Abstract
We created neutral antimalarial prodrugs that deliver bisthiazolium compounds with antimalarial activity in the nanomolar range. These drugs primarily affect early intraerythrocytic stages through rapid, nonreversible cytotoxicity. The compounds are suitable for both parenteral and oral use and plasma promotes rapid conversion of the prodrug into the drug. We demonstrate that very low doses offer protection in a murine model of malaria. The drugs show great potential for curing high parasitemia with short-course treatments. Oral administration of the TE3 prodrug completely cures Plasmodium cynomolgi infection in rhesus monkeys. The drugs specifically accumulate inside infected erythrocytes, block phosphatidylcholine biosynthesis, and interact with hemozoin. To our knowledge, this class of compounds represents one of the most potent antimalarials tested to date. These unique properties signal a promising future for this class of antimalarial.
Keywords: malaria, chemotherapy, phospholipid metabolism, drug resistance, Plasmodium
Malaria is a public health problem affecting >40% of the world's population. It causes up to 2 million deaths, mostly young children, in Africa, and >300 million clinical cases each year (1), with major consequent impact on economic productivity and livelihood (2). Currently, there are no licensed vaccines and the spread of drug-resistant parasites and insecticide-resistant mosquitoes is an increasing problem. New antimalarial compounds, particularly those based on compounds structurally unrelated to existing antimalarial drugs with new mechanisms of action, are urgently needed (3-5).
We have developed classes of antimalarial drugs targeting membrane biogenesis during intraerythrocytic Plasmodium falciparum development. We have focused on mono- and bisquaternary ammonium compounds for their potent anti-malarial activity in vitro and in vivo (6, 7). These compounds mimic choline structure; they potently inhibit the low-affinity choline carrier related to phospholipid biosynthesis in eukaryotic cells and the high-affinity carrier involved in biosynthesis of the neurotransmitter acetylcholine in the CNS (8, 9). These compounds have exceptional in vitro and in vivo antimalarial properties devoid of mutagenic activity (10-12).
G25 [1,16-hexadecamethylenebis(N-methylpyrrolidinium) dibromide] and other drugs in this class possess a permanently charged cationic group (7, 13) that is essential for activity but detrimental to oral absorption. This action prejudiced development for the clinic. Oral administration is essential for treatment in dispensaries in endemic countries and for prophylactic or curative treatment for travelers. A targeting carrier system is unsuitable because, in the absence of carrier-mediated specific processes, quaternary ammonium compounds are intrinsically incapable of crossing bilayered cellular membranes. Designing drugs that mask the ionizable groups was therefore the most attractive solution. This was the rationale behind development of a chemically modified form of drug that is converted into an active ionized form by enzymes present in plasma. Here, we report on the design of bisthiazolium precursors. The resulting compounds are quantitatively converted to the active drug form and show impressive antimalarial activities through mechanisms involving the phosphatidylcholine (PC) biosynthesis pathway and a probable interaction with heme.
Materials and Methods
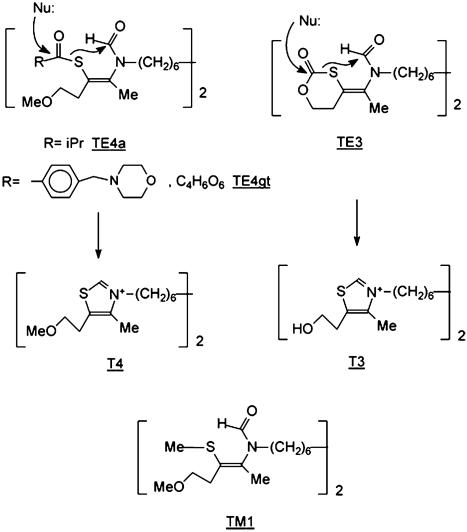
Chemistry. The bisthiazolium iodide salts T3 and T4 (named drugs), TE3, TE4a, and TE4gt (named prodrugs), and TM1 were synthesized in-house. The methods for the synthesis of the compounds and their structural analysis can be found in Supporting Text, which is published as supporting information on the PNAS web site.
Drug Inhibition of in Vitro-Cultured P. falciparum. Chloroquine (CQ)-sensitive (3D7 and Nigerian) or -resistant (FCB1 and FCM29) strains of P. falciparum (12) were asexually cultured in human blood (14) and synchronized by using two successive 5% sorbitol treatments 26 h apart (15). Antimalarial activity of the compounds was routinely determined at 1.5% final hematocrit and 0.6% parasitemia (16).
Bioconversion and Accumulation Studies. After a solid-phase extraction procedure, compounds were identified and quantified by liquid chromatography/electrospray ionization MS (LC/ESI-MS) and validated according to U.S. Food and Drug Administration Good Laborator y Practices guidelines (17, 18).
