Abstract
Introduction
Introduced originally to stratify risk for developing decubitus ulcers, the Waterlow scoring system is recorded routinely for surgical admissions. It is a composite score, reflecting patients’ general condition and co-morbidities. The aim of this study was to investigate whether the Waterlow score can be used as an independent surrogate marker to predict severity and adverse outcome in acute pancreatitis.
Methods
In this retrospective analysis, a consecutive cohort was studied of 250 patients presenting with acute pancreatitis, all of whom had their Waterlow score calculated on admission. Primary outcome measures were length of hospital stay and mortality. Secondary outcome measures included rate of intensive care unit (ICU) admission and development of complications such as peripancreatic free fluid, pancreatic necrosis and pseudocyst formation. Correlation of the Waterlow score with some known markers of disease severity and outcomes was also analysed.
Results
The Waterlow score correlated strongly with the most commonly used marker of disease severity, the Glasgow score (analysis of variance, p=0.0012). Inpatient mortality, rate of ICU admission and length of hospital stay increased with a higher Waterlow score (Mann–Whitney U test, p=0.0007, p=0.049 and p=0.0002 respectively). There was, however, no significant association between the Waterlow score and the incidence of three known complications of pancreatitis: presence of peripancreatic fluid, pancreatic pseudocyst formation and pancreatic necrosis. Receiver operating characteristic curve analysis demonstrated good predictive power of the Waterlow score for mortality (area under the curve [AUC]: 0.73), ICU admission (AUC: 0.65) and length of stay >7 days (AUC: 0.64). This is comparable with the predictive power of the Glasgow score and C-reactive protein.
Conclusions
The Waterlow score for patients admitted with acute pancreatitis could provide a useful tool in prospective assessment of disease severity, help clinicians with appropriate resource management and inform patients.
Keywords: Waterlow score, Pancreatitis
Acute pancreatitis is a common worldwide surgical presentation with an annual incidence of 150–420 cases per million population.1 The spectrum of disease severity ranges from a mild condition (no local or systemic complications or secondary organ failure), through moderate (local or systemic complications and/or transient organ failure) to severe (persistent single or multiple organ failure with consequent mortality).2 Although the aetiology and pathophysiology of this condition have been studied extensively, outcome still remains unpredictable and it imposes a large burden on current clinical practice.3 While mild pancreatitis usually resolves spontaneously with basic supportive care alone, moderate and severe disease typically requires significantly more intensive management, often necessitating admission to critical care units.4 Mortality is uncommon in mild pancreatitis but may reach up to 50% in severe episodes.5–7
Clearly, early prediction of disease severity in acute pancreatitis is important as this will guide management and determine optimum escalation of care. However, this has proved to be difficult in the absence of any established single reliable disease and patient specific surrogate markers. Several parameters have been studied including haematocrit, arterial pH, base deficit, glomerular filtration rate, blood urea nitrogen, melatonin and C-reactive protein (CRP), with conflicting results.8–11 None of these have shown strong predictive power for estimating the severity of acute pancreatitis reliably.5,12
In order to address this problem, a number of composite scoring systems have been developed. Ranson, Glasgow and Acute Physiology and Chronic Health Evaluation II (APACHE II) scores are well established clinical tools used for diagnosing disease severity.10,13 Of these, the latter was originally introduced to assess conditions for patients being admitted to the intensive care unit (ICU) and is a generalised system for recognising any critically ill patient.13 The limitations of these scoring systems include their complexity, which makes them difficult to deal with in acute situations, and their relative lack of clinical effectiveness in the presence of other patient specific, pre-existing confounding factors.5 Owing largely to its cumbersome nature, APACHE II scoring has often been omitted in acute situatons.13
Developed in 1985, the Waterlow scoring system is used by nursing staff to determine the risk of developing pressure sores in many hospitals.14 Use of this score has been implemented widely in the National Health Service.14,15 In our institution, there is a protocol driven target of 100% of patients having their scores recorded within six hours of admission. Previous reports have suggested that the Waterlow score correlates with disease severity and outcome in acute surgical emergencies.15–17 This study sought to establish whether this score might be an effective surrogate tool for assessing the severity of acute pancreatitis, risk of associated disease specific complications, need for escalation of treatment and, finally, mortality risk.
