Abstract
The molecular details relevant to dietary supplementation of the omega-3 fatty acid DHA in mothers as well as in their offspring are not clear. The PUFA elongase, elongation of very long-chain fatty acid (ELOVL)2, is a critical enzyme in the formation of DHA in mammals. In order to address the question regarding the origin of DHA during perinatal life, we have used DHA-deficient Elovl2-ablated mice as a model system to analyze the maternal impact on the DHA level in their offspring of various genotypes. Elovl2−/− mothers maintained on control diet had significantly lower systemic levels of DHA compared with the Elovl2+/− and Elovl2+/+ mothers. Dietary DHA administration during the pregnancy and lactation periods led to increased DHA accretion in maternal tissues and serum of all genotypes. The proportion of DHA in the liver and serum of the Elovl2−/− offspring was significantly lower than in the Elovl2+/+ offspring. Remarkably, the DHA level in the Elovl2+/− offspring nursed by DHA-free-fed Elovl2−/− mothers was almost as high as in +/+ pups delivered by +/+ mothers, suggesting that endogenous synthesis in the offspring can compensate for maternal DHA deficiency. Maternal DHA supplementation had a strong impact on offspring hepatic gene expression, especially of the fatty acid transporter, Mfsd2a, suggesting a dynamic interplay between DHA synthesis and DHA uptake in the control of systemic levels in the offspring.
Keywords: docosahexaenoic acid synthesis, polyunsaturated fatty acid, elongation of very long-chain fatty acid 2, supplementation, pregnancy, lactation, docosahexaenoic acid
The omega-3 PUFA DHA (22:6n-3) is suggested to be one of the most important and essential nutrients for proper perinatal development (1, 2). Pregnancy and lactation are characterized as critical periods when high physiological demands of DHA supply into the fetus and neonate are required, which principally can be fulfilled by maternal DHA transfer via the placenta and the milk, respectively (3, 4). The DHA source in the mother can, in turn, arise from dietary intake of, e.g., fish oil-rich products, or from either endogenous synthesis or mobilization from tissue storage.
Bioconversion of the essential omega-3 and omega-6 fatty acids, α-linolenic acid (ALA, 18:3n-3) and linoleic acid (18:2n-6), respectively, into C22 PUFAs is carried out via the enzymatic machinery consisting of the Δ5 fatty acid desaturase (FADS)1 and the Δ6 FADS2, and the fatty acid elongases, elongation of very long-chain fatty acid (ELOVL)2 and ELOVL5. To obtain physiological levels of DHA, a β-oxidation step of C24:6n-3 into C22:6n-3 is required (5). It has been proposed that the activities of the enzymes involved in PUFA metabolism are higher in females due to a positive regulation by estrogen (6, 7) and that the PUFA levels are upregulated especially during pregnancy (8–10).
The existence of the PUFA synthesizing machinery has also been detected in the fetus and neonates (11–13) and studies including fatty acid tracers have described endogenous omega-3 PUFA synthesis in the developing offspring (14–16).
To improve the accretion of DHA in the offspring, a majority of the performed studies have focused on the effects of dietary supply of DHA during pregnancy and lactation (2, 17). In addition, supplementation with the omega-3 precursor, ALA, during pregnancy has also been recognized as a potential source for in vivo (endogenous) DHA synthesis in the mother, as well as in the offspring (18–20).
Nonetheless, one of the major issues discussed today is which factors contribute most to the DHA status in the offspring, i.e., what is the most important DHA source during perinatal development and does dietary supplementation matter?
Until now, studies regarding DHA deficiency during perinatal development have been limited to maternal depletion of dietary omega-3 PUFAs. We have previously reported that ablation of the ELOVL2 enzyme in Elovl2−/− mice leads to a systemic DHA deficiency by about 90% (21). By the use of our Elovl2−/− animal model, we here bring novel data to the field by presenting results showing that endogenous PUFA synthesis in the offspring is the major source of DHA in neonates under normal conditions. We also present data on how dietary intervention during gestation and lactation affects the DHA status in both mothers and offspring under normal and genetically acquired DHA-deficient conditions.
