Abstract
Background
It is important to understand the distribution of 2-dimensional strain values in normal population. We performed a multicenter trial to measure normal echocardiographic values in the Korean population.
Methods
This was a substudy of the Normal echOcardiogRaphic Measurements in KoreAn popuLation (NORMAL) study. Echocardiographic specialists measured frequently used echocardiographic indices in healthy people according to a standardized method at 23 different university hospitals. The strain values were analyzed from digitally stored images.
Results
Of a total of 1003 healthy participants in NORMAL study, 2-dimensional strain values were measured in 501 subjects (265 females, mean age 47 ± 15 years old) with echocardiographic images only by GE echocardiographic machines. Interventricular septal thickness, left ventricular (LV) posterior wall thickness, systolic and diastolic LV dimensions, and LV ejection fraction were 7.5 ± 1.0 mm, 7.4 ± 1.0 mm, 29.9 ± 2.8 mm, 48.9 ± 3.6 mm, and 62 ± 4%, respectively. LV longitudinal systolic strain (LS) values of apical 4-chamber (A4C) view, apical 3-chamber (A3C) view, apical 2-chamber (A2C) view, and LV global LS (LVGLS) were −20.1 ± 2.3, −19.9 ± 2.7, −21.2 ± 2.6, and −20.4 ± 2.2%, respectively. LV longitudinal systolic strain rate (LVLSR) values of the A4C view, A3C view, A2C view, and LV global LSR (LVGLSR) were −1.18 ± 0.18, −1.20 ± 0.21, −1.25 ± 0.21, and −1.21 ± 0.21−s, respectively. Females had lower LVGLS (−21.2 ± 2.2% vs. −19.5 ± 1.9%, p < 0.001) and LVGLSR (−1.25 ± 0.18−s vs. −1.17 ± 0.15−s, p < 0.001) values than males.
Conclusion
We measured LV longitudinal strain and strain rate values in the normal Korean population. Since considerable gender differences were observed, normal echocardiographic cutoff values should be differentially applied based on sex.
Keywords: Echocardiography, Strain, Normal population, Reference value
Introduction
Echocardiography is the single most important imaging modality and provides comprehensive information about cardiac structures and hemodynamic parameters.1) Markers of left ventricular (LV) systolic function can be used as good prognostic parameters in many cardiovascular diseases.2) However, the accurate and reproducible measurement of LV systolic function remains a difficult goal to achieve. Although LV ejection fraction (LVEF) is a widely available echocardiographic marker of LV systolic function, this measurement has some limitations.3),4) For instance, LVEF is a volumetric parameter that can be affected by cardiac loading condition and heart rate, and it demands good visualization of the endocardial borders.5) Moreover, LVEF provides little information regarding regional function or intrinsic myocardial function.
Two-dimensional (2D) speckle tracking echocardiography (STE) can measure myocardial mechanics by tracking tiny echo-dense speckles and measurement of LV deformation.6),7) The strain measurement calculated by STE represents the magnitude of myocardial deformation; this measurement can objectively assess both global and regional myocardial function.
Importantly, these strain values can detect subclinical diseases prior to the development of overt clinical features8) and can also provide additional prognostic information in many cardiovascular diseases.9),10),11),12) Since the clinical application of strain values requires the definition of a normal range, it is important to define this range using a normal population with standardized echocardiographic methods. We performed a nationwide multicenter trial for the measurement of normal echocardiographic values in the Korea population. This trial was supported by the Korean Society of Echocardiography and was called the Normal echOcardiogRaphic Measurements in KoreAn popuLation (NORMAL) study.13),14) In this substudy, we aimed to define the normal values of LV strain.
Methods
Study population
The NORMAL study was a prospective multicenter study performed from January 2011 to March 2014 to establish normal echocardiography reference values in a Korean population.13) A total of 23 tertiary teaching hospitals participated in this study. Normal Korean adult subjects were evaluated using comprehensive echocardiography. We prospectively included normal adult subjects (aged 20–79 years old) who did not have any significant cardiac disorders or clinical illnesses that might affect cardiac structure and function, such as hypertension and diabetes. We excluded subjects with an evident structural or functional abnormality on the cardiac valve or cardiac chamber during echocardiographic examination. All study participants agreed to provide their information for research purposes. If the subject refused to participate in this study, the person was excluded. The requirement for written informed consent was waived. The study protocol was approved by the Institutional Review Boards of all included institutes.
