Abstract
In 1998 we published a perspective review describing how drug-induced neuroadaptations might serve towards understanding drug craving. We proposed experimental perspectives to help discern data relevant to long-lasting brain changes, and to distinguish dopamine-related changes that were largely pharmacological from glutamatergic changes that were based on drug-environment associations. These perspectives are embedded in drug abuse research, and the last 18 years has witnessed marked development in understanding addiction-associated corticostriatal glutamate plasticity. Here we propose three new perspectives on how the field might approach integrating and using the emerging data on glutamatergic adaptations. 1) Consider adaptations produced in kind across drug classes as most useful towards understanding shared characteristics of addiction, such as relapse. 2) Consider how drug-induced changes in glia and the extracellular matrix may contribute to synaptic alterations. 3) Make measurements not only at late withdrawal, but also during drug-seeking events to capture transient changes that mediate active drug seeking that are shared across drug classes.
Keywords: Addiction, Glutamate, Prefrontal Cortex, Nucleus Accumbens, Astroglia, Extracellular Matrix
Introduction
In the 1998 article we proposed a critical role for glutamate transmission in cocaine addiction, and summarized the relatively scant data supporting this view. In this brief update, we are pleased to report that substantial progress has been made in testing and supporting this hypothesis. At that time, we were concerned that the field of addiction was becoming confused with a variety of seemingly contradictory data generated due to high variability of experimental protocols. We proposed two criteria to help organize the literature and energize future studies. The first criterion was to recognize that there were transient adaptations that could be identified within minutes to days after the last drug administration that were being conflated with enduring adaptations measured after weeks of withdrawal. Thus, to understand the long-lasting disorder of drug addiction it was best to focus on the enduring changes measureable after longer withdrawal periods. We reasoned that while short-lived adaptations are likely important for establishing addiction, the actual disorder is enduring, and so should the corresponding neurobiological underpinnings.
The second criterion was derived from the fact that at that time most data had been generated using noncontingent cocaine injection and focused on the pharmacological actions of the drug. In contrast, addiction is a disorder that is derived from a combination of drug pharmacology and learned associations made between the drug experience and the environment. Moreover, we proposed that studying only the pharmacology of the drug created a bias towards prepotent involvement of dopamine transmission in addiction, when in fact the learned associations ultimately driving craving and relapse depend more on corticofugal glutamate transmission. Did these approaches bear fruit over 18 years?
The good news is that the brain circuitry involving cortical and allocortical (amygdala and hippocampus) glutamate transmission has become central to our understanding of the long-term changes produced by cocaine, and are now studied using a variety of contingent drug use models that incorporate a withdrawal period. For example, it is generally accepted that the glutamatergic projection from the medial prefrontal cortex to the nucleus accumbens core mediates reinstated drug-seeking (Kalivas, 2009; Luscher and Malenka, 2011). For this reason, many in the field have expended great effort to characterize the neurobiological adaptations produced at this synapse after drug use.
The bad news is that as we have come to measure more subtle adaptations, glutamatergic plasticity has proven to be even more dependent on experimental conditions than we proposed 18 years ago. Thus, beyond simply differences between contingent and noncontingent drug administration, different drugs, durations of contingent drug administration, and varying withdrawal or abstinence conditions seem to produce distinct adaptations in glutamate transmission. Thus, the capacity of our field to identify a coherent role for glutamate that can be used as a rationale for developing drugs to treat addiction continues to be challenged by long-term neuroadaptations in corticofugal glutamate projections that are distinct between different classes of addictive drugs, and between dissimilar dosing and treatment protocols.
Here we briefly assess the status of glutamate transmission in drug addiction. In doing so, we suggest criteria and perspectives on how we might organize the rapidly emerging surfeit of data on changes in glutamatergic circuitry that may underpin drug addiction.
