Abstract
A simple generic peptide-based vaccine structure that targets Toll-like receptor 2-expressing dendritic cells and causes their activation is described. The vaccines are totally synthetic, serve as their own adjuvant, and are composed of (i) a single helper T cell epitope, (ii) a target epitope that is either recognized by CD8+ T cells or B cells, and (iii) a Toll-like receptor 2-targeting lipid moiety, S-[2,3-bis(palmitoyloxy)propyl]cysteine, that is situated between the peptide epitopes to form a branched configuration. The different CD8+ T cell epitopes examined were from (i) influenza virus, (ii) the intracellular bacterium Listeria monocytogenes, and (iii) ovalbumin as a model tumor antigen. Vaccines containing a B cell epitope from gastrin or luteinizing hormone-releasing hormone as a B cell epitope were also examined for their ability to elicit antibody against the parent hormones. Each of the vaccines was capable of inducing either CD8+ T cell or antibody-mediated immune responses. The lipidated vaccines, but not the nonlipidated vaccines, were able to mediate protection against viral or bacterial infection and mediate prophylactic and therapeutic anticancer activity. The two hormone-based vaccines induced high antibody titers, which in the case of luteinizing hormone-releasing hormone resulted in abrogation of reproductive function. These results highlight the utility of simple, totally synthetic, epitope-based vaccines.
Dendritic cells (DCs) take up antigen, generate peptide epitopes from it, and then load these epitopes into molecules that are encoded by the major histocompatibility complex (MHC). After export to the cell surface, MHC molecule-epitope complexes are presented to T cells, leading to their activation. Activated CD4+ helper T (Th) cells are now able to deliver signals to DCs, enabling them to activate naive CD8+ T cells more efficiently (1, 2) and also improve the CD8+ T cell's ability to assume memory cell status, providing the ability to clear pathogens when subsequently encountered (3). Activated Th cells can also interact directly with B cells, providing them with signals that control differentiation, expansion, and shaping of the antibody isotype that they secrete.
Because epitope sequences determine the specificity of the ensuing immune response they have attracted considerable attention as a basis for vaccine design (for review see ref. 4). Despite several potential advantages (5, 6), no totally synthetic peptide epitope-based vaccines are yet licensed for human or animal use. The poor immunogenicity of peptides in the absence of coadministered adjuvants and the paucity of adjuvant systems suitable for human use has limited the development of viable epitope-based vaccines.
The “danger signal” concept (7, 8) goes some way to explain the poor immunogenicity of epitopes when administered out of the context of the whole antigen; epitopes lack the ability to provide the appropriate signals for DC maturation and inflammatory cytokine release, which we now understand to be a critical property of the more potent adjuvants. In recent years it has emerged that many adjuvants provide danger signals to DCs by engagement of one or more Toll-like receptors (TLRs; for reviews see refs. 9 and 10). The discovery that some lipid structures are powerful adjuvants has driven the development of lipopeptides as potential vaccines (for review see ref. 11). Furthermore, certain of the TLRs present on the surface of DCs specifically recognize particular lipid structures (12), and ligands bound to these receptors are transported into DCs (13).
We have designed a simple synthetic vaccine structure composed of a Th epitope, a target epitope, and the lipid moiety S-[2,3-bis(palmitoyloxy)propyl]cysteine (Pam2Cys) that serves as its own adjuvant and provides TLR2 targeting, DC maturation, and induction of antibody or cytotoxic T lymphocyte (CTL) responses depending on the choice of target epitope. Here we test the generic applicability of this approach in a number of models using class I MHC-restricted epitopes from virus, intracellular bacteria, or model tumors, or by using antibody epitopes from peptide hormones. In each case biologically relevant responses were induced. The striking biological effectiveness of these totally synthetic vaccines, their ease of assembly and enhanced solubility, afforded by placement of the lipid molecule between the epitopes to create a branched structure (14), makes them highly attractive for the development of vaccines for humans and animals.
Materials and Methods
Synthesis and Assembly of Lipidated and Nonlipidated, Epitope-Based Vaccines. The method of assembling, purifying, and characterizing synthetic peptides and synthetic lipopeptides has been described in detail elsewhere (14). The vaccines consisted of a Th epitope synthesized contiguously with and N-terminally to either a class I MHC-restricted epitope or an epitope recognized by antibody. In most cases the Th epitope, sequence KLIPNASLIENCTKAEL, derived from the fusion protein of the morbillivirus canine distemper virus (15), was used, but in the case of the anti-influenza vaccine the I-Ed-restricted Th epitope, sequence GALNNRFQIKGVELKS, derived from the light chain of the influenza virus hemagglutinin (16), was used. The Th epitope and target epitope were separated in sequence by a single lysine residue. The lipid moiety Pam2Cys, corresponding to the lipid component of macrophage-activating lipopeptide 2 (MALP-2) isolated from mycoplasma (17), was attached to the intervening lysine through two serine residues. A diagrammatic representation of this generic structure is shown in Fig. 1. The sequences of the various class I MHC-restricted target epitopes used were as follows: influenza virus, TYQRTRALV (H-2Kd-restricted) (18); the epitope GYKDGNEYI (H-2Kd-restricted) from listeriolysin O of Listeria monocytogenes (19), and SIINFEKL (H-2Kb-restricted) from ovalbumin expressed by B16-OVA melanoma cells (20) and Lewis lung-OVA tumor cells (21). The two antibody epitopes examined were luteinizing hormone-releasing hormone (LHRH) with sequence EHWSYGLRPG and pentagastrin, which represents the C-terminal five residues GWMDF from the hormone gastrin (sequence EGPWLEEEEEAYGWMDF).
