Abstract
Spinal cord injury (SCI) is an extremely serious type of physical trauma observed in clinics. Neuropathic pain resulting from SCI has a lasting and significant impact on most aspects of daily life. Thus, a better understanding of the molecular pathways responsible for the cause of neuropathic pain observed in SCI is important to develop effective therapeutic agents and treatment strategies. Mammalian target of rapamycin (mTOR) is a serine/threonine protein kinase that is well known for its critical roles in regulating protein synthesis and growth. Furthermore, compelling evidence supports the notion that widespread dysregulation of mTOR and its downstream pathways are involved in neuropathic pain. Thus, in this study we specifically examined the underlying mechanisms by which mTOR and its signaling pathways are involved in SCI-evoked neuropathic pain in a rat model. Overall, we demonstrated that SCI increased the protein expression of p-mTOR, and mTORmediated- phosphorylation of 4E–binding protein 4 (4E-BP1) and p70 ribosomal S6 protein kinase 1 (S6K1) in the superficial dorsal horn of the spinal cord. Also, we showed that blocking spinal mTOR by intrathecal injection of rapamycin significantly inhibited pain responses induced by mechanical and thermal stimulation. In addition, blocking spinal phosphatidylinositide 3-kinase (p-PI3K) pathway significantly attenuated activities of p-mTOR pathways as well as mechanical and thermal hyperalgesia in SCI rats. Moreover, blocking mTOR and PI3K decreased the enhanced levels of substance P and calcitonin gene-related peptide (CGRP) in the dorsal horn of SCI rats. We revealed specific signaling pathways leading to SCI-evoked neuropathic pain, including the activation of PI3K, mTOR and its downstream signaling pathways. Targeting one or more of these signaling molecules may present new opportunities for treatment and management of neuropathic pain often observed in patients with SCI.
Keywords: Mammalian target of rapamycin (mTOR) protein kinase, Neuropathic pain, Spinal cord injury
Introduction
Spinal cord injury (SCI) is an extremely serious type of physical trauma observed in medical practice and affects the quality of life of more than 2 million people worldwide [1]. One of the most common and distressing symptoms suffered by patients with SCI is chronic neuropathic pain. Thus, it is clinically important to develop effective therapeutic agents and treatment strategies for SCI patients suffering from neuropathic pain. In general, treatment options for these abnormal sensations have been restricted, partly due to a poor understanding of the underlying mechanisms responsible for neuropathic pain induced by SCI.
Notably, SCI leads to obvious changes in the synaptic circuits in the dorsal horn and the areas rostral to the site of injury through a variety of mechanisms [2], such as abnormalities in the expression and activity of receptors and ion channels, release of local inflammatory cytokines and reactive oxygen species, activation of the immune response in microglia and other immune cells, and the activation of intracellular cascades. Nonetheless, the precise mechanisms remain to be elucidated.
Mammalian target of rapamycin (mTOR) is a serine/threonine protein kinase. Activation of mTOR, in particular mTOR complex 1 (mTORC1), which is more sensitive to rapamycin, leads to promotion of the phosphorylation of downstream effectors, such as p70 ribosomal S6 protein kinase (p70S6K) and this further governs mRNA translation [3]. Thus, mTORC1 is well known to play a critical role in regulating protein synthesis and growth [4, 5].
In addition, prior studies have strongly supported the notion that mTOR plays an important role in the modulation of longterm neuronal plasticity [6, 7]. Specifically, mTOR and its downstream effectors have been identified in dorsal root ganglion and spinal cord dorsal horn as major regions in the process of nociception and recent studies further suggest that mTOR contributes to transmission and modulation of nociceptive information [8]. For example, intrathecal administration of rapamycin, a specific inhibitor of mTOR, produces antinociception in models of inflammation [8-10]. Local perfusion of rapamycin into the spinal cord significantly attenuates formalin-induced neuronal hyperexcitability in the dorsal horn [11]. These findings indicate that mTOR and its downstream effectors are likely activated and play an important role in the development of spinal central sensitization under persistent pain conditions.
