Abstract
Objectives
Alzheimer’s disease (AD) and the behavioral variant of frontotemporal dementia (bvFTD) are the most common causes of dementia; however, their overlapping clinical syndromes and involved brain regions make a differential diagnosis difficult. We aimed to identify the differences in the cognition and motor cortex excitability between AD and bvFTD patients.
Methods
Twenty-seven AD patients and 30 bvFTD patients were included in the study. Each participant received a neurological evaluation. Cognitive event-related potentials (P300) were recorded during an auditory oddball task. Next, the excitability of the motor cortex, including the resting, facilitated motor threshold (RMT and FMT) and cortical silent period (CSP), were assessed during transcranial magnetic stimulation (TMS).
Results
The bvFTD patients exhibited significantly longer P300 latencies compared with AD patients. There was a significant negative correlation between cognition and P300 latency in the bvFTD group. The AD patients showed significantly reduced RMT and FMT values compared to the bvFTD group; however, no significant correlation was found between AD severity and the excitability of the motor cortex.
Conclusions
Cognition and motor cortical functions are different between AD and bvFTD patients. Noninvasive electrophysiological examinations have the potential to identify unique pathophysiological features that can be used to differentially diagnose AD and bvFTD patients.
Keywords: Alzheimer’s disease (AD), Behavioral variant of frontotemporal dementia (bvFTD), Cortical function, Event-related potentials (ERP), Transcranial magnetic stimulation (TMS)
Introduction
With medical advances, mortality rates among older people are decreasing, and the average life expectancy is increasing worldwide. As people live longer, chronic diseases become more prevalent, and dementia is one of the most common chronic diseases in older people. According to the World Alzheimer Report of 2015, there are over 46 million people with dementia worldwide [1]. Alzheimer’s disease (AD) and frontotemporal dementia (FTD) are the most common primary causes of dementia syndrome [1-3]. These two diseases are characterized by distinct clinical syndromes and the involved brain regions. However, these characteristics begin to overlap as the diseases progress. Clinically, episodic memory loss is typically the primary clinical symptom of AD. Changes in language, orientation, mood, motivation and behavior are also frequent, particularly as the disease progresses [4]. The behavioral variant of FTD (bvFTD) is the most common subtype of FTD and is characterized by marked personality changes and behavioral problems, as well as cognitive changes that affect executive function and episodic memory[5]. A recent longitudinal AD study reported that structural changes occur early in the bilateral parietal, hippocampal and association occipital regions; furthermore, these regions continue to atrophy over time, and temporal lobe changes are observed in later stages [6]. Conversely, bvFTD patients show early degradation of the medial prefrontal cortex followed by changes in the anterior temporal and frontal cortices[6]. Owing to the overlap in clinical symptoms and affected regions between bvFTD and AD [7-9], an early differential diagnosis can be challenging. To improve diagnostic accuracy and early differential diagnoses, there is a strong need for markers of the unique brain changes associated with each type of dementia.
Cognitive deficits are typically the first symptoms of dementia to be recognized and a common reason for patients to go to the hospital. The P300 wave is a prominent event related potential (ERP) that indicates changes in the association cortex [10], working memory and attention functioning [11, 12]. The P300 wave is the most commonly recorded potential and can be elicited using the oddball paradigm. In this method, the subject is instructed to only attend to an infrequent target stimulus embedded in a series of frequent background stimuli [13]. Many studies reported that AD patients showed diminished P300 amplitudes and enlarged P300 latencies when compared with age-matched controls [14-18]. Furthermore, some studies have suggested P300 as a biomarker to distinguish between AD patients and age-matched controls [17, 19-21]. Few studies have analyzed the P300 component in FTD patients as compared to age-related controls or AD patients, and the results were not consistent enough to draw a conclusion. Jimenez-Escrig and colleagues found that the P300 wave did not significantly differ between FTD patients and age-matched controls; however, AD patients showed a delayed P300 latency that significantly differed from FTD patients [22]. Another study found that bvFTD patients displayed a longer P300 latency and smaller amplitude than the control group; however, no difference was found between the AD and bvFTD groups [23]. These contrasting P300 results between AD and FTD patients prompted us to investigate if bvFTD patients present abnormalities in P300 that can be used as a diagnostic tool to differentiate between bvFTD and AD patients.
In addition to the progressive deterioration of memory and cognitive functions, motor manifestations, such as bilateral spasticity in the lower limbs, myoclonus, gait disturbances and the presence of an extensor plantar response, are frequently observed in the advanced phases of AD and FTD [24-26]. Transcranial magnetic stimulation (TMS) is a safe, noninvasive and painless technique that is widely employed to explore brain motor functions [27, 28]. Many TMS studies have demonstrated a hyperexcitability in the motor cortex that relates to AD severity [29-32]. In contrast, there are few TMS studies on FTD patients. Alberici et al. suggested that TMS may help distinguish differences across the FTD clinical spectrum [33]. However, Pierantozzi et al. suggested that the motor cortex is not involved in FTD [34]. These inconsistent findings require further verification.
We hypothesized that there are differences in the cognition and motor cortex excitability between AD and bvFTD patients that explain the different behavioral and cognitive features of each type of dementia. Therefore, we used ERPs and TMS to assess the cortical features of AD and bvFTD. We recruited 27 AD and 30 bvFTD patients. We evaluated their global cognition using the Mini-Mental State Examination (MMSE) and Montreal Cognitive Assessment (MoCA) scores. In addition, we assessed their clinical severity using the Clinical Dementia Rating (CDR), determined their cognitive cortical functioning based on the P300 latency and amplitude, and measured their motor cortex excitability using the resting motor threshold (RMT), facilitated motor threshold (FMT) and cortical silent period (CSP).
Experimental procedures
Standard protocol approvals, registration, and patient consent
This study was approved by the Medical Ethics Committee of Tianjin Huanhu Hospital. Written informed consent was obtained from each enrolled subject or his/her authorized guardian. The participants underwent general physical, psychological and laboratory examinations prior to enrollment in the study. At the time of recruitment, none of the subjects were taking cholinomimetic agents, antidepressants, neuroleptics, or sedative-hypnotic drugs for at least one week prior to the assessment, and all patients received professional suggestions for further treatment.