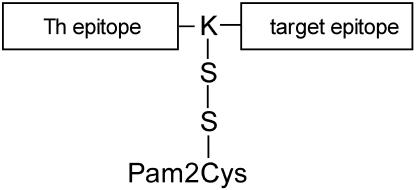
Fig. 1.
Schematic representation of the epitope-based vaccine candidates examined during this study. Each vaccine contains a Th epitope and a target epitope that is either a CTL-inducing epitope or an antibody-inducing epitope. In all cases the Th epitope occupies the N-terminal position and is separated from the target epitope by a single lysine (K) residue. Where lipid is attached, this was done through the ε-amino group of the lysine residue such that the self-adjuvant lipid, linked through two serine residues (S), forms a branch between the Th and target epitopes.
NF-κB Reporter Gene Assay. Cells of the human embryonic epithelial kidney cell line HEK293 were transiently transfected as described (22, 23) with 100 ng of an NF-κB-luciferase reporter gene (five NF-κB sites upstream of luciferase), 70 ng of a β-galactosidase-expressing plasmid, and 5 ng of human TLR2-expressing plasmid by using the FuGENE6 method (Roche Diagnostics, Mannheim, Germany). The total amount of DNA (250 ng) was kept constant by supplementation with empty vector. Lipidated or nonlipidated peptides were added into the wells 24 h after transfection and lysates were prepared 6 h after stimulation. By using enzyme assay kits (Promega) the luciferase and β-galactosidase activities in the cell lysates were determined (23). The relative stimulation of NF-κB activity was calculated by normalizing luciferase activity with β-galactosidase activity.
Maturation of DCs. The maturation of D1 cells, a line of immature DCs derived in this laboratory from spleen cells by the method of Winzler et al. (24), was assessed by flow cytometric determination of surface MHC class II expression.
Influenza Virus System. Inbred 6- to 8-week-old female BALB/c mice (H-2d) were anesthetized with Penthrane (Abbott) and inoculated intranasally (i.n.) with lipidated or nonlipidated peptide in 50 μl of PBS. Mice were infected i.n. with 104.5 plaque-forming units (pfu) of influenza virus, strain A/Memphis/1/71-A/Bellamy/42 (H3N1) under Penthrane anesthesia. Methods for the determination of lung viral titers by plaque assay and of pulmonary IFN-γ-producing CD8+ T cells specific for peptide TYQRTRALV by ELISPOT assay have been previously described (25). In vivo cytotoxicity was determined by the method of Coles et al. (26), using naive BALB/c splenocytes as target cells. Targets were either pulsed with 9 μM CTL peptide TYQRTRALV at 37°C for 90 min or incubated without peptide. After washing, the peptide-pulsed population was labeled with a high concentration (3 μM) and the nonpulsed population with a low concentration (0.5 μM) of 5-(and 6)-carboxyfluorescein diacetate, succinimidyl ester (CFSE; Molecular Probes). The cells were washed, mixed together at a 1:1 ratio, and inoculated into previously immunized or naive mice (3 × 107 cells in 100 μl of PBS by i.v. injection). The mice were killed 16 h later and spleen cell suspensions were analyzed by flow cytometry. One million lymphocytes were analyzed and each population was detected by its differential CFSE fluorescence intensity. The following formula was used to calculate specific lysis: ratio = (% lymphocytes with low CSFE fluorescence/% lymphocytes with high CSFE fluorescence), and the percentage of specific lysis = [1 - (ratio obtained for naive mice/ratio obtained for primed mice)] × 100.
L. monocytogenes System. Male BALB/c mice 6-8 weeks old were immunized by i.v. inoculation with 1 × 103 colony-forming units (cfu) of L. monocytogenes EGD or with lipidated or nonlipidated peptide KLIPNASLIENCTKAEL-K-GYKDGNEYI or PBS s.c. Seven days later, the number of IFN-γ-producing CD8+ T cells was determined after 48-h incubation with GYKDGNEYI by using an ELISPOT assay, as previously described (27). To test antibacterial resistance, the immunized mice were rested for 28 days before i.v. challenge with 5 × 103 L. monocytogenes EGD. Two days later, mice were killed, livers were removed and homogenized, and the number of cfu per organ was determined (28).
Tumor Systems. (i) B16-OVA challenge. C57BL/6 (H-2b) mice (six per group) received a single dose (20 nmol) s.c. in the base of tail with lipidated or nonlipidated peptide KLIPNASLIENCTKAEL-K-SIINFEKL or PBS. After 14 days, mice were challenged with B16 tumor cells expressing the SIINFEKL determinant from ovalbumin, B16-OVA (2 × 105 cells per mouse s.c.), and growth was monitored as described previously (20). Animals were killed when tumors reached 100 mm2.
(ii) Lewis lung-OVA therapy. C57BL/6 mice (six per group) were inoculated with Lewis lung-OVA tumor cells (3 × 104 cells per mouse s.c.) according to Nelson et al. (21). Four and 15 days after receiving the tumor cells, mice were inoculated with the lipidated vaccine, the control nonlipidated vaccine, or PBS and were monitored for tumor growth as above.
