Abstract
Background
Geographic variations in cardiovascular mortality are substantial, but descriptions of geographic variations in major cardiovascular risk factors have relied on data aggregated to counties. Herein, we provide the first description of geographic variation in the prevalence of hypertension, diabetes, and smoking within and across US counties.
Methods and Results
We conducted a cross-sectional analysis of baseline risk factor measurements and latitude/longitude of participant residence collected from 2003 to 2007 in the REasons for Geographic And Racial Differences in Stroke (REGARDS) study. Of the 30,239 participants, all risk factor measurements and location data were available for 28,887 (96%). The mean (±SD) age of these participants was 64.8(±9.4) years, 41% were black, 55% were female, 59% were hypertensive, 22% were diabetic, and 15% were current smokers. In logistic regression models stratified by race, the median(range) predicted prevalence of the risk factors were: for hypertension, 49% (45 - 58%) among whites and 72% (68 - 78%) among blacks; for diabetes, 14% (10 - 20%) among whites and 31% (28 - 41%) among blacks; and for current smoking, 12% (7 - 16%) among whites and 18% (11 - 22%) among blacks. Hypertension was most prevalent in the central Southeast among whites, but in the west Southeast among blacks. Diabetes was most prevalent in the west and central Southeast among whites but in south Florida among blacks. Current smoking was most prevalent in the west Southeast and Midwest among whites and in the north among blacks.
Conclusions
Geographic disparities in prevalent hypertension, diabetes, and smoking exist within states and within counties in the continental US, and the patterns differ by race.
Keywords: risk factors, cardiovascular diseases, epidemiology
Geographic disparities in risk factors for cardiovascular disease (CVD) have been documented by county for hypertension,1 diabetes,2 and obesity.3 Identifying variation of key risk factors between regions can help direct prevention efforts. County-level maps are often created because data are available only on a per-county basis. However, county-level maps of risk factor prevalences cannot identify disparities within a county and can distort the true patterns of disparities by using somewhat arbitrary administrative boundaries. Therefore, we used data from a national cohort study of geographic disparities in stroke mortality, which collected the street addresses of participants’ residences, to describe geographic disparities in the prevalences of hypertension, diabetes, and smoking by creating heat maps for the continental US that showed geographic disparities below the county level.
Methods
Study sample
For our analysis we used the REasons for Geographic and Racial Differences in Stroke (REGARDS) study, which is a longitudinal, population-based cohort study designed to identify factors associated with the excess stroke mortality among blacks and residents of the southeast US. REGARDS was designed to be a near-national cohort of individuals age 45 years and older, randomly selected with approximately equal representation of whites and blacks, men and women, with oversampling from the “Stroke Belt” (8 southeast states of North Carolina, South Carolina, Georgia, Tennessee, Alabama, Mississippi, Louisiana, and Arkansas) and “Stroke Buckle” (coastal plains of North Carolina, South Carolina, and Georgia). The study enrolled 30,239 participants from across the continental US from 2003 to 2007, and has continued to follow them prospectively.4 Participants were recruited by mail and telephone, and a baseline computer assisted telephone interview (CATI) was used to obtain demographic variables and self-reported health information (e.g., pre-existing history of stroke). The CATI was followed by an in-home visit from a trained health professional, who collected anthropometric measurements (e.g., height, weight, and waist circumference), blood pressure, an electrocardiogram, and blood and urine samples. All participants provided written informed consent, and the study protocol was approved by the Institutional Review Boards of all participating institutions.