Stability studies were performed in 0.16 M sodium phosphate buffer (pH 7.4) and simulated gastric and intestinal fluids (19) at 37°C for 8 h. Bioconversion of TE3 was assessed at 37°C in human plasma diluted to 80% with 0.16 M sodium phosphate buffer (pH 7.4).
Accumulation was studied in both uninfected and trophozoite-infected erythrocytes. Cells were suspended in RPMI medium 1640 supplemented with 0.5% albumax containing 50 nM T4. After 2 h at 37°C, cells were washed and isolated by passage through a dibutyl phtalate cushion (10). The cellular accumulation ratio is defined as the ratio of the amount of T4 in the same volume of suspending solution.
In Vivo Studies. All animal studies followed relevant laws and institutional guidelines. They were performed either at the Centre d'Elevage et de Conditionnement Experimental des Modèles Animaux, Montpellier, under permission no. A34370 (Centre National de la Recherche Scientifique), or at the Biomedical Primate Research Centre (Rijswijk) after approval by the animal experimenting commission.
Antimalarial activities were determined against the Plasmodium vinckei petteri (279BY) strain in female Swiss mice (11). Drugs were injected i.p. in 100 μl of 0.9% NaCl whereas prodrugs were administered i.p. or orally in 100 μl of DMSO. Parasitemia levels were monitored in Giemsa-stained blood smears, and blood samples were collected for determination on a fluorescence-activated cell sorter (20). All results are expressed as mean ± SEM.
Four groups of four Macaca mulatta were infected by i.v. inoculation with Plasmodium cynomolgi M strain blood-stage parasites obtained from an infected donor monkey. The monkeys were randomly divided over four groups according to treatment (with one control group receiving vehicle only and three treatment groups receiving 3, 9, and 27 mg/kg TE3, respectively). Treatment was initiated on the day when parasitemia was between 0.01% and 0.2% in all monkeys; TE3 (solubilized in 2.5 ml of DMSO) was given by gavage once a day for 4 consecutive days, parasitemia was monitored by examination of Giemsa-stained thin films prepared from finger-prick blood, and all parasites were counted whether they appeared to be viable or not viable. By using an outcome of the time at which parasitemia was reduced to zero, data were subject to Kaplan-Meier analysis and statistical significance between treated and untreated groups was assessed by log-rank test. To monitor parasite survival, on the last 3 days of dosing, immediately before drug administration, red blood cell samples collected from two animals from each group were washed extensively before overnight in vitro cultivation and subsequent thin-film examination. TE3 and T3 were quantified by LC/ESI-MS in plasma samples from all monkeys drawn just before and 30 min after each dosing.
Results
Thiazolium Compounds and Their Bioprecursors Exert Potent in Vitro Antimalarial Activities. We determined the in vitro antimalarial activity of bisthiazolium compounds T3 and T4 and their respective potential bioprecursors TE3 and TE4a (Fig. 1) after one blood cycle (48 h) contact with P. falciparum. The bisthiazolium drugs, T3 and T4, exhibited potent activity showing half-maximal inhibition concentration (IC50) of 2.3-9 and 0.65-2.9 nM, respectively (Table 1). TE4a and TE4gt, which both possess cleavable thioester bonds enabling formation of T4, also had an IC50 in the same low nanomolar range. Similarly TE3, a proT3 compound, had an IC50 similar to or lower than that of T3, possibly due to a better cellular penetration of the neutral prodrugs. In contrast, the parent compound, TM1 (Fig. 1), which does not possess a labile thioester bond, was inactive <1 μM. All compounds had very steep dose-response curves with IC50/IC90 ratios of <2.
Fig. 1.
Structure of the compounds. Bioprecursors are TE4a, TE4gt, and TE3. Upon action of esterases (Nu:), S-CO bonds of thioester (TE4a and TE4gt) and of thiocarbonate (TE3) of prodrugs break and generate stable thiazolium cycle with loss of hydroxyl ion. TM1 is a compound that is stable under esterase action.
Table 1. In vitro and in vivo antimalarial activity of the compounds.
|
In vitro activity against P. falciparum IC50, nM
|
In vivo activity against P. vinckei (rodent model)
|
|||||
|---|---|---|---|---|---|---|
| CQ-sensitive
|
CQ-resistant
|
|||||
| Compounds | Nigerian | 3D7 | FCB1 | FCM29 | ED50 i.p., mg/kg | ED50 p.o., mg/kg |
| T4 | 0.65 ± 0.15 | 1.3 ± 0.23 | 2.4 | 2.0 ± 0.3 | 0.14 ± 0.01 | ND |
| TE4gt (proT4) | 2.5 ± 0.21 | 7.0 | 3.2 | 4.5 | 3.4 | 90 |
| TE4a (proT4) | 1.1 ± 0.03 | 4.8 | 3.6 | 5.5 | 0.12 | 11 ± 0.77 |
| T3 | 3.0 ± 0.03 | 2.3 ± 0.75 | 6.3 | 4.7 ± 1.1 | 0.2 ± 0.02 | >10 |
| TE3 (proT3) | 2.25 ± 0.38 | 4.8 ± 1.62 | 3.2 | 5.7 | 0.25 | 5 ± 0.28 |
| TM1 | 3,533 ± 897 | 1,033 ± 484 | ND | ND | ND | ND |
| CQ | 20.0 ± 2.5 | 20.0 ± 1.6 | 160.0 | 400.0 ± 57 | 1.1 | 3.4 |
IC50 values were assessed after 48-hr contact of infected RBCs with the drug. ED50 values against P. vinckei were determined after i.p. administration of the compounds once daily for 4 days in infected mice, and parasitemia levels were determined on the day after the last administration. Values are the means of at least three independent experiments performed in triplicate (SEM is indicated). Otherwise, values are the means of two independent experiments (each performed in triplicate) differing by <15%. There was no recrudescence after 20 days at 4- to 5-fold ED50. ND, not determined; p.o., orally.