Subjects
The subjects were recruited from the outpatients of Tianjin Huanhu Hospital between 2011 and 2014. The recruited AD patients were diagnosed using the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association criteria for probable AD [35, 36]. The diagnosis of bvFTD was based on the clinical criteria proposed by McKhann et al. and Neary et al. [37, 38]. The criteria for exclusion in the study included any significant neurological or psychiatric illness that can influence cognitive function and the presence of a significant unstable systemic illness or organ failure. All patients were subjected to evaluation on the severity of their cognitive deterioration and dementia by using the Mini-Mental State Examination (MMSE), Montreal Cognitive Assessment (MoCA), and Clinical Dementia Rating (CDR) scales. The MMSE is a brief measure of cognitive functioning, and it has high test retest reliability, internal consistency and interobserver reliability [39]. The MMSE consists of 11 items and has a maximum score of 30 and a cut-off score of 24 with a sensitivity of 87% and specificity of 82% [40]. The MoCA is a 30-point cognitive test consisting of executive function and attention tasks that were designed for individuals who scored 24-30 on the MMSE [41,42]. Thus, we used the MMSE and MoCA as measures of general cognition. The CDR offers a global characterization of everyday functions that may be affected by the neurodegenerative disease and is a clinical scale developed to assess the presence and severity of dementia [43]. The CDR is a five-point scale in which CDR-0 denotes no cognitive impairment. The remaining four points represent various stages of dementia (0.5 = mild cognitive impairment, 1 = mild dementia, 2 = moderate dementia, 3 = severe dementia). In our study, all of the patients with dementia scored 1-2 on the CDR. In other words, we chose participants with mild-to moderate cognitive impairment. Due to the limited sample size, we did not create subgroups for disease severity.
A total of 80 subjects with cognitive impairment were enrolled in the study. Sixty-five subjects met the criteria for AD or bvFTD, and 15 subjects had other types of dementia. Eight subjects did not complete the ERP experiments. Thus, 27 AD patients and 30 bvFTD patients were included in the final analysis. The demographic and neuropsychological details of the participants are listed in Tables 1 and 2. The participants first underwent the ERP examination, and then completed the TMS examination 30 min later.
Table 1.
Demographic and clinical information of the study subjects.
| AD (27) | bvFTD (30) | t value | P value | |
|---|---|---|---|---|
| Sex, M/F (n) | 12/15 | 13/17 | 0.933 | |
| Age (year) | 72.07 ± 1.62 | 68.13 ± 1.43 | 1.83 | 0.073 |
| Education (year) | 10.96 ± 0.77 | 11.10 ± 0.62 | −0.14 | 0.887 |
| MMSE | 19.85 ± 0.73 | 21.70 ± 0.87 | −0.16 | 0.113 |
| MoCA | 13.93 ± 0.90 | 14.47 ± 1.03 | −0.39 | 0.697 |
AD, Alzheimer’s disease; bvFTD, behavioral variant of frontotemporal dementia; M/F, male/female; MMSE, MiniMental State Examination; MoCA, Montreal Cognitive Assessment.
Table 2.
Normality of demographic, clinical and electrophysiological data for the AD and bvFTD groups.
| AD | bvFTD | Kolmogorov-Smirnov | P value | |
|---|---|---|---|---|
| Age (years) | 72.07 ± 1.62 | 68.13 ± 1.43 | 0.254 | 0.616 |
| Education (years) | 10.96 ± 0.77 | 11.10 ± 0.62 | 0.770 | 0.384 |
| MMSE | 19.85 ± 0.73 | 21.70 ± 0.87 | 1.290 | 0.261 |
| MoCA | 13.93 ± 0.90 | 14.47 ± 1.03 | 0.419 | 0.520 |
| P300-Pz | ||||
| Latency (ms) | 377.44 ± 7.35 | 400.97 ± 6.33 | 0.383 | 0.538 |
| Amplitude (gV) | 9.66 ± 1.71 | 8.86 ± 1.24 | 0.859 | 0.358 |
| P300-Cz | ||||
| Latency (ms) | 378.63 ± 7.65 | 398.90 ± 6.33 | 0.804 | 0.374 |
| Amplitude (gV) | 9.87 ± 1.79 | 7.68 ± 1.25 | 0.832 | 0.366 |
| RMT (%) | 44.37 ± 1.27 | 49.83 ± 1.43 | 3.521 | 0.066 |
| FMT (%) | 30.89 ± 0.95 | 34.71 ± 0.97 | 1.177 | 0.283 |
| CSP (ms) | 157.10 ± 8.17 | 155.14 ± 4.93 | 3.103 | 0.084 |
AD, Alzheimer’s disease; bvFTD, behavioral variant of frontotemporal dementia; MMSE, Mini-Mental State Examination; MoCA, Montreal Cognitive Assessment; Cz, central; Pz, parietal; RMT, resting motor threshold; FMT, facilitated motor threshold; CSP, cortical silent period.
ERP procedure
The auditory oddball stimuli used were simple and easily gained the attention of the subjects. The oddball P300 is an indicator of memory function, and many studies have investigated the utility of auditory P300 for the assessment of AD [44-46]. A simple auditory two-tone discrimination (oddball paradigm) was used to elicit ERP responses. Five hundred tones (25-millisecond duration with a 1.5-second interstimulus interval) were binaurally presented through headphones in a pseudorandomized order. Targeted (2000 Hz, 100 dB) and non-targeted (1000 Hz, 100 dB)tones appeared with a probability of 20% and 80%, respectively. The targeted stimulation superposition was 100 times. The stimulus display used was a DantecTMKEYPOINT® G4 Workstation. Electroencephalograms (EEG) were recorded from two scalp derivations (central, Cz, and parietal, Pz) according to the international 10-20 standard. The guidelines written by Picton et al. state that ERPs can be adequately examined for clinical purposes using simple recording channels [13]. In support of a previous study [47], these authors also suggested that analyzing ERPs collected at the Cz and Pz provides a good initial view of cognitive processes. Based on the involved regions in dementia, we limited our study to ERPs at the Pz and Cz. Two linked electrodes were attached to the left and right earlobes (A1-A2) as a reference. Other electrodes were placed above and below the left eye to monitor eye movements, and one ground electrode was attached to the middle of the forehead.
Subjects were comfortably seated in an armchair in a sound-attenuated room and asked to relax, close their eyes, and minimize eye and mandibular movements during the recording. Subjects were requested to press a hand-held button when they detected a target stimulus. They were also requested to silently count the target tones, and report the total number of tones at the end of the session. The test was initiated only when the subject demonstrated a complete understanding of the task. All datasets with a 95% concordance between the number of stimuli presented and the total number of tones reported were used for further analysis.
The data were initially band-pass filtered between 0.2 and 20 Hz. The recording was initiated at 100 ms before stimulation to capture a baseline measurement. The recording was maintained for 900 ms thereafter. Next, eye movement components were removed using an algorithm [48]. After their removal, the remaining components were back-projected onto the EEG channels. The P300 amplitude was defined relative to the baseline period, which was set at the 100 ms level prior to stimulus onset. An automated peak-picking procedure was used to determine peak amplitudes and latencies. The P300 wave was defined as the maximum point between 300 and 600 ms after stimulus onset. Reaction times were not recorded for the oddball paradigm.