Discerning criteria for identifying relevant cocaine-induced changes in brain
Over the last 10 years, most investigators with a goal of elucidating the neurological basis of relapse to drug use have adopted a substantial withdrawal period (typically weeks). Unfortunately, even by using self-administration paradigms and extended withdrawal, marked differences between some studies remains, especially if different addictive drugs are employed. Here, we suggest new criteria that may help disentangle the literature and assist in distilling the neuroadaptations critical to the uncontrollable motivation to seek drug.
Criterion #1: Can the adaptation be observed after withdrawal from self-administration of different classes of addictive drug?
Since the vulnerability to relapse is a shared endophenotype of addiction to all drugs, the underlying biology should contain shared circuits and cellular plasticity. The exemplar of a need for this criterion is the immense amount of work conducted to discern the signaling pathways underpinning the increased spine density produced in the nucleus accumbens after withdrawal from contingent or noncontingent cocaine administration (Russo et al., 2010). Unfortunately, this morphological adaptation is not produced by opioids (Robinson and Kolb, 2004; Shen et al., 2011), yet both cocaine and heroin engender an enduring vulnerability to relapse. This is not to say that the knowledge obtained with cocaine does not have great value in understanding mechanisms of synaptic plasticity, just that it seems unlikely that this work will reveal molecular targets for neuropathologies of drug addiction that are shared by psychostimulants and opioids.
Criterion #2: Consider involvement of the entire tetrapartite synapse
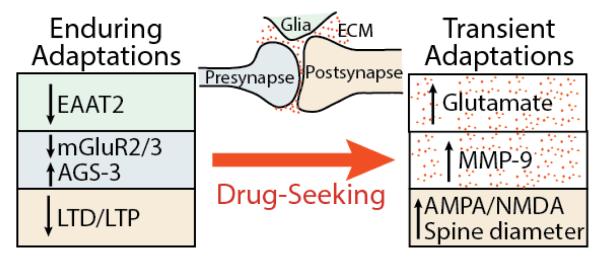
The study of neuroadaptations produced by drugs has focused on both pre- and postsynaptic changes in neurotransmission. However, over the last 20 years it has become clear that glia are involved in synaptic plasticity and regulating synaptic transmission. Accordingly, neuroadaptations in the patterned expression of glial proteins, such as glutamate transporters, need to be considered to understand how drugs of abuse may provoke enduring or transient neuroadaptations in glutamate transmission (Vijayaraghavan, 2009; Bridges et al., 2012). More recently, involvement of signaling in the extracellular space has come into focus as a fourth component of the synapse where drug-induced neuroadaptations may affect glutamate transmission (Smith et al., 2015). The extracellular space is composed of proteins secreted by neurons and glia and constitutes ~20% of neuropil volume. The protein extracellular matrix (ECM) serves an important role in intra- and extracellular signaling that is catalyzed by synaptic activation of matrix metalloproteases (MMPs) (Senkov et al., 2014; Huntley, 2012). Thus, synaptic plasticity arises from coordination amongst all four components of the tetrapartite synapse: presynapse, postsynapse, astroglia and ECM (Figure 1).
Figure 1. Adaptations in glutamate synapses are shared across drug classes and produced in the NAcore of withdrawn animals (enduring adaptations) and in animals undergoing cue-induced drug-seeking (transient adaptations).
The arrows indicate direction of change from control animals (enduring adaptations) or from withdrawn animals (transient adaptations). The transient adaptions appear within 15 min after cue initiation and return to baseline by 120 min. The boxes are color-coded according to where in the tetrapartite synapse they are measured. (ECM= extracellular matrix).
Criterion #3: Make measurements during a highly motivated drug-seeking event
In addition to training rats to self-administer drug, and measuring enduring changes after a withdrawal period, one can also make important measurements during a highly motivated state of drug seeking. Understanding the neurobiological consequences of the drug-seeking event is critical to understanding the uncontrollable desire elicited when addicts are presented with stimuli associated with the drug experience. Indeed the strong drive to use drug and relapsing to drug use are cardinal endophenotypes shared by addiction to all drug classes.
