Abstract
LPL hydrolyzes triglycerides in plasma lipoproteins. Due to the complex regulation mechanism, it has been difficult to mimic the physiological conditions under which LPL acts in vitro. We demonstrate that isothermal titration calorimetry (ITC), using human plasma as substrate, overcomes several limitations of previously used techniques. The high sensitivity of ITC allows continuous recording of the heat released during hydrolysis. Both initial rates and kinetics for complete hydrolysis of plasma lipids can be studied. The heat rate was shown to correspond to the release of fatty acids and was linearly related to the amount of added enzyme, either purified LPL or postheparin plasma. Addition of apoC-III reduced the initial rate of hydrolysis by LPL, but the inhibition became less prominent with time when the lipoproteins were triglyceride poor. Addition of angiopoietin-like protein (ANGPTL)3 or ANGPTL4 caused reduction of the activity of LPL via a two-step mechanism. We conclude that ITC can be used for quantitative measurements of LPL activity and interactions under in vivo-like conditions, for comparisons of the properties of plasma samples from patients and control subjects as substrates for LPL, as well as for testing of drug candidates developed with the aim to affect the LPL system.
Keywords: lipolysis, apolipoproteins, angiopoietin-like proteins, triglycerides, very low density lipoprotein, enzymology
The hydrolytic breakdown of plasma triglycerides by LPL at the capillary endothelium is a crucial event that contributes to control of the levels of triglycerides in plasma (1, 2). Many recent studies support the view that an elevated level of triglycerides in plasma is an independent risk factor for development of atherosclerosis (3–5). Therefore, the LPL system is considered to be an interesting target for drug design (6, 7).
LPL is produced and secreted from parenchymal cells like adipocytes and myocytes for transport to the luminal side of the endothelium via interaction with the glycosylphosphatidylinositol-anchored high density lipoprotein binding protein 1 (GPIHBP1) (8). Several plasma components have been shown to directly or indirectly modulate the activity of LPL. apoC-II and apoA-V increase the activity of LPL, while apoC-I, apoC-III, angiopoietin-like protein (ANGPTL)3, ANGPTL4, and ANGPTL8 decrease the activity (1, 9). The expression of each of these proteins depends on nutritional and hormonal factors, so that lipid uptake in tissues to a large extent is regulated by posttranslational effects on LPL (1, 9). It is possible that the macromolecular environment in plasma itself may be an influence on the interaction of LPL with its ligands. The protein concentration of plasma (80 g/l) has been shown to cause significant crowding effects (10). It is also possible that some plasma regulators of LPL activity have not been identified yet.
LPL activity can be measured in vitro by using artificial, usually emulsified, systems of radiolabeled, fluorogenic, or chromogenic substrates, or isolated triglyceride-rich lipoproteins (TRLs). The reaction products are detected at certain time points by chemical quantification or by determination of radioactivity or fluorescence. These methods have been used to unravel important aspects of the action of LPL and also to quantitate the levels of LPL activity in cells and tissues. Only small amounts of LPL activity are normally present in the circulating blood (11). Therefore, intravenous injections of heparin are made to release LPL from its endothelial binding sites. Determination of LPL activity in postheparin plasma, using artificial substrate systems, is considered to give an estimation of the amount of active LPL at the vascular endothelium (12).
Lack of a suitable technique for continuous monitoring of triglyceride hydrolysis in plasma has hampered the understanding of the action of LPL under physiological conditions. The properties of the substrate lipoproteins are likely to change during the lipolysis. When core triglycerides are removed, lipolysis products like monoglycerides and fatty acids may accumulate on the surface, the particle size will decrease, the surface pressure will increase and there will be an exchange of apolipoproteins between TRLs and other lipoproteins in plasma (13). Fatty acids can affect LPL either directly or through binding to some of its ligands, like ANGPTL4 (14, 15). Because the physical properties of the lipid substrate are sensed by LPL, there are several examples demonstrating that the activity of LPL on nonphysiological substrates is less affected by its regulator proteins (16) and is more resistant to thermal inactivation (17) or proteolytic cleavage (18) than when lipoproteins are used as substrates. Therefore, it is unlikely that determination of LPL activity in samples of postheparin plasma using artificial substrate systems will give a sufficiently good image of the lipolysis event in vivo.
In the present study, we demonstrate that isothermal titration calorimetry (ITC) overcomes a number of the limitations of other techniques for measurement of LPL activity. ITC provides a continuous assay using the observable heat rate that is directly proportional to the rate of the lipolysis (19). Raw data from ITC experiments are presented as thermograms in which the changes in the heat rate (also named heat flow, thermal power or heat flux) are monitored at a constant temperature. The method can be easily automated. We demonstrate that ITC can be used for determination of LPL activity on lipoproteins in human plasma. Both initial rates (zero-order kinetics) and kinetics for complete lipolysis can be measured. ITC can also be used for investigations of the effects of activating and inhibiting proteins on LPL activity. The ITC-based approach proposed in this report should be suitable for testing of drug candidates that are developed for targeting LPL activity.
MATERIALS AND METHODS
Reagents
Bovine LPL was purified from milk (20) and dialyzed to buffer containing 10 mM TRIS (pH 8.5, 4°C) and 4 mM sodium deoxycholate. Stock solutions of 0.5 mg LPL per milliliter were stored at −80°C. The N-terminal coiled-coil domain of human ANGPTL4, residues 26-184, was expressed in Escherichia coli and purified as described (14). Full-length human ANGPTL3, expressed in Sf 21 cells, was obtained from R&D Systems (USA). apoC-III0 was purified from human plasma (21). Human apoC-II and human apoA-V were expressed in E. coli and purified as described (22, 23). A synthetic peptide corresponding to the N-terminal domain of human GPIHBP1, residues 23-51, was purchased from Caslo (Denmark). The sequence of the peptide was as follows: QQEEEEEDEDHGPDDYDEEDEDEVEEEET. Antibodies to human HL were raised in a goat against HL isolated from human postheparin plasma (24). The IgG fraction was isolated using Protein-A columns and the final preparation contained 5 mg protein per milliliter in 20 mM Na-phosphate buffer and 0.15 M NaCl (pH 7.4).
Decoded samples of human plasma (treated with EDTA) were obtained from the Tallinn Blood Centrum. Blood was taken by forearm vein puncture from healthy 20- to 30-year-old volunteers 2 h after they had eaten a normal meal. Cells were removed from plasma by centrifugation for 30 min at 2,000 g at 4°C. The plasma samples were aliquoted and stored at −80°C and were only thawed once. EnzyChrom triglyceride assay kit (BioAssay Systems, USA) or Triglyceride Colorimetric assay kit (Cayman, USA) were used for determination of triglyceride concentrations. Fatty acids were quantified by the NEFA-HR (2) kit (Wako Chemicals). A sample of human postheparin plasma (used as LPL standard for many years in the Olivecrona group at Umeå University) was from a male volunteer that had received 100 IU heparin per kilogram body weight by intravenous injection in one forearm. After 15 min, blood was collected from the other arm into heparinized tubes and plasma was collected by centrifugation (11, 12). Human VLDLs were purified from normal plasma by floatation in the ultracentrifuge at d = 1.006 g/ml (25). The final preparation contained 1.3 mg protein per milliliter and 2.5 mM triglycerides. Commercial human VLDL was purchased from Kalen Biomedical (USA). This preparation contained 1 mg protein per milliliter and 4.2 mM triglycerides. Goat serum was from Invitrogen (product code 10000C). Intralipid (a 20% phospholipid-stabilized emulsion of soy bean triglycerides used for parenteral nutrition of patients) was obtained from Sigma. Heparin was purchased from LEO Pharma (Denmark).