TMS procedure
All participants were tested while lying comfortably in order to achieve complete relaxation. The EMG was monitored in the background using acoustic feedback before and during all TMS recordings. Magnetic stimulation was performed with a butterfly-shaped coil (loop diameter of 50 mm) connected with a single Maglite Pro 30 Stimulator (MagVenture Inc., Alpharetta, GA, USA) through a MC-BT0 that discharged a maximum output of 2.5 T. Motor-evoked potentials (MEP) during the TMS were recorded from the abductor pollicis brevis (APB) via surface electrodes applied in a belly tendon pattern. The coil was placed 6 cm lateral to the Cz along the interlobe line, over the scalp region corresponding to the primary hand motor area and contralateral to the target muscle. In both AD and bvFTD groups, the TMS procedures were performed bilaterally.
The resting motor threshold (RMT) was defined as the minimal intensity required to elicit MEP with a 50 μV peak-to-peak amplitude in five out of ten consecutive trials. The facilitated motor threshold (FMT) was determined using the same method while subjects made their strongest muscle contraction. FMT was defined as the minimal intensity eliciting an MEP larger than 50 μV in five out of ten consecutive trials. All subjects were asked to perform approximately 100% of their maximal contraction while the electromyographic activity was recorded. Ten magnetic stimuli were applied at an intensity of 140% FMT. The cortical silent period (CSP) was defined as the time between the end of the MEP and the return of voluntary electromyographic activity, and ten consecutive responses were averaged.
Statistical analysis
Statistical analysis was performed using SPSS (version 18.0, released 2009, SPSS Inc., Chicago, IL, USA). For RMT, FMT, CSP, and P300 amplitude and latency, the Kolmogorov test was performed to evaluate continuity. Afterwards, t-tests were conducted to evaluate differences between the AD and bvFTD groups. P-values less than 0.05 were considered significant. To evaluate the relationship between cognitive performance (MMSE and MoCA) and electrophysiological parameters, Pearson’s correlation coefficients were calculated.
Results
Subject characteristics
The AD and bvFTD groups did not statistically differ in age (t = 1.83, p = 0.073), the number of years of education (t = −0.14, p = 0.887) or cognitive ability (MMSE: t = −1.61, p = 0.113; MoCA: t = −0.39, p = 0.697). The clinical data are provided in Table 1.
Cognitive cortical cunction
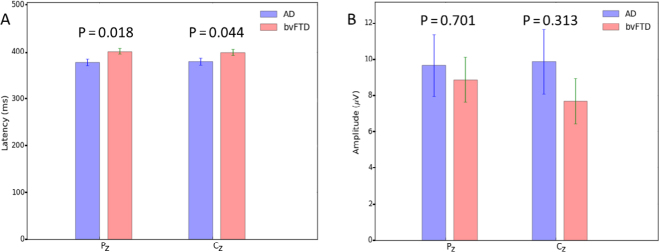
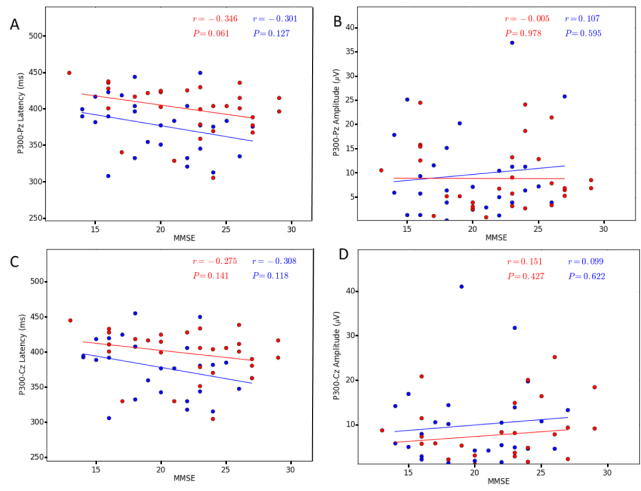
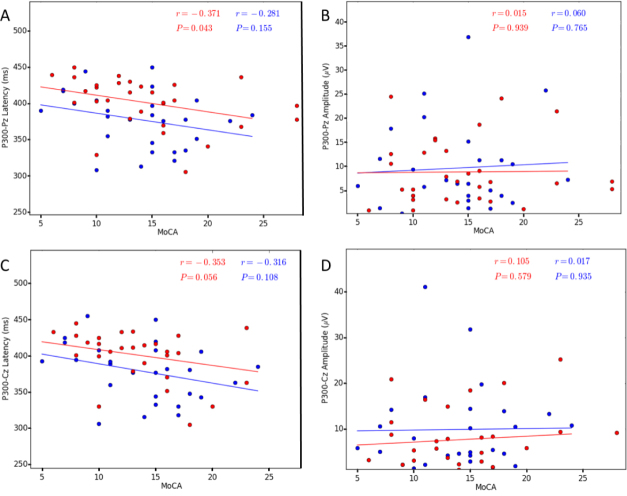
All subjects responded to the target tones of the auditory ERPs with greater than 95% accuracy. Among the ERP parameters measured in this study, the P300 latency in the bvFTD group was significantly longer than in the AD group at the Pz site (AD: 377.44 ± 7.35, bvFTD: 400.97 ± 6.33, t = −2.439, p = 0.018) and Cz site (AD: 378.63 ± 7.65, bvFTD: 398.90 ± 6.33, t = −2.057, p = 0.044). The AD group did not significantly differ from the bvFTD group in P300 amplitude at either site (Pz: t = 0.385, p = 0.701 and Cz: t = 1.102, p = 0.313). The data are shown in Table 3 and Fig. 1. Furthermore, we correlated the ERP parameters with the cognition scores (Supplementary Table 2, and Figs. 2 and 3). A negative correlation was found between the MoCA score and P300 latency within the bvFTD group (Pz: r = −0.371, p = 0.043 and Cz: r = −0.353, p = 0.056) but not the AD group. No correlation was found between the MMSE score and P300 latency. No correlations between the P300 amplitude and cognitive scores were found.
Table 3.
Event-related potential latencies and amplitudes at PZ and CZ for the AD and bvFTD groups.
| AD (n = 27) | bvFTD (n = 30) | t value (two-tailed) | P value | |
|---|---|---|---|---|
| P300-PZ | ||||
| Latency (ms) | 377.44 ± 7.35 | 400.97 ± 6.33 | -2.439 | 0.018* |
| Amplitude (pV) | 9.66 ± 1.71 | 8.86 ± 1.24 | 0.385 | 0.701 |
| P300-CZ | ||||
| Latency (ms) | 378.63 ± 7.65 | 398.90 ± 6.33 | -2.057 | 0.044* |
| Amplitude (pV) | 9.87 ± 1.79 | 7.68 ± 1.25 | 1.102 | 0.313 |
AD, Alzheimer’s disease; bvFTD, the behavioral variant of frontotemporal dementia; Cz, central; Pz, parietal; *P, significant group differences (P & 0.05).