Therefore, we suspected that mTOR is likely an important player for the induction and maintenance of SCI induced-neuropathic pain. As a key region of pain regulation [12], the spinal cord dorsal horn is likely engaged in the effects of mTOR on SCI-induced pain [10]. We specifically hypothesized that SCI increases the protein expression of mTOR and its downstream pathways in the superficial dorsal horn, resulting in mechanical and thermal hypersensitivity. Blocking mTOR pathways attenuates pain responses evoked by mechanical and thermal stimulation. We also hypothesized that blocking spinal phosphatidylinositide 3-kinase (PI3K) attenuates activities of mTOR pathways, thereby leading to a reduction in mechanical and thermal sensitivity.
Materials and methods
Animals
All animal protocols were approved by the Animal Care and Use Committee of our Medical Research Institute, and were carried out in accordance with the guidelines of the International Association for the Study of Pain. Male Wistar rats weighing 200-250 g were obtained from the Center for Experimental Animal Sciences. The rats were housed in individual cages with free access to food and water and were kept in a temperature-controlled room (25°C) on a 12/12 h light/dark cycle.
A model of spinal cord injury
There are a number of animal models generally used to study the mechanisms of spinal cord injury. For example, SCI is induced by epidural balloon inflation and by application of an impactor on the spinal cord of the rat [14-16]. In the current study, all the rats were anesthetized by sodium pentobarbital (60 mg/kg, intraperitoneally) and a laminectomy was then performed to expose spinal segment T10. The Infinite Horizon impactor (Precision Systems and Instrumentation, Lexington, KY, USA, 0.0015 N, 1 s dwell time) was used to produce a contusive spinal injury [14]. Following the injury, the musculature and skin were sutured. The animals were allowed to recover from the surgery. A subcutaneous injection of 0.3 mL of enrofloxacin (25 mg/mL twice daily) was given for 7 days and bladders were manually expressed twice daily. Behavioral test to examine mechanical allodynia and thermal hyperalgesia was performed 4 weeks following SCI.
Intrathecal catheter for administration of drugs
The rats were anesthetized by sodium pentobarbital (60 mg/kg, intraperitoneally) in order to implant an intrathecal catheter for administration of drugs 3 days prior to each experiment. Briefly, one end of polyethylene-10 tubing was inserted intrathecally through an incision in the cisternal membrane and advanced 7-9 cm caudal until the tip of the catheter was positioned at the lumbar spinal level (L5 to L6). The other end of the intrathecal tubing was sutured to the musculature and skin at the incision site and externalized to the back of the rat. In each experiment, a Hamilton microsyringe (250 μL) was connected to the intrathecal tubing and used to deliver 100 μl of dimethyl sulfoxide (DMSO) as vehicle control, antagonists to mTOR (rapamycin, 10 μg, obtained from Selleck Chem, Houston, TX, USA) and inhibitor of PI3K (LY294002, 10 μM, obtained from Sigma-Aldrich, St. Louis, MO, USA).
In a subset of studies, in order to examine the effects of mTOR and PI3K on the levels of substance P and CGRP and the effects of blocking PI3K on mTOR expression, rapamycin (10 μg) and LY294002 (10 μM) were intrathecally given using an infusion pump in control rats and rats following SCI, respectively. The pump was set to constantly deliver vehicle or the drugs over a period of 3 h. At the end of infusion, the superficial dorsal horn tissues were obtained under an anatomical microscope for Western Blot and ELISA experiments.
Behavioral test
To quantify the mechanical sensitivity of the hind paw, rats were placed in individual plastic boxes and allowed to acclimate for > 30 min. Mechanical paw withdrawal threshold (PWT) of a rat hind paw in response to the stimulation of von Frey filaments was determined. A series of calibrated von Frey filaments (ranging from 0.5 to 18.0 g) were applied perpendicularly to the plantar surface of the hind paw with a sufficient force to bend the filaments for 60 s or until paw withdrew. In the presence of a response, the filament of next lower force was applied. In the absence of a response, the filament of next greater force was applied. To avoid injury during tests, the cutoff strength of the von Frey filament was 18 g. The tactile stimulus producing a 50% likelihood of withdrawal was determined using the “up-down” method [17]. Each trial was repeated two times at approximately 2 min intervals. The mean value was used as the force producing a withdrawal response [18].
