Abstract
Introduction:
The public health impact of vaporized nicotine products (VNPs) such as e-cigarettes is unknown at this time. VNP uptake may encourage or deflect progression to cigarette smoking in those who would not have otherwise smoked, thereby undermining or accelerating reductions in smoking prevalence seen in recent years.
Methods:
The public health impact of VNP use are modeled in terms of how it alters smoking patterns among those who would have otherwise smoked cigarettes and among those who would not have otherwise smoked cigarettes in the absence of VNPs. The model incorporates transitions from trial to established VNP use, transitions to exclusive VNP and dual use, and the effects of cessation at later ages. Public health impact on deaths and life years lost is estimated for a recent birth cohort incorporating evidence-informed parameter estimates.
Results:
Based on current use patterns and conservative assumptions, we project a reduction of 21% in smoking-attributable deaths and of 20% in life years lost as a result of VNP use by the 1997 US birth cohort compared to a scenario without VNPs. In sensitivity analysis, health gains from VNP use are especially sensitive to VNP risks and VNP use rates among those likely to smoke cigarettes.
Conclusions:
Under most plausible scenarios, VNP use generally has a positive public health impact. However, very high VNP use rates could result in net harms. More accurate projections of VNP impacts will require better longitudinal measures of transitions into and out of VNP, cigarette and dual use.
Implications:
Previous models of VNP use do not incorporate whether youth and young adults initiating VNP would have been likely to have been a smoker in the absence of VNPs. This study provides a decision-theoretic model of VNP use in a young cohort that incorporates tendencies toward smoking and shows that, under most plausible scenarios, VNP use yields public health gains. The model makes explicit the type of surveillance information needed to better estimate the effect of new products and thereby inform public policy.
Introduction
The 2014 US Surgeon General’s Report stated that the use of combustible tobacco, primarily cigarettes, is by far the dominant cause of the preventable deaths from tobacco use, but also noted that e-cigarettes, and variations in this class of noncombustible products, may help to speed the decline of lethal combustible tobacco use.1,2 Vaporized nicotine products (VNPs), including e-cigarettes and heat-not-burn cigarettes, represent a new generation of nicotine delivery products. Although the long-term health risks have yet to be thoroughly characterized, VNPs are likely to be much safer than cigarettes3–5 and are generally perceived by smokers as less risky than cigarettes.6–9 In addition, some types of VNPs have been shown to deliver nicotine more efficiently than nicotine replacement products10–13 and provide sensorimotor experiences similar to smoking.
The recent upsurge in VNP awareness and past 30-day use,14,15 especially among youth, appears to have flattened.16–18 Future uptake and use of VNPs will also be influenced by the regulatory context in which it is brought to market.19–23 VNPs have been banned in some countries while being subject to few or no regulations in other countries.24 With “deeming” regulation,25 the US Food and Drug Administration (FDA) is now confronting the challenge of how to regulate VNPs in ways that would be beneficial to public health, while recognizing that “sufficient data about e-cigarettes to determine (VNPs) effect on the public health”25 are not yet available.
If used instead of smoking cigarettes, VNPs provide the potential to reduce harm and thereby improve population health.1,26 However, VNPs have the potential to increase population-level harm if youth who would not have otherwise smoked become cigarette smokers as a direct consequence of using VNPs27–32 (ie, a hypothesized gateway) or if current smokers who would otherwise have quit smoking cigarettes instead delay or fail to quit.33,34
In the absence of the requisite data, modeling can provide a structure to analyze the public health impact under different assumptions about how VNPs might be used. This paper presents a decision-theoretic model of the public health impact of VNP use in the United States. Unlike previous models of e-cigarette use,34–36 our model is cohort- rather than age-based. While cohorts may differ in terms of awareness, perceived risks, products available (eg, flavorings, ability to satisfy nicotine cravings), and user characteristics (eg, early vs. late adopters, high vs. low income), we model the potential impact of VNP initiation on VNP and cigarette use using a recent cohort of young people.
The model improves on previous work by explicitly modeling the decisions that individuals make at each age regarding VNP use and comparing that use to a no-VNP use scenario based on an age-period-cohort analysis.37 We project the likely future impact of VNPs based on the best available evidence. Sensitivity analysis is used to discern the trade-off between harm-reducing and harm-increasing effects of VNP use on net public health and to identify the specific parameters that contribute to the net effect of VNPs. Our analysis also identifies the kind of data that are needed to better evaluate the public health impact of VNP use.
Methods
The analysis is confined to patterns of VNP and cigarette use, since cigarette smoking is the dominant threat to public health.1,2 Using data on smoking rates prior to when VNPs were introduced, we describe a hypothetical “No-VNP scenario” as the projection of future smoking rates in the absence of VNPs. We then consider a hypothetical, data-informed VNP use scenario where patterns of VNP use and associated cigarette smoking interact to influence health outcomes. Public health implications are derived in terms of the change in smoking-related mortality and life years gained or lost between the two scenarios.
The “No-VNP Scenario”
To estimate smoking rates in the absence of VNP use, we analyze a cohort of current, former, and never smokers in the United States using data through 2012. The data were developed38 by applying an age-period-cohort statistical technique37 to National Health Interview Surveys (NHIS) from 1965–2012 while correcting for bias due to higher mortality among smokers. Since sustained VNP use was still low in 2012,14 the NHIS data are used to approximate cigarette smoking trends prior to VNPs.
A representative cohort of individuals aged 15 years in 2012 (born in 1997) was chosen, since most initiation into smoking takes place beginning at age 15.39 Projected initiation and cessation rates are used to calculate current, former, and never smoker prevalence through the year 2083 in the absence of VNPs. Never smokers become current smokers reflecting their initiation at each age, with some percentage of smokers who are alive at a given age becoming former smokers as a result of cessation. Cessation is measured as the percent quit for 2 years to approximate net cessation, taking into account relapse rates.38
VNP Scenarios
The VNP model includes (1) established use of VNPs alone, (2) cigarettes alone, (3) dual use of VNPs and cigarettes, and (4) nonuse of either. “Established use” is conceptualized as long enough for measureable harms to accumulate, which typically requires years of use for cigarettes.40 Established dual user refers to frequent use of both products (eg, at least weekly), since occasional use of one (eg, once in the past month) and regular use of the other is unlikely to materially affect risk profiles. While established use of VNPs and cigarettes is used to determine health outcomes, short-term (“trial”) use of VNPs is incorporated as a direct pathway into established use, since most estimates of VNP use in the literature (eg use at least once in the past 30 days) are likely to reflect primarily short-term rather than long-term use.41,42
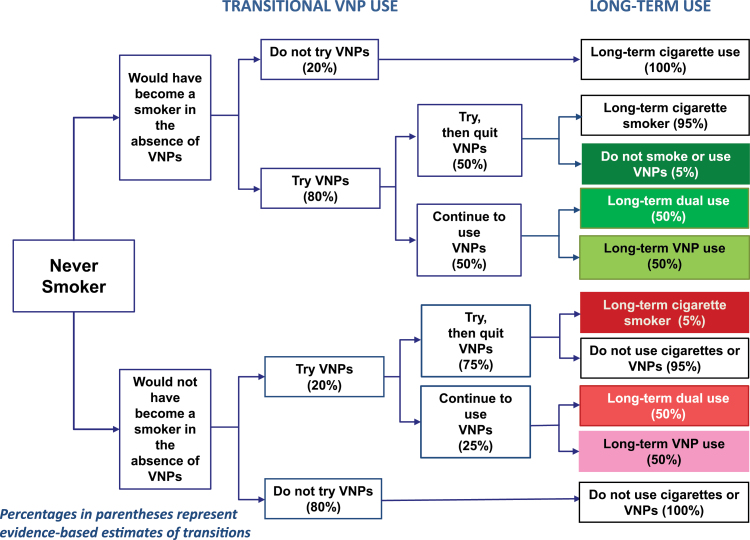
Potential transitions and public health impacts are distinguished relative to the No-VNP scenario of otherwise smokers (ie, those who would have become smokers without VNPs being on the market) and otherwise nonsmokers. As shown in Figure 1, green endpoints indicate harm-reducing and red endpoints indicating harm-increasing endpoints. VNP use is harm-reducing for those who would have otherwise smoked and instead become exclusive or dual VNP users, or use and then quit VNPs without smoking. Harm is increased for those who would not have otherwise smoked cigarettes and transition to long-term cigarette use as a direct result of VNP use or transition to established exclusive VNP cigarette or dual use.
