Abstract
The identification of immunogenic glycotopes that render glycoconjugate vaccines protective is key to improving vaccine efficacy. Synthetic oligosaccharides are an attractive alternative to the heterogeneous preparations of purified polysaccharides that most marketed glycoconjugate vaccines are based on. To investigate the potency of semi- synthetic glycoconjugates, we chose the least efficient serotype in the current pneumococcal conjugate vaccine Prevnar 13™, Streptococcus pneumoniae serotype 3 (ST3). Glycan arrays containing synthetic ST3 repeating unit oligosaccharides were used to screen a human reference serum for antibodies and to define the recognition site of two ST3-specific protective monoclonal antibodies. The glycan array screens identified a tetrasaccharide that was selected for in-depth immunological evaluation. The tetrasaccharide-CRM197 carrier protein conjugate elicited protective immunity as evidenced by opsonophagocytosis assays and protection against pneumonia caused by ST3 in mice. Formulation of the defined protective lead candidate glycotope has to be further evaluated to elicit optimal long-term immunity.
Keywords: Streptococcus pneumoniae, Synthetic glycans, Glycan arrays, Glycoconjugate vaccines, Epitope mapping, Opsonophagocytosis
eTOC entry
Parameswarappa et al. describe a synthetic immunogenic tetrasaccharide hapten of the capsular polysaccharide of Streptococcus pneumoniae serotype 3. Glycoconjugates of this antigen are able to protect mice from pneumococcal pneumonia in a lethal transnasal challenge model.
Introduction
Streptococcus pneumoniae is an encapsulated bacterium that accounts for the majority of bacterial upper and lower respiratory tract infections and is responsible for millions of deaths each year (Feikin et al., 2000; World Health Organization, 2007). To protect from morbidity and mortality caused by S. pneumoniae, pneumococcal carbohydrate vaccines were introduced. Vaccine-induced immunoprotection against encapsulated bacteria is primarily mediated by opsonophagocytic antibodies that bind capsular polysaccharides (CPSs) (Song et al., 2013). CPS antigens are thymus-independent type 2 antigens and often induce low-affinity antibodies with a restricted isotype distribution and evoke only insignificant B-cell memory particularly in the very young and the very old (Mond et al., 1995). To overcome these deficiencies of polysaccharide vaccines, current conjugate vaccines against pneumococci consist of CPSs that are chemically linked to a carrier protein to form glycoconjugates that promote T cell-dependent antibody responses and long-lasting memory (Pletz et al., 2008). Only a minority of the more than 90 S. pneumoniae serotypes cause the vast majority of pneumococcal infections (Gruber et al., 2012). Seven serotypes were included in the first S. pneumoniae glycoconjugate vaccine (PCV7) that drastically reduced invasive pneumococcal disease. However, the overall efficacy of PCV7 was partly offset by serotype replacement and subsequent increase in invasive pneumococcal disease caused by non-vaccine serotypes (Hicks et al., 2007; Yildirim et al., 2012). Serotypes that emerged after the introduction of PCV7 have been included in the current anti-pneumococcal glycoconjugate vaccine (PCV13, Prevnar 13™) (Gruber et al., 2012). S. pneumoniae serotype 3 (ST3) causes invasive pneumococcal infections in adults and is covered by PCV13. ST3 strains are often used in experimental models of pneumonia, meningitis, and otitis media owing to their high virulence in animals (Chiavolini et al., 2008) and as a model to study protective immunogenic properties of candidate vaccines (Jakobsen et al., 1999a; Jakobsen et al., 1999b). The ST3 glycoconjugates contained in PCV13 are of rather low efficacy leading to hyporesponsiveness (Gruber et al., 2012; Song et al., 2013). ST3 CPS conjugates showed an atypical immunogenicity and boostability pattern. PCV13 is insufficient at limiting acute otitis media infections caused by ST3. Altered CPS expression, capsule thickness, and an impaired booster response may all contribute to the low efficacy of ST3 conjugates (Poolman et al., 2009).
Current glycoconjugate vaccine preparations contain CPS isolated from fermentation broths after inactivation of the cultured pathogen (Schumann et al., 2013). The resulting products are ill-defined and may generate artificial neoglycotopes when subjected to processing steps such as depolymerization and activation prior to conjugation (Schumann et al., 2013). Polysaccharides contain multiple coupling sites and consist of a complex glycan pool of different chain lengths. Contamination with other highly immunogenic broth components such as cell wall polysaccharide is sometimes observed and may lead to hyporesponsiveness. Glycoconjugates of synthetic oligosaccharides are a promising alternative to vaccines derived from isolated glycans (Astronomo and Burton, 2010; Anish et al., 2014). The immunogenic properties of synthetic glycoconjugates can be optimized by designing antigenic structures based on the binding specificities and structural features of protective antibodies. Synthetic oligosaccharides based on ST3 CPS repeating units have been shown to protect immunized mice against lethal systemic challenge with ST3 pneumococci via non-mucosal routes (Benaissa-Trouw et al., 2001). It remains unclear whether glycoconjugates containing synthetic oligosaccharide can confer protection against bacterial pneumonia, the most common form of severe pneumococcal infection. Moreover, the structural characteristics of ST3 CPS antigenic components that confer protection or drive non-specific responses are still poorly understood.
Here, we define a highly immunogenic tetrasaccharide glycotope based on the disaccharide repeating unit of ST3 CPS (Figure 1). We show that synthetic oligosaccharide-based glycoconjugates are immunoprotective against experimental pneumonia caused by transnasal infection with ST3 strain PN36. Antigenic specificities of anti-ST3 antibodies were dissected by glycan arrays displaying oligosaccharide fragments including the ST3 CPS repeating unit. An ST3 oligosaccharide antigen lead for vaccine development was identified by combining organic synthesis, glycan arrays, glycoconjugation, in vivo vaccination and challenge experiments.
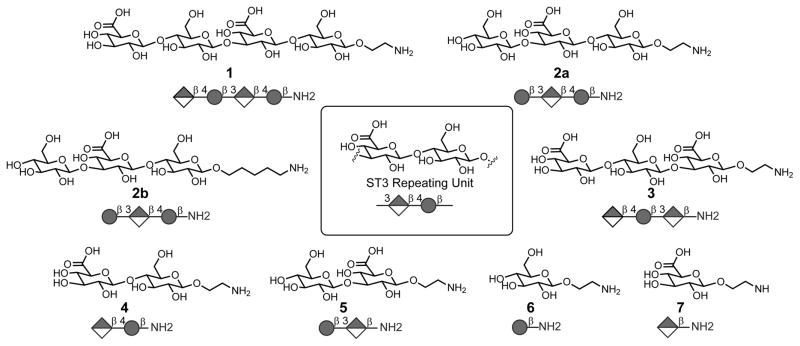
Figure 1. Synthetic ST3 glycans 1–7 based on the repeating unit of ST3-CPS.
Center: Repeating unit of ST3 CPS that is produced by the bacterium in a synthase-dependent pathway by repetitive addition of monosaccharides (Yother, 2011). Surrounding: Chemical structures of synthetic ST3 glycans.