Participants were considered to have hypertension if: (1) they had a systolic blood pressure >= 140 mmHg; (2) they had a diastolic blood pressure >= 90 mmHg; or (3) they self-reported currently taking antihypertensive medications. Blood pressure was estimated as the average of two blood pressure measurements after the participant had been seated for 5 minutes. Participants were considered to have diabetes if: (1) they had a fasting glucose level >= 126 mg/dL (or a glucose level >= 200 mg/dL if they failed to fast); or (2) they self-reported currently taking medication for diabetes management. Participants were considered current smokers if they had positive responses to the questions: (1) “Have you smoked at least 100 cigarettes in your lifetime?”; and (2) “Do you smoke cigarettes now, even occasionally?,” which is similar to the definition used by the Behavioral Risk Factors Surveillance System (BRFSS). Past smokers were grouped with never smokers. We initially considered dyslipidemia as well, but we found no evidence of spatial clustering during preliminary testing (see Methods in Supplemental Material). Participant residential locations were geocoded using SAS (SAS Institute, Cary, NC).
Statistical methods
We calculated descriptive statistics of the sample by race. To generate heat maps, we used logistic regression models stratified by race with smooth functions of latitude and longitude of residence, adjusting for age and gender as standard potential confounders, which were chosen a priori. In other words, separate smooth maps were created for blacks and whites. We did not include other variables such as lifestyle factors that were available from the REGARDS study in our models because our goal was to identify potential geographic variation in risk factor prevalence below state and county levels, not to explain its potential causes. Spline-based techniques were used to model the smooth functions of location, as well as smooth functions of age. We chose the spline for age to be a restricted cubic spline with 3 knots, and the spline for location to be a second order thin plate spline (TPS), fit using thin plate regression splines (TPRS).5 The functions of location were essentially 1,000 smooth planes joined together in order to provide estimates of prevalence that were seamless from one location to another. Model fit was assessed using percent of deviance of null model (intercept only) explained by fitted model. We then used the estimated model to provide predicted probabilities of the risk factor at the intersections of a 10 km x 10 km grid across the continental US. Maps were created for “typical” REGARDS participants for each race by assuming populations of the average age and percent of women of each race in the REGARDS sample. Model fitting and prediction were performed using the mgcv package (v. 1.8-14)6 in R (v. 3.3.1),7 and map creation was done using ggplot2 (v. 2.1.0)8 in R. Technical details of the analyses are presented in the Supplemental Methods.
Results
After excluding participants with anomalous data (n = 56), missing hypertension status (n = 74), missing smoking status (n = 116), missing diabetes status (n = 1,095), and missing location (n = 11), our final sample size for this study was 28,887 (96% of the original sample).
The mean(sd) age of the sample was 64.8(9.4) years. The sample was 41% black and 55% female. The sampling frame of the REGARDS study is reflected in the geographic distribution of participants: 35% of the participants lived in the Stroke Belt, 21% lived in the Stroke Buckle, and 45% lived in the rest of the continental US. Fifty-nine percent of the participants had hypertension, 22% had diabetes, and 15% were current smokers. Summary statistics by race are presented in Table 1. Of the 17,107 participants with hypertension, 61% did not have an elevated SBP or DBP but reported taking medication to lower blood pressure, and 11% had an elevated SBP or DBP but did not report taking medication to lower blood pressure. Of the 6,362 participants with diabetes, 45% did not have elevated blood glucose but reported using pills or insulin to treat diabetes, and 11% had elevated blood glucose but did not report using pills or insulin to treat diabetes. Figure 1 shows the participant residential locations by race.
Table 1.
Characteristics of the study sample by race. All statistics are numbers (percentages) unless otherwise noted.
| Variable | Level | Blacks | Whites |
|---|---|---|---|
| Sample size | 11,896 (41.2%) | 16,991 (58.8%) | |
| Age, years* | 64.0 (9.3) | 65.4 (9.5) | |
| Women | 7,360 (61.9%) | 8,472 (49.9%) | |
| Region | Rest of US | 5,821 (48.9%) | 7,032 (41.4%) |
| Stroke Belt | 3,947 (33.2%) | 6,054 (35.6%) | |
| Stroke Buckle | 2,128 (17.9%) | 3,905 (23.0%) | |
| Hypertension | 8,497 (71.4%) | 8,610 (50.7%) | |
| Diabetes | 3,671 (30.9%) | 2,691 (15.8%) | |
| Smoking | Current | 2,064 (17.4%) | 2,140 (12.6%) |
| Never | 5,414 (45.5%) | 7,639 (45.0%) | |
| Past | 4,418 (37.1%) | 7,212 (42.4%) |
mean(standard deviation)
Figure 1. Point map of REGARDS participant locations (n = 28,879).