Sample preparation
Before the experiments, the plasma samples were diluted 1.2 times with TRIS buffer (pH 7.4) or with the additions specified for each experiment. The final concentration of TRIS was 20 mM in all cases. The stock solution of bovine LPL was diluted in cold 10 mM TRIS (pH 8.5) containing 4 mM sodium deoxycholate. In this buffer, LPL is stable for a long period of time, even at low protein concentrations. The final concentration of deoxycholate during incubations with plasma or lipoproteins was 10 to 100 times lower than the initial. Control experiments showed that these levels had no influence on the enzymatic reaction. For inhibition of HL activity in postheparin plasma, 0.5 vol of goat anti-human HL IgG or the corresponding volume of PBS were added to the plasma. The mixture was then incubated for 2 h on ice prior to the experiments (24). All samples were degassed under vacuum for 15 min before the ITC experiments.
ITC measurements
Most of the experiments were performed on a Nano ITC model 5300 (TA Instruments, USA) at 25°C. A MicroCal Auto-iTC200 (GE Healthcare) instrument was used for experiments on the relationship between total heat production and released fatty acids. In a typical experiment performed by Nano ITC, the lipase substrate (plasma, Intralipid 20%, or VLDL) with or without added ligands was placed in the calorimetric cell (1,035 μl) and the syringe (250 μl) was filled with LPL-containing solution (bovine LPL or postheparin plasma) (see Fig. 1A). The reference cell contained MilliQ water (1,032 μl). The stirring speed in the sample cell was 400 rpm. The baseline was left to stabilize for at least 1 h before LPL or postheparin plasma was injected. The first injection was 2 μl and after that single or sequential injections of 10 to 25 μl were made. The interval between the injections was from 200 to 500 s. In experiments performed by MicroCal Auto-iTC200, 400 μl of lipase substrate (plasma), 400 μl of equilibration buffer (20 mM TRIS, pH 7,4), and a sufficient volume of LPL (0.8 μM) were placed on the loading plate. The calorimetric cell (200 μl) was rinsed with equilibration buffer and loaded with 200 μl of substrate in an automated fashion. The syringe was loaded with 40 μl of LPL-containing solution. The stirring speed in the sample cell was 600 rpm and the baseline was stabilized automatically. The first injection of LPL was 0.2 μl and the second injection was 2 μl. The experiment time was 1 h to allow full hydrolysis of plasma triglycerides. After each experiment the Nano ITC sample cell was washed with the following solutions, one after the other: MilliQ water, 2% SDS, 40 mM NaOH, and finally 95% ethanol. The MicroCal Auto-iTC200 was washed with MilliQ water, 10% Decon90 (Decon Laboratories Ltd.), and 100% methanol. Raw ITC data were analyzed using the NanoAnalyze (TA Instruments) or MicroCal Origin (GE Healthcare).
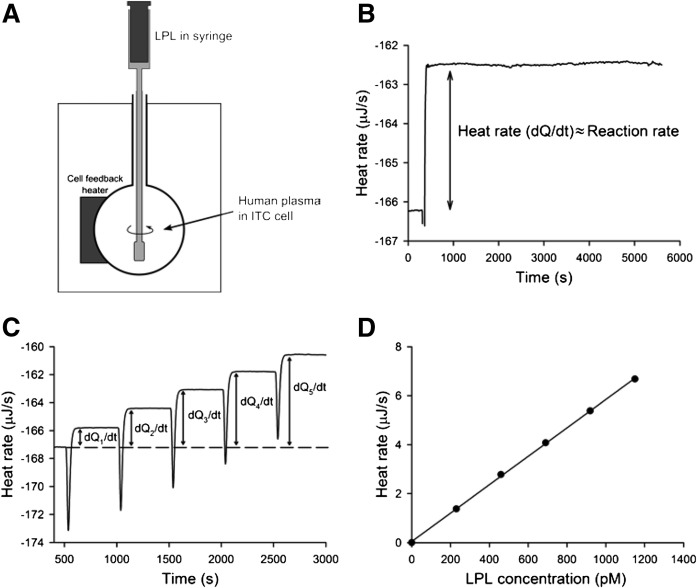
Fig. 1.
Enzymatic activity of LPL recorded by ITC. A: Schematic model of the ITC system. The ITC cell is filled with human plasma. LPL is injected to the cell from the syringe-stirrer. B: Heat rate change as a result of a single injection of LPL into ITC cell containing 1 ml of human plasma. Triglyceride and LPL concentrations in the ITC cell were 2.7 mM and 500 pM, respectively. The change of heat rate, dQ/dt, is proportional to the reaction rate. C: Sequential injections of LPL into the same aliquot of plasma as in (B). Each injection increased LPL concentration in the ITC cell by 230 pM. D: Data obtained from (C) presented as relationship of heat rate versus LPL concentration.
HL assay
Measurements of HL activity were performed using a gum arabic-stabilized emulsion of 3H-triolein and soy bean triglycerides (24). For measurement of HL activity, samples corresponding to 10 μl of postheparin plasma were incubated (in triplicates) in a total volume of 200 μl emulsion mixture for 40 min at 25°C. One unit of enzyme activity corresponds to release of 1 μmol fatty acid per minute.
RESULTS
ITC can be used for measurements of LPL activity in human plasma
To evaluate whether ITC could be used for studies of LPL action, we first tested the stability of LPL during the ITC experiments. The ITC cell was filled with human plasma and LPL was injected (Fig. 1A). As can be seen in Fig. 1B, this resulted in an increase of the heat rate, which then remained constant for the duration of the experiment (5,500 s). This demonstrated, as expected, that the reaction was exothermic, and also that the catalytic activity of LPL was unchanged during the whole experiment. The reaction followed zero-order kinetics, meaning that consumption of the substrate (presumably triglycerides and phospholipids in plasma lipoproteins) or changes of the physical properties of the substrate due to lipolysis did not influence the reaction rate. Sequential injections of LPL into the ITC cell led to a step-wise increase of the heat rate (Fig. 1C). The heat rate level increased almost equally with each injection, indicating a proportional relationship between the concentration of LPL and the reaction rate (Fig. 1D).
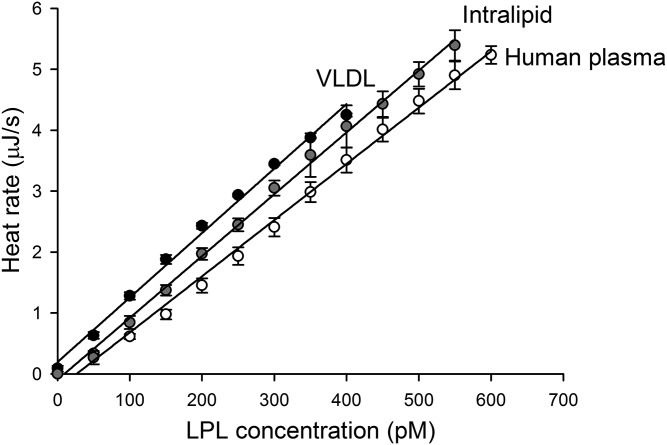
Next we compared the activity of LPL in plasma to that recorded with isolated human VLDL or with the synthetic lipid emulsion, Intralipid (with goat serum as a source of apoC-II), as substrate (Fig. 2). At the same initial triglyceride concentration (1 mM), the heat rate level was proportional to the concentration of LPL in all three systems, at least up to 400 pM (Fig. 2). The observed heat production rate was almost the same, suggesting that the total heat production was mainly due to hydrolysis of triglycerides. The calculated P values (unpaired, two-tailed distribution; pairwise comparison between slopes of the lines) were as follows: plasma/VLDL, P = 0.006; plasma/Intralipid, P = 0.07; VLDL/Intralipid, P = 0.2. The lowest detectable concentration of LPL differed only slightly between the substrate systems. It is generally accepted that a measurement is reliable when the determined parameter is at least ten times over the noise level. The noise level, calculated as the standard deviation of the heat rate level, was found to be 24.6 ± 2.1 nJ/s. Hence, for reliable determinations of LPL activity the change of heat rate must be over 250 nJ/s. Based on the slope of the relationship between heat rate and LPL concentration (Fig. 2, human plasma), it was possible to determine that 50 pM is the lowest concentration of LPL that can be reliably measured by the ITC instrument when the triglyceride concentration is 1 mM. The broad linearity range demonstrates that the ITC technique is suitable for quantitative measurements of LPL activity.
Fig. 2.