Results and Discussion
Synthesis of ST3 oligosaccharides
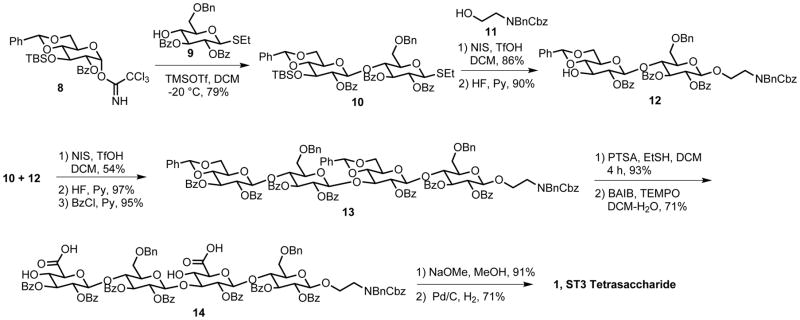
ST3 glycans ranging from mono- to tetrasaccharides containing different terminal sugars (Figure 1, 1–7) were prepared to include an alkylamine linker at the reducing end for glycan array immobilization and conjugation to carrier proteins (Figure 2). Two differentially protected glucose building blocks (8 and 9) were prepared using previously established methods (Hällgren and Widmalm, 1993; Eichler et al., 1997; Yu and Ensley, 2003). Selective activation of glycosyl trichloroacetimidate 8 and coupling with thioglycoside 9 yielded β-linked disaccharide 10 (Figure 2) (Yu and Ensley, 2003; Mo et al., 2009). Placement of the ethanolamine linker 11 by thioglycoside activation and silyl removal provided disaccharide acceptor 12 (Beshore and Dinsmore, 2002). Glycosylation of disaccharides 10 and 12 using NIS/TfOH furnished the fully protected tetrasaccharide 13 after TBS removal and benzoylation. Benzylidene cleavage followed by selective oxidation of the primary C6- hydroxyl groups resulted in the dicarboxylic acid derivative 14. Removal of all protective groups under Zemplen conditions followed by hydrogenation furnished tetrasaccharide 1. Overall yield was 13% from building blocks 8 and 9. Glycans 2a and 3–7 were synthesized in a similar manner (see Supplemental Information). Trisaccharide 2b with a pentanolamine linker was synthesized using automated glycan assembly (Weishaupt et al., 2016).
Figure 2. Synthesis of ST3 tetrasaccharide 1.
TMSOTf: trimethylsilyl trifluoromethanesulfonate, DCM: dichloromethane, NIS = N-iodosuccinimide, TfOH = trifluoromethanesulfonic acid, HF: hydrofluoric acid, Py: pyridine, BzCl: benzoylchloride, PTSA: p-toluenesulfonic acid, EtSH: ethanethiol, BAIB: bis(acetoxy)iodobenzene, TEMPO: 2,2,6,6-tetramethylpiperidine 1-oxyl, NaOMe: sodium methoxide.
Analysis of monoclonal antibodies and hyper immune sera against ST3-CPS by glycan array screening
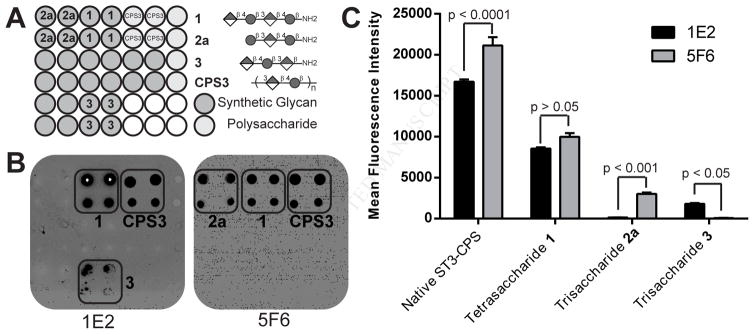
Sera and antibodies against the native antigen were analyzed to identify key antigenic components that are recognized by the immune system. Glycan arrays containing the synthetic ST3 carbohydrate antigens 1–7 as well as ST3-CPS were used to screen monoclonal antibodies (mAbs) and polyclonal hyper immune serum for epitope mapping (Geissner et al., 2014). Pooled sera of individuals that received the polysaccharide vaccine Pneumovax 23 contain antibodies that bind tetrasaccharide 1 and trisaccharide 2b (Figure S2) (Goldblatt et al., 2011). Two protective mAbs, 1E2 and 5F6, that had been raised against ST3 CPS were found to bind ST3 CPS on the glycan array (Figure 3) (Tian et al., 2009). Both antibodies bound tetrasaccharide 1 better than any of the smaller synthetic glycans, however, the mAbs differed in binding to trisaccharides 2a and 3. Apparently, distinct and shifted epitopes are being recognized by the two antibodies that differ from the known cellubiuronic acid minimal protective ST3 epitope as none of the disaccharides on the array were recognized (Benaissa-Trouw et al., 2001). Tetrasaccharide 1 contains the protective epitope of both mAbs and was selected for further immunogenicity studies. Glycan array screening of polyclonal and monoclonal antibodies recently allowed us to identify a lead tetrasaccharide glycotope for S. pneumoniae serotype 8 (ST8) that also presents a capsule lacking non-mammalian monosaccharides (Schumann et al., unpublished). The glycan array screening approach therefore seems to be widely applicable for this type of CPS antigens.
Figure 3. Glycan array analysis of protective murine mAbs raised against ST3-CPS.
(A) Simplified printing pattern only showing bound compounds. Complete printing pattern is shown in Figure S1. (B) Representative glycan array fields showing binding pattern of mAbs 1E2 and 5F6. (C) Comparison of binding strengths of mAbs at 16 μg/mL represented by fluorescence intensities. Each bar is the mean of four spots with the error bar representing the standard error of mean (SEM). Significance levels were calculated by pairwise Bonferroni comparison after two-way analysis of variance (ANOVA). A glycan array analysis of human reference serum 007sp is shown in Figure S2.
Preparation and characterization of ST3 tetrasaccharide 1-CRM197 glycoconjugates
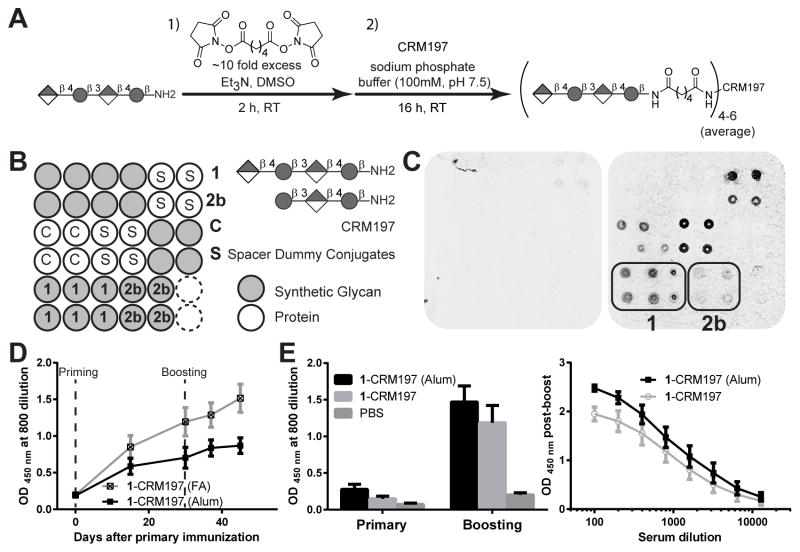
Carbohydrates generally act as T cell-independent B cell antigens (Brodeur and Wortis, 1980; Mond et al., 1995). Covalent attachment of carbohydrate antigens to carrier proteins produces neoglycoconjugates that, unlike native carbohydrates, induce a T cell-dependent immune response (Avery and Goebel, 1931; Pozsgay, 2000). Synthetic tetrasaccharide 1 was conjugated to the carrier protein CRM197, a widely used detoxified mutant of diphtheria toxin that is a constituent of licensed vaccines and induces little carrier-mediated suppression of anti-hapten responses (Tontini et al., 2013; Pecetta et al., 2015; Pecetta et al., 2016). Di-(N-succinimidyl) adipate (DSAP) was used as cross-linker. The amine group of the linker in tetrasaccharide 1 was reacted with one N-hydroxy succinimide (NHS) ester present in DSAP to form the spacer-appended oligosaccharide (Figure 4A). Unreacted DSAP was subsequently extracted with chloroform. The neoglycoconjugate was formed by coupling the spacer-activated glycan with the amino groups of the lysines present in CRM197. Coupling was confirmed by SDS-PAGE. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) revealed that on average four to six oligosaccharide haptens were loaded on each CRM197 molecule (Figure S3). The oligosaccharide/protein ratio is an important immunogenicity determinant of glycoconjugates and effects of loading on our antigen will eventually have to be determined, but no loading effects for ST3 oligosaccharides obtained by depolymerization of native CPS have been observed (Laferrière et al., 1997). A recent detailed MS analysis identified the surface amine groups that were preferentially modified on CRM197 (Möginger et al., 2016).