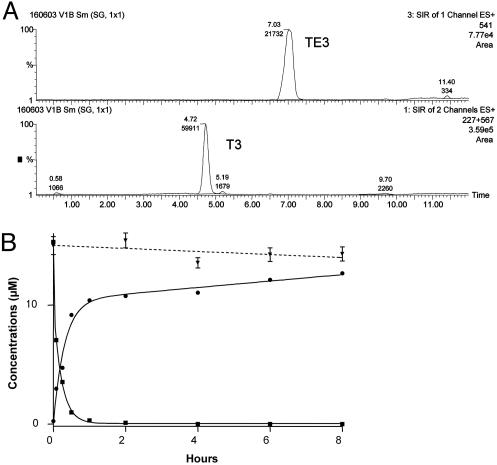
Bioprecursors Are Rapidly Converted into Active Compounds in Plasma. Conversion of TE3 into T3 was assayed by LC/ESI-MS (Fig. 2A). TE3 did not undergo significant conversion in phosphate buffer (pH 7.4) (Fig. 2B), or in medium simulating intestinal fluid (data not shown), for at least 8 h at 37°C. In medium simulating gastric fluid, TE3 disappeared with first-order process (elimination half-time of 8 h). Because gastric residence half-time is 20 min under fasting conditions (21), TE3 appears sufficiently stable for oral administration. In contrast, human plasma promoted rapid conversion of TE3 into T3. At 15 μM, TE3 decreased concomitantly with the appearance of T3, with an initial conversion half-life of ≈5 min and nearly complete bioconversion after 8 h (Fig. 2B). After a 1-h incubation in the presence of 10% plasma, >50% of the prodrug was converted into the drug. Heat-denatured plasma (70°C for 2 h) did not bioconvert TE3, indicating the involvement of a protein or an enzymatic process in the bioconversion.
Fig. 2.
Prodrug stability and conversion into drugs at 37°C. (A) LC/ESI-MS chromatograms (single-ion monitoring mode) of samples obtained after 5 min of incubation of 15 μM TE3 in 80% human plasma. Peaks were normalized to 100%, and concentrations were computed from a calibration curve by using verapamil as internal standard. Indicated TE3 and T3 peaks correspond to concentrations of 7.1 and 3.0 μM, respectively. The traces are obtained for the two different analytes at the same time. (B) Stability of TE3 (15 μM) in phosphate buffer (▾) and bioconversion of TE3 (▪) into T3 (•) by 80% plasma.
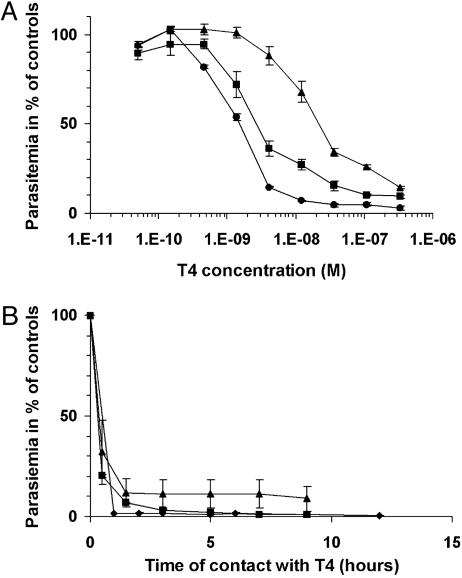
Stage-Specific Susceptibility and Time Course of P. falciparum Growth Inhibition. P. falciparum susceptibility to thiazolium compounds differed according to parasite developmental stage. Four-hour treatment with T4 during schizont development resulted in an IC50 of 21 nM; the IC50 was 1.4 and 2.5 nM when the 4-h treatment was applied during ring and trophozoite stages, respectively (Fig. 3A).
Fig. 3.