ITC-based assays for determination of LPL activity using different substrate systems. Sequential injections of LPL into human plasma, substrate mixture containing human VLDL, or substrate mixture containing Intralipid. The initial triglyceride concentration was 1 mM in all cases. Each injection increased the LPL concentration by 50 pM. The slopes of the lines were as follows: human plasma 0.0092, Intralipid 0.0102, and VLDL 0.0106. The human plasma was diluted to contain 20 mM TRIS (pH 7.4). The substrate mixture used for VLDL contained 10 mg BSA/ml, 10 IU heparin/ml, 20 mM TRIS (pH 7.4), and 0.15 M NaCl. The substrate mixture used for Intralipid contained 63% inactivated serum from goat (as source for apoC-II) in addition to the mentioned constituents. The values are mean ± SD of three different measurements.
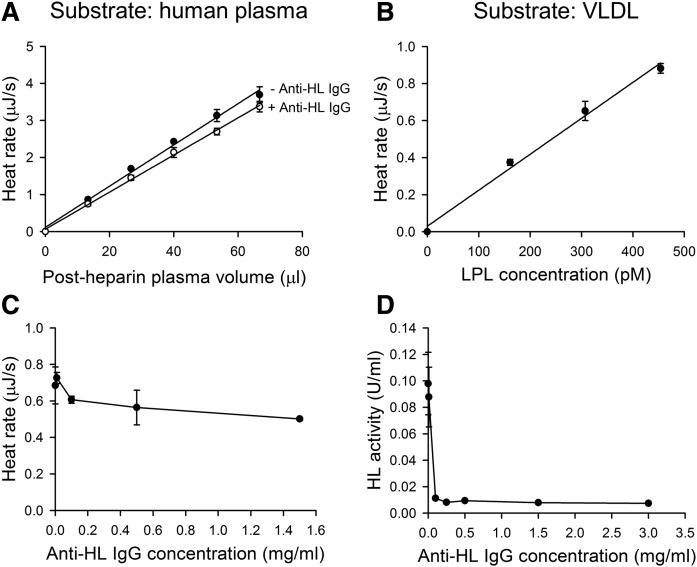
To investigate whether ITC could be used for measurement of LPL activity in human postheparin plasma, we injected small amounts of postheparin plasma from the syringe into the ITC cell containing triglyceride-rich normal human plasma. As with purified LPL, a linear relationship between the amount of postheparin plasma and the heat rate level was observed (Fig. 3A). Because postheparin plasma contains another triglyceride lipase in addition to LPL, named HL based on its tissue origin (26), we pretreated a sample of postheparin plasma with antibodies known to specifically inhibit HL (12, 24). The antibody concentration used in these experiments (1.5 mg/ml) had been shown to be sufficient to completely inhibit activity of HL (12, 24). After injection to the ITC cell, the slope of the heat rate versus plasma volume was only slightly decreased with plasma containing the antibodies compared with the original postheparin plasma diluted to the same extent with buffer. Analysis by t-test revealed that the differences between the slopes of the two lines (post-heparin plasma with and without the inhibitory antibody) were not significantly different (P = 0.13; paired, two-tailed distribution). To verify that the anti-HL IgG was able to fully inhibit HL, we made concentration curves with different amounts of IgG using ITC (Fig. 3C). We also used a specific HL assay with radiolabeled substrate to measure the remaining HL activity (Fig. 3D). These results demonstrate that the heat rate detected with injection of postheparin plasma to the ITC cell was almost fully due to LPL. Based on comparison of the activity of the purified LPL with that detected by injection of postheparin plasma (Fig. 2, human plasma and Fig. 3A), we estimated that the sample of postheparin plasma contained 7.3 pmol LPL per milliliter. This equals about 0.8 μg LPL/ml.
Fig. 3.
LPL activity in postheparin plasma as measured by ITC. A: Five sequential injections of human postheparin plasma with antibodies to HL (+ Anti-HL IgG) or without (− Anti-HL IgG) were made to 1 ml of normal human plasma (the final concentration of triglyceride was 1 mM). The final concentration of anti-HL IgG was 1.5 mg/ml. B: Three sequential injections of human postheparin plasma were made into 1 ml of substrate mixture containing commercial VLDL (the final concentration of triglyceride and protein were 0.42 mM and 0.1 mg/ml, respectively), and 10 mg BSA/ml, 10 IU heparin/ml, 20 mM TRIS (pH 7.4), and 0.15 M NaCl. C: Single injections of postheparin plasma preincubated with IgG to HL were made to normal human plasma. The concentrations of anti-HL IgG during preincubations were as follows: 0 mg/ml; 0.01 mg/ml; 0.1 mg/ml; 0.5 mg/ml; and 1.5 mg/ml. D: Measurements of remaining HL activity after preincubation of human postheparin plasma with anti-HL antibodies using a radiolabeled triolein/gum arabic emulsion incubated at 1 M NaCl. Postheparin plasma was preincubated with antibodies to HL at the following concentrations: 0 mg/ml; 0.01 mg/ml; 0.1 mg/ml; 0.25 mg/ml; 0.5 mg/ml; 1.5 mg/ml; and 3.0 mg/ml. The values are mean ± SD of three determinations.
To demonstrate that the ITC assay for LPL activity could be adopted for general use, measurements were performed with a commercial preparation of human VLDL (Fig. 3B). The triglyceride concentration was lower (0.42 mM) in this preparation than in the previously used plasma samples (1 mM), and the recorded LPL activity was lower than that obtained in Fig. 3A (about one-fourth). The activity was, however, sufficiently high for a linear determination based on sequential injections of postheparin plasma to the ITC cell (Fig. 3B).
ITC can be used to record complete hydrolysis of plasma lipids by LPL
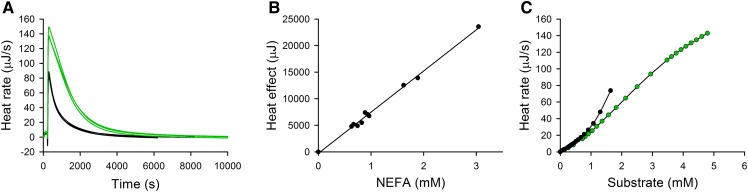
In the next experiments, ITC was used to monitor the kinetics for complete hydrolysis of available substrate lipids in plasma by LPL. For practical reasons, the amounts of LPL used were 40–100 times higher than those used for determination of initial rates. Examples of hydrolysis curves (run in triplicates) for two plasma samples, that differed in their initial triglyceride concentrations by a factor of 2.7, are shown in Fig. 4A. The areas under the curves correspond to the total heat production. The areas differed by a factor of 2.6, indicating a good correlation between total heat production and the initial triglyceride concentration of the plasma samples. The amounts of fatty acids released, as determined by the NEFA kit at the end of each reaction, were approximately 2-fold higher than the initial triglyceride concentrations in the plasma samples. This is in agreement with LPL being known to catalyze hydrolysis of the ester bonds at positions sn-1 and sn-3 of triglycerides (27) and that isomerization of acyl groups from the sn-2 position to the sn-1(3) positions is slow at pH 7.4. Thus, the actual substrate concentration can be considered to be equal to the concentration of hydrolyzable ester bonds, which is two times higher than the triglyceride concentration. Using results from several experiments, with plasma samples from different individuals, a linear correlation was found between the total heat production and the amounts of fatty acids released (Fig. 4B). The slope of this relationship was used for calculation of the apparent enthalpy ΔH, using the equation: Q = [P]VΔH, where Q is the total heat production, P is the concentration of released fatty acids, and V is the volume of the ITC cell (19). This calculation resulted in a ΔH value equal to 38.8 kJ/mol. To further analyze the curves for complete hydrolysis in Fig. 4A, the data were transformed into a plot of reaction rate versus remaining substrate concentration (Fig. 4C). This was obtained by subtracting the amount of hydrolyzed substrate at a chosen time point from the concentration of hydrolyzable ester bonds (the total area). This transformation enabled us to examine how the reaction rate depended on the substrate concentration, using data from a single hydrolysis curve. As can be seen, the reaction rate for the samples differed when the substrate concentration was high, but the rates were overlapping in the lower substrate range. This indicates that there might be detectable differences in the properties of plasma samples with regard to their ability to undergo hydrolysis by LPL.