Blue indicates black race and red indicates white race.
High-resolution maps of hypertension, diabetes, and smoking prevalence
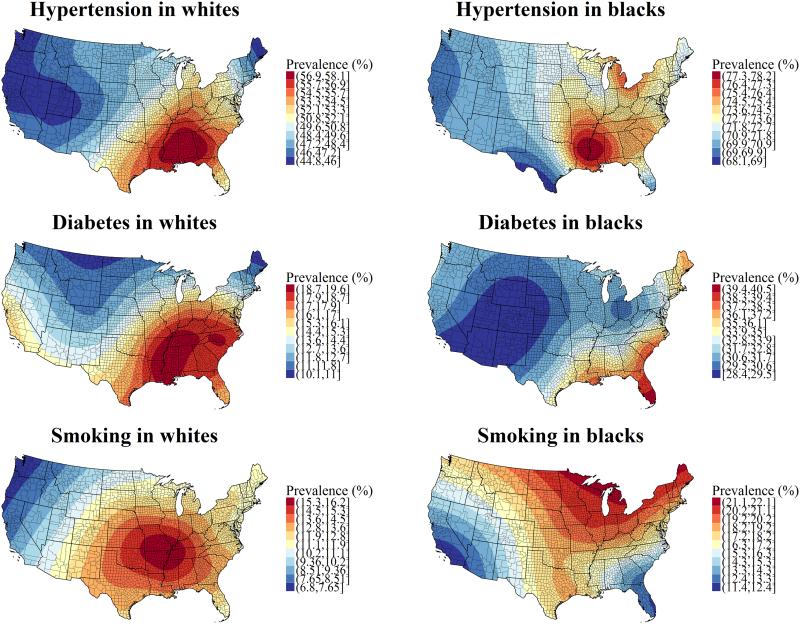
Table 2 shows the age- and location-adjusted odds ratios (95% confidence intervals) for women versus men for each risk factor and race. Age was significantly associated (p < 0.001) with odds of hypertension, diabetes, and current smoking in both blacks and whites, after adjustment for gender. The smoothed maps for age- and gender-adjusted hypertension prevalence, diabetes prevalence, and smoking prevalence, stratified by race, are presented in Figure 2. The percent deviance explained by the fitted models for whites was 3.9% for hypertension, 1.5% for diabetes, and 3.8% for smoking. The percent deviance explained by the fitted models for blacks was 2.5% for hypertension, 1.3% for diabetes, and 3.7% for smoking. Hypertension, diabetes, and current smoking proved to have significant geographic variation in both blacks and whites (p < 0.01). Predicted prevalences for whites assumed a population age 65 and 50% female (mean age and proportion of women among white REGARDS participants), and predicted prevalences for blacks assumed a population age 64 and 62% female (mean age and proportion of women among black REGARDS participants). Thus, predicted prevalences for each risk factor represent the typical black and white participant in the REGARDS study. The median(range) predicted prevalence of hypertension was 49% (45 - 58%) among whites and 72% (68 - 78%) among blacks. The median(range) predicted prevalence of diabetes was 14% (10 - 20%) among whites and 31% (28 - 41%) among blacks. The median(range) predicted prevalence of current smoking was 12% (7 - 16%) among whites and 18% (11 - 22%) among blacks. Hypertension was most prevalent in the central Southeast among whites (Mississippi, Alabama, and Georgia), but in the west Southeast among blacks (Louisiana, Arkansas, and Mississippi). Diabetes was most prevalent in the west and central Southeast among whites (Louisiana, Arkansas, Mississippi, Alabama, Tennessee, and south Kentucky, as well as parts of North Carolina and South Carolina), but in south Florida among blacks. Current smoking was most prevalent in the west Southeast and east Midwest among whites (Arkansas, Missouri, Illinois, Kentucky, Tennessee, and Mississippi) and in the north among blacks (north Minnesota, north Wisconsin, and central and north Michigan).
