Abstract
Evaluate laser acupuncture as an adjuvant therapy in pain management during percutaneous kidney biopsy procedure in children and adolescents. This prospective, double-blinded, randomized controlled trial enrolled patients aged 7-26 years admitted to a children’s hospital for percutaneous kidney biopsy. Patients received laser acupuncture to treatment points (acupuncture group) or sham points (control group) prior to the procedure. Laser delivered a dose of 42 J/cm2 over 10 acupoints. Patients and parents rated the pain during and after the biopsy, and change in pain scores were calculated for each patient. Anxiety, vital signs, sedation medication, and patient’s biopsy experience were secondary outcomes. Sixty-nine treatments (33 acupuncture group, 36 control group) were eligible for analysis. Patients in the acupuncture group reported a significantly improved change in pain score after the biopsy compared to the controls (0.8 vs −0.5, ρ=0.044). Patients in the acupuncture group had a statistically significant decrease in procedure vital signs including heart rate (−1.8 vs 5.6, ρ=0.043) and respiratory rate (−2.4 vs 0.4, ρ=0.045) when compared to controls. Parents also perceived a correspondingly greater improvement in their child’s pain for those in the acupuncture group compared to the controls (2.3 vs 0.3, ρ=0.04). Adjunctive laser acupuncture significantly improved pain after pediatric percutaneous kidney biopsies.
Keywords: Acupuncture, Laser, Kidney Biopsy, Pediatrics
Introduction
Acupuncture has long been recognized as effective in treating pain [55,60]. The 1998 NIH consensus statement endorsed acupuncture for postoperative dental pain [1]; other groups have described its benefits in procedure pain treatment [18,21,38]. Laser acupuncture (LA) is a noninvasive, painless alternative to needles using low-level intensity light. There is evidence for use in myofacial pain [4,36] and numerous other conditions [4]. In children, LA reduces intensity and number of migraine headaches [23], decreases vomiting in post-operative settings [51], and reduces duration of morphine therapy and hospital stay in neonatal abstinence syndrome infants [44]. Several studies have shown laser acupuncture is as effective as traditional acupuncture using needles. When treating musculoskeletal pain, laser stimulation of acupoints is clinically equivalent, and potentially superior, to the same treatment points using needles [15]. Baxter et al. concluded in a systemic review that laser acupuncture was a viable alternative to other forms of acupuncture point stimulation, including needles [4]. The lack of needles and the safety profile of LA is appealing, especially in pediatrics. This atraumatic treatment reduces the risk of erythema or granuloma formation [41] and presents no bleeding risk [3]. Infection concerns (Hepatitis-B [29], HIV [58], abscess, and pathogen spread [41]) also favor laser [3].
Acupuncture is of particular interest in procedural medicine given the time-course of action. In studies as early as 1970, acupuncture has shown to reduce pain immediately and provide benefit for hours to days after treatment. This suggests a central mechanism of action rather than solely via local, neural inhibitory mechanisms [42,57]. Acupuncture is thought to modulate neural signals in central pain-pathways through diverse signaling molecules including opioid peptides, glutamate, endorphins, adenosine, dopamine, serotonin, and dynorphins [7,22,27,32,62]. Supporting a neuronal mediated mechanism of action, many acupressure points correspond to the location where small nerve bundles penetrate the fascia as well as nerve bundles that surround major blood vessels [7]. Autonomic nervous system stimulation as well as somatic signaling has been demonstrated, and the interplay between these systems may further help to facilitate acupuncture effects [7]. Acupoint signals conduct to the brain via the ventolateral funiculus to activate and deactivate different nuclei and regions [27,62]. Functional MRI studies demonstrate specific activations and deactivations in needle [61] and laser [52] treatments. Acupuncture analgesia is the manifestation of integrative processes at different levels of the central nervous system between afferent impulses of pain and signals from acupoints.
While kidney biopsies are integral in the management of nephrology patients, in pediatrics, this procedure carries high levels of anxiety in parents and patients. Sedation medication is used to address anxiety and pain, and non-pharmacologic adjuncts are desired to reduce medication dosage. The kidney biopsy procedure was chosen given high concern for pain, an overnight admission [33,56] to facilitate completion of post-procedure surveys, and well-defined acupoints associated with kidney energy [28,46].
The aim of our study was to determine if the use of LA as an adjuvant therapy with standard sedation medication would reduce patients’ overall pain during and after a kidney biopsy.
Methods
Design
This double-blinded, randomized controlled trial evaluated the effects of LA on pain levels in patients undergoing a kidney biopsy procedure.
Setting and Participants
This study enrolled patients aged 7-26 years admitted to University of California Benioff Children’s Hospital, San Francisco for a native or transplant kidney biopsy between September 2013 and April 2015. Sample size was determined by power calculation to detect a 1-point difference (9%) [30,43] on the 0-10 point Wong-Baker FACES™ Pain Rating Scale (WBFPRS), using a standard deviation of 1.5 [40,47]. With a power of 0.8 and an acceptable type I error size of 0.05, 38 patients per group was estimated to achieve significance. Patients requiring repeat biopsies during the study period were allowed to participate in the study with each subsequent biopsy. Patients were not approached for enrollment if an acupuncturist was not available (to ensure blinding) or if the biopsy was scheduled without the knowledge or availability of the study team. Patients were excluded from study enrollment if they were <7 years, were unable to use the Wong-Baker FACES™ Pain Rating Scale, or required general anesthesia.
Ethical Considerations
The study was approved by the Institutional Committee on Human Research. Written informed consent was obtained from patients or parents (as appropriate for age) along with assent for children aged 7-17 years. Registered on ClinicalTrials.gov as study NCT01879826.
Randomization
Patients were randomly allocated into the treatment group (TG) or control group (CG) using a 50/50 distribution, random number generator. TG patients received a LA session using treatment acupoints; CG patients received a LA session using different, sham points. The sequentially numbered, sealed envelopes containing the randomization were opened immediately prior to the acupuncture session and only viewed by the acupuncturist performing the treatment. Patients presenting for repeat biopsy were randomized without regard to their prior group for blinding integrity.
Blinding
The medical acupuncturist performed the LA session without any procedure staff present. Patient was positioned prone, and acupoints used were not visible to patient or parent. Laser emitted a light and sound that was identical for both groups. Patient then underwent a standard kidney biopsy protocol. Physician performing the acupuncture was not involved in the clinical treatment or pain/anxiety score assessments in any way. Clinical providers, patients, and parents were all blinded as to patient’s assigned group.
