Abstract
Objectives:
Safranal is a pharmacologically active component of saffron and is responsible for the unique aroma of saffron. The hypotensive effect of safranal has been shown in previous studies. This study evaluates the mechanism for the vasodilatory effects induced by safranal on isolated rat aortas.
Methods:
To study the vasodilatory effects of safranal (0.2, 0.4 and 0.8 mM), we contracted isolated rat thoracic aorta rings by using 10-6-M phenylephrine (PE) or 80-mM KCl. Dimethyl sulfoxide (DMSO) was used as a control. The vasodilatory effect of safranal was also evaluated both on intact and denuded endothelium aortic rings. Furthermore, to study the role of nitric oxide and prostacyclin in the relaxation induced by safranal, we incubated the aortic rings by using L-NAME (10-6 M) or indomethacin (10-5 M), each for 20 minutes.
Results:
Safranal induced relaxation in endothelium-intact aortic rings precontracted by using PE or KCl in a concentration-dependent manner, with a maximum relaxation of more than 100%. The relaxant activity of safranal was not eliminated by incubating the aortic rings with L-NAME (EC50 = 0.29 vs. EC50 = 0.43) or with indomethacin (EC50 = 0.29 vs. EC50 = 0.35), where EC50 is the half maximal effective concentration. Also, the vasodilatory activity of safranal was not modified by endothelial removal.
Conclusion:
This study indicated that relaxant activity of safranal is mediated predominantly through an endothelium- independent mechanism.
Keywords: aorta ring, indomethacin, L-NAME, saffron, vasodilatory effect
1. Introduction
The endothelium produces a variety of substances that play important roles in the regulation of circulation and vascular wall homeostasis. Among those substances, vasodilatory factors, such as nitric oxide (NO), prostacyclin (PGI2) and endothelium-derived hyperpolarizing factor (EDHF), or vasoconstrictive factors, such as thromboxane (TXA2) and endothelin-1 (ET-1), have critical roles in the control of blood-vessel-wall homeostasis [1]. Evidence exists for abnormal endothelium- dependent relaxation in hypertension [2], which is a highly prevalent cardiovascular disease and is regarded as an important risk factor for developing other diseases, such as endothelial dysfunction, metabolic syndrome, diabetes, renal dysfunction, congestive heart failure, coronary artery disease, and stroke [3]. Although many antihypertensive drugs are available, some adverse effects have been reported following their use [4]. Therefore, natural plants and their active components need to be considered as possible new antihypertensive drugs that can be used with safety, efficacy, and cultural acceptability and that may have fewer side effects [5].
Saffron (Crocus sativus L.) is a plant belonging to the Iridaceae family and has been used from ancient times as a herbal medicine, spice, food colorant, and flavoring agent [6]. Saffron and its active components have valuable pharmacological properties, including anticonvulsant [7], antidepressant [8-10], anti-inflammatory and antinociceptive [11], antioxidant [12], antitumor [13, 14], and hypotensive [15, 16] properties. Furthermore, saffron extract or its active components have been shown to have protective effects against some toxic agents, including diazinon and acrylamide, in the brain, liver, and cardiovascular system of rats [17]. Research has identified more than 150 chemicals in saffron stigma [18], and the pharmacological effects of saffron are related to its three major components: crocins, which are considered as coloring agents [19], glycoside picrocrocin, which is responsible for saffron’s bitter taste, and safranal, a monoterpene aldehyde, which is responsible for saffron’s unique aroma [13, 20].
Safranal has been shown to possess various pharmacological activities, including antioxidant [21], anticonvulsant [22], anti-anxiety and antidepressant [8], antitussive [23], antinociceptive [24], and anti-ischemia [25] activities. Safranal has also been shown to be able to improve impaired memory and learning ability in rats [26]. The cardiovascular protective effects of safranal have been demonstrated in several studies. Safranal decreased myocardial contractility through a calcium channel-blocking property in guinea pigs [27]. In addition, safranal protected the cardiovascular system against isoproterenol-induced myocardial infarction in Wistar rats [28]. Moreover, the hypotensive effects of safranal have been demonstrated in anaesthetized normotensive and hypertensive rats [15]. In another study, safranal reduced the mean systolic blood pressure in rats treated chronically with desoxycorticosterone acetate (DOCA) salt [29]. Regarding the hypotensive effect of safranal, the aims of this study are to determine whether safranal exhibits vasodilatory effects on isolated rat aortas and to evaluate the mechanism for the vasodilatory effects, if any, induced by safranal in isolated rat aortas.