Inhibition of in vitro-cultured P. falciparum (3D7). (A) Stage-dependent susceptibility. T4 was added at various concentrations to synchronized parasites in culture at ring (4 h) (•), trophozoite (22 h) (▪), or schizont (33 h) (▴) stages. Incubation was continued for 4 h, cells were washed twice and resuspended in fresh complete medium. (B) Time course of P. falciparum growth inhibition. T4 was added at 40 nM to synchronized cultures at ring, trophozoite, and schizont stages (see symbols above). After incubation for the indicated times, cells were washed twice and resuspended in fresh complete medium. For both of the experiments, [3H]hypoxanthine (0.7 μCi per well; 1 Ci = 37 GBq) was added at 52 h to monitor parasite viability. Reactions were stopped at 76 h, and parasitemia were evaluated for each stage and expressed as a percentage of the control (without drug). The results are expressed as means ± SEM (n ≥ 3).
Synchronized cultures of infected erythrocytes were briefly pulsed with 40 nM T4 (Fig. 3B). Short exposure of ring and trophozoite stages to T4 blocked parasite development; remarkably, 1 h was sufficient to obtain 99% and 85% growth inhibition, respectively, and 85% inhibition required <2 h during schizont development, indicating a potent nonreversible cytotoxic effect. Similar patterns of activity were observed with other compounds, although subtle differences related to the structure may occur (data not shown).
T4 Accumulates Specifically in Infected Cells and Selectively Inhibits de Novo PC Biosynthesis. During a 2-h incubation, T4 accumulated strongly within infected erythrocytes, reaching levels 230- to 310-fold higher (n = 2) than in the incubation medium. T4 was not significantly accumulated within uninfected erythrocytes (cellular accumulation ratio of 1:3), indicating that the process causing drug accumulation is linked to the parasite. Measurement of T4 intracellular distribution into parasitized erythrocytes revealed that 60% of total uptake was within the parasite. Once inside the parasite, analysis using a discontinuous sucrose gradient revealed that 20% of T4 associated with the heme recovered from the 0.2 M fraction (Fig. 4A).
Fig. 4.
Mechanism of action of the compounds. (A) Intracellular drug accumulation and drug interaction with hemozoin evaluated by LC/ESI-MS. Uninfected (RBC) or trophozoite-infected red blood cells (IRBC) (19% parasitemia) were cultured with T4 (50 nM for 2 h) and washed before analysis of total incorporation into RBC and IRBC (summed white, gray, and black bars). IRBCs were then treated to obtain free parasites [streptolysin (39) at 15 hemolytic per ml for 6 min at 20°C] that were analyzed for T4 (summed gray and black bars). Free parasites were sonicated and were overlaid onto a discontinuous sucrose gradient (34) from which the 2M gradient fraction (where hemozoin is recovered) was also analyzed for T4 (black bar). (B) Effect of T4 on the incorporation of 20 μM [3H]choline (2 μCi per well) (•) or 2 μM [3H]ethanolamine (1 μCi per well) (□) into macromolecules. Effects were monitored in microtiter plates (40). After 30 min at 37°C, radioactive precursors were added (50 μl) for 3 h by using the candle jar method (14). Incorporation of [3H]hypoxanthine (0.7 μCi per well) into nucleic acids (▴) was monitored in the same experiment to determine specific effects.
Because bisquaternary ammoniums are among the most potent choline antagonists, we evaluated the effect of T4 on biosynthesis of the two major plasmodial phospholipids, PC and phosphatidylethanolamine, and on nucleic acid biosynthesis. After drug treatment (3 h), T4 specifically inhibited choline incorporation into PC (Fig. 4B). The effect was substantial at 0.4 μM and the amount of biosynthesized PC was reduced by 50% at 0.9 μM. T4 had no significant effect on ethanolamine and hypoxantine incorporation below 10 μM.
Prodrugs Are Highly Effective in Vivo. Antimalarial activity was first tested in mice infected with erythrocytic P. vinckei parasites. Drugs were given i.p. once a day for 4 consecutive days. T3, T4, and their respective bioprecursors, TE3 and TE4a, all had outstanding antimalarial activity (ED50 of 0.1-0.25 mg/kg; Table 1). TE4gt had somewhat lower activity (ED50 of 3.4 mg/kg). Prodrugs were also tested after oral administration. TE4gt completely cleared the parasitemia, but the ED50 was high (90 mg/kg). TE4a and TE3 had much higher activity (ED50 of 11 and 5 mg/kg, respectively). Irrespective of the compound and mode of administration, very steep dose-response curves were obtained; activities occurred over <2 log-dose concentrations. Drug treated (4- to 5-fold ED50) parasites did not recrudesce.