Protocol
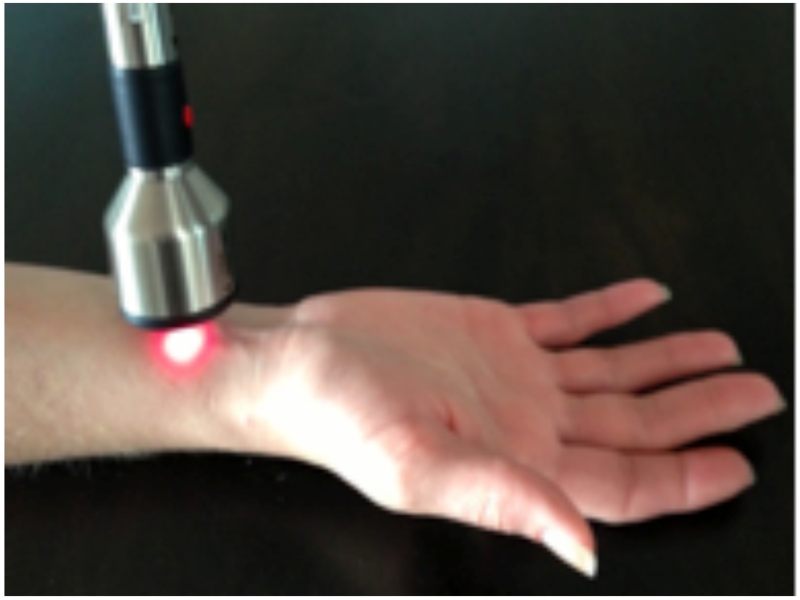
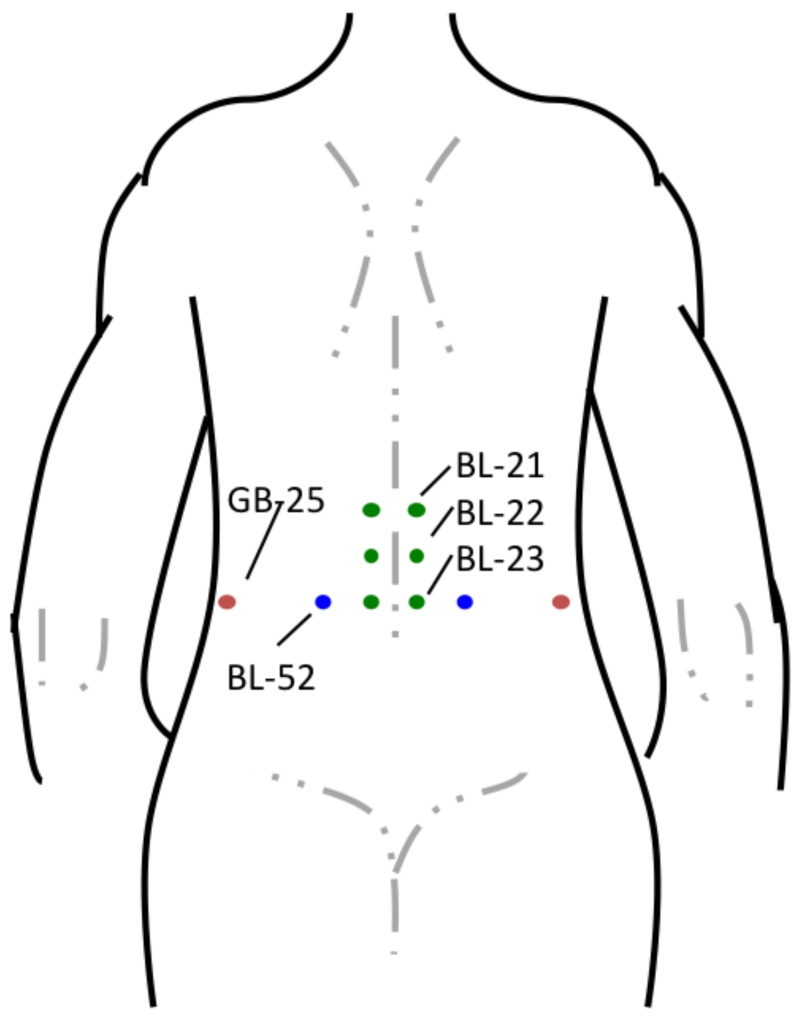
Five licensed, experienced, medical acupuncturists with over a decade of collective acupuncture experience performed one session of LA 15-30 minutes prior to the procedure. LA was delivered to all patients using a single, FDA-approved, class 3b, hand-held, Microlight ML830 Acupuncture Laser (Microlight Corporation of America, 2935 Highland Lakes Dr., Missouri City, TX) (Figure 1). The gallium-aluminum-arsenide laser, continuous wave mode (wavelength 830nm), delivers 3 separate, 1mm diameter, beams in a triangular arrangement. Pulse duration is 33s with 0.99J and 30mW of output per beam [26,37,49]. A visible red light was added to the beam for safety. All patients were offered protective goggles, and the laser emitted a beep when activated. Acupoints are described according to the 1989 Geneva Standard International Acupuncture Nomenclature [25], and LA was delivered to gall bladder (GB)-25, bladder (BL)-52, BL-23, BL-22, BL-21 acupoints in the TG (Figure 2). Patients in the sham group received LA to different points than the TG. Points 2 cuns (patient finger breadths) above GB-25, and 2 cuns lateral to BL-52, BL-23, BL-22, BL-21 were used as the corresponding sham points. Patients in both groups received 33s of active laser to each described point, for a total session length of 5 ½ min.
Figure 1.
Microlight ML830 Acupuncture Laser. Laser treatment prescription used wavelength 830nm, power output 90mW, power density 3.8 W/cm2, and dose 42 J/cm2.
Figure 2.
Location of Acupoints. Both groups received a LA session. TG patients had LA to treatment acupoints, noted above. For sham points, points 2 cuns (patient finger breadths) above GB-25, and 2 cuns lateral to BL-52, BL-23, BL-22, BL-21 were used as the corresponding points.
Kidney biopsies were preformed in the hospital treatment room; all patients underwent the same biopsy protocol regardless of group assignment. Patients were positioned prone or supine as appropriate for native or transplant biopsy respectively. The area was cleaned and draped in sterile fashion. Local anesthesia (1:1:0.2 mixture of lidocaine, bupivacaine, and sodium bicarbonate) was given subcutaneously and extending to the kidney capsule. A 3mm incision was made over the kidney, and using a spring loaded, 16-gauge needle with direct ultrasound guidance, core biopsies were taken. Additional cores were taken if more tissue was needed for clinical evaluation. A pediatric sedation attending was present throughout the procedure and prescribed pharmacologic sedation. Pressure was held to the site for at least 5 minutes following the procedure.