To determine thermal hyperalgesia, rat paw withdrawal latency (PWL) to a radiant heat was measured as described previously [19]. Rats were placed individually in plastic cages on an elevated glass platform and allowed for 30 min acclimation. Each hind paw received three stimuli with a 10 min interval, and the mean of the three withdrawal latencies was defined as PWL. The heat was maintained at a constant intensity. To prevent tissue damage, the cut-off latency was set at 20 s. All the behavioral tests were performed in a blind style.
Western blot analysis
The rats were euthanized and the superficial dorsal horn (lumbar 4-6) tissues were dissected under an anatomical microscope. The tissues were then homogenized in ice-cold radioimmunoprecipitation assay buffer containing 25 mM Tris·HCl (pH 7.6), 150 mM NaCl, 1% Nonidet P-40, 1% sodium deoxycholate, and 0.1% sodium dodecyl sulfate (SDS) with protease inhibitor cocktail kit (Sigma-Aldrich, St. Louis, MO). Then the tissues were centrifuged. The supernatant was then diluted to the same volume and applied to SDS-PAGE. Membranes were incubated with the rabbit anti-p-mTOR/p-S6K1/p-4EBP1antibodies; rabbit anti-mTOR /S6K1/4EBP1 antibodies; rabbit anti-p-PI3K p85/ anti- PI3K p85, respectively. All these primary antibodies were purchased from the Abcam Company (Cambridge, UK); and goat antirabbit secondary antibodies were purchased from Santa Cruz Biotechnology (Dallas, TX, USA). Immunoreactive proteins were detected by enhanced chemiluminescence. The membrane was also processed to detect β-actin for equal loading. The optical density of protein bands was first analyzed using the NIH Scion image Software ImageJ (http://rsb. info.nih.gov/ij/), and values for densities of immunoreactive bands/β-actin band densities from the same lane were determined. Each of the values was then normalized to a control sample.
ELISA measurements
To examine the levels of substance P and CGRP in the superficial dorsal horn of the spinal cord (L4-L6), enzyme-linked immunosorbent assay (ELISA) methods were employed. Substance P was measured using substance P ELISA kit following the manufacturer’s instructions (Abcam Company, Cambridge, UK). Briefly, the diluted tissue supernatant (100 μl) was placed in a 96-well goat anti-mouse IgGcoated plate and incubated for 2 hours. After incubation, the plate was washed using the provided washing buffer, and the color was developed by adding p-nitrophenyl phosphate (pNPP, 200 μl) substrate after 45 min and determined by an ELISA plate reader. The amount of substance P was calculated by using a substance P standard curve. In a similar way, the CGRP content of the samples (100 μl supernatant) was determined using a commercial CGRP ELISA kit (Cayman Chemical Company, Ann Arbor, MI, USA). Briefly, the diluted samples were placed in a 96-well plate incubated with pre-coated anti-rat IgG antibody overnight, washed and developed, and quantified [20].
Statistical analysis
All data were analyzed using a one-way analysis of variance. Values were presented as means ± standard deviation (SD). For all analyses, differences were considered significant at level of α < 0.05. All statistical analyses were performed by using SPSS for Windows version 13.0 (SPSS Inc., Chicago, IL, USA).
Results
First, we examined responses to the mechanical and thermal stimulation prior to SCI surgery in order to obtain baseline values (number of rats = 79). Basal PWT was 15.5 ± 4.5 g (means ± SD) and basal PWL was 13.3 ± 3.2 s (means ± SD). Mechanical allodynia and thermal hyperalgesia began to appear 2 weeks after induction of SCI and lasted for 4 weeks. In those rats, PWT was 5.4 ± 1.5 g (means ± SD; P = 0.0085 vs. baseline) and PWL was 5.5 ± 1.7 s (means ± SD; P = 0.0078 vs. baseline) 4 weeks following SCI. Animals (~5%) that did not display increases in mechanical and thermal sensitivity of at least 40% of baseline values were excluded from the experiment [14]. In general, about 90-95% of rats that receive SCI procedures display pain responses and they are considered to be included in the study [14, 21].