Fig. 4.
Complete hydrolysis of lipids in human plasma by LPL as measured by ITC. A: Raw thermograms of total hydrolysis of human plasma lipids by LPL. Experiments were performed with plasma samples that contained 1 mM (black lines) or 2.7 mM (green lines) triglycerides. The concentration of added LPL was 22 nM. Three measurements for each plasma sample are presented. B: Relationship between the total heat production and the amounts of released fatty acids. Heat rates were obtained from full hydrolysis of 10 different human plasma samples on MicroCal Auto-iTC200. In these experiments, 200 μl of plasma was hydrolyzed by 8 nM LPL for 1 h. The surface areas of the curves for full hydrolysis were calculated in the MicroCal Origin and compared with the release of free fatty acids (final concentration minus initial concentration) measured using a NEFA kit. C: Transformation of the hydrolysis data presented in (A) into the relationship between heat rate and remaining substrate concentration (expressed as concentration of hydrolyzable ester bonds).
ITC can be used to study the influence of added regulators on the LPL reaction in plasma
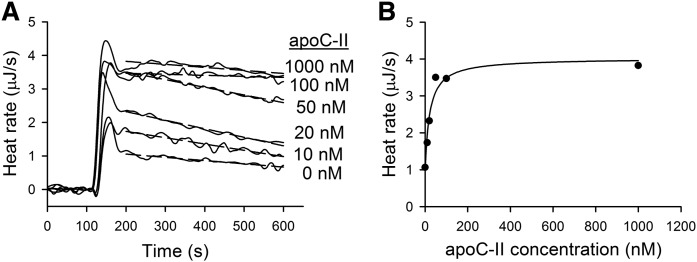
In the next set of experiments, we examined how the LPL activity in plasma was influenced by addition of apoC-II, apoC-III, apoA-V, ANGPTL3, or ANGPTL4. Experiments with apoC-II were carried out using Intralipid as substrate, because plasma normally contains sufficient amounts of apoC-II for full activation of LPL (28). The LPL concentration was held constant, while the apoC-II concentration was varied from 0 to 1,000 nM. ITC thermograms are presented in Fig. 5A. As expected, addition of apoC-II caused increased activity of LPL. To estimate the activation factor and the affinity of apoC-II for LPL, the heat rate values at 200 s were plotted against the apoC-II concentration (Fig. 5B). As can be seen, apoC-II activated LPL in a saturating fashion. For the analysis of the apoC-II activation data, we used a model proposed by Quinn, Shirai, and Jackson (29). The estimated maximal activation factor was 4.2 ± 1.2 and the Kd value was 22 ± 8 nM. The real time recordings demonstrated that LPL was unstable in the absence of apoC-II, or when the concentration of apoC-II was below 100 nM. Thus, in addition to activation, apoC-II also stabilized LPL. This is in accord with results from previous studies (30). The stabilizing effect of apoC-II is usually not recognized with other substrate systems used for measurements of LPL activity. The advantage with ITC is that the continuous monitoring of the reaction rate provides detailed information about the first minutes of the reaction.
Fig. 5.
Activation of LPL by apoC-II as studied by ITC. A: Raw ITC thermograms of hydrolysis of Intralipid by LPL in the absence or presence of apoC-II. The substrate mixture composition in the ITC cell was as follows: Intralipid corresponding to 1 mg triglyceride/ml, 10 mg/ml BSA, 10 IU/ml heparin, 0.15 M NaCl, and 10 mM TRIS (pH 7.4). Single injections of LPL were made in experiments that contained or did not contain human apoC-II. The concentration of LPL was 1.2 nM in all experiments. B: Hydrolysis heat rate as a function of apoC-II concentration. Heat rate points at 200 s were obtained from (A). The data were fitted using the following equation: v = kcat · L · [(β · C + Kd)/(C + Kd)], where v is the reaction rate, kcat is catalytic rate constant, L is the concentration of LPL, C is the concentration of apoC-II, β is the activation factor that indicates how much more active the LPL/apoC-II complex is compared with LPL alone, and Kd is the equilibrium dissociation constantQ11 (29).
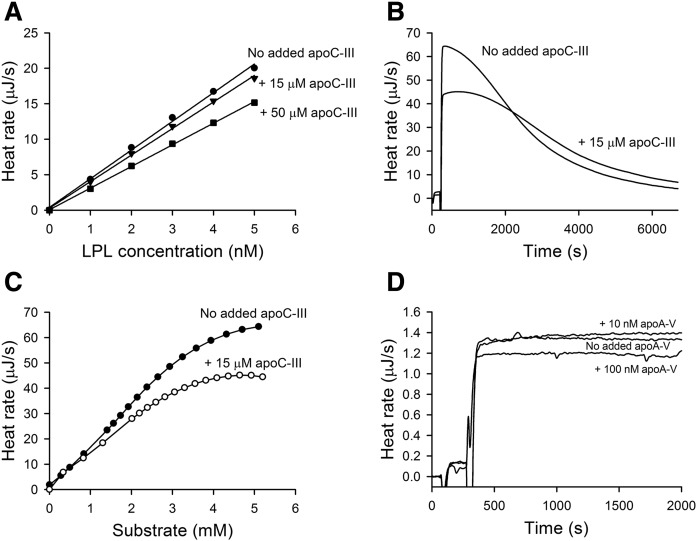
For studies of the effects of apoC-III on the activity of LPL, experiments were conducted: 1) under zero-order conditions when the LPL concentration was so low that the reaction rate was constant because the substrate was not significantly depleted (Fig. 6A); or 2) under conditions with higher concentrations of LPL resulting in complete hydrolysis of the substrate (Fig. 6B). Under zero-order conditions, a reduction of the reaction rate by apoC-III was observed at the start of the reaction and the reduction remained unchanged for more than 1 h (data not shown). This indicated that apoC-III affected the activity, rather than the stability of the enzyme. On sequential injections of LPL into plasma under zero-order conditions, without and with added apoC-III (15 or 50 μM), the presence of apoC-III clearly restricted the activity of LPL (Fig. 6A). Under conditions for complete lipolysis, addition of apoC-III caused an initial inhibition of lipolysis (Fig. 6B). With time, when much of the available substrate had been degraded, the heat rates without and with added apoC-III became similar. Transformation of the ITC data from Fig. 6B into plots of reaction rate versus calculated remaining substrate concentration illustrated that apoC-III inhibited LPL activity only when lipoproteins with high triglyceride content still remained (Fig. 6C). The added apoC-III (15 μM) corresponded approximately to a doubling of the concentration of apoC-III in normal human plasma (31).
Fig. 6.
Effect of apoC-III and apoA-V on LPL activity in plasma as studied by ITC. A: Effect of apoC-III on LPL activity under zero-order reaction conditions. Sequential injections of LPL were made into plasma samples in which the apoC-III concentration was increased by 15 μM, 50 μM, or not increased. The LPL concentration was increased by 1 nM per injection. The concentration of triglycerides in the ITC cell was 2 mM. B: Thermograms for complete hydrolysis by LPL in plasma when the concentration of apoC-III was not increased or was increased by 15 μM. The final concentration of LPL was 22 nM and the concentration of triglycerides was 2.7 mM. One representative curve from three different measurements is presented for each condition. C: Dependence of heat rate on substrate concentration as calculated from the curves presented in (B). D: LPL activity was measured in plasma in the absence or presence of added apoA-V corresponding to 10 nM or 100 nM. Single injections of LPL into the plasma were made. The concentrations of triglycerides and LPL in the ITC cell were 1 mM and 0.6 nM, respectively.
It was interesting to test the effect of apoA-V in the ITC system. Evidence in vivo point to a stimulation of LPL activity by apoA-V, but the effect has been difficult to reproduce in vitro (32). Using ITC and zero-order conditions, low concentrations of apoA-V (10 nM) did not stimulate the activity of LPL (Fig. 6D). On the contrary, higher concentrations of apoA-V (100 nM) seemed to inhibit LPL activity. It should be noted that the normal concentration of apoA-V in human plasma is considerably lower, about 4 nM (33).