Induction of Anti-LHRH and Anti-Gastrin Antibody Responses. Groups of five female BALB/c mice, 6-8 weeks old, were inoculated s.c. in the base of the tail on day 0 and on day 28 with 100 μl (20 nmol) of peptide vaccine in saline. For comparison, nonlipidated peptides were formulated as an emulsion in an equal volume of complete Freund's adjuvant (CFA) for the primary injection and incomplete Freund's adjuvant for the secondary inoculation. Sera were prepared from blood taken 4 weeks after primary inoculation and 2 weeks after secondary inoculation and assayed by ELISA as described in ref. 29, using either LHRH or gastrin as the coating antigen. The titers of antibody are expressed as the reciprocal of the highest dilution of serum to achieve an OD at 405 nm of 0.2, which represents ≈5 times the OD in the absence of antibody.
Results and Discussion
Lipopeptide-Based Vaccines of Branching Structure Target TLR2 and Cause the Maturation of DCs. The peptide and lipopeptide-based vaccines assembled for this study ranged in length from 23 amino acid residues (KLIPNASLIENCTKAEL-K-GWMDF, the morbillivirus CD4 epitope and pentagastrin B cell epitope vaccine) to 26 residues (GALNNRFQIKGVELKS-K-TYQRTRALV, the influenza virus CD4 and CD8 epitope-based vaccine). These are relatively short peptide sequences and posed no difficulties during the synthesis procedures. All peptide products eluted as a single peak on analytical HPLC and had the expected mass when analyzed by ion spray mass spectrometry (data not shown). The use of a branched geometry in the construction of these vaccines (Fig. 1) is based on our earlier observations that synthetic peptide epitopes presented to the immune system as branched structures are significantly more immunogenic than the same epitopes in a tandem linear arrangement (14). Branched peptides are also presented 10-fold more efficiently in vitro to T cell clones by splenic CD8- DCs and exhibit a greater resistance to proteolysis in serum compared with linear peptides, a fact that may further enhance the differences in their in vivo potency (30).
The creation of a branched lipopeptide structure through attachment of the lipid moiety from the ε-amino group of an internal lysine residue has also shown advantages in the solubility of the construct, which is significantly improved compared with N-terminal attachment of the lipid (14). The improvement to solubility and the ease with which these epitope-based vaccines can be prepared are attractive characteristics for any manufacturing process and address some of the challenges identified by BenMohamed et al. (11) for the use of lipopeptide vaccines.
The Pam2Cys group used in the construction of these vaccines is a synthetic version of the lipid moiety from the 2-kDa macrophage-activating lipopeptide 2 derived from Mycoplasma fermentans. The Pam2Cys moiety has been shown to be a ligand for TLR2s (12, 31). These receptors are present on DCs, monocytes, and lung epithelia. The interaction of Pam2Cys with DCs induces their maturation (32), suggesting that vaccines containing this lipid moiety may interact directly with DCs to promote immune responses. We first determined whether Pam2Cys, when covalently attached to a synthetic peptide immunogen in a branched configuration, provides a lipopeptide that retains TLR2 binding capacity. The results in Fig. 2A demonstrate the ability of such a lipopeptide to stimulate NF-κB-dependent gene activation in TLR2-transfected HEK293 cells. The lipopeptide did not stimulate cells lacking TLR2, nor are cells expressing TLR2 stimulated by the nonlipidated peptide, indicating that the lipopeptide-induced NF-κB signaling was TLR2-dependent and mediated by the Pam2Cys moiety.
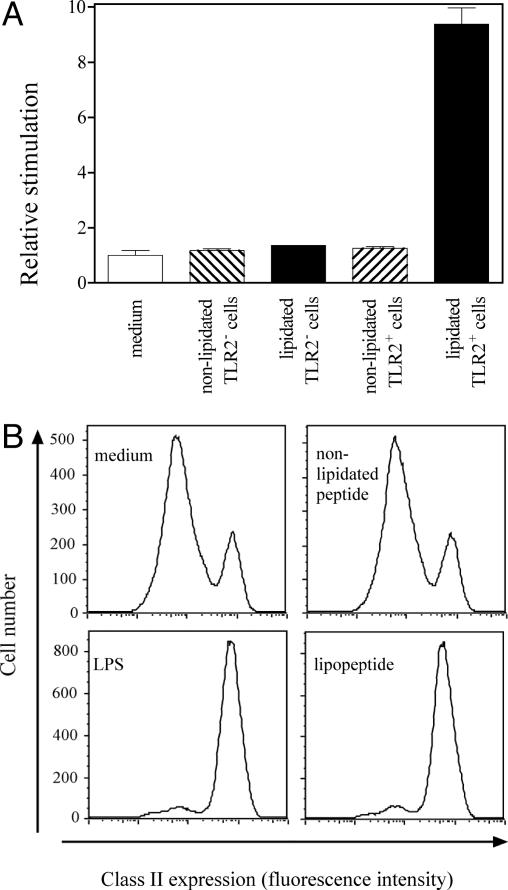
Fig. 2.
Pam2Cys-containing lipopeptides are ligands for TLR2 and act as potent stimulants for DC maturation. (A) The ability of the lipidated and nonlipidated forms of influenza peptide GALNNRFQIKGVELKS-K-TYQRTRALV to signal through TLR2 was tested by using HEK293 cells transfected with an NF-κB-luciferase reporter gene, with or without cotransfection with a TLR2-expressing plasmid. Transfected cells (2 × 104 in 200 μl) were exposed for 6 h to 1.4 μM lipopeptide, nonlipidated peptide, or medium alone and then harvested into lysis buffer, and luciferase activity was determined. Data are expressed as relative stimulation activity calculated from the mean of triplicate samples. (B) DC maturation was examined by using D1 cells (2 × 105 cells per well) incubated overnight with 9 nM lipidated or nonlipidated influenza peptide or 5 μg/ml Escherichia coli serotype O111:B4 lipopolysaccharide (LPS). Cells were recovered and examined for surface expression of MHC class II molecules by flow cytometry. Mature D1 cells were identified by their high expression of MHC class II molecules. The degree of spontaneous maturation was monitored by incubating the cells with medium alone.
