Abstract
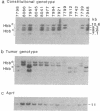
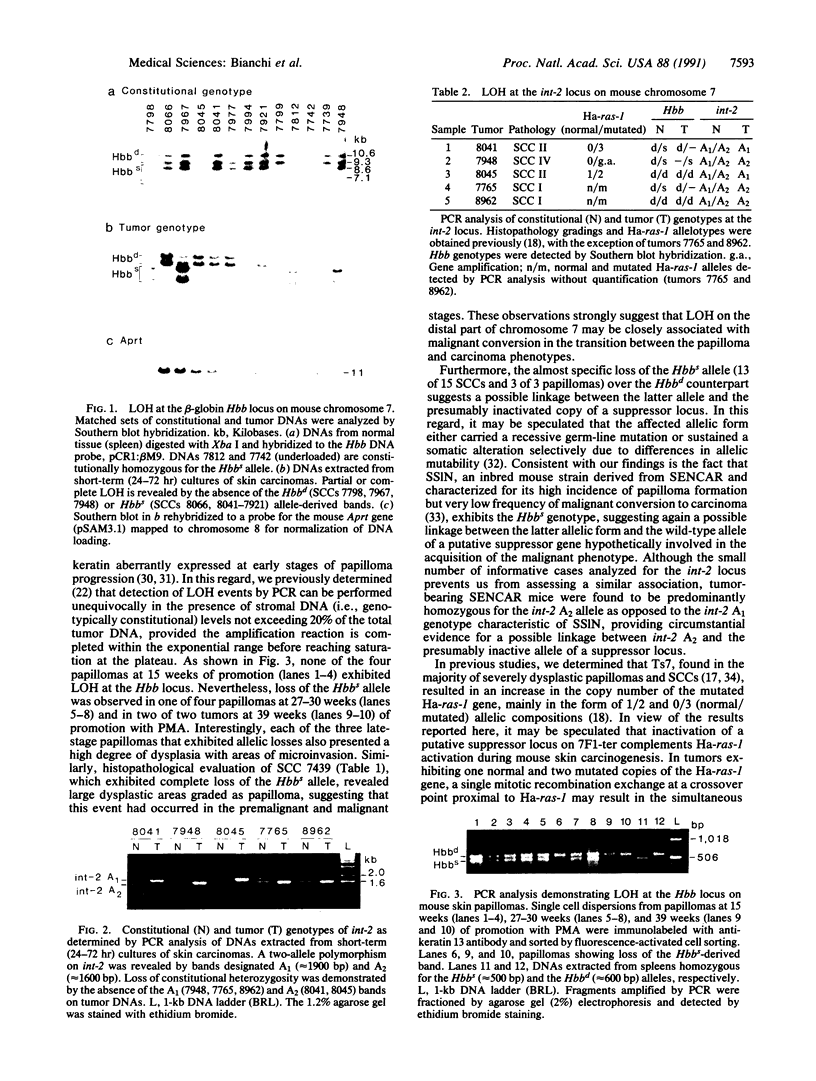
A significant role for mouse chromosome 7 abnormalities during chemically induced skin carcinogenesis has been advanced based on previous cytogenetic and molecular studies. To determine the frequency of allelic losses at different loci of chromosome 7 in skin tumors induced in the outbred SENCAR mouse stock by a two-stage initiation-promotion protocol, we compared the constitutional and tumor genotypes of premalignant papillomas and squamous cell carcinomas for loss of heterozygosity at different informative loci. In a previous study, these tumors had been analyzed for their allelic composition at the Harvey ras-1 (Ha-ras-1) locus and it was found that 39% of squamous cell carcinomas had lost the normal Ha-ras-1 allele exhibiting 3 or 2 copies of the mutated counterpart or gene amplification. In the present study, by combining Southern blot and polymerase chain reaction fragment length polymorphism analyses, we detected complete loss of heterozygosity at the beta-globin (Hbb) locus, distal to Ha-ras-1, in 15 of 20 (75%) skin carcinomas. In addition, 5 of 5 informative cases attained homozygosity at the int-2 locus, 27 centimorgans distal to Hbb. Polymerase chain reaction analysis of DNA extracted from papillomas devoid of stromal contamination by fluorescence-activated sorting of single cell dispersions immunolabeled with anti-keratin 13 antibody revealed loss of heterozygosity at the Hbb locus, demonstrating that this event occurs during premalignant stages of tumor development. Interestingly, loss of heterozygosity was only detected in late-stage lesions exhibiting a high degree of dysplasia and areas of microinvasion. Analysis of allelic ratios by densitometric scanning of tumors that had become homozygous at Hbb but retained heterozygosis at Ha-ras-1 indicated mitotic recombination as the mechanism underlying loss of heterozygosity on mouse chromosome 7 during chemically induced skin carcinogenesis. These findings are consistent with the presence of a putative tumor suppressor gene linked to the Hbb locus in the 7F1-ter region of mouse chromosome 7, the functional inactivation of which may constitute a critical event in skin tumor progression, possibly during the malignant conversion stage.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldaz C. M., Conti C. J., Klein-Szanto A. J., Slaga T. J. Progressive dysplasia and aneuploidy are hallmarks of mouse skin papillomas: relevance to malignancy. Proc Natl Acad Sci U S A. 1987 Apr;84(7):2029–2032. doi: 10.1073/pnas.84.7.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldaz C. M., Trono D., Larcher F., Slaga T. J., Conti C. J. Sequential trisomization of chromosomes 6 and 7 in mouse skin premalignant lesions. Mol Carcinog. 1989;2(1):22–26. doi: 10.1002/mc.2940020104. [DOI] [PubMed] [Google Scholar]
