SUMMARY
Male germline stem cells—spermatogonial stem cells (SSCs)—self-renew and produce large numbers of differentiating germ cells that become spermatozoa throughout postnatal life and transmit genetic information to the next generation. SSCs are the only germline stem cells in adults, because all female germline stem cells cease proliferation before birth. In this article, we first summarize development of SSCs, and then the relation of SSCs to somatic stem cells in tissues and pluripotent stem cells in vitro, such as embryonic stem cells. Next, we describe a transplantation technique in which donor testis cells from a fertile male can be transplanted to the testes of an infertile male where they re-establish spermatogenesis and restore fertility. The transplantation technique has been used to study the biology of SSCs, which made possible the identification of external factors that support in vitro self-renewal and proliferation of mouse and rat SSCs. Since SSCs of all mammalian species examined, including human, can replicate in mouse seminiferous tubules following transplantation, the growth factors required for SSC self-renewal are probably conserved among mammalian species. Culture techniques should therefore soon be available for human SSCs. In the final section, we discuss current and potential approaches for using the transplantation technique and in vitro culture of SSCs in human medicine. Because assisted reproductive techniques to fertilize oocytes with round or elongated spermatids are available, clinical use of cultured human SSCs will be greatly facilitated by development of techniques for in vitro differentiation of SSCs to mature germ cells.
Keywords: germline stem cells, spermatogonial stem-cell culture, spermatogonial stem-cell therapy, spermatogonial stem cells, testis-cell transplantation
INTRODUCTION
Germ cells are responsible for transmission of the genetic information of an individual to the next generation and through this process assure the continuity of a species. Manipulation of germ cells therefore offers the opportunity to study the mechanisms underlining continuation of the germline and to develop novel techniques for germline modification or therapy. Recent research with spermatogonial stem cells (SSCs)—male germline stem cells—in the mouse 1–3 demonstrates the potential of these stem cells to be the centerpiece in a new era of clinical application for treatment of infertility and regenerative medicine.
An application of human SSCs in medicine is transplantation of SSCs into the seminiferous tubules of infertile men to restore fertility. A clinical trial of testicular-cell transplantation into testes of patients after treatment for cancer was reported several years ago,4 and results should be available in the next few years.5 Another potential clinical application using human SSCs is GERMLINE GENE THERAPY. Numerous studies on the molecular genetics of endocrine diseases and metabolic disorders have identified germline gene mutations.6 These studies raise the possibility of gene therapy to correct the nonfunctional, hypofunctional, or hyperfunctional genes that cause these endocrine or metabolic abnormalities. Some defects lead to slow injury that results in life-threaten ing metabolic failure in middle age or later; however, the most severe metabolic diseases can be lethal if not treated immediately after birth. To prevent those lethal or life-threatening diseases caused by inborn errors, germline gene therapy using SSCs will become a promising and feasible approach, although considerable ethical concerns exist. The approach of germline gene therapy could be applicable to many genetic disorders, such as cystic fibrosis, hemophilia, sickle-cell anemia, phenylketonuria, Tay-Sachs disease, or severe combined immunodeficiency disease. Although couples carrying gene mutations may often choose prenatal genetic diagnosis of embryos and selective abortion, others may be unwilling to sacrifice any fetus. For those couples, germline gene therapy using SSCs to correct the defect could provide an alternative option. To pursue these novel therapeutic approaches, in vitro culture of human SSCs is a prerequisite, and development of in vitro differentiation techniques from SSCs to functional male germ cells will be enormously valuable.
SSCS AND THEIR RELATION TO OTHER STEM CELLS
Following fertilization of the oocyte by a spermatozoon, the zygote begins proliferation, and then differentiates to produce the many cell types that constitute an individual. The zygote is considered totipotent because it gives rise to all cells of both the fetus and extraembryonic tissues. Although studies of human embryos are limited because of technical difficulties and ethical concerns, early development of the mammalian embryo has been examined using other species, particularly mice, as models.7
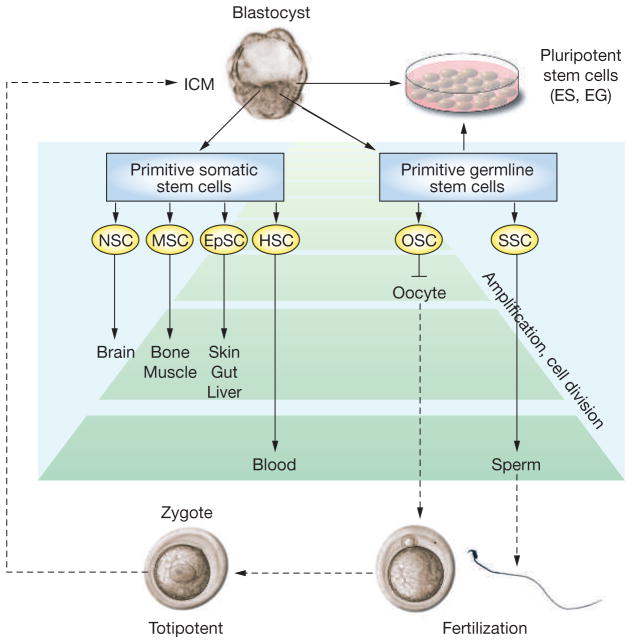
During the first 5 days after fertilization in the mouse, the zygote becomes a blastocyst, and then begins the process of implantation. At this time, the embryo consists of an inner cell mass (ICM), primitive endoderm, and trophectoderm. The ICM cells are the precursors for epiblast cells that give rise to the three germ layers (ectoderm, endoderm and mesoderm), which produce stem cells for all tissues of the fetus. Epiblast cells gradually commit to specific cell lineages and lose pluripotent developmental potential, and some of these committed cells become cell-lineage-specific primitive somatic stem cells, while others become primitive germline stem cells (Figure 1). In the fetus, extrinsic signals provided by surrounding cells, the extracellular matrix, or soluble factors cause these primitive somatic and germline stem cells to develop into adult tissue-specific stem cells. These adult tissue-specific stem cells, which have the capacity for SELF-RENEWAL and produce daughter cells for differentiation, are the foundation cells that maintain and regenerate tissues. Timing of self-renewal and differentiation as well as characteristics of somatic stem cells of each tissue are determined by interaction of their intrinsic gene expression and extrinsic signals from the environment through endocrine, paracrine, and autocrine pathways (Figure 1).8,9
Figure 1.