Data Collection
Heart rate, blood pressure, and respiratory rate were measured at baseline and every 15 minutes during the procedure. Sex, weight, age, number of samples, procedure and post-procedure medications, and hospital course were collected from the hospital chart. Patients and their parents were independently surveyed on the pain and anxiety scored with separate survey forms at 2 different time points. Fifteen minutes prior to the procedure, patients and parents independently assessed baseline-pain and baseline-anxiety scores on the pre-procedure form. Post-procedure forms were completed 15 minutes to 24 hours after the procedure to assess procedure and post-procedure pain, description of pain, post-procedure anxiety, overall procedure experience (“Better”, “Same”, or “Worse”), and desire for future laser (“Yes” or “No”). Survey forms used the WBFPRS to assess pain (“0-no hurt“ to “10-hurts worst”) and a visual analog scale to assess anxiety (“0-not at all” to “10-worst imaginable”). The WBFPRS has been validated in children [18,39,54,59] and was chosen given the simplicity, patient preference, and speed in assessing pain [10,39,54]. The anxiety scale [9,14] was chosen for consistency and given the same feasibility reasons. For further rigor, study design incorporated as many elements from the IMMPACT recommendations for chronic pain clinical trials as feasible [16-17].
Data Analysis
All variables were reported as mean with mean standard error or as numbers and percentages. Vital sign and pain/anxiety score changes were calculated as a difference in procedure to post-procedure values. Differences between the groups were tested using the Mann-Whitney U-test and Fisher’s exact test. A ρ-value of <0.05 was considered statistically significant. All statistical analyses were performed with Statistical Package for the Social Sciences 22 (IBM SPSS Statistics Inc., Chicago, IL).
Results
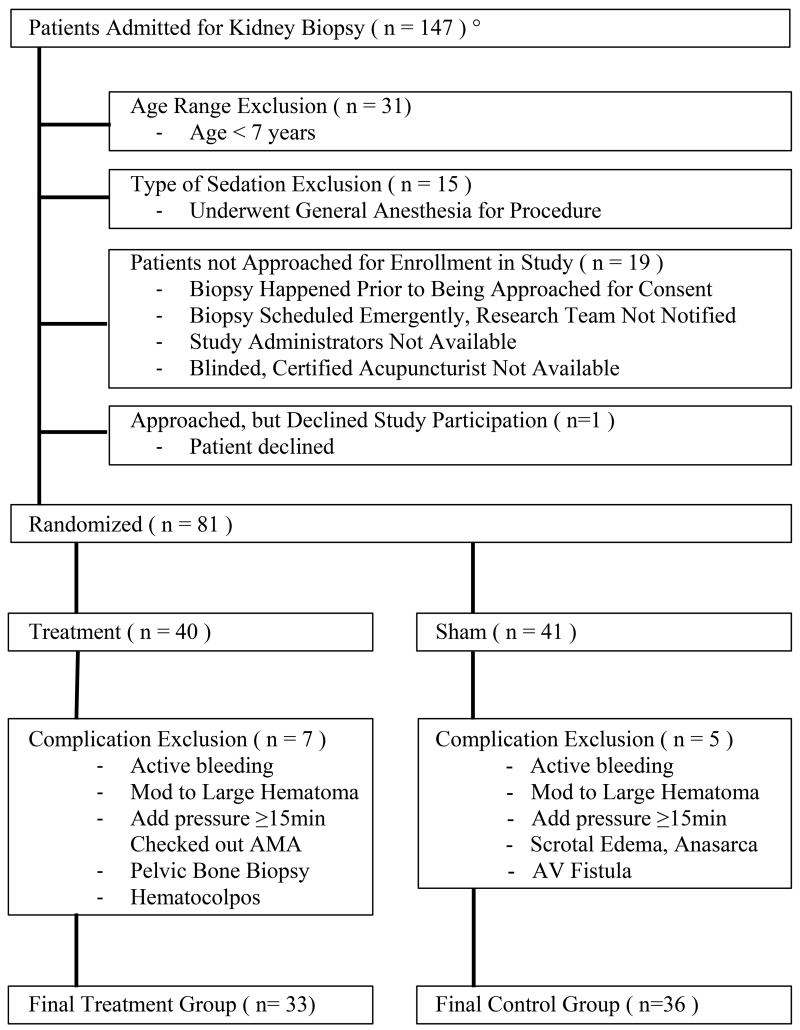
One hundred forty-seven kidney biopsies were performed during the study period, September 2013 to April 2015 (Figure 3). Sixty-six patients were not approached for enrollment due to age (n=31), general anesthesia (n=15), consent was not obtained (n=19), or declined to participate (n=1). Eleven patients had multiple biopsies. Eighty-one patient encounters were randomized into two groups, with 7 excluded from the TG and 5 from the CG because of post-procedure events. These events occurred as a complication of the procedure or later in the hospitalization. Given the potential to confound the assessments of pain and anxiety, they were excluded from the analysis. Biopsy complications necessitating exclusion included active bleeding, large hematoma (hematoma >10cm in one dimension), arterial venous fistula, pressure >15 minutes, enlarging hematoma, or involvement of the pelvic bone. One patient with recurrent hematocolpos pain, one with scrotal edema and anasarca, and one who left against medical advice were excluded. Our procedure complication rate was similar to published rates [33].
Figure 3.
Biopsy Patient Flow Chart. °Study Period from September 01, 2013 – April 30, 2015 (period of 20 months).
Sixty-nine patient encounters were included in the final analysis, 33 in TG and 36 in CG. Baseline characteristics were similar between the groups regarding age, sex, weight, baseline-anxiety scores, and prior biopsies (Table 1). Baseline-pain was significantly higher in the CG with a score of 0.9 (0.3) than in the TG with a score of 0.2 (0.1), ρ=0.046. Both groups shared a similar distribution of native and transplant biopsies, presence of parents and/or a child life specialist, and received similar medication with fentanyl, midazolam, and/or ketamine. Child life specialists are professionally certified and trained in working with children in a medical setting. Their participation, as available, is part of standard of care for procedures, like kidney biopsies, at our institution.
Table 1.
Characteristics of Patients and Biopsies. Numbers expressed as mean with standard error of the mean or as number with percentages.
| Treatment Group |
Sham Group |
ρ value | |
|---|---|---|---|
|
|
|||
| Total Patient Encounters: | n=33, (47.8%) | n=36, (52.2%) | |
| Patient Characteristics: | |||
| Mean age, years | 16.1 (0.6) | 14.9 (0.8) | 0.224 |
| Female | n=17, (47.2%) | n=14, (42.4%) | 0.810 |
| Mean Weight, kg | 63.5 (4.1) | 57.3 (3.4) | 0.250 |
| Mean Number of Prior Biopsies | 1.67 (0.32) | 1.81 (0.34) | 0.768 |
| Mean Baseline-Pain Score | 0.2 (0.1) | 0.9 (0.3) | 0.046* |
| Mean Baseline-Anxiety Score | 2.5 (0.5) | 2.6 (0.4) | 0.846 |
| Biopsy Characteristics: | |||
| Transplant Biopsy | n=22, (66.7% | n=26, (72.2%) | 0.794 |
| Parent in Room | n=19, (57.6%) | n=21, (58.3%) | 1.000 |
| Child Life in Room | n=24, (42.9%) | n=32, (57.1%) | 0.203 |
| Mean Number of Biopsy Passes | 2.4 (0.1) | 2.2 (0.1) | 0.285 |
| Mean Fentanyl Given, mg/kg | 0.95 (0.1) | 0.91 (0.08) | 0.788 |
| Mean Midazolam Given, mg/kg | 0.03 (0.01) | 0.03 (0.01) | 0.468 |
| Mean Ketamine Given, mg/kg | 1.33 (0.19) | 1.34 (0.08) | 0.985 |
| Acetaminophen Used Post Biopsy | n=4, (12.1%) | n=4, (11.4%) | 1.000 |
Statistical difference between TG and CG.
