Abstract
Introduction
Active surveillance (AS) is an option for management of low-risk prostate cancer (PCa). However, grade and stage progression is an important consideration. Neutrophil-to-lymphocyte ratio (NLR) is a useful marker of cancer-related inflammation. In this study, we aimed to identify the roles of neutrophil count (NC), lymphocyte count (LC), and NLR to predict Gleason score (GS) upgrading, disease upstaging, and biochemical recurrence rates (BCR) in low-risk PCa patients.
Methods
We retrospectively evaluated data of 210 low-risk PCa patients eligible for AS, but who underwent radical prostatectomy. The roles of NC, LC, and NLR on the GS upgrading, disease upstaging, and BCR rates were investigated. Univariate and multivariate models were used to determine the effect of these parameters.
Results
There were 104 and 106 patients in the NLR <2.5 and NLR ≥2.5 groups, respectively. GS upgrading in the NLR ≥2.5 group was more common than in the NLR<2.5 group (p=0.04). The NLR ≥2.5 group had significantly higher GS (8–10; p=0.03). With regard to NLR, the groups were found to have similar rates of disease upstaging (9/104 in NLR <2.5 vs. 16/106 in NLR ≥2.5; p=0.200). BCR rates were also significantly different between groups (p=0.033). NC an LC were not found to be associated with GS upgrading, disease upstaging, or BCR.
Conclusions
NLR is a predictor of GS upgrading and BCR, but not disease upstaging in patients with low-risk PCa. Furthermore, higher NLR was found to be associated with higher GS PCa. NLR is a cost-effective and easily accessible tool that can be used in the decision-making process for treatment of low-risk PCa cases.
Introduction
Prostate cancer (PCa) is the second most common cancer and the fifth leading cause of cancer death among men worldwide.1 In the era of prostate-specific antigen (PSA)-based screening, PCa incidence has increased markedly over time, especially low-risk cases.2 Low-risk PCa is defined as stage T1–T2a, PSA <10ng/ml and Gleason score (GS) ≤6 by the National Comprehensive Cancer Network (NCCN) guidelines.3 Management options for low-risk PCa are curative treatment or active surveillance (AS); when comparing the advantages and disadvantages of the two options, deciding between them is a dilemma for patients and physicians. Treatment can lead to significant morbidity and functional impairment, such as incontinence and erectile dysfunction.4,5 Therefore, delaying curative treatment until the disease progresses to a more aggressive category without losing the chance for active treatment seems to be favourable.
However, grade and stage progression has been reported from cohorts of men who underwent curative treatment despite being eligible for AS. Rates of upgrading were found to be as high as 23–35%, which may be either due to disease progression over time or insufficiency of basic diagnostic biopsy.6–9 We need some other biochemical or biological markers to better stratify patients into risk groups.
Neutrophil-to-lymphocyte ratio (NLR) is a useful marker of cancer-related inflammation and has been shown to be associated with poor prognosis in various types of cancers.10,11 In the case of PCa, the role of NLR on outcomes was validated, particularly in metastatic PCa, where higher NLR indicates more aggressive disease.12 However, inflammatory process is mediated by different levels of subtypes of cells in different phases. For instance, tumour-infiltrating lymphocytes are needed in the early stages of cancers, whereas with increasing stage, systemic inflammation through the increased levels of neutrophils is needed. This has been supported by the findings from a recent study by Kwon et al.13 In another recent study, NLR prior to prostate biopsy was found to be associated with the presence of PCa and higher GS as well.14
In this study, we aimed to identify the roles of neutrophil count (NC), lymphocyte count (LC), and NLR to predict disease upgrading, disease upstaging, and biochemical recurrence rates (BCR) in a cohort of low-risk PCa patients eligible for AS, but who underwent radical prostatectomy.
