Abstract
Background
Severe aortic stenosis (AS), leads to pathological left ventricular remodeling that may worsen with concomitant overweight and obesity (OW/O).
Methods
We aimed to prospectively analyze the impact of OW/O on ventricular remodeling in severe AS, by evaluating the percentage of intraendomyocardial fibrosis (PIEF) and the percentage of infiltrating intraendocardial lipid vacuoles (PIELV) and its relationship to global longitudinal strain (GLS) in patients with OW/O.
Results
44 patients with severe AS were included, 13 non-obese (29%) and 31 OW/O (71%), all of them with left ventricular ejection fraction ≥ 55%. GLS was evaluated with 2D speckle tracking. During valve replacement, an endocardial biopsy was obtained, where PIEF and PIELV were analyzed. Patients with higher PIEF and PIELV had greater body mass index (p < 0.0001) and worse GLS (p < 0.0053). A GLS cut-off point < -14% had a sensitivity of 75%, and a specificity of 92.8% to detect important PIEF (AUC: 0.928, 95% confidence interval: 0.798–1.00). On multivariate analysis, OW/O and PIELV were independently associated to the PIEF, and OW/O and PIEF were independently associated to GLS. A high correlation between the amount of PIELV and PIEF were found.
Conclusion
Patients with severe AS and OW/O have greater PIEF and PIELV, suggesting more pathological remodeling. GLS is useful to detect subclinical myocardial injury and is potentially useful for endomyocardial fibrosis detection. The presence of higher PIELF may be a trigger factor for the development of intraendomyocardial fibrosis.
Keywords: Severe aortic stenosis, Overweight and obesity, Intraendomyocardial fibrosis, Global longitudinal strain
Introduction
The prevalence of overweight and obesity (OW/O) is increasing worldwide.1) Epidemiological studies have shown that both excess weight and obesity are independent factors associated to heart failure.2),3),4),5) OW/O generate a myocardiopathic state resulting from cardiac steatosis; this phenomenon increases and saturate the beta-oxidation and produces an enhanced release of reactive oxygen species resulting in fibrosis, hypertrophy and myocardial apoptosis.6),7)
Patients with OW/O develop ventricular remodeling has been shown that histological features such as: myocyte hypertrophy, fibrosis, tissue degeneration and inflammation, are proportional to the degree of obesity.8) Hypertrophy in the OW/O context is generated under a range of stimuli including insulin resistance, hyperleptinemia, galectin-3 and high levels of TNFα and interleukins.9),10)
Severe aortic stenosis (AS), is also associated with the development of pathological ventricular hypertrophy and fibrosis, resulting from maladaptation to systolic wall stress.11) Moreover, the hypertrophied heart undergoes a shift in substrate utilization, with a preference for glucose metabolism and downregulation of fatty acid oxidation, favoring its accumulation.12)
Both entities are thus associated with myocardial fibrosis and fatty acid accumulation, which in turn have been proven to be related to ventricular dysfunction. The introduction of novel echocardiographic techniques has made it possible to detect subclinical ventricular dysfunction in patients with normal ejection fraction, in both severe AS and OW/O, using global longitudinal strain (GLS).13),14),15)
We aimed to analyze the impact of OW/O in ventricular remodeling in patients with severe AS by evaluating the percentage of intraendomyocardial fibrosis (PIEF) and the percentage of infiltrating intraendomyocardial lipid vacuoles (PIELV) and its relationship to GLS.
Methods
We prospectively enrolled 104 adult patients from March 2012 to February 2014, in whom aortic valve surgery was planned with a diagnosis of degenerative severe AS. Severe AS was defined as a peak aortic jet velocity ≥ 4 m/s, a mean gradient above 40 mm Hg, and aortic valve area ≤ 1 cm2, who had a tridimensional ejection fraction ≥ 55%. All patients agreed to participate and informed consent was obtained. 32 patients were excluded due to the presence of other valve lesions, previous heart surgery, significant coronary artery lesions, segmental wall motion abnormalities, any kind of arrhythmia, hypothyroidism and/or poor acoustic window. Twenty-eight patients withdrew their consent at the previous day of the surgery and were excluded too. The study was approved by the Scientific and Bioethical Committees of the National Institute of Cardiology of Mexico “Ignacio Chávez” (No. 13-796) and it is in accordance with the Helsinki Declaration.
Overweight was defined when the patients had a body mass index (BMI) > 25 kg/m2 and less than 30 kg/m2, Obesity was present in patients with BMI > 30 kg/m2. We designed a comparative study; patients with a BMI ≥ 25 kg/m2 were assigned to the OW/O group and patients with a BMI < 25 kg/m2 were allocated to the non-obese group.