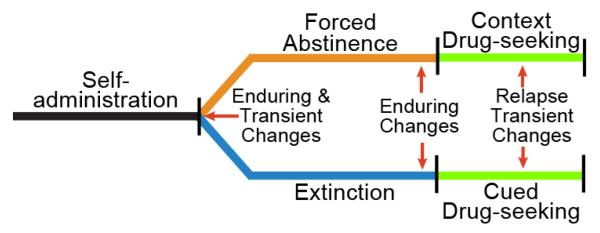
Figure 2 illustrates two popular routes for studying relapse (Venniro et al., 2016). The first, forced abstinence, is withdrawal from daily drug use in a non-drug environment (usually the home cage), then returning the animals to the drug-paired context to elicit a dramatic drug-seeking response (typically pressing the lever or nose-poking without drug delivery). The second model, extinction, involves pairing drug delivery during self-administration with a discrete cue, such as a light and tone. During withdrawal the drug-seeking response elicited by the drug context is extinguished by daily exposure to the context without drug. Later, restoring drug-associated cue(s) to the extinguished context motivates animals to aggressively seek drug without drug delivery. Using either model it is possible to measure changes in the glutamatergic corticostriatal system that parallel the time-course of the drug-seeking event, and are produced in animals trained to self-administer different drug classes.
Figure 2. Self-administration animal models for inducing a drug-free state of highly motivated drug-seeking and measuring neuroadaptations.
Following self-administration of an addictive drug, animals are placed in an extended withdrawal period, either with extinction training or without (forced abstinence). Both enduring and transient adaptations will be expected if measures are made shortly (0.5-72 hours) after discontinuing self-administration (red arrow). Transient changes may be part of a signaling sequence that establishes enduring changes. Enduring neuroadaptations are measured at the end of an extended (weeks) withdrawal period (red arrows). Highly motivated drug-seeking can then be induced by either placing the animal in an un-extinguished, drug-paired context, or within an extinguished context by presenting the animal with drug-conditioned discrete cues, such as a light or tone. To estimate neurological adaptations elicited by entering a highly motivated drug-seeking state, animals are examined at different times after initiating drug seeking with context or cues. Importantly, while the extent of self-administration training and withdrawal period varies, for studying motivated drug seeking these two approaches are the most simple to interpret and widely used.
Enduring corticostriatal adaptations in glutamate transmission that are shared between classes of addictive drug
The most complete literature on enduring adaptations in corticostriatal glutamate synapses after withdrawal from drug self-administration has been obtained with cocaine (Wolf, 2010; Luscher and Malenka, 2011; Dong and Nestler, 2014). Enduring adaptations have been reported in all tetrapartite compartments, including changes that are indicative of synaptic potentiation, such as increased presynaptic release probability, increases in AMPA currents and increases in dendritic spine density and/or head diameter (Conrad et al., 2008; Dietz et al., 2012; Moussawi et al., 2011). Unfortunately, none of these adaptations are produced after withdrawal from opioid self-administration (Robinson and Kolb, 2004; Shen et al., 2011), and thus do not meet criterion #1 listed above.
However, by considering the entire tetrapartite synapse, certain adaptations have been discovered that span across drug classes (Figure 1). In the core subcompartment of the nucleus accumbens (NAcore), astrocytes show reduced glutamate transport via EAAT2 after withdrawal from addictive drugs, including cocaine, nicotine, heroin and alcohol (Mulholland et al., 2016). In the cortico-accumbens projection there is also reduced mGluR2/3 presynaptic signaling due to a down-regulation of the receptor and/or an increase in the Gi subunit binding protein Activator of G-protein Signaling-3 (AGS-3). Increased AGS-3 reduces mGluR2/3 coupling to Gi by binding to Gi and preventing the reformation of the G-protein heterotrimer. AGS-3 is increased after withdrawal from cocaine, heroin and alcohol, and mGluR2/3 is down-regulated after cocaine or alcohol (Bowers et al., 2004; Bowers et al., 2008; Yao et al., 2005; Mulholland et al., 2016). A final enduring change in corticostriatal synapses that crosses drug classes is a loss in the ability to induce LTP or LTD. An enduring loss of corticostriatal synaptic plasticity has been shown for alcohol, cocaine and heroin (Shen and Kalivas, 2013; Moussawi et al., 2009; Martin et al., 2006; Pascoli et al., 2014; Marty and Spigelman, 2012).