Lineage development of somatic and germline stem cells in vivo and pluripotent stem cells in vitro. Inner-cell-mass cells of the blastocyst generate all somatic and germ cells of the fetus. Primitive fetal somatic stem cells develop into adult somatic tissue-specific stem cells. Primitive germline stem cells, which are called primordial germ cells, generate male or female germ cells depending on the sex of the gonad. In the fetal ovary, oogonial stem cells cease proliferation before birth and enter the first meiotic division; therefore, they have no stem-cell potential after birth. In the fetal testis, primordial germ cells develop into gonocytes, which soon enter mitotic arrest; however, they become spermatogonial stem cells after birth and self-renew as well as produce daughter cells that commit to differentiate into spermatozoa throughout life. During spermatogenesis, one spermatogonium undergoes up to 12 cell divisions before forming mature spermatozoa.8,15 The number of amplification cell divisions of spermatogonial stem cells and hematopoietic stem cells is greater than for other adult stem cells, and they are considered the most productive adult stem-cell systems.8 Pluripotent stem cells, embryonic stem cells and embryonic germ cells, can be derived in vitro from inner-cell-mass or primordial germ cells, respectively. The developmental potential of these pluripotent stem cells, when transplanted to a blastocyst, is similar to epiblast cells in vivo. Embryonic stem cells and embryonic germ cells self-renew in vitro under appropriate culture conditions.
EG cell, embryonic germ cell; EpSC, epithelial stem cell; ES cell, embryonic stem cell; HSC, hematopoietic stem cell; ICM, inner cell mass; MSC, mesenchymal stem cell; NSC, neuronal stem cell; OSC, oogonial stem cell; SSC, spermatogonial stem cell.
In mammals, the germ-cell lineage is not predetermined in the early embryo before germ-cell formation; instead, current experimental evidence indicates that the germ-cell lineage is induced by instructive signals, which have not been identified.10,11 About 7 days postcoitum in the mouse, specification of the germ-cell lineage occurs in the proximal epiblast under the influence of surrounding extraembryonic tissues.11 The primitive germline cells are called pri mordial germ cells (PGCs), and they migrate to the female or male genital ridge and differentiate into oogonia or gonocytes, respectively. The processes of migration and differentiation of germ cells, as well as gonadal tissue formation in embryos, are largely regulated by growth factors and hormones, such as Steel factor, stromal cell-derived factor 1, anti-Müllerian hormone, or testosterone.7,11
Oogonia in the female gonad quickly enter meiotic prophase as oocytes, which are all arrested in meiosis I before birth. The adult ovary therefore does not contain germline stem cells.7 Although recent studies identified putative proliferating female germ cells in the ovary of postnatal mice,12,13 there is no direct evidence of either the existence of germline stem cells in adult ovaries or the fertilizability of the oocytes generated from the putative post-natal pro liferating female germ cells. It therefore remains highly controversial whether mammalian female germline stem cells exist in postnatal ovaries.14 In the male, gonocytes cease mitosis in the fetal seminiferous tubules of the testes and resume mitotic activity after birth.7,15 In the fetus, gonocytes are located in the center of the tubules, but during the first few days after birth in the mouse and at later times in other species, they either undergo apoptosis or migrate to the basement membrane and become SSCs.15,16
Once SSCs are formed, they continue to self-renew and produce spermatogonia that are committed to differentiate into spermatozoa, as determined by their gene expression and environmental cues. The balance of self-renewal and differentiation of SSCs must be regulated strictly to maintain normal spermatogenesis; however, the mechanisms governing the fate decision—self-renewal or differentiation—are largely unknown. Several lines of evidence demonstrate hormonal regulation of spermatogenesis. Although most studies focused on the post-spermatogonial stage of spermatogenesis and testicular somatic cells, including Sertoli cells, Leydig cells, and myoid cells,17 it is possible that hormonal factors play a role in SSC self-renewal through the surrounding testicular somatic cells.18 In the testis, only SSCs, not other germ cells, can self-renew; therefore, they are responsible for maintaining spermatogenesis throughout life in the male.15,18,19 Since female germ cells are not capable of self-renewal after birth, SSCs are the only stem cells in the adult that continue to proliferate and are capable of transmitting genetic information to the next generation under normal circumstances (Figure 1).
Embryonic stem (ES) cells are pluripotent and self-renew in vitro when cultured under appropriate conditions.20–22 ES cell lines can be established by culturing blastocysts of the mouse, human and monkey and are believed to arise from ICM cells of the blastocyts during in vitro differentiation (Figure 1).23 The developmental potential of ES cells is similar to that of epiblast cells. In addition, ES-cell-like pluripotent cells can be formed following culture of mouse or human fetal gonad,24–26 and are called embryonic germ (EG) cells (Figure 1). Recently, ES-cell-like pluripotent cells were isolated from cultured neonatal mouse testes.27 Such ES-cell-like pluripotent cells could not, however, be generated from adult normal testes, and only occasionally from adult p53-knockout mouse testes, which also frequently develop terato-carcinomas.28 The ES-cell-like pluri potent cells could therefore arise from residual fetal germ cells in neonatal testes, which are thought to be the origin of terato carcinomas.29 The absence of p53 must generate an abnormal SSC prone to develop into teratocarcinoma or an ES-cell-like phenotype in vitro. All these in vitro pluripotent cell types have therefore been generated with the use of cells from only a few species and closely related tissues.
ES cells or ES-cell-like cells do not exist in vivo, but are in vitro derivatives from primitive cells found in the embryo or fetus. PGCs do not continuously self-renew in the fetal gonad; however, they generate EG cells that self-renew in vitro like ES cells. Both ES and EG cells are able to differentiate into germ cells as well as all three germ layers when injected into blasto-cysts.30,31 PGCs are also the natural precursors of gonocytes that give rise to SSCs.15 ES cells, EG cells and SSCs are therefore self-renewing cells with the ability to contribute to the germ-line, and PGCs appear to be the common precursors for in vitro development of EG cells and in vivo development of SSCs. In the context of origin, cell lineage, and biological characteristics, ES cells, EG cells, PGCs and SSCs are closely related (Figure 1).