Methods
In this single centre retrospective study, data were reviewed from all patients admitted to our institution with acute pancreatitis between December 2010 and September 2013. Primary outcome measures were length of hospital stay and mortality. Secondary outcome measures included ICU admission and development of complications such as presence of peripancreatic inflammation, free intraperitoneal fluid, necrosis of the pancreas and pancreatic pseudocyst formation. All patients admitted with a clinical diagnosis of pancreatitis were included except those with a previous or current diagnosis of chronic pancreatitis. With these criteria, a consecutive series of 250 patients was identified.
The Waterlow score is recorded routinely by nursing staff on the day of admission and stored electronically. Mortality data and blood results (CRP 48 hours following admission) were taken from patients’ electronic records. Computed tomography (CT) is carried out routinely at 7–14 days after admission to screen for complications and the presence or absence of disease specific complications (presence of peripancreatic free fluid, pancreatic pseudocyst formation and pancreatic necrosis) was extracted from radiologists’ reports. The Glasgow score is not stored routinely in patient records but was available for some patients (106/250, 42.4%).
Statistical analysis
Statistical analysis was carried out with Prism® version 6 (GraphPad Software, La Jolla, CA, US) and Excel® (Microsoft, Redmond, WA, US). Non-parametric tests (Mann–Whitney U test, analysis of variance [ANOVA]) were used to analyse continuous score data. Categorical data were compared with Fisher’s exact test. The predictive power of the Waterlow score was investigated with receiver operating characteristic (ROC) curves, using area under the curve (AUC) and likelihood ratios.
Results
Our cohort included 250 patients admitted with acute pancreatitis. The median age was 66.5 years (interquartile range: 50.0–77.8 years). Patients had a median length of stay of 7 days (range: 1–156 days); 8.4% of patients were admitted to the ICU and the overall inpatient mortality rate was 8.0% (Table 1). CT data were available for 223 patients, data on ICU admission for 226 patients, CRP levels for 233 patients and Glasgow scores for 106 patients.
Table 1.
Patient characteristics (n=250)
| Age | |
| Median | 66.5 years |
| Range | 18–92 years |
| IQR | 50.0–77.8 years |
| Length of stay | |
| Median | 7 days |
| Range | 1–156 days |
| IQR | 4.0–11.0 days |
| Inpatient mortality | 8.0% |
| Admitted to ICU | 8.4% |
IQR = interquartile range; ICU = intensive care unit
The relationship was examined between the Waterlow score and the Glasgow score, the most widely used marker of pancreatitis severity. A strong correlation was found (ANOVA, p=0.0012; Fig 1). Inpatient mortality, rate of ICU admission and length of hospital stay increased with a higher Waterlow score (Fig 2). Mann–Whitney U tests demonstrated a statistically significant association between the Waterlow score and inpatient mortality (p=0.0007), ICU admission (p=0.049) and length of stay (p=0.0002) (Fig 3). There was no significant association between the Waterlow score and any of the CT findings studied (peripancreatic free fluid, pancreatic pseudocyst formation or pancreatic necrosis).
Figure 1.
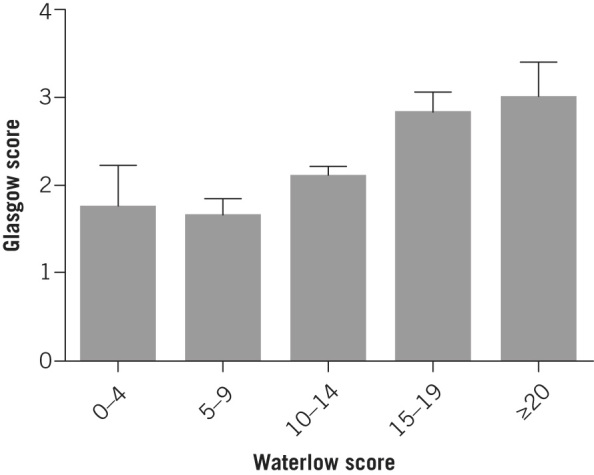
Relationship between Waterlow score and Glasgow score
Figure 2.