Figure 4. Immune response towards neoglycoconjugates 1-CRM197.
(A) Conjugation protocol to obtain neoglycoconjugates 1-CRM197 of tetrasaccharide 1. See Figure S3 for conjugate characterization. (B) Simplified glycan array printing pattern only showing bound compounds. Complete printing pattern is shown in Figure S4. (C) Glycan array analysis of reactivity of IgG in polyclonal mouse serum using pooled serum diluted 1:200 of FA group as example. No binding to synthetic glycans is seen in pre-immune serum (left) while tetrasaccharide 1 binds high levels of antibodies in post-boost serum (right). Antibodies against the carrier protein and the spacer were also detected. (D) Comparison of serum IgG reactivity against native ST3-CPS measured by ELISA at various time points post vaccination of mice. (E) ELISA comparison of antibody levels in short term challenge experiments between control group and 1-CRM197 immunized mice and titer comparison of groups with and without Alum adjuvant on day 28.
Immunogenicity evaluation of glycoconjugates
To test the immunogenicity of tetrasaccharide hapten 1, six female C57BL/6 mice per group were each immunized subcutaneously (s.c.) 28 days apart with two doses of glycoconjugate 1-CRM197 formulated either with Freund’s Adjuvant (FA) or Alum (Alhydrogel, aluminum hydroxide). Aluminum hydroxide was preferred over aluminum phosphate that is part of approved glycoconjugate vaccines due to its positive charge at neutral pH allowing better absorption of the CRM modified with the negatively charged ST3 oligosaccharides (Lindblad, 2004). Each dose contained 5 μg of tetrasaccharide 1.Control animals received PBS. The anti-hapten 1 antibody titers were monitored using glycan array analysis. The immunized mice elicited IgG antibodies that specifically bound to tetrasaccharide 1, demonstrating that hapten 1 is immunogenic (Figure 4C). Isotype switching to IgG and boosting effects indicated a T cell-dependent immune response (Figure 4C,D). The serum from immunized mice reacted against both the carrier protein as well as the glycoconjugate spacer (Figure 4C), possibly due to some lysine residues modified only with the spacer (Möginger et al., 2016). Serum antibody titers against ST3-CPS from immunized mice were measured by ELISA using purified ST3-CPS as the coating antigen. Antibodies raised against synthetic hapten 1 significantly cross-reacted with the natural ST3-CPS, thus highlighting the vaccine potential of hapten 1 (Figure 4D). The immunogenicity of tetrasaccharide 1 was strongly dependent on the adjuvant formulation. Conjugates formulated in FA induced higher antibody titers in mice compared to Alum formulated conjugates (Figure 4D).
Protective effects of the ST3 tetrasaccharide 1-CRM197 conjugate
Protective antibodies often function by opsonization and promotion of complement-mediated lysis of target pathogens (Romero-Steiner et al., 2006; Tian et al., 2009). Therefore, we tested sera from 1-CRM197 immunized mice for their opsonophagocytic potential in a standardized opsonophagocytosis assay (OPA) (Johnson et al., 1999; Romero-Steiner et al., 2006). Human promyelocytic leukemia cells (HL-60) were differentiated into neutrophil-like cells in the presence of dimethylformamide (DMF) for 6–7 days. Next, S. pneumoniae serotype 3 (PN36, strain NCTC7978) were incubated with test sera from naïve (pre-immune) or 1-CRM197 immunized mice and then added to differentiated HL-60 cells in the presence of complement. After one hour, remaining viable S. pneumoniae were quantified by plating and subsequent counting of colonies (cfu-assay). Serum from 1-CRM197 immunized mice mediated dose-dependent killing of pneumococci, demonstrating that the synthetic conjugate vaccine elicited opsonophagocytic antibodies (Figure 5). OPA titers of ≥1:8 are considered efficient in individuals immunized with existing 7-valent or 13-valent conjugate vaccines (Schuerman et al., 2011; Song et al., 2013). Anti-pneumococcal serum antibody titers as well as opsonophagocytic activity were strongly dependent on the adjuvant used for formulation. FA elicited higher antibody titers (Figure 4D) with higher killing-activity in the OPA assay (Figure 5) compared to Alum adjuvanted 1-CRM197 glycoconjugate.
Figure 5. OPA responses mediated by 1-CRM197 induced antibodies.
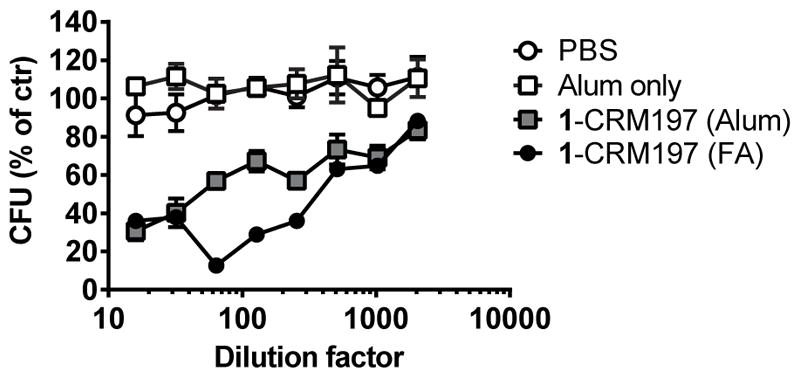
Mice were immunized with PBS, Alum or 1-CRM197 formulated with Alum s.c. on days 0, 14 and 28. Mice were bled on day 35 for serum collection and OPA-titers were determined. Values from three independent experiments with pooled sera are given as mean and SEM. OPA titers using sera of mice that received two doses of 1-CRM197 formulated with FA in the pre-study (no replicates) were included for comparison.