Table 2.
Odds ratios for women versus men from logistic regression models for each risk factor, stratified by race. Models were adjusted for age and spatial location.
| Risk factor | Race |
Odds ratio (95% confidence
interval) for women versus men |
|---|---|---|
| Hypertension | Blacks | 1.23 (1.14 - 1.34) |
| Whites | 0.92 (0.87 - 0.98) | |
| Diabetes | Blacks | 0.94 (0.87 - 1.02) |
| Whites | 0.7 (0.65 - 0.76) | |
| Current smoking | Blacks | 0.74 (0.67 - 0.81) |
| Whites | 1.10 (1.00 - 1.20) |
Figure 2. Maps of estimated hypertension, diabetes, and current smoking prevalence among whites and blacks, adjusted for age and gender.
High prevalence is indicated by red, while low prevalence is indicated by blue. Predicted prevalences assumed a population with the same proportion of women for each race and the same age as the mean age of each race. Thus, the prevalences reflect the gender and age composition of REGARDS participants of each race.
Discussion
We found that the age- and gender-adjusted prevalence of hypertension, diabetes, and current smoking among both black and white REGARDS participants varied across the continental US. The areas with the highest prevalence of hypertension were fairly concordant among whites and blacks, with the highest prevalence for blacks slightly more concentrated around northeast Louisiana, southeast Arkansas, and central Mississippi. Higher than average prevalence of hypertension was found not only in the Southeast, but also in the Midwest and parts of the Northeast. Higher than average prevalence of diabetes was present throughout the Southeast in whites, but was limited to the coastal regions of the Southeast among blacks. Although portions of the Southeast had higher than average prevalence of current smokers, current smokers were most prevalent in the Midwest among whites. These maps revealed geographic disparities for major CVD risk factors not only within states, but also within counties.
Compared to previous studies, we provided smooth heat maps of prevalence as opposed to maps of prevalence by county. Because of the high resolution, we were able to identify high hypertension prevalence in north Louisiana among blacks, which was not identified in a previous study.1 A previous study by the Centers for Disease Control using data from BRFSS identified a “Diabetes Belt”2 in the Southeast, which had higher levels of prevalence of self-reported diabetes compared to the rest of the US. While our maps showed high prevalences of diabetes in the same region, we also identified an area in the west and central Southeast that had higher prevalences of diabetes than the rest of the Diabetes Belt. The high smoking prevalence identified among whites in the midwest was similar to previous maps for smoking prevalence among women in 2012.9 The map for smoking for men in 2012 in that study included the same counties as for women, but also extended throughout much of the Southeast. By creating smooth maps, we were able to identify subregions of increased prevalence for hypertension and diabetes that were previously unidentified using county-level maps.
Geographic disparities in the estimated prevalence of hypertension and diabetes in our study could have arisen from three main sources: (1) variation in the prevalence of factors that led to the development of hypertension or diabetes (e.g., diet, physical activity, or chronic kidney disease); (2) variation in the treatment of hypertension or diabetes, given that our definition of these risk factors included self-reported use of medications for these conditions; or (3) variation in the degree of self-report bias of use of medications for these conditions. Geographic disparities in socioeconomic status (SES) could explain the geographic disparities in these risk factors, but SES explains less than 16% of the excess stroke mortality in the Stroke Belt compared to the rest of the US.10 Because hypertension, diabetes, and smoking are key risk factors for stroke,11 it is unclear whether disparities in SES were a major contributor to the geographic disparities in these risk factors observed in our study. The contribution of SES is an area we hope to pursue in future work. Given that we included measured blood pressures and blood glucose levels, variation in degree of self-report bias for medication use would affect our results only if, among participants with normal blood pressures or blood glucose levels, there was large-scale over-reporting of use of these medications. Geographic variation in smoking prevalence could have been due to geographic variation in other lifestyle risk factors, but could also have been influenced by state-, county-, or city-level political and economic policies regarding tobacco. The multiple potential causes of geographic variation require further investigation to determine the contribution of each cause.
