199709000-00009. [DOI] [PubMed] [Google Scholar]
- Fernández-Klett F, Offenhauser N, Dirnagl U, Priller J, Lindauer U. Pericytes in capillaries are contractile in vivo, but arterioles mediate functional hyperemia in the mouse brain. Proc. Natl. Acad. Sci. USA. 2010;107:22290–22295. doi: 10.1073/pnas.1011321108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrits RJ, Raczynski C, Greene AS, Stein EA. Regional cerebral blood flow responses to variable frequency whisker stimulation: an autoradiographic analysis. Brain Res. 2000;864:205–212. doi: 10.1016/s0006-8993(00)02142-9. [DOI] [PubMed] [Google Scholar]
- Goense J, Logothetis NK, Merkle H. Flexible, phase-matched, linear receive arrays for high-field MRI in monkeys. Magn. Reson. Imaging. 2010;28:1183–1191. doi: 10.1016/j.mri.2010.03.026. [DOI] [PubMed] [Google Scholar]
- Goense JBM, Logothetis NK. Laminar specificity in monkey V1 using high-resolution SE-fMRI. Magn. Reson. Imaging. 2006;24:381–392. doi: 10.1016/j.mri.2005.12.032. [DOI] [PubMed] [Google Scholar]
- Goense JBM, Zappe AC, Logothetis NK. High-resolution fMRI of macaque V1. Magn. Reson. Imaging. 2007;25:740–747. doi: 10.1016/j.mri.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Grubb RL, Jr, Hernandez-Perez MJ, Raichle ME, Phelps ME. The effects of iodinated contrast agents on autoregulation of cerebral blood flow. Stroke. 1974;5:155–160. doi: 10.1161/01.str.5.2.155. [DOI] [PubMed] [Google Scholar]
- Harel N, Lee SP, Nagaoka T, Kim DS, Kim SG. Origin of negative blood oxygenation level-dependent fMRI signals. J. Cereb. Blood Flow Metab. 2002;22:908–917. doi: 10.1097/00004647-200208000-00002. [DOI] [PubMed] [Google Scholar]
- Harel N, Lin J, Moeller S, Ugurbil K, Yacoub E. Combined imaging-histological study of cortical laminar specificity of fMRI signals. Neuroimage. 2006;29:879–887. doi: 10.1016/j.neuroimage.2005.08.016. [DOI] [PubMed] [Google Scholar]
- He X, Raichle ME, Yablonskiy DA. Transmembrane dynamics of water exchange in human brain. Magn. Reson. Med. 2012;67:562–571. doi: 10.1002/mrm.23019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hlushchuk Y, Hari R. Transient suppression of ipsilateral primary somatosensory cortex during tactile finger stimulation. J Neurosci. 2006;26:5819–5824. doi: 10.1523/JNEUROSCI.5536-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Plyka I, Li H, Eisenstein EM, Volkow ND, Springer CS., Jr Magnetic resonance imaging (MRI) detection of the murine brain response to light: temporal differentiation and negative functional MRI changes. Proc. Natl. Acad. Sci. USA. 1996;93:6037–6042. doi: 10.1073/pnas.93.12.6037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin T, Kim SG. Improved cortical-layer specificity of vascular space occupancy fMRI with slab inversion relative to spin-echo BOLD at 9.4 T. Neuroimage. 2008;40:59–67. doi: 10.1016/j.neuroimage.2007.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EN, Hawken MJ, Shapley R. The spatial transformation of color in the primary visual cortex of the macaque monkey. Nat. Neurosci. 2001;4:409–416. doi: 10.1038/86061. [DOI] [PubMed] [Google Scholar]
- Kennan RP, Zhong J, Gore JC. Intravascular susceptibility contrast mechanisms in tissues. Magn. Reson. Med. 1994;31:9–21. doi: 10.1002/mrm.1910310103. [DOI] [PubMed] [Google Scholar]
- Kim SG. Quantification of relative cerebral blood flow change by flow-sensitive alternating inversion recovery (FAIR) technique: application to functional mapping. Magn. Reson. Med. 1995;34:293–301. doi: 10.1002/mrm.1910340303. [DOI] [PubMed] [Google Scholar]
- Koopmans PJ, Barth M, Orzada S, Norris DG. Multi-echo fMRI of the cortical laminae in humans at 7 T. Neuroimage. 2011;56:1276–1285. doi: 10.1016/j.neuroimage.2011.02.042. [DOI] [PubMed] [Google Scholar]