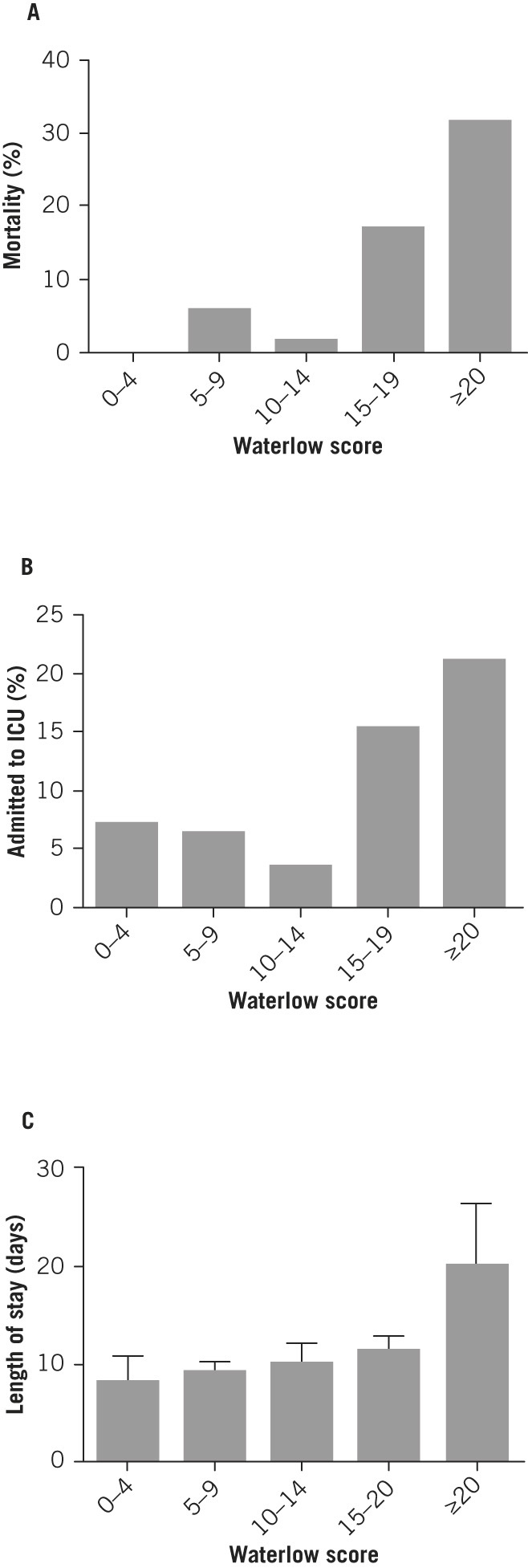
Relationship between Waterlow score and inpatient mortality rate (A), rate of intensive care unit (ICU) admission (B) and length of hospital stay (C)
Figure 3.
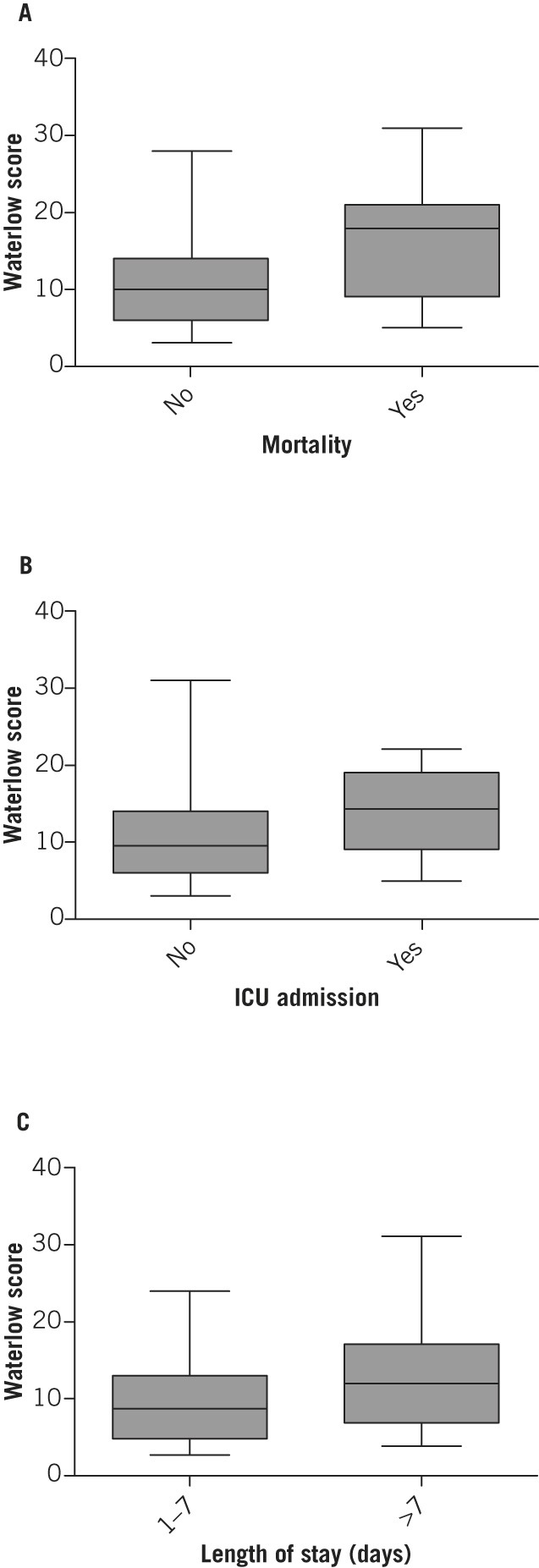
Association between Waterlow score and inpatient mortality (A), intensive care unit (ICU) admission (B) and length of stay (C)
In order to determine whether Waterlow score on admission can be used to predict severity of pancreatitis, ROC curves were analysed for mortality, ICU admission and length of stay (Fig 4). This demonstrated good predictive power for mortality (AUC: 0.73, 95% confidence interval [CI]: 0.59–0.86, p=0.0007), ICU admission (AUC: 0.65, 95% CI: 0.50–0.80, p=0.049) and length of stay >7 days (AUC: 0.64, 95% CI: 0.57–0.71, p=0.0002).