The ability of an activating ligand to trigger a DC allows the cell to attain a state of maturation in which MHC products and costimulatory molecules are up-regulated on the cell surface. The ability of lipopeptides to cause DC maturation is shown in Fig. 2B. The influenza virus lipopeptide triggered >90% of D1 cells to increase MHC class II expression, a level similar to that achieved with lipopolysaccharide. Similar up-regulation of the costimulatory molecule CD86 on lipopeptide-treated DCs was also observed (data not shown). The nonlipidated peptide, however, showed no significant increase in the level of maturation markers above the spontaneous rate occurring in culture with medium alone.
Ligation of TLR2 on human DCs has recently been shown (13) by using mouse anti-TLR2 antibody, to trigger receptor-mediated endocytosis of the antibody ligand, resulting in highly efficient presentation of epitopes present on the antibody by MHC class II molecules. The lipidated vaccines that we have constructed for this study therefore have the potential to induce efficient immune responses through their ability to target TLR2s on the surface of DCs, leading to antigen uptake by the DCs and provision of signals for the maturation of the cells. We therefore evaluated the induction of both humoral and cellular immunity by lipopeptides containing the appropriate epitopes in different model systems.
Lipopeptides Are Effective in Diverse Models of Protection Requiring the Induction of CD8+ T Cell Immunity. Pam2Cys has not previously been used for the construction of lipopeptides designed for the induction of CD8+ T cell immunity. However, the related lipid moiety Pam3Cys, N-palmitoyl-S-[2,3-bis(palmitoyloxy)propyl]-cysteine, has been successfully used for this purpose (33). The two ester-bonded palmitoyl side chains are thought to be responsible for the powerful adjuvanticity of this lipid moiety for HLA class I-restricted CD8+ T cell expansion (34), and it is these particular lipid chains that are also present in Pam2Cys.
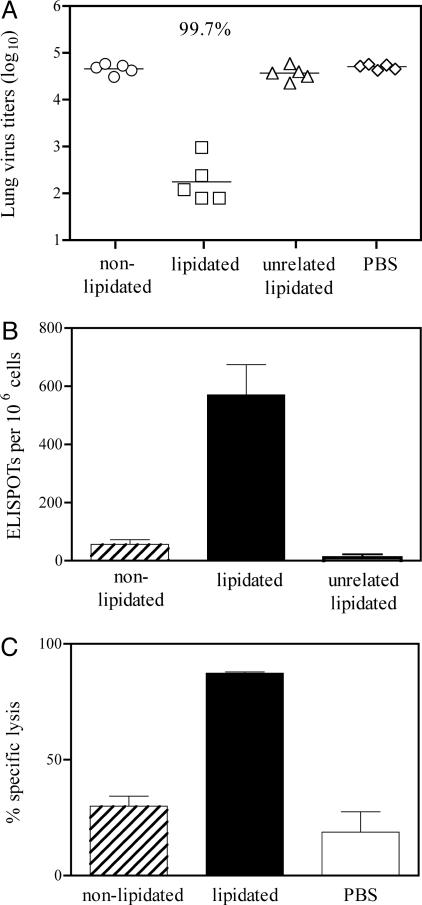
Lipidated and nonlipidated forms of peptide GALNNRFQIKGVELKS-K-TYQRTRALV and the lipidated form of the unrelated peptide KLIPASLIENCTKAEL-K-EHWSYGLRPG were used to inoculate BALB/c mice, which were challenged 28 days later with live influenza virus. Determination of virus in the lungs of mice after challenge (Fig. 3A) indicated that the influenza virus-specific lipidated peptide afforded 99.7% reduction in pulmonary viral titers compared with the PBS control group. This level of viral clearance was significantly greater than that induced by the corresponding nonlipidated peptide or the unrelated lipopeptide, where virtually no virus clearance was observed. This result indicates that the lipid moiety increases peptide-induced specific protective immunity rather than inducing a nonspecific effect on viral clearance. The number of TYQRTRALV-specific IFN-γ-producing CD8+ T cells found in the lungs of lipopeptide-primed mice on day 7 after vaccination was also significantly elevated compared with those in nonlipidated peptide or unrelated lipopeptide-primed mice (Fig. 3B), implicating peptide-specific CD8+ T cells in the clearance of virus. The lytic capacity of the lipopeptide-induced CD8+ T cells was confirmed in an in vivo lysis experiment (Fig. 3C). This experiment tested the ability of those memory CD8+ T cells induced by the vaccine to rapidly gain cytolytic function upon encounter with infectious virus. Mice were inoculated with the lipopeptide, nonlipidated peptide, or PBS, and 28 days later the memory T cell population was recalled by viral challenge. Four days after challenge, before the time at which effector T cells generated in response to the virus infection itself can be detected in this assay, splenocytes pulsed with the peptide TYQRTRALV and labeled with CFSE were transferred to the immunized animals. The disappearance of these target cells from the spleen 16 h later, relative to a second population of cotransferred unpulsed cells, was assessed by flow cytometry. This procedure provides a measure of the lytic activity of the memory CD8+ T cells upon recall. The results (Fig. 3C) demonstrate that in influenza virus lipopeptide-primed mice, very high levels of specific lysis of the target cells were observed. This observation was in contrast to mice that had been inoculated with nonlipidated peptide, where the level of specific lysis of target cells was not significantly increased above that seen in mice that had been inoculated with PBS before viral challenge. Together these results indicate that the influenza virus lipopeptide but not the corresponding nonlipidated peptide was capable of inducing specific CD8+ memory that mediated enhanced clearance of the virus upon recall, providing high levels of pulmonary protection.