2. Material and Methods
Adult male Wistar rats (weights: 200 - 250 g) were provided by the Animal Center, School of Pharmacy, Mashhad University of Medical Sciences. They were kept on a 12-hour light/dark cycle at a temperature of 23 ± 1°C with free access to food and water. These conditions were constant throughout the experiments. All animal experiments were carried out in accordance with the requirements established by the Ethics Committee, Mashhad University of Medical Sciences (Verification number: 87124; date of approval: Nov 9, 2009).
Safranal was obtained from Fluka (Switzerland). Phenylephrine (PE) hydrochloride, acetylcholine chloride (ACh), indomethacin, and N-nitro-L-arginine methylester (L-NAME) were purchased from Sigma-Aldrich (Germany). The dimethyl sulfoxide (DMSO) (a solvent of safranal) and other chemicals used in this study were obtained from Merck (Germany).
Rats were killed by using intraperitoneal (IP) injection of ketamine/xylazine. Then, the thorax was opened, after which the thoracic aorta was quickly removed, cleaned from adherent connective tissues, and cut into rings (4 - 5 mm in length). Special care was taken to ensure that endothelial damage did not occur during tissue preparation. Two stainless-steel stirrups were passed through the lumen of each ring. One stirrup was connected to an isometric force transducer (POWERLAB, AD Instrument, Australia) to measure the tension in the vessels. The rings were placed in a 25-mL organ chamber (Organ Bath, 4 channels, AD Instrument, Australia) containing Krebs solution gassed with 95% O2/5% CO2 and maintained at 37°C. The composition of the Krebs solution was as follows: 118.0- mM NaCl, 4.7-mM KCl, 1.2-mM NaH2PO4, 1.2-mM MgSO4, 25.0-mM NaHCO3, 11.1-mM glucose, and 2.5-mM CaCl2 (pH = 7.4) [30]. Rings were placed under a resting tension of 2 g, which had been determined in preliminary studies to be optimal, and allowed to equilibrate for 1 hour. During this equilibration period, the physiological salt solution was replaced every 15 minutes. The Labchart 7.3 (AD-Instruments) software was used for this study.
For the study of the vasodilatory effects of safranal, the thoracic aorta rings isolated from rats were contracted by using 10-6-M PE or 80-mM KCl in two separate experiments. When the vasoconstriction curves for the rings reached the plateau phase of maximum tension, safranal (0.2, 0.4 and 0.8 mM) was added, and the tensions were recorded. The vasodilatory effect of safranal was expressed as a percentage of relaxation to maximum constriction induced by 10-6-M PE or 80-mM KCl. DMSO was used as a control. The vasodilatory effect of safranal on constriction induced by PE in rat thoracic aortas was also evaluated for both intact endothelium rings and denuded endothelium rings. To confirm that the endothelial layer had been removed, we tested with a single dose of acetylcholine (10-6 M) after phenylephrine (10-6 M) in denuded vessels. The maximum relaxation induced by acetylcholine was less than 30%.
To examine whether the safranal induced vasorelaxation was mediated by the NO, we incubated the aortic rings in L-NAME (10-5 M) for 20 minutes, after which the thoracic aortic rings were contracted by using 10-6-M PE. Then, the vasorelaxant effect of safranal (0.2, 0.4 and 0.8 mM) was examined. To examine the role of the cyclooxygenase pathway in safranal-mediated vasorelaxation, we incubated the aortic rings in indomethacin (10-5 M) for 20 minutes. Then, the thoracic aortic rings were contracted by using 10-6-M PE, and the vasorelaxant effect of safranal (0.2, 0.4 and 0.8 mM) was examined.
All results are expressed as means ± standard errors of the mean (SEMs). The mean of maximum effects at each safranal concentration was used for comparing the results by using analysis of variance (ANOVA) analyses, followed by Tukey-Kramer tests. The software Pharm-PCS was used to calculate of the half maximal effective concentration (EC50). P-values less than 0.05 were considered as significant.