Our study had some notable limitations. Because the study used simple random sampling within race-sex-region strata, there was a lack of uniform data coverage across the entire continental US, which increased the uncertainty of our predictions in Western states (where the US population is more sparse), particularly for blacks (see Supplemental Figure 2). However, when we restricted the sample to participants living in the east US, where the majority of REGARDS participants were sampled, there were not any meaningful differences in the predicted geographic patterns of each risk factor (see Supplemental Figure 3). Other spatial prediction methods could have potentially identified smaller scale variation than the spline-based approach we used. Spline-based models are known to capture long-range variation quite well, but often fail to effectively capture short-range variation.12 We attempted to overcome this limitation by using the largest number of planes joined together that balanced goodness of fit for the statistical model and computation time. However, we could not completely overcome the limited ability of spline-based methods to capture short-range trends. The data presented in this analysis were collected approximately 10 years ago, and the prevalences of these risk factors might have changed. However, the patterns we observed in our study would only be affected if there were important differences in the temporal trends of these risk factors among regions. Such a trend was not obvious in a previous analysis of hypertension prevalence from 2001 to 2009.1 Finally, the REGARDS study oversampled blacks and residents of the Stroke Belt and Stroke Buckle, which likely affected our estimates of prevalence of these conditions. However, the patterns of geographic disparities depicted in the heat maps should not have depended on the estimated prevalences. While we could have used sampling weights to produce estimates of prevalence that reflected the US population, this would have required the derivation of new statistical methods that correctly estimate standard errors for a TPRS, which was outside the scope of this manuscript. The estimated prevalences were also not representative of the general US population because we excluded adults younger than age 45. This exclusion criterion may not have been overly negative given that hypertension and diabetes tend to affect the older US population more than the younger population. However, most current smokers are under 45 years of age.13
Despite its limitations, our study also had important strengths. Because of the sampling strategy of the REGARDS study, we were able to obtain dense coverage in areas currently purported to have high prevalences of CVD risk factors. While previous studies have generally relied on only self-reported risk factors (e.g., BRFSS), we were able to use both measured blood pressures and blood glucose levels, in addition to self-reported medication use, to identify those with hypertension or diabetes. The sensitivity of self-reported antihypertensive medication use was 94% in a sample of approximately 36,000 men and women from the Monitoring Project on Cardiovascular Disease risk factors in the Netherlands.14 The sensitivity and specificity of self-reported hypertension have been reported to be 71% and 90%,15 respectively, and the sensitivity and specificity of self-reported diabetes have been reported to be 58% - 71% and 96% - 97%,16 respectively. By using self-reported medication use instead of self-reported risk factor status, we were able to more reliably identify participants with these risk factors. In addition to potentially missing undiagnosed hypertension and diabetes, self-report bias can also vary geographically and prevent valid comparisons of estimated prevalences between regions, as noted in a previous study of geographic variation in diabetes.2 While we do not have direct evidence of such geographic variation in misreporting for hypertension, diabetes, or smoking status, self-report of obesity has been shown to have such a bias in the continental US.17 Finally, the heat maps produced in this analysis were on an 10 km x 10 km grid, which allows for public distribution of maps of risk factor prevalence on a fine scale without compromising participant confidentiality. These maps could then be combined with heat maps of other risk factors for cardiovascular disease already available, such as ambient air pollution.18,19
Conclusions and Future Directions
We have shown evidence that the prevalences of hypertension, diabetes, and smoking varied on a finer scale than the state or county level. We have also shown that the patterns of these geographic disparities differed between blacks and whites. This information could inform prevention efforts targeted at particular populations within the US. A clear future direction of this research is to determine the degree to which geographic disparities in these risk factors contribute to geographic disparities in CVD, included coronary heart disease (CHD) and stroke, which are the first and fifth leading causes of death in the US, respectively.20 CHD mortality rates are known to be higher in the Mississippi and Ohio River valleys compared to the rest of the country,21,22 and stroke mortality rates are highest in the Stroke Belt23 and the Stroke Buckle.10 Knowledge of the degree to which geographic disparities in risk factors contribute to geographic disparities in CVD could lead to focused efforts that reduce the burden of CVD in the most affected regions of the US.
