Abstract
Purpose
The main goals of this study were to investigate the expression of anti-Müllerian hormone (AMH) and its receptor (AMHR2) during follicular development in primates, and to evaluate the potential of AMH as a biomarker for follicle growth and oocyte maturation in vitro.
Methods
The mRNA and protein expression of AMH and AMHR2 were determined using isolated follicles and ovarian sections from rhesus macaques (n = 4) by real-time PCR and immunohistochemistry, respectively. Isolated secondary follicles were cultured individually. Follicle growth and media AMH concentrations were assessed by ELISA. The mRNA expression profiles, obtained from RNA sequencing, of in vitro- and in vivo-developed antral follicles were compared. Secondary follicles from additional animals (n = 35) were cultured. Follicle growth, oocyte maturation, and media AMH concentrations were evaluated for forecasting follicular development in vitro by AMH levels.
Results
AMH immunostaining was heterogeneous in the population of preantral follicles that were also stained for AMHR2. The mRNA expression profiles were comparable between in vivo- and in vitro-developed follicles. AMH levels produced by growing follicles were higher than those of nongrowing follicles in culture. With a cutoff value of 1.40 ng/ml, 85 % of nongrowing follicles could be identified while eliminating only 5 % of growing follicles. Growing follicles that generated metaphase II-stage oocytes secreted greater amounts of AMH than did those yielding immature germinal vesicle-stage oocytes.
Conclusions
AMH, co-expressed with AMHR2, was produced heterogeneously by preantral follicles in macaques with levels correlated positively with follicle growth and oocyte maturation. AMH may serve as a biomarker for primate follicular development in vitro.
Keywords: Anti-Müllerian hormone, Three-dimensional follicle culture, RNA sequencing, Preantral follicle, Antral follicle
Introduction
Anti-Müllerian hormone (AMH), an intraovarian peptide produced by adult mammals, is dynamically expressed in developing ovarian follicles. AMH messenger RNA (mRNA) and/or protein is detectable in preantral follicles, peaks in antral follicles, and decreases following subsequent follicle growth to the preovulatory stage in rodents [1], domestic animals [2], and women [3]. Physiologically, AMH may be a gatekeeper for follicular development as suggested by clinical studies [4]. AMH is also considered as a promising biomarker of ovarian function in a large array of clinical situations including assessing the need for fertility preservation for patients experiencing cancer therapies or surgeries that may cause ovarian damage [4]. As a member of the transforming growth factor beta (TGFβ) family, AMH signals by binding to a specific type II transmembrane serine/threonine kinase receptor, AMHR2 [5]. AMHR2 appears to follow a similar expression pattern as that of AMH in the growing follicle, at least in rodents [6]. However, AMH signaling via AMHR2 during specific stages of follicular development has not been reported in primates.
Although hundreds of preantral follicles activated from the primordial follicle pool continually exist in the ovary, much fewer numbers grow to the antral stage during each menstrual cycle in women [7]. The majority of preantral follicles either remains at the primary/secondary stage or become atretic. The molecular mechanisms that determine the survival and growth potential of preantral follicles are not clear. Studies in mice suggest that AMH plays an essential role during follicular development by inhibiting preantral follicle growth and antral follicle maturation [8]. Although not documented in studies using rodents and domestic animals, the levels of AMH protein varied in marmoset preantral follicles based on immunohistochemical analysis [9]. Thus, one can hypothesize that AMH production by preantral follicles portends the potential for further follicle growth and maturation in primates.
An encapsulated three-dimensional culture system was developed to support rhesus macaque preantral (primary and secondary) follicle growth to the small antral stage with function in steroidogenesis and oocyte maturation [10]. Notably, in vitro-developed macaque follicles produced AMH with expression patterns similar to those observed in women by immunohistochemistry [11]. Media concentrations of AMH protein were detectable during the preantral stage and peaked after antrum formation [10]. The data also indicated that, although the diameters were similar at onset of culture, macaque preantral follicles differ in their ability to produce AMH. Media AMH concentrations correlate positively with follicle growth rates during monkey follicle culture [10]. Thus, macaque follicles grown in vitro may mimic those developed in vivo in terms of AMH production. This follicle culture technique offers a powerful tool to study individual follicles for AMH expression and regulation at preantral and small antral stages of follicular development in primates.
Therefore, the present study was designed to examine the expression of AMH and AMHR2 by macaque preantral and small antral follicles. The mRNA expression profiles were compared between in vivo- and in vitro-developed small antral follicles to assess whether encapsulated three-dimensional follicle culture allows coordinated follicular development in vitro similar to that in vivo. Experiments were also performed to evaluate the potential of AMH in noninvasively predicting further follicle growth and oocyte maturation in vitro.
Materials and methods
Animal use and ovary collection
The general care and housing of rhesus macaques (Macaca mulatta) were provided by the Division of Comparative Medicine at the Oregon National Primate Research Center (ONPRC), Oregon Health & Science University (OHSU). Animals were pair-caged in a temperature-controlled (22 °C), light-regulated (12L:12D) room. Diet consisted of Purina monkey chow (Ralston-Purina, Richmond, IN, USA) provided twice a day, supplemented with fresh fruit or vegetables once a day and water ad libitum. Animals were treated according to the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals. Protocols were approved by the ONPRC Institutional Animal Care and Use Committee [10].
Ovary pairs were collected from adult, female macaques exhibiting regular menstrual cycles, with the first day of menstruation considered day 1 of the cycle. Ovariectomies were conducted on anesthetized monkeys at early follicular phase (days 1–4 of the cycle). Ovaries were immediately transferred into HEPES-buffered holding media (CooperSurgical, Inc., Trumbull, CT, USA) and kept at 37 °C for subsequent processing [12]. Four monkeys (11–12 years old) provided ovaries for experiment I, and 35 (4–12 years old) for experiment II.
Experiment I
Immunohistochemistry for AMH and AMHR2
Half of one ovary from each monkey was fixed in 4 % paraformaldehyde-PBS (137 mM NaCl, 10 mM Na2HPO4, 2.7 mM KCl, 1.8 mM KH2PO4; Invitrogen, Carlsbad, CA, USA) solution at 4 °C overnight, dehydrated in ascending concentrations of ethanol (70–100 %), and embedded in paraffin. Five-micrometer sections were cut by the Histopathology-Morphology Research Core at ONPRC [10]. Ovarian sections were deparaffinized and hydrated through xylene (Thermo Fisher Scientific Inc., Grand Island, NY, USA) and a graded series of ethanol. Sections were rehydrated in PBS, followed by incubation in 3 % hydrogen peroxide/60 % methanol to quench endogenous peroxidase activity. Adjacent sections were incubated at 4 °C overnight with primary antibodies (1:75 for mouse anti-human AMH antibody, MAB1737 from R&D Systems, Inc., Minneapolis, MN, USA; 1:300 for goat anti-human AMHR2 antibody, sc-66546 from Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA). Antibodies preabsorbed with blocking peptides (1737-MS from R&D Systems, Inc. for AMH antibody; sc-66546P from Santa Cruz Biotechnology, Inc. for AMHR2) at 4 °C overnight were used as negative controls. Slides were then incubated with the appropriate secondary antibodies and processed using a VECTASTAIN Elite ABC Kit from Vector Laboratories, Inc. (Burlingame, CA, USA; PK-6102 biotinylated anti-mouse IgG for AMH; PK-4005 biotinylated anti-goat IgG for AMHR2). The antigen-antibody complex was visualized by incubation with 3,3′-diaminobenzidine. Sections were counterstained using hematoxylin and images were captured via an Olympus BX40 inverted microscope and an Olympus DP72 digital camera (Olympus Imaging America Inc., Center Valley, PA, USA) [10].
