Abstract
Several lines of evidence suggest the importance of phonological working memory (PWM) in language acquisition. We investigated the neural correlates of PWM in young adults who were under compelling social pressure to be bilingual. Equal bilinguals had high proficiency in English and Chinese as measured by a standardized examination, whereas unequal bilinguals were proficient in English but not Chinese. Both groups were matched on several measures of nonverbal intelligence and working memory. In-scanner behavioral results did not show between-group differences. Of the regions showing load-dependent increments in activation, the left insula showed greater activation in equal bilinguals. Unequal bilinguals showed greater task-related deactivation in the anterior medial frontal region and greater anterior cingulate activation. Although unequal bilinguals kept apace with equal bilinguals in the simple PWM task, the differential cortical activations suggest that more optimal engagement of PWM in the latter may correlate with better second-language attainment.
Keywords: phonological working memory, functional MRI, intergroup differences
In an increasingly global world, facility with two or more languages is a capability that confers competitive advantages. Of specific interest to the current investigation is why, despite being immersed in the same environment, some individuals have difficulty acquiring a second language even though they have an excellent command of their first language.§ Prior neuroimaging studies have characterized the effects of age of acquisition (1, 2) and amount of linguistic experience in bilinguals (3) on brain activation but, to our knowledge, none have examined functional anatomy underlying differences in second-language acquisition ability.
Several lines of evidence suggest that phonological working memory¶ (PWM) plays a crucial role in determining language acquisition ability (4). It has been proposed that the phonological loop, consisting of a short-term phonological store and a subvocal rehearsal system, exists to facilitate language acquisition (5, 6). Specifically, the phonological loop is thought to temporarily store unfamiliar sound patterns, whereas more permanent representations are being constructed for long-term memory storage (4).
In support of these postulates, a number of behavioral studies have shown that measures of PWM, such as digit span and word and nonword repetition, predict the outcome of native language acquisition in children (6-8) and foreign language acquisition in both children (9) and adults (10, 11). It has also been observed that polyglots have a larger PWM capacity than nonpolyglots (12). Further, neuropsychological studies of patients with defective short-term memory (13) and children with specific language impairment and low achievement (14, 15) provide additional support for the hypothesis that the PWM plays a crucial role in language acquisition. Taken together, these findings point to a compelling link between PWM and language acquisition and suggest that PWM is important for successful language acquisition.
In this study, we investigated how the neural correlates of PWM might differ in young adults who achieved excellent grades in English but who differed in attainment as regards their second language (Chinese). Language proficiency was indexed by scores in standardized language examinations. These evaluated both oral and written language skills. To reduce the confounding effects of factors that could influence second-language acquisition, volunteers were matched for educational environment, scholastic performance, and performance in a number of standardized neuropsychological tests of nonverbal and verbal skills.
To evaluate the neural correlates of PWM, participants were scanned while performing an auditory n-back test. Successful performance of this task requires continuous updating and temporal reordering of phonological information. We used stimuli that were phonologically unfamiliar to the volunteers to ensure that task performance would be minimally facilitated by the use of lexical and sublexical information. Item load was varied to reveal PWM load-dependent effects on activation. We expected areas engaged by PWM to show a monotonic increase in activation (16, 17). Previous theoretical and behavioral work points to the existence of two dissociable components in PWM: a subvocal rehearsal system and a short-term phonological store. Functional imaging results suggest that the subvocal rehearsal system is located in Broca's area 44 (BA 44), left premotor cortex (BA 6), and supplementary motor area (BA 6), whereas the phonological store resides in the left inferior parietal cortex (BA 40) (17, 18). The obligatory role of the frontal opercular areas in phonological processing has been recently demonstrated by using repetitive transcranial magnetic stimulation (19).
We predicted that there would be group-level differences in activation in brain regions involved in PWM and that this difference would be more prominent with increasing PWM load. We made no specific prediction concerning the direction of the effect as previous studies have shown both increases (20) and decreases (21, 22) in brain activation after a linguistic task is learned.
Methods
Participants. Thirty neurologically normal, right-handed English-Chinese bilinguals participated in this study after giving informed consent. They were selected on the basis of responses provided to a questionnaire. All volunteers had been exposed to both English and Chinese before the age of 5 years. Each underwent 10 years of formal training in English and Chinese following a common syllabus. English was the primary language of instruction, and Chinese was the second language. None of the subjects were familiar with French, which was used in the test stimuli. All participants scored excellent grades for English in standardized high school examinations. The highest grade on this scale is 1 and the lowest 9. To qualify for this study, volunteers scored either 1 or 2 for English. Further detail regarding volunteers' language background is provided in Table 4, which is published as supporting information on the PNAS web site.