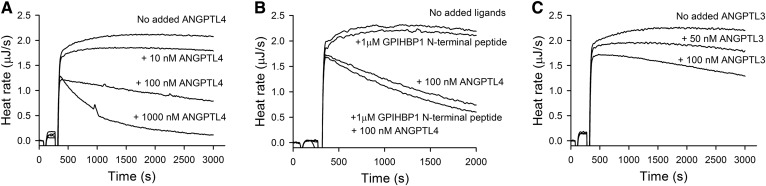
ANGPTL4 is considered to be a major regulator of LPL activity in vivo (9). The normal range for ANGPTL4 in plasma is between 0.04 and 3 nM (34). In experiments with ITC, suppression of LPL activity was seen by the presence of ANGPTL4 (the N-terminal fragment). A 10% drop of LPL activity was detected almost from the start of the experiment by the presence of added ANGPTL4 corresponding to an increase of the plasma concentration by only 10 nM. The decrease in activity persisted for the duration of the experiment (Fig. 7A). With higher concentrations of added ANGPTL4 (corresponding to 100 nM or 1,000 nM), the activity of LPL initially dropped more, and the heat rate continued to decrease after the first drop (Fig. 7A). These observations indicated that ANGPTL4 may affect LPL activity in plasma through two different mechanisms, one immediate that may involve inhibition of activity, but likely not irreversible inactivation. The other, seen at higher concentrations of ANGPTL4, is likely reflecting continuous irreversible inactivation of LPL. This occurred even though the enzyme was stable in plasma under the experimental conditions and the measurements were performed under the zero-order conditions, when the decrease of available substrates should not influence on the activity of LPL.
Fig. 7.
Effects of ANGPTL4 or ANGPTL3 on LPL activity in plasma as studied by ITC. In (A) and (C), the LPL activity was measured in the absence or presence of different added concentrations of ANGPTL4 (A) or ANGPTL3 (C). In (B), the effect of ANGPTL4 on LPL was tested in the presence of the N-terminal peptide of GPIHBP1. In all experiments a single injection of LPL into the plasma sample was made. The concentrations of triglycerides and LPL in the ITC cell were 1 mM and 0.6 nM, respectively.
Finally we wanted to study the effects of the LPL-binding protein, GPIHBP1, shown by others to stabilize LPL against inactivation by ANGPTL4 (35). Also, the acidic N-terminal peptide of GPIHBP1 is known to bind and stabilize LPL (36, 37). When added to the ITC system under zero-order conditions, the peptide did not protect LPL from inactivation by ANGPTL4 (Fig. 7B). Studies with full-length ANGPTL3 demonstrated that it had similar effects on LPL as the N-terminal fragment of ANGPTL4, but ANGPTL3 appeared to be less potent than ANGPTL4 (Fig. 7C).
DISCUSSION
We have demonstrated that ITC can be used to obtain reliable and reproducible data illustrating the action of LPL on plasma lipoproteins. The advantage with ITC is the possibility to perform continuous measurements of LPL activity in plasma, using changes in heat rate for determination of the reaction rate. There is, in general, a linear relationship between the reaction rate and the heat rate (19). However, in the case of lipolysis in plasma, the heat rate includes heat effects of at least four different processes: hydrolysis of ester bonds, structural changes of the lipoproteins due to removal of lipids, neutralization of the released fatty acids by the buffer, and binding of the fatty acids to albumin. In spite of this complexity, several observations suggest that the heat rate is a suitable quantitative parameter for measurement of catalytic activity of LPL in plasma. The arguments are: 1) that the heat produced by the action of LPL was proportional to the amount of fatty acids released; 2) that LPL was stable in the ITC cell for a long period of time; 3) that there was a linear dependency between the amount of LPL added to the ITC cell and the heat rate; 4) that on complete hydrolysis of the plasma lipids, the total heat production was linearly dependent on the initial triglyceride concentration; and 5) that the heat effect was nearly the same with the synthetic triglyceride emulsion Intralipid as with isolated VLDL or with triglyceride-rich plasma as substrate for LPL. Based on this, it is reasonable to assume that heat effects due to structural changes of the lipoproteins, or to other interactions established in the system, are negligible in comparison with those due to hydrolysis of ester bonds.
The ITC assay used for measurement of initial rates was sensitive enough to determine the levels of lipase activity in human postheparin plasma, but the activity present in preheparin human plasma was too low to be detected. The level of LPL activity in plasma before injection of heparin is only around 1% of that in postheparin plasma (12). In addition to LPL, heparin injection also releases endothelial lipase (EL) and HL from the vascular endothelium. Like LPL, these enzymes catalyze hydrolysis of triglycerides and phospholipids, and they may contribute to the ITC measurements. However, their substrate specificity is different from that of LPL. In the case of HL, we used inhibiting antibodies to demonstrate that HL did not significantly contribute to the lipase activity during the ITC assay. HL acts preferably on phospholipids in HDL and smaller VLDL, but it can also hydrolyze ester bonds in triglycerides (24, 38). Although it does not seem necessary in the ITC assay, we still advocate the use of inhibiting antibodies to HL for specific analyses of LPL activity in postheparin plasma. Slight variations in the substrate lipoproteins, or in the assay conditions, could otherwise allow HL to contribute to the hydrolysis. In the case of EL, it has been shown that TRLs are poor substrates (38). Furthermore, Badellino et al. (39) have shown that heparin administration increases the EL concentration in plasma only 3-fold. For comparison, LPL increases it more than 100-fold. In our ITC experiments, postheparin plasma was diluted 1.5 times with PBS or antibodies. Then 20 μl of this diluted sample was injected into the ITC cell, which contained 1 ml triglyceride-rich plasma. It follows that the concentration of EL was not significantly increased in the ITC cell after injection of postheparin plasma. Inhibiting antibodies to EL could be used to verify this conclusion.
The ITC assay is an alternative to assays that use radiolabeled (40) or fluorogenic substrates (41, 42), or directly measure released fatty acids by NEFA kits (43). Because plasma contains free fatty acids at high concentrations, [0.3–1 mM (44)], the NEFA kit is applicable only when significant amounts of fatty acids have been produced by the added LPL. In addition, most of the mentioned assays cannot be used for continuous registration of the lipolysis, and they are more laborious and time consuming than the ITC measurements. The use of plasma as substrate source is an advantage because plasma contains the natural substrate for LPL in the form of TRLs. The lipoproteins do not need to be isolated, meaning that the possible risk of damaging their native structure or changing their apolipoprotein composition can be avoided. For measurements of LPL activity, or functional studies of LPL and its controller proteins, it is important to use native substrates to be able to pick up functional effects that might be important in vivo. In addition, we demonstrate that commercially available human VLDL, or a synthetic emulsion of triglycerides like Intralipid, can be used as substrate in the ITC.
The broad linearity range, the high sensitivity, and the possibility to use an automatic setup are arguments for ITC for routine measurements of LPL activity. The continuous monitoring of the reaction rate allows accurate zero-order recordings of true initial rates. The stabilization of LPL by addition of apoC-II, as seen in the present study, is usually not detected in other substrate systems. Therefore the magnitude of the activation of LPL by apoC-II can easily be overestimated (30). ITC provided a method to carefully investigate possible effects of apoA-V on the catalytic activity of LPL. Animal experiments (45) and population studies in humans (46, 47) have demonstrated that apoA-V is an important regulator of triglyceride levels in plasma (48). apoA-V lowers triglyceride concentrations in vivo, but its effect has been difficult to reproduce in vitro using studies of LPL activity in different substrate systems (49). Our data from ITC adds to the notion that apoA-V does not directly stimulate the activity of LPL.