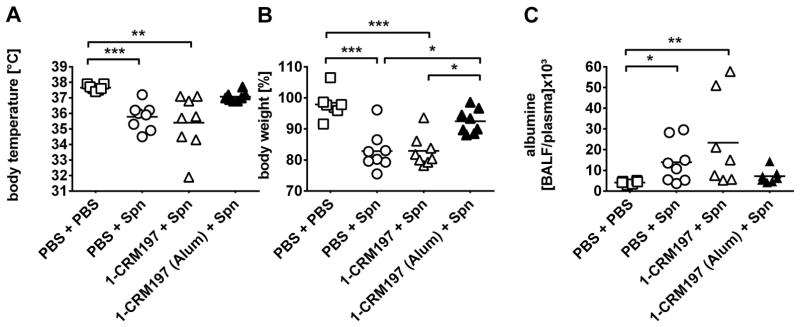
Having established 1-CRM197 glycoconjugate immunogenicity, we next evaluated its immunoprotective properties against pneumococcal pneumonia in an established mouse model (Reppe et al., 2009; Witzenrath et al., 2011). As FA is not suitable for vaccine use, we administered the glycoconjugate formulated with Alum or unadjuvanted. Alum (aluminum phosphate) is the adjuvant in current commercial pneumococcal conjugate vaccines such as PCV13. Mice were immunized three times (days 0, 14, 28) with 1-CRM197 containing 5 μg of tetrasaccharide 1 per dose and antibody titers were measured (Figure 4E). Naïve and 1-CRM197 immunized C57BL/6 mice were infected transnasally on day 35 with 1x106 cfu S. pneumoniae (PN36, NCTC 7978) and clinical signs of disease (weight loss, body temperature) were monitored over the course of 48 h. Immunization with Alum-adjuvanted 1-CRM197 significantly reduced disease activity as evidenced by a less pronounced drop in body temperature and lower weight loss (Figure 6A,B). As a surrogate for organ damage and barrier disruption, we measured pneumonia-induced lung permeability. Whereas pneumococcal pneumonia strongly increased lung permeability in naïve and 1-CRM197 immunized mice, vaccination with 1-CRM197 adjuvanted with Alum preserved alveolar barrier integrity, demonstrated by low albumin BALF/plasma ratio (Figure 6C).
Figure 6. Effect of vaccination on pneumonia severity.
After immunization on days 0, 14 and 28 mice were infected transnasally with 1x106 cfu S. pneumoniae (Spn) or sham infected with PBS on day 35. (A) Body temperature (n=7–8), (B) body weight (n=7–8), and (C) lung permeability (n=6–8) were assessed 48 h after infection.
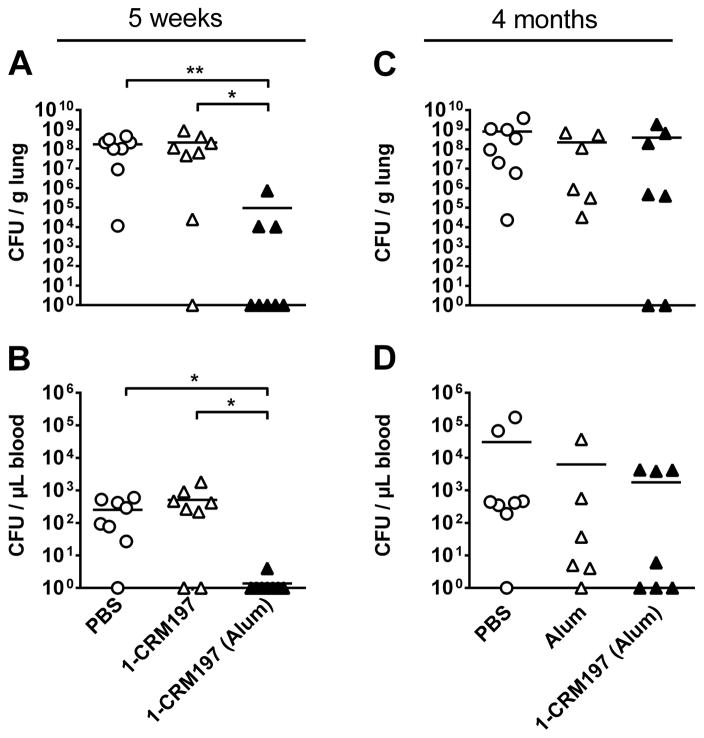
As a direct indicator of antibacterial defense in naïve and immunized mice, we quantified bacterial burdens 48 hours post infection to assess the protective effects of 1-CRM197 immunization on bacterial growth. Immunization with 1-CRM197 and Alum significantly reduced pulmonary bacterial loads when compared to PBS-treated mice or animals immunized with the glycoconjugate without adjuvant (Figure 7A). Moreover, immunization almost completely prevented bacteremia that was frequently observed in vaccine-naïve control animals (Figure 7B). The improved antibacterial defense was accompanied by increased lymphocyte numbers (Figure S5) and reduced cytokine levels (Figure S6) in the alveolar compartment of S. pneumoniae-infected mice. These results provide a solid proof-of-concept for the efficiency of a semi-synthetic pneumococcal carbohydrate conjugate vaccine strategy against ST3.
Figure 7. Short term effects (5 weeks after initial immunization) and long term effects (4 months after initial immunization) of vaccination on pneumonia severity.
(A,B) Mice were immunized s.c. on days 0, 14, and 28, and were transnasally infected with 1x106 cfu S. pneumoniae on day 35. Bacterial burden was assessed in lung tissue (A) and blood (B) 48 h post infection (n=8). Pulmonary leukocyte recruitment and cytokine release in S. pneumoniae-infected lungs are shown in Figures S5 and S6, respectively. (C,D) Mice immunized on days 0, 14 and 28 were transnasally infected with 1x106 cfu S. pneumoniae 112 d after initial immunization. Bacterial burden was assessed in lung tissue (C) and blood (D) 48 h post infection (n=6–8).
Induction of long-lived immunity is an important feature of successful vaccination. Therefore, we assessed long-term effects of ST3-tetrasaccharide immunization. In contrast to the robust protection at 35 days post immunization, we observed minor effects four months after the initial vaccination (Figure 7C,D), which is being evaluated further and will be reported elsewhere. The deficiency of the synthetic ST3-tetrasaccharide vaccine to generate long-lasting immunity may not be a matter of antigenic potential, even though only a small tetrasaccharide antigen was employed as hapten. More studies have suggested that tetrasaccharides or smaller glycans are enough to elicit protective immunity, such as for Neisseria meningitidis serogroup W135 (Wang et al., 2013) as well as S. pneumoniae serotypes 6 (Jansen, W. T. M. et al., 2001) and 14 (Safari et al., 2008), but long term memory is not regularly tested. However, sometimes larger glycans seem to be necessary, as devised in case of lipopolysaccharide O-antigens of Salmonella typhimurium O12 (Svenson and Lindberg, 1981) and Shigella flexneri serotype 2a (Phalipon et al., 2006). Additionally, oligosaccharides can be fine-tuned for ideal antigenicity by minor structural modifications compared to the natural sequence as we have recently shown for an ST8 tetrasaccharide hapten (Schumann et al., unpublished). Carbohydrates generally elicit a weaker immunity when compared to peptide-antigens. Studies on Prevnar-13 and during the development of Synflorix showed that ST3 is an especially challenging pneumococcal serotype to achieve high titers and memory even when conjugates of the large purified polysaccharide are injected (Poolman et al., 2009; Gruber et al., 2012; Prymula and Schuerman, 2014). Rather than using larger antigens, formulation effects like antigen loading, carrier protein and adjuvant adjustment to mobilize protective T cell and subsequent B cell memory responses may be required to increase the immunogenicity of semi-synthetic glycoconjugate vaccines (Snippe et al., 1983; Sander, 2012). Having a protective synthetic oligosaccharide lead antigen in hand will allow in-depth studies for hapten and formulation optimization.