Figure 1.
Transitional vaporized nicotine product (VNP) use. Percentages in parentheses represent evidence-based estimates of transitions.
To distinguish public health implications, we differentiate transitions by those who would have otherwise initiated smoking at each age from those who would have remained nonsmokers. To avoid double counting of those individuals who would not have otherwise have smoked, we use the smoking prevalence at the peak age (age 25 with smoking prevalence 19%) in the No-VNP model to determine the population of nonsmokers who engage in trial use.
We assume that individuals progress from trial use into a single state of exclusive cigarette, exclusive VNP, dual, or no use. While this assumption is made for model tractability, transitions to and from smoking abstinence are rarely smooth; many experiment with a product and do not transition to established use, or have periods of experimentation alternating with periods of use before transitioning to a stable state.43,44 The model could be extended to incorporate multiple transitions, but the resultant use rates are critical, rather than the details of how they occur. After age 25, cessation of exclusive cigarette, VNP, and dual use is assumed to occur at the rate of smoking cessation under the No-VNP scenario. However, we consider cases where cessation rates of dual and/or exclusive smokers increase (eg, due to VNP availability) or decrease.
Assumptions and their justifications are shown in Supplementary Table 1.
“Base Case” VNP Scenario
The transitions in Figure 1 under the VNP scenario were developed using estimates from recent literature. We chose estimates that imply more conservative effects (ie, outcomes that yield less harm reduction), with the percent following each path shown in Figure 1 and described in Supplementary Table 2.
In a large 2014 nationally representative US sample of 17- to 18-year-old students,42 7% of those who never smoked had used an e-cigarette in the last month compared to about 50% of those who had regularly or occasionally smoked. In other US studies, the odds of current youth e-cigarette use by smokers was 7.9 compared to nonsmokers,45 young adult current e-cigarette use was 31% among smokers compared to 10% among former smokers and 2% among never smokers,46 and the odds of college student e-cigarette use relative to never smokers was 3.1 for ever smokers and 6.6 for current smokers.47 VNP users have also been found to be those who are more susceptible to smoking.48–50 Based on the combined use of youth and young adults, we estimate that 80% of otherwise smokers try VNPs and 20% of otherwise nonsmokers try VNPs, a 4:1 ratio.
Of 17- to 18-year-old past month e-cigarette users,42 32% of never smokers used e-cigarettes 6 or more days compared to 50% among those who occasionally or regularly smoked cigarettes. Similar results were found in a recent study of 17–18 year olds51 and studies of adults.46,52,53 While these studies do not distinguish otherwise smokers from otherwise nonsmokers, VNP users have been found to have similar profiles to cigarette smokers (eg, common risk behaviors47,54,55 and higher rates of executive function deficits, parental and peer cigarette use56). Based on these studies, particularly the large scale 2014 sample of 17–18 year olds,42 we estimate that 50% of smokers and 25% of nonsmokers who try VNPs progress to established use, implying that 40% (80% * 50%) of all smokers and 5% (20% * 25%) of nonsmokers become established VNP users, an 8:1 ratio.
Of 17–18 year olds, 24% of those currently smoking at least half a pack per day used VNPs 6 or more days compared to 31% of never smokers.42 Another study54 obtained a prevalence of 17% for exclusive VNP use compared to 12% for dual use, with both group exhibiting similar risk factors to exclusive cigarette users. Among adults who used VNPs in the last month, one study52 found that 41% of current, but 51% of former, smokers were regular users, and another17 found close to 70% of recent former smokers were daily users compared to about 23% of current smokers and less than 1% of never smokers. Since none of the studies indicated that more than 50% of regular users were current smokers and the studies do not provide evidence to explicitly distinguish otherwise smokers from otherwise nonsmokers, we estimate that established VNP users are split between dual users and exclusive VNP users for both otherwise smokers and otherwise nonsmokers.
In the absence of evidence on VNP users who do not continue to established use, we estimate that 5% of otherwise nonsmokers become smokers and 5% of otherwise smokers become nonsmokers. Among those not trying VNPs, we assume no change in status among otherwise smokers and nonsmokers.
We consider variations for each of the potential transitions by otherwise smokers and otherwise nonsmokers in the VNP base case to gauge sensitivity. Holding all other parameters constant, we consider trial use rates among otherwise smokers to vary between 0% and 100%. We then individually consider variations for trial use rates among otherwise smokers holding all other parameters constant. Similarly, we consider transitions from trial to established use and transitions from established use to dual use for both otherwise nonsmokers and otherwise smokers.
Health Outcomes
All-cause cohort life tables by age, cohort, and sex were calculated by cigarette smoking status (never, former, and current) using mortality relative risk estimates by sex and smoking status derived from the American Cancer Society Cancer Prevention Studies I and II partitioned by smoking status.38 Excess risk was calculated by age and gender as the mortality rate for smokers—mortality rate for never smokers and similarly for former smokers.
Although the long-term implications are not yet known and will likely vary by product,3,57–59 VNPs contain substantially lower levels of toxic substances59–62 and are thus likely to be much lower risk than cigarettes.3–5 A multidecision analysis estimated that exclusive VNP use is associated with 5% of the risks of exclusive cigarette use,5 similar to the risks of low-nitrosamine smokeless tobacco (snus) use.63 Since lung cancer and chronic obstructive pulmonary disease risk are sensitive to smoking duration and intensity,64,65 dual users may have lower mortality risk than exclusive cigarette smokers if VNP use delays smoking initiation or reduces the average quantity smoked. While some studies of VNP use report reductions in cigarette use of more than 50%,9,66–70 others indicate smaller reductions, especially among nondaily VNP users.71,72 A review found 75%–80% lower cigarette consumption among dual cigarette and snus users than among exclusive smokers.73
We estimate that among those who are exclusive VNP users the excess health risk is 5% whereas among dual users of cigarettes and VNPs the excess risk is 70% of cigarette only users. We also consider risks at 2.5% and 50% (low), 10% and 85% (medium), and 25% and 100% (high) for exclusive and dual use, respectively. Excess risks for former dual and exclusive VNP users are assumed to decline by the same percentages as applied to the difference in risk between current and former smokers.
Calculation of Lives and Life Years Lost
For the No-VNP scenario, the number of smoking-attributable deaths (SADs) is calculated for current and former smokers for each age in the 1997 cohort as the product of excess risks and the corresponding population (projected US population74 multiplied by the prevalence rate). The number of life years lost (LYL) at each age is estimated as the product of number of premature deaths and the expected years of life remaining for a never smoker. For each VNP scenario, SADs and LYL are calculated for current and former exclusive smokers, exclusive VNP users and dual users, at each age and then summed. The public health impact of VNP use is measured by the difference in SADs and LYL under the No-VNP and VNP scenario.