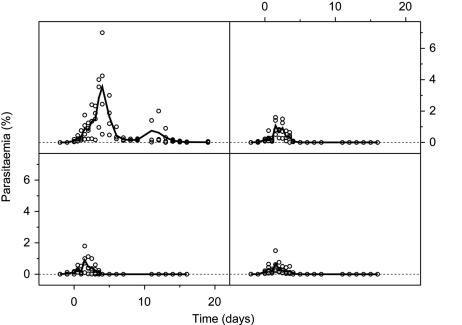
We subsequently tested TE3 against P. cynomolgi in the rhesus monkey (10). Oral dosages (27, 9, and 3 mg/kg once a day for 4 consecutive days) were based on ED50 values obtained in mice (5 mg/kg). Parasite development (an initial increase in parasite numbers followed by a decrease and subsequent recrudescence) was similar to that previously reported (10). All doses were rapidly and completely curative (P = 0.001); recrudescence occurred in all untreated animals but was absent in all TE3-treated animals (Fig. 5). Blood from control and experimental monkeys on each of the last 3 days of drug treatment was washed and used to initiate in vitro parasite cultures. Parasites in treated animals did not develop further, whereas those from control monkeys did, indicating accumulation inside infected erythrocytes and irreversible drug effects.
Fig. 5.
In vivo antimalarial efficacy of TE3 against P. cynomolgi-infected rhesus monkeys. The parasitemia of individual monkeys in each group (○) is shown along with a line depicting the weighted average. (Left Upper) No treatment. (Right Upper) Treatment with 3 mg/kg. (Left Lower) Treatment with 9 mg/kg. (Right Lower) Treatment with 27 mg/kg.
Compounds Are Suitable for Curing High Parasitemia and for Short-Course Treatment. The high potency of this class of compounds prompted us to assess whether they would be curative at high parasitemia. Mice were infected with 107 or 108 P. vinckei parasites and treated i.p. with T3 1 day later. At 0.4 ± 0.002% (n = 20) initial parasitemia, ED50 and ED90 were 0.2 and 0.49 mg/kg, respectively. Interestingly, at high initial parasitemia (9.7 ± 0.02%, n = 25), T3 still exerted antimalarial activity over a narrow concentration range, with ED50 and ED90 of 0.5 and 1.1 mg/kg, respectively (Fig. 6). In pilot studies, we determined the ED50 of T3 and T4 after short-course daily treatments. ED50 of T3 ranged from 0.2 (4 days) to 0.55 and 0.6 mg/kg (3 and 2 days, respectively). After a single administration of T3 and T4, parasite clearance also occurred with an ED50 of 1.1 and 0.4 mg/kg (Fig. 6). Remarkably, a single administration also cured very high initial parasitemias without recrudescence [ED50 of 2.9 and 1.7 mg/kg for T3 (Fig. 6) and T4].
Fig. 6.
In vivo antimalarial properties of TE3 against P. vinckei-infected mice. Mice were i.v.-infected with 107 or 108 parasites, leading to parasitemia of 0.5% (▪ and □)or9%(• and ○) on day 1. Treatments consisted in either a one daily i.p. injection for 4 consecutive days (closed symbols) or one single injection on day 1 (open symbols). Results are the mean of at least four mice per dosage ± SEM.
Thiazolium Compounds Have Promising Pharmacokinetic and Toxicological Properties. The absolute bioavailability of the TE3 compound after oral administration to Sprague-Dawley rat was 16% (F.B. and H.J.V., unpublished work). In rhesus monkeys, TE3 was rapidly absorbed from the gastrointestinal tract and rapid prodrug/drug conversion occurred. The prodrug was not detected in any plasma samples. Thirty minutes after the first oral dose, T3 plasma concentrations ranged from 53 to 80 ng/ml. The last day of treatment, low trough concentrations were observed (35.5 ng/ml after 27 mg/kg); 30 min after the final dose, plasma concentrations ranged from 85 to 171 ng/ml. These results suggest that absorption is not a simple first-order process (22). The accumulation ratio (23) was ≈1.8, indicating an elimination half-life of 15-20 h in monkey. The drugs were well tolerated at all doses given to rhesus monkeys and mice in the above experiments. Preliminary toxicological tests were performed in mice to determine acute toxicity after a single administration in a stepwise procedure. No sign of clinical toxicity was observed after i.p. administration of drugs and prodrugs. Similarly, no clinical signs of toxicity were observed after oral administration of at least 1 g/kg of the prodrugs.
Discussion
Clinical malaria is caused by the asexual intraerythrocytic stages of parasite development, and membrane biogenesis is critical to this development. In the quasiabsence of cholesterol, the phospholipid erythrocyte content is increased >5-fold, constituting the bulk of parasite lipids. PC, the major lipid, is mainly synthesized endogenously (24). Bisquaternary ammonium compounds such as G25, which mimic choline structure (7) and lead to subsequent inhibition of de novo PC synthesis, have received recent attention due to their potent antimalarial activity (10, 11). However, intrinsic low oral permeability has been a major, seemingly insurmountable barrier to their oral use. This finding prompted us to create a system to deliver orally administered ammonium compounds to the blood (25). As a first step, exploratory research led to the design of thiazolium salts in which the quaternary compounds are modified by nitrogen substitution, a duplication of the molecule, linkage by one long lipophilic chain, and removal of the choline hydroxyethyl group, which proved to be a nonessential feature (data not shown). T3 and T4 are two examples of this approach (Fig. 1B) that exerts potent antimalarial activity. However, the oral antimalarial ED50 for T3 could not be determined because it was >10 mg/kg (data not shown).