Transient neuroadaptations during induced drug-seeking that are present across drug classes
A typical relapse event consists of two stages, a highly motivated drug-seeking phase, which if successful terminates in a drug-taking phase. Surprisingly, neurobiological changes produced during the relapse event itself are not widely studied. Recently, we have used the animal model in Figure 2 to examine changes associated with drug-seeking that are initiated by a drug-associated cue. We have identified a number of transient synaptic alterations that are shared across drug classes and involve the entire tetrapartite synapse. Importantly, these measurements are made during a state of highly motivated drug seeking, but without drug access.
The down-regulation of EAAT2, combined with reduced negative regulation of presynaptic glutamate release probability by mGluR2/3 increases the spillover of synaptic glutamate in the NAcore during reinstated drug seeking in animals extinguished from alcohol, cocaine, heroin, methamphetamine and nicotine self-administration (Mulholland et al., 2016). Prelimbic cortex projections to the NAcore are critical contributors to the spillover since inhibiting activity in prelimbic cortex prevents spillover. The spillover is transient and triggers activation of MMP-9, which is a necessary mediator of the induction of transient synaptic potentiation (t-SP) in medium spiny neurons (MSNs) (Smith et al., 2014). Transient-SP has been shown ex vivo using brain slices obtained after initiating reinstatement to measure increases in AMPA glutamate receptor currents and spine head diameter, or in vivo using evoked responses in the NAcore elicited by electrical stimulation of the prelimbic cortex (Gipson et al., 2013a; Gipson et al., 2013b; Shen et al., 2011; Famous et al., 2008). Transient-SP returns to pre-reinstatement baseline by 120 min after initial presentation of the drug-associated cue. Importantly, the extent of t-SP (AMPA/NMDA ratio and spine head diameter) is positively correlated with the intensity of the drug-seeking response (lever pressing) over the first 15 min after initiating cue presentation (Gipson et al., 2013a).
Potential therapeutic utility of using the three criteria and conclusions
Addiction to all classes of abused drugs shares a defining characteristic of being a chronic, relapsing disorder typified by an overwhelming motivation to use the drug in spite of negative consequences. As a shared endophenotype of all drug addiction, Criterion #1 poses that the most significant neurological adaptations to study in animal models of addiction will be those shared by different drug classes. In studying shared adaptations, Criterion #2 poses the need to consider not just classic synaptic transmission and plasticity, but to include adaptations in astroglia and the extracellular space, which together with the pre- and postsynapse constitute the tetrapartite synapse. Figure 1 outlines four such adaptations measured in the NAcore that are enduring and involve tetrapartite synaptic activity. Directly or indirectly manipulating any four of these adaptations inhibits drug seeking in animal models (Pascoli et al., 2014; Moussawi et al., 2009; Bowers et al., 2008; Bossert et al., 2006), and two are readily drugable targets, EAAT2 and mGluR2/3. Indeed, trials have been conducted with N-acetylcysteine to restore EAAT2 that show good efficacy at reducing the motivation to seek drug (craving), and more modest efficacy in reducing relapse to drug use (McClure et al., 2014; Rushworth and Megson, 2014). This profile may help explain why N-acetylcysteine is also modestly effective in treating a variety of psychiatric disorders including major depression, post-traumatic stress disorder, gambling and trichotillomania (Kalivas and Kalivas, 2016). Similar to craving in addiction, in all of the disorders where N-acetylcysteine has shown efficacy intrusive thoughts can initiate psychiatric symptoms of the disorder.