Fig. 3.
Lipopeptides can induce strong anti-influenza CD8+ T cell-mediated viral clearing responses. (A) Groups of six mice were inoculated i.n. with 9 nmol of lipidated or nonlipidated influenza virus peptide GALNNRFQIKGVELKS-K-TYQRTRALV, the unrelated lipopeptide based on the sequence KLIPASLIENCTKAEL-K-EHWSYGLRPG, or PBS. Twenty-eight days after priming, mice were challenged i.n. with influenza virus, and 5 days later infectious virus present in lung homogenates was measured. Symbols represent data for individual mice and the line represents the geometric mean titer. The reduction in infectious virus in influenza lipopeptide-primed mice relative to the PBS control is indicated. Other groups were not significantly different from the PBS group (P > 0.05). (B) The number of TYQRTRALV-specific IFN-γ-secreting cells in the lungs of BALB/c mice inoculated i.n. with 9 nmol of lipidated or nonlipidated influenza virus peptide or lipidated unrelated peptide was determined 7 days after vaccination. Data are ELISPOTs per 106 lung cells and represent triplicate determinations of pooled lungs from three mice per group. The number of ELISPOTs in cultures lacking antigen have been subtracted. (C) Ability of immunogens to elicit CTLs. Groups of three mice were inoculated i.n. with 9 nmol of lipidated or nonlipidated peptide or with PBS and infected with influenza virus 28 days later. Equal numbers of (i) syngeneic spleen cells pulsed with epitope TYQRTRALV and labeled with a high concentration of CFSE and (ii) syngeneic spleen cells labeled with a low concentration of CFSE but not exposed to epitope, were injected into these mice and into naive mice 4 days later. After 16 h mice were killed and CFSE-labeled splenocytes were enumerated. Specific lysis of epitope-pulsed targets was calculated from the ratios of nonpulsed to epitope-pulsed targets remaining in vaccinated and infected mice compared with naive animals. Bars show the mean and SD for the three mice in each group.
In a second model of protection against pathogen challenge, mice were inoculated with live L. monocytogenes, lipidated or nonlipidated forms of the peptide KLIPNASLIENCTKAEL-K-GYKDGNEYI, or PBS. Table 1 shows the numbers of spleen cells capable of secreting IFN-γ in response to peptide GYKDGNEYI 7 days later. Only those animals that were inoculated with bacteria or lipidated vaccine showed significant numbers of IFN-γ-secreting cells, indicating that these antigens were able to elicit CD8+ T cell responses. Furthermore, only those animals receiving bacteria or lipidated peptide exhibited any degree of protection from challenge with live bacteria 28 days after immunization (Table 1). The lipopeptide induced levels of protection in the liver comparable with prior exposure to the live intracellular bacterium.
Table 1. Immunogenicity and protective capacity of lipopeptides containing a CTL epitope from L. monocytogenes.
| Immunization* | IFN-γ-producing cells per 106 splenocytes at day 7† | log10(listeria/liver) after challenge at day 28‡ | % protection§ |
|---|---|---|---|
| PBS | 11 ± 19 | 6.06 ± 0.50 | |
| 103 live Listeria | 258 ± 51 | 4.28 ± 0.42 | 98 |
| Nonlipidated peptide | 1 ± 2 | 6.83 ± 0.44 | Nil |
| Lipidated peptide | 206 ± 79 | 4.85 ± 0.18 | 94 |
Groups of five BALB/c mice were inoculated with 9 nmol of lipidated or nonlipidated peptide vaccine s.c. or with 103 live bacteria or PBS i.v. The peptide vaccines contained the MHC class I-restricted epitope GYKDGNEYI from the listeriolysin O protein of L. monocytogenes
The number of IFN-γ-producing cells per 106 splenocytes was measured on day 7 after in vitro stimulation with the CTL epitope. Numbers in cultures without antigen were uniformly less than 40. Results are mean ± SD
On day 28 mice were injected with 5 × 103 live Listeria. The number of colony-forming units present in liver was measured 2 days later; results are mean ± SD
Data are expressed as the percentage reduction in live bacteria relative to the PBS control group
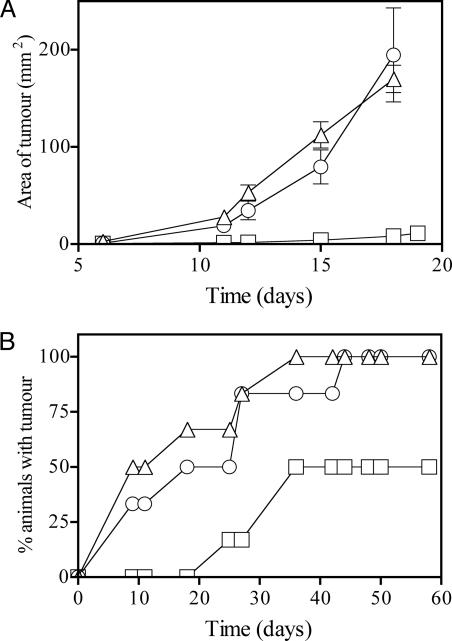
CD8+ T cells are also known to have potent anticancer activity. We tested the lipopeptide vaccines in two tumor models using tumors expressing ovalbumin, which contains the CD8+ T cell epitope SIINFEKL. Animals vaccinated with lipidated or nonlipidated peptide KLIPNASLIENCTKAEL-K-SIINFEKL were challenged, on day 14 after immunization, with melanoma B16-OVA. Animals vaccinated with the lipidated peptide showed substantially reduced tumor growth (Fig. 4A). To test the efficacy of the vaccine in a therapeutic setting, Lewis lung-OVA carcinomas were established in mice 4 days before receiving the first of two doses (20 nmol) of vaccine. All mice therapeutically vaccinated with lipidated peptide remained tumor free for 18 days, whereas mice inoculated with the nonlipidated peptide had the same frequency of tumor appearance as the control PBS-treated animals. By 60 days, when all control and nonlipidated peptide-inoculated mice had measurable tumors, half of the lipopeptide-immunized mice remained tumor free, indicating that lipidated peptide vaccination mediated a significant therapeutic benefit.