Real-time PCR
The other half of the same ovary from each monkey was used for secondary follicle isolation as described previously [10, 13]. Briefly, the ovarian cortex was cut into 1 × 1 × 1 mm cubes for follicle isolation mechanically using 30-gauge needles. Secondary follicles (diameter 125–225 μm) met the criteria for study if they exhibited an intact basement membrane, 2–4 layers of granulosa cells, and a healthy, centrally located oocyte. Follicles were pooled by monkey (30 follicles/monkey) and transferred into the lysis buffer of an Absolutely RNA Nanoprep Kit (Agilent Technologies, Santa Clara, CA, USA) for RNA isolation based on the manufacturer’s instruction. RNA was reverse-transcribed into complementary DNA (cDNA) using a GoScript™ Reverse Transcription System (Promega Corporation, Madison, WI, USA) based on the manufacturer’s instruction. Real-time PCR was performed for AMH and AMHR2 using TaqMan® Gene Expression Assays (Thermo Fisher Scientific Inc.; AMH Assay ID: Hs03986144_s1; AMHR2 Assay ID: Rh01086649_m1) and Applied Biosystems 7900HT Fast Real-time PCR System (Thermo Fisher Scientific Inc.) as previously described [14].
Follicle culture
The cortex region of the second ovary from each monkey was used for secondary follicle (diameter 125–225 μm) isolation as described above. Isolated follicles were encapsulated and cultured individually as reported previously [10]. Briefly, follicles (24 follicles/monkey) were transferred into 5 μl 0.25 % (w/v) sterile sodium alginate (FMC Health and Nutrition, Philadelphia, PA, USA)-PBS. The droplets were cross-linked in 50 mM CaCl2, 140 mM NaCl, 10 mM HEPES solution (pH 7.2). Encapsulated follicles were placed in wells of 48-well plates containing 300 μl alpha minimum essential medium (Thermo Fisher Scientific Inc.) supplemented with 1 ng/ml recombinant human follicle-stimulating hormone (FSH) (NV Organon, Oss, Netherlands), 6 % (v/v) human serum protein supplement (Cooper Surgical, Inc.), 0.5 mg/ml bovine fetuin, 5 μg/ml insulin, 5 μg/ml transferrin, 5 ng/ml sodium selenite, and 10 μg/ml gentamicin (Sigma-Aldrich, St Louis, MO, USA). Follicles were cultured individually at 37 °C in a 5 % O2 environment (in 6 % CO2/89 % N2) for 5 weeks [10]. Media (150 μl) was replaced every other day. Media samples collected weekly were analyzed for AMH concentrations by ELISA using an SKU: AL-105 kit (AnshLabs, Webster, TX, USA) based on the manufacturer’s instruction. The AMH ELISA assay has an analytical measure range of 0.06–14.2 ng/ml and a sensitivity of 23 pg/ml.
Follicle growth and antrum formation were assessed weekly using an Olympus CK-40 inverted microscope and an Olympus DP11 digital camera (Olympus Imaging America Inc.) as described previously [10]. Follicle sizes were determined by measuring the distance from the outer layer of cells at the widest diameter and then the diameter perpendicular to the first measurement. The mean of the two values was considered the follicle’s overall diameter. The measurements were performed using ImageJ 1.48 software (National Institutes of Health, Bethesda, MD, USA). Follicles were considered atretic if the oocyte was dark or not surrounded by a layer of granulosa cells, the granulosa cells appeared dark or fragmented, or the follicle diameter decreased. At the end of week 5, healthy, small antral follicles (diameter = 0.5–1.5 mm) developed in vitro were pooled by monkey (10 follicles/monkey) for RNA isolation, as described above, and for the subsequent RNA sequencing experiment.
RNA sequencing
The medulla region of the second ovary from each monkey was used for small antral follicle (diameter = 0.5–1.5 mm) isolation as described previously [15]. Isolated follicles were pooled by monkey (10 follicles/monkey) for RNA isolation as described above. Illumina sequencing was performed by the OHSU Massively Parallel Sequencing Shared Resource. Short read RNA sequencing was used to map and quantify transcriptomes in small antral follicles developed in vivo and in vitro. Total RNA integrity was assessed using an Agilent 2100 Bioanalyzer (Agilent Technologies). RNA sequencing libraries were constructed using a TruSeq RNA Library Preparation Kit v2 (Illumina, Inc., San Diego, CA, USA). Poly(A)+ RNA was isolated using oligo‐dT bound to magnetic beads. Chemically fragmented RNA was converted to double-stranded cDNA using random hexamer priming. Overhanging nucleotides were removed and a single adenine was added to the 3′ end of each strand. Illumina adaptors with barcode sequences were ligated to the fragments. Resulting libraries was amplified using PCR. Unincorporated nucleotides and adaptor dimers were removed using an Agencourt AMPure XP system (Beckman Coulter, Inc., Indianapolis, IN, USA). Libraries were applied for multiplexing and paired-end sequencing using a HiSeq 2000 sequencer (Illumina). Base call data were assembled into FASTQ files using a CASAVA suite (Illumina).
RNA aliquots from the same samples were reverse-transcribed into cDNA as described above. Quantitative real-time PCR was performed, as described above, to validate RNA sequencing results for AMH and AMHR2, as well as for selected genes that are critical for antral follicle function, e.g., gonadotropin signaling (FSH receptor, FSHR, Assay ID: Hs00174865_m1; luteinizing hormone/choriogonadotropin receptor, LHCGR, Assay ID: Hs00896336_m1), steroidogenesis (cytochrome P450 family 17 subfamily A polypeptide 1, CYP17A1, Assay ID: Hs01124136_m1; cytochrome P450 family 19 subfamily A polypeptide 1, CYP19A1, Assay ID: Hs00903413_m1), and oocyte quality (bone morphogenetic protein 15, BMP15, Assay ID: Hs00193764_m1; growth differentiation factor 9, GDF9, Assay ID: Hs03986126_s1). The 18S ribosomal RNA (rRNA) served as internal control.
Experiment II
This was a retrospective study including follicles obtained from animals reported in previous studies [10, 12, 16, 17]. As described in experiment I, secondary follicles (diameter 125–225 μm; 12–24 follicles/monkey) were isolated from ovaries of each monkey and cultured individually for 5 weeks. Follicle growth and antrum formation were assessed. Media samples collected at the end of weeks 1 and 2 were analyzed for AMH.
At the end of week 5, healthy, selected small antral follicles (diameter = 0.5–1.5 mm) developed in vitro were treated with 100 ng/ml recombinant human chorionic gonadotropin (hCG; Merck Serono, Geneva, Switzerland) for 34 h. Oocyte harvest and evaluation were performed on a 37 °C warming plate as previously described [10]. Briefly, cumulus-oocyte complexes were released in Tyrode’s albumin lactate pyruvate (TALP)-HEPES-BSA (0.3 % v/v) medium and were pipetted to dissociate cumulus cells and obtain denuded oocytes. Harvested oocytes were transferred to TALP medium and photographed. Oocyte meiotic status was assessed using the same camera described above.