Standard conventional echocardiography
Echocardiographic images were acquired and measured at each institute according to a standard method outlined by the American Society of Echocardiography.15) All conventional echocardiographic parameters were measured and averaged from 3 cardiac cycles. Briefly, M-mode echocardiography was performed on parasternal views. LV end-diastolic dimension (LVEDD), interventricular septal wall thickness (IVST), and LV posterior wall thickness (LVPWT) values were measured at end-diastole. The LV end-systolic dimension (LVESD) and left atrial anteroposterior dimension values were measured at endsystole. LVEDD and LVESD were indexed to body surface area (BSA). The LV mass (LVM) was calculated using a linear method using both measurement values from M-mode and 2D images, as follows: LVM (gm) = 0.8 × {1.04 × [(IVST + LVEDD + LVPWT)3 − LVEDD3]} + 0.6 (gm). LVM was also indexed to BSA. LV end-diastolic volume (LVEDV) and LV end-systolic volume (LVESV) values were measured and indexed to BSA. LVEF was measured using the biplane Simpson's method on apical 4-chamber (A4C) and 2-chamber (A2C) views.
Echocardiographic images were stored in DICOM format and electronically transferred to the Echocardiographic Core Laboratory (ECL) at Samsung Medical Center. Trained ECL staff reviewed and reanalyzed all echocardiographic images with commercially available software (EchoPAC PC software, GE Medical Systems, Horten, Norway).
Two-dimensional strain echocardiography
We analyzed longitudinal strain values from 2D echocardiographic images of A4C, apical 3-chamber (A3C), and A2C views in the Strain Core Lab at Chungnam National University Hospital and all 2D strain was analyzed by one researcher (PJH). We only analyzed values from images acquired with GE Medical Systems echocardiographic machines. For the offline analysis, we used images that were digitally stored in cineloop format at approximately 60 frames/sec. Strain and strain rate values were measured using offline software (EchoPAC PC 13.0.0, GE Medical Systems). After selecting the best digital 2D echocardiographic image of the stored cardiac cycles, the LV endocardial border was manually traced at the end-systolic frame. After this tracing, a speckle-tracking region of interest was automatically selected to approximate the myocardium between the endocardium and epicardium. The width of the region of interest was adjusted as necessary to accommodate the total thickness of the LV wall, while excluding blood and pericardial tissue. The software automatically tracked stable echo-dense speckles in each frame using the sum of absolute differences algorithm. Each LV wall was divided into 3 segments (apical, mid, and basal), and the tracing quality of each myocardial segment was evaluated. If the segment exhibited poor tracing quality, the endocardial border or the region of interest was readjusted to obtain good tracing results; alternatively, different echocardiographic images were used. Strain analysis was feasible in 99% of all analyzed segments. Myocardial velocity was derived as the ratio between frame-to-frame displacement in all apical views. Longitudinal peak systolic strain (LS) and longitudinal peak systolic strain rate (LSR) values were calculated from an average value of 6 segments, whereas LV global LS (LVGLS) and LV global LSR (LVGLSR) values were measured from an average of 3 apical view global values. Since the Lagrangian strain measurement expresses deformation relative to the initial length, systolic shortening in the longitudinal orientation is expressed as a negative value. Lower LVGLS and LVGLSR values indicate better LV systolic function.
Statistical analysis
Data are expressed as mean ± standard deviation, and 95% confidence intervals (CIs) for each parameter are provided for continuous variables. The independent t-test was used to compare mean values between males and females, and a one-way analysis of variance test was performed to evaluate whether mean values differed according to age. The gender difference of LVGLS with adjustment of other variables was calculated with analysis of covariance (ANCOVA). Pearson's method was used to evaluate significant correlations among clinical and measurement variables. Intraobserver and interobserver variabilities were analyzed from 20 randomly selected cases, and intraclass correlation coefficients (ICCs) were calculated. Also, mean of two measurements and limit of agreement were calculated with Bland-Altman plot. One researcher repeated measurements at least 2 weeks after the first measurements for the assessment of intraobserver variability testing, and another researcher who was blinded to the first measurement value performed the same measurements for the assessment of interobserver variability. p values < 0.05 were considered statistically significant. All statistical analyses were performed using SPSS Statistics version 22 (SPSS Inc., Chicago, IL, USA) or MedCalc (version 12.3.0.0, MedCalc Software, Mariakerke, Belgium).