DONOR-DERIVED SPERMATOGENESIS IN SSC-TRANSPLANT RECIPIENTS
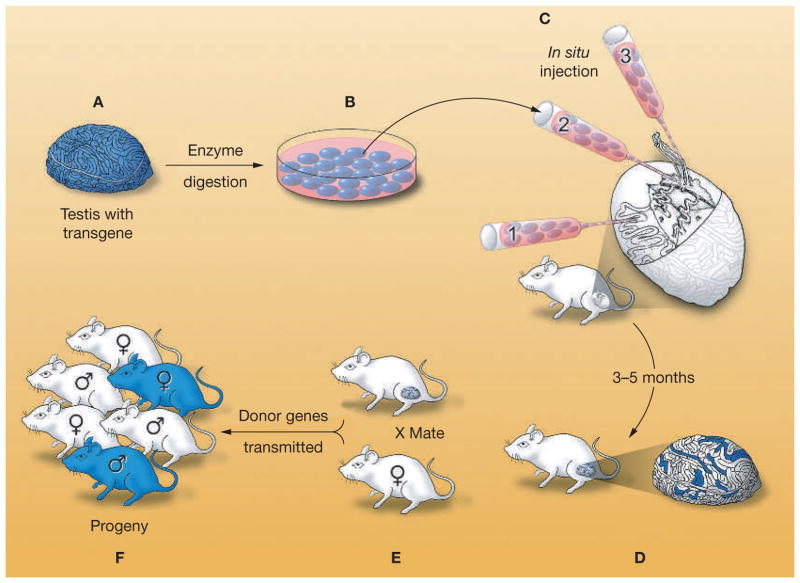
A decade ago, a technique to transplant testis cells into seminiferous tubules of recipient males was developed in mice.1,2 In this procedure, donor cells are harvested from the testes of fertile mice that express a reporter transgene, and a single-cell suspension of the cells is microinjected into seminiferous tubules of recipient infertile adult mice (Figure 2).19 Three different methods to introduce donor cells into the recipient tubules were developed using mice and rats (Figure 2).32 In the first method, cells are injected directly into the seminiferous tubules using a micropipette, and the cells flow to other tubules through the rete testis, to which all tubules are connected. A second method is to insert a micropipette directly into the rete testis. The third method is to inject donor cells into a large efferent duct, which leads to the rete testis. Because all tubules access the rete testis, a cell suspension entering the rete testis can potentially fill a whole system of seminiferous tubules. Whereas all three methods have been used for mice and rats,1,2,32,33 only injection of donor cells directly into the rete testis has been used for pigs,34 goats,35 cattle,36 sheep,37 cynomolgus monkeys,38 and humans.4
Figure 2.
Procedure for testis-cell transplantation as developed in the mouse. (A) A single-cell suspension is prepared from the testes of a fertile male that expresses a reporter transgene, Escherichia coli lacZ. (B) The testis cells can be cultured with appropriate conditions. (C) Cells are microinjected into the seminiferous tubules of an infertile recipient male. There are three methods for microinjection: the micropipette can be inserted (1) directly into the seminiferous tubules, (2) into the rete testis, or (3) into an efferent duct. (D) Spermatogonial stem cells colonize the basement membrane of the tubules and generate donor-cell-derived spermatogenesis, which can be stained blue using a substrate for the reporter gene product (β-galactosidase). Each blue stretch of cells in the seminiferous tubules of the recipient testis represents a spermatogenic colony derived from a single donor stem cell. (E) Mating the recipient male to a wild-type female results in donor-cell-derived spermatozoa fertilizing wild-type oocytes. (F) Progeny with the donor haplotype are produced. Modified with permission from references 19 © (2002) American Association for the Advancement of Science and 32 © (1997) University of the Basque Country Press.
Following microinjection using any of the three methods, only a percentage of the donor SSCs colonize the basement membrane of the seminiferous tubules and begin proliferating. This percentage probably varies among species, but for the mouse is estimated to be 5–12%.39,40 In the mouse model, during the first month after transplantation, a majority of proliferating donor-derived cells are still located on the basement membrane.41 By 2 months after transplantation, however, differentiated germ cells begin to fill the tubule from the basement membrane toward the lumen, and spermatozoa appear in the lumen of the donor-cell-derived colonies. The reconstituted spermatogenic colonies continuously produce spermatozoa throughout the remaining life of the recipient males. It has been demonstrated that each donor-derived spermatogenic colony generally arises from a single SSC.41–43 The recipient male mice become fertile and produce progeny with donor-cell haplotype, demonstrating normal function of the spermatozoa originating from transplanted stem cells.2,44 These results clearly demonstrate that the spermatogenic colony-forming cells are SSCs.
Stem cells are defined by their biological function; they have the ability to both self-renew and produce daughter cells that undergo differentia tion. Using the transplantation technique as a functional assay, SSCs can be detected unequivocally in any donor-cell population. The method of testis-cell transplantation therefore paved the way to identify and study SSCs on the basis of their biological activity. Although many adult tissues are maintained by tissue-specific stem cells, only a few types of stem cells (hematopoietic, spermatogonial and epidermal) can be unequivocally identified by a functional assay in which complete replacement of the dependent tissue or system occurs.1,2,45,46 SSCs and the functional transplantation assay are therefore important not only to study and modify the cells of the male germline, but these stem cells and assay also form the basis of a unique experimental system potentially relevant to all stem cells.19,47
Many adult tissues contain a stem-cell population; in most cases the number of stem cells present is low, and they proliferate slowly. Previous studies indicate that the concentration of SSCs in the adult mouse testis is only 1 in 3,000–4,000 cells,48 and no morphologic or biochemical markers exist for the stem cells. However, FLUORESCENCE-ACTIVATED CELL SORTING (FACS), in combination with the transplantation assay, has allowed determination of the surface-antigenic profile of mouse SSCs, which is MHC class I− Thy-1lo/+ c-Kit− αv-integrin−/dim α6-integrin+ throughout postnatal life.49–52 Using these surface markers, a highly enriched SSC population could be obtained; the final concentration of stem cells can be up to 1 in 15.51 Rat SSCs have a similar antigenic profile,53 and evidence suggests that the surface-antigenic phenotype of SSCs in most mammalian species will be similar. Although surface markers for human SSCs have not yet been identified, the SSCs of the baboon share several antigens (e.g. Thy-1, α6-integrin, Ep-CAM) with the mouse and rat (H Kubota, unpublished data).