- Leite FP, Tsao D, Vanduffel W, Fize D, Sasaki Y, Wald LL, Dale AM, Kwong KK, Orban GA, Rosen BR, et al. Repeated fMRI using iron oxide contrast agent in awake, behaving macaques at 3 Tesla. Neuroimage. 2002;16:283–294. doi: 10.1006/nimg.2002.1110. [DOI] [PubMed] [Google Scholar]
- Li B, Freeman RD. Neurometabolic coupling differs for suppression within and beyond the classical receptive field in visual cortex. J. Physiol. 2011;589:3175–3190. doi: 10.1113/jphysiol.2011.205039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Freeman RD. Spatial summation of neurometabolic coupling in the central visual pathway. Neuroscience. 2012;213:112–121. doi: 10.1016/j.neuroscience.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK, Guggenberger H, Peled S, Pauls J. Functional imaging of the monkey brain. Nat. Neurosci. 1999;2:555–562. doi: 10.1038/9210. [DOI] [PubMed] [Google Scholar]
- Lu H, Golay X, Pekar JJ, Van Zijl PC. Functional magnetic resonance imaging based on changes in vascular space occupancy. Magn. Reson. Med. 2003;50:263–274. doi: 10.1002/mrm.10519. [DOI] [PubMed] [Google Scholar]
- Mandeville JB, Marota JJ, Ayata C, Moskowitz MA, Weisskoff RM, Rosen BR. MRI measurement of the temporal evolution of relative CMRO2 during rat forepaw stimulation. Magn. Reson. Med. 1999a;42:944–951. doi: 10.1002/(sici)1522-2594(199911)42:5<944::aid-mrm15>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Mandeville JB, Marota JJ, Ayata C, Zaharchuk G, Moskowitz MA, Rosen BR, Weisskoff RM. Evidence of a cerebrovascular postarteriole windkessel with delayed compliance. J. Cereb. Blood Flow Metab. 1999b;19:679–689. doi: 10.1097/00004647-199906000-00012. [DOI] [PubMed] [Google Scholar]
- McCasland JS, Hibbard LS. GABAergic neurons in barrel cortex show strong, whisker-dependent metabolic activation during normal behavior. J Neurosci. 1997;17:5509–5527. doi: 10.1523/JNEUROSCI.17-14-05509.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta AD, Ulbert I, Schroeder CE. Intermodal selective attention in monkeys II: physiological mechanisms of modulation. Cereb. Cortex. 2000;10:359–370. doi: 10.1093/cercor/10.4.359. [DOI] [PubMed] [Google Scholar]
- Moskalenko YE, Woolsey TA, Rovainen C, Weinstein GB, Liu D, Semernya VN, Mitrofanov VF. Blood flow dynamics in different layers of the somatosensory region of the cerebral cortex on the rat during mechanical stimulation of the vibrissae. Neurosci. Behav. Physiol. 1998;28:459–467. doi: 10.1007/BF02464807. [DOI] [PubMed] [Google Scholar]
- Nagaoka T, Zhao F, Wang P, Harel N, Kennan RP, Ogawa S, Kim SG. Increases in oxygen consumption without cerebral blood volume change during visual stimulation under hypotension condition. J. Cereb. Blood Flow Metab. 2006;26:1043–1051. doi: 10.1038/sj.jcbfm.9600251. [DOI] [PubMed] [Google Scholar]
- Nasr S, Liu N, Devaney KJ, Yue X, Rajimehr R, Ungerleider LG, Tootell RB. Scene-selective cortical regions in human and nonhuman primates. J Neurosci. 2011;31:13771–13785. doi: 10.1523/JNEUROSCI.2792-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norup Nielsen A, Lauritzen M. Coupling and uncoupling of activity-dependent increases of neuronal activity and blood flow in rat somatosensory cortex. J. Physiol. 2001;533:773–785. doi: 10.1111/j.1469-7793.2001.00773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudo RJ, Masterton RB. Stimulation-induced [14C]2-deoxyglucose labeling of synaptic activity in the central auditory system. J. Comp .Neurol. 1986;245:553–565. doi: 10.1002/cne.902450410. [DOI] [PubMed] [Google Scholar]