Results
Clinical characteristics of the study patients
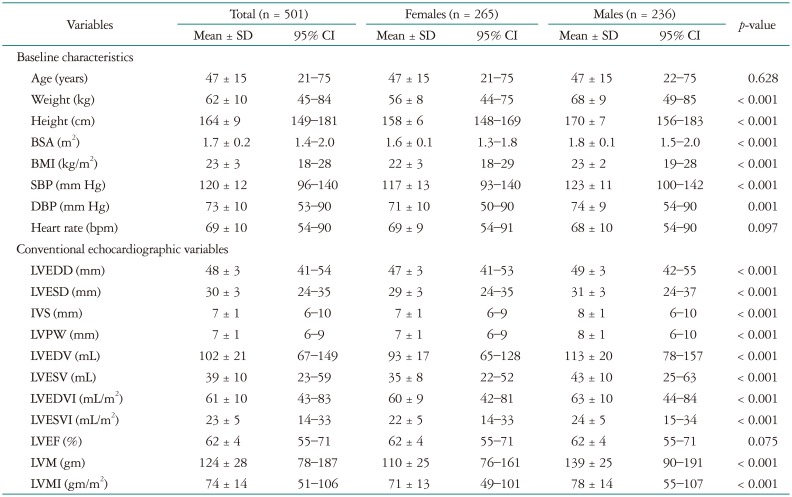
Initially, a total of 1003 normal subjects from 23 centers were evaluated in the current study. After exclusion of 315 echocardiographic images done with other echocardiographic machines and 183 echocardiographic images with different DICOM format unable to calculate strain, we initially tried to analyze 2D strain values from 505 of these subjects. However, 4 of the images were unable to measure speckle tracking strain value, total 501 patients were included in this analysis. Strain measurement was available in 99.2% of stored echocardiographic images. Patient demographic and clinical data are provided in Table 1 according to sex. The mean age was 47 ± 15 years (20–78). Physical parameters of weight, height, and BSA were significantly lower in females than in males (p < 0.001 for all variables). However, the blood pressure values in males were higher than in females. Heart rate was not significantly different between males and females.
Table 1. Demographic and conventional echocardiographic variables.
SD: standard deviation, CI: confidence interval, BSA: body surface area, BMI: body mass index, SBP: systolic blood pressure, DBP: diastolic blood pressure, LVEDD: left ventricular end-diastolic dimension, LVESD: left ventricular end-systolic dimension, IVS: interventricular septum, LVPW: left ventricular posterior wall, LVEDV: left ventricular end-diastolic volume, LVESV: left ventricular end-systolic volume, LVEDVI: left ventricular end-diastolic volume index, LVESVI: left ventricular end-systolic volume index, LVEF: left ventricular ejection fraction, LVM: left ventricular mass, LVMI: left ventricular mass index
Conventional echocardiographic data
Conventional echocardiographic variables are presented in Table 1 according to sex. Also, clinical characteristics and conventional echocardiographic values are presented in Supplementary Table 1 according to age group. Significant differences in conventional echocardiographic variables were observed between males and females. Specifically, the LVEDD, LVESD, IVST, LVPWT, LVEDV, LVESV, and LVM values were greater in males than in females. However, the LVEF values of the two groups were not significantly different.
Two-dimensional strain echocardiographic data
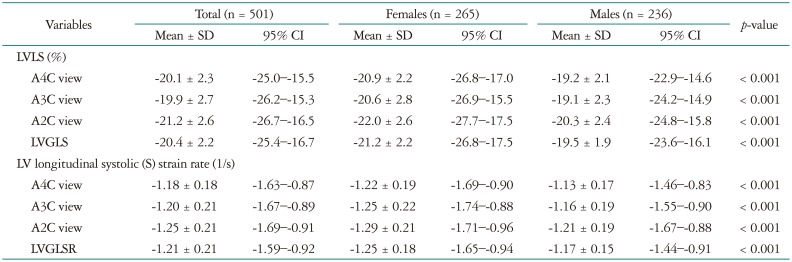
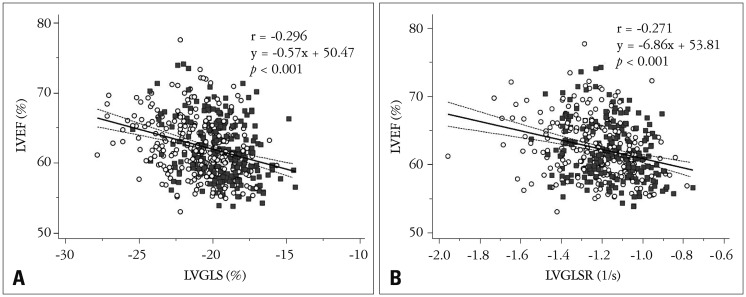
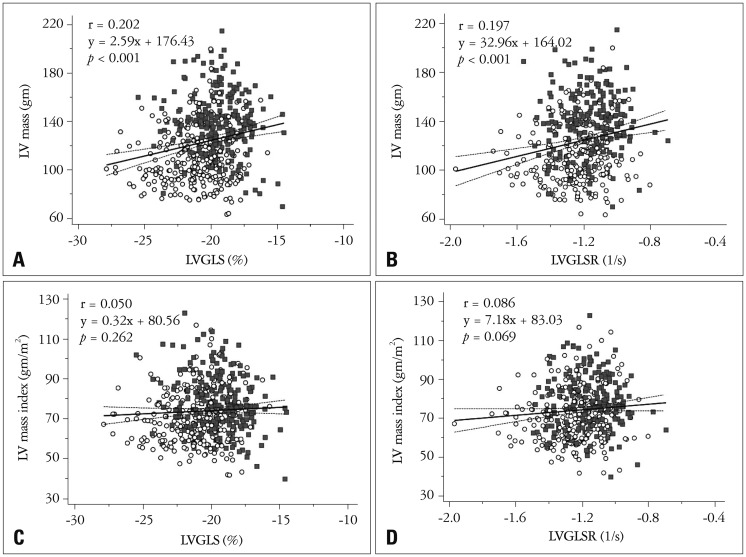
The LV 2D longitudinal strain values are listed in Table 2. Specifically, the LVGLS was −20.4 ± 2.2% (95% CI = −25.4−−16.7%), the LVGLSR was −1.21 ± 0.21−S (95% CI = −1.59−−0.92−S), the peak early diastolic strain rate (E) was 1.55 ± 0.39−S (95% CI = 0.94−2.36−S), and the peak late diastolic strain rate (A) was 0.84 ± 0.29−S (95% CI = 0.39−1.47−S). The LVGLS and LVGLSR values showed significant correlations with the LVEF value (both p < 0.001) (Fig. 1). These systolic parameters also demonstrated significant correlations with LVM. However, the correlations with indexed LVM were not significant (Fig. 2).