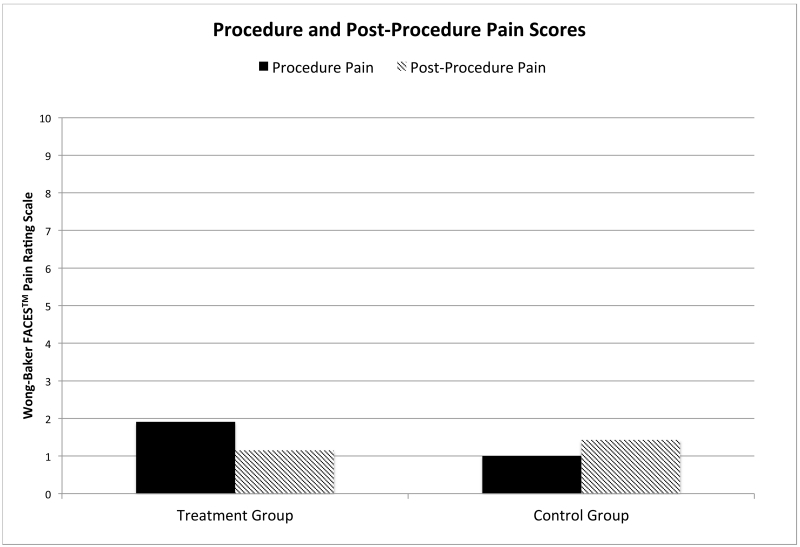
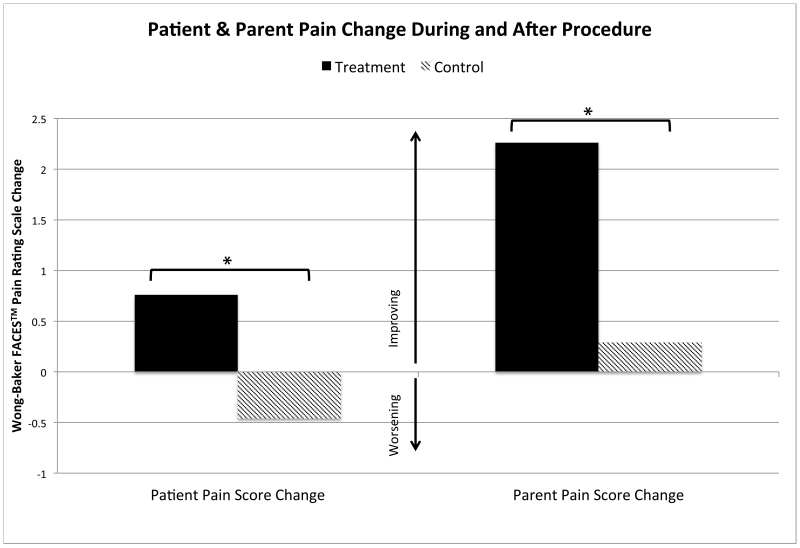
Procedure (ρ=0.078) and post-procedural pain (ρ=0.946) scores were similar in both groups with the TG reporting a pain score of 1.9 (0.41) and 1.2 (0.2), respectively and the CG reporting a pain score of 1.0 (0.23) and 1.4 (0.35), respectively (Figure 4). Both groups used a similar amount of acetaminophen post-procedure, with 12.1% (n=4) of those in the TG receiving one dose and 11.4% (n=4) in the CG receiving one dose, ρ=1.0. Neither group received more than one dose of acetaminophen, nor did any patient use an opioid pain medication post-procedure. TG patients reported a significant improvement in pain score change from procedure to post-procedure when compared to the CG (Figure 5). TG patients reported an improvement in pain scores of 0.8 (0.4), and CG patients reported a worsening of pain scores of −0.5 (0.5), ρ=0.044. This was mirrored in the parents’ perceptions of the patients’ pain. Parents of patients in the TG reported pain improvement with a pain score change of 2.3 (0.8), vs the CG with a pain score change of 0.3 (0.5), ρ=0.04. Patients reported a degree of improvement in their pain of 1.2 points on the pain scale (11%) in the TG vs the CG; parents reported an even larger improvement of 2.0 points on the pain scale (18%).
Figure 4.
Patient Pain Scores During Procedure and Post-Procedure. Mean pain scores rating procedure and post-pocedure pain on a scale of 0 to 10. There was no statistical difference seen between the groups comparing patient reported procedure or post-procedure pain scores.
Figure 5.
Mean Pain Score Change During Procedure Compared to Post-Procedure. Pain change was calculated as a difference in procedure pain score as compared to post-procedure pain score. Improving pain after the procedure is reflected as a positive value. *Statistically significant with ρ value < 0.05.
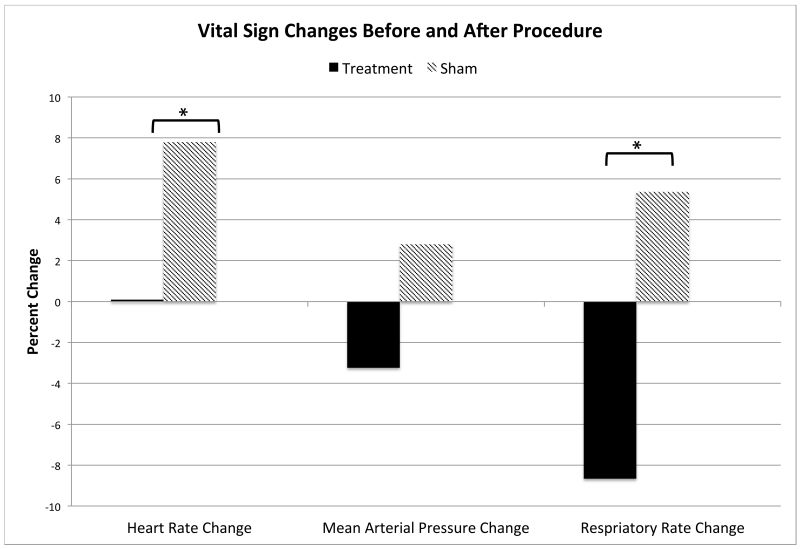
Patients were observed to have a significant decrease in intra-procedure vital signs including heart rate change of −1.8 (2.7) vs 5.6 (2.4), ρ=0.043 and respiratory rate change of −2.4 (1.1) vs 0.4 (0.9), ρ=0.045 when comparing TG vs CG. Expressed as a percent change, the heart rate change was 0.1% vs 7.8% and the respiratory rate change was −8.7% vs 5.4%, respectively (Figure 6). The mean arterial pressure change of −3.4 (2.2) vs 2.0 (2.6) in the TG vs CG was not significant, ρ=0.118.