Effects of mTOR and PI3K on SCIinduced hyperalgesia
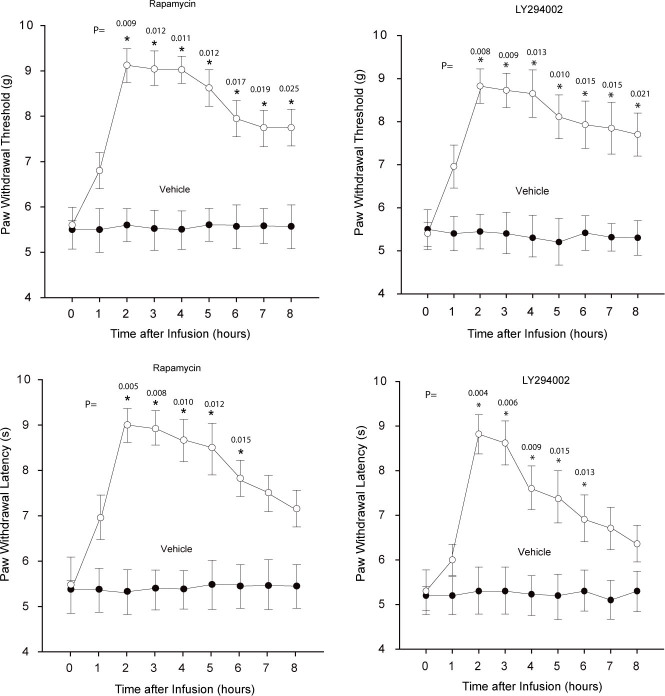
After mechanical and thermal hyperalgesia were well established in rats, rapamycin and LY294002 were intrathecally injected. Mechanical and thermal sensitivities were examined 0, 1, 2, 3, 4, 5, 6, 7 and 8 h after injection of the drugs. Figure 1 (left panel) showed that rapamycin (number of rats =15) significantly attenuated SCI-induced mechanical and thermal hyperalgesia (all P values < 0.05 vs. vehicle control, number of rats = 10). The inhibitory effects of rapamycin on mechanical and thermal hyperalgesia began from about 60 min after its administration, peaked at 2-3 h and lasted for 8 h (PWT) and 6 h (PWL). Similarly, Figure 1 (right panel) demonstrated that LY294002 had significant attenuating effects on SCI-induced mechanical and thermal hyperalgesia in a time manner (all P values < 0.05, LY294002 vs. vehicle, number of rats = 12 for each group). At this dose, the effects of LY294002 appeared at about 60 min, peaked at 2-3 h and lasted for about 8 h (PWT) and ~6 h (PWL) after injection.
Figure 1.
Effects of blocking mTOR and PI3K pathways on SCI-evoked mechanical and thermal hyperalgesia. Intrathecal administration of rapamycin (left panel) and LY294002 (right panel) increased PWT and PWL in SCI rats. *all P values < 0.05, indicated vs. vehicle control. Number of rats = 10 in vehicle controls; and number of rats = 15 in group of rapamycin; number of rats = 12 in each of vehicle control LY294002 groups.
Expression of mTOR signaling pathways engaged in SCI-induced hyperalgesia
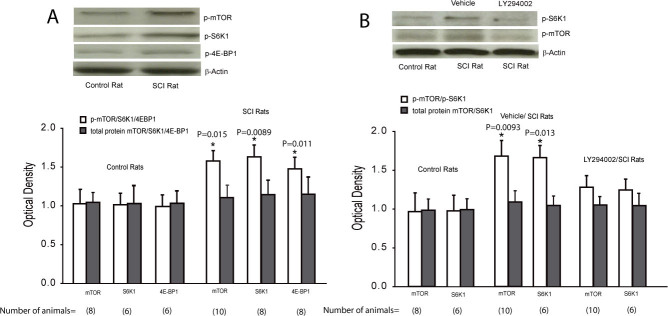
Figure 2A showed the protein expression of p-mTOR, p-S6K1 and p-4E-BP1 as well as mTOR, S6K1 and 4E-BP1 in control rats and SCI rats. SCI significantly increased the protein levels of p-mTOR and mTOR-mediated p-S6K1 and p-4E-BP1 in the superficial dorsal horn as compared with control rats (all P values < 0.05, SCI rats vs. control rats, number of rats = 6-10 in each group). Note that total protein of mTOR, S6K1 and 4E-BP1 levels was not significantly increased in SCI rats. We also examined the effects of blocking PI3K on expression of p-mTOR and p-S6K1. Figure 2B demonstrated that the protein expression of p-mTOR and p-S6K1 was significantly increased in SCI rats (number of rats = 6-10) as compared with control rats (number of rats = 6-8). When LY294002 was infused into the spinal cord of SCI rats via a pump, the amplified activities of p-mTOR and p-S6K1 evoked by SCI were significantly attenuated (number of rats = 6-10 in each group).