Nevertheless, this design gave a platform from which we attempted to overcome the low bioavailability of these drugs when delivered orally by creating prodrugs with a hydrolyzable thioester bond that could be bioconverted to the active form in the blood (26, 27). One compound (TM1) lacking this hydrolyzable thioester, is devoid of antimalarial activity. In contrast, prodrugs TE3 and TE4a (neutral molecules) and TE4gt (a tertiary amine) undergo esterase-mediated rearrangement that leads to stable, cyclic thiazolium compounds with antimalarial activity. Importantly, all three prodrugs had in vitro activity similar to the parent drugs, indicating that prodrug-drug conversion was nearly quantitative. Both drugs and prodrugs had very potent antimalarial activity in vivo. Evaluation against the lethal rodent malaria P. vinckei revealed an ED50 as low as 0.1 mg/kg after i.p. administration and complete cure without recrudescence at doses < 1 mg/kg. Thus, this class of compounds appears ≈10-fold more active than CQ (28, 29) or artemisinin derivatives (30, 31) and as active as atovaquone (28) in the rodent model.
These encouraging results led us to increase the stringency of our tests. Significantly, T3 and T4 had an ED50 ≤1 mg/kg after a single administration. Drug activities were also evaluated at high parasitemia. To sidestep the mouse immune response against the parasite, these experiments were achieved by means of i.v. injections of 108 parasitized erythrocytes, mice being treated 24 h later at 9-10% parasitemia. Notably, a single administration of T3 or T4 was able to completely clear this high parasitemia without recrudescence with an ED50 of <3 mg/kg.
To demonstrate that the prodrug approach was effective in primates, we evaluated TE3 in rhesus monkeys infected with P. cynomolgi. We observed a complete cure without recrudescence (i. e., ED100) after four daily oral doses of 3 mg/kg; notably, the ED50 in mice was 5 mg/kg. After the second dose, parasites were unable to develop further ex vivo, suggesting that fewer than four doses were required for complete cure. Other results are equally encouraging and indicate that, in terms of curative dose, P. falciparum and P. cynomolgi infections in primates are 10- to 20-fold more sensitive to our compounds than malaria in rodents (data not shown). Quantitative assays on blood from infected and noninfected monkeys indicated an elimination half-life of 10-20 h and suggested the presence of parasites had no effect on drug pharmacokinetics. TE3 promises to have a very good therapeutic index, with an absence of symptoms in mice at doses of 20 mg/kg i.p. and 1,000 mg/kg orally.
The promise of this approach is enhanced because of the following. (i) Bisthiazolium compounds specifically accumulate inside their target cells, i.e., infected erythrocytes. (ii) Biosynthesis of PC, the main parasite phospholipid, was highly inhibited by drug concentrations that did not effect DNA or synthesis of other phospholipids (Fig. 4B). We have already reported a positive relationship between the ability of the drugs to inhibit parasite growth and inhibition of PC biosynthesis (6, 11). Use of yeast as a surrogate system to identify the targets of our antimalarial compounds indicate that G25 specifically targets PC synthetic pathways (32). We have also characterized the choline carrier of P. falciparum parasite. T16, a radiolabeled compound very similar to T3 and T4, potently inhibits parasite choline uptake with an IC50 of 140 nM (33). Increasing the choline concentration in the medium causes a dose-dependent inhibition of T16 uptake that is directly proportional to the inhibition of antimalarial activity. (iii) Bisthiazolium drugs associated with parasite hamozoin (Fig. 4A). We recently showed interaction of a thiazolium-bisquaternary ammonium with parasite-derived heme; this interaction was critical for drug accumulation and antimalarial activity (34). Thus, there is evidence that this class of compounds has a dual mode of action on the intracellular parasite, affecting PC biosynthetic activity and interacting with the toxic hemoglobin metabolite. This dual compound would mimic a drug combination, as corroborated by the fact that resistance of the malarial parasite to G25, a compound that shares the same mechanism of action as T3/T4, could not be induced under drug pressure of several months (ref. 35 and data not shown). The interaction with heme might suggest that the modes of action of this class of compounds resemble those of other heme-interaction antimalarials (36-38). However, they have shown high efficacy against multiresistant P. falciparum field isolates and genetically modified P. falciparum clones expressing mutant forms of the CQ resistance determinant pfcrt (P. Ringwald and D. A. Fidock, unpublished work), and we have evidence of distinct action toward heme polymerization (H.J.V. and S. A. Ward, unpublished work).