RNA sequencing analyses
Approximately 817 million 101-base pair (bp) outputted reads were evaluated for elevated duplication levels and sequence quality using FastQC v0.10.1 software (Babraham Institute, Cambridge, Cambridgeshire, UK). Potential PCR duplicates were marked using the MarkDuplicates v1.98 utility (Broad Institute, Cambridge, MA, USA). Adaptive quality trimming was performed using a Trimmomatic v0.30 algorithm (The Usadel lab, Aachen, Germany) [18]. About 83.7 % reads were retained at length of >75 bp. Remaining reads were aligned using GSnap v2013-10-28 (Genentech, Inc., South San Francisco, CA, USA) [19] with the most current rhesus macaque genome build and gene annotation (BioProject, accession number: PRJNA214746). The default parameters for the GSnap aligner were used, with the exception of increasing the allowed mismatch percentage to 5 % of a given read to aid in the extension of alignment beyond ends of exons. Approximately 360 million read pairs (88 %) aligned uniquely. All reads were retained. HTSeq v0.5.4p3 software (Python Software Foundation, Portland, OR, USA) was used to count unique reads for differential expression analysis [20]. Count data were based on exon boundaries within the latest gene annotation containing 15,236 annotated genes (BioProject, accession number: PRJNA214746). Output from each sample was collected in a matrix of counts corresponding to each gene identifier.
The R v3.0.2 package DESeq2 v1.2.5 (Bioconductor, Seattle, WA, USA) was used in further differential expression analysis of the count data [21]. A DESeq2 clustering algorithm revealed that the samples were correctly clustered according to experimental condition (in vitro versus in vivo). Raw counts were normalized internally. DESeq2 makes use of the negative binomial distribution to fit generalized linear models of the count data and compares potential fits to infer whether specification of additional factors has improved the significance of the treatment effect. A single-factor design was completed comparing in vitro versus in vivo samples at a common follicle stage. Gene Set Enrichment Analysis (Broad Institute) was used for gene ontological analysis on the differentially expressed gene sets [21].
Statistical analyses
Statistical analysis was performed using SAS V9.4 software (SAS Institute Inc., Cary, NC, USA). Due to the limited sample size (n = 4) for formal statistical hypothesis testing, for real-time PCR experiments, a permutation test was used to compare mRNA levels between groups. Pairwise comparison between groups was performed to generate pseudo distributions of group differences. Differences were considered significant at permutation P < 0.05 and values are presented as the mean ± SEM.
For follicle culture experiments, weekly media AMH concentrations were analyzed using a two-way ANOVA with repeated measures. Contrast t tests under repeated measures ANOVA framework were used to compare between groups at each time point. Data represent individual follicles from four animals. To compare media AMH concentrations produced by different follicle cohorts based on follicle growth or oocyte meiotic status at week 5, the mixed effect model with first-order autoregressive as a within-group covariance was used with follicle cohorts as the between-group factor and monkeys nested within the groups as the random effect. Data represent individual follicles from 35 animals. Contrast t tests were used to compare between two groups at each time point as described above. Differences were considered significant at P < 0.05 and values are presented as the mean ± SEM. To estimate a 95 % cutoff value for media AMH concentrations between follicle cohorts, a bootstrapping method was used to establish the reference intervals [22].
Results
Experiment I
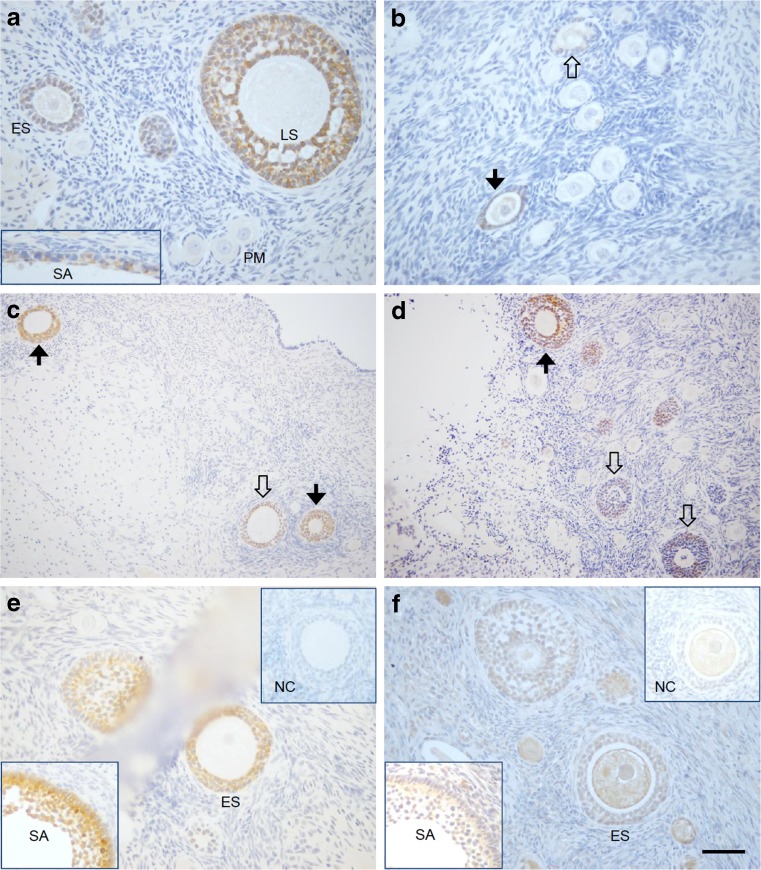
Specific immunostaining for AMH concentrated in the cytoplasm of the granulosa cells in growing follicles, but not in either the theca or surrounding stroma cells (Fig. 1a). In the early follicular phase, AMH staining was not evident in primordial follicles (PM, Fig. 1a). Positive staining became obvious as follicles progressed to the primary (Fig. 1b), early secondary (ES, Fig. 1a, c), and late secondary (LS, Fig. 1a, d) stages. AMH staining was also present in the granulosa layer of small antral follicles (SA, Fig. 1a). However, staining intensity of AMH varied among preantral follicles at the same developmental stages. AMH staining was detectable in some, but not all, primary follicles (Fig. 1b), and was either modest or intense in early (Fig. 1c) and late (Fig. 1d) secondary follicles within the same ovarian section. In preantral and small antral follicles with positive staining of AMH (Fig. 1e), immunostaining of AMHR2 was also evident in granulosa cells (Fig. 1f). The variation of AMHR2 staining intensity followed a similar pattern as that of AMH in preantral follicles (data not shown). Immunostaining for AMHR2 in the oocyte (Fig. 1f) appeared to be nonspecific since similar staining was also observed in the oocyte when the ovarian section was incubated with primary antibody preabsorbed with the blocking peptide.
Fig. 1.