Supplementary Material
What is Known.
The prevalence of self-reported hypertension, diabetes, and smoking vary by county in the US.
What this Study Adds
A description of the geographic heterogeneity of hypertension including directly measured blood pressure and diabetes including glucose assessment.
The prevalence of hypertension, diabetes, and self-reported smoking vary on a scale across the continental US.
The prevalence of hypertension, diabetes, and smoking are not uniformly high across the Southeast US, and regions of high prevalence of the risk factors differ.
Public health interventions for CVD prevention should be mindful of geographic heterogeneity and be planned according to local data and factors.
Acknowledgments
The authors thank the anonymous reviewers for their helpful comments, as well as the other investigators, the staff, and the participants of the REGARDS study for their valuable contributions.
Funding Sources: This research project is supported by a cooperative agreement U01 NS041588 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, Department of Health and Human Service. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health. Representatives of the funding agency have been involved in the review of the manuscript but not directly involved in the collection, management, analysis or interpretation of the data. A full list of participating REGARDS investigators and institutions can be found at http://www.regardsstudy.org. This research project was also partially funded by an American Heart Association 2014 Predoctoral Fellowship (14PRE18830073) and 5T32HL00745734 from the National Heart, Lung, and Blood Institute, NIH, Department of Health and Human Services.
Footnotes
Conflict of Interest Disclosures: Dr. Loop has received salary support from Amgen Inc., and Dr. Levitan receives salary support from Amgen, Inc. Dr. Safford has received salary support from Amgen, Inc. for an investigator initiated projected and has served as a consultant for diaDexus. Dr. Levitan has received research grants from Amgen, Inc., serves on the Amgen, Inc. advisory board, and is a consultant to Robinson Calcagnie Robinson Shapiro Davis.
References
- 1.Olives C, Myerson R, Mokdad AH, Murray CJL, Lim SS. Prevalence, awareness, treatment, and control of hypertension in United States counties, 2001-2009. PLoS One. 2013;8:e60308. doi: 10.1371/journal.pone.0060308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barker LE, Kirtland KA, Gregg EW, Geiss LS, Thompson TJ. Geographic distribution of diagnosed diabetes in the U.S.: a diabetes belt. Am J Prev Med. 2011;40:434–439. doi: 10.1016/j.amepre.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 3.Gregg EW, Kirtland KA, Cadwell BL, Rios Burrows N, Barker LE, Thompson TJ, Geiss L, Pan L. Estimated County-Level Prevalence of Diabetes and Obesity --- United States, 2007. MMWR Surveill Summ. 2009;58:1259–1263. [PubMed] [Google Scholar]
- 4.Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ, Graham A, Moy CS, Howard G. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology. 2005;25:135–143. doi: 10.1159/000086678. [DOI] [PubMed] [Google Scholar]
- 5.Wood SN. Thin plate regression splines. J R Stat Soc Series B Stat Methodol. 2003;65:95–114. [Google Scholar]
- 6.Wood SN. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J R Stat Soc Series B Stat Methodol. 2010;73:3–36. [Google Scholar]
- 7.R Core Team R: A language and environment for statistical computing [Internet] 2016 Available from: https://cran.r-project.org/
- 8.Wickham H. ggplot2: elegant graphics for data analysis. Springer; New York: 2009. [Google Scholar]