Figure 2.
Expression of mTOR pathways in the superficial dorsal horn of the spinal cord of control rats and SCI rats. A. Typical bands and averaged data showing that p-mTOR, p-S6K1 and p-4E-BP1 in the dorsal horn of the spinal cord were upregulated in SCI rats. *all P values <0.05 vs. control rats (number of rats = 6-10 in control and SCI). There were insignificant differences in total protein expression of mTOR, S6K1 and 4E-BP1 in control rats and SCI rats (number of rats = 6-10). B. Typical bands and averaged data showing expression of p-mTOR/mTOR and p-S6K1/S6K1 were amplified in the dorsal horn of SCI rats with vehicle treatment (number of rats = 6-10) as compared with control rats (number of rats = 6-8). Blocking PI3K pathways by LY294002 attenuated increases in p-mTOR and p-S6K1 in SCI rats. *all P values <0.05 vs. control animals and SCI animals infused with LY294002 (number of rats = 6-10 in each group).
The levels of substance P and CGRP
In additional experiments, we examined the effects of SCI on the levels of substance P and CGRP in the superficial dorsal horn of the spinal cord. Figure 3 showed that substance P and CGRP were significantly increased in SCI rats (number of rats = 15) as compared with control rats (number of rats = 15). Furthermore, blocking individual mTOR and PI3K signaling pathways by intrathecal injection of rapamycin and LY294002 (number of rats = 10 in each group) significantly attenuated amplifications in substance P and CGRP evoked by SCI. Note that a greater inhibitory effect on substance P and CGRP was observed by LY294002 compared with rapamycin.
Figure 3.
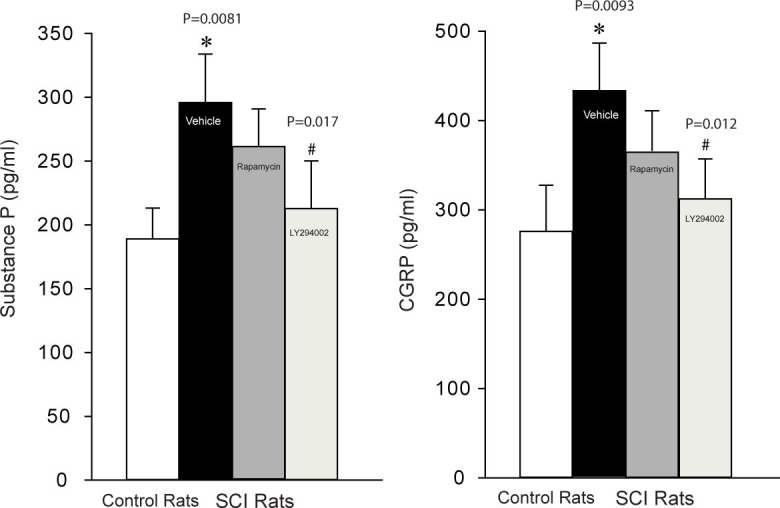
Effects of blocking mTOR and PI3K pathways on the levels of substance P and CGRP in the superficial dorsal horn of the spinal cord. SCI significantly increased substance P and CGRP as compared with controls and blocking mTOR and PI3K by rapamycin and LY294002 significantly attenuated the enhancement in substance P and CGRP evoked by SCI. Note that a greater inhibitory effect on substance P and CGRP was observed by LY294002. *P = 0.0081 (for substance P) and P = 0.0093 (for CGRP), indicated SCI rats (number of rats = 15) vs. control rats (number of rats =15) and SCI rats with rapamycin (n=10) and LY294002 (number of rats =10) injection. #P = 0.017 (for substance) and P = 0.012 (for CGRP), indicated SCI rats with rapamycin injection vs. with LY294002 injection
Discussion
There are two distinct mTOR forms of protein complexes, mTOR complex 1 (mTORC1) and mTORC2. In general, mTORC1 is composed of raptor, mLST8, and mTOR, and is known to function by gate translation of most proteins by phosphorylation of specific downstream effectors including, 4E-BPs and p70 ribosomal S6Ks [22]. mTOR, S6K1, and 4E-BP1 are expressed in the mammalian nervous system, particularly in spinal cord dorsal horn [7, 22]. Because the superficial dorsal horn is the first synaptic site from peripheral afferent nerves to the central nervous system[23, 24] and plays an important role in modulating pain and morphine tolerance [12, 25], in this study we determined the role played by mTOR in the superficial dorsal horn in regulating mechanical and thermal hyperalgesia following development of SCI.