Table 2. Left ventricular 2-dimensional longitudinal strain and strain rates in the normal population.
SD: standard deviation, CI: confidence interval, LV: left ventricle, LVLS: left ventricular longitudinal strain, A4C: apical 4-chamber, A3C: apical 3-chamber, A2C: apical 2-chamber, LVGLS: left ventricular global longitudinal systolic strain, LVGLSR: left ventricular global longitudinal systolic strain rate
Fig. 1. Correlations between LVEF and LVGLS (A) and LVEF and LVGLSR (B) (open circle: female, filled quadrangle: male). LVEF: left ventricular ejection fraction, LVGLS: left ventricular global longitudinal strain, LVGLSR: left ventricular global longitudinal systolic strain rate.
Fig. 2. Correlation between LV mass and LV longitudinal systolic strain. LVGLS and LVGLSR show significant correlations with LV mass (A and B). However, LVGLS and LVGLSR were not significantly correlated with LV mass index (C and D) (open circle: female, filled quadrangle: male). LV: left ventricular, LVGLS: LV global longitudinal strain, LVGLSR: LV global longitudinal systolic strain rate.
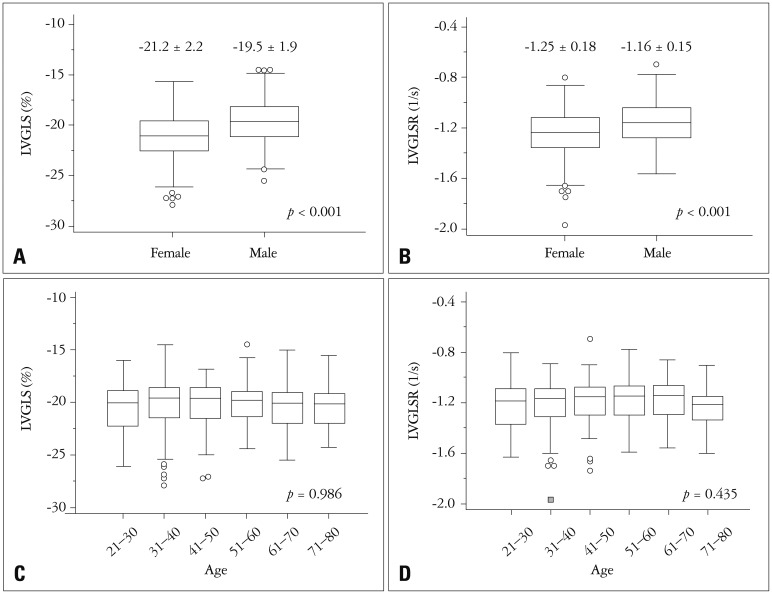
The 2D STE variables are presented in Table 2 according to sex. Females had significantly lower peak systolic strain (better systolic function) and systolic strain rate values compared to those of males (Fig. 3A and B). After ANCOVA analysis, female has better LVGLS (p < 0.001) and LVGLSR (p < 0.001) after adjustment of systolic blood pressure (SBP), LVM, and body mass index (BMI). Also, the peak early diastolic strain rate was higher in females. However, the peak late diastolic strain rates of males and females were similar.
Fig. 3. Females have significantly lower (better) LVGLS (A) and LVGLSR (B) values than those of males. No significant differences were observed among the different age groups (C and D). LVGLS: left ventricular global longitudinal strain, LVGLSR: left ventricular global longitudinal systolic strain rate.