Immunohistochemical staining of macaque ovarian sections for anti-Müllerian hormone (AMH) and its type II receptor (AMHR2). Preantral and small antral (SA; bottom-left corner) follicles were stained for AMH (a; no staining in primordial follicles). Heterogeneous staining of AMH was evident among follicles at the primary (b) and secondary (c, d) stages. Positive staining of AMH (e) and AMHR2 (f) was evident in the same preantral and small antral (SA; bottom-left corner) follicles on adjacent ovarian sections, with staining associated with the preabsorbed corresponding antibodies illustrated in the upper-right corners. Solid arrows, intense staining of AMH in preantral follicles. Open arrows, negative or modest staining of AMH in preantral follicles. Scale bar = 100 μm for a, b, e, and f; 200 μm for c and d. PM primordial follicle, ES early secondary follicle, LS late secondary follicle, SA small antral follicle, NC negative control
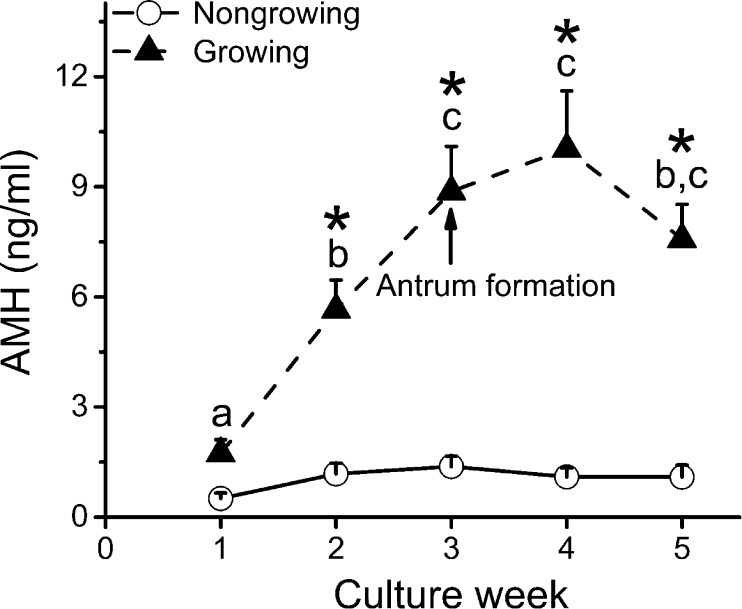
Though follicle diameters did not differ at the beginning of culture, distinct cohorts of viable follicles were observed based on their growth rates and antrum formation [23]. One cohort (26.5 %) remained similar in size to the initial secondary follicles without significant change in diameters or antrum formation throughout 5 weeks of culture and was termed “nongrowing” follicles. Another cohort (73.5 %) increased their diameters with antrum formation around week 3 and was termed “growing” follicles. During the first week of culture, media AMH concentrations produced by individual secondary follicles varied from 0.1 to 9.2 ng/ml. AMH levels increased (P < 0.05) in growing follicles during the first 3 weeks of culture and plateaued after antrum formation (Fig. 2). Growing follicles produced higher (P < 0.05) levels of AMH at weeks 2–5 of culture compared with those of nongrowing follicles (Fig. 2).
Fig. 2.
Media anti-Müllerian hormone (AMH) concentrations produced by nongrowing (n = 9) and growing (n = 25) follicles during 5 weeks of culture. Data are presented as the mean ± SEM with differences considered significant at P < 0.05. Different letters (a–c) indicate differences between culture weeks for growing follicles. Asterisks, differences between growing and nongrowing follicles at each culture week
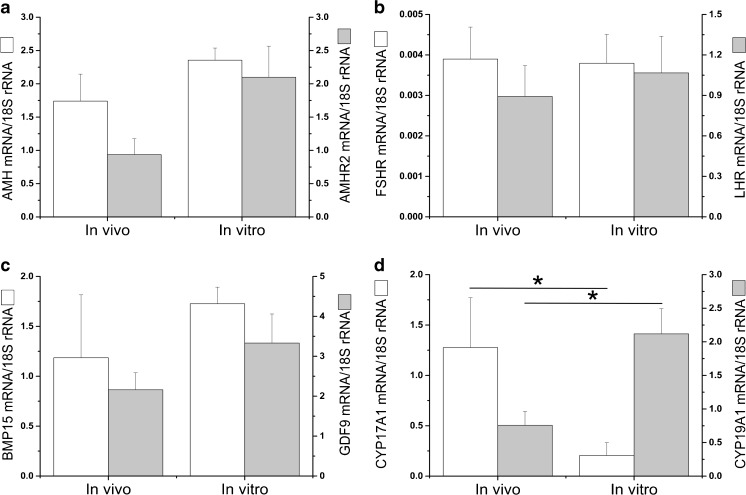
The mRNA expression of both AMH and AMHR2 was detectable in all pooled samples of isolated secondary (AMH mRNA/18S rRNA = 2.8 ± 0.2; AMHR2 mRNA/18S rRNA = 2.5 ± 0.6) and small antral follicles (Fig. 3a) from four monkeys by real-time PCR. Only 917 out of 15,236 (6.0 %) annotated genes were identified as differentially expressed with a >2-fold change (log2FoldChange; P < 0.05) between small antral follicles developed in vivo and in vitro by RNA sequencing. There were no differences in mRNA levels for genes that are involved in AMH signaling (e.g., AMH, AMHR2, and activin receptor-like kinase), gonadotropin signaling (e.g., LHCGR and FSHR), or oocyte growth (e.g., BMP15 and GDF9). Real-time PCR confirmed that mRNA levels were comparable between small antral follicles developed in vivo and in vitro for AMH, AMHR2 (Fig. 3a.), FSHR, LHCGR (Fig. 3b), BMP15, and GDF9 (Fig. 3c). However, compared with in vivo-developed follicles, mRNA levels of genes involved in ovarian steroidogenesis were altered in small antral follicles developed in vitro (Table 1). Lower (P = 0.04) mRNA levels for CYP17A1 and higher (P = 0.01) mRNA levels for CYP19A1 in in vitro-, relative to in vivo-, developed small antral follicles were identified (Fig. 3d). In addition, RNA sequencing revealed differences in mRNA levels of genes involved in follicular function in mural granulosa cells and cumulus cells between in vitro- and in vivo-developed small antral follicles. The majority of differentially expressed genes that promote ovarian angiogenesis or cell apoptosis were downregulated, while those that facilitate cumulus cell glycolysis or cell survival were upregulated, in small antral follicles grown in vitro relative to those developed in vivo (Table 2). RNA sequencing data were submitted to the Sequence Read Archive at http://www.ncbi.nlm.nih.gov/sra (accession number: SRP044327).
Fig. 3.
Real-time PCR validation of mRNA levels for selected genes in small antral follicles developed in vivo and in vitro based on RNA sequencing data. Data are presented as the mean ± SEM with 4 animals per group. AMH anti-Müllerian hormone, AMHR2 AMH receptor II (a); FSHR follicle-stimulating hormone receptor, LHCGR luteinizing hormone/choriogonadotropin receptor (b); BMP15 bone morphogenetic protein 15, GDF9 growth differentiation factor 9 (c); CYP17A1 cytochrome P450 family 17 subfamily A polypeptide 1, CYP19A1 cytochrome P450 family 19 subfamily A polypeptide 1 (d); 18S rRNA, internal control. *, significant difference with P < 0.05
Table 1.