Figure 1.
The latency and amplitude of P300. A. A bar chart of the latency of P300 between the AD and bvFTD groups. The latency of the bvFTD group is significantly different from that of the AD group at both Pz and Cz (P & 0.05). B. A bar chart of the amplitude of P300 between the AD and bvFTD groups. The amplitude of the bvFTD group is not different from that of the AD group at both Pz and Cz (P > 0.05). Abbreviations: AD, Alzheimer’s disease; bvFTD, behavioral variant of frontotemporal dementia.
Figure 2.
Correlation between the P300 parameters and MMSE in both AD and bvFTD patients (P & 0.05). A. P300 Pz latency, B. P300 Pz amplitude, C. P300 Cz latency, D. P300 Cz amplitude. The AD patients are indicated by the blue circles and the bvFTD patients by the red circles. Abbreviations: AD, Alzheimer’s disease; bvFTD, behavioral variant of frontotemporal dementia; MMSE, Mini-Mental State Examination.
Figure 3.
Correlation between the P300 parameters and MoCA in both AD and bvFTD patients (P & 0.05). A. P300 Pz latency, B. P300 Pz amplitude, C. P300 Cz latency, D. P300 Cz amplitude. The AD patients are indicated by the blue circles and the bvFTD patients by the red circles. Abbreviations: AD, Alzheimer’s disease; bvFTD, behavioral variant of frontotemporal dementia; MoCA, Montreal Cognitive Assessment.
Motor cortical function
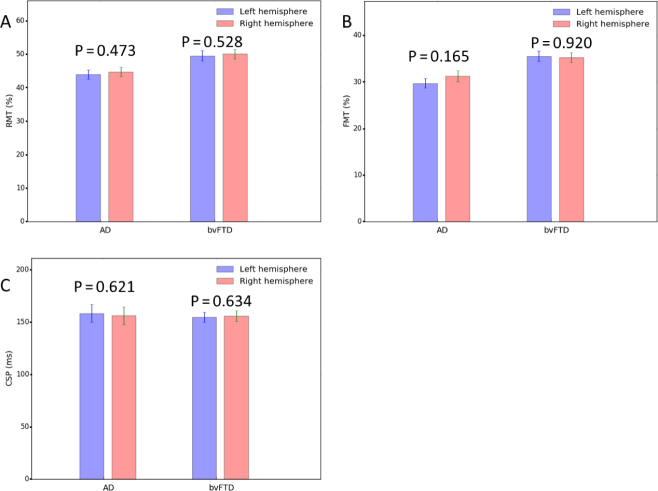
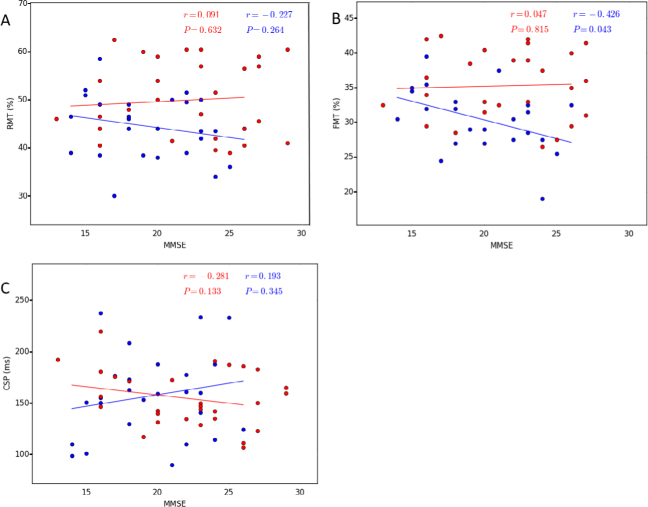
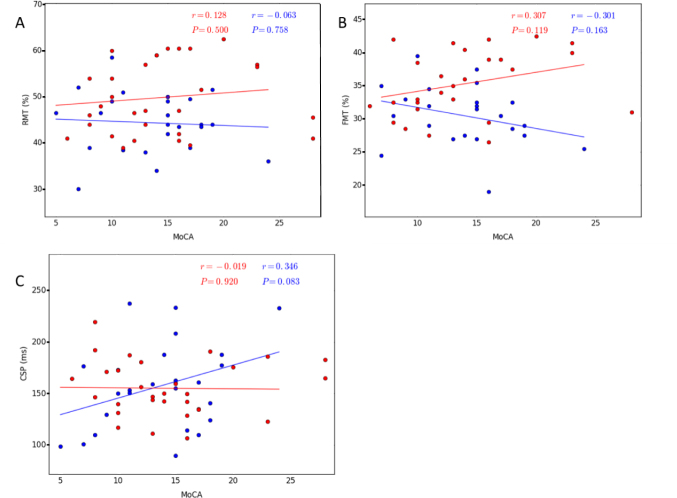
There were no significant differences between the right and left hemispheres in any of the motor cortical excitability parameters (Table 4 and Fig. 4). Thus, the average of both hemispheres was used for the AD and bvFTD groups. The RMT and FMT were significantly lower in the AD group than the bvFTD group (RMT: 44.37 vs 49.83, t = −2.818, p = 0.007 and FMT: 30.84 vs 34.71, t = −3.548, p = 0.001) (Table 5). The CSP was not significantly different between groups (t = 0.212, p = 0.833). There was no correlation between MMSE/MoCA scores and the RMT and FMT in either group (Table 6, and Figs. 4 and 5).
Table 4.
Differences in TMS parameters in the left and right hemispheres within the AD and bvFTD groups.
| Left hemisphere | Right hemisphere | t value | P value | |
|---|---|---|---|---|
| AD | ||||
| RMT (%) | 43.96 ± 1.34 | 44.77 ± 1.44 | -0.728 | 0.473 |
| FMT (%) | 29.70 ± 1.00 | 31.26 ± 1.19 | -1.435 | 0.165 |
| CSP (ms) | 158.23 ± 8.47 | 155.97 ± 8.48 | 0.501 | 0.621 |
| FTD | ||||
| RMT (%) | 49.57 ± 1.54 | 50.10 ± 1.44 | -0.638 | 0.528 |
| FMT (%) | 35.33 ± 1.12 | 35.22 ± 1.08 | 0.102 | 0.920 |
| CSP (ms) | 154.55 ± 4.88 | 155.73 ± 5.26 | -0.481 | 0.634 |
AD, Alzheimer’s disease; bvFTD, the behavioral variant of frontotemporal dementia; CSP, cortical silent period; FMT, facilitated motor threshold; RMT, resting motor threshold; TMS, transcranial magnetic stimulation.