Because spermatogenesis is a highly conserved process among mammalian species,54 it should not be surprising to find shared phenotypic and metabolic characteristics of the stem cells of this remarkable system. Findings from testis-cell transplantation studies support this hypothesis, because mouse seminiferous tubules can maintain SSCs from many different species.19 SSCs from all mammals examined, including rats,55 rabbits,56 dogs,56 pigs,57 cattle,57,58 horses,57 baboons,59 and humans,60 colonized and proliferated for 1–12 months in the seminiferous tubules of immunodeficient mice. This indicates that factors necessary to support and maintain proliferation of SSCs are conserved among many species, including human.
SSCS SELF-RENEW AND INCREASE IN NUMBER DURING CULTURE
Development of a culture system that supports self-renewal and proliferation of SSCs in vitro is enormously valuable to understand their biological characteristics and possibly modify their genes.3,61 Xenotransplantation experiments55–60 suggested that external factors to support self-renewal of SSCs would be conserved among various mammalian species. Development of a defined culture system for mouse SSCs would therefore probably be applicable to other species, including humans, with minor modifications.
To pursue this objective, we developed a culture system for mouse SSCs that consisted of enriched stem cells, serum-free defined medium and mitotically inactivated STO feeder cells (a mouse embryonic feeder cell line).3,52 Each of these characteristics proved to be important. First, an enriched stem-cell population was critical because large numbers of contaminating testis somatic cells have an adverse effect on SSC maintenance in vitro.52,62 Second, a hormonally and chemically defined serum-free medium was developed based on defined media for other stem cells or other progenitor cells.63 Serum contains complex undefined materials, and considerable variation occurs among serum sources. Since serum-free culture systems demonstrated that most mammalian cells require specific growth factors and hormones to proliferate in vitro, use of serum-free defined medium is crucial to identify these exogenous factors.64 In addition, serum is toxic for many cells.64 In fact, when SSCs were cultured in medium containing only 0.1% fetal bovine serum, proliferation of SSCs was dramatically decreased compared with cells cultured in a serum-free medium.3 Third, mitotically in activated STO feeders were used rather than freshly isolated embryonic fibroblast feeders, because the former are homogeneous, whereas the latter are heterogeneous depending on fetal age and tissue of origin.65 In addition, STO feeder cells have been used as feeder cells for many types of stem cells.20,21,24–26,63 Because the culture conditions were well defined, it was possible to identify the specific factor requirements for continuous self-renewal of mouse SSCs as glial-cell-line-derived neurotrophic factor (GDNF), soluble GDNF-family receptor α1 and basic fibroblast growth factor.3
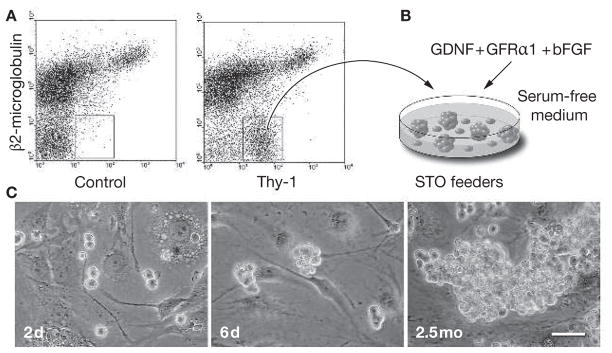
In the mouse culture experiments, an anti-Thy-1 (CD90) antibody was used to obtain an enriched SSC population using FACS or magnetically activated cell sorting (Figure 3A).51,52 When the Thy-1lo/+ cells were placed on STO feeders in a serum-free medium supplemented with GDNF, soluble GDNF-family receptor α1 and basic fibroblast growth factor (Figure 3B), SSCs formed densely packed cell clumps (Figure 3C). The germ-cell clumps continuously proliferated under these culture conditions and, at periodic intervals, the cells were transplanted into infertile recipient mouse testes to determine the stem-cell activity (the ability to form donor-cell-derived colonies of spermatogenesis) of the clump-forming cells. The results of the functional transplantation assay clearly demonstrated that proliferating clump-forming cells are SSCs.3 During culture, the number of stem cells doubled every 5.6 days, and they continuously proliferated over 6 months. Like ES cells, SSCs seem to have the ability to proliferate indefinitely in culture. Transplantation of the cultured SSCs into infertile mouse testes restored fertility, and progeny were produced with the cultured-cell haplotype, demonstrating that the cultured stem cells are functionally normal.3 Furthermore, the cultured SSCs expressed molecular markers similar to undifferentiated ES cells or PGCs, including Oct-4 and tissue nonspecific alkaline phos-phatase.3 These results indicate that SSCs, ES cells and PGCs share molecular characteristics with pluripotent cells.66
Figure 3.
In vitro proliferation of mouse spermatogonial stem cells. (A) A highly enriched spermatogonial stem-cell population is obtained from adult cryptorchid mouse testes by fluorescence-activated cell sorting using antibodies against β2-microglobulin (the light chain of the MHC class I molecule) and Thy-1.51,52 The concentration of stem cells in the cryptorchid testes is 20-fold to 25-fold higher than in wild-type testes.76 (B) The enriched stem-cell population is placed on STO feeder cells in a serum-free defined medium supplemented with glial-cell-line-derived neurotrophic factor, soluble glial-cell-line-derived neurotrophic factor-family receptor α1 and basic fibroblast growth factor. The MHC class I− Thy-1+ cells form germ-cell clumps and proliferate. (C) Microscopic appearance of germ-cell clump formation and continuous proliferation of clump-forming cells is shown (2 days, 6 days and 2.5 months after in vitro culture of spermatogonial stem cells sorted using fluorescence-activated cell sorting; scale bar = 50 μm). The clump-forming germ cells can reconstitute normal spermatogenesis and restore fertility following transplantation into recipient testes of infertile male mice, indicating that they are spermatogonial stem cells. Under these culture conditions, spermatogonial stem cells continuously proliferate over 6 months.3 bFGF, basic fibroblast growth factor; d, days; GDNF, glial-cell-line-derived neurotrophic factor; GFRα1, glial-cell-line-derived neurotrophic factor-family receptor α1; mo, months.