Results
No-VNP Versus VNP Scenarios
Table 1 contains male and female smoking and VNP prevalence, smoking-attributable deaths and LYL under the No-VNP scenario and the evidence-informed VNP scenarios. The 1997 cohort at age 15 years in 2012 includes 2 118 400 males and 2 025 700 females (Supplementary Table 3).
Table 1.
Male and Female Smoking and Vaporized Nicotine Product (VNP) Prevalence, Smoking-attributable Deaths, and Life Years Lost, Under No-VNP and Base VNP Scenario, By Risk Levels and With Cessation Rate Effects
| Age | 15 years | 25 years | 45 years | 65 years | 85 years | Cumulative: Age 15–85 years | Reduced LYL and SADsa | % Gaina | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Male | ||||||||||
| No-VNP scenario | Prevalence | Smoker | 4.6% | 20.4% | 12.7% | 5.6% | 1.1% | |||
| SADs | — | — | 581 | 2116 | 2816 | 79 322 | ||||
| LYL | — | — | 23 573 | 46 335 | 16 706 | 1 539 242 | ||||
| VNP best estimate risk | Prevalence | Smoker | 2.8% | 12.4% | 7.7% | 3.4% | 0.6% | |||
| VNP | 1.3% | 5.9% | 3.7% | 1.6% | 0.3% | |||||
| Dual | 1.3% | 5.9% | 3.7% | 1.6% | 0.3% | |||||
| SADs | — | — | 480 | 1653 | 2041 | 61 058 | 18 264 | 23.0% | ||
| LYL | — | — | 19 465 | 36 184 | 12 108 | 1 208 000 | 331 242 | 21.5% | ||
| Variation in levels of VNP and dual risks | ||||||||||
| Low risk | SADs | — | — | 442 | 1522 | 1879 | 56 213 | 23 109 | 29.1% | |
| LYL | — | — | 17 921 | 33 313 | 11 147 | 1 112 151 | 427 091 | 27.7% | ||
| Medium risk | SADs | — | — | 514 | 1769 | 2185 | 65 365 | 13 958 | 17.6% | |
| LYL | — | — | 20 838 | 38 736 | 12 962 | 1 293 200 | 246 042 | 16.0% | ||
| High risk | SADs | — | — | 565 | 1944 | 2401 | 71 824 | 7498 | 9.5% | |
| LYL | — | — | 22 898 | 42 564 | 14 243 | 1 421 000 | 118 242 | 7.7% | ||
| Changes in cessation rate with best estimate risks | ||||||||||
| 10% Decrease smoker only | SADs | — | — | 481 | 1656 | 2046 | 61 187 | 18 135 | 22.9% | |
| LYL | — | — | 19 507 | 36 255 | 12 138 | 1 210 475 | 328 766 | 21.4% | ||
| 10% Decrease smoker and dual user | SADs | — | — | 481 | 1657 | 2047 | 61 221 | 18 101 | 22.8% | |
| LYL | — | — | 19 521 | 36 275 | 12 143 | 1 211 182 | 328 060 | 21.3% | ||
| 10% Increase Smoker only | SADs | — | — | 479 | 1650 | 2039 | 60 962 | 18 360 | 23.1% | |
| LYL | — | — | 19 425 | 36 124 | 12 095 | 1 205 958 | 333 284 | 21.7% | ||
| 10% Increase smoker and dual user | SADs | — | — | 479 | 1649 | 2038 | 60 928 | 18 395 | 23.2% | |
| LYL | — | — | 19 410 | 36 103 | 12 090 | 1 205 231 | 334 011 | 21.7% | ||
| Female | ||||||||||
| No-VNP scenario | Prevalence | Smoker | 2.7% | 14.3% | 8.8% | 3.5% | 0.5% | |||
| SADs | — | — | 66 | 609 | 987 | 21 609 | ||||
| LYL | — | — | 2844 | 14 635 | 6955 | 419 076 | ||||
| VNP best estimate risk | Prevalence | Smoker | 1.7% | 8.9% | 5.5% | 2.2% | 0.3% | |||
| VNP | 0.9% | 4.9% | 3.0% | 1.2% | 0.2% | |||||
| Dual | 0.9% | 4.9% | 3.0% | 1.2% | 0.2% | |||||
| SADs | — | — | 58 | 517 | 726 | 17 561 | 4048 | 18.7% | ||
| LYL | — | — | 2502 | 12 432 | 5111 | 349 551 | 69 525 | 16.6% | ||
| Variation in levels of VNP and dual risks | ||||||||||
| Low risk | SADs | — | — | 53 | 472 | 662 | 16 023 | 5587 | 25.9% | |
| LYL | — | — | 2283 | 11 343 | 4663 | 318 929 | 100 147 | 23.9% | ||
| Medium risk | SADs | — | — | 62 | 557 | 782 | 18 929 | 2681 | 12.4% | |
| LYL | — | — | 2696 | 13 401 | 5508 | 376 771 | 42 306 | 10.1% | ||
| High risk | SADs | — | — | 69 | 618 | 867 | 20 980 | 629 | 2.9% | |
| LYL | — | — | 2989 | 14 853 | 6105 | 417 600 | 1476 | 0.4% | ||
| Changes in cessation rate with best estimate risks | ||||||||||
| 10% Decrease smoker only | SADs | — | — | 58 | 519 | 728 | 17 616 | 3994 | 18.4% | |
| LYL | — | — | 2507 | 12471 | 5128 | 350 585 | 68 491 | 16.1% | ||
| 10% Decrease smoker and dual user | SADs | — | — | 58 | 519 | 729 | 17 634 | 3975 | 18.3% | |
| LYL | — | — | 2509 | 12 485 | 5132 | 350 950 | 68 126 | 16.0% | ||
| 10% Increase smoker only | SADs | — | — | 58 | 516 | 724 | 17 516 | 4093 | 18.9% | |
| LYL | — | — | 2497 | 12 397 | 5101 | 348 638 | 70 438 | 16.6% | ||
| 10% Increase smoker and dual user | SADs | — | — | 58 | 515 | 724 | 17 499 | 4111 | 19.0% | |
| LYL | — | — | 2495 | 12 383 | 5097 | 348 291 | 70 786 | 16.7% | ||
LYL = life years lost; SADs = smoking-attributable deaths.
aReduced life years lost and reduced SADs are measured in terms of the difference from the no-VNP scenario.
Under the No-VNP scenario, male smoking prevalence at age 15 is 4.6% increasing to 20.4% at age 25, and declining to 5.6% at age 65, while female prevalence at age 15 is 2.7% increasing to 14.3% at age 25, and declining to 3.5% at age 65. A cumulative total of 79 300 SADs and 1 539 200 LYL are estimated for males and 21 600 SADs and 419 100 LYL for females, a total of 100 900 deaths and 1 958 300 LYL from smoking in the 1997 birth cohort.