Comparison of mRNA levels for genes involved in steroidogenesis between in vitro- and in vivo-developed small antral follicles
| Gene name | Synonym | Gene symbol | GenBank accession number | Fold changea (in vitro/in vivo) |
|---|---|---|---|---|
| Low-density lipoprotein receptor | – | LDLR | NM_000527 | 2.6 ↑ |
| Steroidogenic acute regulatory protein | – | STAR | NM_000349 | |
| Cytochrome P450 family 11 subfamily A polypeptide 1 | Cholesterol side-chain cleavage enzyme | CYP11A1 | NM_000781 | 4.2 ↑ |
| Hydroxy-delta-5-steroid dehydrogenase, 3 beta- and steroid delta-isomerase 1 | 3 beta-hydroxysteroid dehydrogenase/Delta 5→4-isomerase | HSD3B1 | NM_000862 | – |
| Cytochrome P450 family 17 subfamily A polypeptide 1 | Steroid 17-alpha-hydroxylase/17,20 lyase | CYP17A1 | NM_000102 | – |
| Cytochrome P450 family 19 subfamily A polypeptide 1 | Cytochrome P-450 aromatase | CYP19A1 | NM_000103 | 4.3 ↑ |
| Hydroxysteroid (17-beta) dehydrogenase 1 | Estradiol 17-beta-dehydrogenase 1 | HSD17B1 | NM_000413 |
↑ mRNA levels increased in in vitro- versus in vivo-developed small antral follicles
aLog2FoldChange in mRNA levels that is greater than 2 by RNA sequencing; n = 4 animals
Table 2.
Comparison of mRNA levels for selected genes involved in antral follicle function between in vitro- and in vivo-developed small antral follicles
| Gene name | Synonym | Gene symbol | GenBank accession number | Fold changea (in vitro/in vivo) |
|---|---|---|---|---|
| Angiogenesis | ||||
| Vascular endothelial growth factor A | – | VEGFA | NM_003376 | 2.3 ↑ |
| Phospholipase A2, group IVF | Cytosolic phospholipase A2 zeta | PLA2G4F | NM_000413 | 3.1 ↑ |
| Fms-related tyrosine kinase 1 | Vascular endothelial growth factor receptor 1 | FLT1 | NM_002019 | 4.5 ↓ |
| Kinase insert domain receptor | Vascular endothelial growth factor receptor 2 | KDR | NM_002253 | 4.5 ↓ |
| Nitric oxide synthase 3 | – | NOS3 | NM_000603 | 3.9 ↓ |
| Thrombospondin 1 | – | THBS1 | NM_003246 | 3.7 ↓ |
| Runt-related transcription factor 1 | – | RUNX1 | NM_001754 | 3.5 ↓ |
| Serpin peptidase inhibitor, clade E, member 1 | Nexin/plasminogen activator inhibitor 1 | SERPINE1 | NM_000602 | 2.6 ↓ |
| Phosphoinositide-3-kinase, regulatory subunit 5 | – | PIK3R5 | NM_014308 | 2.2 ↓ |
| Cumulus cell glycolysis | ||||
| Fibroblast growth factor 8 | – | FGF8 | NM_006119 | 3.1 ↑ |
| Enolase 2 | Gamma-enolase | ENO2 | NM_001975 | 6.5 ↑ |
| Aldolase C, fructose-bisphosphate | – | ALDOC | NM_005165 | 3.7 ↑ |
| Lactate dehydrogenase A | L-lactate dehydrogenase | LDHA | NM_005566 | 2.9 ↑ |
| Phosphoglycerate mutase 1 | – | PGAM1 | NM_002629 | 2.8 ↑ |
| Hexokinase 2 | – | HK2 | NM_000189 | 2.7 ↑ |
| Phosphoglycerate kinase 1 | – | PGK1 | NM_000291 | 2.4 ↑ |
| Triosephosphate isomerase 1 | – | TPI1 | NM_000365 | 2.4 ↑ |
| Pyruvate kinase, muscle | Pyruvate kinase isozymes M1/M2 | PKM | NM_002654 | 2.3 ↑ |
| Glyceraldehyde-3-phosphate dehydrogenase | – | GAPDH | NM_002046 | 2.2 ↑ |
| Fructose-1,6-bisphosphatase | – | FBP1 | NM_000507 | 2.1 ↓ |
| Apoptosis | ||||
| Insulin-like growth factor 2 | Somatomedin A | IGF2 | NM_000612 | 2.9 ↑ |
| V-kit Hardy-Zuckerman 4 feline sarcoma viral oncogene homolog | Mast/stem cell growth factor receptor | KIT | NM_000222 | 2.9 ↑ |
| Integrin, alpha 6 | – | ITGA6 | NM_000210 | 2.1 ↑ |
| Tumor necrosis factor (ligand) superfamily, member 10 | – | TNFSF10 | NM_003810 | 4.0 ↓ |
| Growth arrest and DNA-damage-inducible, beta | – | GADD45B | NM_015675 | 3.1 ↓ |
| Tumor necrosis factor | – | TNF | NM_000594 | 2.4 ↓ |
| Phosphoinositide-3-kinase, regulatory subunit 5 | – | PIK3R5 | NM_014308 | 2.4 ↓ |
| Heparin-binding EGF-like growth factor | – | HBEGF | NM_001945 | 2.3 ↓ |
↑/↓ mRNA levels increased/decreased in in vitro- versus in vivo-developed small antral follicles
aLog2FoldChange in mRNA levels that is greater than 2 by RNA sequencing; n = 4 animals
Experiment II
As reported in experiment I, nongrowing and growing follicles were also observed during individual follicle culture in experiment II. Metaphase II (MII) oocytes were only produced by growing follicles.