Figure 4.
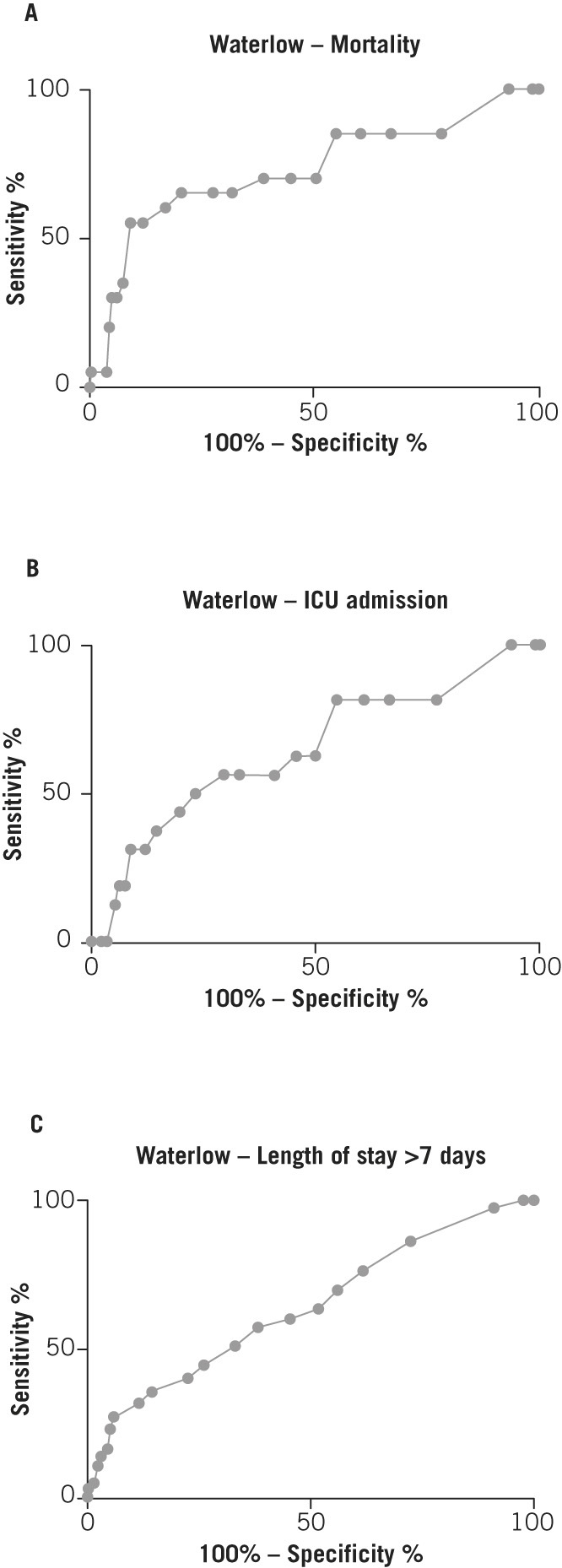
Receiver operating characteristic curves for Waterlow score and inpatient mortality (A), intensive care unit (ICU) admission (B) and length of stay (C)
A Waterlow score of 15 has been used previously as a cut-off point to categorise patients into high and low risk groups for pressure ulcer development.14 This threshold was also adopted to split our population for subsequent analysis. Fisher’s exact test was used to compare these two groups (Table 2). A Waterlow score of ≥15 was associated with significantly higher mortality (odds ratio [OR]: 7.23, p<0.0001), a higher chance of ICU admission (OR: 3.68, p=0.011) and a higher chance of a hospital stay of more than 7 days (OR: 3.11, p=0.0003).