In addition to the usual determinations of the initial rates of hydrolysis, ITC allowed monitoring of the kinetics for complete lipolysis of the plasma samples by LPL. Transformation of a single lipolysis time course into a plot of heat rate versus remaining substrate concentration enabled us to follow how the reaction rate was changing during the degradation of TRLs to remnant particles. Comparison of plasma samples from two randomly selected volunteers demonstrated that individual differences could easily be picked up by ITC. This suggests that analyses of curves for total hydrolysis of plasma lipids by LPL can be used to search for possible causes for hypertriglyceridemia in individual subjects. We have not yet systematically explored this possibility.
The proteins apoC-III, ANGPTL3, and ANGPTL4 are known to suppress LPL activity in vivo and are all regarded as potential targets for drug development with the aim to lower triglyceride levels in plasma (6, 7). We found that addition of apoC-III to plasma could only moderately affect the activity of LPL. An increase of apoC-III by 50 μM reduced the activity of LPL by about 30%. These results are in line with the recent results of Gordts et al. (50), but are different from the data obtained with isolated chylomicrons as substrate for LPL where the inhibitory effect of apoC-III was stronger (51). In that case, complete inhibition was observed already by addition of apoC-III to a concentration of 10 μM. Our analyses of the ITC curves for complete hydrolysis of plasma lipids demonstrated that apoC-III affected LPL activity only when there were still TRLs in the system (at low degrees of lipolysis), but not later, when only triglyceride-poor lipoproteins remained (at high degrees of lipolysis). This can possibly be explained by expulsion of apoC-III from the surface of TRLs when their triglyceride content, or their size, is reduced. The distribution of apoC-III between plasma lipoproteins is known to be affected by the total triglyceride content in plasma, so that in plasma from subjects with elevated triglyceride levels, more apoC-III will be found with the TRLs and less with HDLs (52, 53). apoC-III was reported to displace LPL from triglyceride-rich emulsion particles (51). No direct interaction between LPL and apoC-III has so far been detected, meaning that the inhibition probably reflects competition between apoC-III and LPL for binding to the TRLs. Differences in the physical properties of the TRLs, as well as the levels of other plasma components, are likely to determine the binding of LPL to the TRLs, and thereby the lipolysis rate. HDLs are known to sequester apoC-III and other inhibiting apolipoproteins in an exchangeable fashion. ITC provides an easy method to study lipolysis directly in plasma, with all relevant components present, and to compare results from many different subjects.
Studies in recent years have identified some of the ANGPTLs as important regulators of lipid metabolism, and in particular as inhibitors/inactivators of LPL (9). There is as yet no consensus about by what mechanisms ANGPTL3 and ANGPTL4 suppress the activity of LPL. Results from several studies show that ANGPTL4 induces irreversible dissociation of active LPL dimers to inactive monomers (54–56). Shan et al. (57) have proposed that ANGPTL3 and ANGPTL4 inhibit LPL through different mechanisms and that only ANGPTL4 induces irreversible inactivation of LPL. Others have proposed that ANGPTL4 acts as a noncompetitive inhibitor by forming a reversible complex with LPL (58). Using ITC, addition of nanomolar concentrations of ANGPTL4 to plasma was required to see effects on LPL activity. This is at least one order of magnitude higher than the concentrations normally found in human plasma (34), and is in line with LPL being stable in plasma for the duration of the ITC experiments. We conclude that the concentrations of ANGPTL4 and ANGPTL3 in blood are too low to affect the activity of LPL. Our data confirm the previous results of Nilsson et al. (59) that were obtained using other techniques to measure effects on LPL activity. In the ITC experiments, ANGPTL4 was more efficient in lowering of LPL activity compared with ANGPTL3, but the time courses for the inhibition of LPL were similar for the two proteins. In both cases, it was possible to distinguish an initial fast phase followed by a slow phase. This suggests that the ANGPTLs may use similar inhibition mechanisms in the plasma environment, including both inhibition of LPL activity and irreversible inactivation. It is likely that a reversible complex is first formed between LPL and the ANGPTL, and that this may cause inhibition of the catalytic activity against TRLs and lipoprotein-like substrates. The interaction may then lead to irreversible inactivation of LPL by formation of LPL monomers and dissociation of the ANGPTLs from LPL, as previously suggested (54). It is tempting to speculate that both mechanisms, inhibition and inactivation, may operate on LPL in vivo.
Development of drugs that reduce the inhibitory effect of apoC-III, ANGPTL3 (60), or ANGPTL4 (61) on LPL activity is currently ongoing, with the aim to reduce plasma triglyceride levels in patients at high risk for coronary heart disease. In addition, an apoC-II mimetic peptide was tested to lower plasma triglycerides in subjects with mild to moderate hypertriglyceridemia or in patients with severe hypertriglyceridemia due to deficiency of apoC-II (62, 63). ITC offers a convenient method to study the effects of new drug candidates in a plasma environment. With ITC, it is also possible to directly investigate the cause for the hypertriglyceridemia by direct incubation of plasma samples with LPL.
In the clinical laboratory, ITC can be used to find out whether hypertriglyceridemia in patients is caused by reduced amounts of active heparin-releasable LPL or by an unfavorable composition of the patient’s plasma. In the first case, ITC would be an alternative to the standard radioisotope or fluorometric methods previously described. In the second case, ITC can be used to characterize plasma samples according to their properties as LPL substrates. This is a new way to analyze dysfunction of plasma lipoprotein metabolism, not easily attainable by other techniques.
In summary, ITC can be used for quantitative measurements of LPL activity in human postheparin plasma, for real-time recording of complete lipolysis of human plasma lipoproteins by LPL, and for investigations of the effects of some different control proteins on LPL activity in a plasma environment. We propose that studies of the action of LPL by ITC can be used for diagnostic purposes and for basic research, as well as tools in drug development.
Acknowledgments
The authors thank Annamaria Rahumeel, Piret Rospu, Valeria Saar, and Eva-Lotta Andersson for technical support.
Footnotes
Abbreviations:
- ANGPTL
- angiopoietin-like protein
- EL
- endothelial lipase
- GPIHBP1
- glycosylphosphatidylinositol-anchored high density lipoprotein binding protein 1
- ITC
- isothermal titration calorimetry
- TRL
- triglyceride-rich lipoprotein
This work was supported by Estonian Ministry of Education and Research Grant IUT 19-9, Swedish Research Council for Health, Working Life and Welfare Grant 12203, and Swedish Heart and Lung Foundation Grant 20130684.