MATERIAL AND METHODS
Diet
Control diet (D12450H) and DHA-enriched diet (D13021002) were formulated in the form of food pellets by Research Diets, New Brunswick, NJ. Both diets were low-fat diets where the fat content was equal to 10% of calories (Table 1). The DHA content in the DHA-enriched diet was 14.5% of total fatty acids and there was no trace amount of this fatty acid in the control diet (Table 2). Moreover, both diets had a similar linoleic acid (18:2n-6)/ALA (18:3n-3) ratio of 8/1. Both diets were provided ad libitum during the whole time of the experiment. In order to reduce the risk of oxidation, new food pellets were provided every 7 days.
TABLE 1.
Diet composition
| Ingredient | Control Diet(10% kcal fat; D12450H) | DHA-Enriched Diet(10% kcal fat, 1% DHA; D13021002) |
| Casein | 200 | 200 |
| L-cystine | 3 | 3 |
| Cornstarch | 452.2 | 452.2 |
| Maltodextrin 10 | 75 | 75 |
| Sucrose | 172.8 | 172.8 |
| Cellulose, BW200 | 50 | 50 |
| Soybean oil | 25 | 25 |
| DHA | 0 | 10 |
| Lard | 20 | 10 |
| Mineral mix S10026 | 10 | 10 |
| Dicalcium phosphate | 13 | 13 |
| Calcium carbonate | 5.5 | 5.5 |
| Potassium citrate, 1H20 | 16.5 | 16.5 |
| Vitamin mix V10001 | 10 | 10 |
| Choline bitartrate | 2 | 2 |
| FD&C yellow dye #5 | 0.04 | 0 |
| FD&C red dye #40 | 0.01 | 0.025 |
| FD&C blue dye #1 | 0 | 0.025 |
| Total (g) | 1,055.5 | 1,055.05 |
Diet composition expressed in grams of mass for each ingredient of control diet (not DHA enriched) (10% kcal fat, D12450H; Research Diets) and DHA-enriched diet (10% kcal fat, 1% DHA, D13023002; Research Diets).
TABLE 2.
Diet fatty acid composition
| Fatty Acid | Control Diet(10% kcal fat; D12450H) | DHA-Enriched Diet(10% kcal fat, 1% DHA; D13021002) |
| C12:0 | 0.09 | 0.05 |
| C14:0 | 0.80 | 0.48 |
| C15:0 | 0.05 | 0.05 |
| C16:0 | 16.83 | 12.48 |
| C16:1n-9 | 0.17 | 0.09 |
| C16:1n-7 | 0.86 | 0.51 |
| C18:0 | 8.43 | 5.35 |
| C18:1n-9 | 27.87 | 21.35 |
| C18:1n-7 | 1.66 | 1.29 |
| C18:2n-6 | 37.43 | 35.64 |
| C18:3n-6 | 0.16 | 0.19 |
| C18:3n-3 | 4.24 | 4.46 |
| C20:0 | 0.30 | 0.23 |
| C18:4n-3 | 0.05 | 0 |
| C20:1n-9 | 0.37 | 0.25 |
| C20:2n-6 | 0.31 | 0.19 |
| C20:4n-6 | 0.11 | 0.24 |
| C22:0 | 0.24 | 0.20 |
| C20:4n-3 | 0 | 0.03 |
| C20:5n-3 | 0 | 0.71 |
| C22:3n-3 | 0 | 0.08 |
| C22:4n-6 | 0 | 1.04 |
| C22:5n-3 | 0 | 0.57 |
| C22:6n-3 | 0 | 14.53 |
| SFA | 26.74 | 18.86 |
| MUFA | 30.93 | 23.49 |
| PUFA | 42.3 | 57.68 |
| SFA/MUFA/PUFA | 1/1.16/1.58 | 1/1.25/3.06 |
| Omega-6 | 38.01 | 37.3 |
| Omega-3 | 4.29 | 20.38 |
| Omega-6/omega-3 | 8.86 | 1.83 |
Fatty acids expressed as percent of total fatty acids of control diet (not DHA enriched) (10% kcal fat, D12450H; Research Diets) and DHA-enriched diet (10% kcal fat, 1% DHA, D13023002; Research Diets).