- Ozeki H, Finn IM, Schaffer ES, Miller KD, Ferster D. Inhibitory stabilization of the cortical network underlies visual surround suppression. Neuron. 2009;62:578–592. doi: 10.1016/j.neuron.2009.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasley BN, Inglis BA, Freeman RD. Analysis of oxygen metabolism implies a neural origin for the negative BOLD response in human visual cortex. Neuroimage. 2007;36:269–276. doi: 10.1016/j.neuroimage.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ress D, Glover GH, Liu J, Wandell B. Laminar profiles of functional activity in the human brain. Neuroimage. 2007;34:74–84. doi: 10.1016/j.neuroimage.2006.08.020. [DOI] [PubMed] [Google Scholar]
- Ringach DL, Shapley RM, Hawken MJ. Orientation selectivity in macaque V1: diversity and laminar dependence. J Neurosci. 2002;22:5639–5651. doi: 10.1523/JNEUROSCI.22-13-05639.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer K, Blankenburg F, Kupers R, Grüner JM, Law I, Lauritzen M, Larsson HB. Negative BOLD signal changes in ipsilateral primary somatosensory cortex are associated with perfusion decreases and behavioral evidence for functional inhibition. Neuroimage. 2012;59:3119–3127. doi: 10.1016/j.neuroimage.2011.11.085. [DOI] [PubMed] [Google Scholar]
- Schridde U, Khubchandani M, Motelow JE, Sanganahalli BG, Hyder F, Blumenfeld H. Negative BOLD with large increases in neuronal activity. Cereb. Cortex. 2008;18:1814–1827. doi: 10.1093/cercor/bhm208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmuel A, Yacoub E, Pfeuffer J, Van de Moortele PF, Adriany G, Hu XP, Ugurbil K. Sustained negative BOLD, blood flow and oxygen consumption response and its coupling to the positive response in the human brain. Neuron. 2002;36:1195–1210. doi: 10.1016/s0896-6273(02)01061-9. [DOI] [PubMed] [Google Scholar]
- Shmuel A, Augath M, Oeltermann A, Logothetis NK. Negative functional MRI response correlates with decreases in neuronal activity in monkey visual area V1. Nat. Neurosci. 2006;9:569–577. doi: 10.1038/nn1675. [DOI] [PubMed] [Google Scholar]
- Silva AC, Lee SP, Iadecola C, Kim SG. Early temporal characteristics of cerebral blood flow and deoxyhemoglobin changes during somatosensory stimulation. J. Cereb. Blood Flow Metab. 2000;20:201–206. doi: 10.1097/00004647-200001000-00025. [DOI] [PubMed] [Google Scholar]
- Smirnakis SM, Schmid MC, Weber B, Tolias AS, Augath M, Logothetis NK. Spatial specificity of BOLD versus cerebral blood volume fMRI for mapping cortical organization. J. Cereb. Blood Flow Metab. 2007;27:1248–1261. doi: 10.1038/sj.jcbfm.9600434. [DOI] [PubMed] [Google Scholar]
- Smith AT, Williams AL, Singh KD. Negative BOLD in the visual cortex: evidence against blood stealing. Hum. Brain. Mapp. 2004;21:213–220. doi: 10.1002/hbm.20017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanovic B, Warnking JM, Pike GB. Hemodynamic and metabolic responses to neuronal inhibition. Neuroimage. 2004;22:771–778. doi: 10.1016/j.neuroimage.2004.01.036. [DOI] [PubMed] [Google Scholar]
- Takano T, Tian GF, Peng W, Lou N, Libionka W, Han X, Nedergaard M. Astrocyte-mediated control of cerebral blood flow. Nat. Neurosci. 2006;9:260–267. doi: 10.1038/nn1623. [DOI] [PubMed] [Google Scholar]
- Tootell RB, Silverman MS, Hamilton SL, De Valois RL, Switkes E. Functional anatomy of macaque striate cortex III Color. J Neurosci. 1988a;8:1569–1593. doi: 10.1523/JNEUROSCI.08-05-01569.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tootell RB, Switkes E, Silverman MS, Hamilton SL. Functional anatomy of macaque striate cortex II Retinotopic organization. J Neurosci. 1988b;8:1531–1568. doi: 10.1523/JNEUROSCI.08-05-01531.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tootell RB, Hadjikhani N, Hall EK, Marrett S, Vanduffel W, Vaughan JT, Dale AM. The retinotopy of visual spatial attention. Neuron. 1998;21:1409–1422. doi: 10.1016/s0896-6273(00)80659-5. [DOI] [PubMed] [Google Scholar]