Figure 4.
Bar graphs of TMS parameters in the left and right hemispheres. The bar graphs show no differences between the hemispheres within the AD and bvFTD groups (P > 0.05). A. RMT, B. FMT, C. CSP. Abbreviations: AD, Alzheimer’s disease; bvFTD, behavioral variant of frontotemporal dementia; CSP, cortical silent period; FMT, facilitated motor threshold; RMT, resting motor threshold; TMS, transcranial magnetic stimulation.
Table 5.
Excitability of the motor cortex in AD and bvFTD patients.
| AD (n = 27) | FTH (n = 30) | t value | P value | |
|---|---|---|---|---|
| RMT (%) | 44.37 ± 1.27 | 49.83 ± 1.43 | -2.818 | 0.007* |
| FMT (%) | 30.89 ± 0.95 | 34.71 ± 0.97 | -3.548 | 0.001* |
| CSP (ms) | 157.10 ± 8.17 | 155.14 ± 4.93 | 0.212 | 0.833 |
AD, Alzheimer’s disease; bvFTD, the behavioral variant of frontotemporal dementia; CSP, cortical silent period; FMT, facilitated motor threshold; RMT, resting motor threshold; *, P & 0.05
Table 6.
Relationships between cognition and electrophysiological parameters in the AD and bvFTD groups.
| AD | bvFTD | |||
|---|---|---|---|---|
| MMSE | MoCA | MMSE | MoCA | |
| P300-PZ | ||||
| Latency (ms) | -0.0301 | -0.281 | -0.346 | -0.371* |
| Amplitude (pV) | 0.107 | 0.060 | -0.005 | 0.015 |
| P300-Cz | ||||
| Latency (ms) | -0.308 | -0.316 | -0.275 | -0.353 |
| Amplitude (pV) | 0.099 | 0.017 | 0.151 | 0.105 |
| RMT (%) | -0.227 | -0.063 | 0.091 | 0.128 |
| FMT (%) | 0.426* | -0.301 | 0.047 | 0.307 |
| CSP (ms) | 0.193 | 0.346 | -0.287 | -0.019 |
AD: Alzheimer’s disease; bvFTD, behavioral variant of frontotemporal dementia; CSP: cortical silent period; Cz, central; FMT, facilitated motor threshold; MMSE, Mini-Mental State Examination; MoCA, Montreal Cognitive Assessment; Pz, parietal; RMT, resting motor threshold; *, P & 0.05.
Figure 5.
Correlation between the TMS parameters and MMSE in both AD and bvFTD patients (P & 0.05). A. RMT, B. FMT, C. CSP. The AD patients are indicated by the blue circles and the bvFTD patients by the red circles. Abbreviations: AD, Alzheimer’s disease; bvFTD, behavioral variant of frontotemporal dementia; CSP, cortical silent period; FMT, facilitated motor threshold; RMT, resting motor threshold; TMS, transcranial magnetic stimulation.
Discussion
This study investigated two aspects of brain function involving the association cortex and motor cortex. The results showed that ERPs were decreased in bvFTD patients, as indicated by the longer P300 latency in the oddball task. In addition, the P300 latency may be useful to assess the severity of bvFTD. Our study confirmed that the excitability of the motor cortex during TMS was increased in AD patients, as demonstrated by the reduction in RMT and FMT. We suggest that the combination of ERP and TMS techniques may provide the key to understanding the pathophysiological mechanisms of AD and bvFTD.
The P300 wave is the most frequently recorded potential. The P300 latency is considered a measure of stimulus classification speed and is sensitive to task processing demands and cognitive abilities. The amplitude of the P300 is considered to be the manifestation of brain activity that reflects attention to incoming stimulus information[12]. Previous studies have shown decreased P300 amplitudes and increased P300 latencies in AD patients [3, 18, 23, 49, 50]. In recent years, the utility of P300 in bvFTD patients has been studied. Chen and colleagues found decreased P300 amplitudes and increased P300 latencies in FTD patients when compared with agematched controls [23]. The abnormal P300 components may be due to changes in the brain regions associated with the P300. P300 generation involves a widespread network of cortical structures that overlap in AD and FTD, including parietal, temporal, and prefrontal cortices [51,52].
Furthermore, recent studies analyzed the P300 component in FTD and AD patients. Jimenez-Escrig and colleagues found that the P300 latency of FTD patients was significantly longer compared with the AD group [22]. However, another study showed no difference between AD and bvFTD patients [23]. Interestingly, our study found that bvFTD patients had a longer P300 latency compared with AD patients. One reason for this discrepancy may be due to subject selection. FTD is rather heterogeneous clinical entity and shows variable clinical and neuropathological manifestations. We assessed only bvFTD patients. In contrast, patients with primary progressive aphasia and semantic dementia were included in the study by Jimenez-Escrig. The specific molecular pathologies and involved brain regions of different dementia subtypes may affect ERP parameters to some extent. Another explanation is that clinically diagnosed FTD can involve a mixed pathology of both FTD and AD or turn out to be another FTD subtype based on postmortem neuropathological analysis. Thus, the results of these studies are not sufficiently robust to firmly support the use of the P300 paradigm to distinguish between AD and bvFTD patients. Longitudinal studies are needed to clarify this point.
Our study identified increased excitability of the motor cortex in AD patients when compared with bvFTD patients. These findings are consistent with previous studies [29, 53-56]. Recent neuropathological studies showed that the density of neurofibrillary tangles and senile plaques in the motor cortex was approximately equivalent to other areas considered to be specific targets for AD abnormalities [57, 58]. Secondly, the primary motor cortex expresses muscarinic receptors and receives widespread inputs from cholinergic pathways. Therefore, some researchers have suggested that the cholinergic deficits in AD modify the excitability and function of the motor system [59-61]. Clinically, the excitability changes occur long before clinical signs of motor deficits are detected [62]. We speculate that the observed hyperexcitability is a compensatory mechanism to execute voluntary movements. In contrast, the excitability of the motor cortex was preserved in the bvFTD group. Thus, TMS has the potential to be used as a noninvasive tool for reaching an early differential diagnosis between cholinergic (AD) and non-cholinergic forms of dementia (FTD). FTD includes a wide spectrum of heterogeneous clinical and anatomical conditions. Alberici and colleagues found that TMS might help in distinguishing differences among the FTD clinical spectrum [33].
Furthermore, we found a lack of correlation between AD severity and cortical excitability parameters. These findings are consistent with the study by Ferreri et al. [54]. In contrast, Alagona et al. [63] found a significant correlation between RMT and MMSE, indicating that the lower the MMSE score, the lower the RMT (cortical hyperexcitability). However, this may be ascribed partly to the clinical homogeneity of their patients (all of them had mild dementia). In contrast, our participants showed different levels of disease severity. In the early stages of AD, a decrease in RMT (cortical hyperexcitability) may be a compensatory mechanism for the loss of cortical neurons involved in motor functions. However, in the advanced stages of AD, the excitability is decreased owing to cortical atrophy. Although TMS does not represent a specific diagnostic tool for AD, it may provide the key to understanding the pathophysiological mechanisms of AD.