Animal handling
Female mice of Elovl2+/+, Elovl2+/−, and Elovl2−/− genotypes, backcrossed into the 129/Sv strain for five generations (21), were kept for 14 days together with males of either Elovl2+/+ or Elovl2+/− genotype. Mice were given either control or DHA-enriched diet a few days prior to conception and throughout the whole experiment. A few days prior to delivery, the animals were separated into single cages and, within 24 h after spontaneous delivery, litter size for each female was recorded. Pups were kept together with lactating mothers for about 2 weeks postpartum and no correction of the litter size was performed. At the end of the study, offspring were euthanized together with mothers or only the offspring were euthanized and −/− mothers were used for a subsequent breeding. Maternal body weight was measured at the start and at the end of the study and offspring body weight was recorded after they had been euthanized. A nonpregnant control group was composed of females that did not give birth. Mothers and control females were euthanized by CO2 administration and cervical dislocation. Blood was collected by direct heart puncture. Offspring were euthanized by cervical dislocation and blood was collected after decapitation. All blood samples were kept at 4°C and thereafter centrifuged at 10,000 g at 4°C for 5 min and serum was collected. Each pup was genotyped postmortem and, due to small volumes, serum from single pups of each genotype was pooled. Tissues samples were dissected out from each animal and immediately put in liquid nitrogen and then stored at −80°C until being further analyzed. For a detailed experimental set-up, see supplemental Fig. S1. All animals were housed at room temperature, maintained on a 12 h light/dark cycle, had free access to water, and were fed ad libitum. All studies were carried out with ethical permission from the Animal Ethics Committee of the North Stockholm region, Sweden.
MRI measurement
In order to measure body fat and lean content of the offspring, in vivo MRI using EchoMRI-100TM (Echo Medical Systems, Houston, TX) was performed after the animals had been euthanized and liver and brain dissected out.
Fatty acid analysis
Tissues and diets were extracted with hexane-isopropanol (3:2, v/v), following the method of Hara and Radin (22). Total lipids in diets and tissues were methylated with boron trifluoride and dry methanol, following the procedure of Appelqvist (23) and the fatty acid methyl esters were analyzed with a gas chromatograph CP-3800 (Varian AB, Stockholm, Sweden) equipped with flame ionization detector and split injector and an autosampler mode (Combi PAL AutoSampler; Varian AB). The column was a fused silica capillary column BPX 70 (SGE, Austin, TX), length 50 m id. 0.22 mm, 0.25 μm film thickness. The column temperature was programmed to start at 158°C and kept for 5 min and then increased by 2°C/min up to 220°C where it remained for 8 min. The carrier gas was helium (0.8 ml/min) and the make-up gas was nitrogen. The injector and detector temperatures were 230°C and 250°C, respectively. Fatty acids were identified by comparison with the standard fatty acid mixture, GLC-68 (Nu-check Prep, Inc., Elysian, MO), and retention times. Peak areas were integrated using Star chromatography workstation software version 5.5 (Varian AB). All solvents and other chemicals used for analysis were purchased from Merck and were used without further purification.
Quantitative RT-PCR
RNA was isolated from similar pieces of offspring livers with TRI Reagent (Sigma-Aldrich) following the manufacturer’s procedure. For real-time PCR, 500 ng of total RNA for each sample was reverse transcribed using random hexamer primers, dNTPs, multiscript, and RNase inhibitor (Applied Biosystems, Foster City, CA). cDNA samples were diluted 1:10 and duplicate aliquots of 2 μl of each sample cDNA were mixed with SYBR Green JumpStart Taq ReadyMix (Sigma-Aldrich), prevalidated primers, and DEPC-treated water. PCR-primers were used for Fas, steroyl-CoA desaturase 1 (Scd1), sterol regulatory element-binding protein 1c (Srebp-1c), major facilitator superfamily domain containing 2a (Mfsd2a), Elovl2, Fads1, Fas, and Elovl5. For primer sequences, see supplemental Table S1. Expression analysis was performed using the Bio-Rad detection system. Data were normalized to the ribosomal 18S RNA.