- 9.Dwyer-Lindgren L, Mokdad AH, Srebotnjak T, Flaxman AD, Hansen GM, Murray CJ. Cigarette smoking prevalence in US counties: 1996-2012. Popul Health Metr. 2014;12:5. doi: 10.1186/1478-7954-12-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Howard G, Anderson R, Johnson NJ, Sorlie P, Russell G, Howard VJ. Evaluation of social status as a contributing factor to the stroke belt region of the United States. Stroke. 1997;28:936–940. doi: 10.1161/01.str.28.5.936. [DOI] [PubMed] [Google Scholar]
- 11.D’Agostino RB, Wolf PA, Belanger AJ, Kannel WB. Stroke risk profile: adjustment for antihypertensive medication. The Framingham Study. Stroke. 1994;25:40–43. doi: 10.1161/01.str.25.1.40. [DOI] [PubMed] [Google Scholar]
- 12.Sang H, Huang JZ. A full scale approximation of covariance functions for large spatial data sets. J R Stat Soc Series B Stat Methodol. 2012;74:111–132. [Google Scholar]
- 13.Jha P, Ramasundarahettige C, Landsman V, Rostron B, Thun M, Anderson RN, McAfee T, Peto R. 21st-century hazards of smoking and benefits of cessation in the United States. N Engl J Med. 2013;368:341–350. doi: 10.1056/NEJMsa1211128. [DOI] [PubMed] [Google Scholar]
- 14.Klungel OH, de Boer A, Paes AH, Herings RM, Seidell JC, Bakker A. Agreement between self-reported antihypertensive drug use and pharmacy records in a population-based study in The Netherlands. Pharm World Sci. 1999;21:217–220. doi: 10.1023/a:1008741321384. [DOI] [PubMed] [Google Scholar]
- 15.Vargas CM, Burt VL, Gillum RF, Pamuk ER. Validity of Self-Reported Hypertension in the National Health and Nutrition Examination Survey III, 1988–1991. Prev Med. 1997;26:678–685. doi: 10.1006/pmed.1997.0190. [DOI] [PubMed] [Google Scholar]
- 16.Schneider ALC, Pankow JS, Heiss G, Selvin E. Validity and reliability of self-reported diabetes in the Atherosclerosis Risk in Communities Study. Am J Epidemiol. 2012;176:738–743. doi: 10.1093/aje/kws156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Le A, Judd SE, Allison DB, Oza-Frank R, Affuso O, Safford MM, Howard VJ, Howard G. The geographic distribution of obesity in the US and the potential regional differences in misreporting of obesity. Obesity. 2014;22:300–306. doi: 10.1002/oby.20451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Hamdan MZ, Crosson WL, Limaye AS, Rickman DL, Quattrochi DA, Estes MG, Jr, Qualters JR, Sinclair AH, Tolsma DD, Adeniyi KA, Niskar AS. Methods for characterizing fine particulate matter using ground observations and remotely sensed data: potential use for environmental public health surveillance. J Air Waste Manag Assoc. 2009;59:865–881. doi: 10.3155/1047-3289.59.7.865. [DOI] [PubMed] [Google Scholar]
- 19.Al-Hamdan MZ, Crosson WL, Economou SA, Estes MG, Estes SM, Hemmings SN, Kent ST, Mark P, Quattrochi DA, Rickman DL, Wade GM, McClure LA. Environmental public health applications using remotely sensed data. Geocarto Int. 2014;29:85–98. doi: 10.1080/10106049.2012.715209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kochanek KD, Murphy SL, Xu J, Arias E. Mortality in the United States, 2013. NCHS Data Brief. 2014:1–8. [PubMed] [Google Scholar]
- 21.Narevic E, Schoenberg NE. Lay Explanations for Kentucky’s “Coronary Valley.”. J Community Health. 2002;27:53–62. doi: 10.1023/a:1013832326912. [DOI] [PubMed] [Google Scholar]
- 22.Gillum RF, Mehari A, Curry B, Obisesan TO. Racial and geographic variation in coronary heart disease mortality trends. BMC Public Health. 2012;12:410. doi: 10.1186/1471-2458-12-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borhani NO. Changes and Geographic Distribution of Mortality from Cerebrovascular Disease. Am J Public Health Nations Health. 1965;55:673–681. doi: 10.2105/ajph.55.5.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.