We recognize the overall limitations of our work. First, all of the AD and bvFTD diagnoses were made clinically without neuropathological confirmation. Based on postmortem neuropathological analyses, some studies suggested that clinically diagnosed AD or bvFTD involves a mixed pathology of other degenerative diseases. Secondly, we chose simple and easily available parameters, such as RMT and FMT, to assess motor cortex excitability. Further study of the pathophysiology should include additional parameters such as short-latency afferent inhibition, intracortical facilitation, and intracortical inhibition.
Figure 6.
Correlation between the TMS parameters and MoCA in both AD and bvFTD patients (P & 0.05). A. RMT, B. FMT, C. CSP. The AD patients are indicated by the blue circles and the bvFTD patients by the red circles. Abbreviations: AD, Alzheimer’s disease; bvFTD, behavioral variant of frontotemporal dementia; CSP, cortical silent period; FMT, facilitated motor threshold; MoCA, Montreal Cognitive Assessment; RMT, resting motor threshold; TMS, transcranial magnetic stimulation.
Conclusions
The novel findings of the present study concern the differences in cortical functioning between patients with AD and bvFTD. The results showed that bvFTD patients displayed a significantly longer P300 latency compared with AD patients. Simultaneously, AD patients displayed a hyperexcitability of the motor cortex, which may be a compensatory mechanism for the execution of voluntary movements. We suggest that combining different electrophysiological tools will help determine the unique pathophysiological mechanisms in AD and bvFTD.
Acknowledgments
Conflict of interest statement: All authors have no conflicts of interest to declare. All authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Yuying Zhou and Pan Wang. Acquisition of data: Huihong Zhang and Lu Han. Analysis and interpretation of data: Yuying Zhou. Writing the manuscript draft: Pan Wang. Critical revision of the manuscript for intellectual content: Yuying Zhou. Statistical analysis: Pan Wang and Lu Han. Study supervision: Yuying Zhou. This work was supported by the technology fund of Tianjin City Department of Health (Grant No. 13KG121).
Reference
- [1].Prince M., Wimo A., Guerchet M., Ali G.C., Wu Y.T., Prina M. Alzheimer’s Disease International. London: 2015. World Alzheimer Report 2015. The Global Impact of Dementia: an analysis of prevalence, incidence, cost and trends. [Google Scholar]
- [2].Snowden J.S., Neary D., Mann D.M.. Frontotemporal dementia. Br. J. Psychiat. 2002;180:140–143. doi: 10.1192/bjp.180.2.140. [DOI] [PubMed] [Google Scholar]
- [3].Ratnavalli E., Brayne C., Dawson K., Hodges J.R.. The prevalence of frontotemporal dementia. Neurology. 2002;58:1615–1621. doi: 10.1212/wnl.58.11.1615. [DOI] [PubMed] [Google Scholar]
- [4].Querfurth H.W., LaFerla F.M.. Alzheimer's disease. Engl N. Med J. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- [5].Piguet O., Hornberger M., Mioshi E., Hodges J.R.. Behaviouralvariant frontotemporal dementia: diagnosis, clinical staging, and management. Lancet Neurol. 2011;10:162–172. doi: 10.1016/S1474-4422(10)70299-4. [DOI] [PubMed] [Google Scholar]
- [6].Landin-Romero R., Kumfor F., Leyton C.E., Irish M., Hodges J.R., Piguet O.. Disease-specific patterns of cortical and subcortical degeneration in a longitudinal study of Alzheimer's disease and behaviouralvariant frontotemporal dementia. Neuroimage. 2016 doi: 10.1016/j.neuroimage.2016.03.032. doi: 10.1016/j.neuroimage.2016.03.032 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- [7].Galton C.J., Patterson K., Xuereb J.H., Hodges J.R.. Atypical and typical presentations of Alzheimer's disease: a clinical, neuropsychological, neuroimaging and pathological study of 13 cases. Brain. 2000;123:484–498. doi: 10.1093/brain/123.3.484. [DOI] [PubMed] [Google Scholar]
- [8].Hornberger M., Wong S., Tan R., Irish M., Piguet O., Kril J.. In vivo and post-mortem memory circuit integrity in frontotemporal dementia and Alzheimers disease. Brain. 2012;135:3015–3025. doi: 10.1093/brain/aws239. [DOI] [PubMed] [Google Scholar]
- [9].Pleizier C.M., van derVlies A.E., Koedam E., Koene T., Barkhof F., vander Flier W.M.. Episodic memory and the medial temporal lobe: not all it seems. Evidence from the temporal variants of frontotemporaldementia. J. Neurol. Neurosurg. Psychiatry. 2012;83:1145–1148. doi: 10.1136/jnnp-2012-302437. [DOI] [PubMed] [Google Scholar]
- [10].Bennys K., Portet F., Touchon J., Rondouin G.. Diagnostic value of event-related evoked potentials N200 and P300 subcomponents inearly diagnosis of Alzheimers disease and mild cognitive impairment. j. Clin.Neurophysiol. 2007;24:405–412. doi: 10.1097/WNP.0b013e31815068d5. [DOI] [PubMed] [Google Scholar]
- [11].Donchin E., Coles M.G.H.. Is the P300 component a manifestation of context updating? Behav. Brain Sci. 1988;11:357–427. [Google Scholar]
- [12].Polich J.. Updating P300: an integrative theory of P3a and P3b. Clin. Neurophysiol. 2007;118:2128–2148. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Picton T.W., Bentin S., Berg P., Donchin E., Hillyard S.A., Johnson R. Jr.. et al. Guidelines for using human event-related potentialsto study cognition: recording standards and publication criteria. Psychophysiology. 2000;37:127–152. [PubMed] [Google Scholar]
- [14].Filipovic S.R., Kostic V.S.. Utility of auditory P300 in detection of presenile dementia. J. Neurol Sci. 1995;131:150–155. doi: 10.1016/0022-510x(95)00093-h. [DOI] [PubMed] [Google Scholar]
- [15].Hedges D., Janis R., Mickelson S., Keith C., Bennett D., Brown B.L.. P300 amplitude in Alzheimer's disease: a meta-analysis and metaregression. Clin. EEG Neurosci. 2016;47:48–55. doi: 10.1177/1550059414550567. [DOI] [PubMed] [Google Scholar]