In summary, we demonstrate that synthetic ST3 tetrasaccharide-conjugates are viable vaccine candidates to protect from bacterial pneumonia. The formulation including the employed adjuvants of the semi-synthetic vaccine needs further refinement to achieve long-lived immune memory. Activation of innate immune responses capable of instructing robust adaptive immunity may be crucial in this regard (Blander and Sander, 2012; Sander, 2012).
Significance
Pneumonia and other invasive infections induced by Streptococcus pneumoniae are associated with significant morbidity and mortality particularly in toddlers and in elderly people. While current pneumococcal conjugate vaccines protect against severe infections induced by most of the included serotypes, the immune response against serotype 3 is often too low for efficient immunoprotection. Alternative approaches are needed to improve vaccines for this highly invasive pneumococcal serotype. One promising strategy involves replacement of the ill-defined isolated polysaccharides in current vaccines with synthetic designer carbohydrate antigens. We show that glycoconjugates of a synthetic carbohydrate antigen can confer protection against serotype 3 in a mouse model of pneumococcal pneumonia, the most common form of pneumococcal infection. The investigated glycan structure, its glycoconjugates and formulation can be optimized and refined to newly establish semi-synthetic and fully synthetic vaccine candidates with the potential to overcome the limitations of the currently used pneumococcal glycoconjugates. This study is therefore an important step toward improving efficacy and availability of pneumococcal conjugate vaccines.
Experimental Methods
This section gives an overview of the methods used in this study. Detailed experimental procedures can be found in the Supplemental Information.
Glycan arrays
Glycan arrays were obtained by robotically spotting carbohydrates onto CodeLink NHS activated glass slides (Surmodics) using an S3 microarray printer (Scienion) as described previously (Pereira et al., 2015; Reinhardt et al., 2015; Geissner et al., 2016). Printing patterns are described in Figures S1 and S4. Slides were incubated overnight at high humidity, quenched and stored dry until use. Before the assay, slides were blocked with 1% BSA-PBS. After drying, a 64 well incubation grid (Grace Biolabs) was attached and the primary antibody or serum diluted in 1% BSA-PBS was applied to individual wells. After incubation and washing, dilutions of secondary antibodies were applied. Following incubation, washing and drying, slides were scanned with a 4300A microarray scanner (Molecular Devices).
Preparation of neoglycoconjugates
Neoglycoconjugates 1-CRM197 were prepared as described previously (Anish et al., 2013a; Anish et al., 2013b). Tetrasaccharide 1 was reacted with di-(N-succinimidyl) adipate in DMSO catalyzed by triethylamine for 2 h at RT. Addition of coupling buffer (100 mM sodium phosphate buffer, pH 7.5) and extraction of unreacted linker with chloroform was followed by reaction of linker appended tetrasaccharide with recombinant CRM197 (Pfenex Inc.) overnight in coupling buffer. After desalting with 10 kDa Amicon ultrafiltration devices (Millipore), neoglycoconjugates were characterized by SDS-PAGE and MALDI-TOF MS.
ELISA
Antibody titers were determined on Immulon HBX (Thermo Scientific) high binding plates coated with isolated ST3 CPS (SSI Diagnostica) and blocked with 1% BSA-PBS. Subsequently, plates were incubated with the serum diluted in 1% BSA-PBS, washed, incubated with goat anti-mouse IgG HRP (Dianova) diluted 1:10,000, washed and incubated with 1-Step Ultra TMB (Thermo Scientific Pierce). After stopping the reaction with 2% sulfuric acid, absorbance at 450 nm was determined.
Immunizations and challenge
All animal experiments were approved by the institutional and regulatory authorities and conducted according to the legal regulations. In the pre-study, six female C57BL/6 mice (Charles River, Sulzfeld, Germany) per group were immunized twice s.c. with 1-CRM197 containing 5 μg tetrasaccharide 1 formulated either with Alum (Alhydrogel, Brenntag) or Freund’s Adjuvant (Sigma Aldrich; Complete Freund’s Adjuvant for priming, Incomplete Freund’s Adjuvant for boosting). Primary and boosting immunizations were 28 days apart. Serum samples were taken regularly to follow immunization success.
For the short-term challenge study, female C57BL/6N mice (n=10–11 per group, eight weeks, Charles River, Sulzfeld, Germany) were immunized s.c. with 1-CRM197 containing 5 μg tetrasaccharide formulated with or without Alhydrogel (Alum) at a total volume of 100 μL. Control mice received 100 μL of sterile phosphate-buffered saline (PBS) subcutaneously. Booster doses were given on days 14 and 28. Blood (20 μL) for serum separation was collected from the tail vein on days 14 and 28. For the long-term challenge study, mice (n=11 per group) were immunized s.c. with Alhydrogel alone, 1-CRM197 containing 5 μg tetrasaccharide formulated with Alhydrogel or PBS (control) at a total volume of 100 μL on days 0, 14 and 28. Serum was collected on days 0 and 35.
OPA
HL-60 cells differentiated to pseudogranulocytes were resuspended in Hank’s buffer without Ca2+ or Mg2+ (OPA-buffer) to an effector-to-target cell ratio of 400:1 and kept in ice bath until usage. For the assay, 5 μL aliquots of serum (run in quadruplicate for each assay) were added to OPA buffer in a microtiter plate and serially diluted for a titer range of 1:32 to 1:4096. Twenty μL of S. pneumoniae suspension (serotype 3, strain NCTC7978) taken directly from thawed cryostocks were added to each well. The plate was then incubated for 15 min at 37 °C and 5% CO2. Afterward, phagocytosis was initiated by addition of 10 μL baby rabbit complement (Cedar Lane / BIOZOL) to each well, followed immediately by 40 μL of differentiated HL-60 cells. After incubation for 45 minutes at 37 °C in a horiz ontal shaker (220 rpm), the plate was kept on ice and 5 μL of suspension from each condition was plated onto 5% sheep blood agar plates and incubated for 10–12 h at 37 °C to manually count colony-forming units.
Challenge
In pneumococcal challenge experiments analyzing bacterial elimination, pulmonary barrier dysfunction and inflammation, a total of seven to eight mice per immunized group were anaesthetized intraperitoneally (i.p.) with ketamine (80 mg/kg, Ketavet®, Pfizer, Berlin, Germany) and xylazine (25 mg/kg, Rompun®, Bayer, Leverkusen, Germany) on day 35 (short-term effect) or 112 (long-term effect) and were transnasally infected with 1x106 cfu S. pneumoniae (serotype 3, strain NCTC7978) in 20 μL PBS as described previously (Schmeck et al., 2004; Witzenrath et al., 2006). Control mice received 20 μL PBS transnasally. Health status was monitored at 12 h intervals post infection.
Dissection and sampling
Forty eight hours post infection, mice were anesthetized with ketamine (160 mg/kg) and xylazine (75 mg/kg), intubated, ventilated and heparinized (Dames et al., 2014), and blood was drawn from the inferior vena cava. Lungs were flushed with 0.9% saline via the pulmonary artery. Bronchoalveolar lavage (BAL) was performed twice using 800 μL PBS and lungs were removed.
Bacterial burden in lung tissue and blood
Serial dilutions of homogenized lungs and blood were plated on blood agar and incubated at 37 °C under 5% CO2 for 24 h to count colony-forming units.