Fig. 4.
Ability of lipopeptide containing an epitope expressed by tumor cells to afford prophylactic and therapeutic protection. (A) Protection against B16-OVA challenge. Mice (six per group) were immunized once with lipidated (□) or nonlipidated (○) peptide vaccine KLIPNASLIENCTKAEL-K-SIINFEKL (20 nmol), or with PBS (▵). Two weeks later, animals were challenged with B16-OVA, and tumor area (±SE) was monitored over time. (B) Lewis lung-OVA therapy. Mice (six per group) were inoculated with Lewis lung-OVA tumor cells, then on days 4 and 15 inoculated with lipidated (□) or nonlipidated (○) peptide vaccine (20 nmol in each case) or with PBS (▵). The number of animals developing measurable tumors (>1 × 1 mm) over time is represented as a Kaplan-Meier plot.
Lipopeptides also Induce Potent Antibody Responses. We also determined whether this simple vaccine structure could be used for efficient induction of antibody responses. We chose the two hormones LHRH and gastrin as the target antibody epitopes because each has attracted attention in the treatment of prostate (35) or pancreatic (36, 37) cancers. LHRH-based vaccines have also been considered as a means of controlling reproduction (38).
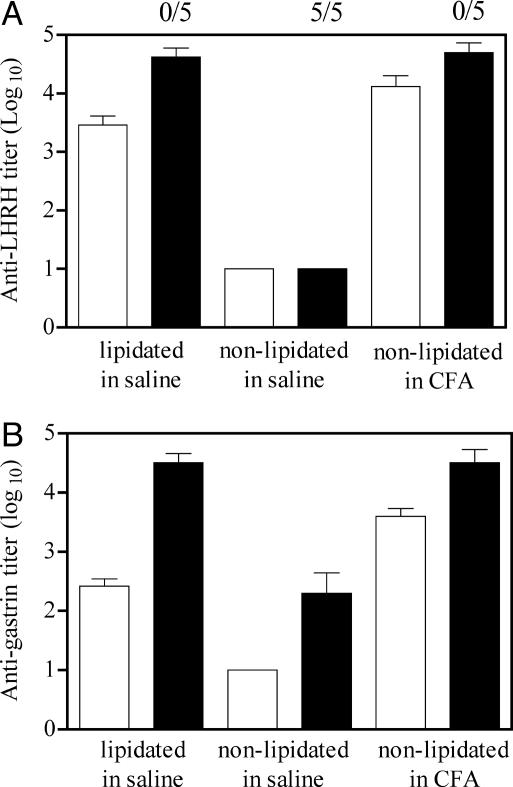
When mice were inoculated with lipopeptides based on LHRH (Fig. 5A) or a 5-aa epitope from gastrin (Fig. 5B), high-titer antibodies (rivaling those obtained when nonlipidated peptides were administered in CFA) were induced. In the case of the lipidated LHRH immunogen, none of the female mice inoculated with lipidated peptide demonstrated reproductive function (Fig. 5A), illustrating the biological effectiveness of the vaccine. The ability to induce antibodies against these two self hormones demonstrates that these simple epitope-based structures can be used to break tolerance.
Fig. 5.
Anti-hormone antibody responses to synthetic peptide-based vaccines containing the LHRH sequence HWSYGLRPG (A) or the pentagastrin sequence GWMDF (B). Groups of BALB/c mice received lipidated peptide in saline, nonlipidated peptide in saline, or nonlipidated peptide in CFA. In all cases animals received 20 nmol of immunogen. Animals were bled 4 weeks after receiving the first dose of vaccine and then inoculated with a second dose (20 nmol) and bled after a further 2 weeks. Antibody titers to the two hormones present in the primary (white bars) and secondary (black bars) responses were determined by ELISA. The numbers above the bars in A refer to the number of mice that dropped litters during the course of a fertility study carried out after the second inoculation of PBS or LHRH-based vaccine.