We consistently observed development of mechanical and thermal hyperalgesia in SCI rats. We also demonstrated that expression of p-mTOR, p-S6K1 and p-4E-BP1 in the superficial dorsal horn of SCI rats was upregulated, and mTOR antagonist, rapamycin, injected in the dorsal horn attenuated mechanical and thermal hyperalgesia evoked by SCI (Figures 1 and 2).
The PI3K/Akt pathway is an intracellular signaling pathway in regulating the cell cycle. This important mechanism is directly related to cellular quiescence, proliferation, cancer, and longevity. PI3K can phosphorylate and activate Akt in the plasma membrane [26]. The Akt leads to several downstream effects which alters transcription of p70 ribosomal S6K1 or 4E-BP1 and activating cAMP response elementbinding protein (CREB) and inhibition of p27 etc. [3, 4, 6, 26]. Our study demonstrated that blocking PI3K attenuated p-mTOR and p-S6K1 expression, but also that intrathecal injection of PI3K inhibitor attenuated mechanical and thermal hyperalgesia evoked by SCI (Figures 1 and 2). This suggests that PI3K is necessary to play a regulatory role in mediating the effects of mTOR on SCI evoked-pain responses.
It is well known that stimulation of nociceptive receptors in the sensory nerves leads to the releases of substance P and CGRP in the superficial dorsal horn [27, 28]. In our current study, the levels of substance P and CGRP, as two important neurotransmitters engaged in the neuropathic pain, were significantly increased in the superficial dorsal horn of SCI rats. Moreover, the increased substance P and CGRP were significantly attenuated after respective injection of rapamycin and LY294002. Thus, our data suggests that amplified expression of spinal mTOR and its downstream pathways S6K1 or 4E-BP1 are likely engaged in SCI-induced mechanical and thermal hyperalgesia via the releases of substance P and CGRP. Notably, we observed that LY294002 had a greater effect on substance P and CGRP than rapamycin did, indicating the role played by PI3K pathways in regulating mTOR in hypersensitive pain responses observed in SCI rats. Nevertheless, to the best of our knowledge there is a lack of evidence specifically showing the role played by mTOR and PI3K in regulating the releases of spinal substance P and CGRP in a neuropathic pain model induced by SCI. Results of the present report suggest that substance P and CGRP regulated by mTOR and PI3K at the spinal level contribute to SCI-induced neuropathic pain.
It should be noted that in general, about 90- 95% of rats that receive SCI procedures display pain responses and they are considered to be included in the study [14, 21]. Accordingly, in our current report, 5% of rats that did not display increases in responses to mechanical and thermal stimulation were excluded from the experiment.
In conclusion, in SCI, spinal p-mTOR and mTOR-mediated p-S6K1 and p-4E-BP1 are upregulated, which results in mechanical and thermal hypersensitivity. Moreover, blocking PI3K decreases amplified expression of p-mTOR pathways and thereby leads to blunting of SCI-evoked neuropathic pain. Results of our study will provide a base for the mechanisms responsible for SCI-induced neuropathic pain and further potentially offer a strategy to target the spinal neuronal levels for treatment and management of neuropathic pain often observed in patients with SCI. In addition, targeting one or more of these signaling molecules involved in activation of mTOR evoked by SCI may present new opportunities for treatment and management of neuropathic pain.
Acknowledgments
Conflict of interest statement: The authors declare that they have no conflict of interest.