The STE values are presented in Supplementary Table 2 according to age group. No significant differences were observed among any of the age groups (Fig. 3C and D). However, younger females (< 40 years old) had better LVGLS (−21.8 ± 2.4% vs. −20.8 ± 2.1%, p < 0.001) and LVGLSR (−1.3 ± 0.2 vs. −1.2 ± 0.2, p = 0.03) values than older females (> 60 years old). No significant differences were observed between younger males and older males.
Intraobserver and interobserver variability
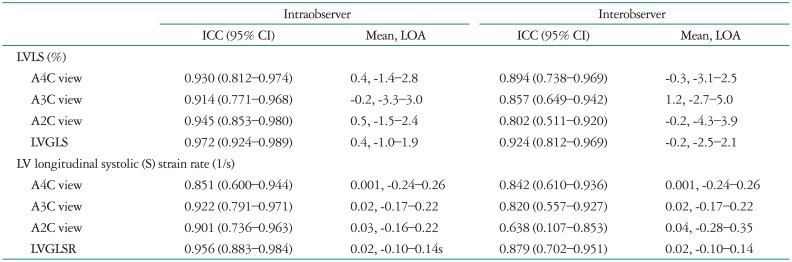
The ICCs for intraobserver, inter-observer, mean of two measurements and limit of agreement by Bland-Altman plot are listed in Table 3. The intraobserver and inter-observer ICCs for LVGLS-total were 0.972 and 0.924, respectively; similarly, the intraobserver and inter-observer ICCs for LVGLSR-total were 0.956 and 0.879, respectively.
Table 3. Intraobserver and interobserver variability of strain values.
ICC: intraclass correlation coefficient, CI: confidence interval, LOA: limit of agreement, LV: left ventricle, LVLS: left ventricular longitudinal strain, A4C: apical 4-chamber, A3C: apical 3-chamber, A2C: apical 2-chamber, LVGLS: left ventricular global longitudinal systolic strain, LVGLSR: left ventricular global longitudinal systolic strain rate
Discussion
In this study, we identified normal reference values of LV longitudinal strain and strain rate using data from the NORMAL study, which prospectively evaluated normal Korean patients from 23 centers nationwide. Females showed more negative (better) peak systolic strain and strain rate values than males. However, no significant differences were observed among different age groups.
‘Myocardial strain’ is a term used to describe local shortening, thickening, and lengthening of the myocardium. Myocardial strain is also considered to be a marker of regional LV function. This strain can be measured on three cardiac axes: longitudinal, radial, and circumferential.16) A negative strain value indicates tissue shortening, whereas a smaller value (that is, a higher absolute value) indicates better ventricular systolic function. Strain can be measured by tissue Doppler imaging (TDI) or by STE. Although TDI can be used to calculate natural myocardial strain, this technique has a number of limitations including angle-dependency, influence of region of interest, and lack of information of global systolic function.17) Currently, STE is the most widely used modality for measuring strain. Importantly, this technique can objectively measure myocardial strain, independent of the angle. Strain rate can be also calculated by STE; these values show good correlation with ventricular contractility.18),19) Many echocardiographic laboratories measure LV strain in the longitudinal direction and use GLS, which is calculated as the average of all apical view segments, as a marker of global LV systolic function.16) Moreover, LVGLS and LVGLSR show significant correlations with pressure-volume loop-derived contractility indices.20)
In our study, we found that the LV longitudinal strain of the A4C view, the A3C view, the A2C view, and the LVGLS were −20.1 ± 2.3, −19.9 ± 2.7, −21.2 ± 2.6, and −20.4 ± 2.2%, respectively. These values are similar to the results of previous studies.21),22),23),24) In a meta-analysis including 2597 subjects from 24 studies, the normal 2D LVGLS value was found to range from −15.9 to −22.1%, with the mean value of −19.7%.21) Kocabay et al.23) reported a normal LVGLS of −21.5 ± 2.0%, calculated from 247 normal Italian subjects. In the Japanese ultrasound speckle tracking of the left ventricle (JUSTICE) study,22) the GLS as assessed by GE machines was −21.3 ± 2.1%; notably, this value was different from the GLS values calculated when using other machines. Saito et al.24) compared 2D strain and 3D strain values from 46 Japanese volunteers and found that the normal 2D LVGLS was −19.9 ± 6.7%. Since LVGLS can be affected by SBP, machine manufacturer, and BMI,19),21),22) we included only subjects with echocardiographic images obtained on GE machines. Moreover, we included normal subjects without hypertension and obesity (mean SBP and BMI were 120 ± 12 mm Hg and 23 ± 3, respectively). We also identified the normal LVLSR range. Specifically, the LVLSR values of the A4C view, the A3C view, the A2C view, and the LVGLSR were −1.18 ± 0.18, −1.20 ± 0.21, −1.25 ± 0.21, and −1.21 ± 0.21−S, respectively. Our calculated mean LVGLSR is similar to those of previous studies with healthy subjects.25),26)
Females had lower (better) LVGLS (−21.2 ± 2.2% vs. −19.5 ± 1.9%, p < 0.001) and LVGLSR (−1.25 ± 0.18−S vs. −1.17 ± 0.15−S, p < 0.001) values than males. This finding is similar to those of a previous study based on strain echocardiography26) and a previous study based on cardiac magnetic resonance.27) We hypothesize that this difference might be a consequence of the different LVM values between the two sexes. Specifically, because males have higher LVM values than females, the LVGLS and LVGLSR values might be lower in females.