Finally, following on the logic of Criterion #1 that the motivation to seek drug is a shared endophenotype of all addictive drugs, Criterion #3 is that transient adaptations seen across drug classes during a state of high motivation to seek drug may be possible targets for treating addiction. Figure 1 outlines three shared, transient events produced by presenting a drug-conditioned cue to initiate drug seeking. Of these, the most readily available for drug development in treating addiction is MMP-9. Unfortunately, earlier trials with MMP inhibitors for other disorders have been largely abandoned due to unacceptable side-effects (Vafadari et al., 2015). Nonetheless, research in addiction adopting Criterion #3 is nascent, and other potentially drugable targets may emerge as new neuroadaptations produced during a relapse event are discovered.
Funding Acknowledgements
This work was supported in part by USPHS grants DA003906, DA012513 and DA015369.
Footnotes
Conflict of Interest Disclosure: The authors have no conflict of interest to disclose.
References
- Bossert JM, Poles GC, Sheffler-Collins SI, et al. The mGluR2/3 agonist LY379268 attenuates context- and discrete cue-induced reinstatement of sucrose seeking but not sucrose self-administration in rats. Behav Brain Res. 2006;173:148–152. doi: 10.1016/j.bbr.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Bowers MS, Hopf FW, Chou JK, et al. Nucleus accumbens AGS3 expression drives ethanol seeking through G betagamma. Proc Natl Acad Sci U S A. 2008;105:12533–12538. doi: 10.1073/pnas.0706999105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers MS, McFarland K, Lake RW, et al. Activator of G-protein signaling 3: a gatekeeper of cocaine sensitization and drug-seeking. Neuron. 2004;42:269–281. doi: 10.1016/s0896-6273(04)00159-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges R, Lutgen V, Lobner D, et al. Thinking outside the cleft to understand synaptic activity: contribution of the cystine-glutamate antiporter (System xc-) to normal and pathological glutamatergic signaling. Pharmacological Reviews. 2012;64:780–802. doi: 10.1124/pr.110.003889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, et al. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz DM, Sun H, Lobo MK, et al. Rac1 is essential in cocaine-induced structural plasticity of nucleus accumbens neurons. Nature Neuroscience. 2012;15:891–896. doi: 10.1038/nn.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Nestler EJ. The neural rejuvenation hypothesis of cocaine addiction. Trends Pharmacol Sci. 2014;35:374–383. doi: 10.1016/j.tips.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famous KR, Kumaresan V, Sadri-Vakili G, et al. Phosphorylation-dependent trafficking of GluR2-containing AMPA receptors in the nucleus accumbens plays a critical role in the reinstatement of cocaine seeking. J Neurosci. 2008;28:11061–11070. doi: 10.1523/JNEUROSCI.1221-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson CD, Kupchik YM, Shen H, et al. Relapse induced by cues predicting cocaine depends on rapid, transient synaptic potentiation. Neuron. 2013a;77:867–872. doi: 10.1016/j.neuron.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson CD, Reissner KJ, Kupchik YM, et al. Reinstatement of nicotine seeking is mediated by glutamatergic plasticity. Proceedings of the National Academy of Sciences of the United States of America. 2013b;110:9124–9129. doi: 10.1073/pnas.1220591110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntley GW. Synaptic circuit remodelling by matrix metalloproteinases in health and disease. Nat Rev Neurosci. 2012;13:743–757. doi: 10.1038/nrn3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas BC, Kalivas PW. Corticostriatal circuitry in regulating diseases characterized by intrusive thinking. Dialogues Clin Neurosci. 2016;18:65–76. doi: 10.31887/DCNS.2016.18.1/pkalivas. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- Luscher C, Malenka RC. Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron. 2011;69:650–663. doi: 10.1016/j.neuron.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M, Chen BT, Hopf FW, et al. Cocaine self-administration selectively abolishes LTD in the core of the nucleus accumbens. Nat Neurosci. 2006;9:868–869. doi: 10.1038/nn1713. [DOI] [PubMed] [Google Scholar]