Echocardiographic study
Echocardiograms were obtained at hospital admission following current echocardiographic guidelines,16),17) using a Philips iE33 ultrasound system (Philips Healthcare, Andover, MA, USA). Myocardial speckle-tracking strain quantification was performed offline using Q-Lab version 10.3, on 2-dimensional (2D) B-mode (grayscale) images. GLS was measured in the apical long axis, apical 4-, and 2-chambers views as previously described.17) Left ventricular mass was calculated using the linear method, and indexed to height to avoid bias in obese patients.18) The endocardial and epicardial contours were manually traced. Contours were only accepted when visual inspection and the software indicated adequate tracking. GLS was calculated as the average peak strain from 16 segments. All strain measurements were performed by the same investigator to avoid interobserver variability that was blinded to biopsy results. All the patients had a normal blood pressure at the time of the study. Standard parasternal long-axis and short-axis views from 2D images were used for to measure epicardial fat as have been described previously.19)
Procurement and handling of tissue samples
The procedure was performed only if the patient’s septum had a thickness ≥ 12 mm. During surgery subendocardial tissue was obtained from the interventricular septum at a maximal depth of 2 mm, in the shape of a wedge (triangular shape, endocardial base of 4 mm, maximum distance to the vertex of 2 mm to prevent the risk of perforation). The technique used aimed to obtain mainly endocardial tissue. The biopsy was divided into 2 segments and transferred in a saline and 10% formalin solution.
Using Masson trichrome stain in myocardial biopsies, we measured the percentage of myocardial interstitial fibrosis. After analyzing 5 fields, the extent was calculated by dividing the sum of the fibrotic areas seen in the section by the total tissue area, and classified as: mild = < 25%, moderate = 25 to 50%, or severe = > 50%. Fatty infiltration was assessed according to the percentage of PIELV observed with oil red staining; using the same percentages as in the fibrosis classification. We also searched for the presence of adipocytes in all the fields of the myocardium samples.
Histopathological analysis was conducted by the same pathologist blinded to the patients’ clinical and echocardiographic data.
Statistical analysis
Continuous variables are expressed as means and standard deviations or medians and interquartile ranges per their distribution. Categorical variables are presented as percentages; between-group comparisons of continuous variables were analyzed with Student’s t-test for independent samples or Wilcoxon rank sum test according to their distribution. Receiver operating characteristic (ROC) curves were constructed to establish the optimal cut-off point of GLS with the highest sensibility and specificity associated with myocardial fibrosis and fatty infiltration. We then used this cut-off to defined significant subclinical systolic dysfunction and compare the two groups.
One-way ANOVA was used to compare the fatty and fibrous myocardial infiltration and corrected post hoc with Bonferroni’s test. Categorical variables were compared with Fisher’s exact test. Bivariate correlation was obtained with the Pearson or Spearman method, as required. The risk of developing subclinical severe systolic dysfunction per unit of change in body mass was analyzed by logistic regression yielding odds ratios and 95% confidence intervals (CIs). We used multiple linear regression to determine whether any factors were independently associated with subclinical systolic dysfunction. The multivariable model was generated with the variables showing statistical significance in the bivariate analysis. All analyses were two-tailed and a p value < 0.05 was considered significant. We used the STATA 12.1 statistical program.
Results
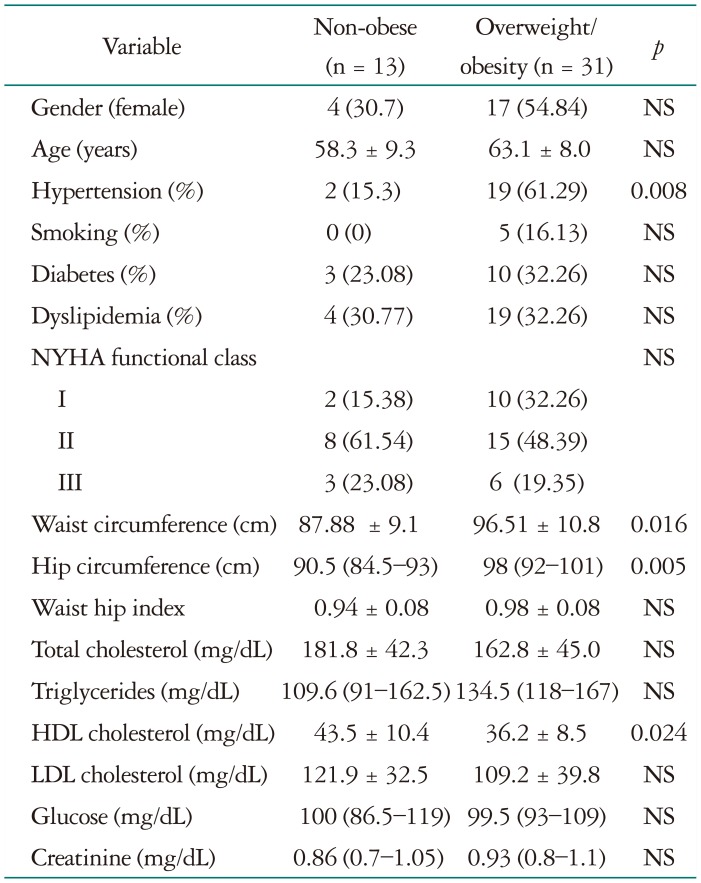
A total of 44 patients were included in the study; 13 were non-obese (29%) and 31 had OW/O (71%). Demographic and laboratory data are shown in Table 1. Patients with OW/O were significantly more hypertensive, and had greater waist and hip circumferences than non-obese subjects. high-density lipoprotein cholesterol was lower in the OW/O group.