Table 2.
Discriminatory power of Waterlow score (cut-off ≥15) in predicting inpatient mortality, ICU admission and LOS
| Outcome | Sensitivity (95% CI) | Specificity (95% CI) | Relative risk (95% CI) | Odds ratio (95% CI) | Likelihood ratio | p-value |
|---|---|---|---|---|---|---|
| Inpatient mortality | 0.80 | 0.65 | 1.23 | 7.23 | 2.27 | <0.0001 |
| (0.74–0.85) | (0.41–0.85) | (1.07–1.41) | (2.73–19.14) | |||
| ICU admission | 0.95 | 0.17 | 1.62 | 3.68 | 1.14 | 0.011 |
| (0.90–0.98) | (0.09–0.29) | (1.00–2.62) | (1.41–9.58) | |||
| LOS >7 days | 0.85 | 0.35 | 1.79 | 3.11 | 1.32 | 0.0003 |
| (0.78–0.90) | (0.27–0.45) | (1.25–2.57) | (1.70–5.71) |
ICU = intensive care unit; LOS = length of stay; CI = confidence interval
Finally, the predictive power of the Waterlow score was compared with two known measures of pancreatitis severity: CRP at 48 hours following admission and Glasgow score. ROC curve analysis (Figs 5 and 6) and comparison of AUC (Table 3) showed that the Waterlow score is comparable with CRP and the Glasgow score in predicting mortality (AUC 0.73 vs 0.66 vs 0.62 respectively), ICU admission (AUC 0.65 vs 0.87 vs 0.68 respectively) and length of hospital stay >7 days (AUC 0.64 vs 0.79 vs 0.65 respectively).
Figure 5.
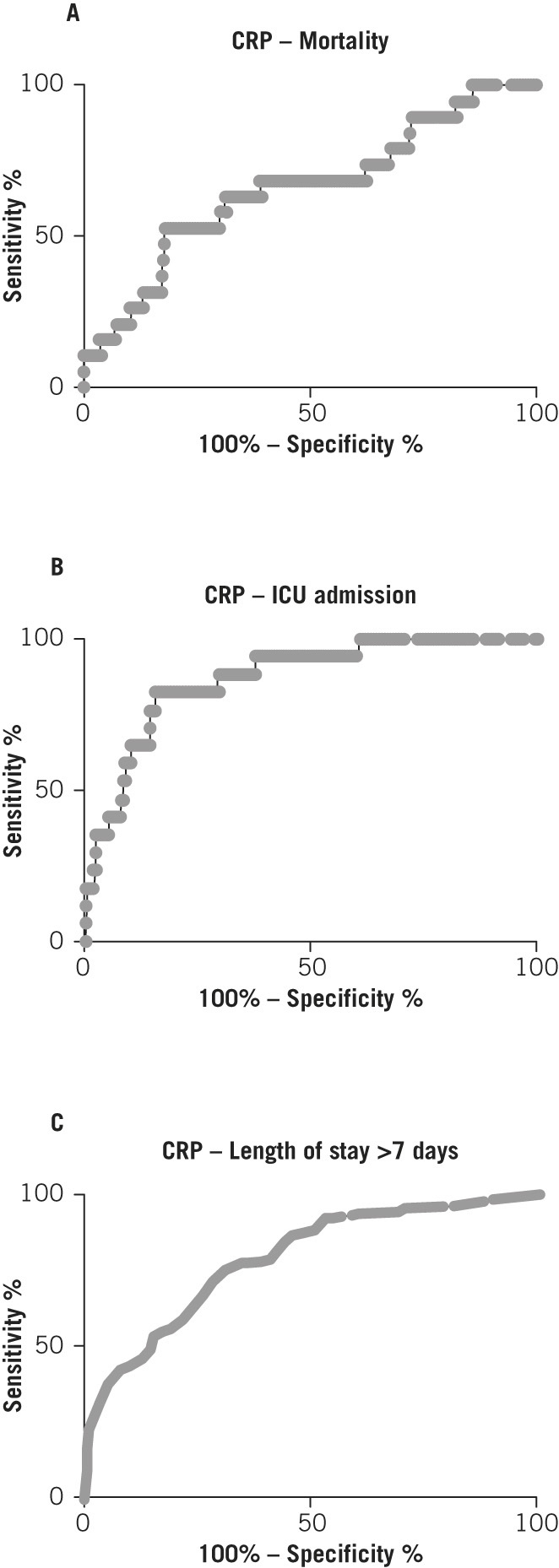
Receiver operating characteristic curves for C-reactive protein (CRP) levels and inpatient mortality (A), intensive care unit (ICU) admission (B) and length of stay (C)
Figure 6.