We did not identify any significant differences between different age groups. However, LVGLS was higher in younger females (< 40 years old) than in older females (> 60 years old, p < 0.001). Moreover, younger females showed significantly lower LVGLS (−21.8 ± 2.4 vs. −19.2 ± 1.7, p < 0.001) and LVGLSR (−1.30 ± 0.19 vs. −1.16 ± 0.15, p < 0.001) values than younger males. However, no significant differences for LVGLS (−20.8 ± 2.1 vs. −20.1 ± 2.3, p = 0.113) or LVGLSR (−1.22 ± 0.17 vs. −1.19 ± 0.13, p = 0.334) were observed with respect to sex in the older group. In females, the younger group showed lower LVM values than the older group (100.1 ± 19.2 vs. 124.2 ± 23.1, p < 0.001). However, no significant differences were observed in males (136.5 ± 25.8 vs. 142.3 ± 26.6, p = 0.198).
We also identified significant correlations between LVGLS, LVGLSR, and LVM. Because we included normal subjects, these correlations were not strong. These findings are consistent with a cardiac magnetic resonance imaging based study.28) In a previous report of patients with severe aortic stenosis (AS), patients with AS showed markedly decreased GLS prior to aortic valve replacement (AVR), while GLS improved significantly after AVR with decrease of LVM.
Limitations
Several limitations of this study should be acknowledged. First, we included only normal Korean subjects in the NORMAL study; therefore, our results might not be generalizable to other populations. However, this study provides useful LVGLS and LVGLSR reference values and also had much strength, such as the relatively good imaging quality and the fact that it was a prospective study with a reasonably large number of participants. Second, our study included only data acquired with GE echocardiographic machines. Since machines made by different manufacturers could lead to different results, other strain algorithms need to be used to calculate LVGLS and LVGLSR in order to overcome this limitation. Third, patients with significant disease, such as hypertension or diabetes, were excluded based only on past medical histories obtained from the study subjects, while bloodwork and/or other clinical tests were not obtained. Therefore, patients with preclinical hypertension or subclinical coronary artery disease might have been included in the current study. However, the effects of these conditions on heart structure are unlikely to be significant.
Conclusion
Using data from the NORMAL study, we identified reference values for the normal ranges of LVGLS and LVGLSR as assessed by STE. These values can be used as references in the evaluation of LV systolic function.
Acknowledgements
NORMAL study was supported by a research fund from Korean Society of Echocardiography.
Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.4250/jcu.2016.24.4.285.
Baseline characteristics and conventional echocardiographic values according to sex and age (mean ± SD)
Left ventricular 2-dimensional longitudinal strain and strain rates according to sex and age (mean ± SD)
References
- 1.Wolk MJ, Bailey SR, Doherty JU, Douglas PS, Hendel RC, Kramer CM, Min JK, Patel MR, Rosenbaum L, Shaw LJ, Stainback RF, Allen JM American College of Cardiology Foundation Appropriate Use Criteria Task Force. ACCF/AHA/ASE/ASNC/HFSA/HRS/SCAI/SCCT/SCMR/STS 2013 multimodality appropriate use criteria for the detection and risk assessment of stable ischemic heart disease: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2014;63:380–406. doi: 10.1016/j.jacc.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 2.Joyce E, Hoogslag GE, Leong DP, Debonnaire P, Katsanos S, Boden H, Schalij MJ, Marsan NA, Bax JJ, Delgado V. Association between left ventricular global longitudinal strain and adverse left ventricular dilatation after ST-segment-elevation myocardial infarction. Circ Cardiovasc Imaging. 2014;7:74–81. doi: 10.1161/CIRCIMAGING.113.000982. [DOI] [PubMed] [Google Scholar]
- 3.White HD, Norris RM, Brown MA, Takayama M, Maslowski A, Bass NM, Ormiston JA, Whitlock T. Effect of intravenous streptokinase on left ventricular function and early survival after acute myocardial infarction. N Engl J Med. 1987;317:850–855. doi: 10.1056/NEJM198710013171402. [DOI] [PubMed] [Google Scholar]