Clearly, the identification of growth-factor requirements and development of a long-term culture system for mouse SSCs has established a foundation for in vitro manipulation of SSCs; moreover, we recently applied a similar culture system to rat SSCs and demonstrated that the same three factors are required to support their in vitro self-renewal and proliferation.67 Because SSCs of many mammalian species proliferate for 6 months or longer in the seminiferous tubules of immunodeficient mice, it is likely that SSCs of all these species require the same growth-factor support now identified for mouse and rat SSCs; therefore, similar culture systems for SSCs of other mammalian species, including humans, can probably be established in the next few years.
SSC TRANSPLANTATION AND CULTURE IN HUMAN MEDICINE
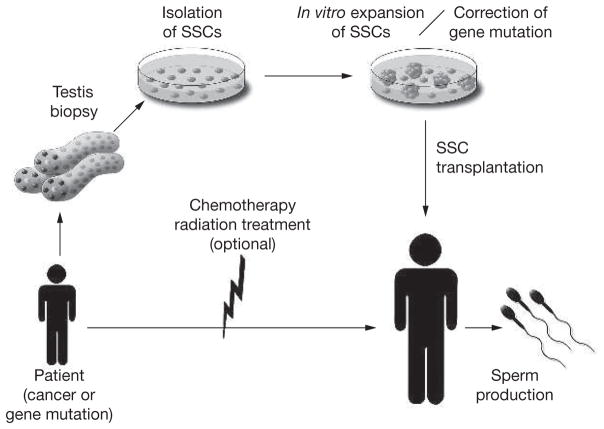
At present, many individuals with cancer are treated with chemotherapy, radiation, or both. Although the survival rate is increasing, restoration of fertility in patients after cancer treatment is an important issue for quality of life. Since the first reports of spermatogonial transplantation in the mouse1,2 and successful cryopreservation of SSCs,68 autologous spermatogonial transplantation of cryopreserved SSCs of patients has been considered as a feasible technique for restoration of infertility after chemotherapy or radiation. Although semen cryopreservation allows subsequent artificial insemination or in vitro fertilization for men, the success rate of this approach is not high—in one study, approximately 25%.69 In addition, these methods are not applicable to patients before puberty. For prepubertal patients and perhaps adult men, SSC cryopreservation and transplantation provides a promising option to restore spermatogenesis after cancer treatment (Figure 4). In addition, when culture methods to achieve in vitro proliferation of human SSCs become available, one would be able to greatly increase the number of SSCs before cryo preservation or transplantation to maximize coloniza tion of recipient testes by the injected SSCs.
Figure 4.
Germline stem-cell therapy. Proposed outline for isolation of human spermatogonial stem cells, expansion by proliferation in culture, with possible genetic modification, and transplantation into recipient testes. Spermatogonial stem cells may be cryopreserved at any point between isolation and transplantation. In cancer patients, spermatogonial stem cells could be isolated by testis biopsy before treatment with chemotherapy or radiation, and the stem-cell number increased in culture. After successful treatment, the spermatogonial stem cells would be transplanted to the patient’s testes to restore fertility. This approach is particularly valuable for prepubertal patients because they do not have mature spermatozoa that can be cryopreserved for future use. Before transplantation of spermatogonial stem cells into a recovered patient, contamination by cancer cells must be ruled out. This could be accomplished by a combination of several techniques: first, by enrichment of testis cells and/or cultured cells using antibodies to specific surface antigens for spermatogonial stem cells; second, by culturing spermatogonial stem cells under conditions that will not support cancer cells; and third, by testing an aliquot of the cells in immunodeficient mice to determine if cancer cells are present. Development of an in vitro differentiation system to allow intracytoplasmic spermatid injection to fertilize oocytes would eliminate any problem with contaminating cancer cells. For patients carrying a genetic defect, spermatogonial stem cells could be isolated and cultured in vitro, the defective gene corrected, and the spermatogonial stem cells with the corrected gene transplanted into the testes of the patient. To enhance colonization of corrected spermatogonial stem cells, local irradiation could be used to destroy endogenous spermatogenesis. Although the transplantation of gene-corrected spermatogonial stem cells back to the patient is feasible, the approach is more likely to find use when in vitro differentiation of spermatogonial stem cells to spermatids or spermatozoa can be achieved. SSC, spermatogonial stem cell.
A clinical trial of testis-cell transplantation using cryopreserved samples of testis tissue obtained from human males treated for lymphoma was reported in 1999.4 The testicular cells were harvested before chemotherapy and cryopreserved. Following treatment, the cells were reinfused into the rete testis. Final results of the trial will appear over the coming years,5 but it will be difficult to evaluate the outcome because some endogenous spermatogenesis can return following radiation or chemotherapy.
One concern of this approach is that transplanted testis-cell suspensions could be contaminated with cancerous cells that could cause recurrence of the cancer.70 Recently, using a mouse lymphoma model, cancer cells were eliminated from the testis-cell suspension by FACS before transplantation.71 This approach resulted in no recurrence of lymphoma, suggesting the feasibility for application in human medicine. The identification of unique SSC surface phenotypic markers49–52 not only facilitates the enrichment of stem cells for study and culture, but also these markers can be used to concentrate SSCs and remove malignant cells before transplantation.
Two advances will facilitate clinical use of SSC therapies: the culture of human SSCs and the in vitro differentiation of SSCs to round or elongated spermatids that can be used for intracytoplasmic spermatid injection to fertilize oocytes. The second objective is dependent on the first, because successful in vitro differentiation will require a continuous supply of stem cells proliferating in vitro for use in developing methods for achieving differentiation and then to provide stem cells to differentiate for clinical use. Although culture of human SSCs appears to be an attainable goal in the next few years, a timetable for in vitro differentiation is less predictable. Recently, mature haploid male germ cells were isolated from embryonic bodies generated by mouse ES cells.72 When the nuclei from the most mature of these germ cells were injected into oocytes, blastocysts formed but no fetuses or pups were reported. This approach might allow generation of human mature haploid germ cells, but ethical problems exist regarding the use of human ES cells; moreover, haploid germ cells derived from ES cells cannot be autologous for an adult unless nuclear transplantation techniques are employed.73
There are many genetic disorders that cause human disease. Despite the existence of considerable ethical concerns, germline gene therapy would be a potential clinical application using in vitro manipulation of SSCs and transplantation. For men who carry gene mutations, and their female partners, whether to choose treatment by germ-cell gene therapy to correct a genetic mutation will be a difficult decision. Because long-term culture of SSCs is possible in mice and rats, and self-renewal signals are likely to be conserved among many mammals, human SSC culture will soon be possible. Once human SSCs are proliferating in vitro, all the sophisticated techniques that have been developed to make subtle genetic modifications in mouse ES cells or human somatic cells will be immediately applicable. For example, a point mutation of a specific gene could be corrected by several recently developed methods, 74 such as engineered zinc-finger nucleases.75 A potential problem exists, however, following injection of SSCs with the corrected gene into the testis because endogenous uncorrected SSCs will still be present. Competition between spermato zoa arising from corrected and endogenous un corrected SSCs will therefore occur at the time of fertilization. Development of in vitro differentiation from corrected SSCs will, therefore, be a crucial step.