Figure 6.
Mean Vital Sign Changes Prior to Procedure and Following Procedure. Vital sign changes were calculated as a difference in initial procedure vital signs as compared to post-procedure vital signs. Values reported as a percent change. *Statistically significant with ρ value < 0.05.
When comparing TG vs CG, no significant difference in patient reported anxiety score changes from before or after the procedure −1.8 (0.5) vs −2.0 (0.5), ρ = 0.682 or in parent reported perception of score changes −0.9 (0.4) vs −1.1 (0.4), ρ = 0.812 were seen. Regardless of TG vs CG, the majority of patients and their parents desired future laser treatments (90.6% vs 94.3%, ρ=0.292; 93.5% vs 87.9%, ρ=0.625), reported the same or better pain compared to past biopsies (100% vs 95%, ρ=0.697; 86.6% vs 95.7%, ρ=0.488), and thought the patient had received treatment LA (84.4% vs 90.0%, ρ=0.709; 92.9% vs 96.4%, ρ=1.0).
All patients tolerated the LA treatments well without any clinically visible changes to the skin, nor was there any patient distress or negative feedback reported during their hospital course related to the LA.
Discussion
To our knowledge, this is the first blinded RCT of procedure pain management with LA in adults or children, and one of the first trials of LA for acute procedure pain. This study showed a clear improvement in self-reported pain scores in the TG vs CG. We believe this reduction in pain is of clinical significance given the degree in pain improvement [30,43], as well as the accompanying vital sign changes, the corresponding parental perceptions of pain improvement, and the overwhelmingly positive experience ratings. The improvements in pain were of a moderate effect size reported by both patients (d=0.50) and parents (d=0.63) [13]. The finding of similar anxiety levels between the groups suggests that LA had a specific effect on pain.
The technical specifications of the LA prescription are important when evaluating efficacy [26,37]. A systematic review of 18 LA studies, noted only 2 of the 5 high quality studies used clinically appropriate parameters of at least 10mW and 0.5 J per point [4]. In a review of placebo-controlled trials, Bjordal et al. recommends that >5 J is needed to treat acute pain [6]. Our laser treatment parameters followed the mean output recommendation from the World Association of Laser Therapy [34]. A study of blood flow velocity in the ophthalmic artery showed that a threshold of 1.3 W/cm2 power density was important to stimulate effects [37]. It is worth noting that the power density needed to reach an acupoint in the back is likely higher than for the ophthalmic artery, although the exact difference is unknown. Our study’s laser prescription (wavelength 830nm, power output 90mW, power density 3.8 W/cm2, and dose 42 J/cm2) met the highest standard of technical parameters to affect the desired outcomes while balancing safety. Similar device technical parameters have shown to improve headache pain in children [23]. Given safety concerns, the calculated energy density [49] of this study’s treatment was 0.042 kJ/cm2, much less than the 2.3-4.6 kJ/cm2 energy density treatments showing no adverse skin effects [34].
Acupoint Selection
For patients with nephrolithiasis, Resim et al. used acupoints BL-20, BL-21, BL-22, BL-23, and BL-52 to relieve pain during shockwave lithotripsy [46]. For the same procedure, Rogenhofer et al. used acupoints BL-23, BL-53, Large Intestine (LI)-4, Governor Vessel (GV)-20, Spleen (SP)-6, and Kidney (KI)-3 [48]. Recently, a study comparing diclofenac, acupuncture, and acetaminophen on the treatment of renal colic, used acupoints BL-21, BL-22, BL-23, BL-24, BL-45, BL-46, BL-47, and BL-48 [28]. Of note, none of these studies utilized LA. Our study used similar points with the addition of GB-25 in hopes of reducing pain during kidney biopsies. This acupuncture point, GB-25, is used to target local lumbar pain specific to the kidney, and it works well with the other acupuncture points to complete the kidney meridian. While there is some debate in the literature as to appropriate CG treatment for LA studies, we chose to use sham points with an active laser treatment for the CG. This enabled us to maintain blinding as both groups experienced the laser’s light and sound activation. The sham points used in this study are unrecognized in the acupuncture field as being related to the kidney meridian or pain. While the laser used in this study operated a cluster of 3 diodes, care was taken to ensure there was no overlap of the diode target area between the treatment and sham points. There is no literature comparing the use of single vs cluster diodes for the treatment of kidney meridian pain.
Procedure and Acute Pain
There is limited data on LA for the treatment of acute pain. For heel lancing pain in infants, LA was shown to be less effective than oral sucrose [2]. However, this study had numerous limitations including using only one acupoint, no comparison as an adjunct pain tool in the control group, and the low 0.3 J dose per point. The timing of the treatment, <2 minutes prior to the procedure, also may have affected the results. Two different studies compared acute neck pain from undefined causes and from whiplash respectively [12,53], with improvement in relative risk of pain when comparing LA to placebo. Soriano et al. reported an improvement in pain in the laser group in both the acute treatment period and reduction in pain recurrence [53]. Although in the whiplash study, no difference was seen in mobility or cervical collar duration. Neither study used LA prophylactically. Fleckenstein et al. showed a reduction in pain using LA for tonsillitis and pharyngitis [19], however there was similar pain improvement in both the treatment and control groups. In that study, the sham acupuncture was done to the same acupoints (instead of to sham points). The palpation of the acupoints during this process may have resulted in a similar result for both groups. Again, LA was not used prophylactically. We believe our study provides additional insight into the therapeutic uses of LA in the management of procedure and acute pain.
Limitations
Three limitations of this study warrant discussion. First, despite randomization, there was a statistical difference in baseline-pain between the groups, with CG reporting higher baseline-pain than TG. We did not have detail on the acute or chronic sources of this baseline-pain, nor did we have access to any comorbid factors such as depression or anxiety. However, there was no correlation seen between baseline-pain and the post-procedure pain scores (r = 0.095). Using baseline-pain as a covariant in the analysis similarly shows it was not a significant factor (ρ = 0.743). Adjusted pain scores in the TG vs CG remain meaningfully unchanged when using baseline-pain as a covariant. Although chronic pain, depression, and anxiety were not used in the statistical analysis for the above reasons, it is well understood that these factors all have a role in modulating pain [8,24,50]. Second, given patients and parents evaluated their procedure pain in the post-procedure survey, and not at the biopsy, their response for procedure pain was subject to recall bias. The timing of post-procedure surveys also varied (15 minutes post-procedure to the following day) as the patients were not always available at a standard time. Future studies are needed to evaluate the time-course of pain relief from LA. Third, the modest sample size may limit the generalizability of these findings.