Methods
We retrospectively evaluated the data of 611 men diagnosed with PCa and who underwent radical prostatectomy in our institution between May 2005 and March 2015. Patients were stratified to risk groups of low, intermediate, and high according to the NCCN guidelines.3 We further defined the patients who were suitable for AS based on the following criteria: PSA <10 ng/mL, GS ≤6, clinical stage ≤T2a, ≤ 2 positive cores, and ≤50% cancer involvement in each positive core.
Patient charts were reviewed and data regarding age, PSA level, biopsy GS, radical prostatectomy GS, NC, and LC were collected. NLR was calculated by dividing NC by LC. Additionally, data of positive surgical margins, pathological stage, and BCR were collected. BCR was defined as an increase in PSA level on two consecutive measurements after radical prostatectomy, with the last PSA value >0.2 ng/mL.
Patients with history of autoimmune or inflammatory diseases that may modify the levels of neutrophils and lymphocytes, clinical suspicion or laboratory signs of bacterial or viral infection at the time of blood collection, and those with history of chemotherapy or radiation therapy at any time of the study were excluded from analysis. Patients with history of regular use of anti-inflammatory drugs or systemic steroids were also excluded from the study.
As there is no universally accepted value of abnormal NLR, NC, and LC, the median values of our low-risk group were accepted as the cutoff values: 2.5 for NLR; 4.2 × 1019/L for NC; and 1.4 × 1019/L for LC.
For statistical analysis, SPSS version 21 (IBM Corp, Armonk, NY, U.S.) was used. P value of 0.05 was considered statistically significant. Comparisons between groups were performed using Chi-square tests for categorical variables, and t-tests and analysis of variance were used for continuous variables, depending on the distribution of the data. Univariate and multivariate logistic regression analyses were conducted to identify variables predictive of upgrading and upstaging. Survival analyses were performed using the Kaplan-Meier method with a log-rank test.
Results
Of the 611 men diagnosed with localized PCa and treated with radical prostatectomy, 210 patients were found to meet the defined AS criteria. The mean age of this population was 59.2 ± 7.1 years and the mean PSA value 5.4 ± 1.1 ng/ml. When these patients are grouped with regard to NLR cutoff level of 2.5, there were 104 and 106 patients in the NLR <2.5 and NLR ≥2.5 groups, respectively. These groups were found to be similar with regard to preoperative demographic and tumour characteristics, with the exception of the NC, LC, and NLR levels (Table 1). Rates of positive surgical margins were also similar in the NLR <2.5 and NLR ≥2.5 groups (11.5% vs. 14.1%, respectively; p=0.68).
Table 1.
Demographic and PCa-related characteristics of the patients
| Parameters | General population (n=210) | NLR<2.5 (n=104) | NLR ≥2.5 (n=106) | p value |
|---|---|---|---|---|
| Age, mean ± SD | 59.2 ± 8.1 | 59.7 ± 8.8 | 58.6 ± 7.9 | 0.855 |
| PSA, mean ± SD | 5.4 ± 1.1 | 5.28 ± 1.0 | 5.5 ± 1.1 | 0.122 |
| No. of positive cores | 0.179 | |||
| One core positive, n (%) | 98 (46.7) | 47 (45.2) | 51 (48.1) | |
| Two cores positive, n (%) | 112 (53.3) | 57 (54.8) | 55 (51.9) | |
| Maximum percentage of cancer in a core, mean ± SD | 22.1 ± 9.9 | 22.02 ± 9.6 | 22.17 ± 10.6 | 0.749 |
| Clinical stage | 0.568 | |||
| cT1c, n (%) | 197 (93.8) | 99 (95.2) | 98 (92.5) | |
| cT2a, n (%) | 13 (6.2) | 5 (4.8) | 8 (7.5) |
PCa: prostate cancer; PSA: prostate-specific antigen; NLR: neutrophil-to-lymphocyte ratio; SD: standard deviation.