There were 15 unequal bilinguals and 15 equal bilinguals. The standardized language examinations referred to here form the basis of entry to local universities, and failure to achieve a passing grade had a negative impact on admission to college. The unequal bilinguals (six females; mean age of 26.07; SD = 5.05) had scores of 5 or lower for Chinese and also reported that they were able to comprehend and express themselves in English much better than in Chinese. In contrast, the equal bilinguals (seven females; mean age of 22.40; SD = 2.53) scored either 1 or 2 for Chinese and reported comparable fluency in English and Chinese.
Functional MRI (fMRI) Experiment. Participants performed a block design, auditory n-back fMRI experiment involving bisyllabic French words or pseudowords (Fig. 1). French was selected, as it was a foreign language with which participants were unfamiliar. Extremely common French words, such as “bonjour,” were excluded.
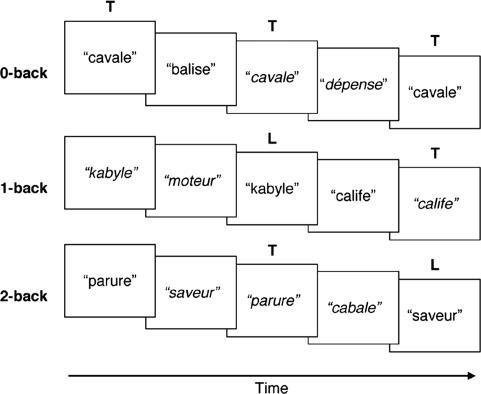
Fig. 1.
Schematic showing the sequence of presentation of stimuli in the 0-back, 1-back, and 2-back tasks. The auditory stimuli consisted of French words or pseudowords, spoken either by a male or a female (marked in italics). T, targets; L, lures.
Each stimulus was presented by either a male or a female native French speaker. Whenever a particular stimulus was repeated in the 1-back and 2-back conditions, it was spoken by a speaker who was of a different gender. In these conditions, the same stimulus was never repeated more than once during the entire experiment. These two measures were undertaken to encourage the engagement of PWM as opposed to acoustic matching when determining whether a stimulus was presented previously.
There were four experimental runs, each containing two blocks of each condition. Each of the three conditions was presented in a pseudorandom order. The order of task block presentation was counterbalanced across subjects. Experimental task blocks were separated by 30 s of silence during which volunteers looked at a central crosshair. At the beginning of each experimental task block, a visual cue (0-back, 1-back, or 2-back) appeared for 2 s, indicating the task to be performed. Each task block consisted of 12 auditory stimuli, presented with a stimulus onset asynchrony of 3 s. Each stimulus was presented for <900 ms in the 1-s silent interval between functional scans. Participants achieved performance accuracy of at least 80% in a practice session that took place before imaging. Volunteers were instructed to press a button whenever they heard a target stimulus.
In the 0-back task, the target stimulus was specified at the beginning of each experimental block. Each block comprised four target and eight nontarget stimuli.
In the 1-back condition, participants responded when the current and immediately prior stimulus matched. Each block consisted of four target pairs, a pair of lures and two nontarget stimuli. Lures for the 1-back task were presented in either the 2-back or 3-back position. A similar organization of targets, lures, and nontarget stimuli was used for the 2-back condition. Lures for the 2-back condition were presented in either the 1-back or 3-back position.
Repeated-measures ANOVA was performed on the response time and the proportion of correct responses, with group (equal and unequal bilinguals) as the between-subject variable, and PWM load (0-back, 1-back and 2-back) as the within-subject variable by using SPSS 11 (SPSS, Chicago).
Psychometric Tests. Participants were evaluated by using several measures of working memory to ensure that they were matched in cognitive domains other than second-language attainment and PWM. Visuospatial working memory was evaluated by using forward and backward spatial span tests; PWM was evaluated by forward and backward digit span tests and foreign word repetition. Nonverbal intelligence was evaluated by using Raven's Advanced Progressive Matrices Set II (APM) (The Test Agency, Oxford). Psychometric tests apart from the APM were conducted in a separate session from the fMRI experiment. Twenty-five of 30 participants were tested. Between-group differences in raw scores obtained from each test were compared by using independent-samples t tests.
The foreign word repetition task involved 10 uncommon French words and required participants to repeat the word, immediately after each presentation. Participant's verbal responses were recorded by using a microphone and digitized by using praat (www.praat.org). A blinded, native French speaker rated the phonetic quality of word repetition according to a four-point scale (where 1 represented the highest rating).
MRI and Analysis. MR images were acquired with a 3.0-T Siemens Allegra scanner (Siemens Medical Systems, Erlangen, Germany). Head motion was minimized by the use of a bite bar. Auditory stimuli were presented through a MR-compatible headset (Resonance Technology, Northridge, CA). Gradient noise occupied 2 s, leaving a quiet interval of 1 s for the presentation of auditory stimuli.