- Angel J. M., Morizot D. C., Richie E. R. Genetics of N-methyl-N-nitrosourea induction of thymic lymphomas in AKR/J mice: assignment of a susceptibility gene to mouse chromosome 7. J Natl Cancer Inst. 1989 Nov 1;81(21):1652–1655. doi: 10.1093/jnci/81.21.1652. [DOI] [PubMed] [Google Scholar]
- Bianchi A. B., Aldaz C. M., Conti C. J. Nonrandom duplication of the chromosome bearing a mutated Ha-ras-1 allele in mouse skin tumors. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6902–6906. doi: 10.1073/pnas.87.17.6902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi A. B., Navone N. M., Conti C. J. Detection of loss of heterozygosity in formalin-fixed paraffin-embedded tumor specimens by the polymerase chain reaction. Am J Pathol. 1991 Feb;138(2):279–284. [PMC free article] [PubMed] [Google Scholar]
- Bizub D., Wood A. W., Skalka A. M. Mutagenesis of the Ha-ras oncogene in mouse skin tumors induced by polycyclic aromatic hydrocarbons. Proc Natl Acad Sci U S A. 1986 Aug;83(16):6048–6052. doi: 10.1073/pnas.83.16.6048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner R., Balmain A. Genetic changes in skin tumor progression: correlation between presence of a mutant ras gene and loss of heterozygosity on mouse chromosome 7. Cell. 1990 May 4;61(3):407–417. doi: 10.1016/0092-8674(90)90523-h. [DOI] [PubMed] [Google Scholar]
- Conti C. J., Aldaz C. M., O'Connell J., Klein-Szanto A. J., Slaga T. J. Aneuploidy, an early event in mouse skin tumor development. Carcinogenesis. 1986 Nov;7(11):1845–1848. doi: 10.1093/carcin/7.11.1845. [DOI] [PubMed] [Google Scholar]
- Fearon E. R., Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990 Jun 1;61(5):759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- Gimenez-Conti I., Aldaz C. M., Bianchi A. B., Roop D. R., Slaga T. J., Conti C. J. Early expression of type I K13 keratin in the progression of mouse skin papillomas. Carcinogenesis. 1990 Nov;11(11):1995–1999. doi: 10.1093/carcin/11.11.1995. [DOI] [PubMed] [Google Scholar]
- Greenhalgh D. A., Welty D. J., Player A., Yuspa S. H. Two oncogenes, v-fos and v-ras, cooperate to convert normal keratinocytes to squamous cell carcinoma. Proc Natl Acad Sci U S A. 1990 Jan;87(2):643–647. doi: 10.1073/pnas.87.2.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhalgh D. A., Yuspa S. H. Malignant conversion of murine squamous papilloma cell lines by transfection with the fos oncogene. Mol Carcinog. 1988;1(2):134–143. doi: 10.1002/mc.2940010209. [DOI] [PubMed] [Google Scholar]
- Hansen M. F., Cavenee W. K. Genetics of cancer predisposition. Cancer Res. 1987 Nov 1;47(21):5518–5527. [PubMed] [Google Scholar]
- Holdener-Kenny B., Weaver S. A naturally occurring deletion in the mouse Hbbs beta-globin gene cluster. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4374–4378. doi: 10.1073/pnas.83.12.4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T. Cooperation between oncogenes. Cell. 1991 Jan 25;64(2):249–270. doi: 10.1016/0092-8674(91)90637-e. [DOI] [PubMed] [Google Scholar]