CONCLUSIONS
Extraordinary advances have been made during the past 10 years in our understanding of male germline stem-cell biology. This understanding, as well as the transplantation and culture methods developed, holds great promise in treating male infertility and perhaps in germline gene therapy. The next critical advances will be human SSC culture and in vitro differentiation of the cultured stem cells. The first should occur in 2–3 years, the second in perhaps 5–10 years.
REVIEW CRITERIA.
All articles from journals for this review were obtained using PubMed. The search terms, used in different combinations, were “testis”, “male germline stem cells”, “spermatogonial stem cells”, “spermatogonia”, “stem cells”, “germ cells”, “pluripotent stem cells”, “self-renew”, “transplantation”, “gene therapy”, “inborn errors of metabolism”, “serum-free culture” and “assisted reproductive technique”. All referenced articles were in English.
KEY POINTS.
Spermatogonial stem cells are the only cells in postnatal mammals that undergo self-renewal and transmit genes to subsequent generations
In vitro growth-factor requirements for mouse spermatogonial stem cells have been identified using defined culture conditions
Because extrinsic factors for self-renewal of spermatogonial stem cells appear to be conserved among many mammalian species, including humans, in vitro culture techniques for human spermatogonial stem cell will probably be developed in the near future
Spermatogonial stem-cell transplantation could be used to restore fertility in men following chemotherapy or radiation treatment
Development of techniques for the in vitro differentiation of spermatogonial stem cells to functional spermatozoa is a crucial step for the treatment of infertility or germline gene therapy
Acknowledgments
We thank Jon Oatley for helpful comments on the manuscript and James Hayden for assistance in developing the figures. Financial support for the research was from the National Institute of Child Health and Human Development Grant 044445; and the Robert J Kleberg Jr, and Helen C Kleberg Foundation.
GLOSSARY
- GERMLINE GENE THERAPY
An approach to prevent disease in descendants by engineering genes of parent germ cells
- SELF-RENEWAL
The ability of a stem cell to divide and give rise to one or more stem cells with identical developmental potential
- FLUORESCENCE-ACTIVATED CELL SORTING
A method for isolation of specific types of live cells by their response to light signals while flowing in a stream past a light beam
Footnotes
Competing interests
The authors declared they have no competing interests.
References
- 1.Brinster RL, Zimmermann JW. Spermatogenesis following male germ-cell transplantation. Proc Natl Acad Sci USA. 1994;91:11298–11302. doi: 10.1073/pnas.91.24.11298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brinster RL, Avarbock MR. Germline transmission of donor haplotype following spermatogonial transplantation. Proc Natl Acad Sci USA. 1994;91:11303–11307. doi: 10.1073/pnas.91.24.11303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kubota H, et al. Growth factors essential for self-renewal and expansion of mouse spermatogonial stem cells. Proc Natl Acad Sci USA. 2004;101:16489–16494. doi: 10.1073/pnas.0407063101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Radford J, et al. Fertility after treatment for cancer. Questions remain over ways of preserving ovarian and testicular tissue. BMJ. 1999;319:935–936. doi: 10.1136/bmj.319.7215.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Radford J. Restoration of fertility after treatment for cancer. Horm Res. 2003;59(Suppl 1):21–23. doi: 10.1159/000067840. [DOI] [PubMed] [Google Scholar]
- 6.Barzon L, et al. New perspectives for gene therapy in endocrinology. Eur J Endocrinol. 2000;143:447–466. doi: 10.1530/eje.0.1430447. [DOI] [PubMed] [Google Scholar]
- 7.Nagy A, et al. Manipulating the Mouse Embryo, A Laboratory Manual. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 2003. [Google Scholar]
- 8.Potten CS, Morris RJ. Epithelial stem cells in vivo. J Cell Sci Suppl. 1988;10:45–62. doi: 10.1242/jcs.1988.supplement_10.4. [DOI] [PubMed] [Google Scholar]
- 9.Reid LM. Stem cell biology, hormone/matrix synergies and liver differentiation. Curr Opin Cell Biol. 1990;2:121–130. doi: 10.1016/s0955-0674(05)80042-0. [DOI] [PubMed] [Google Scholar]
- 10.Tam PP, Zhou SX. The allocation of epiblast cells to ectodermal and germ-line lineages is influenced by the position of the cells in the gastrulating mouse embryo. Dev Biol. 1996;178:124–132. doi: 10.1006/dbio.1996.0203. [DOI] [PubMed] [Google Scholar]
- 11.McLaren A. Primordial germ cells in the mouse. Dev Biol. 2003;262:1–15. doi: 10.1016/s0012-1606(03)00214-8. [DOI] [PubMed] [Google Scholar]
- 12.Johnson J, et al. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature. 2004;428:145–150. doi: 10.1038/nature02316. [DOI] [PubMed] [Google Scholar]
- 13.Johnson J, et al. Oocyte generation in adult mammalian ovaries by putative germ cells in bone marrow and peripheral blood. Cell. 2005;122:303–315. doi: 10.1016/j.cell.2005.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Telfer EE, et al. On regenerating the ovary and generating controversy. Cell. 2005;122:821–822. doi: 10.1016/j.cell.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 15.de Rooij DG. Stem cells in the testis. Int J Exp Pathol. 1998;79:67–80. doi: 10.1046/j.1365-2613.1998.t01-1-00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Orwig KE, et al. Male germ-line stem cell potential is predicted by morphology of cells in neonatal rat testes. Proc Natl Acad Sci USA. 2002;99:11706–11711. doi: 10.1073/pnas.182412099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holdcraft RW, Braun RE. Hormonal regulation of spermatogenesis. Int J Androl. 2004;27:335–342. doi: 10.1111/j.1365-2605.2004.00502.x. [DOI] [PubMed] [Google Scholar]