A gradient-echo planar image sequence was used to acquire functional images [repetition time (TR) 3,000 ms; echo time (TE) 30 ms; field of view 192 × 192 mm, 64 × 64 matrix]. Twenty oblique axial slices of 4 mm thick, approximately parallel to the line between the anterior and posterior commissures (AC-PC line), were acquired. High-resolution coplanar T2 anatomical images were acquired in the identical orientation to the functional data set. A further high-resolution anatomical reference image was acquired by using a T1 3d-mprage sequence.
Image Analysis. Functional image analysis was performed by using brain voyager 2000 (Version 4.9.6, Brain Innovation, Maastricht, The Netherlands). Functional image preprocessing was performed as described (23).
Functional data were modeled by using a general linear model with three predictors: 0-back, 1-back, and 2-back. Voxels activated above a threshold of P < 0.001 (uncorrected) were considered for further analysis by using a region of interest approach based on group-level data that were subjected to a random-effects analysis.
Regions of interest were identified by using two between-groups random-effects contrast maps. The first map contrasted the total contribution of all load predictors between the two groups and was meant to identify any group differences in activation. The second stage of analysis involved contrasting equal 2-back vs. 0-back with unequal 2-back vs. 0-back conditions. This procedure identified areas that showed a parametric increase in activation as a function of working memory load. We chose this analysis strategy because prior studies have shown sigmoid (24), quadratic (25), and linear (26) patterns of blood oxygenation level-dependent signal modulation in response to working memory load. The chosen approach does not make assumptions concerning the specific pattern of how load modulates activation, allowing for these to be detected without bias.∥
Estimates of blood oxygenation level-dependent signal change from 0-back, 1-back, and 2-back conditions were compared across volunteers by using repeated-measures ANOVA with group (equal and unequal bilinguals) as the between-subject variable and PWM load (0-back, 1-back, and 2-back) as the within-subject variable (SPSS).
Results
Behavioral Results. The two groups of participants were matched for nonverbal intelligence as indexed by the scores in the Raven's progressive matrices [t(28) < 1, not significant (n.s.)] (Table 1). The groups were also matched in measures of working memory indexed by backward spatial span [t(23) < 1, n.s.], forward [t(23) < 1, n.s.] and backward [t(23) = 1.35, P = 0.190] digit span, and foreign word repetition scores [t(23) < 1, n.s.]. Unequal bilinguals attained higher scores in forward spatial span compared to equal bilinguals [t(23) = 2.29, P < 0.05].
Table 1. Demographic and psychometric test score data.
| Data subject | Equal bilinguals | Unequal bilinguals |
|---|---|---|
| Age of acquisition for Chinese, years | 3.13 (2.00) | 4.07 (1.7) |
| Years of education in Chinese | 12.20 (0.86) | 12.67 (0.90) |
| High school grade for Chinese* | 1.13 (0.35) | 5.13 (1.64) |
| Raven's Advanced Progressive Matrices | 29.33 (4.48) | 28.53 (3.80) |
| Forward spatial span† | 9.46 (1.27) | 10.75 (1.55) |
| Backward spatial span | 9.69 (1.32) | 9.50 (1.45) |
| Forward digit span | 11.15 (2.30) | 10.75 (2.53) |
| Backward digit span | 10.15 (2.30) | 8.83 (2.59) |
| Foreign word repetition | 2.71 (0.59) | 2.66 (0.65) |
Numbers in parentheses represent 1 SD. Raw test scores are provided.
P < 0.001; range of score was 1 to 9, with 1 denoting highest performance.
P < 0.05.
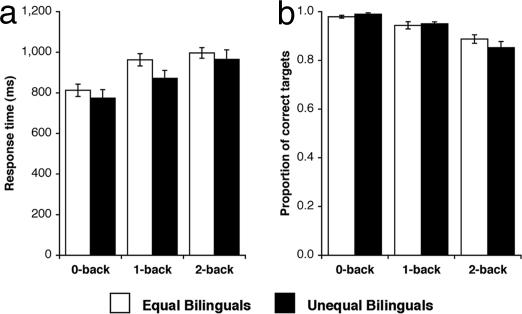
With both equal and unequal bilinguals, response time increased [F(2,56) = 62.24, P < 0.001], and target identification accuracy decreased [F(2,56) = 35.08, P < 0.001] as PWM load increased (Fig. 2). The two groups did not differ in terms of response time [F(1,28) = 1.29, P = 0.266] or target identification accuracy [F(1,28) < 1, n.s.]. There was no task by group interaction for either response time [F(2,56) = 1.81, P = 0.179] or target identification accuracy [F(2,56) = 1.65, P = 0.209].
Fig. 2.
Behavioral performance in the n-back task. (a) Mean response time (ms). (b) Mean proportion of correct targets. Error bars indicate 1 SEM.