- Johnson D. K., Hand R. E., Jr, Rinchik E. M. Molecular mapping within the mouse albino-deletion complex. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8862–8866. doi: 10.1073/pnas.86.22.8862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudson A. G., Jr Genetics of human cancer. Annu Rev Genet. 1986;20:231–251. doi: 10.1146/annurev.ge.20.120186.001311. [DOI] [PubMed] [Google Scholar]
- Knudson A. G., Jr Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci U S A. 1971 Apr;68(4):820–823. doi: 10.1073/pnas.68.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koi M., Morita H., Yamada H., Satoh H., Barrett J. C., Oshimura M. Normal human chromosome 11 suppresses tumorigenicity of human cervical tumor cell line SiHa. Mol Carcinog. 1989;2(1):12–21. doi: 10.1002/mc.2940020103. [DOI] [PubMed] [Google Scholar]
- Koufos A., Grundy P., Morgan K., Aleck K. A., Hadro T., Lampkin B. C., Kalbakji A., Cavenee W. K. Familial Wiedemann-Beckwith syndrome and a second Wilms tumor locus both map to 11p15.5. Am J Hum Genet. 1989 May;44(5):711–719. [PMC free article] [PubMed] [Google Scholar]
- Larsson C., Skogseid B., Oberg K., Nakamura Y., Nordenskjöld M. Multiple endocrine neoplasia type 1 gene maps to chromosome 11 and is lost in insulinoma. Nature. 1988 Mar 3;332(6159):85–87. doi: 10.1038/332085a0. [DOI] [PubMed] [Google Scholar]
- Marshall C. J. Tumor suppressor genes. Cell. 1991 Jan 25;64(2):313–326. doi: 10.1016/0092-8674(91)90641-b. [DOI] [PubMed] [Google Scholar]
- Miller D. R., Viaje A., Aldaz C. M., Conti C. J., Slaga T. J. Terminal differentiation-resistant epidermal cells in mice undergoing two-stage carcinogenesis. Cancer Res. 1987 Apr 1;47(7):1935–1940. [PubMed] [Google Scholar]
- Moore R., Casey G., Brookes S., Dixon M., Peters G., Dickson C. Sequence, topography and protein coding potential of mouse int-2: a putative oncogene activated by mouse mammary tumour virus. EMBO J. 1986 May;5(5):919–924. doi: 10.1002/j.1460-2075.1986.tb04304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y., Larsson C., Julier C., Byström C., Skogseid B., Wells S., Oberg K., Carlson M., Taggart T., O'Connell P. Localization of the genetic defect in multiple endocrine neoplasia type 1 within a small region of chromosome 11. Am J Hum Genet. 1989 May;44(5):751–755. [PMC free article] [PubMed] [Google Scholar]
- Nischt R., Roop D. R., Mehrel T., Yuspa S. H., Rentrop M., Winter H., Schweizer J. Aberrant expression during two-stage mouse skin carcinogenesis of a type I 47-kDa keratin, K13, normally associated with terminal differentiation of internal stratified epithelia. Mol Carcinog. 1988;1(2):96–108. doi: 10.1002/mc.2940010205. [DOI] [PubMed] [Google Scholar]