GS upgrading and disease upstaging rates with regard to NLR
GS upgrading was observed in a total of 69 patients (32.8%). When the groups were compared with regard to NLR, there were 27 (25.9%) and 42 (39.6%) patients who had GS upgrading in the NLR <2.5 and NLR ≥2.5 groups, respectively (p=0.04). When the groups were compared for their upgraded GS, the NLR ≥2.5 group had significantly higher GS (8–10) compared to the NLR <2.5 group (1/104 vs. 7/106; p=0.03). The results are summarized in Table 2. Disease upstaging was observed in a total of 25 patients (11.9%). With regard to NLR, the two groups were found to have similar rates of disease upstaging (9/104 vs. 16/106 patients; p=0.20) (Table 2).
Table 2.
GS upgrade and disease upstage rates of the groups
| Parameters | NLR 2.5 (n=104) | NLR ≥2.5 (n=106) | p value | NC <4.2 × 1019/L (n=103) | NC ≥4.2 × 1019/L (n=107) | p value | LC <1.4 × 1019/L (n=106) | LC ≥1.4 × 1019/L (n=104) | p value |
|---|---|---|---|---|---|---|---|---|---|
| GS upgrade, n (%) | 0.04 | 0.25 | 0.59 | ||||||
| Yes | 27 (25.9) | 42 (39.6) | 30 (29.1) | 39 (36.4) | 33 (31.1) | 36 (34.6) | |||
| No | 77 (74.1) | 64 (60.4) | 73 (70.9) | 68 (63.6) | 73 (68.9) | 68 (65.4) | |||
| GS distribution | 0.03 | 0.28 | 0.85 | ||||||
| GS 6 | 77 (74.1) | 64 (60.4) | 73 (70.9) | 68 (63.6) | 73 (68.9) | 68 (65.4) | |||
| GS 7 | 26 (25.0) | 35 (33.0) | 28 (27.2) | 33 (30.8) | 29 (27.4) | 32 (30.8) | |||
| GS 8–10 | 1 (0.9) | 7 (6.6) | 2 (1.9) | 6 (5.6) | 4 (3.7) | 4 (3.8) | |||
| Disease upstaging | 0.20 | 0.59 | 0.12 | ||||||
| Yes | 9 (8.6) | 16 (15.1) | 11 (10.7) | 14 (13.1) | 9 (8.5) | 16 (15.4) | |||
| No | 95 (91.4) | 90 (84.9) | 92 (89.3) | 93 (86.9) | 97 (91.5) | 88 (84.6) |
GS: Gleason score; LC: lymphocyte count; NC: neutrophil count; NLR: neutrophil-to-lymphocyte ratio.
GS upgrading and disease upstaging rates with regard to NC
The population was divided into two with regard to median NC of 4.2 × 1019/L; there were 103 and 107 patients in the NC<4.2 × 1019/L and ≥4.2 × 1019/L groups, respectively. Rates of GS upgrade (GS 8–10) and disease upstage were found to have tendency to be higher in NC ≥4.2 × 1019/L group, but none of the differences were statistically significant. Results are summarized in Table 2.
GS upgrading and disease upstaging rates with regard to LC
The population was divided in to two with regard to median LC of 1.4 × 1019/L; there were 106 and 104 patients in the LC <1.4 × 1019/L and ≥1.4 × 1019/L groups, respectively. Rates of GS upgrade (GS 8–10) and disease upstage were found to be similar in both groups. Results are summarized in Table 2.
Univariate analysis was performed to determine factors associated with GS upgrading and disease upstaging. Preoperative serum PSA level and NLR were found to be associated with increased risk of GS upgrading. However, none of the factors were found to be associated with higher risk of disease upstaging. The results are summarized in Table 3. Multivariate analysis was performed to determine the independent predictors of GS upgrading. Both higher PSA (odds ratio [OR] 1.384, 95% confidence interval [CI] 1.116–2.722; p=0.01) and higher NLR (OR 1.821, 95% CI 1.246–3,255; p=0.007) were found to be significant predictors of GS upgrading.
Table 3.