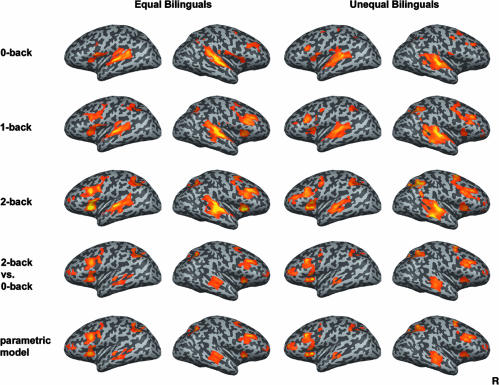
fMRI Results. Both equal and unequal bilinguals showed increasing activation with increasing load in the middle frontal gyrus, anterior middle frontal gyrus, insula, superior temporal gyrus, and inferior parietal lobule bilaterally (Table 2). Similar results were obtained by using the 2-back vs. 0-back contrast and the linear parametric model (Fig. 3).
Table 2. Brain regions activated in each group in the contrast between 2-back vs. 0-back conditions (random effects analysis, threshold P < 0.001).
| Unequal bilinguals
|
Equal bilinguals
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Area | BA | x | y | z | Peak t | x | y | z | Peak t |
| Activation | |||||||||
| L middle frontal gyrus | 9 | −39 | 13 | 27 | 10.71 | −46 | 21 | 30 | 11.88 |
| R middle frontal gyrus | 9 | 38 | 25 | 30 | 12.68 | 44 | 25 | 30 | 11.38 |
| L ant. middle frontal gyrus | 10 | −31 | 54 | 12 | 8.07 | −31 | 49 | 15 | 10.64 |
| R ant. middle frontal gyrus | 10 | 29 | 49 | 9 | 7.98 | 35 | 51 | 13 | 7.18 |
| L insula | 13 | −31 | 19 | 8 | 10.78 | −34 | 16 | 3 | 14.55 |
| R insula | 13 | 26 | 18 | 3 | 10.64 | 32 | 20 | 0 | 8.75 |
| L superior temporal gyrus | 21/22 | −60 | −20 | 0 | 8.93 | −52 | −23 | −1 | 9.39 |
| R superior temporal gyrus | 21/22 | 59 | −23 | 1 | 8.10 | 53 | −23 | 0 | 8.43 |
| L inferior parietal lobule | 7/40 | −35 | −59 | 42 | 11.66 | −34 | −51 | 39 | 7.51 |
| R inferior parietal lobule | 7/40 | 38 | −50 | 36 | 12.79 | 32 | −60 | 39 | 11.58 |
| Deactivation | |||||||||
| < bottom-border> Ant. medial frontal gyrus | 10/32 | 8 | 37 | 3 | −13.16 | −4 | 32 | −7 | −8.50 |
L, left; R, right; ant., anterior.
Fig. 3.
Activation associated with the performance of the 0-back, 1-back, and 2-back task and the contrast between 2-back vs. 0-back and activation is demonstrated with parametric model with linear predictors (n = 15 in each group, random effects analysis, threshold P < 0.001).
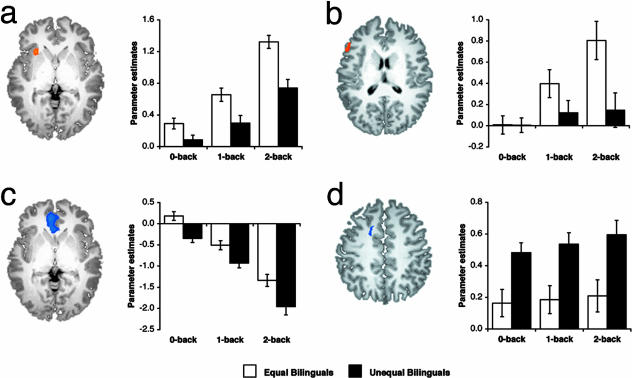
Comparisons Between Equal and Unequal Bilinguals. In the between-group contrast that considered all load predictors together, equal bilinguals showed greater activation in the left insula and right inferior parietal lobule (Table 3). Activation in the left insula showed the main effects of load [F(2,56) = 75.10, P < 0.001] and group [F(1,28) = 19.00, P < 0.001], and an interaction between load and group [F(2,56) = 3.67, P < 0.05] (Fig. 4a). This finding is consistent with the hypothesis that the left insula is sensitive to working memory load. Further, there was a positive relationship between left insula activation and Chinese test score performance (Fig. 5). There was a main effect of group but not load in the right inferior parietal lobule [F(1,28) = 13.64, P < 0.005].