References
- [1].Fehlings M.G., Wilson J.R., O’Higgins M.. Introduction: Spinal cord injury at the cutting edge of clinical translation: a focus issue collaboration between NACTN and AOSpine North America. J. Neurosurg. Spine. 2012;17:1–3. doi: 10.3171/2012.6.AOSPINE12632. [DOI] [PubMed] [Google Scholar]
- [2].Kumru H., Vidal J., Kofler M., Portell E., Valls-Sole J.. Alterations in excitatory and inhibitory brainstem interneuronal circuits after severe spinal cord injury. J. Neurotrauma. 2010;27:721–728. doi: 10.1089/neu.2009.1089. [DOI] [PubMed] [Google Scholar]
- [3].Hay N., Sonenberg N.. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- [4].Shor B., Gibbons J.J., Abraham R.T., Yu K.. Targeting mTOR globally in cancer: thinking beyond rapamycin. Cell Cycle. 2009;8:3831–3837. doi: 10.4161/cc.8.23.10070. [DOI] [PubMed] [Google Scholar]
- [5].Hess K.R., Varadhachary G.R., Taylor S.H., Wei W., Raber M.N., Lenzi R.. Metastatic patterns in adenocarcinoma. Cancer. 2006;106:1624–1633. doi: 10.1002/cncr.21778. [DOI] [PubMed] [Google Scholar]
- [6].Banko J.L., Poulin F., Hou L., DeMaria C.T., Sonenberg N., Klann E.. The translation repressor 4E-BP2 is critical for eIF4F complex formation. synaptic plasticity, and memory in the hippocampus, J. Neurosci. 2005;25:9581–9590. doi: 10.1523/JNEUROSCI.2423-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Costa-Mattioli M., Sossin W.S., Klann E., Sonenberg N.. Translational control of long-lasting synaptic plasticity and memory. Neuron. 2009;61:10–26. doi: 10.1016/j.neuron.2008.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Geranton S.M., Jimenez-Diaz L., Torsney C., Tochiki K.K., Stuart S.A., Leith J.L.. A rapamycin-sensitive signaling pathway is essential for the full expression of persistent pain states. J. Neurosci. 2009;29:15017–15027. doi: 10.1523/JNEUROSCI.3451-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Price T.J., Rashid M.H., Millecamps M., Sanoja R., Entrena J.M., Cervero F.. Decreased nociceptive sensitization in mice lacking the fragile X mental retardation protein: role of mGluR1/5 and mTOR. J. Neurosci. 2007;27:13958–13967. doi: 10.1523/JNEUROSCI.4383-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Xu Q., Fitzsimmons B., Steinauer J., O’Neill A., Newton A.C., Hua X.Y.. Spinal phosphinositide 3-kinase-Akt-mammalian target of rapamycin signaling cascades in inflammation-induced hyperalgesia. J. Neurosci. 2011;31:2113–2124. doi: 10.1523/JNEUROSCI.2139-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Asante C.O., Wallace V.C., Dickenson A.H.. Formalin-induced behavioural hypersensitivity and neuronal hyperexcitability are mediated by rapid protein synthesis at the spinal level. Mol. Pain. 2009;5:27. doi: 10.1186/1744-8069-5-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hua X.-Y., Yaksh T.L. Malcangio M. Synaptic plasticity in pain. Springer, Berlin; 2009. Dorsal horn substance P and NK1 receptors: study of a model system in spinal nociceptive processing; pp. 109–138. [Google Scholar]
- [13].Ma W., Eisenach J.C.. Intraplantar injection of a cyclooxygenase inhibitor ketorolac reduces immunoreactivities of substance P. calcitonin gene-related peptide, and dynorphin in the dorsal horn of rats with nerve injury or inflammation, Neuroscience. 2003;121:681–690. doi: 10.1016/s0306-4522(03)00497-4. [DOI] [PubMed] [Google Scholar]