Our study strengths are also worth emphasizing, including a rigorous design with strict randomization and concealment of the intervention or control conditions from patients, parents, and providers.
Conclusions
Patients, especially the vulnerable pediatric population, undergo many painful procedures ranging from venipunctures to lumbar punctures and biopsies. Many different, non-invasive, non-pharmacologic interventions have been used successfully to ameliorate pain during these procedures [11,35]. Our findings from this trial suggest that LA is also an effective therapeutic adjuvant to reduce pain. LA is inexpensive and portable to multiple clinical settings with a negligible risk profile when thoughtful treatment energy dosages and acupoints are chosen.
Supplementary Material
Summary.
This first, randomized controlled trial of laser acupuncture in procedural pain management for kidney biopsies suggests it can reduce pain and improve the procedure experience.
Acknowledgements
The authors wish to thank Barbara Schrader and Michael Acree for their assistance with initial data analysis. The study was in part funded by University of California, San Francisco, and the NIH training grant, T32 DK007219.
Abbreviations
- LA
Laser Acupuncture
- WBFPRS
Wong-Baker FACES™ Pain Rating Scale
- TG
Treatment Group
- CG
Control Group
Footnotes
Financial Disclosure: The authors have no financial relationships relevant to the article to disclose.
Conflicts of Interest: The authors have no conflicts of interest relevant to this article to disclose.
Clinical Trial Registry: Registered on ClinicalTrials.gov. Pediatric Laser Acupuncture and Renal Biopsy; NCT01879826. https://clinicaltrials.gov/ct2/show/NCT01879826
References
- 1.NIH Consensus Conference. Acupuncture. JAMA. 1998 Nov 4;280(17):1518–24. [No authors listed] [PubMed] [Google Scholar]
- 2.Abbasoglu A, Cabioglu MT, Tugcu AU, Yapakci E, Tekindal MA, Tarcan A. Laser acupuncture before heel lancing for pain management in healthy term newborns: a randomised controlled trial. Acupunct Med. 2015 Dec;33(6):445–50. doi: 10.1136/acupmed-2015-010765. [DOI] [PubMed] [Google Scholar]
- 3.Adams D, Cheng F, Jou H, Aung S, Yasui Y, Vohra S. The safety of pediatric acupuncture: a systematic review. Pediatrics. 2011 Dec;128(6):e1575–87. doi: 10.1542/peds.2011-1091. [DOI] [PubMed] [Google Scholar]
- 4.Baxter GD, Bleakley C, McDonough S. Clinical effectiveness of laser acupuncture: a systematic review. J Acupunct Meridian Stud. 2008 Dec;1(2):65–82. doi: 10.1016/S2005-2901(09)60026-1. [DOI] [PubMed] [Google Scholar]
- 5.Bjordal JM. Low level laser therapy (LLLT) and World Association for Laser Therapy (WALT) dosage recommendations. Photomed Laser Surg. 2012 Feb;30(2):61–2. doi: 10.1089/pho.2012.9893. [DOI] [PubMed] [Google Scholar]
- 6.Bjordal JM, Johnson MI, Iversen V, Aimbire F, Lopes-Martins RA. Low-level laser therapy in acute pain: a systematic review of possible mechanisms of action and clinical effects in randomized placebo-controlled trials. Photomed Laser Surg. 2006 Apr;24(2):158–68. doi: 10.1089/pho.2006.24.158. [DOI] [PubMed] [Google Scholar]
- 7.Bowsher David, Filshie Jacqueline, White Adrian. Medical Acupuncture: A Western Scientific Approach. Churchill Livingstone; London: 1998. Mechanisms of Acupuncture; pp. 69–82. Chapter 6. [Google Scholar]
- 8.Bras M, Dordević V, Gregurek R, Bulajić M. Neurobiological and clinical relationship between psychiatric disorders and chronic pain. Psychiatr Danub. 2010 Jun;22(2):221–6. [PubMed] [Google Scholar]
- 9.Bringuier S, Dadure C, Raux O, Dubois A, Picot MC, Capdevila X. The perioperative validity of the visual analog anxiety scale in children: a discriminant and useful instrument in routine clinical practice to optimize postoperative pain management. Anesth Analg. 2009 Sep;109(3):737–44. doi: 10.1213/ane.0b013e3181af00e4. [DOI] [PubMed] [Google Scholar]
- 10.Chambers CT, Giesbrecht K, Craig KD, Bennett SM, Huntsman E. A comparison of faces scales for the measurement of pediatric pain: children’s and parents’ ratings. Pain. 1999 Oct;83(1):25–35. doi: 10.1016/s0304-3959(99)00086-x. [DOI] [PubMed] [Google Scholar]
- 11.Chen E, Joseph MH, Zeltzer LK. Behavioral and cognitive interventions in the treatment of pain in children. Pediatr Clin North Am. 2000 Jun;47(3):513–25. doi: 10.1016/s0031-3955(05)70223-6. [DOI] [PubMed] [Google Scholar]
- 12.Chow RT, Johnson MI, Lopes-Martins RA, Bjordal JM. Efficacy of low-level laser therapy in the management of neck pain: a systematic review and meta-analysis of randomised placebo or active-treatment controlled trials. Lancet. 2009 Dec 5;374(9705):1897–908. doi: 10.1016/S0140-6736(09)61522-1. [DOI] [PubMed] [Google Scholar]
- 13.Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Lawrence Earlbaum Associates; Hillsdale, NJ: 1988. [Google Scholar]
- 14.Crandall M, Lammers C, Senders C, Savedra M, Braun JV. Initial validation of a numeric zero to ten scale to measure children’s state anxiety. Anesth Analg. 2007 Nov;105(5):1250–3. doi: 10.1213/01.ane.0000284700.59088.8b. [DOI] [PubMed] [Google Scholar]
- 15.Dorsher PT. Clinical Equivalence of Laser Needle to Metal Acupuncture Needle in Treating Musculoskeletal Pain: A Pilot Study. Medical Acupuncture. 2010 Mar;22(1):11–17. [Google Scholar]
- 16.Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP, Kerns RD, Stucki G, Allen RR, Bellamy N, Carr DB, Chandler J, Cowan P, Dionne R, Galer BS, Hertz S, Jadad AR, Kramer LD, Manning DC, Martin S, McCormick CG, McDermott MP, McGrath P, Quessy S, Rappaport BA, Robbins W, Robinson JP, Rothman M, Royal MA, Simon L, Stauffer JW, Stein W, Tollett J, Wernicke J, Witter J. IMMPACT. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005 Jan;113(1-2):9–19. doi: 10.1016/j.pain.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 17.Dworkin RH, Turk DC, McDermott MP, Peirce-Sandner S, Burke LB, Cowan P, Farrar JT, Hertz S, Raja SN, Rappaport BA, Rauschkolb C, Sampaio C. Interpreting the clinical importance of group differences in chronic pain clinical trials: IMMPACT recommendations. Pain. 2009 Dec;146(3):238–44. doi: 10.1016/j.pain.2009.08.019. [DOI] [PubMed] [Google Scholar]