Table 3. Brain regions showing differential activation between equal and unequal bilinguals (random effects analysis, threshold P < 0.001).
| Area | BA | x | y | z | Peak t |
|---|---|---|---|---|---|
| Equal vs. unequal bilinguals | |||||
| Activation | |||||
| 0-back, 1-back, and 2-back | |||||
| L insula | 13 | −28 | 19 | 2 | 3.74 |
| R inferior parietal lobule | 40 | 55 | −38 | 23 | 3.49 |
| 2-back minus 0-back | |||||
| L inferior frontal gyrus | 45 | −50 | 23 | 16 | 3.48 |
| Unequal vs. equal bilinguals | |||||
| Activation | |||||
| 0-back, 1-back, and 2-back | |||||
| Cingulate gyrus | 32 | −16 | 7 | 41 | 3.51 |
| Deactivation | |||||
| 0-back, 1-back, and 2-back | |||||
| Ant. medial frontal gyrus | 10/32 | −7 | 40 | 1 | 3.69 |
| R Cuneus | 19 | 29 | −74 | 27 | 3.33 |
| 2-back minus 0-back | |||||
| Precuneus | 7 | 1 | −41 | 51 | 4.21 |
L, Left; R, right; ant., anterior.
Fig. 4.
Regions showing robust between-group differences in activation. Parameter estimates of activation as a function of PWM load are those obtained from individual regions of interest in left insula (a, -28, 19, 2), left inferior frontal gyrus (b, -53, 23, 16), anterior medial frontal gyrus (c, -7, 40, 1), and cingulate gyrus (d, -16, 7, 41) for equal bilinguals and unequal bilinguals. Error bars indicate 1 SEM.
Fig. 5.
Relationship between left insula activation and standardized Chinese test score in individual subjects. Volunteers shown in black are those whose subjective ratings of home language usage were between 3 and 5. The rating scale had a range from 1 to 7 (1, Chinese only; 2, mainly Chinese, English rarely; 3, mostly Chinese, with English used at least 25% of the time; 4, equal use of English and Chinese; 5, mostly English, with Chinese used at least 25% of the time; 6, mainly English, Chinese rarely; 7, English only).
Relative to equal bilinguals, unequal bilinguals showed greater activation in the anterior cingulate gyrus and greater deactivation in the anterior medial frontal gyrus and the right cuneus. There was an effect of group in the cingulate gyrus [F(1,28) = 11.00, P < 0.005] (Fig. 4d), the anterior medial frontal gyrus [F(1,28) = 14.13, P < 0.005] (Fig. 4c), and the right cuneus [F(1,28) = 10.15, P < 0.005]. In addition, the magnitude of deactivation increased with load in the anterior medial frontal gyrus [F(2,56) = 18.59, P < 0.001]. Interaction between load and group was found in the right cuneus [F(2,56) = 3.45, P < 0.05].
In the contrast-comparing activation in 2-back vs. 0-back between-groups, equal bilinguals showed greater activation in the left inferior frontal gyrus, whereas unequal bilinguals showed greater deactivation in the precuneus (Table 3). Subsequent region of interest-based analysis revealed the main effects of load [F(2,56) = 21.66, P < 0.001] and an interaction between load and group [F(2,56) = 10.61, P < 0.001] in the left inferior frontal gyrus (Fig. 4b). The main effect of group did not reach statistical significance [F(1,28) = 3.58, P = 0.069]. The precuneus showed increasing deactivation with load [F(2,56) = 97.97, P < 0.001] and an interaction between load and group [F(2,56) = 11.45, P < 0.001].
Discussion
The key finding in the present study relates to people who have excellent first-language attainment and who despite having comparable impetus to be bilingual differ in second-language proficiency. We found that these individuals show differences in cortical activation that suggest an important contribution of PWM to language attainment. There were two critical sets of observations. In equal bilinguals, there was increased activation in cortical areas that participate in PWM. In unequal bilinguals there was greater activation in areas that are engaged in goal-directed processing. These differences in cortical activation suggest that a more optimal engagement of PWM or allied processes in equal bilinguals is linked to higher second-language attainment.
Group Differences in Cortical Activation. In addition to production or motor aspects of language, the insula and inferior frontal gyrus have been shown to participate in processes relevant to language and working memory (27-31). Many attempts have been made to segregate the function of this region. For example, the more anterior part of the inferior frontal gyrus has been imputed with semantic processing, whereas the more posterior portion has been imputed with phonological processing (30). However, such a view is not uncontroversial because other investigators have shown that this more anterior region is activated whenever controlled processing, phonological or semantic, is required (29). In the present work, we do not discount the possibility that the anterior insula and the rest of the inferior frontal gyrus make distinct contributions to the performance of the novel language auditory n-back task. However, as blood oxygenation level-dependent signal in both regions is modulated in a similar fashion with increasing working memory load and in the absence of explicit contrary evidence, we have grouped them together for the purpose of discussion.
Of interest to the present study is the role of the left inferior frontal region in tasks engaging PWM (18). Within this region, the left anterior insula has been linked to subvocal rehearsal (17, 32), and the left inferior frontal gyrus has been linked to sublexical phonological processes (33). Superior PWM as it relates to the ability to repeat unfamiliar phonotactic constructs correlates with vocabulary development (34). Thus, the observation that equal bilinguals activate this region more readily with increased working memory demands relative to unequal bilinguals may be interpreted as denoting a more facile engagement of the neural circuitry required to incorporate novel speech-like sounds into long-term phonological representations. The successful engagement of such neural circuitry may correspond to vocabulary growth (6).