Univariate analysis for GS upgrading and disease upstaging
| GS upgrading | Disease upstaging | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Variables | OR | 95% CI | p value | OR | 95% CI | p value |
| Age | 1.066 | 0.645–1.316 | 0.844 | 1.010 | 0.326–1.174 | 0.997 |
| PSA | 1.534 | 1.078–2.544 | 0.008 | 1.045 | 0.555–1.872 | 0.851 |
| Two positive cores vs. one positive core | 1.399 | 0.680–3,102 | 0.428 | 1.148 | 0.677–1.842 | 0.812 |
| Maximum percentage of cancer in a core | 1.147 | 0.792–2.889 | 0.814 | 1.209 | 0.804–2.114 | 0.572 |
| Clinical stage (cT2a vs. cT1c) | 1.115 | 0.398–2.012 | 0.838 | 1.015 | 0.417–1.887 | 0.894 |
| NLR ≥2.5 | 2.234 | 1.131–5.712 | 0.003 | 1.122 | 0.571–1.874 | 0.667 |
| NC ≥4.2 × 1019/L | 1.266 | 0.603–2.655 | 0.781 | 1.117 | 0.566–1.767 | 0.821 |
| LC ≥1.4 × 1019/L | 1.205 | 0.497–2.667 | 0.821 | 1.085 | 0.381–1.884 | 0.965 |
CI: confidence interval; GS: Gleason score; LC: lymphocyte count; neutrophil count; NLR: neutrophil-to-lymphocyte ratio; OD: odds ratio; PSA: prostate-specific antigen.
Results of biochemical recurrence-free survival rates
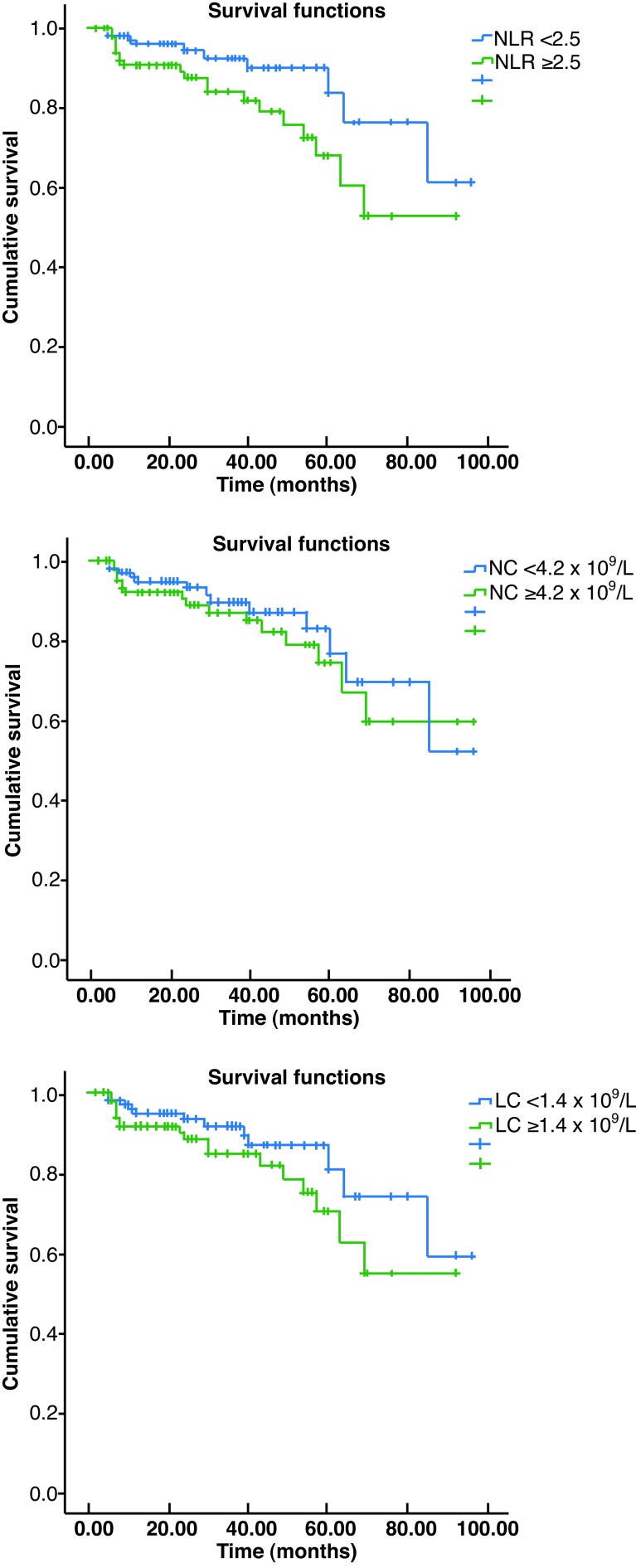
PSA recurrence developed in 30 patients after a median followup of 27 months (range 3–98). When the groups were compared with regard to NLR, NC, and LC, the only significant difference was observed with regard to NLR. The three- and five-year recurrence-free survival rates in the NLR ≥2.5 group were 81.9% and 60.4%, respectively, and these survival rates were significantly lower than their counterparts in the NLR<2.5 group (89.9% and 76.5%, respectively; p=0.033). The three- and five-year recurrence-free survival rates in the NC <4.2 × 1019/L and ≥4.2 × 1019/L groups were 87.0% and 76.4% vs. 84.9% and 67.0%, respectively; the difference was not statistically significant (p=0.442). The three- and five-year recurrence-free survival rates in the LC <1.4 × 1019/L and ≥1.4 × 1019/L groups were 89.4% and 73.9% vs. 81.8% and 62.5%, respectively; the difference was not statistically significant (p=0.143). The Kaplan-Meier curves of the groups are given in Figs. 1A, 1B, and 1C.
Fig. 1.
Kaplan-Meier curves for biochemical recurrence-free survival with regard to: (A) neutrophil-to-lymphocyte ratio; (B) neutrophil count; and (C) lymphocyte count.