3. Results
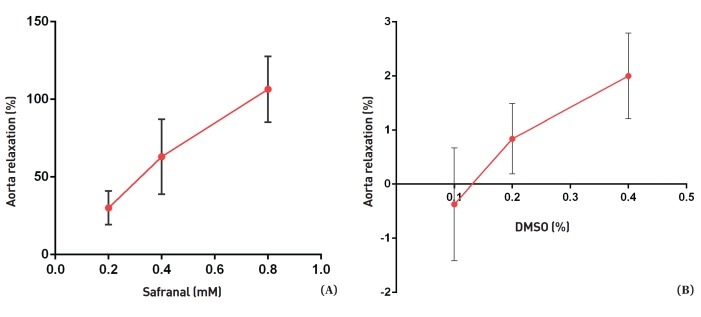
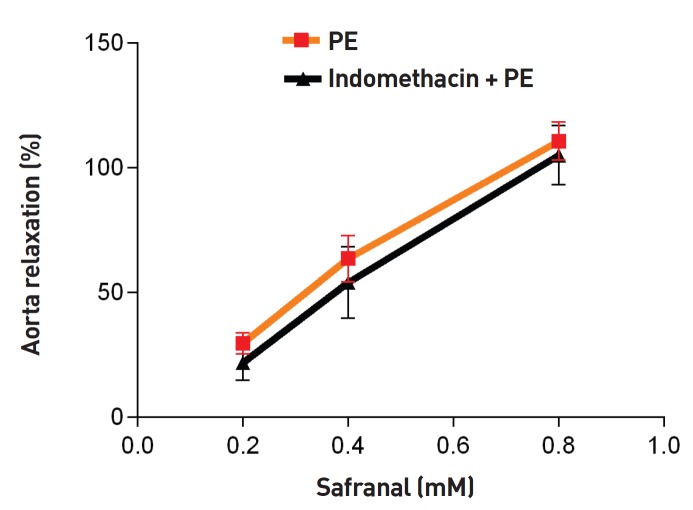
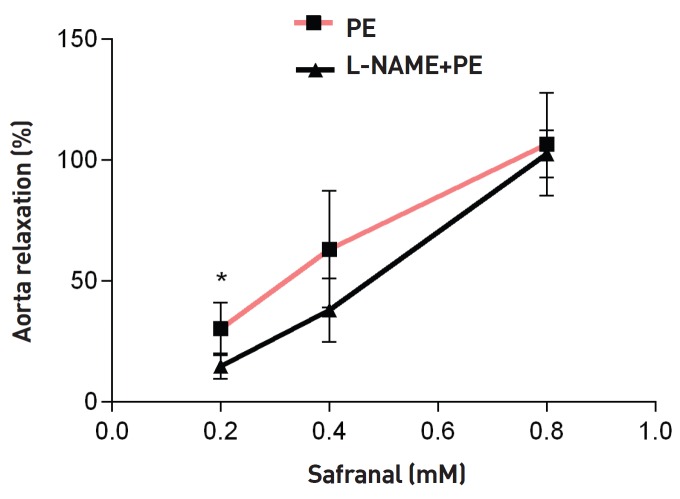
As shown in Fig. 1, safranal (0.2, 0.4 and 0.8 mM) induced relaxation in endothelium-intact aortic rings precontracted with PE (10-6 M) in a concentration-dependent manner. The maximum relaxation response induced by the highest concentration of safranal (0.8 mM) was more than 100%. DMSO had no relaxation effect in endothelium-intact aortic rings precontracted with PE (10-6 M). As shown in Fig. 2, no significant difference was noted in the vasorelaxant responses induced by safranal in endothelium-intact aortic rings precontracted with PE (10-6 M) in the absence (EC50 = 0.29) or the presence of indomethacin (10-5 M) (EC50 = 0.35) (Table 1). Relaxation induced by safranal (0.4 and 0.8 mM) incubated with L-NAME for 20 minutes in endothelium- intact aortic rings precontracted with PE (10-6 M) (EC50 = 0.43) was not significantly different from that induced by safranal in the absence of L-NAME (EC50 = 0.29). However, a significant difference between the relaxations induced by safranal in the presence and the absence of L-NAME was observed for the lowest concentration of safranal (0.2 mM) (P < 0.05) (Fig. 3).
Fig. 1. (A) Effect of safranal in endothelium-intact aortic rings precontracted by using PE (10-6 M). The vasodilatory effect of safranal was expressed as a percentage of relaxation to maximum constriction induced by 10-6-M PE. Values are expressed as means ± SEMs. (B) Effect of the final amount of DMSO (%) in the organ chamber for each safranal concentration on aorta rings precontracted by using PE. DMSO was used as a control.