Statistical analyses
All statistical analyses, except for mRNA analysis that was performed by using GraphPad PRISM (San Diego, CA), and the statistical differences were calculated with Student’s unpaired t-test and were performed under R, a programming language and software environment for statistical computing and graphics. Pearson’s chi-squared test was used to evaluate differences in successful pregnancies and offspring survival within and across different genotypes. Kruskal-Wallis rank sum test was used to test differences between litter sizes. The P values were adjusted for multiple comparisons using the Benjamini-Hochberg method, and P < 0.05 was used as a significance cut-off. Principal component (PC) analysis (PCA) was used to visualize the high dimensional data on the two-component space, along PC1 and PC2, which explains the most variance in the data. The consensus dataset was prepared prior to the PCA by including fatty acids (P = 30) with complete measurements across all the tissues and samples (n = 199). The ellipses on the PCA score plots were drawn at 75% confidence level and the loading plots are based on the absolute loading values. The differences in DHA level in tissues between the groups and within the same genotype were assessed by nonparametric test, Kruskal-Wallis rank sum test (as for differences between litter sizes above), and for comparisons of mothers and females, log2 absolute fold change was calculated. In addition, multiple linear regression models were fitted to assess the relationship between weight and groups adjusted by litter size. Pearson’s correlation coefficient was calculated between DHA measurements across different tissues in both mothers and nonpregnant females or only across tissues in mothers. All the statistical significances are listed in supplemental Tables S2–S15.
RESULTS
Effects of female Elovl2 genotype and DHA supplementation on breeding outcome
It is well-known that the genotype of the female influences the possibility of being pregnant as well as the pregnancy outcome. In our experimental set-up (supplemental Fig. S1), breeding of wild-type females fed control diet resulted in 100% successful pregnancies, whereas Elovl2+/− and Elovl2−/− females showed, independently of the genotype of the male, a tendency toward, but not significantly, a lower pregnancy success rate (Table 3). Likewise, litter size was somewhat, but not significantly, smaller for the Elovl2+/− and Elovl2−/− mothers than for the wild-type mother group (Table 3).
TABLE 3.
Effect of genotype and diet on breeding outcomes
| Female: Elovl2+/+; Male: Elovl2+/+ | Female: Elovl2+/−; Male: Elovl2+/+ | Female: Elovl2+/−; Male: Elovl2+/− | Female: Elovl2−/−; Male: Elovl2+/+ | Female: Elovl2−/−; Male: Elovl2+/− | ||||||
| Parameters | Control | DHA | Control | DHA | Control | DHA | Control | DHA | Control | DHA |
| Successful pregnancies | 100% (4/4) | 100% (5/5) | 85.7% (6/7) | 87.5% (7/8) | 66.7% (4/6) | 83.3% (5/6) | 87.5% (7/8) | 40% (4/10) | 100% (6/6) | 23.8% (5/21) |
| Litter size (average ± SEM) | 7.5 ± 1.0 | 7.2 ± 0.7 | 4.8 ± 1.0 | 5.7 ± 1.1 | 4.0 ± 1.3 | 3.2 ± 1.2 | 4.9 ± 0.9 | 6.8 ± 0.9 | 3.8 ± 1.0 | 2.8 ± 1.0 |
| Pups survival | 96.7% (29/30) | 61.1% (22/36) | 55.2% (16/29) | 80% (32/40) | 81.3% (13/16) | 81.3% (13/16) | 91.2% (31/34) | 63% (17/27) | 87% (20/23) | 71.4% (10/14) |
| Distribution (Elovl2 genotype) | 29 (+/+) | 22 (+/+) | 10 (+/+), 6 (+/−) | 20 (+/+), 12 (+/−) | 6 (+/+), 2 (+/−), 5 (−/−) | 2 (+/+), 8 (+/−), 3 (−/−) | 31 (+/−) | 17 (+/−) | 11 (+/−), 9 (−/−) | 6 (+/−), 4 (−/−) |
Breeding outcomes of mating pairs consisting of Elovl2+/+, Elovl2+/−, and Elovl2−/− females and Elovl2+/+ and Elovl2+/− males presented as percent of successful pregnancies (amount of pregnancies/amount of mating pairs × 100%), litter size (amount of offspring delivered by each pregnant female), offspring survival rate (number of offspring that were euthanized at the end of experiment/number of offspring recorded for the litter size right after delivery × 100%), distribution (genotype of offspring that were euthanized at the end of the experiment).