In the multivariate analysis, GS upgrading (OR1 664, 95% CI 1.108–3.118; p=0.006), disease upstaging (OR 1.274, 95% CI 1.005–2.216; p=0.01), and having positive surgical margins (OR 1.815, 95% CI 1.380–4.633; p=0.001) were found to be associated with increased BCR rates. Having a NLR ≥2.5 was not found to be an independent risk factor for BCR in the multivariate analysis (OR 1.209, 95% CI 0.804–2.114; p=0.572).
Discussion
AS prevents or at least delays adverse effects related to the treatment of low-risk PCa; however, GS upgrading is an important concern, with rates of as high as 23–35% shown in contemporary radical prostatectomy series.6–9 GS upgrading results in delay of definitive treatment in a patient who is actually not in the low-risk group.
Current imaging and biopsy techniques are insufficient to determine low-risk group, therefore, some biochemical or biological markers to better stratify patients into risk groups are needed. Recently, some biochemical markers, such as PCA3 and prostate health index (PHI) (which combines free and total PSA with [−2]proPSA), as well as four kallikrein (4K) protein biomarkers (total PSA, free PSA, intact PSA, and human kallikrein-related peptidase 2) were developed to predict the presence of aggressive PCa.15–17 However, these tests are expensive and cannot be applied widely, especially in developing countries. Therefore, less expensive and more easily accessible tests are needed. In the current study, we examined the roles of NC, LC, and NLR in the prediction of GS upgrading, disease upstaging, and BCR rates. Our results revealed NLR as a significant predictor of GS upgrading and BCR, but not of disease upstaging.
The immune system responds differently in distinct phases of carcinogenesis and cancer growth.18 Higher NLR indicates more aggressive disease and poor response to treatment in metastatic PCa patients.12 In a study in early-stage, low-risk PCa patients, Kwon et al found that LC was associated with GS upgrading and NC was associated with BCR.13 In their analysis, NLR was not associated with any of the study endpoints. The authors explained this by the hypothesis of significant involvement of tumour-infiltrating lymphocytes in the early phase of PCa.13 Our results revealed that NLR was associated with GS upgrading and BCR, but neither NC nor LC were associated with any of the study endpoints. NLR and preoperative serum PSA levels were also found to be associated with GS upgrading in the multivariate analysis.