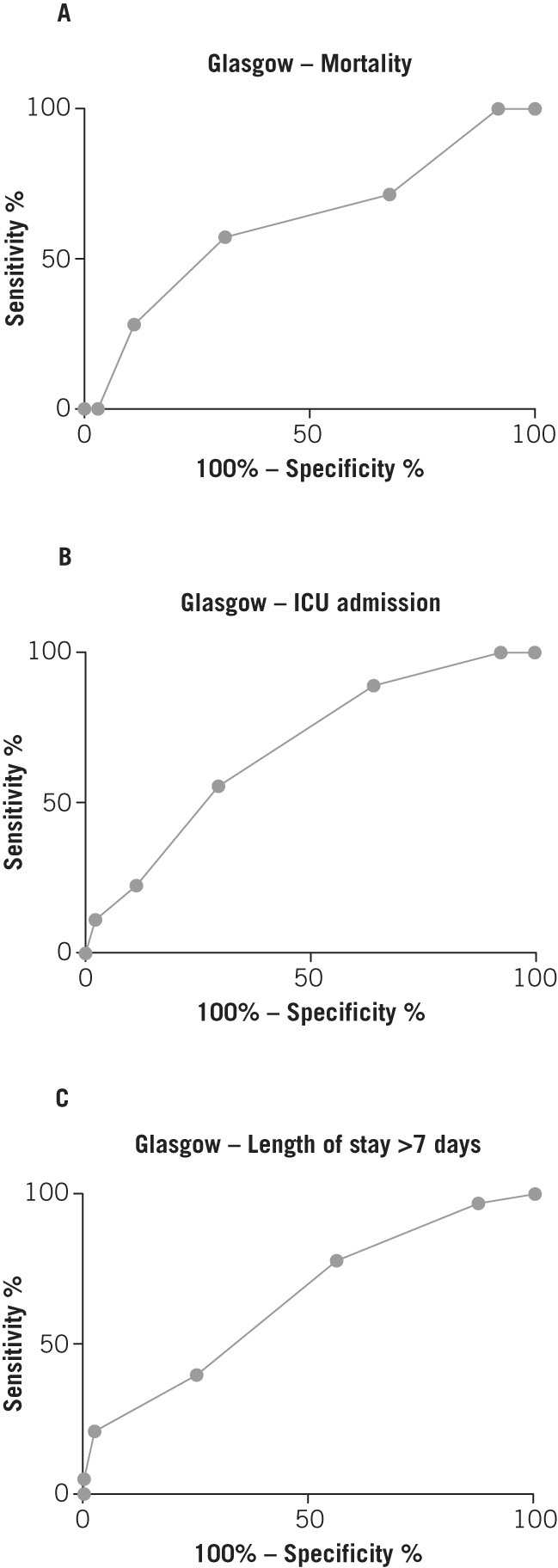
Receiver operating characteristic curves for Glasgow score and inpatient mortality (A), intensive care unit (ICU) admission (B) and length of stay (C)
Table 3.