Table 1. Demographic data by groups.
Continuos variables are expresed as mean ± SD or mediana and intercuartilar range according to its distributiion. Categorical variables are presented as percentages. NYHA: New York Heart Association, NS: non significant, HDL: high-density lipoprotein, LDL: low-density lipoprotein
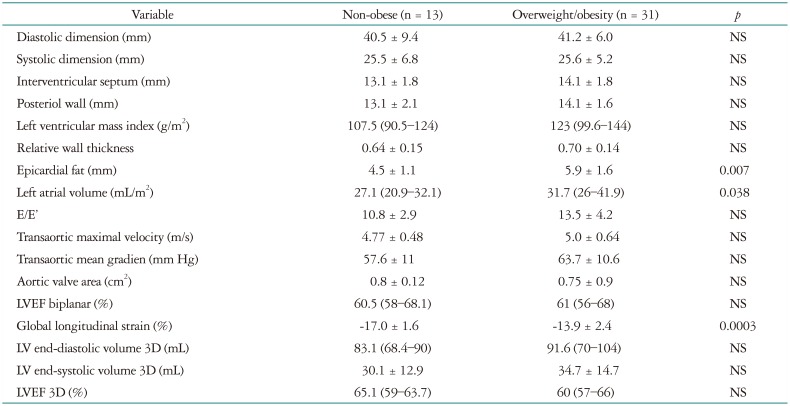
Echocardiographic findings are shown in Table 2. Lineal epicardial fat, left ventricular mass and left atrial volumes were greater in the OW/O group. GLS analysis revealed that patients with OW/O had worse GLS than non-obese patients.
Table 2. Echocardiographic parameters by group.
Continuous variables are expresed as mean ± SD or median and interquartile range according to its distributiion. Categorical variables are presented as percentages. NS: non significant, LVEF: left ventricular ejection fraction
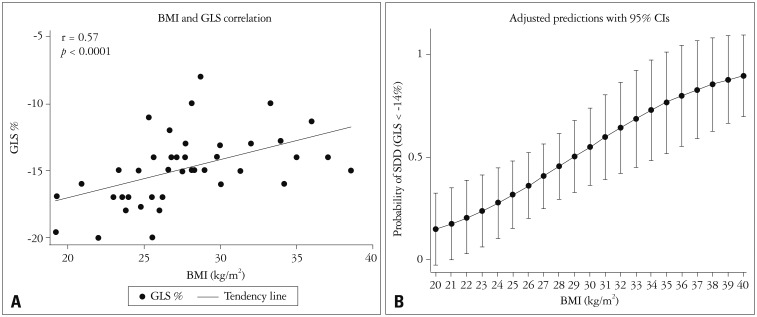
Subclinical systolic dysfunction was evaluated using GLS. Based on ROC curve analysis, a GLS less negative than -14%, was selected to define significant subclinical systolic dysfunction we found correlation between BMI and GLS (r = 0.57, p < 0.0001) (Fig. 1A). The risk of developing significant subclinical systolic dysfunction increases for every BMI unit [odds ratio (OR) 1.2 95% CI: 1.03–1.42, p= 0.019] (Fig. 1B).
Fig. 1. Left ventricular function and obesity. A: Scatter plot showing the body mass index (BMI) and global longitudinal strain (GLS) as continuous variables. B: Increased risk of systolic subclinical dysfunction (SSD) by unit of BMI, odds ratios are shown with its 95% confidence intervals (CIs).
Intraobserver and interobserver agreement for GLS measurements were assessed in 18 randomly selected patients finding a intraclass correlation coefficient for GLS intraobserver of 0.86 (95% CI: 0.65–0.9786), interobserver 0.78 (95% CI: 0.59–0.89). Intraobserver variability for the assessment of endomyocardial fibrosis and fat infiltration showed a substantial agreement with a cohen’s Kappa coefficient of 0.65.
Histopathology
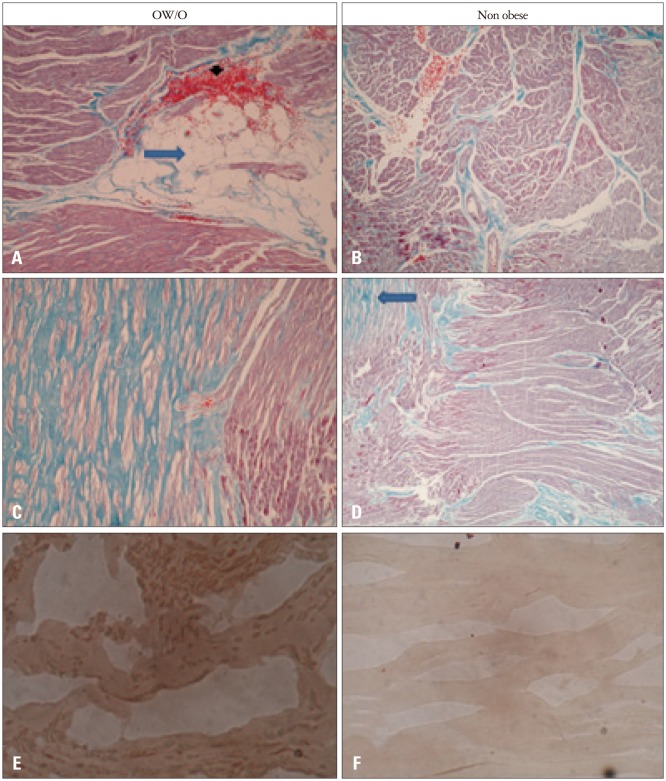
During surgery, according to the surgeon’s criterion and considering patient safety, 18 myocardial biopsies were obtained (40.9%). Representative microphotographs of myocardial biopsies of both groups are shown in Fig. 2.