- 18.Ecevit A, Ince DA, Tarcan A, Cabioglu MT, Kurt A. Acupuncture in preterm babies during minor painful procedures. J Tradit Chin Med. 2011 Dec;31(4):308–10. doi: 10.1016/s0254-6272(12)60009-0. [DOI] [PubMed] [Google Scholar]
- 19.Fleckenstein J, Lill C, Lüdtke R, Gleditsch J, Rasp G, Irnich D. A single point acupuncture treatment at large intestine meridian: a randomized controlled trial in acute tonsillitis and pharyngitis. Clin J Pain. 2009 Sep;25(7):624–31. doi: 10.1097/AJP.0b013e3181a49e35. [DOI] [PubMed] [Google Scholar]
- 20.Garra G, Singer AJ, Taira BR, Chohan J, Cardoz H, Chisena E, Thode HC., Jr. Validation of the Wong-Baker FACES Pain Rating Scale in pediatric emergency department patients. Acad Emerg Med. 2010 Jan;17(1):50–4. doi: 10.1111/j.1553-2712.2009.00620.x. [DOI] [PubMed] [Google Scholar]
- 21.Gilbey P, Bretler S, Avraham Y, Sharabi-Nov A, Ibrgimov S, Luder A. Acupuncture for posttonsillectomy pain in children: a randomized, controlled study. Paediatr Anaesth. 2015 Jun;25(6):603–9. doi: 10.1111/pan.12621. [DOI] [PubMed] [Google Scholar]
- 22.Goldman N, Chen M, Fujita T, Xu Q, Peng W, Liu W, Jensen TK, Pei Y, Wang F, Han X, Chen JF, Schnermann J, Takano T, Bekar L, Tieu K, Nedergaard M. Adenosine A1 receptors mediate local anti-nociceptive effects of acupuncture. Nat Neurosci. 2010 Jul;13(7):883–8. doi: 10.1038/nn.2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gottschling S, Meyer S, Gribova I, Distler L, Berrang J, Gortner L, Graf N, Shamdeen MG. Laser acupuncture in children with headache: a double-blind, randomized, bicenter, placebo-controlled trial. Pain. 2008 Jul 15;137(2):405–12. doi: 10.1016/j.pain.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 24.Han C, Pae CU. Pain and depression: a neurobiological perspective of their relationship. Psychiatry Investig. 2015 Jan;12(1):1–8. doi: 10.4306/pi.2015.12.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jenkins M. A new standard international Acupuncture Nomenclature. Acupunct Med. 1990;7(1):21–22. [Google Scholar]
- 26.Jenkins PA, Carroll JD. How to report low-level laser therapy (LLLT)/photomedicine dose and beam parameters in clinical and laboratory studies. Photomed Laser Surg. 2011 Dec;29(12):785–7. doi: 10.1089/pho.2011.9895. [DOI] [PubMed] [Google Scholar]
- 27.Kawakita K, Okada K. Acupuncture therapy: mechanism of action, efficacy, and safety: a potential intervention for psychogenic disorders? Biopsychosoc Med. 2014 Jan 20;8(1):4. doi: 10.1186/1751-0759-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaynar M, Koyuncu F, Buldu I, Tekinarslan E, Tepeler A, Karatag T, Kstnbulluoglu MO, Ceylan K. Comparison of efficacy of diclofenac, acupuncture, and acetaminophen in the treatment of renal colic. Am J Emerg Med. 2015 Jun;33(6):749–53. doi: 10.1016/j.ajem.2015.02.033. [DOI] [PubMed] [Google Scholar]
- 29.Kent GP, Brondum J, Keenlyside RA, LaFazia LM, Scott HD. A large outbreak of acupuncture-associated hepatitis B. Am J Epidemiol. 1988 Mar;127(3):591–8. doi: 10.1093/oxfordjournals.aje.a114834. [DOI] [PubMed] [Google Scholar]
- 30.Kelly AM. Does the clinically significant difference in visual analog scale pain scores vary with gender, age, or cause of pain? Acad Emerg Med. 1998 Nov;5(11):1086–90. doi: 10.1111/j.1553-2712.1998.tb02667.x. [DOI] [PubMed] [Google Scholar]
- 31.Khin Hla T, Hegarty M, Russell P, Drake-Brockman TF, Ramgolam A, von Ungern-Sternberg BS. Perception of pediatric pain: a comparison of postoperative pain assessments between child, parent, nurse, and independent observer. Paediatr Anaesth. 2014 Nov;24(11):1127–31. doi: 10.1111/pan.12484. [DOI] [PubMed] [Google Scholar]
- 32.Kong JT, Schnyer RN, Johnson KA, Mackey S. Understanding central mechanisms of acupuncture analgesia using dynamic quantitative sensory testing: a review. Evid Based Complement Alternat Med. 2013;2013:187182. doi: 10.1155/2013/187182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Korbet SM, Volpini KC, Whittier WL. Percutaneous renal biopsy of native kidneys: a single-center experience of 1,055 biopsies. Am J Nephrol. 2014;39(2):153–62. doi: 10.1159/000358334. [DOI] [PubMed] [Google Scholar]
- 34.Kurath-Koller S, Litscher G, Gross A1, Freidl T, Koestenberger M, Urlesberger B, Raith W. Changes of locoregional skin temperature in neonates undergoing laser needle acupuncture at the acupuncture point large intestine 4. Evid Based Complement Alternat Med. 2015;2015:571857. doi: 10.1155/2015/571857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Landier W, Tse AM. Use of complementary and alternative medical interventions for the management of procedure-related pain, anxiety, and distress in pediatric oncology: an integrative review. J Pediatr Nurs. 2010 Dec;25(6):566–79. doi: 10.1016/j.pedn.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Law D, McDonough S, Bleakley C, Baxter GD, Tumilty S. Laser acupuncture for treating musculoskeletal pain: a systematic review with meta-analysis. J Acupunct Meridian Stud. 2015 Feb;8(1):2–16. doi: 10.1016/j.jams.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 37.Litscher G, Opitz G. Technical Parameters for Laser Acupuncture to Elicit Peripheral and Central Effects: State-of-the-Art and Short Guidelines Based on Results from the Medical University of Graz, the German Academy of Acupuncture, and the Scientific Literature. Evid Based Complement Alternat Med. 2012;2012:697096. doi: 10.1155/2012/697096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu XL, Tan JY, Molassiotis A, Suen LK, Shi Y. Acupuncture-Point Stimulation for Postoperative Pain Control: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Evid Based Complement Alternat Med. 2015;2015:657809. doi: 10.1155/2015/657809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luffy R, Grove SK. Examining the validity, reliability, and preference of three pediatric pain measurement tools in African-American children. Pediatr Nurs. 2003 Jan-Feb;29(1):54–9. [PubMed] [Google Scholar]