Alternatively, the group difference in the left inferior frontal region could be attributed to other phonological processes relating to phonological segmentation (35). For example, this region has been activated in tasks requiring explicit phonemic segmentation (33). However, phonemic segmentation alone cannot account for the parametric increase in activation in this region as participants heard the same number of sounds presented at the same rate across task loads. The increased activation in response to increasing load is congruent with the subvocal rehearsal of test items, although this does not rule out the participation of other working memory processes.
On the surface, the observation that equal bilinguals engage this region more than unequal bilinguals might seem to contradict a recent finding that a greater reduction in insula activation was observed when “good learners” (relative to “poor learners”) were able to master the discrimination of a nonnative phonological contrast (21). However, in that experiment, only relative changes in activation before and after learning were documented. An explanation parsimonious with the results of the present study is that good learners started off with greater insula activation pretraining (as in the current experiment) and ended up with native-language levels of activation, whereas poor learners activated the insula to the same extent for nonnative sounds irrespective of training.
In support of this proposition, greater activation of a number of brain regions, including the left anterior insula and BA, was observed in the course of learning a difficult speech contrast (20). Activity in both of these regions was correlated with better performance in learning this difficult speech contrast (36). To account for these findings, it was postulated that in the course of learning unfamiliar speech contrasts, orosensory-articulatory mappings** that engage speech-planning areas (anterior insula and BA) facilitate the identification of unfamiliar speech contrasts. This explanation could contribute to explaining the greater activation of the left anterior insula in equal bilinguals in the present study. Our results also add weight to a proposal that traditionally “motor” areas are involved in sensory-perceptual processing (37).
Interestingly, the left parietal region that has been implicated in phonological storage showed increasing activation in response to increasing PWM load, in accordance with its putative role as a storage area (38, 39). However, the absence of group differences in activation in this region suggests some working memory process other than storage correlates with the difference in language attainment between the equal and unequal bilinguals.
Group Differences in Cortical Deactivation. Deactivation, referring to a reduction of blood oxygenation level-dependent signal during task performance relative to the baseline (40), was more pronounced with increasing load in both groups. The deactivated anterior medial frontal regions we observed are part of a “default network” (40) that is more active during passive (baseline) than active (task) conditions in a wide variety of experiments (41, 42). The present findings are consistent with the notion that these regions are disengaged during the performance of cognitive tasks and that the magnitude of deactivation may increase in accordance with processing demands (23, 42). We interpret the more pronounced anterior medial frontal deactivation in unequal bilinguals as an indication of a greater need to allocate attentional resources to perform the PWM task at higher levels of load. This notion is supported by the observation that cingulate activation, a marker of having to deal with response conflict (43), was greater in unequal bilinguals.
The Links Between Imaging Findings, Behavioral Findings, and Second-Language Attainment. Although the functional imaging results and the level of second-language attainment are clear enough, the equivalence of working memory and phonological test scores might prompt questions as to the construct validity of the experiment. In this regard, it is critical to appreciate the objectivity of standardized scores of language attainment and the relationship between the left insula and PWM. We might therefore attribute the absence of significant group differences in behavioral scores to the possibility that although these tests engage PWM, they are relatively insensitive and that a greater number (typical for behavioral experiments) of volunteers might be necessary to uncover such effects. The greater sensitivity of functional neuroimaging in uncovering intermediate phenotypes has been highlighted in recent studies on attention (44) and working memory (45). The notion of “intermediate phenotype” suggests the existence of people who are genetically predisposed to certain traits but who may not express overt phenotypic abnormality.
It is important to point out that although we observed a strong correlation between left insula activation and a reduced anterior cingulate and anterior frontal activation and high second-language attainment, this finding does not imply causality. Even if the home-language backgrounds of the two groups were matched, we would not be able to discern whether the observed patterns of activation predate or are a consequence of better second-language attainment.
Conclusion
Taken together, these observations support the overall construct that unequal bilinguals, although able to keep apace with equal bilinguals in a simple PWM task, show differences in neural activation patterns that may belie a less efficient processing strategy that correlates with poorer second-language attainment. The extent to which such processing differences are the cause or consequence of impaired second-language attainment remains to be explored.
Supplementary Material
Acknowledgments
This work was supported by National Medical Research Council Grant 2000/0477, Biomedical Research Council Grant 014, and the Shaw Foundation.
Author contributions: M.W.L.C., C.S.S., C.P., and H.L.L. designed research; H.L.L. performed research; M.W.L.C., C.S.S., and H.L.L. analyzed data; M.W.L.C., C.S.S., and H.L.L. wrote the paper; and C.P. provided stimuli.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: PWM, phonological working memory; BA, Broca's area; fMRI, functional MRI; n.s., not significant.