- 18.Meistrich ML, van Beek MEAB. Spermatogonial stem cells. In: Desjardins C, Ewing LL, editors. Cell and Molecular Biology of the Testis. New York: Oxford University Press; 1993. pp. 266–295. [Google Scholar]
- 19.Brinster RL. Germline stem cell transplantation and transgenesis. Science. 2002;296:2174–2176. doi: 10.1126/science.1071607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 21.Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci USA. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thomson JA, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 23.Smith AG. Embryo-derived stem cells: of mice and men. Annu Rev Cell Dev Biol. 2001;17:435–462. doi: 10.1146/annurev.cellbio.17.1.435. [DOI] [PubMed] [Google Scholar]
- 24.Matsui Y, et al. Derivation of pluripotential embryonic stem cells from murine primordial germ cells in culture. Cell. 1992;70:841–847. doi: 10.1016/0092-8674(92)90317-6. [DOI] [PubMed] [Google Scholar]
- 25.Resnick JL, et al. Long-term proliferation of mouse primordial germ cells in culture. Nature. 1992;359:550–551. doi: 10.1038/359550a0. [DOI] [PubMed] [Google Scholar]
- 26.Shamblott MJ, et al. Derivation of pluripotent stem cells from cultured human primordial germ cells. Proc Natl Acad Sci USA. 1998;95:13726–13731. doi: 10.1073/pnas.95.23.13726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kanatsu-Shinohara M, et al. Generation of pluripotent stem cells from neonatal mouse testis. Cell. 2004;119:1001–1012. doi: 10.1016/j.cell.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 28.Harvey M, et al. Genetic background alters the spectrum of tumors that develop in p53-deficient mice. FASEB J. 1993;7:938–943. doi: 10.1096/fasebj.7.10.8344491. [DOI] [PubMed] [Google Scholar]
- 29.Stevens LC. The origin and development of testicular, ovarian, and embryo-derived teratomas. In: Silver LM, et al., editors. Teratocarcinoma Stem Cells. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 1983. pp. 23–36. [Google Scholar]
- 30.Bradley A, et al. Formation of germ-line chimaeras from embryo-derived teratocarcinoma cell lines. Nature. 1984;309:255–256. doi: 10.1038/309255a0. [DOI] [PubMed] [Google Scholar]
- 31.Labosky PA, et al. Mouse embryonic germ (EG) cell lines: transmission through the germline and differences in the methylation imprint of insulin-like growth factor 2 receptor (Igf2r) gene compared with embryonic stem (ES) cell lines. Development. 1994;120:3197–3204. doi: 10.1242/dev.120.11.3197. [DOI] [PubMed] [Google Scholar]
- 32.Ogawa T, et al. Transplantation of testis germinal cells into mouse seminiferous tubules. Int J Dev Biol. 1997;41:111–122. [PubMed] [Google Scholar]
- 33.Ogawa T, et al. Recipient preparation is critical for spermatogonial transplantation in the rat. Tissue Cell. 1999;31:461–472. doi: 10.1054/tice.1999.0060. [DOI] [PubMed] [Google Scholar]
- 34.Honaramooz A, et al. Germ cell transplantation in pigs. Biol Reprod. 2002;66:21–28. doi: 10.1095/biolreprod66.1.21. [DOI] [PubMed] [Google Scholar]
- 35.Honaramooz A, et al. Germ cell transplantation in goats. Mol Reprod Dev. 2003;64:422–428. doi: 10.1002/mrd.10205. [DOI] [PubMed] [Google Scholar]
- 36.Izadyar F, et al. Autologous and homologous transplantation of bovine spermatogonial stem cells. Reproduction. 2003;126:765–774. [PubMed] [Google Scholar]
- 37.Oatley JM, et al. Changes in spermatogenesis and endocrine function in the ram testis due to irradiation and active immunization against luteinizing hormone-releasing hormone. J Anim Sci. 2005;83:604–612. doi: 10.2527/2005.833604x. [DOI] [PubMed] [Google Scholar]
- 38.Schlatt S, et al. Germ cell transplantation into X-irradiated monkey testes. Hum Reprod. 2002;17:55–62. doi: 10.1093/humrep/17.1.55. [DOI] [PubMed] [Google Scholar]
- 39.Nagano MC. Homing efficiency and proliferation kinetics of male germ line stem cells following transplantation in mice. Biol Reprod. 2003;69:701–707. doi: 10.1095/biolreprod.103.016352. [DOI] [PubMed] [Google Scholar]
- 40.Ogawa T, et al. Expansion of murine spermatogonial stem cells through serial transplantation. Biol Reprod. 2003;68:316–322. doi: 10.1095/biolreprod.102.004549. [DOI] [PubMed] [Google Scholar]
- 41.Nagano M, et al. Pattern and kinetics of mouse donor spermatogonial stem cell colonization in recipient testes. Biol Reprod. 1999;60:1429–1436. doi: 10.1095/biolreprod60.6.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dobrinski I, et al. Computer assisted image analysis to assess colonization of recipient seminiferous tubules by spermatogonial stem cells from transgenic donor mice. Mol Reprod Dev. 1999;53:142–148. doi: 10.1002/(SICI)1098-2795(199906)53:2<142::AID-MRD3>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 43.Zhang X, et al. Genetic analysis of the clonal origin of regenerating mouse spermatogenesis following transplantation. Biol Reprod. 2003;69:1872–1878. doi: 10.1095/biolreprod.103.019273. [DOI] [PubMed] [Google Scholar]
- 44.Ogawa T, et al. Transplantation of male germ line stem cells restores fertility in infertile mice. Nat Med. 2000;6:29–34. doi: 10.1038/71496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Till JE, McCulloch E. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res. 1961;14:213–222. [PubMed] [Google Scholar]
- 46.Blanpain C, et al. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 2004;118:635–648. doi: 10.1016/j.cell.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 47.Morrison SJ, et al. Regulatory mechanisms in stem cell biology. Cell. 1997;88:287–298. doi: 10.1016/s0092-8674(00)81867-x. [DOI] [PubMed] [Google Scholar]
- 48.Tegelenbosch RA, de Rooij DG. A quantitative study of spermatogonial multiplication and stem cell renewal in the C3H/101 F1 hybrid mouse. Mutat Res. 1993;290:193–200. doi: 10.1016/0027-5107(93)90159-d. [DOI] [PubMed] [Google Scholar]