- 40.Moadad N, Kozman K, Shahine R, Ohanian S, Badr LK. Distraction Using the BUZZY for Children During an IV Insertion. J Pediatr Nurs. 2016 Jan-Feb;31(1):64–72. doi: 10.1016/j.pedn.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 41.Park SM, Kim WJ, Mun JH, Kim HS, Ko HC, Kim BS, Kim MB, Song M. Adverse events associated with acupuncture: a clinicopathologic review. Int J Dermatol. 2015 Sep 4; doi: 10.1111/ijd.12914. [DOI] [PubMed] [Google Scholar]
- 42.Pomeranz B. Brain’s Opiates at work in acupuncture? New Sci. 1997 Jan 6;:12–13. [Google Scholar]
- 43.Powell CV, Kelly AM, Williams A. Determining the minimum clinically significant difference in visual analog pain score for children. Ann Emerg Med. 2001 Jan;37(1):28–31. doi: 10.1067/mem.2001.111517. [DOI] [PubMed] [Google Scholar]
- 44.Raith W, Schmölzer GM, Resch B, Reiterer F, Avian A, Koestenberger M, Urlesberger B. Laser Acupuncture for Neonatal Abstinence Syndrome: A Randomized Controlled Trial. Pediatrics. 2015 Nov;136(5):876–84. doi: 10.1542/peds.2015-0676. [DOI] [PubMed] [Google Scholar]
- 45.Rajasagaram U, Taylor DM, Braitberg G, Pearsell JP, Capp BA. Paediatric pain assessment: differences between triage nurse, child and parent. J Paediatr Child Health. 2009 Apr;45(4):199–203. doi: 10.1111/j.1440-1754.2008.01454.x. [DOI] [PubMed] [Google Scholar]
- 46.Resim S, Gumusala Y, Ekerbicer HC, Sahim MA, Sahinkanat T. Effectiveness of electro-acupuncture compared to sedo-analgesics in relieving pain during shockwave lithotripsy. Urol Res. 2005;33:285–290. doi: 10.1007/s00240-005-0473-7. [DOI] [PubMed] [Google Scholar]
- 47.Reza N, Ali S, Saeed K, Abul-Qasim A, Reza TH. The impact of music on postoperative pain and anxiety following cesarean section. Middle East J Anaesthesiol. 2007 Oct;19(3):573–86. [PubMed] [Google Scholar]
- 48.Rogenhofer S, Wimmer K, Blana A, Roessler W, Wieland WF, Filbeck T. Acupuncture for pain in extracorporeal shockwave lithotripsy. J Endourol. 2004 Sep;18(7):634–7. doi: 10.1089/end.2004.18.634. [DOI] [PubMed] [Google Scholar]
- 49.Round R, Litscher G, Bahr F. Auricular acupuncture with laser. Evid Based Complement Alternat Med. 2013;2013:984763. doi: 10.1155/2013/984763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Salama-Hanna J, Chen G. Patients with chronic pain. Med Clin North Am. 2013 Nov;97(6):1201–15. doi: 10.1016/j.mcna.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 51.Schlager A, Offer T, Baldissera I. Laser stimulation of acupuncture point P6 reduces postoperative vomiting in children undergoing strabismus surgery. Br J Anaesth. 1998 Oct;81(4):529–32. doi: 10.1093/bja/81.4.529. [DOI] [PubMed] [Google Scholar]
- 52.Siedentopf CM, Golaszewski SM, Mottaghy FM, Ruff CC, Felber S, Schlager A. Functional magnetic resonance imaging detects activation of the visual association cortex during laser acupuncture of the foot in humans. Neurosci Lett. 2002 Jul 12;327(1):53–6. doi: 10.1016/s0304-3940(02)00383-x. [DOI] [PubMed] [Google Scholar]
- 53.Soriano FA, Rios R, Pedrola M, Giagnorio J, Battagliotti CR. Acute cervical pain is relieved with Gallium Arsenide (GaAs) laser radiation. A double blind preliminary study. Laser Therapy. 1996;8:149–154. [Google Scholar]
- 54.Tomlinson D, von Baeyer CL, Stinson JN, Sung L. A systematic review of faces scales for the self-report of pain intensity in children. Pediatrics. 2010 Nov;126(5):e1168–98. doi: 10.1542/peds.2010-1609. [DOI] [PubMed] [Google Scholar]
- 55.Vickers AJ, Cronin AM, Maschino AC, Lewith G, MacPherson H, Foster NE, Sherman KJ, Witt CM, Linde K. Acupuncture Trialists’ Collaboration. Acupuncture for chronic pain: individual patient data meta-analysis. Arch Intern Med. 2012 Oct 22;172(19):1444–53. doi: 10.1001/archinternmed.2012.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Whittier WL, Korbet SM. Renal biopsy: update. Curr Opin Nephrol Hypertens. 2004 Nov;13(6):661–5. doi: 10.1097/00041552-200411000-00013. [DOI] [PubMed] [Google Scholar]
- 57.Wilkinson J, Faleiro R. Acupuncture in pain management. Contin Educ Anaesth Crit Care Pain. 2007;7(4):135–138. [Google Scholar]
- 58.Wiwanitkit V. HIV infection after Chinese traditional acupuncture treatment. Complement Ther Med. 2003 Dec;11(4):272. doi: 10.1016/S0965-2299(03)00141-9. [DOI] [PubMed] [Google Scholar]
- 59.Wong DL, Baker CM. Pain in children: comparison of assessment scales. Pediatr Nurs. 1988;14(1):9–17. [PubMed] [Google Scholar]
- 60.Woodbury A, Soong SN, Fishman D, García PS. Complementary and alternative medicine therapies for the anesthesiologist and pain practitioner: a narrative review. Can J Anaesth. 2016;63:69–85. doi: 10.1007/s12630-015-0506-9. [DOI] [PubMed] [Google Scholar]
- 61.Yan B, Li K, Xu J, Wang W, Li K, Liu H, Shan B, Tang X. Acupoint-specific fMRI patterns in human brain. Neurosci Lett. 2005 Aug 5;383(3):236–40. doi: 10.1016/j.neulet.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 62.Zhao ZQ. Neural mechanism underlying acupuncture analgesia. Prog Neurobiol. 2008 Aug;85(4):355–75. doi: 10.1016/j.pneurobio.2008.05.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.