Fig. 2. Microphotographs of myocardial biopsies. A: Focal adipose infiltration evidenced by the presence of adipocytes (arrow) and a small area of hemorrhage (arrowhead) resulting from the procedure (Masson × 10). B: Myocardial fascicles pointing in different directions and thin connective tissue septa separating them (blue); no adipocytes are observed (Masson × 4). C: Extensive area of fibrosis in blue contrasting with the red myocardium (Masson × 10). D: Muscle fascicles (red) pointing in different directions and limited by thin connective tissue septa. The myocardium shows evidence of fibrosis in a small subendocardial area (arrow) (Masson × 10). E: Myocardial microphotograph after lipid staining. Abundant reddish vacuoles are observed in the myocyte cytoplasm (Oil Red × 40). F: No red vacuoles are observed within the fibers (Oil Red × 40). OW/O: overweight or obesity.
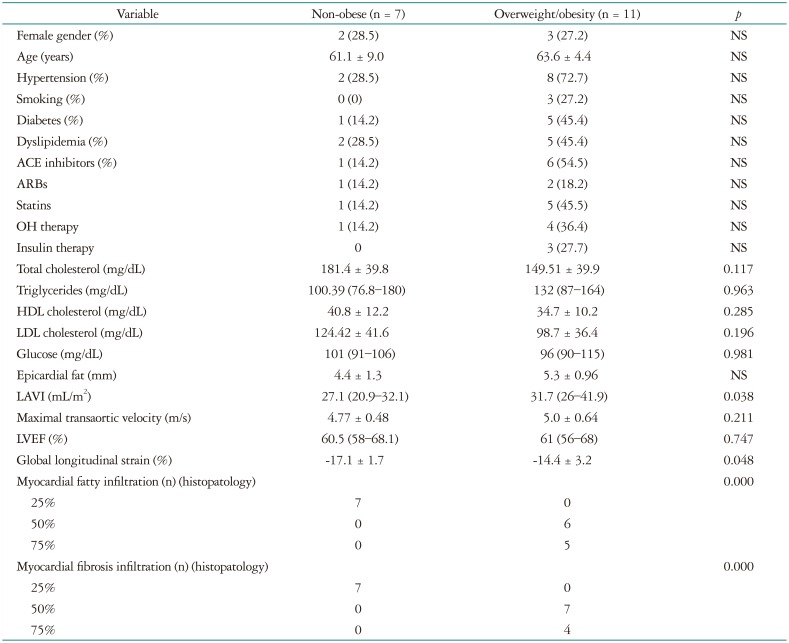
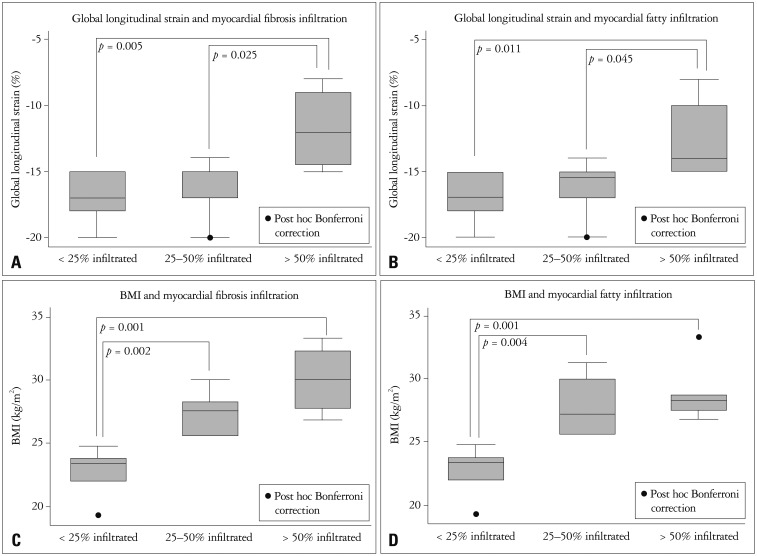
In the group with histological samples there were 7 non-obese patients and 11 with OW/O, their characteristics are shown in Table 3. Interestingly non-obese patients had less PIELV and PIEF, and there was a linear relationship between the amount of PIELV and PIEF and BMI. Patients with more than 50% of PIELV and PIEF had a significantly greater BMI (30.0 ± 2.8 kg/m2 vs. 22.8 ± 1.7 kg/m2, p < 0.0001) and significant subclinical systolic dysfunction (GLS: -11.7 ± 3.3% vs. -17.1 ±1.7%, p < 0.0053) (Fig. 3).