PE, phenylephrine; SEMs, standard errors of the mean; DMSO, dimethyl sulfoxide.
Fig. 2. Relaxation effect of safranal in endothelium-intact aortic rings in the presence of indomethacin (10-5 M). Aortic rings were incubated with indomethacin for 20 minutes, after which the aortic rings were contracted by using 10-6-M PE. The vasorelaxant effect of safranal was then examined. Values are expressed as means ± SEM (Tukey-Kramer).
PE, phenylephrine; SEM, standard error of the mean.
Table. 1. EC50 and Emax values of safranal vasodilatory effects in aortic rings precontracted by using PE and incubated by using L-NAME and indomethacin, in aortic rings precontracted by using KCl, and in denuded vessels.
| PE | Indomethacin + PE | L-NAME + PE | KCl | Denuded | |
|---|---|---|---|---|---|
| EC50 | 0.29 | 0.34 | 0.43 | 0.34 | 0.46 |
| Emax | 110.6 | 105.04 | 102.5 | 101.7 | 97.8 |
EC50, effective concentration 50; PE, phenylephrine; L-NAME, N-nitro-L-arginine methylester.
Fig. 3. Relaxation effect of safranal in endothelium-intact aortic rings in the presence of L-NAME (10-6 M). Aortic rings were incubated by using L-NAME for 20 minutes. Then, they were contracted by using 10-5-M PE, and the vasorelaxant effect of safranal was examined. Values are expressed as means ± SEM based on the Tukey-Kramer test (P < 0.05 vs. L-NAME preincubated rings).
L-NAME, N-nitro-L-arginine methylester; PE, phenylephrine; SEM, standard error of the mean.
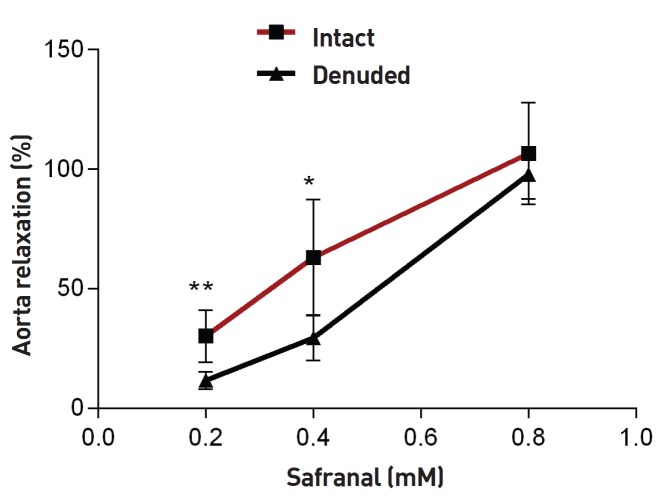
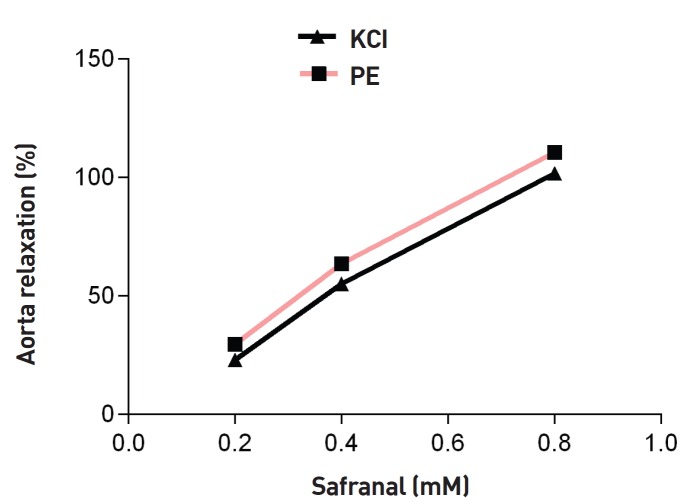
Safranal (0.8 mM) could elicit maximum relaxation in endothelium-denuded aortic rings. Significant differences were observed between relaxation induced by safranal in endothelium-intact and endothelium-denuded aortic rings for the low and the moderate concentrations of safranal (0.2 and 0.4 mM) (P < 0.01 and P < 0.05, respectively) (Fig. 4). As shown in Fig. 5, safranal (0.2, 0.4 and 0.8 mM) induced relaxation in endothelium-intact aortic rings precontracted with KCl (80 mM) in a concentration-dependent manner. No significant difference was seen between the relaxation induced by safranal in endothelium-intact aortic rings precontracted by using KCl and that induced by safranal in endothelium-intact aortic rings precontracted by using PE (Fig. 5).