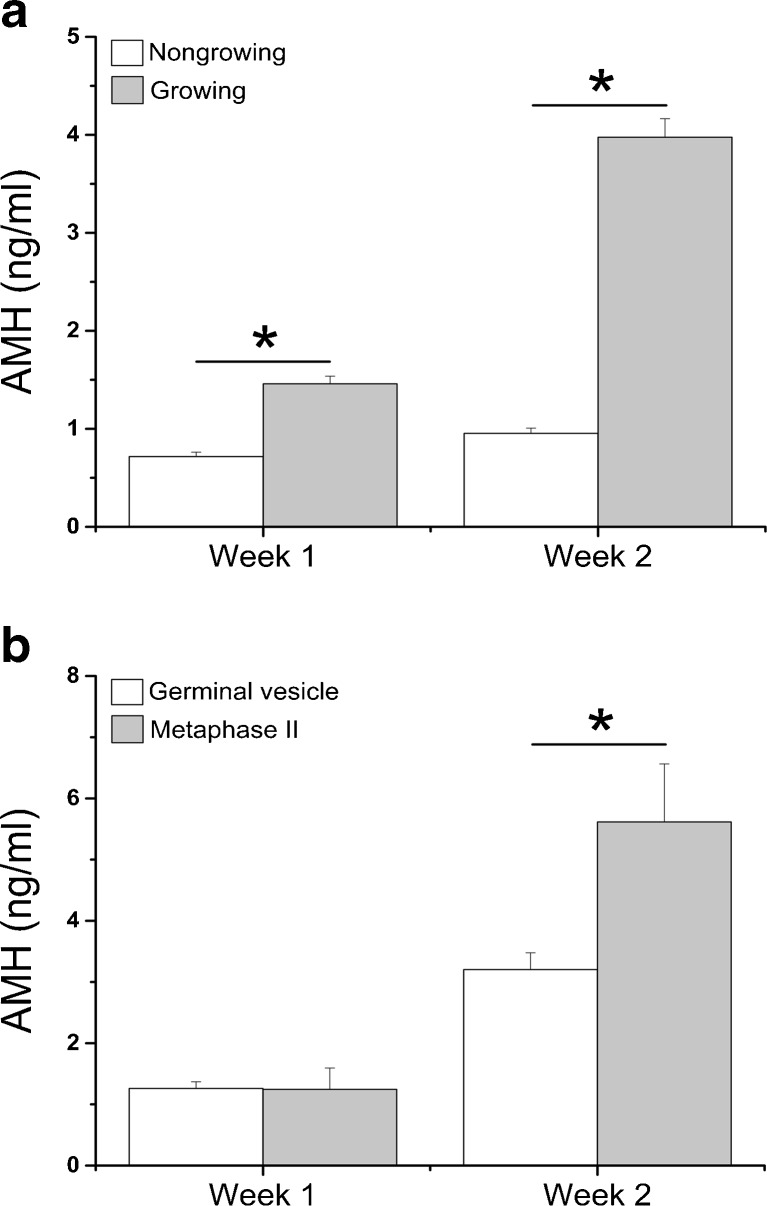
Media AMH concentrations produced by growing follicles (n = 229) were higher (P < 0.0001) than those of nongrowing follicles (n = 102) at culture week 1 and week 2 (Fig. 4a). Over 95 % of secondary follicles producing >1.4 ng/ml AMH at week 2 grew to the small antral stage at week 5. With a cutoff value of 1.4 ng/ml AMH, 85.3 % of nongrowing follicles can be filtered out at week 2 of culture while losing only 5.0 % of growing follicles. There were no differences in media AMH concentrations at week 1 between growing follicles producing germinal vesicle- and MII-stage oocytes at week 5 (Fig. 4b). However, growing follicles that generated MII oocytes (n = 9) secreted greater (P = 0.0001) amounts of AMH at week 2 than did those yielding germinal vesicle (GV) oocytes (n = 67), though follicle diameters at week 2 were comparable between the two follicle cohorts (follicles yielding GV versus MII oocytes—302.1 ± 8.4 versus 310.4 ± 16.5 μm).
Fig. 4.
Media anti-Müllerian hormone (AMH) concentrations produced by nongrowing (n = 102) and growing (n = 229) follicles (a), as well as by growing follicles yielding germinal vesicle (n = 67) and metaphase II (n = 9) oocytes at week 5 (b), during weeks 1 and 2 of culture. Data are presented as the mean ± SEM. *, significant difference with P < 0.05
Discussion
The current study used multiple approaches to investigate the expression of AMH and its type II receptor, AMHR2, in defined follicle types of the primate ovary, which represents a step toward the development of a novel biomarker for in vitro follicular development. The mRNAs for AMH and AMHR2 were identified in isolated preantral and small antral follicles by real-time PCR. Immunohistochemical staining further indicated that AMH and AMHR2 proteins were co-expressed in granulosa cells of growing follicles. AMH was not expressed by primordial follicles, while AMHR2 staining in the primordial follicle oocytes needs further investigation using an antibody without nonspecific oocyte staining. In addition, AMH protein secretion was detected in media during growth of individual macaque follicles in three-dimensional culture. The data are consistent with a previous study co-localizing AMH and AMHR2 mRNAs in granulosa cells of preantral and small antral follicles of the postnatal rat ovary by in situ hybridization [6]. Although AMH shares type I receptors with BMPs, it is generally believed that only the type II receptor (i.e., AMHR2) is specific for AMH signaling through a BMP-like signaling pathway [24]. Therefore, AMH actions via AMHR2 in regulating primate follicular development may be autocrine in nature, specifically within the granulosa cells of growing follicles.
The current study indicated that AMH was expressed as the follicle began to grow at the primary stage, increased during the subsequent development in preantral follicles, and peaked after antrum formation. The patterns are consistent with previous findings in nonprimate [1, 2] and primate [10, 11] species. However, though similar in size and morphological characteristics, macaque preantral follicles at the same developmental stage appeared to differ in AMH biosynthesis and secretion, as distinguished by AMH immunostaining intensity and AMH concentrations in the culture media. Although not reported in rodents and domestic animals, similar findings were demonstrated in marmoset preantral follicles by immunohistochemistry [9] and in macaque preantral follicles during culture [10]. In a recent study, it was hypothesized that AMH promotes preantral follicle growth to the small antral stage in the primate ovary [25]. Since AMH production by individual preantral follicles correlated positively with follicle growth rate in vitro, as reported in the current and previous [10] studies, the heterogeneity of AMH production may indicate differences in the local actions of AMH and in growth capacities of preantral follicles in primates.
The three-dimensional follicle culture system is valuable as an in vitro model for studying indices of primate ovarian folliculogenesis [12, 25, 26]. To date, the transcriptome of in vitro-developed follicles has only been studied in mouse from the secondary to antral stage during culture [27]. In the current study, transcriptomic profiles were compared between macaque antral follicles developed in vivo and in vitro to further evaluate whether the follicle culture system recapitulates in vivo follicular development. The data suggest that mRNA expression is generally comparable between in vivo- and in vitro-developed small antral follicles in terms of AMH signaling, gonadotropin signaling, and oocyte growth. The data are consistent with findings from mouse follicle culture by real-time PCR in which mRNA expression patterns, including BMP15 and GDF9, of oocytes from cultured follicles were similar to those of in vivo-developed follicles [28]. However, mRNA expression for steroidogenic enzymes (e.g., CYP17A1 and CYP19A1) was altered during follicle culture, which may impact follicular capacity for steroid biosynthesis. The lower levels of CYP17A1 may indicate limited development of the theca layer or its steroidogenic function in in vitro-developed antral follicles [29]. The increased CYP19A1 mRNA levels may be due to overexposure of follicles to FSH during culture, which is consistent with the observation that FSH stimulated the proliferation of granulosa cells, as well as their CYP19A1 expression and estradiol secretion [30]. The change in steroid (e.g., progesterone, androgen, and estrogen) production may affect subsequent follicular growth and oocyte maturation in vitro [12, 26]. Therefore, the culture conditions need to be further improved to optimize granulosa and theca cell proliferation and differentiation in antral follicles to facilitate proper steroidogenic function and oocyte maturation that mimics in vivo follicular development.
RNA sequencing revealed that mRNA levels for vascular endothelial growth factor receptors and other angiogenic regulatory factors were downregulated in in vitro-developed small antral follicles. This suggests that, though with similar sizes, in vitro-derived small antral follicles may not achieve the same maturation state as that of follicles developed in vivo which require vascularization for further growth [31]. Or the vascularization process may become less essential, and therefore limited, in antral follicles due to the sufficient O2 and nutrient supply in culture. The oocyte is deficient in utilizing glucose and requires cumulus cell glycolysis to provide energy substrates [32]. Transcripts encoding key enzymes in glycolytic pathway were upregulated in small antral follicles developed in vitro. Because follicles were cultured at 5 % O2 to improve survival and growth rates [16], the hypoxia condition may promote follicular glucose metabolism as reported in mouse follicle culture [33]. The mRNA levels of proapoptotic factors were low, while anti-apoptotic factors were high, in cultured small antral follicles as indicated by the RNA sequencing. During each menstrual cycle, only one of the small antral follicles is selected for further growth to become a dominant follicle in macaques while remaining ones undergo atresia, which is similar to that of women [7]. The process of programmed cell death, e.g., apoptosis, could already be initiated in in vivo-derived small antral follicles at the time of follicle collection. However, follicle atresia may not take place in vitro due to the absence of factors that induce apoptosis, or the presence of exogenous FSH that promotes the expression of anti-apoptotic proteins [34]. Future studies will further validate the RNA sequencing results of these selected genes, and to investigate the causes and effects of differential gene expression as references to improve coordinated follicular development leading to oocyte competence.