Table 3. Characteristic by groups in patients with histopatologic study.
Continuos variables are expresed as mean ± SD or median and intercuartilar range according to its distributiion. Categorical variables are presented as percentages. ACE: angiotensin converting enzyme, ARBs: angiotensin receptor blockers, OH: oral hypoglycemic, LAVI: left atrial volume index, NS: non significant, LVEF: left ventricular ejection fraction, HDL: high-density lipoprotein, LDL: low-density lipoprotein
Fig. 3. Difference in body mass index by % of myocardial fibrosis infiltration (A) and myocardial fatty infiltration (B). Difference in global longitudinal strain according to the % of myocardial fibrosis infiltration (C) and % myocardial fatty infiltration (D), expressed as categorical variables. BMI: body mass index.
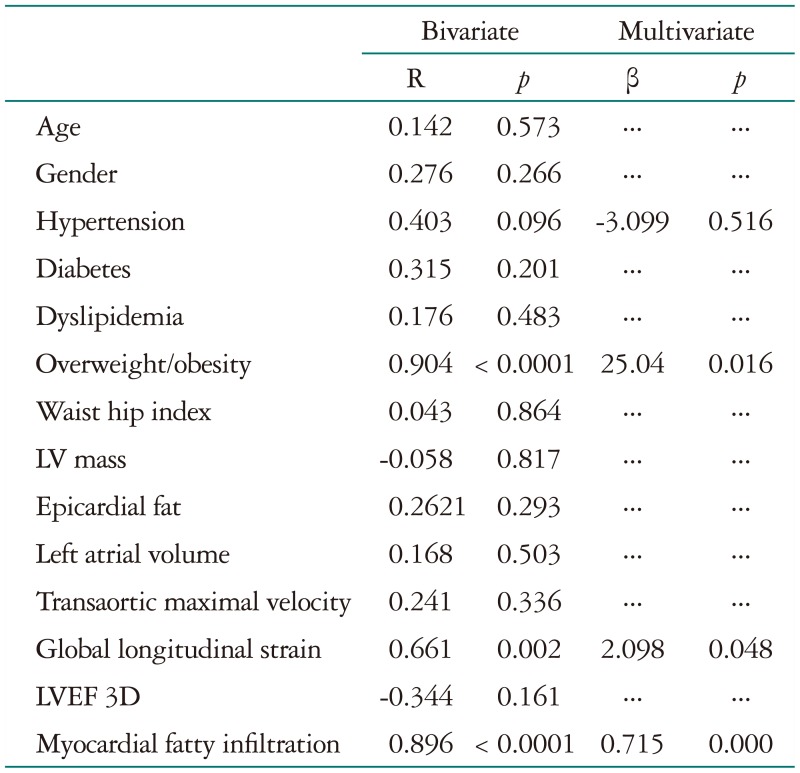
On bivariate analysis, OW/O and PIELV were associated to the PIEF; on multivariate analysis, these variables remained independently associated to endomyocardial interstitial fibrosis (Table 3). On the other hand, bivariate analysis showed that OW/O, LV mass, PIELV and PIEF were associated to GLS, after multivariate analysis only OW/O and PIEF remained significant, Table 4.
Table 4. Association of multiple variables with myocardial interstitial fibrosis.
LVEF: left ventricular ejection fraction
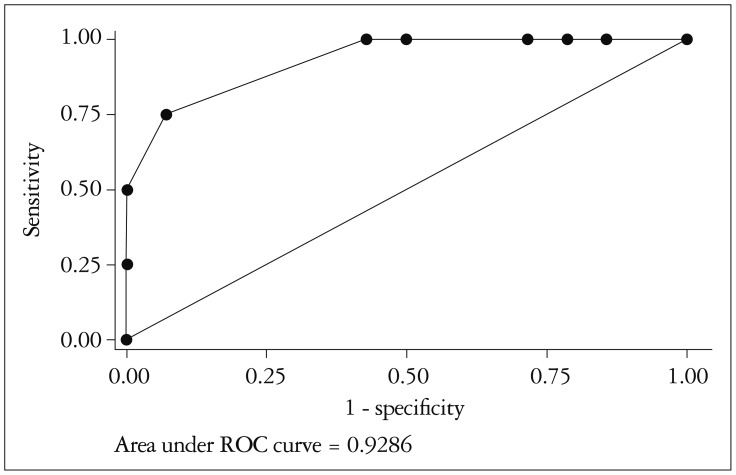
By receiving operating characteristic curve analysis, GLS showed a good discriminative ability to identify patients with important myocardial fibrosis assessed by histopathology (> 50%). The optimal cut-off value GLS of -14% showed a sensitivity of 75%, a specificity of 92.8% and a likelihood ratio of 10.5, with an area under the curve of 0.928 (95% CI: 0.798–1.00) (Fig. 4).