Fig. 4. Relaxation effect of safranal in endothelium-denuded aortic rings precontracted by using PE (10-6 M). The vasodilatory effect of safranal was expressed as a percentage of relaxation to maximum constriction induced by using 10-6-M PE both on the intact endothelium and the denuded endothelium rings (Tukey- Kramer). Values are expressed as means ± SEM.
PE, phenylephrine; SEM, standard error of the mean.
Fig. 5. Effect of safranal in endothelium-intact aortic rings precontracted by using KCl (80 m M). The vasodilatory effect of safranal was expressed as a percentage of relaxation to maximum constriction induced by using KCl. Values are expressed as means ± SEMs (Tukey-Kramer Test).
PE, phenylephrine; SEMs, standard errors of the mean.
4. Discussion
Safranal, the main constituent of Crocus sativus essential oil, is responsible for saffron’s unique odor [20]. Our results showed that safranal could induce vasorelaxation in isolated rat aortic rings via an endothelium-independent mechanism. The vasorelaxant effect of safranal might be due to its effect on vascular smooth muscle cells via L-type voltage-dependent calcium channels. Safranal is also well known to exhibit hypotensive effects. Intravenous administration of safranal (1 mg/kg) has been shown to induce 50 ± 5.2 mmHg reductions in the mean arterial blood pressure in anaesthetized normotensive and hypertensive rats. This hypotensive effect did not induce reflex tachycardia [15]. Furthermore, saffron extract has shown a potent relaxant effect on guinea-pig tracheal chains partly due to the presence of safranal [31]. Another study revealed that chronic administration of safranal could reduce the mean systolic blood pressure in rats treated with DOCA salt [29]. Regarding the effect of safranal in lowering blood pressure, this study was undertaken to evaluate the mechanism of safranal’s hypotensive effect by using aortic rings isolated from rats.
Our results showed that safranal could induce vasorelaxation in endothelium-intact aortic rings precontracted with PE. To investigate whether the relaxing effect of safranal depended on endothelium-mediated mechanisms, we evaluated endothelium-derived vasodilator-relaxing factors such as NO and prostacyclin [32]. NO is is a factor released by the endothelium that is produced by an endothelium- releasing factor which produces by endothelial nitric oxide synthase (eNOS) and induces vascular smooth muscle relaxation through the activation of guanylyl cyclase [33]. NO has an essential role in the regulation of normal and pathological blood pressure [34]. To assess the contribution of NO in relaxation induced by safranal, we incubated the aortic rings with L-NAME, which is an inhibitor of NO synthase. Inhibition of NO by using L-NAME did not eliminate the relaxation induced by safranal. EC50 values (EC50 = 0.29 vs. EC50 = 0.43) indicate that safranal has lower potency when NO is inhibited. Hence, one might conclude that the vasodilatory action of safranal is partially endothelium-dependent.
PGI2 is another factor released by the endothelium that is produced by endothelial cyclooxygenase. PGI2 elevates cyclic AMP, reduces the availability of calcium, and induces smooth muscle cell relaxation [32]. To verify the involvement of prostacyclin in safranal-induced vasorelaxation, we performed another experiment with safranal in the presence of indomethacin. Results showed that the relaxation produced by safranal was not modified by inhibition of cyclooxygenase. Therefore, safranal vasorelaxation is not mediated through the cyclooxygenase pathway. Taken together, our findings demonstrate that the relaxation induced by safranal is partially related to factors derived from the endothelium. In order to verify this hypothesis, we performed another experiment on endothelium-denuded aortic rings. The obtained relaxant responses in the intact and the denuded endothelial were similar, especially at the highest safranal concentration. However, the low and the moderate safranal doses had different relaxant effects in the intact and the denuded endothelial. This suggests that the relaxatory effects of safranal may be partially endothelium-dependent.