Comparison of the ability of three scoring systems to predict inpatient mortality, intensive care unit admission and length of stay
| Number of cases | AUC (95% CI) | p-value | |
|---|---|---|---|
| Inpatient mortality | |||
| Waterlow score | 250 | 0.73 (0.59–0.86) | 0.0007 |
| CRP at 48h | 233 | 0.66 (0.52–0.79) | 0.021 |
| Glasgow score | 106 | 0.62 (0.39–0.85) | 0.288 |
| Intensive care unit admission | |||
| Waterlow score | 222 | 0.65 (0.50–0.80) | 0.049 |
| CRP at 48h | 209 | 0.87 (0.79–0.95) | <0.0001 |
| Glasgow score | 96 | 0.68 (0.51–0.85) | 0.080 |
| Length of stay >7 days | |||
| Waterlow score | 249 | 0.64 (0.57–0.71) | 0.0002 |
| CRP at 48h | 232 | 0.79 (0.73–0.85) | <0.0001 |
| Glasgow score | 106 | 0.65 (0.55–0.75) | 0.0081 |
AUC = area under the curve; CI = confidence interval; CRP = C-reactive protein
Discussion
The incidence of pancreatitis in the UK continues to rise.18 both as a knock-on effect of the increasing prevalence of gallstone disease and as a result of persistently high levels of alcohol consumption. Pancreatitis as a clinical entity can range from a mild, self-limiting condition to a severe illness with marked systemic upset and considerable consequent morbidity and mortality. ICU admission is common (8.4% in our patient group) and the condition is frequently fatal (8.0% in our patient group; reported mortality rates elsewhere range from 5% to 30%).5
While the diagnosis of pancreatitis is relatively straightforward (characteristic clinical picture together with raised serum amylase or lipase, with or without imaging findings), predicting severity is more difficult. The Glasgow scoring system predicts severity by measuring nine variables: age, white cell count, glucose, lactate dehydrogenase, aspartate transaminase/alanine transaminase, urea, calcium, PaO2 and albumin. It has been shown to predict mortality and morbidity accurately10 but it is complex and relatively expensive to measure (requiring an arterial blood gas sample and enzyme assays). It is therefore not measured routinely and it was recorded for only 106 of the 250 patients in this study.
Ranson’s criteria assess age, white cell count, glucose, lactate dehydrogenase and aspartate transaminase on admission, together with haematocrit, urea, calcium, PaO2, base deficit and fluid sequestration at 48 hours.5 This scoring system is similarly difficult to implement. CRP has also been investigated as a means of predicting severity. CRP at 48 hours following admission is the most commonly reported measure, with values of >150mg/l indicating poor prognosis.5 While easier to measure, this involves a delay of 48 hours from admission before severity can be assessed (similar to the Ranson score) as CRP values at earlier timepoints are less well correlated with prognosis. In recent years, there has been a move to introduce simpler systems for severity scoring (eg procalcitonin measurement and the bedside index for severity in acute pancreatitis). None of these have yet gained widespread acceptance.
The Waterlow score was first introduced in 1985 as a tool to screen for patients at risk of developing decubitus ulcers during inpatient stays.14,15 Its use has since become accepted as a standard of care in the majority of UK hospitals.14,19 Pressure ulcer prevention remains a priority for nursing staff and as a result, there is a strong drive to record Waterlow scores for all patients on admission. In our patient group, 100% of patients had their Waterlow score assessed within 24 hours of admission. The Waterlow score is simple to calculate and shows good intraoperator reliability.19
Previous authors have shown that the Waterlow score can be used to predict inpatient mortality and morbidity for selected groups of patients.15,17 We have extended this work by showing that Waterlow score on admission may be used as a severity predication tool for acute pancreatitis, and that it compares favourably with both CRP and the Glasgow score. Consequently, the Waterlow score may represent a useful means of rapidly predicting severity of pancreatitis that could be integrated easily and cheaply into the routine surgical admission process. This information could enable clinicians to prepare resources and inform patients. Identifying a high risk patient group is also helpful as it may allow this group to be given priority when arranging follow-up investigations or procedures (eg listing patients for cholecystectomy in cases of gallstone induced pancreatitis).
The overall inpatient mortality rate in our cohort was 8.0%. Using the Waterlow cut-off of 15 for identifying high risk patients provides a likelihood ratio of 2.27, corresponding to a post-test probability of mortality of 16.5%. This cut-off is clearly useful for identifying patients at significantly increased risk of death, who would therefore be candidates for more intensive monitoring and/or management.