In conclusion, these compounds are at the forefront of therapeutic malarial research. The prodrug approach used to deliver bisthiazolium compounds demonstrated complete protection in murine models, even at high initial parasitemia or by using short-course treatment. Moreover, and very significantly, these compounds provided complete cure of P. cynomolgi infection in rhesus monkeys, showing valuable pharmacokinetic properties. Efficacy and tolerance studies in mice and primates were very promising, indicating that this approach could be applicable for human infection; future detailed toxicological studies are warranted. Importantly, the clinical potential of this series of phospholipid metabolism inhibitors extends to pharmacoresistant malaria treatment and paves the way for a wide range of potentially active compounds. Detailed studies to unravel the dual aspect of their mechanisms of action are also necessary.
Supplementary Material
Acknowledgments
We thank Rob Ridley and P. Olliaro for reading the manuscript; M. L. Roudière, E. Richier, E. Vivien, J. J. Bourguignon, M. Maynadier, M. Dubbeld, and J. Verburgh for their expertise and skillful assistance; E. Remarque for assistance with statistical analyses; and J. L. Aubagnac and C. Enjalbal (Université Montpellier II) for their analytical assistance. This work was supported by European Community Grant QLK2-CT-2000-01166, the Ministère de l'Education Nationale et Recherche Scientifique (PAL+), and the United Nations Development Program/World Bank/World Health Organization special program for Research and Training in Tropical Diseases.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: G25, [1,16-hexadecamethylenebis(N-methylpyrrolidinium) dibromide]; LC/ESI-MS, liquid chromatography/electrospray ionization MS; PC, phosphatidylcholine; CQ, chloroquine.
References
- 1.Breman, J. G. (2001) Am. J. Trop. Med. Hyg. 64, 1-11. [DOI] [PubMed] [Google Scholar]
- 2.Sachs, J. & Malaney, P. (2002) Nature 415, 680-685. [DOI] [PubMed] [Google Scholar]
- 3.Olliaro, P. L. & Yuthavong, Y. (1999) Pharmacol. Ther. 81, 91-110. [DOI] [PubMed] [Google Scholar]
- 4.Ridley, R. G. (2002) Nature 415, 686-693. [DOI] [PubMed] [Google Scholar]
- 5.Biagini, G. A., O'Neill, P. M., Nzila, A., Ward, S. A. & Bray, P. G. (2003) Trends Parasitol. 19, 479-487. [DOI] [PubMed] [Google Scholar]
- 6.Ancelin, M. L., Calas, M., Bompart, J., Cordina, G., Martin, D., Ben Bari, M., Jei, T., Druilhe, P. & Vial, H. J. (1998) Blood 91, 1426-1437. [PubMed] [Google Scholar]
- 7.Calas, M., Ancelin, M. L., Cordina, G., Portefaix, P., Piquet, G., Vidal-Sailhan, V. & Vial, H. (2000) J. Med. Chem. 43, 505-516. [DOI] [PubMed] [Google Scholar]
- 8.Fisher, A. & Hanin, I. (1980) Life Sci. 27, 1615-1634. [DOI] [PubMed] [Google Scholar]
- 9.Holden, J. T., Rossier, J., Beaujouan, J. C., Guyenet, P. & Glowinski, J. (1975) Mol. Pharmacol. 11, 19-27. [PubMed] [Google Scholar]
- 10.Wengelnik, K., Vidal, V., Ancelin, M. L., Cathiard, A. M., Morgat, J. L., Kocken, C. H., Calas, M., Herrera, S., Thomas, A. W. & Vial, H. J. (2002) Science 295, 1311-1314. [DOI] [PubMed] [Google Scholar]
- 11.Ancelin, M. L., Calas, M., Bonhoure, A., Herbute, S. & Vial, H. J. (2003) Antimicrob. Agents Chemother. 47, 2598-2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ancelin, M. L., Calas, M., Vidal-Sailhan, V., Herbute, S., Ringwald, P. & Vial, H. J. (2003) Antimicrob. Agents Chemother. 47, 2590-2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calas, M., Cordina, G., Bompart, J., Ben Bari, M., Jei, T., Ancelin, M. L. & Vial, H. (1997) J. Med. Chem. 40, 3557-3566. [DOI] [PubMed] [Google Scholar]
- 14.Trager, W. & Jensen, J. B. (1976) Science 193, 673-675. [DOI] [PubMed] [Google Scholar]
- 15.Lambros, C. & Vanderberg, J. P. (1979) J. Parasitol. 65, 418-420. [PubMed] [Google Scholar]
- 16.Desjardins, R. E., Canfield, C. J., Haynes, J. D. & Chulay, J. D. (1979) Antimicrob. Agents Chemother. 16, 710-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shah, V. P., Midha, K. K., Dighe, S., McGilveray, I. J., Skelly, J. P., Yacobi, A., Layloff, T., Viswanathan, C. T., Cook, C. E., McDowall, R. D., et al. (1992) J. Pharm. Sci. 81, 309-312. [DOI] [PubMed] [Google Scholar]
- 18.Bressolle, F., Bromet-Petit, M. & Audran, M. (1996) J. Chromatogr. B Biomed. Appl. 686, 3-10. [DOI] [PubMed] [Google Scholar]
- 19.Shah, V. P., Konecny, J. J., Everett, R. L., McCullough, B., Noorizadeh, A. C. & Skelly, J. P. (1989) Pharm. Res. 6, 612-618. [DOI] [PubMed] [Google Scholar]
- 20.Barkan, D., Ginsburg, H. & Golenser, J. (2000) Int. J. Parasitol. 30, 649-653. [DOI] [PubMed] [Google Scholar]
- 21.Amidon, G. L., Lennernas, H., Shah, V. P. & Crison, J. R. (1995) Pharm. Res. 12, 413-420. [DOI] [PubMed] [Google Scholar]
- 22.Mayersohn, M. (2002) in Modern Pharmaceutics, eds. Banker, G. S. & Rhodes, C. T. (Dekker, New York), pp. 23-66.