In this study, the effect of safranal on aortic rings isolated from rats and precontracted by using KCl was also evaluated. KCl is believed to depolarize the cell membrane and to increase intracellular calcium via the voltage-dependent calcium channels of smooth muscle cells, with this process leading to contraction [35]. Our results showed no differences in vasorelaxation induced by safranal in aortic rings precontracted by using PE or KCl. PE (an α1-receptor agonist)- induced contraction involves receptor-operated calcium and voltage-operated calcium channels [35]. Thus, the vasodilatory effect of safranal seems to be associated with inhibition of the voltage-operated calcium channels, leading to a decreased intracellular calcium concentration.
Taken together, our results show that the endothelium has a partial role in safranal-induced vasorelaxation. Safranal-induced relaxation could be due to a direct action on vessel smooth muscle cells. These findings are in agreement with those of Boskabady et al. who showed that the inhibitory effect of safranal on guinea-pig smooth muscle cell contraction was through inhibition of the calcium channel [31]. In addition, our results are consistent with the findings of a previous study by Imenshahidi et al (2010) that demonstrated intravenous administration of safranal caused potent hypotensive activity in normotensive and desoxycorticosterone-acetate-induced hypertensive rats [15].
Endothelial dysfunction has been shown to be induced as a result of DOCA-induced hypertension [36]. Because the differences between the hypotensive activities of normotensive and hypertensive rats were not remarkable statistically, one can conclude that vasorelaxation induced by intravenous administration of safranal is not endothelium-dependent. In a previous study, chronic administration of safranal also reduced blood pressure in DOCA-salt-induced hypertensive rats, probably due to several mechanisms, including its inhibitory effect on smooth muscle cells, blocking of the calcium channel, inhibition of sarcoplasmic reticulum Ca2+ release into cytosol, and/or interaction with GABA(A)-benzodiazepine receptor complex [29].
According to lethal dose (LD)50 values in animal studies, safranal has low-toxicity when administered via the acute intraperitoneal route and is non-toxic for acute oral administration [37]. However, further clinical trials and toxicological studies should be carried out in humans.
5. Conculsion
In summary, the results of the present study indicate that safranal-induced relaxation in isolated rat aortic rings predominantly is due to the inhibition of smooth muscle cell contraction caused by a blocking of the L-type calcium channels and is partially endothelium-dependent.
Acknowledgments
The authors are thankful to the Vice Chancellor of Research, Mashhad University of Medical Sciences, for financial support. The results described in this paper were part of a Doctor of Pharmacy thesis. This research was supported by a grant from the Vice Chancellor of Research (87124) from Mashhad University of Medical Sciences.
Footnotes
Conflict of interest The authors declare that there are no conflicts of interest.
References
- 1.Sandoo A, Veldhuijzen van, Zanten, Metsios GS, Carroll D, Kitas GD. The endothelium and its role in regulating vascular tone. Open Cardiovasc Med J. 2010;4:302–312. doi: 10.2174/1874192401004010302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dharmashankar K, Widlansky ME. Vascular endothelial function and hypertension: insights and directions. Curr Hypertens Rep. 2010;12(6):448–455. doi: 10.1007/s11906-010-0150-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Veeramani C, Al-Numair K, Chandramohan G, Alsaif MA, Alhamdan AA, Pugalendi KV. Antihypertensive effect of Melothria maderaspatana leaf fractions on DOCA-salt-induced hypertensive rats and identification of compounds by GC-MS analysis. J Nat Med. 2012;66(2):302–310. doi: 10.1007/s11418-011-0590-2. [DOI] [PubMed] [Google Scholar]