Data deposition: The neuroimaging data have been deposited with the fMRI Data Center, www.fmridc.org (accession no. 2-2004-116RG).
Footnotes
We use the terms “first language” and “second language” to refer to relative language dominance. In the study population, individuals were exposed to both languages early in life. The common semantic extensions to the term “second language” do not apply here (optional, secondary, spoken by a minority, reduced opportunities for usage; see Methods for details concerning these points).
We use the term “phonological working memory” in preference to “verbal working memory” (which is the term usually used in the imaging of working memory) because it refers directly to the phonological coding (or recoding) that lies at the heart of the latter term.
A separate analysis using a parametric predictor model was also performed involving two predictors for each subject. The first predictor modeled task-related activation for any experimental condition. The second predictor modeled for activation that showed a linear increase with PWM load.
This term means “mentally visualizing” the movement of oral structures to emulate speaking the difficult contrast. This mapping can be performed even though the person may be unable to actually voice the difficult sound contrast.
References
- 1.Perani, D., Abutalebi, J., Paulesu, E., Brambati, S., Scifo, P., Cappa, S. F. & Fazio, F. (2003) Hum. Brain Mapp. 19, 170-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fiebach, C. J., Friederici, A. D., Muller, K., von Cramon, D. Y. & Hernandez, A. E. (2003) NeuroImage 19, 1627-1637. [DOI] [PubMed] [Google Scholar]
- 3.Hsieh, L., Gandour, J., Wong, D. & Hutchins, G. D. (2001) Brain Lang. 76, 227-252. [DOI] [PubMed] [Google Scholar]
- 4.Baddeley, A. D. (2003) J. Commun. Disord. 36, 189-208. [DOI] [PubMed] [Google Scholar]
- 5.Gathercole, S. E. & Baddeley, A. D. (1995) J. Speech Hear. Res. 38, 463-472. [DOI] [PubMed] [Google Scholar]
- 6.Baddeley, A. D., Gathercole, S. E. & Papagno, C. (1998) Psychol. Rev. 105, 158-173. [DOI] [PubMed] [Google Scholar]
- 7.Gathercole, S. E., Willis, C. S., Emslie, H. & Baddeley, A. D. (1992) Dev. Psychol. 28, 887-898. [Google Scholar]
- 8.Gathercole, S. E., Hitch, G. J., Service, E. & Martin, A. J. (1997) Dev. Psychol. 33, 966-979. [DOI] [PubMed] [Google Scholar]
- 9.Service, E. (1992) Q. J. Exp. Psychol. A 45, 21-50. [DOI] [PubMed] [Google Scholar]
- 10.Atkins, P. W. B. & Baddeley, A. D. (1998) Appl. Psychololinguistics 19, 537-552. [Google Scholar]
- 11.Gathercole, S. E., Service, E., Hitch, G. J., Adams, A. M. & Martin, A. J. (1999) Appl. Cognit. Psychol. 13, 65-77. [Google Scholar]
- 12.Papagno, C. & Vallar, G. (1995) Q. J. Exp. Psychol. A 48, 98-107. [DOI] [PubMed] [Google Scholar]
- 13.Vallar, G. & Papagno, C. (2002) in The Handbook of Memory Disorders, eds. Baddeley, A. D., Kopelman, M. D. & Wilson, B. A. (Wiley, London), pp. 249-270.
- 14.Gathercole, S. E. & Baddeley, A. D. (1990) J. Mem. Lang. 29, 336-360. [Google Scholar]
- 15.Gathercole, S. E. (2002) in The Handbook of Memory Disorders, eds. Baddeley, A. D., Kopelman, M. D. & Wilson, B. A. (Wiley, London), pp. 475-500.