REFERENCES
- 1.Olivecrona G. 2016. Role of lipoprotein lipase in lipid metabolism. Curr. Opin. Lipidol. 27: 233–241. [DOI] [PubMed] [Google Scholar]
- 2.Young S. G., and Zechner R.. 2013. Biochemistry and pathophysiology of intravascular and intracellular lipolysis. Genes Dev. 27: 459–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nordestgaard B. G. 2016. Triglyceride-rich lipoproteins and atherosclerotic cardiovascular disease: new insights from epidemiology, genetics, and biology. Circ. Res. 118: 547–563. [DOI] [PubMed] [Google Scholar]
- 4.Musunuru K., and Kathiresan S.. 2016. Surprises from genetic analyses of lipid risk factors for atherosclerosis. Circ. Res. 118: 579–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khetarpal S. A., and Rader D. J.. 2015. Triglyceride-rich lipoproteins and coronary artery disease risk: new insights from human genetics. Arterioscler. Thromb. Vasc. Biol. 35: e3–e9. [DOI] [PubMed] [Google Scholar]
- 6.Rader D. J. 2016. New therapeutic approaches to the treatment of dyslipidemia. Cell Metab. 23: 405–412. [DOI] [PubMed] [Google Scholar]
- 7.Dallinga-Thie G. M., Kroon J., Borén J., and Chapman M. J.. 2016. Triglyceride-rich lipoproteins and remnants: targets for therapy? Curr. Cardiol. Rep. 18: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davies B. S. J., Beigneux A. P., Barnes R. H., Tu Y., Gin P., Weinstein M. M., Nobumori C., Nyrén R., Goldberg I., Olivecrona G., et al. . 2010. GPIHBP1 is responsible for the entry of lipoprotein lipase into capillaries. Cell Metab. 12: 42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dijk W., and Kersten S.. 2016. Regulation of lipid metabolism by angiopoietin-like proteins. Curr. Opin. Lipidol. 27: 249–256. [DOI] [PubMed] [Google Scholar]
- 10.Ellis R. J. 2001. Macromolecular crowding: obvious but underappreciated. Trends Biochem. Sci. 26: 597–604. [DOI] [PubMed] [Google Scholar]
- 11.Karpe F., Olivecrona T., Walldius G., and Hamsten A.. 1992. Lipoprotein lipase in plasma after an oral fat load: relation to free fatty acids. J. Lipid Res. 33: 975–984. [PubMed] [Google Scholar]
- 12.Tornvall P., Olivecrona G., Karpe F., Hamsten A., and Olivecrona T.. 1995. Lipoprotein lipase mass and activity in plasma and their increase after heparin are separate parameters with different relations to plasma lipoproteins. Arterioscler. Thromb. Vasc. Biol. 15: 1086–1093. [DOI] [PubMed] [Google Scholar]
- 13.Eisenberg S., and Olivecrona T.. 1979. Very low density lipoprotein. Fate of phospholipids, cholesterol, and apolipoprotein C during lipolysis in vitro. J. Lipid Res. 20: 614–623. [PubMed] [Google Scholar]
- 14.Robal T., Larsson M., Martin M., Olivecrona G., and Lookene A.. 2012. Fatty acids bind tightly to the N-terminal domain of angiopoietin-like protein 4 and modulate its interaction with lipoprotein lipase. J. Biol. Chem. 287: 29739–29752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peterson J., Bihain B. E., Bengtsson-Olivecrona G., Deckelbaum R. J., Carpentier Y. A., and Olivecrona T.. 1990. Fatty acid control of lipoprotein lipase: a link between energy metabolism and lipid transport. Proc. Natl. Acad. Sci. USA. 87: 909–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olivecrona G., and Beisiegel U.. 1997. Lipid binding of apolipoprotein CII is required for stimulation of lipoprotein lipase activity against apolipoprotein CII-deficient chylomicrons. Arterioscler. Thromb. Vasc. Biol. 17: 1545–1549. [DOI] [PubMed] [Google Scholar]
- 17.Lookene A., Zhang L., Hultin M., and Olivecrona G.. 2004. Rapid subunit exchange in dimeric lipoprotein lipase and properties of the inactive monomer. J. Biol. Chem. 279: 49964–49972. [DOI] [PubMed] [Google Scholar]
- 18.Lookene A., and Bengtsson-Olivecrona G.. 1993. Chymotryptic cleavage of lipoprotein lipase. Identification of cleavage sites and functional studies of the truncated molecule. Eur. J. Biochem. 213: 185–194. [DOI] [PubMed] [Google Scholar]
- 19.Bianconi M. L. 2004. Titration calorimetry as a tool to determining thermodynamic and kinetic parameters of enzymes. In Biocalorimetry 2: Applications of Calorimetry in the Biological Sciences. J. E. Ladbury and M. L. Doyle, editors. John Wiley & Sons, Ltd., Chichester, UK. 175–185. [Google Scholar]
- 20.Bengtsson-Olivecrona G., and Olivecrona T.. 1991. Phospholipase activity of milk lipoprotein lipase. Methods Enzymol. 197: 345–356. [DOI] [PubMed] [Google Scholar]
- 21.Bengtsson-Olivecrona G., and Sletten K.. 1990. Primary structure of the bovine analogues to human apolipoproteins CII and CIII. Studies on isoforms and evidence for proteolytic processing. Eur. J. Biochem. 192: 515–521. [DOI] [PubMed] [Google Scholar]
- 22.Shen Y., Lookene A., Zhang L., and Olivecrona G.. 2010. Site-directed mutagenesis of apolipoprotein CII to probe the role of its secondary structure for activation of lipoprotein lipase. J. Biol. Chem. 285: 7484–7492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beckstead J. A., Oda M. N., Martin D. D. O., Forte T. M., Bielicki J. K., Berger T., Luty R., Kay C. M., and Ryan R. O.. 2003. Structure-function studies of human apolipoprotein A-V: a regulator of plasma lipid homeostasis. Biochemistry. 42: 9416–9423. [DOI] [PubMed] [Google Scholar]
- 24.Olivecrona T., and Olivecrona G.. 2000. Determination and clinical significance of lipoprotein lipase and hepatic lipase. In Handbook of Lipoprotein Testing. N. Rifai, G. R. Warnick, and M. H. Dominiczak, editors. AACC Press, Washington, DC. 479–498. [Google Scholar]
- 25.Havel R. J., Eder H. A., and Bragdon J. H.. 1955. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J. Clin. Invest. 34: 1345–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perret B., Mabile L., Martinez L., Tercé F., Barbaras R., and Collet X.. 2002. Hepatic lipase: structure/function relationship, synthesis, and regulation. J. Lipid Res. 43: 1163–1169. [PubMed] [Google Scholar]
- 27.Olivecrona T., and Olivecrona G.. 2009. The ins and outs of adipose tissue. In Cellular Lipid Metabolism. C. Ehnholm, editor. Springer Berlin, Heidelberg. 315–369. [Google Scholar]
- 28.Kei A. A., Filippatos T. D., Tsimihodimos V., and Elisaf M. S.. 2012. A review of the role of apolipoprotein C-II in lipoprotein metabolism and cardiovascular disease. Metabolism. 61: 906–921. [DOI] [PubMed] [Google Scholar]
- 29.Quinn D., Shirai K., and Jackson R. L.. 1983. Lipoprotein lipase: mechanism of action and role in lipoprotein metabolism. Prog. Lipid Res. 22: 35–78. [DOI] [PubMed] [Google Scholar]
- 30.Bengtsson G., and Olivecrona T.. 1980. Lipoprotein lipase: some effects of activator proteins. Eur. J. Biochem. 106: 549–555. [DOI] [PubMed] [Google Scholar]
- 31.Fredenrich A., Giroux L. M., Tremblay M., Krimbou L., Davignon J., and Cohn J. S.. 1997. Plasma lipoprotein distribution of apoC-III in normolipidemic and hypertriglyceridemic subjects: comparison of the apoC-III to apoE ratio in different lipoprotein fractions. J. Lipid Res. 38: 1421–1432. [PubMed] [Google Scholar]
- 32.Nilsson S. K., Heeren J., Olivecrona G., and Merkel M.. 2011. Apolipoprotein A-V; a potent triglyceride reducer. Atherosclerosis. 219: 15–21. [DOI] [PubMed] [Google Scholar]
- 33.O’Brien P. J., Alborn W. E., Sloan J. H., Ulmer M., Boodhoo A., Knierman M. D., Schultze A. E., and Konrad R. J.. 2005. The novel apolipoprotein A5 is present in human serum, is associated with VLDL, HDL, and chylomicrons, and circulates at very low concentrations compared with other apolipoproteins. Clin. Chem. 51: 351–359. [DOI] [PubMed] [Google Scholar]