- 4.Krum H, Pellizzer AM. New and emerging drug treatments for hypertension. Aust Fam Physician. 1998;27(4):235–237. [PubMed] [Google Scholar]
- 5.Talha J, Priyanka M, Akanksha A. Hypertension and herbal plants. Int Res J Pharm. 2011;2(8):26–30. [Google Scholar]
- 6.Hosseinzadeh H, Nassiri-Asl M. Avicenna's (Ibn Sina) the canon of medicine and saffron (Crocus sativus): a review. Phytother Res. 2013;27(4):475–483. doi: 10.1002/ptr.4784. [DOI] [PubMed] [Google Scholar]
- 7.Hosseinzadeh H, Khosravan V. Anticonvulsant effects of aqueous and ethanolic extracts of Crocus sativus L. stigmas in mice. Arch Iran Med. 2002;5:44–47. [Google Scholar]
- 8.Hosseinzadeh H, Karimi G, Niapoor M. Antidepressant effect of Crocus sativus L. Stigma extracts and their constituents, crocin and safranal, in Mice. J Med Plants. 2004;3:48–58. [Google Scholar]
- 9.Vahdati Hassani, F, Naseri V, Razavi BM, Mehri S, Abnous K, Hosseinzadeh H. Antidepressant effects of crocin and its effects on transcript and protein levels of CREB, BDNF, and VGF in rat hippocampus. DARU. DARU. 2014;22(1) doi: 10.1186/2008-2231-22-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghasemi T, Abnous K, Vahdati F, Mehri S, Razavi BM, Hosseinzadeh H. Antidepressant effect of Crocus sativus aqueous extract and its effect on CREB, BDNF, and VGF transcript and protein levels in rat hippocampus. Drug Res. 2015;65(7):337–343. doi: 10.1055/s-0034-1371876. [DOI] [PubMed] [Google Scholar]
- 11.Hosseinzadeh H, Younesi HM. Antinociceptive and anti-inflammatory effects of Crocus sativus L. stigma and petal extracts in mice. BMC Pharmacol. 2002;2 doi: 10.1186/1471-2210-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hosseinzadeh H, Shamsaie F, Mehri S. Antioxidant activity of aqueous and ethanolic extracts of Crocus sativus L. stigma and its bioactive constituents crocin and safranal. Pharmacogn Mag. 2010;5(20):419–424. [Google Scholar]
- 13.Abdullaev FI. Biological effects of saffron. Biofactors. 1993;4(2):83–86. [PubMed] [Google Scholar]
- 14.Hosseinzadeh H, Behravan J, Ramezani M, Ajgan KH. Anti-tumor and cytotoxic evaluation of Crocus sativus L. stigma and petal extracts using brine shrimp and potato disc assays. J Med Plants. 2005;4(15):59–65. [Google Scholar]
- 15.Imenshahidi M, Hosseinzadeh H, Javadpour Y. Hypotensive effect of aqueous saffron extract (Crocus sativus L.) and its constituents, safranal and crocin, in normotensive and hypertensive rats. Phytother Res. 2010;24(7):990–994. doi: 10.1002/ptr.3044. [DOI] [PubMed] [Google Scholar]
- 16.Imenshahidi M, Razavi BM, Faal A, Gholampoor A, Mousavi SM, Hosseinzadeh H. Effects of chronic crocin treatment on desoxycorticosterone acetate (doca)-salt hypertensive rats. Iran J Basic Med Sci. 2014;17(1):9–13. [PMC free article] [PubMed] [Google Scholar]
- 17.Razavi BM, Hosseinzadeh H. Saffron as an antidote or a protective agent against natural or chemical toxicities. DARU. DARU. 2015;23 doi: 10.1186/s40199-015-0112-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bathaie SZ, Mousavi SZ. New applications and mechanisms of action of saffron and its important ingredients. Crit Rev Food Sci Nutr. 2010;50(8):761–786. doi: 10.1080/10408390902773003. [DOI] [PubMed] [Google Scholar]
- 19.Alavizadeh SH, Hosseinzadeh H. Bioactivity assessment and toxicity of crocin: a comprehensive review. Food Chem Toxicol. 2014;64:65–80. doi: 10.1016/j.fct.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 20.Rezaee R, Hosseinzadeh H. Safranal: from an aromatic natural product to a rewarding pharmacological agent. Iran J Basic Med Sci. 2013;16(1):12–26. [PMC free article] [PubMed] [Google Scholar]
- 21.Assimopoulou AN, Sinakos Z, Papageorgiou VP. Radical scavenging activity of Crocus sativus L. extract and its bioactive constituents. Phytother Res. 2005;19(11):997–1000. doi: 10.1002/ptr.1749. [DOI] [PubMed] [Google Scholar]