The GS upgrading (32.8%) and disease upstaging (11.9%) rates of our cohort are concordant with the current literature and slightly higher than the cohort of Kwon et al.13 Also, BCR was observed in 30 of 210 (14.3%) of the patients, which is much higher than the cohort of Kwon et al (6 of 217 patients, 2.8%). This disparity may explain the higher systemic inflammation in our cohort and this might have reflected as increased NLR in patients with BCR.
In our cohort, patients with higher NC and LC have higher rates of GS upgrading and disease upstaging, although this is not statistically significant. This may be due to the fact that in the low-risk, early-stage PCa, significant immune response through these cells might not become obvious yet or our relatively small sample size might have been be underpowered to show a significant association between these parameters. Another important point is the vulnerability of NC and LC to any other cause of inflammation; imunohistochemical studies to show the concordance of systemic reflection of cell counts on the cancer tissue is necessary to validate these results.
NLR showed a significant association not only with GS upgrading, but also with the distribution of GS. Patients with elevated NLR levels were found to have significantly higher GS (8–10) compared to those with lower NLR (6.6% vs. 0.9%; p=0,03). In the study of Kwon et al, similarly all GS 8–10 cases were observed among patients with higher NLR values (p=0.19).13 This finding supports the increased immune response in patients with higher GS, who have higher tendency for systemic dissemination. To verify these findings, further studies comparing the inflammatory status of low-, intermediate-, and high-risk localized PCa patients are needed, along with immunohistochemical evidence.
The most commonly used parameter to show disease progression in low-risk PCa patients is recurrence-free survival. In our cohort, PSA relapse after surgery was observed in 30 of the 210 patients. In the multivariate analysis, GS upgrading, disease upstaging, and having positive surgical margins were found to be associated with increased BCR rates. However, having a NLR ≥2.5 was not found to be an independent risk factor for BCR in the multivariate analysis.
Limitations of the study
The retrospective nature of our study is the most important limitation. Medical charts of the patients were reviewed to exclude presence of any condition that may be associated with alterations in the white blood cell counts. Our study reflects results of a single tertiary academic centre and cannot reflect the results from a general population. Although the pathologists have considerable experience in evaluation of the radical prostatectomy specimens, central pathological review of the specimens was not performed. Also, there is no evidence of immunohistochemical findings that reflect the immunological findings are solely attributed to PCa.
Conclusions
Our results demonstrated that the NLR is a predictor of GS upgrading and BCR, but not disease upstaging in patients with low-risk PCa eligible for AS. Also, higher NLR was found to be associated with higher GS (8–10) PCa following radical prostatectomy. NC and LC may also have roles in the inflammatory process of early-stage PCa. NLR is a cost-effective tool that is easily accessible in different healthcare settings. At our centre, we use it to support decision-making for treatment of low-risk PCa cases. These findings should be further validated with immunohistochemical studies.
Footnotes
Competing interests: The authors report no competing personal or financial interests.
This paper has been peer-reviewed.
References
- 1.Prostate cancer estimated incidence, mortality, and prevalence worldwide in 2012. [Accessed October 4, 2016]. Available at http://globocan.iarc.fr/old/FactSheets/cancers/prostate-new.asp.
- 2.Cooperberg MR, Broering JM, Kantoff PW, et al. Contemporary trends in low-risk prostate cancer: Risk assessment and treatment. J Urol. 2007;178:S14. doi: 10.1016/j.juro.2007.03.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Comprehensive Cancer Network. [Accessed October 4, 2016]. Available at http://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf.