Albumin ELISA
BALF supernatant and plasma albumin concentration were measured by ELISA (Bethyl Laboratories Inc., Montgomery, AL, USA) according to the manufacturer′s instructions. Alveolar edema formation was assessed by calculating the albumin BALF/plasma ratio.
Statistical analysis
Data are expressed as mean+SEM or scatter plots and mean. Monoclonal antibody analysis: Two-way ANOVA with post-hoc pairwise Bonferroni comparison was performed by using GraphPad Prism 5. Challenge studies: One-way ANOVA followed by Dunn′s post hoc test were performed by using GraphPad Prism 4.02 software. *p<0.05, **p<0.01 and ***p<0.001 were considered to be significant.
Supplementary Material
Highlights.
Glycan arrays reveal epitopes of two monoclonal antibodies to S. pneumoniae 3 CPS
CRM197 conjugates of one identified tetrasaccharide antigen are immunogenic in mice
Vaccination with the glycoconjugates protects mice from pneumococcal pneumonia
Acknowledgments
This work was supported in part by the Max-Planck Society (P.H.S.), the German Research Council (DFG), Collaborative Research Centre SFB-TR 84 (C3, C6 to M.W.; C8 to L.E.S., P.H.S.; B1 to N.S.; SA1940/2-1 to L.E.S.) and by the German Ministry for Education and Research (e:Med CAPSyS, TP2 to N.S.; TP4 to M.W.) P.M. acknowledges support by a European Research Council fellowship (Marie Sklodowska-Curie Actions project No. 652745). The authors gratefully thank Sven Hammerschmidt for kindly providing S. pneumoniae serotype 3 (NCTC7978) and Uwe Vogel for the valuable technical supervision of flow cytometry. The excellent technical assistance of Maria Spelling and Denise Barthel is greatly appreciated. Parts of this work will be included in the doctoral theses of R.L.B. and A.G. P.H.S. is a shareholder of Vaxxilon AG, Reinach, Switzerland. S.G.P. and C.L.P. are employees of a Vaxxilon AG subsidiary.
Footnotes
Author Contributions
S.G.P., K.R., L.-A.P., L.E.S., M.W., C.L.P., C.A., P.H.S planned the study. S.G.P., K.R., A.G., P.M., S.G., A.D.J.C., A.W., M.W.W., B.P.M., R.L.B., C.A. performed experiments. S.G.P., K.R., A.G., P.M., S.G., A.D.J.C., M.W.W., R.L.B., L.E.S., M.W., C.L.P., C.A., P.H.S analyzed the data. S.H., L.-A.P. provided reagents. S.G.P, K.R., A.G., L.E.S., M.W., N.S., C.A., C.L.P., P.H.S. wrote the manuscript with input from all authors.
The other authors declare no financial conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anish C, Guo X, Wahlbrink A, Seeberger PH. Plague Detection by Anti-carbohydrate Antibodies. Angew Chem Int Ed. 2013a;52:9524–9528. doi: 10.1002/anie.201301633. [DOI] [PubMed] [Google Scholar]
- Anish C, Martin CE, Wahlbrink A, Bogdan C, Ntais P, Antoniou M, Seeberger PH. Immunogenicity and Diagnostic Potential of Synthetic Antigenic Cell Surface Glycans of Leishmania. ACS Chem Biol. 2013b;8:2412–2422. doi: 10.1021/cb400602k. [DOI] [PubMed] [Google Scholar]
- Anish C, Schumann B, Pereira CL, Seeberger PH. Chemical Biology Approaches to Designing Defined Carbohydrate Vaccines. Chem Biol. 2014;21:38–50. doi: 10.1016/j.chembiol.2014.01.002. [DOI] [PubMed] [Google Scholar]
- Astronomo RD, Burton DR. Carbohydrate vaccines: developing sweet solutions to sticky situations? Nat Rev Drug Discov. 2010;9:308–324. doi: 10.1038/nrd3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery OT, Goebel WF. Chemo-Immunological Studies On Conjugated Carbohydrate-Proteins. V The Immunological Specificity Of An Antigen Prepared By Combining The Capsular Polysaccharide Of Type III Pneumococcus With Foreign Protein J Exp Med. 1931;54:437–447. doi: 10.1084/jem.54.3.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benaissa-Trouw B, Lefeber DJ, Kamerling JP, Vliegenthart JFG, Kraaijeveld K, Snippe H. Synthetic Polysaccharide Type 3-Related Di-, Tri-, and Tetrasaccharide-CRM197 Conjugates Induce Protection against Streptococcus pneumoniae Type 3 in Mice. Infect Immun. 2001;69:4698–4701. doi: 10.1128/IAI.69.7.4698-4701.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beshore DC, Dinsmore CJ. Preparation of Substituted Piperazinones via Tandem Reductive Amination-( N , N ‘-Acyl Transfer)-Cyclization. Org Lett. 2002;4:1201–1204. doi: 10.1021/ol025644l. [DOI] [PubMed] [Google Scholar]
- Blander JM, Sander LE. Beyond pattern recognition: five immune checkpoints for scaling the microbial threat. Nat Rev Immunol. 2012;12:215–225. doi: 10.1038/nri3167. [DOI] [PubMed] [Google Scholar]
- Brodeur PH, Wortis HH. Regulation of thymus-independent responses: unresponsiveness to a second challenge of TNP-Ficoll is mediated by hapten-specific antibodies. J Immunol. 1980;125:1499–1505. [PubMed] [Google Scholar]
- Chiavolini D, Pozzi G, Ricci S. Animal models of Streptococcus pneumoniae disease. Clin Microbiol Rev. 2008;21:666–685. doi: 10.1128/CMR.00012-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dames C, Akyüz L, Reppe K, Tabeling C, Dietert K, Kershaw O, Gruber AD, Meisel C, Meisel A, Witzenrath M, et al. Miniaturized bronchoscopy enables unilateral investigation, application, and sampling in mice. Am J Respir Cell Mol Biol. 2014;51:730–737. doi: 10.1165/rcmb.2014-0052MA. [DOI] [PubMed] [Google Scholar]
- Eichler E, Jennings HJ, Whitfield DM. Synthesis of a Single Repeat Unit of Type VIII Group B Streptococcus Capsular Polysaccharide 1. J Carbohydr Chem. 1997;16:385–411. doi: 10.1016/s0008-6215(99)00103-2. [DOI] [PubMed] [Google Scholar]
- Feikin DR, Schuchat A, Kolczak M, Barrett NL, Harrison LH, Lefkowitz L, McGeer A, Farley MM, Vugia DJ, Lexau C, et al. Mortality from invasive pneumococcal pneumonia in the era of antibiotic resistance, 1995–1997. Am J Public Health. 2000;90:223–229. doi: 10.2105/ajph.90.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissner A, Anish C, Seeberger PH. Glycan arrays as tools for infectious disease research. Curr Opin Chem Biol. 2014;18:38–45. doi: 10.1016/j.cbpa.2013.11.013. [DOI] [PubMed] [Google Scholar]
- Geissner A, Pereira CL, Leddermann M, Anish C, Seeberger PH. Deciphering Antigenic Determinants of Streptococcus pneumoniae Serotype 4 Capsular Polysaccharide using Synthetic Oligosaccharides. ACS Chem Biol. 2016;11:335–344. doi: 10.1021/acschembio.5b00768. [DOI] [PubMed] [Google Scholar]