- 22.Hosseinzadeh H, Talebzadeh F. Anticonvulsant evaluation of safranal and crocin from Crocus sativus in mice. Fitoterapia. 2005;76(7-8):722–724. doi: 10.1016/j.fitote.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 23.Nemati H, Boskabady MH, Ahmadzadeh Vostakolaei., H Stimulatory effect of Crocus sativus (saffron) on β2-adrenoreceptors of guinea pig tracheal chains. Phytomedicine. 2008;15(12):1038–1045. doi: 10.1016/j.phymed.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 24.Amin B, Hosseinzadeh H. Evaluation of aqueous and ethanolic extracts of saffron, Crocus sativus L. and its constituents, safranal and crocin in allodynia and hyperalgesia induced by chronic constriction injury model of neuropathic pain in rats. Fitoterapia. 2012;83(5):888–895. doi: 10.1016/j.fitote.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 25.Hosseinzadeh H, Sadeghnia H. Safranal, a constituent of Crocus sativus (saffron), attenuated cerebral ischemia induced oxidative damage in rat hippocampus. J Pharm Pharm Sci. 2005;8(3):394–399. [PubMed] [Google Scholar]
- 26.Hosseinzadeh H, Ziaei T. Effects of Crocus sativus stigma extract and its constituents, crocin and safranal, on intact memory and scopolamine-induced learning deficits in rats performing the Morris water maze task. J Med Plants. 2006;5:40–50. [Google Scholar]
- 27.Boskabady MH, Shafei MN, Shakiba A, Sefidi HS. Effect of aqueous-ethanol extract from Crocus sativus (saffron) on guinea-pig isolated heart. Phytother Res. 2008;22(3):330–334. doi: 10.1002/ptr.2317. [DOI] [PubMed] [Google Scholar]
- 28.Mehdizadeh R, Parizadeh MR, Khooei AR, Mehri S, Hosseinzadeh H. Cardioprotective effect of saffron extract and safranal in isoproterenol-induced myocardial infarction in wistar rats. Iran J Basic Med Sci. 2013;16(1):56–63. [PMC free article] [PubMed] [Google Scholar]
- 29.Imenshahidi M, Razavi BM, Faal A, Gholampoor A, Mousavi SM, Hosseinzadeh H. The effect of chronic administration of safranal on systolic blood pressure in rats. Iran J Pharm Res. 2015;14(2):585–590. [PMC free article] [PubMed] [Google Scholar]
- 30.Parsaee H, Imenshahidi M, Fatehi Z. Lovastatin incubation improves acetylcholine-induced relaxation in isolated aortic rings of diabetic rat. Iran J Pharm Res. 2006;5(3):191–198. [Google Scholar]
- 31.Boskabady MH, Aslani MR. Relaxant effect of Crocus sativus (saffron) on guinea pig tracheal chains and its possible mechanisms. J Pharm Pharmacol. 2006;58(10):1385–1390. doi: 10.1211/jpp.58.10.0012. [DOI] [PubMed] [Google Scholar]
- 32.Mombouli JV, Vanhoutte PM. Endothelial dysfunction: from physiology to therapy. J Mol Cell Cardiol. 1999;31(1):61–74. doi: 10.1006/jmcc.1998.0844. [DOI] [PubMed] [Google Scholar]
- 33.Dehpour AR, Ghafourifar P, Samenian J, Sadeghipour HR, Sadr SS. The effect of lithium on endothelial-dependent relaxation in rat isolated aorta. Gen Pharmacol. 1995;26(5):1003–1007. doi: 10.1016/0306-3623(94)00286-V. [DOI] [PubMed] [Google Scholar]
- 34.Sausbier M, Schubert R, Voigt V, Hirneiss C, Pfeifer A, Korth M et al. Mechanisms of NO/cGMP-dependent vasorelaxation. Circ Res. 2000;87(9):825–830. doi: 10.1161/01.RES.87.9.825. [DOI] [PubMed] [Google Scholar]
- 35.Mashhoodi T, Zahedi-Asl S, Sarkaki A. Inhibitory effect of aluminium on KCl and phenylephrine induced contraction in isolated rat aorta. Acta Medica Iranica. 2004;42(5):379–382. [Google Scholar]
- 36.Nugent M, Artru A, Michenfclder JD. Cerebral metabolic vascular and protective effects of midazolam maleate: comparison to diazepam. Anesthesiology. 1982;56(3):172–176. doi: 10.1097/00000542-198203000-00003. [DOI] [PubMed] [Google Scholar]
- 37.Hosseinzadeh H, Sadeghi Shakib, S, Khadem Sameni, A, Taghiabadi E. Acute and subacute toxicity of safranal, a constituent of saffron, in mice and rats. Iran J Pharm Res. 2013;12(1):93–99. doi: 10.1016/j.clinbiochem.2011.08.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]