- 4.Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate cancer survivors. N Engl J Med. 2008;358:1250–61. doi: 10.1056/NEJMoa074311. [DOI] [PubMed] [Google Scholar]
- 5.Alemozaffar M, Regan MM, Cooperberg MR, et al. Prediction of erectile function following treatment for prostate cancer. JAMA. 2011;306:1205–14. doi: 10.1001/jama.2011.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooperberg M, Carroll PR, Klotz L. Active surveillance for prostate cancer: Progress and promise. J Clin Oncol. 2011;29:3669–76. doi: 10.1200/JCO.2011.34.9738. [DOI] [PubMed] [Google Scholar]
- 7.Sundi D, Ross AE, Humphreys EB, et al. African-American men with very low-risk prostate cancer exhibit adverse oncologic outcomes after radical prostatectomy: Should active surveillance still be an option for them? J Clin Oncol. 2013;31:2991–7. doi: 10.1200/JCO.2012.47.0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schreiber D, Chhabra A, Rineer J, et al. A population-based study of men with low-volume, low-risk prostate cancer: Does African-American race predict for more aggressive disease? Clin Genitourin Cancer. 2015;13:e259–64. doi: 10.1016/j.clgc.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 9.Behbahani TE, Ellinger J, Caratozzolo DG, et al. Pathological outcomes of men eligible for active surveillance after undergoing radical prostatectomy: Are results predictable? Clin Genitourin Cancer. 2012;10:32–6. doi: 10.1016/j.clgc.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 10.Templeton AJ, McNamara MG, Seruga B, et al. Prognostic role of neutrophilto-lymphocyte ratio in solid tumours: A systematic review and meta-analysis. J Natl Cancer Inst. 2014;106:dju124. doi: 10.1093/jnci/dju124. [DOI] [PubMed] [Google Scholar]
- 11.Ozcan C, Telli O, Ozturk E, et al. The prognostic significance of preoperative leukocytosis and neutrophil-to-lymphocyte ratio in patients who underwent radical cystectomy for bladder cancer. Can Urol Assoc J. 2015;9:E789–94. doi: 10.5489/cuaj.3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Soest RJ, Templeton AJ, Vera-Badillo FE, et al. Neutrophil-to-lymphocyte ratio as a prognostic biomarker for men with metastatic castration-resistant prostate cancer receiving first-line chemotherapy: Data from two randomized phase 3 trials. Ann Oncol. 2015;26:743–9. doi: 10.1093/annonc/mdu569. [DOI] [PubMed] [Google Scholar]
- 13.Kwon YS, Han CS, Yu JW, et al. Neutrophil and lymphocyte counts as clinical markers for stratifying low-risk prostate cancer. Clin Genitourin Cancer. 2016;14:e1–8. doi: 10.1016/j.clgc.2015.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gokce MI, Hamidi N, Suer E, et al. Evaluation of neutrophil-to-lymphocyte ratio prior to prostate biopsy to predict biopsy histology: Results of 1836 patients. Can Urol Assoc J. 2015;9:E761–5. doi: 10.5489/cuaj.3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cary KC, Cooperberg MR. Biomarkers in prostate cancer surveillance and screening: Past, present, and future. Ther Adv Urol. 2013;5:318–29. doi: 10.1177/1756287213495915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Catalona WJ, Partin AW, Sanda MG, et al. A multicentre study of [−2]pro-prostate-specific antigen combined with prostate-specific antigen and free prostate-specific antigen 16 for prostate cancer detection in the 2.0 to 10.0 ng/ml prostate-specific antigen range. J Urol. 2011;185:1650–5. doi: 10.1016/j.juro.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lilja H, Ulmert D, Vickers AJ. Prostate-specific antigen and prostate cancer: Prediction, detection, and monitoring. Nat Rev Cancer. 2008;8:268–78. doi: 10.1038/nrc2351. [DOI] [PubMed] [Google Scholar]
- 18.Kim R, Emi M, Tanabe K. Cancer immunoediting from immune surveillance to immune escape. Immunology. 2007;121:1–14. doi: 10.1111/j.1365-2567.2007.02587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]