- 4.White HD, Norris RM, Brown MA, Brandt PW, Whitlock RM, Wild CJ. Left ventricular end-systolic volume as the major determinant of survival after recovery from myocardial infarction. Circulation. 1987;76:44–51. doi: 10.1161/01.cir.76.1.44. [DOI] [PubMed] [Google Scholar]
- 5.Hoit BD. Strain and strain rate echocardiography and coronary artery disease. Circ Cardiovasc Imaging. 2011;4:179–190. doi: 10.1161/CIRCIMAGING.110.959817. [DOI] [PubMed] [Google Scholar]
- 6.Leitman M, Lysyansky P, Sidenko S, Shir V, Peleg E, Binenbaum M, Kaluski E, Krakover R, Vered Z. Two-dimensional strain-a novel software for real-time quantitative echocardiographic assessment of myocardial function. J Am Soc Echocardiogr. 2004;17:1021–1029. doi: 10.1016/j.echo.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 7.Behar V, Adam D, Lysyansky P, Friedman Z. The combined effect of nonlinear filtration and window size on the accuracy of tissue displacement estimation using detected echo signals. Ultrasonics. 2004;41:743–753. doi: 10.1016/j.ultras.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Sarvari SI, Haugaa KH, Anfinsen OG, Leren TP, Smiseth OA, Kongsgaard E, Amlie JP, Edvardsen T. Right ventricular mechanical dispersion is related to malignant arrhythmias: a study of patients with arrhythmogenic right ventricular cardiomyopathy and subclinical right ventricular dysfunction. Eur Heart J. 2011;32:1089–1096. doi: 10.1093/eurheartj/ehr069. [DOI] [PubMed] [Google Scholar]
- 9.Thomas JD, Popović ZB. Assessment of left ventricular function by cardiac ultrasound. J Am Coll Cardiol. 2006;48:2012–2025. doi: 10.1016/j.jacc.2006.06.071. [DOI] [PubMed] [Google Scholar]
- 10.Geyer H, Caracciolo G, Abe H, Wilansky S, Carerj S, Gentile F, Nesser HJ, Khandheria B, Narula J, Sengupta PP. Assessment of myocardial mechanics using speckle tracking echocardiography: fundamentals and clinical applications. J Am Soc Echocardiogr. 2010;23:351–369. doi: 10.1016/j.echo.2010.02.015. quiz 453-5. [DOI] [PubMed] [Google Scholar]
- 11.Nahum J, Bensaid A, Dussault C, Macron L, Clémence D, Bouhemad B, Monin JL, Rande JL, Gueret P, Lim P. Impact of longitudinal myocardial deformation on the prognosis of chronic heart failure patients. Circ Cardiovasc Imaging. 2010;3:249–256. doi: 10.1161/CIRCIMAGING.109.910893. [DOI] [PubMed] [Google Scholar]
- 12.Kusunose K, Popović ZB, Motoki H, Marwick TH. Prognostic significance of exercise-induced right ventricular dysfunction in asymptomatic degenerative mitral regurgitation. Circ Cardiovasc Imaging. 2013;6:167–176. doi: 10.1161/CIRCIMAGING.112.000162. [DOI] [PubMed] [Google Scholar]
- 13.Choi JO, Shin MS, Kim MJ, Jung HO, Park JR, Sohn IS, Kim H, Park SM, Yoo NJ, Choi JH, Kim HK, Cho GY, Lee MR, Park JS, Shim CY, Kim DH, Shin DH, Shin GJ, Shin SH, Kim KH, Park JH, Lee SY, Kim WS, Park SW. Normal echocardiographic measurements in a Korean population study: part I. Cardiac chamber and great artery evaluation. J Cardiovasc Ultrasound. 2015;23:158–172. doi: 10.4250/jcu.2015.23.3.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi JO, Shin MS, Kim MJ, Jung HO, Park JR, Sohn IS, Kim H, Park SM, Yoo NJ, Choi JH, Kim HK, Cho GY, Lee MR, Park JS, Shim CY, Kim DH, Shin DH, Shin GJ, Shin SH, Kim KH, Park JH, Lee SY, Kim WS, Park SW. Normal echocardiographic measurements in a Korean population study: part II. Doppler and tissue Doppler imaging. J Cardiovasc Ultrasound. 2016;24:144–152. doi: 10.4250/jcu.2016.24.2.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Smiseth OA, Torp H, Opdahl A, Haugaa KH, Urheim S. Myocardial strain imaging: how useful is it in clinical decision making? Eur Heart J. 2016;37:1196–1207. doi: 10.1093/eurheartj/ehv529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kadappu KK, Thomas L. Tissue Doppler imaging in echocardiography: value and limitations. Heart Lung Circ. 2015;24:224–233. doi: 10.1016/j.hlc.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 18.Greenberg NL, Firstenberg MS, Castro PL, Main M, Travaglini A, Odabashian JA, Drinko JK, Rodriguez LL, Thomas JD, Garcia MJ. Doppler-derived myocardial systolic strain rate is a strong index of left ventricular contractility. Circulation. 2002;105:99–105. doi: 10.1161/hc0102.101396. [DOI] [PubMed] [Google Scholar]