DHA supplementation did not affect the breeding outcome of Elovl2+/+ and Elovl2+/− female mice. However, the number of successful pregnancies was reduced for the Elovl2−/− females and, even with a significantly high number of breeding pairs (21), Elovl2−/− females fed DHA-enriched diet mated with +/− males showed a significantly lower percentage of successful pregnancies in comparison with the other genotypes, which indicates an adverse dietary effect (Table 3). There were no statistical differences in pup survival or Mendelian distribution between the genotypes regardless of diet.
Effects of Elovl2 genotype and DHA supplementation on maternal and offspring body weight
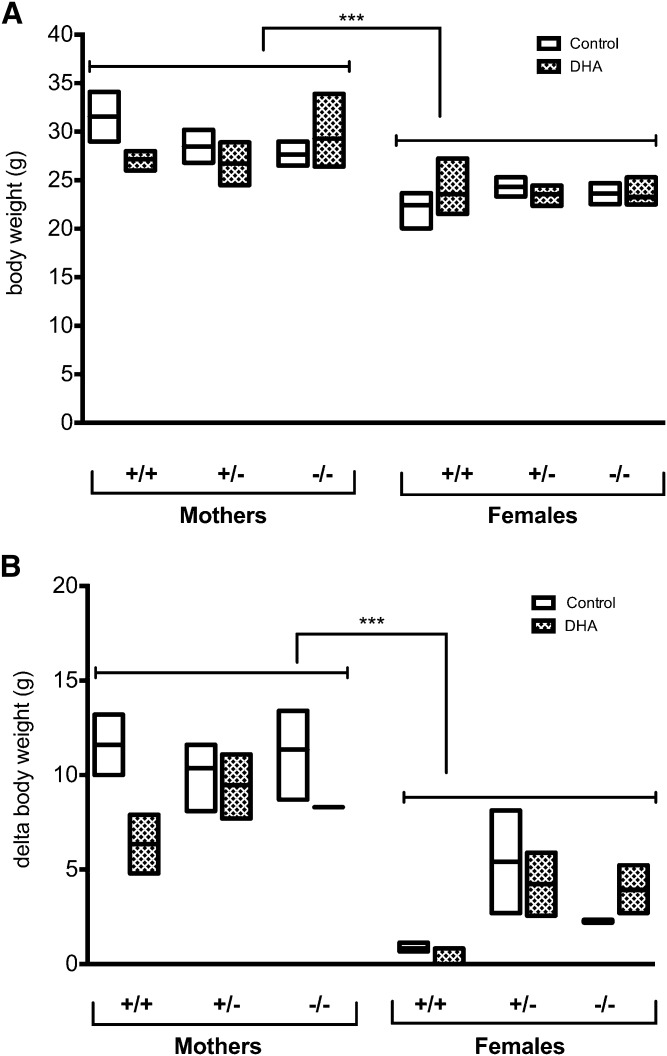
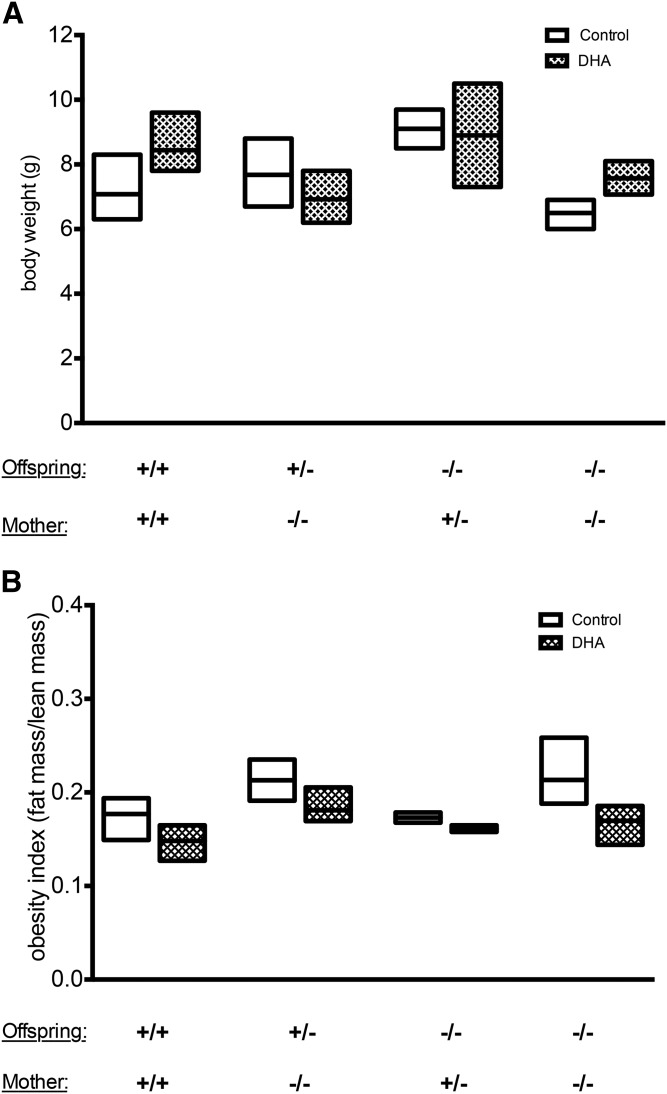
Several studies have reported effects of DHA supplementation during pregnancy and lactation on the length of gestation and, consequently, the birth weight of the offspring. In the present study, the body weights of the mothers at postpartum stage tend to be higher than the weight of control females (Fig. 1A), as they gained weight throughout the gestation and lactation periods (Fig. 1B). There was no significant difference in the body weight between different genotypes and between dietary treatments (Fig. 1A). Maternal DHA supplementation did not show any significant effect on body weight of the suckling pups regardless of genotype (Fig. 2A). Nevertheless, offspring delivered from +/+ mothers supplemented with DHA tended to have higher body weight than those that were supplied by mothers fed control diet (Fig. 2A). Body composition measurements revealed that the observed difference in body weight could be due to a higher lean mass in those animals (Fig. 2B). However, when taking the contribution of litter size into consideration using multiple linear regression models, we were able to conclude that litter size significantly affects the body weight outcome (Pr = 0.0265).
Fig. 1.
Effects of Elovl2 genotype and dietary DHA on the maternal body weight at postpartum stage. Body weight (A) measured at the end of experiment and delta body weight (B) of the weight at the beginning and the end of experiment for Elovl2+/+ (+/+), Elovl2+/− (+/−), and Elovl2−/− (−/−) mothers and control females fed either control diet (Control) or DHA-enriched diet (DHA). Results are presented as mean ± SEM of two to five mice. Statistical significances are shown between groups, ***P < 0.001.
Fig. 2.