Under the VNP base case scenario, exclusive smoking is reduced by age 25 to 12.4% for males and 8.9% for females, but is offset through increased dual (5.9% males and 4.9% for females) and exclusive VNP dual (male 5.9% and female 4.9%) use. The cumulative loss through age 85 is 61 000 SADs and 1 208 000 LYL for males and 17 500 SADs and 350 000 LYL for females. Compared to the No-VNP scenario, a net public health gain of 23% fewer male and 19% fewer female SADs and 21.5% fewer male and 16.6% fewer female LYLs are projected. With low VNP mortality risks, 28% fewer SADs and 26% fewer LYL are projected. The gains decrease to 15% fewer SADs and 14% fewer LYL with medium risks and 6% fewer SADs and 5% fewer LYL with high risks.
If cessation rates are reduced by 10% in later years (Table 1 and Supplementary Table 4), male LYL increase by 2475 (−0.20% lower than with no effect on cessation for the evidence-informed risk estimates) if only smokers are affected and by 3181 (0.26%) if dual users are also affected. A 10% cessation increase yields a male life year gain of 2042 (0.17) if only affecting smokers and 2769 (0.23%) if also affecting dual users. Changes for females are slightly larger, as much as 0.40%.
Sensitivity Analysis
The sensitivity of results is assessed by changing individual parameters while holding constant other parameters at the base VNP levels. Parameter sensitivity is gauged by the variability (from 0% to 100%) in combined male and female LYL as that parameter changes. Gender variations are shown in Supplementary Figures 1–3 and in Supplementary Table 5.
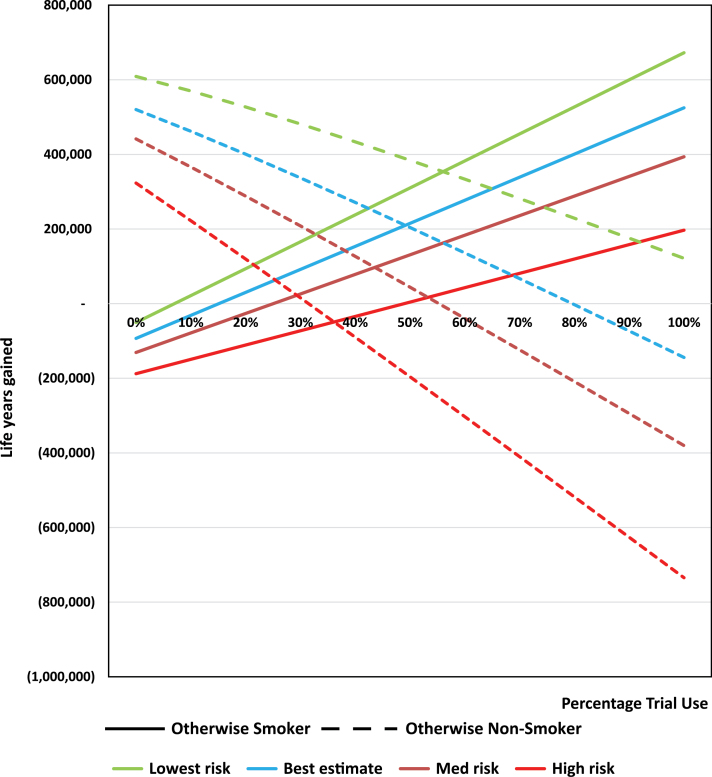
Holding constant the other parameters (including use by otherwise nonsmokers and rates of exclusive and dual VNP use and established use rates), the public health impact on otherwise smokers (Figure 2) for trial use increased from 93 000 LYL at 0% use to a net gain of 525 000 life years gained at 100% use, with breakeven where VNP trial use equals 15%. At less than 15% trial use by otherwise smokers, the LYL due to use by otherwise nonsmokers (held constant at the evidence-informed level) dominates. For otherwise nonsmokers, the public health impact declines from 519 000 life years gained to 134 000 LYL, with breakeven when VNP trial use reaches 80%. Thus, 80% of otherwise nonsmokers would be required to seriously experiment with VNPs for overall public health harm with our best estimate of risks, and 30% of otherwise nonsmokers would be required to seriously experiment with VNPs for the high level of risks. Notably, as gauged by absolute value of their slopes between 0% and 100%, the public health impact is less sensitive among otherwise nonsmokers than otherwise smokers at the lower levels of risk, but becomes less sensitive at the higher levels of VNP risk, as expected.
Figure 2.
Vaporized nicotine product (VNP) trial use sensitivity analysis among never smokers. Sensitivity analysis is conducted holding other use parameters constant, including trial and dual use.
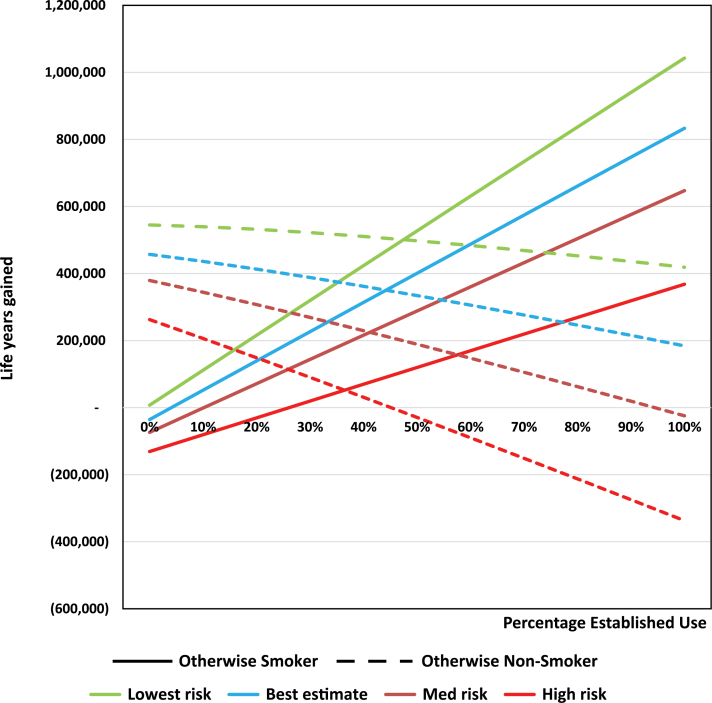
Increasing established use (Figure 3) also increases the gain among otherwise smokers while decreasing the gain among otherwise nonsmokers. Holding constant the other parameters, LYL is 10 000 at 0% established use by those otherwise smokers who try VNPs and reaches over 800 000 life years gained at 100% established use. Breakeven from LYL is at or near zero at the two lower levels of VNP risk, and at 10% for the medium and 25% at the high level of VNP risk. The tipping point with for otherwise nonsmokers is at or near 100% for the three lower levels of risk, and at 45% for the high risk level. The sensitivity to use levels is greater among otherwise smokers than among otherwise nonsmokers at lower risks, but reverses at higher risks.
Figure 3.
Vaporized nicotine product (VNP) established use sensitivity analysis among never smokers. Sensitivity analysis is conducted holding other use parameters constant, including established and dual use.