Fig. 4. Receiver operating characteristic (ROC) curve for the detection of > 50% intramyocardial fibrosis by global longitudinal strain.
Discussion
This study evaluates ventricular remodeling measured with GLS and PIEF in patients with severe AS and its relationship to OW/O. Patients with a greater amount of fibrosis and myocardial fatty infiltration were more often overweight or obese and had lower GLS. Multivariate analysis confirmed these findings by proving that OW/O is an independent factor for the development of myocardial fibrosis and ventricular remodeling in patients with severe AS.
In patients with severe AS, left ventricular hypertrophy and interstitial myocardial fibrosis are known sequels of chronic pressure overload.20),21),22) It is hypothesized that pressure overload may compromise myocardial perfusion leading to ischemia and finally fibrosis. However other mechanisms could be associated with the amount of fibrosis, causing greater ventricular remodelling. Marfella et al.,22) found that in patients with severe AS and metabolic syndrome, systolic function becomes increasingly affected as myocardial steatosis severity increases documented in myocardial biopsies. This supporting the theory that dysmetabolic alterations are crucial factors in ventricular remodeling and function in patients with asymptomatic AS.23) Moreover severe AS itself has been associated with fatty acid intramyocardial accumulation by other possible metabolic processes.24)
In our study we found that all patients with severe AS had fatty acid intramyocardial accumulation being greater in patients with OW/O. Both OW/O were independently associated with steatosis and fibrosis in subendocardium. This could be explained by the saturation of the beta-oxidative system with increasing production of reactive oxygen species7) and excessive fatty acid uptake within cardiomyocytes. Once storage capacity is exceeded, fatty acids enter nonoxidative pathways, producing toxic intermediate products, such as ceramides, leading to apoptosis, and ultimately myocardial fibrosis25),26) altering myocardial structure and function.9),25)
Interestingly myocardial fibrosis and fatty acid accumulation have a linear correlation. This finding suggests that the presence of fat is a possible etiopathogenic mechanism in myocardial fibrosis genesis, this could be supported by the greater degree of myocardial fibrosis present in obese patients.
The progress in echocardiographic techniques has made it possible to assess ventricular function beyond volumetric methods. In our study, we only included patients with preserved ejection fraction, however although they had a good function as measured by traditional parameters, myocardial biopsies revealed variable degrees of fibrosis. Because fibrosis mainly involves the subendocardium and these fibers are predominantly longitudinally oriented; GLS measured by 2D strain is able to detect this subclinical damage.
Investigators have documented GLS abnormalities in OW/O and severe AS as separated groups.13),14) This is the first study to our knowledge that analyzes both OW/O and severe AS. We believe that concomitant occurrence of both conditions, would result in more fibrosis by a double mechanism, ischemic and metabolic.
In our study, we found that a GLS < of -14% showed a sensitivity of 75% and a specificity of 92.8% for the detection of > 50% myocardial fibrosis. Others authors27) identified an optimal cut-off of -11.6% (sensitivity 65% and specify 75%, respectively) to predict fibrosis.
A core strength of our study is that fibrosis was assessed by direct evaluation of endomyocardial tissue (a gold standard for the evaluation of fibrosis). Also, because GLS evaluates mainly subendocardial fibers and our histological samples were taken from this location, fibrosis can be detected at earlier stages. It is important to highlight that the percentage of fibrosis in our study was measured only in the subendocardium. While the others layers (mesocardium and epicardium) may preserve their function explaining why conventional left ventricular function parameters were not altered.
Another factor that may promote myocardial fibrosis and ventricular dysfunction is systemic arterial hypertension,28) in our study, hypertension was more common in patients with OW/O, but when assessing its relationship to myocardial fibrosis it was not independently associated. The OW/O group also had worse GLS, suggesting that this non-invasive parameter measured by echocardiography, may be helpful in defining the type of remodeling that a patient develops and could be useful for follow-up and prognostic purposes. Further evaluation in prospective studies is warranted.
Conclusions
This study shows that patients with concomitant OW/O and severe AS have greater pathological remodeling, as measured by a greater percentage of fibrosis and intra-endomyocardial fat accumulation, when compared to non-obese patients. Fibrosis is present in a linear fashion with fat accumulation and correlates with GLS. GLS is a useful tool capable of detecting subclinical dysfunction and in accordance with our results it could also detect the presence of intra-endomyocardial fibrosis.
Study limitations
Due to the safety implications for patients, biopsies could not be obtained in all the subjects initially included in the study. However, the number of obtained biopsies was sufficient to demonstrate a correlation between non-invasive methods and myocardial tissue changes. The number of patients was limited due to the complexity of sampling and the withdrawal of consent by patients the previous day of the surgery. Although we have used intramyocardial biopsies as the gold standard for myocardial fibrosis, it is an invasive procedure associated with morbidity, sampling error and sampling limitations. The septum was selected for biopsy sampling as it has been shown to be more commonly involved in this patient subset. The cut-off point of GLS < -14% should be validated in other study to confirm our results.