There is an increasing body of literature suggesting that signaling by distinct TLRs can trigger qualitatively different responses (32, 39-41), which may be either Th1- or Th2-biased. However, the TLR2 agonist Pam2Cys appears able to promote the initiation of both humoral and cell-mediated responses. This is in agreement with the previous observation (42) that macrophage-activating lipopeptide 2 treatment of human monocyte-derived DCs that were cocultured with lymphocytes resulted in both IFN-γ and IL-4 and IL-5 production.
In this study we have assembled a number of simple, totally synthetic, and readily characterized vaccines that were effective in a variety of biological systems. In the case of the two antibody-inducing immunogens, the efficacy of the lipidated vaccines rivals that of vaccine administered with CFA but without the disadvantages of this toxic adjuvant. Furthermore, in the case of the influenza virus experiments, the vaccine was administered i.n. Nonparenteral administration provides further incentive for the adoption of this approach for vaccine design. An additional and important advantage of the current strategy is that assembly and analysis of the final product is readily achieved by using established methods such as solid-phase peptide synthesis, HPLC, and mass spectrometry. This simplicity of manufacture and quality assurance is likely to facilitate the commercial development of this type of vaccine, which could be readily adapted to address the problem of antigenic diversity and MHC polymorphism by incorporating a number of simple lipopeptides containing the relevant epitopes.
As the rules governing initiation of immune responses and the role played by DCs are elucidated, we are increasingly able to design rational vaccine strategies. The generic vaccine structure described here is designed to target minimal antigenic epitopes to DCs, resulting in specific immune responses, and could represent a member of a powerful new arsenal of vaccines.
Acknowledgments
We thank Mr. Michael Rizkalla for his invaluable help in synthesizing the peptide-based vaccines. Dr. Ashley Mansell (Monash Institute of Reproduction and Development, Clayton, Victoria, Australia) provided assistance with the TLR2 signaling assays. Drs. Frank Carbone, Jose Villadangos, and Steve Turner provided valuable and constructive criticisms of the manuscript, which have improved its quality. This work was supported by grants from the Cooperative Research Centre for Vaccine Technology, the National Health and Medical Research Council of Australia, and the Queensland Cancer Fund.
Author contributions: D.C.J., Y.F.L., T.L., A.S., G.D., C.C., C.S., W.Z., and L.E.B. designed research, performed research, contributed new reagents/analytical tools, analyzed data, and wrote the paper.
Abbreviations: DC, dendritic cell; TLR, Toll-like receptor; Th, helper T; Pam2Cys, S-[2,3-bis(palmitoyloxy)propyl]cysteine; CTL, cytotoxic T lymphocyte; LHRH, luteinizing hormone-releasing hormone; i.n., intranasally; CFSE, 5-(and 6)-carboxyfluorescein diacetate, succinimidyl ester; CFA, complete Freund's adjuvant.
References
- 1.Bennett, S. R., Carbone, F. R., Karamalis, F., Flavell, R. A., Miller, J. F. & Heath, W. R. (1998) Nature 393, 478-480. [DOI] [PubMed] [Google Scholar]
- 2.Ridge, J. P., Di Rosa, F. & Matzinger, P. (1998) Nature 393, 474-478. [DOI] [PubMed] [Google Scholar]
- 3.Kaech, S. M. & Ahmed, R. (2003) Science 300, 263-265. [DOI] [PubMed] [Google Scholar]
- 4.Sette, A. & Fikes, J. (2003) Curr. Opin. Immunol. 15, 461-470. [DOI] [PubMed] [Google Scholar]
- 5.Jackson, D. C., Purcell, A. W., Fitzmaurice, C. J., Zeng, W. & Hart, D. N. (2002) Curr. Drug Targets 3, 175-196. [DOI] [PubMed] [Google Scholar]
- 6.Purcell, A. W., Zeng, W., Mifsud, N. A., Ely, L. K., Macdonald, W. A. & Jackson, D. C. (2003) J. Pept. Sci. 9, 255-281. [DOI] [PubMed] [Google Scholar]
- 7.Medzhitov, R. & Janeway, C. A., Jr. (2002) Science 296, 298-300. [DOI] [PubMed] [Google Scholar]
- 8.Janeway, C. A., Jr. (1989) Cold Spring Harbor Symp. Quant. Biol. 54, 1-13. [DOI] [PubMed] [Google Scholar]
- 9.Beg, A. A. (2002) Trends Immunol. 23, 509-512. [DOI] [PubMed] [Google Scholar]
- 10.Marciani, D. J. (2003) Drug Discov. Today 8, 934-943. [DOI] [PubMed] [Google Scholar]
- 11.BenMohamed, L., Wechsler, S. L. & Nesburn, A. B. (2002) Lancet Infect. Dis. 2, 425-431. [DOI] [PubMed] [Google Scholar]
- 12.Takeuchi, O. & Akira, S. (2001) Int. Immunopharmacol. 1, 625-635. [DOI] [PubMed] [Google Scholar]