- 16.Braver, T. S., Cohen, J. D., Nystrom, L. E., Jonides, J., Smith, E. E. & Noll, D. C. (1997) NeuroImage 5, 49-62. [DOI] [PubMed] [Google Scholar]
- 17.Smith, E. E., Jonides, J., Marshuetz, C. & Koeppe, R. A. (1998) Proc. Natl. Acad. Sci. USA 95, 876-882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paulesu, E., Frith, C. D. & Frackowiak, R. S. (1993) Nature 362, 342-345. [DOI] [PubMed] [Google Scholar]
- 19.Nixon, P., Lazarova, J., Hodinott-Hill, I., Gough, P. & Passingham, R. (2004) J. Cognit. Neurosci. 16, 289-300. [DOI] [PubMed] [Google Scholar]
- 20.Callan, D. E., Tajima, K., Callan, A. M., Kubo, R., Masaki, S. & Akahane-Yamada, R. (2003) NeuroImage 19, 113-124. [DOI] [PubMed] [Google Scholar]
- 21.Golestani, N. & Zatorre, R. J. (2004) NeuroImage 21, 494-506. [DOI] [PubMed] [Google Scholar]
- 22.Raichle, M. E., Fiez, J. A., Videen, T. O., MacLeod, A. M., Pardo, J. V., Fox, P. T. & Petersen, S. E. (1994) Cereb. Cortex 4, 8-26. [DOI] [PubMed] [Google Scholar]
- 23.Chee, M. W. & Choo, W. C. (2004) J. Neurosci. 24, 4560-4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen, J. D., Perlstein, W. M., Braver, T. S., Nystrom, L. E., Noll, D. C., Jonides, J. & Smith, E. E. (1997) Nature 386, 604-608. [DOI] [PubMed] [Google Scholar]
- 25.Callicott, J. H., Mattay, V. S., Bertolino, A., Finn, K., Coppola, R., Frank, J. A., Goldberg, T. E. & Weinberger, D. R. (1999) Cereb. Cortex 9, 20-26. [DOI] [PubMed] [Google Scholar]
- 26.Rypma, B., Prabhakaran, V., Desmond, J. E., Glover, G. H. & Gabrieli, J. D. (1999) NeuroImage 9, 216-226. [DOI] [PubMed] [Google Scholar]
- 27.Bamiou, D. E., Musiek, F. E. & Luxon, L. M. (2003) Brain Res. Brain Res. Rev. 42, 143-154. [DOI] [PubMed] [Google Scholar]
- 28.Ardila, A. (1999) Aphasiology 13, 79-87. [Google Scholar]
- 29.Gold, B. T. & Buckner, R. L. (2002) Neuron 35, 803-812. [DOI] [PubMed] [Google Scholar]
- 30.Poldrack, R. A., Wagner, A. D., Prull, M. W., Desmond, J. E., Glover, G. H. & Gabrieli, J. D. (1999) NeuroImage 10, 15-35. [DOI] [PubMed] [Google Scholar]
- 31.Wager, T. D. & Smith, E. E. (2003) Cognit. Affect. Behav. Neurosci. 3, 255-274. [DOI] [PubMed] [Google Scholar]
- 32.Fiez, J. A., Raife, E. A., Balota, D. A., Schwarz, J. P., Raichle, M. E. & Petersen, S. E. (1996) J. Neurosci. 16, 808-822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burton, M. W., Small, S. L. & Blumstein, S. E. (2000) J. Cognit. Neurosci. 12, 679-690. [DOI] [PubMed] [Google Scholar]
- 34.Gathercole, S. E. (1995) Mem. Cognit. 23, 83-94. [DOI] [PubMed] [Google Scholar]
- 35.Snowling, M., Chiat, S. & Hulme, C. (1991) Appl. Psychololinguistics 12, 369-373. [Google Scholar]
- 36.Callan, D. E., Jones, J. A., Callan, A. M. & Akahane-Yamada, R. (2004) NeuroImage 22, 1182-1194. [DOI] [PubMed] [Google Scholar]
- 37.Rizzolatti, G. & Craighero, L. (2004) Annu. Rev. Neurosci. 27, 169-192. [DOI] [PubMed] [Google Scholar]
- 38.Becker, J. T., MacAndrew, D. K. & Fiez, J. A. (1999) Brain Cognit. 41, 27-38. [DOI] [PubMed] [Google Scholar]
- 39.Ravizza, S. M., Delgado, M. R., Chein, J. M., Becker, J. T. & Fiez, J. A. (2004) NeuroImage 22, 562-573. [DOI] [PubMed] [Google Scholar]
- 40.Gusnard, D. A. & Raichle, M. E. (2001) Nat. Rev. Neurosci. 2, 685-694. [DOI] [PubMed] [Google Scholar]
- 41.Mazoyer, B., Zago, L., Mellet, E., Bricogne, S., Etard, O., Houde, O., Crivello, F., Joliot, M., Petit, L. & Tzourio-Mazoyer, N. (2001) Brain Res. Bull. 54, 287-298. [DOI] [PubMed] [Google Scholar]
- 42.McKiernan, K. A., Kaufman, J. N., Kucera-Thompson, J. & Binder, J. R. (2003) J. Cognit. Neurosci. 15, 394-408. [DOI] [PubMed] [Google Scholar]
- 43.Carter, C. S., Braver, T. S., Barch, D. M., Botvinick, M. M., Noll, D. & Cohen, J. D. (1998) Science 280, 747-749. [DOI] [PubMed] [Google Scholar]
- 44.Fan, J., Wu, Y., Fossella, J. A. & Posner, M. I. (2001) BMC Neurosci. 2, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Callicott, J. H., Egan, M. F., Mattay, V. S., Bertolino, A., Bone, A. D., Verchinksi, B. & Weinberger, D. R. (2003) Am. J. Psychiatry 160, 709-719. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.