- Oshimura M., Gilmer T. M., Barrett J. C. Nonrandom loss of chromosome 15 in Syrian hamster tumours induced by v-Ha-ras plus v-myc oncogenes. Nature. 1985 Aug 15;316(6029):636–639. doi: 10.1038/316636a0. [DOI] [PubMed] [Google Scholar]
- Ponder B. Cancer. Gene losses in human tumours. Nature. 1988 Sep 29;335(6189):400–402. doi: 10.1038/335400a0. [DOI] [PubMed] [Google Scholar]
- Ponder B. Carcinogenesis. Is imprinting to blame? Nature. 1989 Jul 27;340(6231):264–264. doi: 10.1038/340264a0. [DOI] [PubMed] [Google Scholar]
- Quintanilla M., Brown K., Ramsden M., Balmain A. Carcinogen-specific mutation and amplification of Ha-ras during mouse skin carcinogenesis. Nature. 1986 Jul 3;322(6074):78–80. doi: 10.1038/322078a0. [DOI] [PubMed] [Google Scholar]
- Robbins P. D., Horowitz J. M., Mulligan R. C. Negative regulation of human c-fos expression by the retinoblastoma gene product. Nature. 1990 Aug 16;346(6285):668–671. doi: 10.1038/346668a0. [DOI] [PubMed] [Google Scholar]
- Saunders A. M., Seldin M. F. The syntenic relationship of proximal mouse chromosome 7 and the myotonic dystrophy gene region on human chromosome 19q. Genomics. 1990 Feb;6(2):324–332. doi: 10.1016/0888-7543(90)90573-d. [DOI] [PubMed] [Google Scholar]
- Saxon P. J., Srivatsan E. S., Stanbridge E. J. Introduction of human chromosome 11 via microcell transfer controls tumorigenic expression of HeLa cells. EMBO J. 1986 Dec 20;5(13):3461–3466. doi: 10.1002/j.1460-2075.1986.tb04670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scrable H. J., Witte D. P., Lampkin B. C., Cavenee W. K. Chromosomal localization of the human rhabdomyosarcoma locus by mitotic recombination mapping. Nature. 1987 Oct 15;329(6140):645–647. doi: 10.1038/329645a0. [DOI] [PubMed] [Google Scholar]
- Searle A. G., Peters J., Lyon M. F., Hall J. G., Evans E. P., Edwards J. H., Buckle V. J. Chromosome maps of man and mouse. IV. Ann Hum Genet. 1989 May;53(Pt 2):89–140. doi: 10.1111/j.1469-1809.1989.tb01777.x. [DOI] [PubMed] [Google Scholar]
- Silver J., Buckler C. E. A preferred region for integration of Friend murine leukemia virus in hematopoietic neoplasms is closely linked to the Int-2 oncogene. J Virol. 1986 Dec;60(3):1156–1158. doi: 10.1128/jvi.60.3.1156-1158.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenman G., Sager R. Genetic analysis of tumorigenesis: a conserved region in the human and Chinese hamster genomes contains genetically identified tumor-suppressor genes. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9099–9102. doi: 10.1073/pnas.84.24.9099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman B. E., Saxon P. J., Pasquale S. R., Jones G. R., Geiser A. G., Stanbridge E. J. Introduction of a normal human chromosome 11 into a Wilms' tumor cell line controls its tumorigenic expression. Science. 1987 Apr 10;236(4798):175–180. doi: 10.1126/science.3031816. [DOI] [PubMed] [Google Scholar]