- [14].Hassler S.N., Johnson K.M., Hulsebosch C.E.. Reactive oxygen species and lipid peroxidation inhibitors reduce mechanical sensitivity in a chronic neuropathic pain model of spinal cord injury in rats. J. Neurochem. 2014;131:413–417. doi: 10.1111/jnc.12830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Urdzikova L., Jendelova P., Glogarova K., Burian M., Hajek M., Sykova E.. Transplantation of bone marrow stem cells as well as mobilization by granulocyte-colony stimulating factor promotes recovery after spinal cord injury in rats. J. Neurotrauma. 2006;23:1379–1391. doi: 10.1089/neu.2006.23.1379. [DOI] [PubMed] [Google Scholar]
- [16].Vanicky I., Urdzikova L., Saganova K., Cizkova D., Galik J.. A simple and reproducible model of spinal cord injury induced by epidural balloon inflation in the rat. J. Neurotrauma. 2001;18:1399–1407. doi: 10.1089/08977150152725687. [DOI] [PubMed] [Google Scholar]
- [17].Chaplan S.R., Bach F.W., Pogrel J.W., Chung J.M., Yaksh T.L.. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- [18].Bao Y., Gao Y., Hou W., Yang L., Kong X., Zheng H.. Engagement of signaling pathways of protease-activated receptor 2 and muopioid receptor in bone cancer pain and morphine tolerance. Int. J. Cancer. 2015;137:1475–1483. doi: 10.1002/ijc.29497. [DOI] [PubMed] [Google Scholar]
- [19].Bao Y., Hou W., Liu R., Gao Y., Kong X., Yang L.. PAR2-mediated upregulation of BDNF contributes to central sensitization in bone cancer pain. Mol. Pain. 2014;10:28. doi: 10.1186/1744-8069-10-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wang D., Zhao J., Wang J., Li J., Yu S., Guo X.. Deficiency of female sex hormones augments PGE and CGRP levels within midbrain periaqueductal gray. J. Neurol. Sci. 2014;346:107–111. doi: 10.1016/j.jns.2014.08.002. [DOI] [PubMed] [Google Scholar]
- [21].Crown E.D., Gwak Y.S., Ye Z., Yu Tan H., Johnson K.M., Xu G.Y.. Calcium/calmodulin dependent kinase II contributes to persistent central neuropathic pain following spinal cord injury. Pain. 2012;153:710–721. doi: 10.1016/j.pain.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Bao Y., Gao Y., Hou W., Yang L., Kong X., Zheng H.. Engagement of signaling pathways of protease-activated receptor 2 and muopioid receptor in bone cancer pain and morphine tolerance. Int. J. Cancer. 2015;137:1475–1483. doi: 10.1002/ijc.29497. [DOI] [PubMed] [Google Scholar]
- [23].Morgado C., Terra P.P., Tavares I.. Neuronal hyperactivity at the spinal cord and periaqueductal grey during painful diabetic neuropathy: effects of gabapentin. Eur. J. Pain. 2010;14:693–699. doi: 10.1016/j.ejpain.2009.11.011. [DOI] [PubMed] [Google Scholar]
- [24].Silva M., Amorim D., Almeida A., Tavares I., Pinto-Ribeiro F., Morgado C.. Pronociceptive changes in the activity of rostroventromedial medulla (RVM) pain modulatory cells in the streptozotocin-diabetic rat. Brain Res. Bull. 2013;96:39–44. doi: 10.1016/j.brainresbull.2013.04.008. [DOI] [PubMed] [Google Scholar]
- [25].Bouhassira D., Lantéri-Minet M., Attal N., Laurent B., Touboul C.. Prevalence of chronic pain with neuropathic characteristics in the general population. Pain. 2008;136:380–387. doi: 10.1016/j.pain.2007.08.013. [DOI] [PubMed] [Google Scholar]
- [26].Al-Batran S.E., Ducreux M., Ohtsu A.. mTOR as a therapeutic target in patients with gastric cancer. Int. J. Cancer. 2012;130:491–496. doi: 10.1002/ijc.26396. [DOI] [PubMed] [Google Scholar]
- [27].Bevan S., Quallo T., Andersson D.A.. TRPV1. Handb. Exp. Pharmacol. 2014;222:207–245. doi: 10.1007/978-3-642-54215-2_9. [DOI] [PubMed] [Google Scholar]
- [28].Lin Q., Li D., Xu X., Zou X., Fang L.. Roles of TRPV1 and neuropeptidergic receptors in dorsal root reflex-mediated neurogenic inflammation induced by intradermal injection of capsaicin. Mol. Pain. 2007;3:30. doi: 10.1186/1744-8069-3-30. [DOI] [PMC free article] [PubMed] [Google Scholar]