Perspectives competency in medical knowledge
Other studies including more patients and in other clinical scenarios should be performed to confirm our results, comparing histopathology or cardiac magnetic resonance imaging late gadolinium enhancement to GLS. GLS, constitutes a useful tool capable of detecting subclinical systolic dysfunction due to myocardial fibrosis which can aid for clinical decision making, follow up and prognosis.
Acknowledgements
Dr. Hugo Rodriguez Zanella for his help in the translation of this manuscript.
References
- 1.Mokdad AH, Serdula MK, Dietz WH, Bowman BA, Marks JS, Koplan JP. The spread of the obesity epidemic in the United States, 1991-1998. JAMA. 1999;282:1519–1522. doi: 10.1001/jama.282.16.1519. [DOI] [PubMed] [Google Scholar]
- 2.Hubert HB, Feinleib M, McNamara PM, Castelli WP. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation. 1983;67:968–977. doi: 10.1161/01.cir.67.5.968. [DOI] [PubMed] [Google Scholar]
- 3.Chen YT, Vaccarino V, Williams CS, Butler J, Berkman LF, Krumholz HM. Risk factors for heart failure in the elderly: a prospective community-based study. Am J Med. 1999;106:605–612. doi: 10.1016/s0002-9343(99)00126-6. [DOI] [PubMed] [Google Scholar]
- 4.Murphy NF, MacIntyre K, Stewart S, Hart CL, Hole D, McMurray JJ. Long-term cardiovascular consequences of obesity: 20-year follow-up of more than 15 000 middle-aged men and women (the Renfrew-Paisley study) Eur Heart J. 2006;27:96–106. doi: 10.1093/eurheartj/ehi506. [DOI] [PubMed] [Google Scholar]
- 5.Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, Kannel WB, Vasan RS. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–313. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 6.Ritchie RH. Evidence for a causal role of oxidative stress in the myocardial complications of insulin resistance. Heart Lung Circ. 2009;18:11–18. doi: 10.1016/j.hlc.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 7.Iozzo P, Lautamaki R, Borra R, Lehto HR, Bucci M, Viljanen A, Parkka J, Lepomaki V, Maggio R, Parkkola R, Knuuti J, Nuutila P. Contribution of glucose tolerance and gender to cardiac adiposity. J Clin Endocrinol Metab. 2009;94:4472–4482. doi: 10.1210/jc.2009-0436. [DOI] [PubMed] [Google Scholar]
- 8.Alpert MA. Obesity cardiomyopathy: pathophysiology and evolution of the clinical syndrome. Am J Med Sci. 2001;321:225–236. doi: 10.1097/00000441-200104000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Iozzo P. Myocardial, perivascular, and epicardial fat. Diabetes Care. 2011;34(Suppl 2):S371–S379. doi: 10.2337/dc11-s250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Menini S, Iacobini C, Blasetti Fantauzzi C, Pesce CM, Pugliese G. Role of galectin-3 in obesity and impaired glucose homeostasis. Oxid Med Cell Longev. 2016;2016:9618092. doi: 10.1155/2016/9618092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kupari M, Turto H, Lommi J. Left ventricular hypertrophy in aortic valve stenosis: preventive or promotive of systolic dysfunction and heart failure? Eur Heart J. 2005;26:1790–1796. doi: 10.1093/eurheartj/ehi290. [DOI] [PubMed] [Google Scholar]
- 12.Allard MF. Energy substrate metabolism in cardiac hypertrophy. Curr Hypertens Rep. 2004;6:430–435. doi: 10.1007/s11906-004-0036-2. [DOI] [PubMed] [Google Scholar]
- 13.Carasso S, Cohen O, Mutlak D, Adler Z, Lessick J, Aronson D, Reisner SA, Rakowski H, Bolotin G, Agmon Y. Relation of myocardial mechanics in severe aortic stenosis to left ventricular ejection fraction and response to aortic valve replacement. Am J Cardiol. 2011;107:1052–1057. doi: 10.1016/j.amjcard.2010.11.032. [DOI] [PubMed] [Google Scholar]
- 14.Di Salvo G, Pacileo G, Del Giudice EM, Natale F, Limongelli G, Verrengia M, Rea A, Fratta F, Castaldi B, D’Andrea A, Calabrò P, Miele T, Coppola F, Russo MG, Caso P, Perrone L, Calabrò R. Abnormal myocardial deformation properties in obese, non-hypertensive children: an ambulatory blood pressure monitoring, standard echocardiographic, and strain rate imaging study. Eur Heart J. 2006;27:2689–2695. doi: 10.1093/eurheartj/ehl163. [DOI] [PubMed] [Google Scholar]
- 15.Caputo M, Urselli R, Zacà V, Capati E, Padeletti M, De Nicola S, Navarri R, Antonelli G, Nucci C, Giacomin E, Mondillo S. Detection of early left ventricular and atrial dysfunction in overweight patients with preserved ejection fraction: a speckle tracking analysis. Echocardiography. 2013;30:551–557. doi: 10.1111/echo.12102. [DOI] [PubMed] [Google Scholar]