Effects of maternal and offspring genotype and maternal diet on the offspring body weight. Body weight (A) expressed in grams and obesity index (B) expressed as a ratio of fat mass/lean mass of suckling offspring with Elovl2+/+ (+/+), Elovl2+/− (+/−), and Elovl2−/− (−/−) genotypes delivered by Elovl2+/+ (+/+), Elovl2+/− (+/−), and Elovl2−/− (−/−) mothers fed either control diet (Control) or DHA-enriched diet (DHA) measured at postnatal days 14–18 (moment they were euthanized) (A) and postmortem (B) after dissection of some organs. Results are presented as mean ± SEM of two to five mice.
Effects of Elovl2 genotype on accretion of DHA in maternal tissues and serum throughout pregnancy and lactation
Liver, white adipose tissue (WAT), mammary glands, and serum were isolated from mothers 2 weeks after delivery. Lipid weight obtained from each sample was normalized to tissue wet weight and expressed as percent lipids for each analyzed sample. There was no significant difference in the relative amount of extracted lipids between the various genotype groups or across the dietary treatments within each tissue, with the highest level from WAT (approximately 50%), followed by mammary glands (8%), liver (5%), and serum (0.5%) (supplemental Fig. S2A–D). In addition, we observed a higher level of extracted lipids from mammary glands from control females than from mothers (supplemental Fig. S2C). The relative amount of extracted lipids from offspring liver and serum (supplemental Fig. S2E, F) resembled the amount obtained from corresponding maternal tissues and, similarly, did not differ between genotypes or maternal diet.
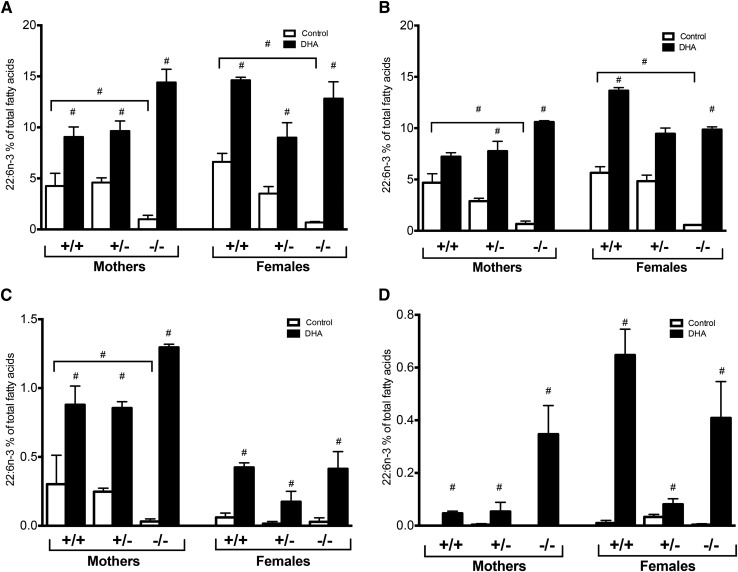
When we analyzed specific fatty acid levels, the Elovl2−/− mothers showed significantly reduced DHA levels in liver and serum compared with Elovl2+/+ and Elovl2+/− mothers fed control diet (Fig. 3A, B). There was no significant difference in DHA levels between mothers and control females of the same genotype (Fig. 3A, B). DHA supplementation resulted in increased DHA concentration in both mothers and nonpregnant females of all genotypes. Interestingly, Elovl2−/− mothers tended to accumulate more DHA than the Elovl2+/+ and Elovl2+/− mothers. This high accretion of DHA was not observed in Elovl2−/− control females fed DHA-enriched diet (Fig. 3A, B). Consistent with liver and serum, the level of DHA in mammary gland was significantly reduced in Elovl2−/− mothers fed control diet and the level increased considerably upon dietary supplementation (Fig. 3C). As for the other tissues, Elovl2−/− mothers exhibited a higher level of DHA accretion in mammary glands than Elovl2+/− or Elovl2+/+ mothers (Fig. 3C). Although DHA supplementation augmented the DHA level in mammary glands of control females, the level was markedly lower than in maternal tissues regardless of genotype.
Fig. 3.