- Marty VN, Spigelman I. Long-lasting alterations in membrane properties, k(+) currents, and glutamatergic synaptic currents of nucleus accumbens medium spiny neurons in a rat model of alcohol dependence. Frontiers in neuroscience. 2012;6:86. doi: 10.3389/fnins.2012.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure EA, Gipson CD, Malcolm RJ, et al. Potential Role of N-Acetylcysteine in the Management of Substance Use Disorders. CNS Drugs. 2014 doi: 10.1007/s40263-014-0142-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussawi K, Pacchioni A, Moran M, et al. N-Acetylcysteine reverses cocaine-induced metaplasticity. Nat Neurosci. 2009;12:182–189. doi: 10.1038/nn.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussawi K, Zhou W, Shen H, et al. Reversing cocaine-induced synaptic potentiation provides enduring protection from relapse. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:385–390. doi: 10.1073/pnas.1011265108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulholland PJ, Chandler LJ, Kalivas PW. Singals from the fourth dimension regulate drug relapse. Trends in Neurosci. 2016 doi: 10.1016/j.tins.2016.04.007. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascoli V, Terrier J, Espallergues J, et al. Contrasting forms of cocaine-evoked plasticity control components of relapse. Nature. 2014;509:459–464. doi: 10.1038/nature13257. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47(Suppl 1):33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Rushworth GF, Megson IL. Existing and potential therapeutic uses for N-acetylcysteine: the need for conversion to intracellular glutathione for antioxidant benefits. Pharmacol Ther. 2014;141:150–159. doi: 10.1016/j.pharmthera.2013.09.006. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Dietz DM, Dumitriu D, et al. The addicted synapse: mechanisms of synaptic and structural plasticity in nucleus accumbens. Trends in Neurosciences. 2010;33:267–276. doi: 10.1016/j.tins.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senkov O, Andjus P, Radenovic L, et al. Neural ECM molecules in synaptic plasticity, learning, and memory. Prog Brain Res. 2014;214:53–80. doi: 10.1016/B978-0-444-63486-3.00003-7. [DOI] [PubMed] [Google Scholar]
- Shen H, Kalivas PW. Reduced LTP and LTD in prefrontal cortex synapses in the nucleus accumbens after heroin self-administration. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2013;16:1165–1167. doi: 10.1017/S1461145712001071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Moussawi K, Zhou W, et al. Heroin relapse requires long-term potentiation-like plasticity mediated by NMDA2b-containing receptors. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:19407–19412. doi: 10.1073/pnas.1112052108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AC, Kupchik YM, Scofield MD, et al. Synaptic plasticity mediating cocaine relapse requires matrix metalloproteinases. Nat Neurosci. 2014;17:1655–1657. doi: 10.1038/nn.3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AC, Scofield MD, Kalivas PW. The tetrapartite synapse: Extracellular matrix remodeling contributes to corticoaccumbens plasticity underlying drug addiction. Brain Res. 2015 doi: 10.1016/j.brainres.2015.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vafadari B, Salamian A, Kaczmarek L. MMP-9 in Translation: From Molecule to Brain Physiology, Pathology and Therapy. J Neurochem. 2015 doi: 10.1111/jnc.13415. [DOI] [PubMed] [Google Scholar]
- Venniro M, Caprioli D, Shaham Y. Animal models of drug relapse and craving: From drug priming-induced reinstatement to incubation of craving after voluntary abstinence. Prog Brain Res. 2016;224:25–52. doi: 10.1016/bs.pbr.2015.08.004. [DOI] [PubMed] [Google Scholar]
- Vijayaraghavan S. Glial-neuronal interactions--implications for plasticity and drug addiction. Aaps J. 2009;11:123–132. doi: 10.1208/s12248-009-9085-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME. The Bermuda Triangle of cocaine-induced neuroadaptations. Trends in Neurosciences. 2010;33:391–398. doi: 10.1016/j.tins.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao L, McFarland K, Fan P, et al. Activator of G protein signaling 3 regulates opiate activation of protein kinase A signaling and relapse of heroin-seeking behavior. Proc Natl Acad Sci U S A. 2005;102:8746–8751. doi: 10.1073/pnas.0503419102. [DOI] [PMC free article] [PubMed] [Google Scholar]