- Goldblatt D, Plikaytis BD, Akkoyunlu M, Antonello J, Ashton L, Blake M, Burton R, Care R, Durant N, Feavers I, et al. Establishment of a new human pneumococcal standard reference serum, 007sp. Clin Vaccine Immunol. 2011;18:1728–1736. doi: 10.1128/CVI.05252-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber WC, Scott DA, Emini EA. Development and clinical evaluation of Prevnar 13, a 13-valent pneumocococcal CRM197 conjugate vaccine. Ann NY Acad Sci. 2012;1263:15–26. doi: 10.1111/j.1749-6632.2012.06673.x. [DOI] [PubMed] [Google Scholar]
- Hällgren C, Widmalm G. Synthesis of a Site-Specific Deuterium Substituted Methyl β-D-Glucan Decasaccharide. J Carbohydr Chem. 1993;12:309–333. [Google Scholar]
- Hicks LA, Harrison LH, Flannery B, Hadler JL, Schaffner W, Craig AS, Jackson D, Thomas A, Beall B, Lynfield R, et al. Incidence of Pneumococcal Disease Due to Non–Pneumococcal Conjugate Vaccine (PCV7) Serotypes in the United States during the Era of Widespread PCV7 Vaccination, 1998–2004. J Infect Dis. 2007;196:1346–1354. doi: 10.1086/521626. [DOI] [PubMed] [Google Scholar]
- Jakobsen H, Saeland E, Gizurarson S, Schulz D, Jónsdóttir I. Intranasal immunization with pneumococcal polysaccharide conjugate vaccines protects mice against invasive pneumococcal infections. Infect Immun. 1999a;67:4128–4133. doi: 10.1128/iai.67.8.4128-4133.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsen H, Schulz D, Pizza M, Rappuoli R, Jónsdóttir I. Intranasal immunization with pneumococcal polysaccharide conjugate vaccines with nontoxic mutants of Escherichia coli heat-labile enterotoxins as adjuvants protects mice against invasive pneumococcal infections. Infect Immun. 1999b;67:5892–5897. doi: 10.1128/iai.67.11.5892-5897.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen WTM, Hogenboom S, Thijssen MJL, Kamerling JP, Vliegenthart JFG, Verhoef J, Snippe H, Verheul AFM. Synthetic 6B Di-, Tri-, and Tetrasaccharide-Protein Conjugates Contain Pneumococcal Type 6A and 6B Common and 6B- Specific Epitopes That Elicit Protective Antibodies in Mice. Infect Immun. 2001;69:787–793. doi: 10.1128/IAI.69.2.787-793.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SE, Rubin L, Romero-Steiner S, Dykes JK, Pais LB, Rizvi A, Ades E, Carlone GM. Correlation of Opsonophagocytosis and Passive Protection Assays Using Human Anticapsular Antibodies in an Infant Mouse Model of Bacteremia for Streptococcus pneumoniae. J Infect Dis. 1999;180:133–140. doi: 10.1086/314845. [DOI] [PubMed] [Google Scholar]
- Laferrière CA, Sood RK, de Muys J-M, Michon F, Jenning HJ. The synthesis of Streptococcus pneumoniae polysaccharide-tetanus toxoid conjugates and the effect of chain length on immunogenicity. Vaccine. 1997;15:179–186. doi: 10.1016/s0264-410x(96)00148-x. [DOI] [PubMed] [Google Scholar]
- Lindblad EB. Aluminium compounds for use in vaccines. Immunol Cell Biol. 2004;82:497–505. doi: 10.1111/j.0818-9641.2004.01286.x. [DOI] [PubMed] [Google Scholar]
- Mo KF, Li H, Mague JT, Ensley HE. Synthesis of the β-1,3-glucan, laminarahexaose: NMR and conformational studies. Carbohydr Res. 2009;344:439–447. doi: 10.1016/j.carres.2008.12.014. [DOI] [PubMed] [Google Scholar]
- Möginger U, Resemann A, Martin CE, Parameswarappa S, Govindan S, Wamhoff EC, Broecker F, Suckau D, Pereira CL, Anish C, et al. Cross Reactive Material 197 glycoconjugate vaccines contain privileged conjugation sites. Sci Rep. 2016;6:20488. doi: 10.1038/srep20488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mond JJ, Vos Q, Lees A, Snapper CM. T cell independent antigens. Curr Opin Immunol. 1995;7:349–354. doi: 10.1016/0952-7915(95)80109-x. [DOI] [PubMed] [Google Scholar]
- Pecetta S, Lo Surdo P, Tontini M, Proietti D, Zambonelli C, Bottomley MJ, Biagini M, Berti F, Costantino P, Romano MR. Carrier priming with CRM197 or diphtheria toxoid has a different impact on the immunogenicity of the respective glycoconjugates: Biophysical and immunochemical interpretation. Vaccine. 2015;33:314–320. doi: 10.1016/j.vaccine.2014.11.026. [DOI] [PubMed] [Google Scholar]
- Pecetta S, Tontini M, Faenzi E, Cioncada R, Proietti D, Seubert A, Nuti S, Berti F, Romano MR. Carrier priming effect of CRM197 is related to an enhanced B and T cell activation in meningococcal serogroup A conjugate vaccination. Immunological comparison between CRM197 and diphtheria toxoid. Vaccine. 2016;34:2334–2341. doi: 10.1016/j.vaccine.2016.03.055. [DOI] [PubMed] [Google Scholar]
- Pereira CL, Geissner A, Anish C, Seeberger PH. Chemical Synthesis Elucidates the Immunological Importance of a Pyruvate Modification in the Capsular Polysaccharide of Streptococcus pneumoniae Serotype 4. Angew Chem Int Ed. 2015;54:10016–10019. doi: 10.1002/anie.201504847. [DOI] [PubMed] [Google Scholar]
- Phalipon A, Costachel C, Grandjean C, Thuizat A, Guerreiro C, Tanguy M, Nato F, Vulliez-Le Normand B, Bélot F, Wright K, et al. Characterization of functional oligosaccharide mimics of the Shigella flexneri serotype 2a O-antigen: implications for the development of a chemically defined glycoconjugate vaccine. J Immunol. 2006;176:1686–1694. doi: 10.4049/jimmunol.176.3.1686. [DOI] [PubMed] [Google Scholar]
- Pletz MW, Maus U, Krug N, Welte T, Lode H. Pneumococcal vaccines: mechanism of action, impact on epidemiology and adaption of the species. Int J Antimicrob Agents. 2008;32:199–206. doi: 10.1016/j.ijantimicag.2008.01.021. [DOI] [PubMed] [Google Scholar]
- Poolman J, Kriz P, Feron C, Di-Paolo E, Henckaerts I, Miseur A, Wauters D, Prymula R, Schuerman L. Pneumococcal serotype 3 otitis media, limited effect of polysaccharide conjugate immunisation and strain characteristics. Vaccine. 2009;27:3213–3222. doi: 10.1016/j.vaccine.2009.03.017. [DOI] [PubMed] [Google Scholar]
- Pozsgay V. Oligosaccharide-protein conjugates as vaccine candidates against bacteria. Adv Carbohydr Chem Biochem. 2000;56:153–199. doi: 10.1016/s0065-2318(01)56004-7. [DOI] [PubMed] [Google Scholar]
- Prymula R, Schuerman L. 10-valent pneumococcal nontypeable Haemophilus influenzae PD conjugate vaccine: Synflorix™. Expert Rev Vaccines. 2014;8:1479–1500. doi: 10.1586/erv.09.113. [DOI] [PubMed] [Google Scholar]