- 13.Schjetne, K. W., Thompson, K. M., Nilsen, N., Flo, T. H., Fleckenstein, B., Iversen, J. G., Espevik, T. & Bogen, B. (2003) J. Immunol. 171, 32-36. [DOI] [PubMed] [Google Scholar]
- 14.Zeng, W., Ghosh, S., Lau, Y. F., Brown, L. E. & Jackson, D. C. (2002) J. Immunol. 169, 4905-4912. [DOI] [PubMed] [Google Scholar]
- 15.Ghosh, S., Walker, J. & Jackson, D. C. (2001) Immunology 104, 58-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jackson, D. C., Drummer, H. E. & Brown, L. E. (1994) Virology 198, 613-623. [DOI] [PubMed] [Google Scholar]
- 17.Muhlradt, P. F., Kiess, M., Meyer, H., Sussmuth, R. & Jung, G. (1997) J. Exp. Med. 185, 1951-1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bodmer, H. C., Pemberton, R. M., Rothbard, J. B. & Askonas, B. A. (1988) Cell 52, 253-258. [DOI] [PubMed] [Google Scholar]
- 19.Harty, J. T. & Bevan, M. J. (1992) J. Exp. Med. 175, 1531-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anraku, I., Harvey, T. J., Linedale, R., Gardner, J., Harrich, D., Suhrbier, A. & Khromykh, A. A. (2002) J. Virol. 76, 3791-3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nelson, D. J., Mukherjee, S., Bundell, C., Fisher, S., van Hagen, D. & Robinson, B. (2001) J. Immunol. 166, 5557-5566. [DOI] [PubMed] [Google Scholar]
- 22.Bowie, A., Kiss-Toth, E., Symons, J. A., Smith, G. L., Dower, S. K. & O'Neill, L. A. (2000) Proc. Natl. Acad. Sci. USA 97, 10162-10167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fitzgerald, K. A., Palsson-McDermott, E. M., Bowie, A. G., Jefferies, C. A., Mansell, A. S., Brady, G., Brint, E., Dunne, A., Gray, P., Harte, M. T., et al. (2001) Nature 413, 78-83. [DOI] [PubMed] [Google Scholar]
- 24.Winzler, C., Rovere, P., Rescigno, M., Granucci, F., Penna, G., Adorini, L., Zimmermann, V. S., Davoust, J. & Ricciardi-Castagnoli, P. (1997) J. Exp. Med. 185, 317-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deliyannis, G., Jackson, D. C., Ede, N. J., Zeng, W., Hourdakis, I., Sakabetis, E. & Brown, L. E. (2002) J. Virol. 76, 4212-4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coles, R. M., Mueller, S. N., Heath, W. R., Carbone, F. R. & Brooks, A. G. (2002) J. Immunol. 168, 834-838. [DOI] [PubMed] [Google Scholar]
- 27.Gilbertson, B., Zhong, J. & Cheers, C. (1999) J. Immunol. 163, 2073-2080. [PubMed] [Google Scholar]
- 28.Liu, Z., Simpson, R. J. & Cheers, C. (1992) Infect. Immun. 60, 4402-4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghosh, S. & Jackson, D. C. (1999) Int. Immunol. 11, 1103-1110. [DOI] [PubMed] [Google Scholar]
- 30.Fitzmaurice, C. J., Brown, L. E., Kronin, V. & Jackson, D. C. (2000) Int. Immunol. 12, 527-535. [DOI] [PubMed] [Google Scholar]
- 31.Takeuchi, O., Kawai, T., Muhlradt, P. F., Morr, M., Radolf, J. D., Zychlinsky, A., Takeda, K. & Akira, S. (2001) Int. Immunol. 13, 933-940. [DOI] [PubMed] [Google Scholar]
- 32.Agrawal, S., Agrawal, A., Doughty, B., Gerwitz, A., Blenis, J., Van Dyke, T. & Pulendran, B. (2003) J. Immunol. 171, 4984-4989. [DOI] [PubMed] [Google Scholar]
- 33.Deres, K., Schild, H., Wiesmuller, K. H., Jung, G. & Rammensee, H. G. (1989) Nature 342, 561-564. [DOI] [PubMed] [Google Scholar]
- 34.Reschner, A., Moretta, A., Landmann, R., Heberer, M., Spagnoli, G. C. & Padovan, E. (2003) Eur. J. Immunol. 33, 2044-2052. [DOI] [PubMed] [Google Scholar]
- 35.Simms, M. S., Scholfield, D. P., Jacobs, E., Michaeli, D., Broome, P., Humphreys, J. E. & Bishop, M. C. (2000) Br. J. Cancer 83, 443-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anonymous (2003) BioDrugs 17, 223-225.12749761 [Google Scholar]
- 37.Brett, B. T., Smith, S. C., Bouvier, C. V., Michaeli, D., Hochhauser, D., Davidson, B. R., Kurzawinski, T. R., Watkinson, A. F., Van Someren, N., Pounder, R. E. & Caplin, M. E. (2002) J. Clin. Oncol. 20, 4225-4231. [DOI] [PubMed] [Google Scholar]
- 38.Talwar, G. P. (1999) Immunol. Rev. 171, 173-192. [DOI] [PubMed] [Google Scholar]
- 39.Re, F. & Strominger, J. L. (2001) J. Biol. Chem. 276, 37692-37699. [DOI] [PubMed] [Google Scholar]
- 40.Toshchakov, V., Jones, B. W., Perera, P. Y., Thomas, K., Cody, M. J., Zhang, S., Williams, B. R., Major, J., Hamilton, T. A., Fenton, M. J. & Vogel, S. N. (2002) Nat. Immunol. 3, 392-398. [DOI] [PubMed] [Google Scholar]
- 41.Ito, T., Amakawa, R., Kaisho, T., Hemmi, H., Tajima, K., Uehira, K., Ozaki, Y., Tomizawa, H., Akira, S. & Fukuhara, S. (2002) J. Exp. Med. 195, 1507-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weigt, H., Muhlradt, P. F., Emmendorffer, A., Krug, N. & Braun, A. (2003) Immunobiology 207, 223-233. [DOI] [PubMed] [Google Scholar]