Study limitations
The results presented here have some limitations. Notably, although the Waterlow score has been shown to be relatively robust to interoperator variability, it does involve an element of subjectivity, which could limit its wider applicability in risk and/or severity assessments. Additionally, this was a relatively small, single centre study and these effects need to be studied in a larger patient group, ideally with a prospective study design. Nevertheless, the findings do suggest that routine consideration of Waterlow scores in patients admitted to hospital with acute pancreatitis could provide a useful adjunct to the clinical and biochemical tools currently used to assess severity.
Conclusions
The Waterlow score for patients admitted with acute pancreatitis could provide a useful tool in prospective assessment of disease severity, help clinicians with appropriate resource management and inform patients.
References
- 1.Gomez D, Addison A, De Rosa A et al. Retrospective study of patients with acute pancreatitis: is serum amylase still required? BMJ Open 2012; : e001471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feng YC, Wang M, Zhu F, Qin RY. Study on acute recent stage pancreatitis. World J Gastroenterol 2014; : 16,138–16,145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lévy P, Domínguez-Muñoz E, Imrie C et al. Epidemiology of chronic pancreatitis: burden of the disease and consequences. United European Gastroenterol J 2014; : 345–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Warndorf MG, Kurtzman JT, Bartel MJ et al. Early fluid resuscitation reduces morbidity among patients with acute pancreatitis. Clin Gastroenterol Hepatol 2011; : 705–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phillip V, Steiner JM, Algül H. Early phase of acute pancreatitis: assessment and management. World J Gastrointest Pathophysiol 2014; : 158–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Werner J, Feuerbach S, Uhl W, Büchler MW. Management of acute pancreatitis: from surgery to interventional intensive care. Gut 2005; : 426–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balthazar EJ. Acute pancreatitis: assessment of severity with clinical and CT evaluation. Radiology 2002; : 603–613. [DOI] [PubMed] [Google Scholar]
- 8.Sharma V, Shanti Devi T, Sharma R et al. Arterial pH, bicarbonate levels and base deficit at presentation as markers of predicting mortality in acute pancreatitis: a single-centre prospective study. Gastroenterol Rep 2014; : 226–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jin Y, Lin CJ, Dong LM et al. Clinical significance of melatonin concentrations in predicting the severity of acute pancreatitis. World J Gastroenterol 2013; : 4,066–4,071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park JY, Jeon TJ, Ha TH et al. Bedside index for severity in acute pancreatitis: comparison with other scoring systems in predicting severity and organ failure. Hepatobiliary Pancreat Dis Int 2013; : 645–650. [DOI] [PubMed] [Google Scholar]
- 11.Khanna AK, Meher S, Prakash S et al. Comparison of Ranson, Glasgow, MOSS, SIRS, BISAP, APACHE-II, CTSI scores, IL-6, CRP, and procalcitonin in predicting severity, organ failure, pancreatic necrosis, and mortality in acute pancreatitis. HPB Surg 2013; 367581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Papachristou GI, Muddana V, Yadav D et al. Comparison of BISAP, Ranson's, APACHE-II, and CTSI scores in predicting organ failure, complications, and mortality in acute pancreatitis. Am J Gastroenterol 2010; : 435–441. [DOI] [PubMed] [Google Scholar]
- 13.Taylor SL, Morgan DL, Denson KD et al. A comparison of the Ranson, Glasgow, and APACHE II scoring systems to a multiple organ system score in predicting patient outcome in pancreatitis. Am J Surg 2005; : 219–222. [DOI] [PubMed] [Google Scholar]
- 14.Thompson D. An evaluation of the Waterlow pressure ulcer risk-assessment tool. Br J Nurs 2005; : 455–459. [DOI] [PubMed] [Google Scholar]
- 15.Thorn CC, Smith M, Aziz O, Holme TC. The Waterlow score for risk assessment in surgical patients. Ann R Coll Surg Engl 2013; : 52–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khambalia HA, Moinuddin Z, Summers AM et al. A prospective study of risk prediction in simultaneous pancreas and kidney transplantation. Ann R Coll Surg Engl 2015; : 445–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanner J, Khan D, Anthony D, Paton J. Waterlow score to predict patients at risk of developing Clostridium difficile-associated disease. J Hosp Infect 2009; : 239–244. [DOI] [PubMed] [Google Scholar]
- 18.UK guidelines for the management of acute pancreatitis Gut 2005; ): iii1–iii9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walsh B, Dempsey L. Investigating the reliability and validity of the Waterlow risk assessment scale: a literature review. Clin Nurs Res 2011; : 197–208. [DOI] [PubMed] [Google Scholar]