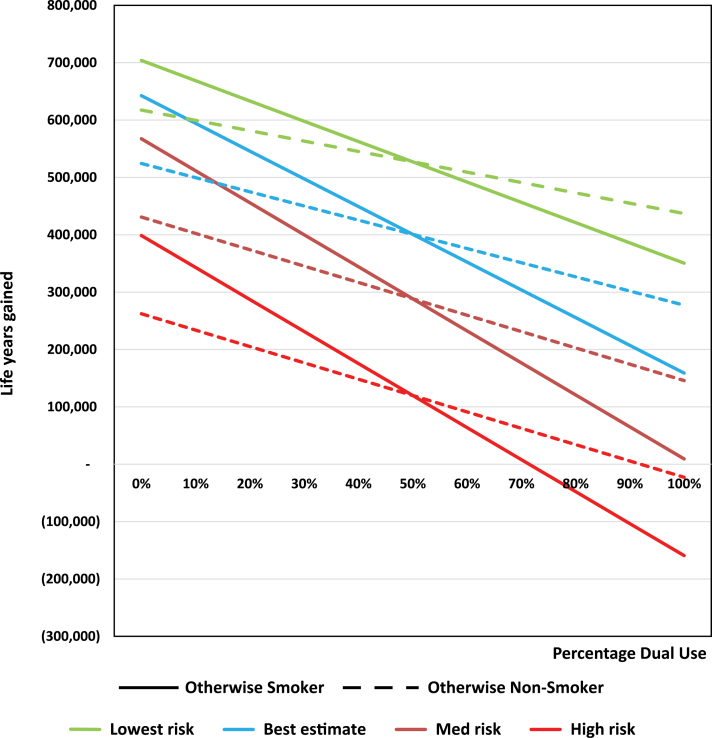
Figure 4 shows that the LYL as dual use among established users increases from 0% to 100% while exclusive VNP use concurrently declines from 100% to 0%. As expected, increasing dual use leads to decreasing health gains from VNP use among both otherwise smokers and otherwise nonsmokers. The sensitivity among otherwise smokers as measured by the absolute value of the slope increases at higher risk levels, but changes little for otherwise nonsmokers. Among otherwise smokers, the tipping point to LYL is slightly less than 100% dual use (ie, no exclusive VNP use) for medium risk and at 75% for high risk. Among otherwise nonsmokers with all other levels of use held constant, public harm (negative life years gained) is projected as dual use increases above 50% for the highest level of risk.
Figure 4.
Dual versus exclusive vaporized nicotine product (VNP) use sensitivity analysis among never smokers. Sensitivity analysis is conducted holding other use parameters constant, including trial and established use.
Discussion
The VNP decision-theoretic model of initiation shows that, while vaping leads to some VNP use and smoking by those who would not have otherwise smoked, the negative public health impact may be offset by the benefits from greater use of VNPs among otherwise smokers who smoke less or not at all. Under evidence-based estimates based on current use patterns, we estimate that 21% of SADs and 20% of LYL would be averted as a result of VNP use for a single cohort. Moreover, public health gains are estimated over a wide range of plausible parameters and use rates. A large proportion of VNP initiation leading directly to more cigarette smoking by otherwise never smokers or an increase in the magnitude of harms from VNPs relative to cigarettes (or both) would be required before a tipping point is reached where harms begin to exceed benefits at the population level.
While the high VNP risk estimates imply less health gains from VNP use, VNPs are likely to be increasingly regulated for quality control, thereby reducing levels and variability in risks. We expect that the highest risk estimates that we modeled are unlikely. Regulations that reduce risks and accurately communicate ingredients and nicotine levels in VNPs can be expected to encourage switching to exclusive VNP use and thus reduce the harms associated with smoking. A regulatory balance is needed that protects the public from avoidable harms while at the same time allowing for innovation in VNPs that encourage the substitution of VNPs for cigarettes.
Sensitivity analysis also showed that those with a higher propensity to smoke (“otherwise smokers”) are especially sensitive to VNP use rates, suggesting the need for stronger tobacco control efforts to discourage combustible use (eg, increase cigarette and cigar taxes, higher minimum purchase age laws,75 targeting combustible use in prevention and cessation media campaigns, and stricter smoke-free air laws). These policies would not only discourage transitions to combustible use but encourage switching to exclusive VNP use by those who otherwise cannot or will not quit combustible product use at early ages. Nevertheless, policies are needed to discourage youth initiation, especially by otherwise nonsmokers into any tobacco or VNP use. These policies may include higher and better enforced minimum purchase age laws marketing restrictions and VNP taxes although proportional to harms of combustible use.75 , 76
Because of our focus on initiation and the recent cohort analyzed, the effects of VNPs through later cessation, as expected, played a minimal role. By assumption, the transitions to VNP use occurred largely at earlier ages. For older cohorts that have initiated smoking before VNPs were more widely used (eg, those aged 26 or older in 2012), VNPs can potentially play a key role in encouraging smoking cessation; smokers who began smoking before VNPs were readily available can benefit from switching to exclusive VNP use, especially at younger ages when cessation from cigarettes is typically low77 and health benefits from cessation are high.78
Thus, a careful balance must be struck that maximizes potential benefits by encouraging VNP use among otherwise smokers while minimizing harms to those who would not have otherwise smoked or who would have otherwise quit.1,2 Given the disproportionate power of the tobacco industry in this market space, there is a strong need for clear and accurate education of the public about the relative harms of each class of products and what behaviors substantially reduce or eliminate those harms. Ongoing longitudinal postmarket surveillance is needed to detect early warning signs of unintended negative consequences especially at early ages.
The decision-theoretic model has several limitations. As with all models of this nature, the projected public health gains depend on assumptions and the parameter estimates chosen for the model. While public health gains depend on the parameters chosen for the model, our aim was to apply conservative estimates of use by otherwise smokers and otherwise nonsmokers. For example, a recent meta-analysis79 found that current smokers had 15 times the odds of current e-cigarette use compared to nonsmokers, increasing to 39 times the odds for adolescents. We also estimated that 25% of otherwise nonsmokers progressed to established VNP use and 50% of those became dual users, both of which we expect are overly pessimistic estimates.80
The empirical estimates we have used to inform the model are from the early stages in the use of these products. It is likely that current estimates underestimate eventual use, but short of a consensus estimate of likely trends, we have taken the conservative approach of using the most current data. If usage declines, then the estimates of benefits will also decline.
We considered cessation by the 1989 cohort, but we limited our analysis to changes in cessation at later ages among dual and exclusive VNP users. Similar effects may be expected among cigarette smokers, suggesting that the estimated gains from VNP use may be understated. In addition, we have assumed a single transition to the final state. In measuring transitions to established use, it will be important for future studies to allow for the possibility that transitions, for example, from dual use to exclusive VNP use, may occur later in life.
The use of other tobacco products, such as cigars, water pipe, and smokeless tobacco, may also influence the patterns of VNP and cigarette use. Cigar, smokeless tobacco, and hookah use has increased in recent years,2 especially among youth.16,18 However, unless long-term use of these products markedly increases, we expect that inclusion of these tobacco products is unlikely to substantially change the results.
Results of our model should not be strictly compared to others that address e-cigarette use.34–36 The various models that have been developed so far differ in their structure, population focus, and modeling methods. Whereas we focused on a specific age cohort, other models34,36 have attempted to generalize to the entire US population. In addition, we model a process dependent on whether the individual is an otherwise smoker or otherwise nonsmoker, while other models have focused on behavioral transitions between tobacco product use states. Nevertheless, our results are broadly consistent with the models of Cobb et al.35 and Cherng et al.36 The differences from Kalkhoran and Glantz model34 appear to result from their higher rates of initiation into smoking implied by VNP use and higher levels of VNP risks.
The model highlights which transitions will need to be studied more closely to more accurately determine the effects of VNPs and evaluate policies affecting VNPs. The initial branch in Figure 1 leads to hypothetical states which cannot be observed and are inferred from past smoking patterns. While some studies have examined the relationship of VNP use to smoking,45,81,82 these have been cross-sectional or relatively short-term and alternative explanations, such as shared common vulnerabilities and other confounders, cannot be omitted.31,83,84 Needed are better measures of trial use taking into account variations in the cigarette-oriented and VNP-oriented policies that are in place.