- [16].Howe A.S., Bani-Fatemi A., De Luca V.. The clinical utility of the auditory P300 latency subcomponent event-related potential in preclinical diagnosis of patients with mild cognitive impairment and Alzheimer's disease. Brain Cogn. 2014;86:64–74. doi: 10.1016/j.bandc.2014.01.015. [DOI] [PubMed] [Google Scholar]
- [17].Lee M.S., Lee S.H., Moon E.O., Moon Y.J., Kim S., Kim S.H.. Neuropsychological correlates of the P300 in patients with Alzheimer’s disease. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2013;40:62–69. doi: 10.1016/j.pnpbp.2012.08.009. [DOI] [PubMed] [Google Scholar]
- [18].Wang P., Zhang X., Liu Y., Liu S., Zhou B., Zhang Z.. et al. Perceptual and response interference in Alzheimer's disease and mild cognitive impairment. Clin. Neurophysiol. 2013;124:2389–2396. doi: 10.1016/j.clinph.2013.05.014. [DOI] [PubMed] [Google Scholar]
- [19].Olichney J.M., Yang J.C., Taylor J., Kutas M.. Cognitive event-related potentials: biomarkers of synaptic dysfunction across the stages of Alzheimer's disease. Alzheimers Dis. J. 2011;26(3):215–228. doi: 10.3233/JAD-2011-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Vecchio F., Määttä S.. The use of auditory event-related potentials in Alzheimer's disease diagnosis. Int. Alzheimers Dis J. 2011;653173 doi: 10.4061/2011/653173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Goodin D.S.. P300 latency as a biologic marker of dementia. Biol. Psychiatry. 1986;21:1111–1113. doi: 10.1016/0006-3223(86)90218-0. [DOI] [PubMed] [Google Scholar]
- [22].Jiménez-Escrig A., Fernandez-Lorente J., Herrero A., Baron M., Lousa M., de Blas G.. Event-related evoked potential P300 infrontotemporal dementia. Dement. Geriatr. Cogn. Disord. 2002;13:27–32. doi: 10.1159/000048630. [DOI] [PubMed] [Google Scholar]
- [23].Chen L., Zhou Y., Liu L., Zhang X., Zhang H., Liu S.. Cortical eventrelated potentials in Alzheimer's disease and frontotemporal lobar degeneration. J. Neurol Sci. 2015;188;359 doi: 10.1016/j.jns.2015.10.040. [DOI] [PubMed] [Google Scholar]
- [24].Chen J.Y., Stern Y., Sano M., Mayeux R.. Cumulative risks of developing extrapyramidal signs, psychosis, or myoclonus in the course of Alzheimer's disease. Arch. Neurol. 1991;48:1141–1143. doi: 10.1001/archneur.1991.00530230049020. [DOI] [PubMed] [Google Scholar]
- [25].Funkenstein H.H., Albert M.S., Cook N.R., West C.G., Scherr P.A., Chown M.J.. Extrapyramidal signs and other neurologic findings in clinically diagnosed Alzheimer's disease. A community-based study. Arch. Neurol. 1993;50:51–56. doi: 10.1001/archneur.1993.00540010045016. [DOI] [PubMed] [Google Scholar]
- [26].Lomen-Hoerth C., Anderson T., Miller B.. The overlap of amyotrophic lateral sclerosis and frontotemporal dementia. Neurology. 2002;59:1077–1079. doi: 10.1212/wnl.59.7.1077. [DOI] [PubMed] [Google Scholar]
- [27].Kobayashi M., Pascual-Leone A.. Transcranial magnetic stimulation in neurology. Lancet Neurol. 2003;2:145–156. doi: 10.1016/s1474-4422(03)00321-1. [DOI] [PubMed] [Google Scholar]
- [28].Rossini P.M., Rossi S.. Transcranial magnetic stimulation: diagnostic, therapeutic, and research potential. Neurology. 2007;68:484–488. doi: 10.1212/01.wnl.0000250268.13789.b2. [DOI] [PubMed] [Google Scholar]
- [29].Ferreri F., Pasqualetti P., Maatta S., Ponzo D., Guerra A., Bressi F.. Motor cortex excitability in Alzheimer’s disease: a transcranial magnetic stimulation follow-up study. Neurosci. Lett. 2011;492:94–98. doi: 10.1016/j.neulet.2011.01.064. [DOI] [PubMed] [Google Scholar]
- [30].Khedr E.M., Ahmed M.A., Darwish E.S., Ali A.M.. The relationship between motor cortex excitability and severity of Alzheimer’s disease: a transcranial magnetic stimulation study. Neurophysiol. Clin. 2011;41:107–113. doi: 10.1016/j.neucli.2011.03.002. [DOI] [PubMed] [Google Scholar]
- [31].Julkunen P., Jauhiainen A.M., Kononen M., Paakkonen A., Karhu J., Soininen H.. Combining transcranial magnetic stimulation and electroencephalography may contribute to assess the severity of Alzheimer’s disease. Int. J. Alzheimers Dis. 2011:654. doi: 10.4061/2011/654794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Guerra A., Assenza F., Bressi F., Scrascia F., Del Duca M., Ursini F.. Transcranial magnetic stimulation studies in Alzheimer’s disease. Int. J. Alzheimers Dis. 2011:263817. doi: 10.4061/2011/263817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Alberici A., Bonato C., Calabria M., Agosti C., Zanetti O., Miniussi C.. The contribution of TMS to frontotemporal dementia variants. Acta Neurol. Scand. 2008;118:275–280. doi: 10.1111/j.1600-0404.2008.01017.x. [DOI] [PubMed] [Google Scholar]
- [34].Pierantozzi M., Panella M., Palmieri M.G., Koch G., Giordano A., Marciani M.G.. et al. Different TMS patterns of intracortical inhibition in early onset Alzheimer dementia and frontotemporal dementia. Clin. Neurophysiol. 2004;115:2410–2418. doi: 10.1016/j.clinph.2004.04.022. [DOI] [PubMed] [Google Scholar]
- [35].Albert M.S., DeKosn S.T., Dickson D., Dubois B., Feldman H.H., Fox N.C.. et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].McKhann G.M., Knopman D.S., Chertkow H., Hyman B.T., Jack C.R. Jr., Kawas C.H.. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Mckhann G.M., Abert M.S., Grossman M., Miller B., Dickson D., Trojanowski J.Q.. Clinical and pathological diagnosis of frontotemporal dementia: report of the Work Group on Frontotemporal Dementia and Pick’s Disease. Arch. Neurol. 2001;58:1803–1809. doi: 10.1001/archneur.58.11.1803. [DOI] [PubMed] [Google Scholar]