- Reinhardt A, Yang Y, Claus H, Pereira CL, Cox AD, Vogel U, Anish C, Seeberger PH. Antigenic potential of a highly conserved Neisseria meningitidis lipopolysaccharide inner core structure defined by chemical synthesis. Chem Biol. 2015;22:38–49. doi: 10.1016/j.chembiol.2014.11.016. [DOI] [PubMed] [Google Scholar]
- Reppe K, Tschernig T, Lührmann A, van Laak V, Grote K, Zemlin MV, Gutbier B, Müller HC, Kursar M, Schütte H, et al. Immunostimulation with Macrophage-Activating Lipopeptide-2 Increased Survival in Murine Pneumonia. Am J Respir Cell Mol Biol. 2009;40:474–481. doi: 10.1165/rcmb.2008-0071OC. [DOI] [PubMed] [Google Scholar]
- Romero-Steiner S, Frasch CE, Carlone G, Fleck RA, Goldblatt D, Nahm MH. Use of Opsonophagocytosis for Serological Evaluation of Pneumococcal Vaccines. Clin Vaccine Immunol. 2006;13:165–169. doi: 10.1128/CVI.13.2.165-169.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safari D, Dekker HAT, Joosten JAF, Michalik D, de Souza AC, Adamo R, Lahmann M, Sundgren A, Oscarson S, Kamerling JP, et al. Identification of the Smallest Structure Capable of Evoking Opsonophagocytic Antibodies against Streptococcus pneumoniae Type 14. Infect Immun. 2008;76:4615–4623. doi: 10.1128/IAI.00472-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander LE. Improved vaccines through targeted manipulation of the body's immunological risk-assessment? Bioessays. 2012;34:876–884. doi: 10.1002/bies.201200057. [DOI] [PubMed] [Google Scholar]
- Schmeck B, Zahlten J, Moog K, van Laak V, Huber S, Hocke AC, Opitz B, Hoffmann E, Kracht M, Zerrahn J, et al. Streptococcus pneumoniae-induced p38 MAPK-dependent phosphorylation of RelA at the interleukin-8 promotor. J Biol Chem. 2004;279:53241–53247. doi: 10.1074/jbc.M313702200. [DOI] [PubMed] [Google Scholar]
- Schuerman L, Wysocki J, Tejedor JC, Knuf M, Kim KH, Poolman J. Prediction of pneumococcal conjugate vaccine effectiveness against invasive pneumococcal disease using opsonophagocytic activity and antibody concentrations determined by enzyme-linked immunosorbent assay with 22F adsorption. Clin Vaccine Immunol. 2011;18:2161–2167. doi: 10.1128/CVI.05313-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann B, Anish C, Pereira CL, Seeberger PH. Carbohydrate Vaccines. In: Jones L, McKnight AJ, editors. Biotherapeutics. CHAPTER 3. Cambridge: Royal Society of Chemistry; 2013. pp. 68–104. [Google Scholar]
- Snippe H, van Houte AJ, van Dam JE, De Reuver MJ, Jansze M, Willers JM. Immunogenic properties in mice of hexasaccharide from the capsular polysaccharide of Streptococcus pneumoniae type 3. Infect Immun. 1983;40:856–861. doi: 10.1128/iai.40.3.856-861.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JY, Moseley MA, Burton RL, Nahm MH. Pneumococcal vaccine and opsonic pneumococcal antibody. J Infect Chemother. 2013;19:412–425. doi: 10.1007/s10156-013-0601-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenson SB, Lindberg AA. Artificial Salmonella vaccines: Salmonella typhimurium O-antigen-specific oligosaccharide-protein conjugates elicit protective antibodies in rabbits and mice. Infect Immun. 1981;32:490–496. doi: 10.1128/iai.32.2.490-496.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian H, Weber S, Thorkildson P, Kozel TR, Pirofski LA. Efficacy of opsonic and nonopsonic serotype 3 pneumococcal capsular polysaccharide-specific monoclonal antibodies against intranasal challenge with Streptococcus pneumoniae in mice. Infect Immun. 2009;77:1502–1513. doi: 10.1128/IAI.01075-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tontini M, Berti F, Romano MR, Proietti D, Zambonelli C, Bottomley MJ, de Gregorio E, Del Giudice G, Rappuoli R, Costantino P, et al. Comparison of CRM197, diphtheria toxoid and tetanus toxoid as protein carriers for meningococcal glycoconjugate vaccines. Vaccine. 2013;31:4827–4833. doi: 10.1016/j.vaccine.2013.07.078. [DOI] [PubMed] [Google Scholar]
- Wang CH, Li ST, Lin TL, Cheng YY, Sun TH, Wang JT, Cheng TJR, Mong Kwok Kong Tony, Wong CH, Wu CY. Synthesis of Neisseria meningitidis Serogroup W135 Capsular Oligosaccharides for Immunogenicity Comparison and Vaccine Development. Angew Chem Int Ed. 2013;52:9157–9161. doi: 10.1002/anie.201302540. [DOI] [PubMed] [Google Scholar]
- Weishaupt MW, Matthies S, Hurevich M, Pereira CL, Hahm HS, Seeberger PH. Automated glycan assembly of a S. pneumoniae serotype 3 CPS antigen. Beilstein J Org Chem. 2016;12:1440–1446. doi: 10.3762/bjoc.12.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witzenrath M, Gutbier B, Hocke AC, Schmeck B, Hippenstiel S, Berger K, Mitchell TJ, de los Toyos Juan R, Rosseau S, Suttorp N, et al. Role of pneumolysin for the development of acute lung injury in pneumococcal pneumonia. Crit Care Med. 2006;34:1947–1954. doi: 10.1097/01.CCM.0000220496.48295.A9. [DOI] [PubMed] [Google Scholar]
- Witzenrath M, Pache F, Lorenz D, Koppe U, Gutbier B, Tabeling C, Reppe K, Meixenberger K, Dorhoi A, Ma J, et al. The NLRP3 Inflammasome Is Differentially Activated by Pneumolysin Variants and Contributes to Host Defense in Pneumococcal Pneumonia. J Immunol. 2011;187:434–440. doi: 10.4049/jimmunol.1003143. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Pneumococcal conjugate vaccine for childhood immunization--WHO position paper. Wkly Epidemiol Rec. 2007;82:93–104. [PubMed] [Google Scholar]
- Yildirim I, Stevenson A, Hsu KK, Pelton SI. Evolving picture of invasive pneumococcal disease in massachusetts children: a comparison of disease in 2007–2009 with earlier periods. Pediatr Infect Dis J. 2012;31:1016–1021. doi: 10.1097/INF.0b013e3182615615. [DOI] [PubMed] [Google Scholar]
- Yother J. Capsules of Streptococcus pneumoniae and Other Bacteria: Paradigms for Polysaccharide Biosynthesis and Regulation. Annu Rev Microbiol. 2011;65:563–581. doi: 10.1146/annurev.micro.62.081307.162944. [DOI] [PubMed] [Google Scholar]
- Yu H, Ensley HE. An efficient method for the preparation of glycosides with a free C-2 hydroxyl group from thioglycosides. Tetrahedron Lett. 2003;44:9363–9366. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.