In conclusion, our analysis suggests the likelihood of public health gains due to VNP use at early ages under most plausible scenarios, and sensitivity analysis provides an indication of tipping points where harms will exceed benefits. The possibility of public health harms exceeding benefits must be carefully monitored via national longitudinal surveillance in order to protect public health via prudent regulation and timely policymaking. Better information is needed for proven measures of trial and established use and on the long-term trajectories of those who try VNPs in terms of whether they would have otherwise initiated or continued to smoke. As better information becomes available, the model presented here can be updated and should be able to provide more accurate estimates of the public health impact of VNP use.
Supplementary Data
Supplementary Tables 1–5 and Supplementary Figures 1–3 can be found online at http://www.ntr.oxfordjournals.org
Funding
Funding was received by DTL, DBA, RN, and ACV from the National Institute on Drug Abuse under grant R01DA036497. TRH and RM received funding from the Cancer Intervention and Surveillance Modeling Network (CISNET) of the Division of Cancer Control and Population Sciences, NCI under grant UO1-CA97450. RB, KMC, GTF, and DTL received funding from the National Cancer Institute under grant P01-CA200512.
Declaration of Interests
KMC has received grant funding from the Pfizer, Inc, to study the impact of a hospital-based tobacco cessation intervention, and has received funding as an expert witness in litigation filed against the tobacco industry. No other conflicts of interest are declared.
Acknowledgments
We thank Eric Lindblom, Georgetown University Law School, for comments on an earlier draft.
References
- 1. Abrams DB. Promise and peril of e-cigarettes: can disruptive technology make cigarettes obsolete? JAMA. 2014;311(2):135–139. doi:10.1001/jama.2013.285347. [DOI] [PubMed] [Google Scholar]
- 2. National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. The Health Consequences of Smoking—50 Years of Progress. A Report of the Surgeon General. Atlanta, GA: Centers for Disease Control and Prevention; 2014. [PubMed] [Google Scholar]
- 3. Goniewicz ML, Knysak J, Gawron M, et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control. 2014;23(2):133–139. doi:10.1136/tobaccocontrol-2012–050859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pellegrino RM, Tinghino B, Mangiaracina G, et al. Electronic cigarettes: an evaluation of exposure to chemicals and fine particulate matter (PM). Ann Ig. 2012;24(4):279–288. [PubMed] [Google Scholar]
- 5. Nutt DJ, Phillips LD, Balfour D, et al. Estimating the harms of nicotine-containing products using the MCDA approach. Eur Addict Res. 2014;20(5):218–225. doi:10.1159/000360220. [DOI] [PubMed] [Google Scholar]
- 6. Pepper JK, Emery SL, Ribisl KM, et al. How risky is it to use e-cigarettes? Smokers’ beliefs about their health risks from using novel and traditional tobacco products. J Behav Med. 2015;38(2):318–326. doi:10.1007/s10865-014-9605-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wackowski OA, Delnevo CD. Young adults’ risk perceptions of various tobacco products relative to cigarettes: results from the National Young Adult Health Survey. Health Educ Beh. 2016;43(3):328–336. doi:10.1177/1090198115599988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhu SH, Gamst A, Lee M, et al. The use and perception of electronic cigarettes and snus among the U.S. population. PloS One. 2013;8(10):e79332. doi:10.1371/journal.pone.0079332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Adkison SE, O’Connor RJ, Bansal-Travers M, et al. Electronic nicotine delivery systems: international tobacco control four-country survey. Am J Prev Med. 2013;44(3):207–215. doi:10.1016/j.amepre. 2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schroeder MJ, Hoffman AC. Electronic cigarettes and nicotine clinical pharmacology. Tob Control. 2014;23(suppl 2):ii30–ii5. doi:10.1136/tobaccocontrol-2013-051469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Spindle TR, Breland AB, Karaoghlanian NV, et al. Preliminary results of an examination of electronic cigarette user puff topography: the effect of a mouthpiece-based topography measurement device on plasma nicotine and subjective effects. Nicotine Tob Res. 2015;17(2):142–149. doi:10.1093/ntr/ntu186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dawkins L, Kimber C, Panwanesarasa Y, et al. First versus second generation electronic cigarettes: predictors of choice and effects on urge to smoke and withdrawal symptoms. Addiction. 2014;110(4):669–677. doi:10.1111/add.12807. [DOI] [PubMed] [Google Scholar]
- 13. Hajek P, Goniewicz ML, Phillips A, et al. Nicotine intake from electronic cigarettes on initial use and after four weeks of regular use. Nicotine Tob Res. 2015;17(2):175–179. doi:10.1093/ntr/ntu153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. King BA, Patel R, Nguyen K, et al. Trends in awareness and use of electronic cigarettes among U.S. adults, 2010–2013. Nicotine Tob Res. 2015;17(2):219–227. doi:10.1093/ntr/ntu191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McMillen RC, Gottlieb MA, Shaefer RM, et al. Trends in electronic cigarette use among U.S. adults: use is increasing in both smokers and nonsmokers. Nicotine Tob Res. 2015;17(10):1195–1202. doi:10.1093/ntr/ntu213. [DOI] [PubMed] [Google Scholar]
- 16. Arrazola RA, Singh T, Corey CG, et al. Tobacco use among middle and high school students—United States, 2011–2014. Morb Mortal Wkly Rep. 2015;64(14):381–385. [PMC free article] [PubMed] [Google Scholar]
- 17. Delnevo CD, Giovenco DP, Steinberg MB, et al. Patterns of electronic cigarette use among adults in the United States. Nicotine Tob Res. 2016;18(5):715–719. doi:10.1093/ntr/ntv237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Johnston LD, O’Malley PM, Miech RA, et al. Monitoring the Future National Survey Results on Drug Use, 1975–2015: Overview, Key Findings on Adolescent Drug Use. Ann Arbor, MI: Institute for Social Research, The University of Michigan; 2016. [Google Scholar]
- 19. Blaser J, Cornuz J. Experts’ consensus on use of electronic cigarettes: a Delphi survey from Switzerland. BMJ Open. 2015;5(4):e007197. doi:10.1136/bmjopen-2014-007197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brandon TH, Goniewicz ML, Hanna NH, et al. Electronic nicotine delivery systems: a policy statement from the American Association for Cancer Research and the American Society of Clinical Oncology. Clin Cancer Res. 2015;21(3):514–525. doi:10.1158/1078-0432.CCR-14–2544. [DOI] [PubMed] [Google Scholar]
- 21. Grana R, Benowitz N, Glantz SA. E-cigarettes: a scientific review. Circulation. 2014;129(19):1972–1986. doi:10.1161/CIRCULATIONAHA.114.007667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kaur J, Rinkoo AV. A call for an urgent ban on E-cigarettes in India-a race against time. Glob Health Promot. 2015;22(2):71–74. doi:10.1177/1757975914537322. [DOI] [PubMed] [Google Scholar]