- 16.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Mor-Avi V, Lang RM, Badano LP, Belohlavek M, Cardim NM, Derumeaux G, Galderisi M, Marwick T, Nagueh SF, Sengupta PP, Sicari R, Smiseth OA, Smulevitz B, Takeuchi M, Thomas JD, Vannan M, Voigt JU, Zamorano JL. Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications endorsed by the Japanese Society of Echocardiography. J Am Soc Echocardiogr. 2011;24:277–313. doi: 10.1016/j.echo.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 18.de Simone G, Daniels SR, Devereux RB, Meyer RA, Roman MJ, de Divitiis O, Alderman MH. Left ventricular mass and body size in normotensive children and adults: assessment of allometric relations and impact of overweight. J Am Coll Cardiol. 1992;20:1251–1260. doi: 10.1016/0735-1097(92)90385-z. [DOI] [PubMed] [Google Scholar]
- 19.Iacobellis G, Willens HJ. Echocardiographic epicardial fat: a review of research and clinical applications. J Am Soc Echocardiogr. 2009;22:1311–1319. doi: 10.1016/j.echo.2009.10.013. quiz 1417-8. [DOI] [PubMed] [Google Scholar]
- 20.Hein S, Arnon E, Kostin S, Schönburg M, Elsässer A, Polyakova V, Bauer EP, Klövekorn WP, Schaper J. Progression from compensated hypertrophy to failure in the pressure-overloaded human heart: structural deterioration and compensatory mechanisms. Circulation. 2003;107:984–991. doi: 10.1161/01.cir.0000051865.66123.b7. [DOI] [PubMed] [Google Scholar]
- 21.Heymans S, Schroen B, Vermeersch P, Milting H, Gao F, Kassner A, Gillijns H, Herijgers P, Flameng W, Carmeliet P, Van de Werf F, Pinto YM, Janssens S. Increased cardiac expression of tissue inhibitor of metalloproteinase-1 and tissue inhibitor of metalloproteinase-2 is related to cardiac fibrosis and dysfunction in the chronic pressure-overloaded human heart. Circulation. 2005;112:1136–1144. doi: 10.1161/CIRCULATIONAHA.104.516963. [DOI] [PubMed] [Google Scholar]
- 22.Marfella R, Di Filippo C, Portoghese M, Barbieri M, Ferraraccio F, Siniscalchi M, Cacciapuoti F, Rossi F, D'Amico M, Paolisso G. Myocardial lipid accumulation in patients with pressure-overloaded heart and metabolic syndrome. J Lipid Res. 2009;50:2314–2323. doi: 10.1194/jlr.P900032-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pagé A, Dumesnil JG, Clavel MA, Chan KL, Teo KK, Tam JW, Mathieu P, Després JP, Pibarot P ASTRONOMER Investigators. Metabolic syndrome is associated with more pronounced impairment of left ventricle geometry and function in patients with calcific aortic stenosis: a substudy of the ASTRONOMER (Aortic Stenosis Progression Observation Measuring Effects of Rosuvastatin) J Am Coll Cardiol. 2010;55:1867–1874. doi: 10.1016/j.jacc.2009.11.083. [DOI] [PubMed] [Google Scholar]
- 24.Mahmod M, Bull S, Suttie JJ, Pal N, Holloway C, Dass S, Myerson SG, Schneider JE, De Silva R, Petrou M, Sayeed R, Westaby S, Clelland C, Francis JM, Ashrafian H, Karamitsos TD, Neubauer S. Myocardial steatosis and left ventricular contractile dysfunction in patients with severe aortic stenosis. Circ Cardiovasc Imaging. 2013;6:808–816. doi: 10.1161/CIRCIMAGING.113.000559. [DOI] [PubMed] [Google Scholar]
- 25.Goldberg IJ, Trent CM, Schulze PC. Lipid metabolism and toxicity in the heart. Cell Metab. 2012;15:805–812. doi: 10.1016/j.cmet.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akki A, Smith K, Seymour AM. Compensated cardiac hypertrophy is characterised by a decline in palmitate oxidation. Mol Cell Biochem. 2008;311:215–224. doi: 10.1007/s11010-008-9711-y. [DOI] [PubMed] [Google Scholar]
- 27.Hoffmann R, Altiok E, Friedman Z, Becker M, Frick M. Myocardial deformation imaging by two-dimensional speckle-tracking echocardiography in comparison to late gadolinium enhancement cardiac magnetic resonance for analysis of myocardial fibrosis in severe aortic stenosis. Am J Cardiol. 2014;114:1083–1088. doi: 10.1016/j.amjcard.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 28.Querejeta R, López B, González A, Sánchez E, Larman M, Martínez Ubago JL, Díez J. Increased collagen type I synthesis in patients with heart failure of hypertensive origin: relation to myocardial fibrosis. Circulation. 2004;110:1263–1268. doi: 10.1161/01.CIR.0000140973.60992.9A. [DOI] [PubMed] [Google Scholar]