- 19.Park JH, Negishi K, Kwon DH, Popovic ZB, Grimm RA, Marwick TH. Validation of global longitudinal strain and strain rate as reliable markers of right ventricular dysfunction: comparison with cardiac magnetic resonance and outcome. J Cardiovasc Ultrasound. 2014;22:113–120. doi: 10.4250/jcu.2014.22.3.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kovács A, Oláh A, Lux Á, Mátyás C, Németh BT, Kellermayer D, Ruppert M, Török M, Szabó L, Meltzer A, Assabiny A, Birtalan E, Merkely B, Radovits T. Strain and strain rate by speckle-tracking echocardiography correlate with pressure-volume loop-derived contractility indices in a rat model of athlete’s heart. Am J Physiol Heart Circ Physiol. 2015;308:H743–H748. doi: 10.1152/ajpheart.00828.2014. [DOI] [PubMed] [Google Scholar]
- 21.Yingchoncharoen T, Agarwal S, Popović ZB, Marwick TH. Normal ranges of left ventricular strain: a meta-analysis. J Am Soc Echocardiogr. 2013;26:185–191. doi: 10.1016/j.echo.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 22.Takigiku K, Takeuchi M, Izumi C, Yuda S, Sakata K, Ohte N, Tanabe K, Nakatani S JUSTICE investigators. Normal range of left ventricular 2-dimensional strain: Japanese Ultrasound Speckle Tracking of the Left Ventricle (JUSTICE) study. Circ J. 2012;76:2623–2632. doi: 10.1253/circj.cj-12-0264. [DOI] [PubMed] [Google Scholar]
- 23.Kocabay G, Muraru D, Peluso D, Cucchini U, Mihaila S, Padayattil-Jose S, Gentian D, Iliceto S, Vinereanu D, Badano LP. Normal left ventricular mechanics by two-dimensional speckle-tracking echocardiography. Reference values in healthy adults. Rev Esp Cardiol (Engl Ed) 2014;67:651–658. doi: 10.1016/j.rec.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 24.Saito K, Okura H, Watanabe N, Hayashida A, Obase K, Imai K, Maehama T, Kawamoto T, Neishi Y, Yoshida K. Comprehensive evaluation of left ventricular strain using speckle tracking echocardiography in normal adults: comparison of three-dimensional and two-dimensional approaches. J Am Soc Echocardiogr. 2009;22:1025–1030. doi: 10.1016/j.echo.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 25.Caselli S, Montesanti D, Autore C, Di Paolo FM, Pisicchio C, Squeo MR, Musumeci B, Spataro A, Pandian NG, Pelliccia A. Patterns of left ventricular longitudinal strain and strain rate in Olympic athletes. J Am Soc Echocardiogr. 2015;28:245–253. doi: 10.1016/j.echo.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 26.Dalen H, Thorstensen A, Aase SA, Ingul CB, Torp H, Vatten LJ, Stoylen A. Segmental and global longitudinal strain and strain rate based on echocardiography of 1266 healthy individuals: the HUNT study in Norway. Eur J Echocardiogr. 2010;11:176–183. doi: 10.1093/ejechocard/jep194. [DOI] [PubMed] [Google Scholar]
- 27.Andre F, Steen H, Matheis P, Westkott M, Breuninger K, Sander Y, Kammerer R, Galuschky C, Giannitsis E, Korosoglou G, Katus HA, Buss SJ. Age- and gender-related normal left ventricular deformation assessed by cardiovascular magnetic resonance feature tracking. J Cardiovasc Magn Reson. 2015;17:25. doi: 10.1186/s12968-015-0123-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Staron A, Bansal M, Kalakoti P, Nakabo A, Gasior Z, Pysz P, Wita K, Jasinski M, Sengupta PP. Speckle tracking echocardiography derived 2-dimensional myocardial strain predicts left ventricular function and mass regression in aortic stenosis patients undergoing aortic valve replacement. Int J Cardiovasc Imaging. 2013;29:797–808. doi: 10.1007/s10554-012-0160-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Baseline characteristics and conventional echocardiographic values according to sex and age (mean ± SD)
Left ventricular 2-dimensional longitudinal strain and strain rates according to sex and age (mean ± SD)