- 23. Paradise J. Electronic cigarettes: smoke-free laws, sale restrictions, and the public health. Am J Pub Health. 2014;104(6):e17–e18. doi:10.2105/ajph.2014.301890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Johns Hopkins Bloomberg School of Public Health. Country Laws Regulating E-cigarettes: A Policy Scan [database on the Internet]. May 2015. http://globaltobaccocontrol.org/e-cigarette/country-laws-regulating-e-cigarettes Accessed October 20, 2015. [Google Scholar]
- 25. Federal Register. Deeming Tobacco Products to Be Subject to the Federal Food, Drug, and Cosmetic Act, as Amended by the Family Smoking Prevention and Tobacco Control Act; Regulations on the Sale and Distribution of Tobacco Products and Required Warning Statements for Tobacco Products. 2014. www.federalregister.gov/articles/2014/04/25/2014–09491/deeming-tobacco-products-to-be-subject-to-the-federal-food-drug-and-cosmetic-act-as-amended-by-the#h-39 Accessed May 15, 2014. [PubMed] [Google Scholar]
- 26. Sweanor D, Yach D. Looking for the next breakthrough in tobacco control and health. S Afr Med J. 2013;103(11):810–811. doi:10.7196/samj.7513. [DOI] [PubMed] [Google Scholar]
- 27. McKee M, Chapman S, Daube M, et al. The debate on electronic cigarettes. Lancet. 2014;384(9960):2107. doi:10.1016/s0140-6736(14)62366-7. [DOI] [PubMed] [Google Scholar]
- 28. Fairchild AL, Bayer R, Colgrove J. The renormalization of smoking? E-cigarettes and the tobacco “endgame”. N Engl J Med. 2014;370:293–295. doi:10.1056/NEJMp1313940. [DOI] [PubMed] [Google Scholar]
- 29. Section On Tobacco Control. Electronic nicotine delivery systems. Pediatrics. 2015;136(5):1018–1026. doi:10.1542/peds.2015–3222. [DOI] [PubMed] [Google Scholar]
- 30. Wasowicz A, Feleszko W, Goniewicz ML. E-cigarette use among children and young people: the need for regulation. Expert Rev Respir Med. 2015;9(5):507–509. doi:10.1586/17476348.2015.1077120. [DOI] [PubMed] [Google Scholar]
- 31. Niaura RS, Glynn TJ, Abrams DB. Youth experimentation with e-cigarettes: another interpretation of the data. JAMA Pediatr. 2014;312(6):1–2. [DOI] [PubMed] [Google Scholar]
- 32. Vanyukov MM, Tarter RE, Kirillova GP, et al. Common liability to addiction and “gateway hypothesis”: theoretical, empirical and evolutionary perspective. Drug Alcohol Depend. 2012;123(suppl 1):S3–S17. doi:10.1016/j.drugalcdep.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kalkhoran S, Glantz SA. E-cigarettes and smoking cessation in real-world and clinical settings: a systematic review and meta-analysis. Lancet Respir Med. 2016;4(2):116–128. doi:10.1016/S2213-2600(15)00521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kalkhoran S, Glantz SA. Modeling the health effects of expanding e-cigarette sales in the United States and United Kingdom: a Monte Carlo analysis. JAMA Internal Med. 2015;175(10):1671–1680. doi:10.1001/jamainternmed.2015.4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cobb CO, Villanti AC, Graham AL, et al. Markov modeling to estimate the population impact of emerging tobacco products: A proof-of-concept study. Tob Reg Sci. 2015;1(2):129–141. [Google Scholar]
- 36. Cherng ST, Tam J, Christine PJ, Meza R. Modeling the effects of e-cigarettes on smoking behavior: implications for future adult smoking prevalence [published online ahead of print April 18, 2016]. Epidemiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Holford TR, Levy DT, McKay LA, et al. Patterns of birth cohort–specific smoking histories, 1965–2009. Am J Prev Med. 2014;46(2):e31–e37. doi:10.1016/j.amepre.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Holford TR, Meza R, Warner KE, et al. Tobacco control and the reduction in smoking-related premature deaths in the United States, 1964–2012. JAMA. 2014;311(2):164–171. doi:10.1001/jama.2013.285112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. USDHHS. Preventing Tobacco Use Among Youth and Young Adults: A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2012. [Google Scholar]
- 40. Pirie K, Peto R, Reeves GK, et al. The 21st century hazards of smoking and benefits of stopping: a prospective study of one million women in the UK. Lancet. 2013; 381(9861):133–141. doi:10.1016/S0140-6736(12)61720-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Warner KE. The remarkable decrease in cigarette smoking by American youth: further evidence. Prev Med Rep. 2015;2:259–261. doi:10.1016/j.pmedr.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Warner KE. Frequency of e-cigarette use and cigarette smoking by American Students in 2014 [published online ahead of print January 25, 2016]. Am J Prev Med. doi:10.1016/j.amepre.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 43. Borland R. Understanding Hard to Maintain Behaviour Change: A Dual-process Approach. Oxford: Wiley-Blackwell; 2004. [Google Scholar]
- 44. Partos TR, Borland R, Thrasher JF, et al. The predictive utility of micro indicators of concern about smoking: findings from the International Tobacco Control Four Country study. Addict Behav. 2014;39(8):1235–1242. doi:10.1016/j.addbeh.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dutra LM, Glantz SA. Electronic cigarettes and conventional cigarette use among US adolescents: a cross-sectional study. JAMA Pediatr. 2014;168(7):610–617. doi:10.1001/jamapediatrics.2013.5488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Biener L, Hargraves JL. A longitudinal study of electronic cigarette use among a population-based sample of adult smokers: association with smoking cessation and motivation to quit. Nic Tob Res. 2015;17(2):127–133. doi:10.1093/ntr/ntu200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Saddleson ML, Kozlowski LT, Giovino GA, et al. Risky behaviors, e-cigarette use and susceptibility of use among college students. Drug Alc Depend. 2015;149:25–30. doi:10.1016/j.drugalcdep.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 48. Bunnell RE, Agaku IT, Arrazola RA, et al. Intentions to smoke cigarettes among never-smoking US middle and high school electronic cigarette users: National Youth Tobacco Survey, 2011–2013. Nicotine Tob Res. 2015;17(2):228–235. doi:10.1093/ntr/ntu166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Coleman BN, Apelberg BJ, Ambrose BK, et al. Association between electronic cigarette use and openness to cigarette smoking among US young adults. Nicotine Tob Res. 2015;17(2):212–218. doi:10.1093/ntr/ntu211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wills TA, Sargent JD, Knight R, et al. E-cigarette use and willingness to smoke: a sample of adolescent nonsmokers. Tob Control. 2016;25(e1):e52–e59. doi:10.1136/tobaccocontrol-2015-052349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Neff LJ, Arrazola RA, Caraballo RS, et al. Frequency of tobacco use among middle and high school students—United States, 2014. Morb Mortal Wkly Rep. 2015;64(38):1061–1065. doi:10.15585/mmwr.mm6438a1. [DOI] [PubMed] [Google Scholar]
- 52. Amato MS, Boyle RG, Levy D. How to define e-cigarette prevalence? Finding clues in the use frequency distribution. Tob Control. 2016;25(e1):e24–e29. doi:10.1136/tobaccocontrol-2015-052236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Agaku IT, King BA, Husten CG, et al. Tobacco product use among adults—United States, 2012–2013. Morb Mortal Wkly Rep. 2014;63(25):542–547. [PMC free article] [PubMed] [Google Scholar]