- 23.Bressolle, F., Galtier, M., Kinowski, J. M., Goncalves, F., Edno, L., Panis, R. & Gomeni, R. (1994) J. Pharm. Sci. 83, 1236-1240. [DOI] [PubMed] [Google Scholar]
- 24.Vial, H. & Ancelin, M. (1998) in Malaria: Parasite Biology, Biogenesis, Protection, ed. Sherman, I. (Am. Soc. Microbiol., Washington DC), pp. 159-175.
- 25.Wermuth, C. G. (1996) in The Practice of Medicinal Chemistry, ed. Wermuth, C. G. (Academic, London), pp. 697-715.
- 26.Bodor, N. & Kaminski, J. J. (1987) Annu. Rep. Med. Chem. 22, 303-313. [Google Scholar]
- 27.Yoshikawa, T., Sakaeda (nee Kakutani), T., Sugawara, T., Hirano, K. & Stella, V. J. (1999) Adv. Drug Delivery Rev. 36, 255-275. [DOI] [PubMed] [Google Scholar]
- 28.Alzeer, J., Chollet, J., Heinze-Krauss, I., Hubschwerlen, C., Matile, H. & Ridley, R. G. (2000) J. Med. Chem. 43, 560-568. [DOI] [PubMed] [Google Scholar]
- 29.Ridley, R. G., Matile, H., Jaquet, C., Dorn, A., Hofheinz, W., Leupin, W., Masciadri, R., Theil, F. P., Richter, W. F., Girometta, M. A., et al. (1997) Antimicrob. Agents Chemother. 41, 677-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peters, W., Li, Z. L., Robinson, B. L. & Warhurst, D. C. (1986) Ann. Trop. Med. Parasitol. 80, 483-489. [DOI] [PubMed] [Google Scholar]
- 31.O'Neill, P. M., Miller, A., Bishop, L. P., Hindley, S., Maggs, J. L., Ward, S. A., Roberts, S. M., Scheinmann, F., Stachulski, A. V., Posner, G. H. & Park, B. K. (2001) J. Med. Chem. 44, 58-68. [DOI] [PubMed] [Google Scholar]
- 32.Roggero, R., Zufferey, R., Nastase, M., Richier, E., Calas, M., Vial, H. & Ben Mamoun, C. (2004) Antimicrob. Agents Chemother. 48, 2816-2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Biagini, G. A., Pasini, E., Hughes, R. H., De Koning, H. P., Vial, H., Ward, S. A. & Bray, P. G. (2004) Blood, in press. [DOI] [PubMed]
- 34.Biagini, G. A., Richier, E., Bray, P. G., Calas, M., Vial, H. & Ward, S. A. (2003) Antimicrob. Agents Chemother. 47, 2584-2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rathod, P. K., McErlean, T. & Lee, P. C. (1997) Proc. Natl. Acad. Sci. USA 94, 9389-9393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bray, P. G., Mungthin, M., Ridley, R. G. & Ward, S. A. (1998) Mol. Pharmacol. 54, 170-179. [DOI] [PubMed] [Google Scholar]
- 37.Kurosawa, Y., Dorn, A., Kitsuji-Shirane, M., Shimada, H., Satoh, T., Matile, H., Hofheinz, W., Masciadri, R., Kansy, M. & Ridley, R. G. (2000) Antimicrob. Agents Chemother. 44, 2638-2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olliaro, P. L., Haynes, R. K., Meunier, B. & Yuthavong, Y. (2001) Trends Parasitol. 17, 122-126. [DOI] [PubMed] [Google Scholar]
- 39.Ansorge, I., Benting, J., Bhakdi, S. & Lingelbach, K. (1996) Biochem. J. 315, 307-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ancelin, M. L., Vialettes, F. & Vial, H. J. (1991) Anal. Biochem. 199, 203-209. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.