The follicle culture system is also considered as a potential option of fertility preservation for women facing fertility-threatening medical treatment [35]. However, the current systems for nonhuman primate [10] and human [36] follicle culture appear to be inefficient with prolonged culture period and low oocyte maturation rates. To date, the viability assessment of cultured follicles is based on rather subjective morphological criteria or molecular parameters requiring termination of follicle growth. AMH is a secreted protein with media concentrations detectable during follicle culture. AMH produced by follicles grown from the preantral to small antral stage in vitro exhibited similar patterns as those of follicles developed in vivo. Therefore, AMH may serve as a noninvasive biomarker indicating follicle conditions at specific stages of development in vitro. In addition, primate preantral follicles appeared to be heterogeneous in terms of their ability to grow, as well as function in AMH production and oocyte maturation, in vitro. Cultured follicles that grew and yielded mature oocytes tended to secrete higher levels of AMH at the secondary stage. Thus, media AMH concentrations may be a marker to predict further follicular development in vitro. The current study identified a cutoff value of 1.4 ng/ml media AMH, which may be used to remove nongrowing from growing follicles during the initial weeks of macaque follicle culture. Future analyses, with increased sample sizes, are warranted to generate a cutoff value of media AMH to identify growing follicles that are most likely to respond to hCG and produce mature oocytes for fertilization.
In summary, the AMH receptor signaling pathway is expressed in growing preantral and small antral follicles in the primate ovary in a stage-dependent manner. Co-expressed with its receptor, AMH appears to be an autocrine factor in regulating primate ovarian follicular development. This stage-dependent expression of a ligand and its receptor has been noted for other TGFβ superfamily members in human follicles [37]. Studies are ongoing to further unravel the molecular mechanisms of AMH signaling in the primate follicle by gene silencing. Due to its stage- and follicle-specific production, AMH has the potential to report follicle condition and growth capacity in vitro. AMH as a noninvasive biomarker may be applied to human follicle culture to eliminate follicles that are not likely to grow and mature, and thereby saving time and resources by aiding in the selection of follicles that will likely produce meiotically competent oocyte.
Acknowledgments
We are thankful for the assistance provided by members of the Division of Comparative Medicine, the Endocrine Technology Support Core, the Histopathology-Morphology Research Core, the Assisted Reproductive Technologies Support Core, the Molecular & Cellular Biology Support Core, and the Biostatistics and Bioinformatics Unit at ONPRC, as well as the OHSU Massively Parallel Sequencing Shared Resource. The valuable assistance of Ms. Maralee Lawson at ONPRC with follicle culture is appreciated. We are grateful to Dr. Richard L. Stouffer at ONPRC for his valuable expertise and critical review of the manuscript.
Research reported in this publication was supported by the National Institutes of Health (NIH) Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD) R01HD082208, NIH Office of Research on Women’s Health/NICHD K12HD043488 (Building Interdisciplinary Research Careers in Women’s Health, BIRCWH), NIH Office of the Director P51OD011092 ONPRC Pilot Grant, Collins Medical Trust, American Society for Reproductive Medicine, and Medical Research Foundation of Oregon. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Compliance with ethical standards
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
This study was funded by the National Institutes of Health (NIH) Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD) R01HD082208, NIH Office of Research on Women’s Health/NICHD K12HD043488 (Building Interdisciplinary Research Careers in Women’s Health, BIRCWH), NIH Office of the Director P51OD011092 ONPRC Pilot Grant, Collins Medical Trust, American Society for Reproductive Medicine, and Medical Research Foundation of Oregon.
Footnotes
Capsule
AMH, co-expressed with AMHR2, was produced heterogeneously by preantral follicles in macaques with levels correlated positively with follicle growth and oocyte maturation. AMH may serve as a biomarker for primate follicular development in vitro.
References
- 1.Durlinger AL, Visser JA, Themmen AP. Regulation of ovarian function: the role of anti-Müllerian hormone. Reproduction. 2002;124:601–9. doi: 10.1530/rep.0.1240601. [DOI] [PubMed] [Google Scholar]
- 2.Rico C, Médigue C, Fabre S, Jarrier P, Bontoux M, Clément F, et al. Regulation of anti-Müllerian hormone production in the cow: a multiscale study at endocrine, ovarian, follicular, and granulosa cell levels. Biol Reprod. 2011;84:560–71. doi: 10.1095/biolreprod.110.088187. [DOI] [PubMed] [Google Scholar]
- 3.Andersen CY, Schmidt KT, Kristensen SG, Rosendahl M, Byskov AG, Ernst E. Concentrations of AMH and inhibin-B in relation to follicular diameter in normal human small antral follicles. Hum Reprod. 2010;25:1282–7. doi: 10.1093/humrep/deq019. [DOI] [PubMed] [Google Scholar]
- 4.Dewailly D, Andersen CY, Balen A, Broekmans F, Dilaver N, Fanchin R, et al. The physiology and clinical utility of anti-Mullerian hormone in women. Hum Reprod Update. 2014;20:370–85. doi: 10.1093/humupd/dmt062. [DOI] [PubMed] [Google Scholar]
- 5.Baarends WM, van Helmond MJ, Post M, van der Schoot PJ, Hoogerbrugge JW, de Winter JP, et al. A novel member of the transmembrane serine/threonine kinase receptor family is specifically expressed in the gonads and in mesenchymal cells adjacent to the Müllerian duct. Development. 1994;120:189–97. doi: 10.1242/dev.120.1.189. [DOI] [PubMed] [Google Scholar]
- 6.Baarends WM, Uilenbroek JT, Kramer P, Hoogerbrugge JW, van Leeuwen EC, Themmen AP, et al. Anti-müllerian hormone and anti-müllerian hormone type II receptor messenger ribonucleic acid expression in rat ovaries during postnatal development, the estrous cycle, and gonadotropin-induced follicle growth. Endocrinology. 1995;136:4951–62. doi: 10.1210/endo.136.11.7588229. [DOI] [PubMed] [Google Scholar]
- 7.Gougeon A. Regulation of ovarian follicular development in primates: facts and hypotheses. Endocr Rev. 1996;17:121–55. doi: 10.1210/edrv-17-2-121. [DOI] [PubMed] [Google Scholar]
- 8.Durlinger AL, Gruijters MJ, Kramer P, Karels B, Kumar TR, Matzuk MM, et al. Anti-Müllerian hormone attenuates the effects of FSH on follicle development in the mouse ovary. Endocrinology. 2001;142:4891–9. doi: 10.1210/endo.142.11.8486. [DOI] [PubMed] [Google Scholar]
- 9.Thomas FH, Telfer EE, Fraser HM. Expression of anti-Mullerian hormone protein during early follicular development in the primate ovary in vivo is influenced by suppression of gonadotropin secretion and inhibition of vascular endothelial growth factor. Endocrinology. 2007;148:2273–81. doi: 10.1210/en.2006-1501. [DOI] [PubMed] [Google Scholar]