- 54. Wills TA, Knight R, Williams RJ, et al. Risk factors for exclusive e-cigarette use and dual e-cigarette use and tobacco use in adolescents. Pediatrics. 2015;135(1):e43–e51. doi:10.1542/peds.2014-0760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hughes K, Bellis MA, Hardcastle KA, et al. Associations between e-cigarette access and smoking and drinking behaviours in teenagers. BMC Public Health. 2015;15:244. doi:10.1186/s12889-015-1618-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Pentz MA, Shin H, Riggs N, et al. Parent, peer, and executive function relationships to early adolescent e-cigarette use: a substance use pathway? Addict Behav. 2015;42:73–78. doi:10.1016/j.addbeh. 2014.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cameron JM, Howell DN, White JR, et al. Variable and potentially fatal amounts of nicotine in e-cigarette nicotine solutions. Tob Control. 2014;23(1):77–78. doi:10.1136/tobaccocontrol-2012–050604. [DOI] [PubMed] [Google Scholar]
- 58. Cheah NP, Chong NW, Tan J, et al. Electronic nicotine delivery systems: regulatory and safety challenges: Singapore perspective. Tob Control. 2014;23(2):119–125. doi:10.1136/tobaccocontrol-2012-050483. [DOI] [PubMed] [Google Scholar]
- 59. Goniewicz ML, Kuma T, Gawron M, et al. Nicotine levels in electronic cigarettes. Nicotine Tob Res. 2013;15(1):158–166. doi:10.1093/ntr/nts103. [DOI] [PubMed] [Google Scholar]
- 60. Goniewicz ML, Hajek P, McRobbie H. Nicotine content of electronic cigarettes, its release in vapour and its consistency across batches: regulatory implications. Addiction. 2013;109(3):500–507. doi:10.1111/add.12410. [DOI] [PubMed] [Google Scholar]
- 61. Goniewicz ML, Knysak J, Gawron M, et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control. 2014;23(2):133–139. doi:10.1136/tobaccocontrol-2012-050859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kosmider L, Knysak J, Goniewicz ML, et al. [Electronic cigarette—a safe substitute for tobacco cigarette or a new threat?]. Przeglad lekarski. 2012;69(10):1084–1089. [PubMed] [Google Scholar]
- 63. Levy DT, Mumford EA, Cummings KM, et al. The relative risks of a low-nitrosamine smokeless tobacco product compared with smoking cigarettes: estimates of a panel of experts. Cancer Epidemiol Biomarkers Prev. 2004;13(12):2035–2042. [PubMed] [Google Scholar]
- 64. Flanders WD, Lally CA, Zhu BP, et al. Lung cancer mortality in relation to age, duration of smoking, and daily cigarette consumption: results from Cancer Prevention Study II. Cancer Res. 2003;63(19):6556–6562. [PubMed] [Google Scholar]
- 65. Burns D, Garfinkel L, Samet J, eds. Changes in Cigarette-Related Disease Risks and Their Implication for Prevention and Control. Bethesda, MD: National Institutes of Health, National Cancer Institute; 1997. [Google Scholar]
- 66. Caponnetto P, Campagna D, Cibella F, et al. EffiCiency and Safety of an eLectronic cigAreTte (ECLAT) as tobacco cigarettes substitute: a prospective 12-month randomized control design study. PloS One. 2013;8(6):e66317. doi:10.1371/journal.pone.0066317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Polosa R, Caponnetto P, Morjaria JB, et al. Effect of an electronic nicotine delivery device (e-Cigarette) on smoking reduction and cessation: a prospective 6-month pilot study. BMC Pub Health. 2011;11:786. doi:10.1186/1471-2458-11-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Polosa R, Rodu B, Caponnetto P, et al. A fresh look at tobacco harm reduction: the case for the electronic cigarette. Harm Reduct J. 2013;10(1):19. doi:10.1186/1477-7517-10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Siegel MB, Tanwar KL, Wood KS. Electronic cigarettes as a smoking-cessation: tool results from an online survey. Am J Prev Med. 2011;40(4):472–475. doi:10.1016/j.amepre.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 70. Bullen C, Howe C, Laugesen M, et al. Electronic cigarettes for smoking cessation: a randomised controlled trial. Lancet. 2013;382(9905):1629–1637. doi:10.1016/s0140-6736(13)61842-5. [DOI] [PubMed] [Google Scholar]
- 71. Brown J, West R, Beard E, et al. Prevalence and characteristics of e-cigarette users in Great Britain: findings from a general population survey of smokers. Addict Behav. 2014;39(6):1120–1125. doi:10.1016/j.addbeh.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hitchman SC, McNeill A, Brose LS. Electronic cigarettes: time for an accurate and evidence-based debate. Addiction. 2014;109(6):867–868. doi:10.1111/add.12550. [DOI] [PubMed] [Google Scholar]
- 73. Lee PN. Health risks related to dual use of cigarettes and snus–a systematic review. Regul Toxicol Pharmacol. 2014;69(1):125–134. doi:10.1016/j.yrtph.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 74. U.S. Bureau of the Census. Annual Estimates of the Resident Population by Single Year of Age and Sex for the United States: April 1, 2010 to July 1, 2014, 2014. Population Estimates. http://factfinder.census.gov/faces/tableservices/jsf/pages/productview.xhtml?src=bkmk.
- 75. Institute of Medicine. Public Health Implications of Raising the Minimum Age of Legal Access to Tobacco Products. Washington, DC: National Academy of Sciences; 2015. [PubMed] [Google Scholar]
- 76. Chaloupka FJ, Sweanor D, Warner KE. Differential taxes for differential risks—toward reduced harm from nicotine-yielding products. N Engl J Med. 2015;373(7):594–597. doi:10.1056/NEJMp1505710. [DOI] [PubMed] [Google Scholar]
- 77. Levy DT, Romano E, Mumford E. The relationship of smoking cessation to sociodemographic characteristics, smoking intensity, and tobacco control policies. Nicotine Tob Res. 2005;7(3):387–396. [DOI] [PubMed] [Google Scholar]
- 78. Jha P, Ramasundarahettige C, Landsman V, et al. 21st-century hazards of smoking and benefits of cessation in the United States. N Engl J Med. 2013;368(4):341–350. doi:10.1056/NEJMsa1211128. [DOI] [PubMed] [Google Scholar]
- 79. Wang M, Wang JW, Cao SS, et al. Cigarette smoking and electronic cigarettes use: a meta-analysis. Int J Environ Res Public Health. 2016;13(1):120. doi:10.3390/ijerph13010120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Bauld L, MacKintosh AM, Ford A, et al. E-cigarette uptake amongst UK youth: experimentation, but little or no regular use in nonsmokers. Nicotine Tob Res. 2016;18(1):102–103. doi:10.1093/ntr/ntv132. [DOI] [PubMed] [Google Scholar]
- 81. Leventhal AM, Strong DR, Kirkpatrick MG, et al. Association of electronic cigarette use with initiation of combustible tobacco product smoking in early adolescence. JAMA. 2015;314(7):700–707. doi:10.1001/jama.2015.8950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Primack BA, Soneji S, Stoolmiller M, et al. Progression to traditional cigarette smoking after electronic cigarette use among US adolescents and young adults. JAMA Pediatr. 2015;169(11):1018–1023. doi:10.1001/jamapediatrics.2015.1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Cortes J, Mohri M, Riley M, et al. Sample selection bias correction theory. Algorithmic Learn Theory. 2008;5254:38–53. [Google Scholar]
- 84. Heckman JJ, Ichimura H, Smith J, Todd P. Sources of selection bias in evaluating social programs: an interpretation of conventional measures and evidence on the effectiveness of matching as a program evaluation method. Proc Natl Acad Sci USA. 1996;93(23):13416–13420. [DOI] [PMC free article] [PubMed] [Google Scholar]