- [38].Neary D., Snowden J.S., Gustafson L., Passant U., Stuss D., Black S.. et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- [39].Folstein M.F., Folstein S.E., McHugh P.R.. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Psychiatr J. Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- [40].Velayudhan L., Ryu S.H., Raczek M., Philpot M., Lindesay J., Lindesay M.. et al. Review of brief cognitive tests for patients with suspected dementia. Int. Psychogeriatr. 2014;26:1247–1262. doi: 10.1017/S1041610214000416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Smith T., Gildeh N., Holmes C.. The Montreal Cognitive Assessment: validity and utility in a memory clinic setting. Can. J. Psychiatry. 2007;52:329–332. doi: 10.1177/070674370705200508. [DOI] [PubMed] [Google Scholar]
- [42].Nasreddine Z.S., Phillips N.A., Bedirian V., Charbonneau S., Whitehead V., Collin I.. et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. Geriatr J. Am. Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- [43].Morris J.C.. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- [44].Ritter W., Vaughan H.G. Jr.. Averaged evoked responses in vigilance and discrimination: a reassessment. Science. 1969;164:326–328. doi: 10.1126/science.164.3877.326. [DOI] [PubMed] [Google Scholar]
- [45].Golob E.J., Starr A.. Age-related qualitative differences in auditory cortical responses during short-term memory. Clin. Neurophysiol. 2000;111:2234–2244. doi: 10.1016/s1388-2457(00)00468-5. [DOI] [PubMed] [Google Scholar]
- [46].Daffner K.R., Rentz D.M., Scinto L.F., Faust R., Budson A.E., Holcomb P.J.. Pathophysiology underlying diminished attention to novel events in patients with early AD. Neurology. 2001;56:1377–1383. doi: 10.1212/wnl.56.10.1377. [DOI] [PubMed] [Google Scholar]
- [47].Chapman R.M., Nowlis G.H., McCrary J.W., Chapman J.A., Sandoval T.C., Guillily M.D.. Brain event-related potentials: diagnosing early-stage Alzheimer’s disease. Neurobiol. Aging. 2007;28:194–201. doi: 10.1016/j.neurobiolaging.2005.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Gratton G., Coles M.G., Donchin E.. A new method for off-line removal of ocular artifact. Electroencephalogr. Clin. Neurophysiol. 1983;55:468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- [49].Ashford J.W., Coburn K.L., Rose T.L., Bayley P.J.. P300 energy loss in aging and Alzheimer’s disease. Alzheimers J. Dis. 2011;26:229–238. doi: 10.3233/JAD-2011-0061. Suppl. 3. [DOI] [PubMed] [Google Scholar]
- [50].Polich J., Corey-Bloom J.. Alzheimer’s disease and P300: review and evaluation of task and modality. Curr. Alzheimer Res. 2005;2:515–525. doi: 10.2174/156720505774932214. [DOI] [PubMed] [Google Scholar]
- [51].Halgren E., Marinkovic K., Chauvel P.. Generators of the late cognitive potentials in auditory and visual oddball tasks. Electroencephalogr. Clin. Neurophysiol. 1998;106:156–164. doi: 10.1016/s0013-4694(97)00119-3. [DOI] [PubMed] [Google Scholar]
- [52].Mochizuki Y., Oishi M., Takasu T.. Correlations between P300 components and regional cerebral blood flows. J. Clin. Neurosci. 2001;8:407–410. doi: 10.1054/jocn.2000.0850. [DOI] [PubMed] [Google Scholar]
- [53].de Carvalho M., de Mendonca A., Miranda P.C., Garcia C., Luis M.L.. Magnetic stimulation in Alzheimer’s disease. J. Neurol., 1997;244:304–307. doi: 10.1007/s004150050091. [DOI] [PubMed] [Google Scholar]
- [54].Ferreri F., Pauri F., P Pasqualetti, Fini R., Dal Forno G., Rossini P.M.. Motor cortex excitability in Alzheimer’s disease: a transcranial magnetic stimulation study. Ann. Neurol. 2003;53:102–108. doi: 10.1002/ana.10416. [DOI] [PubMed] [Google Scholar]
- [55].Di Lazzaro V., Oliviero A., Pilato F., Saturno E., Dileone M., Marra C.. Motor cortex hyperexcitability to transcranial magnetic stimulation in Alzheimer’s disease. J. Neurol Neurosurg. Psychiatry. 2004;75:555–559. doi: 10.1136/jnnp.2003.018127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Pennisi G., Ferri R., Lanza G., Cantone M., Pennisi M., Puglisi V.. Transcranial magnetic stimulation in Alzheimer’s disease: a neurophysiological marker of cortical hyperexcitability. J. Neural Transm., 2011;118:587–598. doi: 10.1007/s00702-010-0554-9. [DOI] [PubMed] [Google Scholar]
- [57].Fisher G.H., D’Aniello A., Vetere A., Padula L., Cusano G.P., Man E.H.. Free D-aspartate and D-alanine in normal and Alzheimer brain. Brain Res. Bull., 1991;26:983–985. doi: 10.1016/0361-9230(91)90266-m. [DOI] [PubMed] [Google Scholar]
- [58].Suva D., Favre I., Kraftsik R., Esteban M., Lobrinus A., Miklossy J.. Primary motor cortex involvement in Alzheimer disease. J. Neuropathol Exp. Neurol. 1999;58:1125–1134. doi: 10.1097/00005072-199911000-00002. [DOI] [PubMed] [Google Scholar]
- [59].Di Lazzaro V., Oliviero A., Tonali P.A., Marra C., Daniele A., P Profice. Noninvasive in vivo assessment of cholinergic cortical circuits in AD using transcranial magnetic stimulation. Neurology. 2002;59:392–397. doi: 10.1212/wnl.59.3.392. [DOI] [PubMed] [Google Scholar]
- [60].Sanger T.D., Garg R.R., Chen R.. Interactions between two different inhibitory systems in the human motor cortex. J. Physiol., 2001;530:307–317. doi: 10.1111/j.1469-7793.2001.0307l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Liepert J., Bar K.J., Meske U., Weiller C.. Motor cortex disinhibition in Alzheimer’s disease. Clin. Neurophysiol. 2001;112:1436–1441. doi: 10.1016/s1388-2457(01)00554-5. [DOI] [PubMed] [Google Scholar]
- [62].Pennisi G., Alagona G., Ferri R., Ferri S., Santonocito D., Pappalardo A.. Motor cortex excitability in Alzheimer disease: one year follow-up study. Neurosci. Lett., 2002;329:293–296. doi: 10.1016/s0304-3940(02)00701-2. [DOI] [PubMed] [Google Scholar]
- [63].Alagona G., Bella R., Bella R., Carnemolla A., Pappalardo A., Costanzo E.. Transcranial magnetic stimulation in Alzheimer disease: motor cortex excitability and cognitive severity. Neurosci. Lett. 2001;314:57–60. doi: 10.1016/s0304-3940(01)02288-1. [DOI] [PubMed] [Google Scholar]