- 10.Xu J, Lawson MS, Yeoman RR, Molskness TA, Ting AY, Stouffer RL, et al. Fibrin promotes development and function of macaque primary follicles during encapsulated three-dimensional culture. Hum Reprod. 2013;28:2187–200. doi: 10.1093/humrep/det093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weenen C, Laven JS, Von Bergh AR, Cranfield M, Groome NP, Visser JA, et al. Anti-Müllerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. Mol Hum Reprod. 2004;10:77–83. doi: 10.1093/molehr/gah015. [DOI] [PubMed] [Google Scholar]
- 12.Rodrigues JK, Navarro PA, Zelinski MB, Stouffer RL, Xu J. Direct actions of androgens on the survival, growth and secretion of steroids and anti-Müllerian hormone by individual macaque follicles during 3-dimensional culture. Hum Reprod. 2015;30:664–74. doi: 10.1093/humrep/deu335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu M, West-Farrell ER, Stouffer RL, Shea LD, Woodruff TK, Zelinski MB. Encapsulated three-dimensional culture supports development of nonhuman primate secondary follicles. Biol Reprod. 2009;81:587–94. doi: 10.1095/biolreprod.108.074732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu F, Stouffer RL, Müller J, Hennebold JD, Wright JW, Bahar A, et al. Dynamics of the 604 transcriptome in the primate ovulatory follicle. Mol Hum Reprod. 2011;17:152–65. doi: 10.1093/molehr/gaq089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peluffo MC, Barrett SL, Stouffer RL, Hennebold JD, Zelinski MB. Cumulus-oocyte complexes from small antral follicles during the early follicular phase of menstrual cycles in rhesus monkeys yield oocytes that reinitiate meiosis and fertilize in vitro. Biol Reprod. 2010;83:525–32. doi: 10.1095/biolreprod.110.084418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu J, Lawson MS, Yeoman RR, Pau KY, Barrett SL, Zelinski MB, et al. Secondary follicle growth and oocyte maturation during encapsulated three-dimensional culture in rhesus monkeys: effects of gonadotrophins, oxygen and fetuin. Hum Reprod. 2011;26:1061–72. doi: 10.1093/humrep/der049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu J, McGee WK, Bishop CV, Park BS, Cameron JL, Zelinski MB, et al. Exposure of female macaques to Western-style diet with or without chronic T in vivo alters secondary follicle function during encapsulated 3-dimensional culture. Endocrinology. 2015;156:1133–42. doi: 10.1210/en.2014-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lohse M, Bolger AM, Nagel A, Fernie AR, Lunn JE, Stitt M, et al. RobiNA: a user-friendly, integrated software solution for RNA-Seq-based transcriptomics. Nucleic Acids Res. 2012;40(Web Server issue):W622–7. doi: 10.1093/nar/gks540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu TD, Nacu S. Fast and SNP-tolerant detection of complex variants and splicing in short reads. Bioinformatics. 2010;26:873–81. doi: 10.1093/bioinformatics/btq057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11:R106. doi: 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–50. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pavlov IY, Wilson AR, Delgado JC. Reference interval computation: which method (not) to choose? Clin Chim Acta. 2012;413:1107–14. doi: 10.1016/j.cca.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 23.Xu J, Bernuci MP, Lawson MS, Yeoman RR, Fisher TE, Zelinski MB, et al. Survival, growth, and maturation of secondary follicles from prepubertal, young, and older adult rhesus monkeys during encapsulated three-dimensional culture: effects of gonadotropins and insulin. Reproduction. 2010;140:685–97. doi: 10.1530/REP-10-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Visser JA. AMH signaling: from receptor to target gene. Mol Cell Endocrinol. 2003;211:65–73. doi: 10.1016/j.mce.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 25.Xu J, Bishop CV, Lawson MS, Park BS, Xu F. Anti-Müllerian hormone promotes preantral follicle growth, but inhibits antral follicle maturation and dominant follicle selection in primates. Hum Reprod. 2016;31:1522–30. doi: 10.1093/humrep/dew100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ting AY, Xu J, Stouffer RL. Differential effects of estrogen and progesterone on development of primate secondary follicles in a steroid-depleted milieu in vitro. Hum Reprod. 2015;30:1907–17. doi: 10.1093/humrep/dev119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skory RM, Bernabé BP, Galdones E, Broadbelt LJ, Shea LD, Woodruff TK. Microarray analysis identifies COMP as the most differentially regulated transcript throughout in vitro follicle growth. Mol Reprod Dev. 2013;80:132–44. doi: 10.1002/mrd.22144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sánchez F, Adriaenssens T, Romero S, Smitz J. Quantification of oocyte-specific transcripts in follicle-enclosed oocytes during antral development and maturation in vitro. Mol Hum Reprod. 2009;15:539–50. doi: 10.1093/molehr/gap051. [DOI] [PubMed] [Google Scholar]
- 29.McNatty KP, Makris A, Osathanondh R, Ryan KJ. Effects of luteinizing hormone on steroidogenesis by thecal tissue from human ovarian follicles in vitro. Steroids. 1980;36:53–63. doi: 10.1016/0039-128X(80)90067-7. [DOI] [PubMed] [Google Scholar]
- 30.Hillier SG. Gonadotropic control of ovarian follicular growth and development. Mol Cell Endocrinol. 2001;179:39–46. doi: 10.1016/S0303-7207(01)00469-5. [DOI] [PubMed] [Google Scholar]
- 31.Stouffer RL, Martínez-Chequer JC, Molskness TA, Xu F, Hazzard TM. Regulation and action of angiogenic factors in the primate ovary. Arch Med Res. 2001;32:567–75. doi: 10.1016/S0188-4409(01)00323-X. [DOI] [PubMed] [Google Scholar]
- 32.Biggers JD, Whittingham DG, Donahue RP. The pattern of energy metabolism in the mouse oocyte and zygote. Proc Natl Acad Sci U S A. 1967;58:560–7. doi: 10.1073/pnas.58.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Makanji Y, Tagler D, Pahnke J, Shea LD, Woodruff TK. Hypoxia-mediated carbohydrate metabolism and transport promote early-stage murine follicle growth and survival. Am J Physiol Endocrinol Metab. 2014;306:E893–903. doi: 10.1152/ajpendo.00484.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang JY, Cheung CK, Wang Y, Tsang BK. Regulation of cell death and cell survival gene expression during ovarian follicular development and atresia. Front Biosci. 2003;8:d222–37. doi: 10.2741/949. [DOI] [PubMed] [Google Scholar]
- 35.Woodruff TK. The emergence of a new interdiscipline: oncofertility. Cancer Treat Res. 2007;138:3–11. doi: 10.1007/978-0-387-72293-1_1. [DOI] [PubMed] [Google Scholar]
- 36.Xiao S, Zhang J, Romero MM, Smith KN, Shea LD, Woodruff TK. In vitro follicle growth supports human oocyte meiotic maturation. Sci Rep. 2015;5:17323. doi: 10.1038/srep17323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Knight PG, Glister C. TGF-beta superfamily members and ovarian follicle development. Reproduction. 2006;132:191–206. doi: 10.1530/rep.1.01074. [DOI] [PubMed] [Google Scholar]