- 34.Robciuc M. R., Tahvanainen E., Jauhiainen M., and Ehnholm C.. 2010. Quantitation of serum angiopoietin-like proteins 3 and 4 in a Finnish population sample. J. Lipid Res. 51: 824–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sonnenburg W. K., Yu D., Lee E-C., Xiong W., Gololobov G., Key B., Gay J., Wilganowski N., Hu Y., Zhao S., et al. . 2009. GPIHBP1 stabilizes lipoprotein lipase and prevents its inhibition by angiopoietin-like 3 and angiopoietin-like 4. J. Lipid Res. 50: 2421–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reimund M., Larsson M., Kovrov O., Kasvandik S., Olivecrona G., and Lookene A.. 2015. Evidence for two distinct binding sites for lipoprotein lipase on glycosylphosphatidylinositol-anchored high density lipoprotein-binding protein 1 (GPIHBP1). J. Biol. Chem. 290: 13919–13934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mysling S., Kristensen K. K., Larsson M., Beigneux A. P., Gårdsvoll H., Fong L. G., Bensadouen A., Jørgensen T. J., Young S. G., and Ploug M.. 2016. The acidic domain of the endothelial membrane protein GPIHBP1 stabilizes lipoprotein lipase activity by preventing unfolding of its catalytic domain. eLife. 5: e12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCoy M. G., Sun G-S., Marchadier D., Maugeais C., Glick J. M., and Rader D. J.. 2002. Characterization of the lipolytic activity of endothelial lipase. J. Lipid Res. 43: 921–929. [PubMed] [Google Scholar]
- 39.Badellino K. O., Wolfe M. L., Reilly M. P., and Rader D. J.. 2006. Endothelial lipase concentrations are increased in metabolic syndrome and associated with coronary atherosclerosis. PLoS Med. 3: e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Briquet-Laugier V., Ben-Zeev O., and Doolittle M. H.. 1999. Determining lipoprotein lipase and hepatic lipase activity using radiolabeled substrates. Methods Mol. Biol. 109: 81–94. [DOI] [PubMed] [Google Scholar]
- 41.Panteghini M., Bonora R., and Pagani F.. 2001. Measurement of pancreatic lipase activity in serum by a kinetic colorimetric assay using a new chromogenic substrate. Ann. Clin. Biochem. 38: 365–370. [DOI] [PubMed] [Google Scholar]
- 42.Basu D., Manjur J., and Jin W.. 2011. Determination of lipoprotein lipase activity using a novel fluorescent lipase assay. J. Lipid Res. 52: 826–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Di Filippo M., Marçais C., Charrière S., Marmontel O., Broyer M., Delay M., Merlin M., Nollace A., Valéro R., Lagarde M., et al. . 2014. Post-heparin LPL activity measurement using VLDL as a substrate: a new robust method for routine assessment of plasma triglyceride lipolysis defects. PLoS One. 9: e99721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dole V. P. 1956. A relation between non-esterified fatty acids in plasma and the metabolism of glucose. J. Clin. Invest. 35: 150–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pennacchio L. A., Olivier M., Hubacek J. A., Cohen J. C., Cox D. R., Fruchart J. C., Krauss R. M., and Rubin E. M.. 2001. An apolipoprotein influencing triglycerides in humans and mice revealed by comparative sequencing. Science. 294: 169–173. [DOI] [PubMed] [Google Scholar]
- 46.Do R., Stitziel N. O., Won H. H., Jørgensen A. B., Duga S., Angelica Merlini P., Kiezun A., Farrall M., Goel A., Zuk O., et al. . 2015. Exome sequencing identifies rare LDLR and APOA5 alleles conferring risk for myocardial infarction. Nature. 518: 102–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Triglyceride Coronary Disease Genetics Consortium and Emerging Risk Factors Collaboration, Sarwar N., Sandhu M. S., Ricketts S. L., Butterworth A. S., Di Angelantonio E., Boekholdt S. M., Ouwehand W., Watkins H., Samani N. J., et al. . Triglyceride-mediated pathways and coronary disease: collaborative analysis of 101 studies. 2010. Lancet 375: 1634–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sharma V., Forte T. M., and Ryan R. O.. 2013. Influence of apolipoprotein A-V on the metabolic fate of triacylglycerol. Curr. Opin. Lipidol. 24: 153–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lookene A., Beckstead J. A., Nilsson S., Olivecrona G., and Ryan R. O.. 2005. Apolipoprotein A-V-heparin interactions: implications for plasma lipoprotein metabolism. J. Biol. Chem. 280: 25383–25387. [DOI] [PubMed] [Google Scholar]
- 50.Gordts P. L. S. M., Nock R., Son N-H., Ramms B., Lew I., Gonzales J. C., Thacker B. E., Basu D., Lee R. G., Mullick A. E., et al. . 2016. ApoC-III inhibits clearance of triglyceride-rich lipoproteins through LDL family receptors. J. Clin. Invest. 126: 2855–2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Larsson M., Vorrsjö E., Talmud P., Lookene A., and Olivecrona G.. 2013. Apolipoproteins C-I and C-III inhibit lipoprotein lipase activity by displacement of the enzyme from lipid droplets. J. Biol. Chem. 288: 33997–34008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gibson J. C., Goldberg R. B., Rubinstein A., Ginsberg H. N., Brown W. V., Baker S., Joffe B. I., and Seftel H. C.. 1987. Plasma lipoprotein distribution of apolipoprotein E in familial hypercholesterolemia. Arteriosclerosis. 7: 401–407. [DOI] [PubMed] [Google Scholar]
- 53.Ooi E. M. M., Barrett P. H. R., Chan D. C., and Watts G. F.. 2008. Apolipoprotein C–III: understanding an emerging cardiovascular risk factor. Clin. Sci. (Lond.). 114: 611–624. [DOI] [PubMed] [Google Scholar]
- 54.Sukonina V., Lookene A., Olivecrona T., and Olivecrona G.. 2006. Angiopoietin-like protein 4 converts lipoprotein lipase to inactive monomers and modulates lipase activity in adipose tissue. Proc. Natl. Acad. Sci. USA. 103: 17450–17455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yau M-H., Wang Y., Lam K. S. L., Zhang J., Wu D., and Xu A.. 2009. A highly conserved motif within the NH2-terminal coiled-coil domain of angiopoietin-like protein 4 confers its inhibitory effects on lipoprotein lipase by disrupting the enzyme dimerization. J. Biol. Chem. 284: 11942–11952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chi X., Shetty S. K., Shows H. W., Hjelmaas A. J., Malcolm E. K., and Davies B. S. J.. 2015. Angiopoietin-like 4 Modifies the Interactions between Lipoprotein Lipase and Its Endothelial Cell Transporter GPIHBP1. J. Biol. Chem. 290: 11865–11877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shan L., Yu X-C., Liu Z., Hu Y., Sturgis L. T., Miranda M. L., and Liu Q.. 2009. The angiopoietin-like proteins ANGPTL3 and ANGPTL4 inhibit lipoprotein lipase activity through distinct mechanisms. J. Biol. Chem. 284: 1419–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lafferty M. J., Bradford K. C., Erie D. A., and Neher S. B.. 2013. Angiopoietin-like protein 4 inhibition of lipoprotein lipase: evidence for reversible complex formation. J. Biol. Chem. 288: 28524–28534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nilsson S. K., Anderson F., Ericsson M., Larsson M., Makoveichuk E., Lookene A., Heeren J., and Olivecrona G.. 2012. Triacylglycerol-rich lipoproteins protect lipoprotein lipase from inactivation by ANGPTL3 and ANGPTL4. Biochim. Biophys. Acta. 1821: 1370–1378. [DOI] [PubMed] [Google Scholar]
- 60.Gusarova V., Alexa C. A., Wang Y., Rafique A., Kim J. H., Buckler D., Mintah I. J., Shihanian L. M., Cohen J. C., Hobbs H. H., et al. . 2015. ANGPTL3 blockade with a human monoclonal antibody reduces plasma lipids in dyslipidemic mice and monkeys. J. Lipid Res. 56: 1308–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Larsson M., Caraballo R., Ericsson M., Lookene A., Enquist P-A., Elofsson M., Nilsson S. K., and Olivecrona G.. 2014. Identification of a small molecule that stabilizes lipoprotein lipase in vitro and lowers triglycerides in vivo. Biochem. Biophys. Res. Commun. 450: 1063–1069. [DOI] [PubMed] [Google Scholar]
- 62.Amar M. J. A., Sakurai T., Sakurai-Ikuta A., Sviridov D., Freeman L., Ahsan L., and Remaley A. T.. 2015. A novel apolipoprotein C–II mimetic peptide that activates lipoprotein lipase and decreases serum triglycerides in apolipoprotein E-knockout mice. J. Pharmacol. Exp. Ther. 352: 227–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sakurai T., Sakurai A., Vaisman B. L., Amar M. J., Liu C., Gordon S. M., Drake S. K., Pryor M., Sampson M. L., Yang L., et al. . 2016. Creation of apolipoprotein C-II (apoC-II) mutant mice and correction of their hypertriglyceridemia with an apoC-II mimetic peptide. J. Pharmacol. Exp. Ther. 356: 341–353. [DOI] [PMC free article] [PubMed] [Google Scholar]