- 49.Shinohara T, et al. β1- and α6-integrin are surface markers on mouse spermatogonial stem cells. Proc Natl Acad Sci USA. 1999;96:5504–5509. doi: 10.1073/pnas.96.10.5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shinohara T, et al. Spermatogonial stem cell enrichment by multiparameter selection of mouse testis cells. Proc Natl Acad Sci USA. 2000;97:8346–8351. doi: 10.1073/pnas.97.15.8346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kubota H, et al. Spermatogonial stem cells share some, but not all, phenotypic and functional characteristics with other stem cells. Proc Natl Acad Sci USA. 2003;100:6487–6492. doi: 10.1073/pnas.0631767100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kubota H, et al. Culture conditions and single growth factors affect fate determination of mouse spermatogonial stem cells. Biol Reprod. 2004;71:722–731. doi: 10.1095/biolreprod.104.029207. [DOI] [PubMed] [Google Scholar]
- 53.Ryu BY, et al. Phenotypic and functional characteristics of spermatogonial stem cells in rats. Dev Biol. 2004;274:158–170. doi: 10.1016/j.ydbio.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 54.Russell LD, et al. Histological and Histopathological Evaluation of the Testis. Clearwater: Cache River Press; 1990. [Google Scholar]
- 55.Clouthier DE, et al. Rat spermatogenesis in mouse testis. Nature. 1996;381:418–421. doi: 10.1038/381418a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dobrinski I, et al. Transplantation of germ cells from rabbits and dogs into mouse testes. Biol Reprod. 1999;61:1331–1339. doi: 10.1095/biolreprod61.5.1331. [DOI] [PubMed] [Google Scholar]
- 57.Dobrinski I, et al. Germ cell transplantation from large domestic animals into mouse testes. Mol Reprod Dev. 2000;57:270–279. doi: 10.1002/1098-2795(200011)57:3<270::AID-MRD9>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 58.Oatley JM, et al. Biological activity of cryopreserved bovine spermatogonial stem cells during in vitro culture. Biol Reprod. 2004;71:942–947. doi: 10.1095/biolreprod.104.028894. [DOI] [PubMed] [Google Scholar]
- 59.Nagano M, et al. Primate spermatogonial stem cells colonize mouse testes. Biol Reprod. 2001;64:1409–1416. doi: 10.1095/biolreprod64.5.1409. [DOI] [PubMed] [Google Scholar]
- 60.Nagano M, et al. Long-term survival of human spermatogonial stem cells in mouse testes. Fertil Steril. 2002;78:1225–1233. doi: 10.1016/s0015-0282(02)04345-5. [DOI] [PubMed] [Google Scholar]
- 61.Kanatsu-Shinohara M, et al. Long-term proliferation in culture and germline transmission of mouse male germline stem cells. Biol Reprod. 2003;69:612–616. doi: 10.1095/biolreprod.103.017012. [DOI] [PubMed] [Google Scholar]
- 62.Nagano M, et al. Maintenance of mouse male germ line stem cells in vitro. Biol Reprod. 2003;68:2207–2214. doi: 10.1095/biolreprod.102.014050. [DOI] [PubMed] [Google Scholar]
- 63.Kubota H, Reid LM. Clonogenic hepatoblasts, common precursors for hepatocytic and biliary lineages, are lacking classical major histocompatibility complex class I antigen. Proc Natl Acad Sci USA. 2000;97:12132–12137. doi: 10.1073/pnas.97.22.12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barnes D, Sato G. Serum-free cell culture: a unifying approach. Cell. 1980;22:649–655. doi: 10.1016/0092-8674(80)90540-1. [DOI] [PubMed] [Google Scholar]
- 65.Chang HY, et al. Diversity, topographic differentiation, and positional memory in human fibroblasts. Proc Natl Acad Sci USA. 2002;99:12877–12882. doi: 10.1073/pnas.162488599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Matzuk MM. Germ-line immortality. Proc Natl Acad Sci USA. 2004;101:16395–16396. doi: 10.1073/pnas.0407344101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ryu BY, et al. Conservation of spermatogonial stem cell self-renewal signaling between mouse and rat. Proc Natl Acad Sci USA. 2005;102:14302–14307. doi: 10.1073/pnas.0506970102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Avarbock MR, et al. Reconstitution of spermatogenesis from frozen spermatogonial stem cells. Nat Med. 1996;2:693–696. doi: 10.1038/nm0696-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Blackhall FH, et al. Semen cryopreservation, utilisation and reproductive outcome in men treated for Hodgkin’s disease. Br J Cancer. 2002;87:381–384. doi: 10.1038/sj.bjc.6600483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jahnukainen K, et al. Intratesticular transplantation of testicular cells from leukemic rats causes transmission of leukemia. Cancer Research. 2001;61:706–710. [PubMed] [Google Scholar]
- 71.Fujita K, et al. Transplantation of spermatogonial stem cells isolated from leukemic mice restores fertility without inducing leukemia. J Clin Invest. 2005;115:1855–1861. doi: 10.1172/JCI24189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Geijsen N, et al. Derivation of embryonic germ cells and male gametes from embryonic stem cells. Nature. 2004;427:148–154. doi: 10.1038/nature02247. [DOI] [PubMed] [Google Scholar]
- 73.Rideout WM, III, et al. Correction of a genetic defect by nuclear transplantation and combined cell and gene therapy. Cell. 2002;109:17–27. doi: 10.1016/s0092-8674(02)00681-5. [DOI] [PubMed] [Google Scholar]
- 74.Kolb AF, et al. Site-directed genome modification: nucleic acid and protein modules for targeted integration and gene correction. Trends Biotechnol. 2005;23:399–406. doi: 10.1016/j.tibtech.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 75.Urnov FD, et al. Highly efficient endogenous human gene correction using designed zinc-finger nucleases. Nature. 2005;435:646–651. doi: 10.1038/nature03556. [DOI] [PubMed] [Google Scholar]
- 76.Shinohara T, et al. Functional analysis of spermatogonial stem cells in Steel and cryptorchid infertile mouse models. Dev Biol. 2000;220:401–411. doi: 10.1006/dbio.2000.9655. [DOI] [PubMed] [Google Scholar]