- Tsao DY, Freiwald WA, Knutsen TA, Mandeville JB, Tootell RB. Faces and objects in macaque cerebral cortex. Nat Neurosci. 2003;6:989–995. doi: 10.1038/nn1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao DY, Moeller S, Freiwald WA. Comparing face patch systems in macaques and humans. Proc. Natl. Acad. Sci.USA. 2008;105:19514–19519. doi: 10.1073/pnas.0809662105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsodyks MV, Skaggs WE, Sejnowski TJ, McNaughton BL. Paradoxical effects of external modulation of inhibitory interneurons. J Neurosci. 1997;17:4382–4388. doi: 10.1523/JNEUROSCI.17-11-04382.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanduffel W, Fize D, Mandeville JB, Nelissen K, Van Hecke P, Rosen BR, Tootell RB, Orban GA. Visual motion processing investigated using contrast agent-enhanced fMRI in awake behaving monkeys. Neuron. 2001;32:565–577. doi: 10.1016/s0896-6273(01)00502-5. [DOI] [PubMed] [Google Scholar]
- Vanduffel W, Fize D, Peuskens H, Denys K, Sunaert S, Todd JT, Orban GA. Extracting 3D from motion: differences in human and monkey intraparietal cortex. Science. 2002;298:413–415. doi: 10.1126/science.1073574. [DOI] [PubMed] [Google Scholar]
- Wade AR, Rowland J. Early suppressive mechanisms and the negative blood oxygenation level-dependent response in human visual cortex. J Neurosci. 2010;30:5008–5019. doi: 10.1523/JNEUROSCI.6260-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber B, Keller AL, Reichold J, Logothetis NK. The microvascular system of the striate and extrastriate visual cortex of the macaque. Cereb. Cortex. 2008;18:2318–2330. doi: 10.1093/cercor/bhm259. [DOI] [PubMed] [Google Scholar]
- Weisskoff RM, Zuo CS, Boxerman JL, Rosen BR. Microscopic susceptibility variation and transverse relaxation: theory and experiment. Magn. Reson. Med. 1994;31:601–610. doi: 10.1002/mrm.1910310605. [DOI] [PubMed] [Google Scholar]
- Whittingstall K, Wilson D, Schmidt M, Stroink G. Correspondence of visual evoked potentials with FMRI signals in human visual cortex. Brain Topogr. 2008;21:86–92. doi: 10.1007/s10548-008-0069-y. [DOI] [PubMed] [Google Scholar]
- Yacoub E, Ugurbil K, Harel N. The spatial dependence of the poststimulus undershoot as revealed by high-resolution BOLD- and CBV-weighted fMRI. J. Cereb. Blood Flow Metab. 2006;26:634–644. doi: 10.1038/sj.jcbfm.9600239. [DOI] [PubMed] [Google Scholar]
- Yang Y, Gu H, Stein EA. Simultaneous MRI acquisition of blood volume, blood flow, and blood oxygenation information during brain activation. Magn. Reson. Med. 2004;52:1407–1417. doi: 10.1002/mrm.20302. [DOI] [PubMed] [Google Scholar]
- Yu X, Glen D, Wang S, Dodd S, Hirano Y, Saad Z, Reynolds R, Silva AC, Koretsky AP. Direct imaging of macrovascular and microvascular contributions to BOLD fMRI in layers IV-V of the rat whisker-barrel cortex. Neuroimage. 2012;59:1451–1460. doi: 10.1016/j.neuroimage.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaharchuk G, Mandeville JB, Bogdanov AA, Jr, Weissleder R, Rosen BR, Marota JJ. Cerebrovascular dynamics of autoregulation and hypoperfusion. An MRI study of CBF and changes in total and microvascular cerebral blood volume during hemorrhagic hypotension. Stroke. 1999;30:2197–2204. doi: 10.1161/01.str.30.10.2197. discussion 2204-2205. [DOI] [PubMed] [Google Scholar]
- Zappe AC, Pfeuffer J, Merkle H, Logothetis NK, Goense JBM. The effect of labeling parameters on perfusion-based fMRI in nonhuman primates. J. Cereb. Blood Flow Metab. 2008;28:640–652. doi: 10.1038/sj.jcbfm.9600564. [DOI] [PubMed] [Google Scholar]
- Zhao F, Wang P, Hendrich K, Ugurbil K, Kim SG. Cortical layer-dependent BOLD and CBV responses measured by spin-echo and gradient-echo fMRI: insights into hemodynamic regulation. Neuroimage. 2006;30:1149–1160. doi: 10.1016/j.neuroimage.2005.11.013. [DOI] [PubMed] [Google Scholar]
- Zhao F, Jin T, Wang P, Kim SG. Improved spatial localization of post-stimulus BOLD undershoot relative to positive BOLD. Neuroimage. 2007;34:1084–1092. doi: 10.1016/j.neuroimage.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.