Maternal DHA accretion through pregnancy and lactation is affected by Elovl2 genotype and dietary DHA. Proportion of DHA (22:6n-3) in the liver (A), serum (B), mammary glands (C), and WAT (D) of Elovl2+/+ (+/+), Elovl2+/− (+/−), and Elovl2−/− (−/−) mothers and control females fed either control diet (Control) or DHA-enriched diet (DHA) expressed as percent of total fatty acids. For the percent of remaining fatty acids, see respectively, supplemental Tables S16–S19. Results are presented as mean ± SEM of two to five mice. #Represents a log2 absolute fold change >1 between control (Control) and DHA-enriched (DHA) diet, unless specified.
The levels of DHA in WAT of mothers of all genotypes fed regular diet was almost undetectable (Fig. 3D). However, DHA supplementation elevated the proportion of DHA, especially in Elovl2−/− mothers. In control females, we observed a significant increase of DHA also in WAT of Elovl2+/+ mice (Fig. 3D).
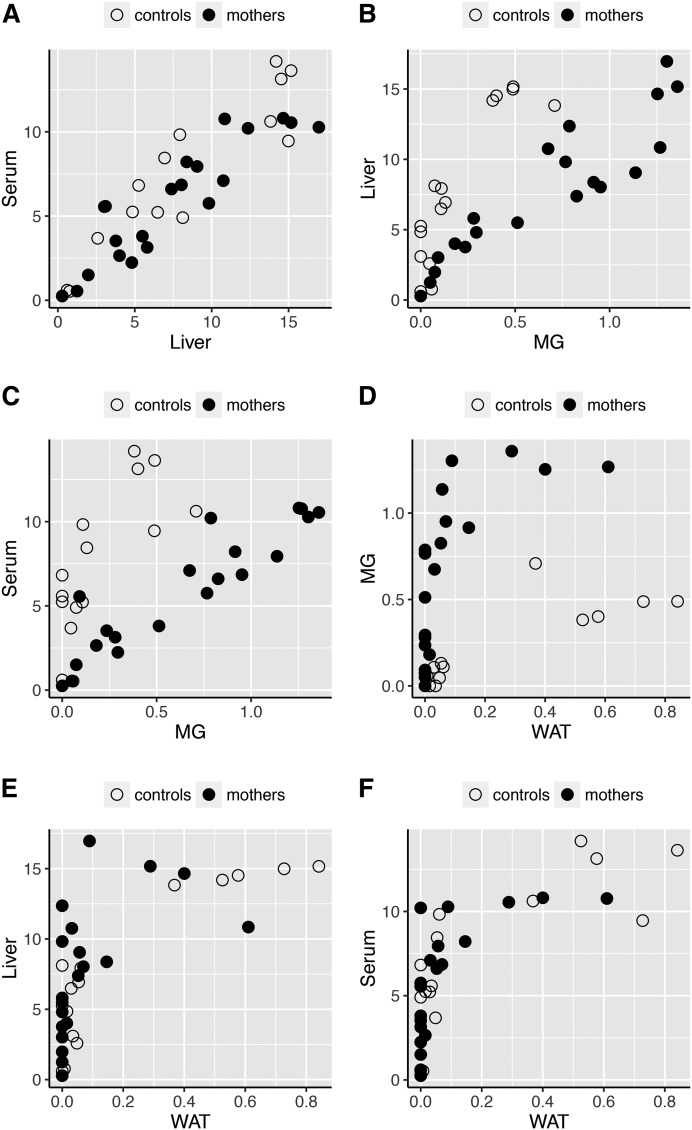
When we performed DHA scatter plots across different tissues of mothers and control females of all genotypes, we found a high Pearson correlation between the percentage of DHA in the liver and serum (0.90) (Fig. 4A), liver with mammary glands (0.72) (Fig. 4B), and serum with mammary glands (0.57) (Fig. 4C). When samples from control females were excluded, the correlation coefficient became stronger between mammary glands and liver (0.92) and between mammary glands and serum (0.92), which clearly indicates that the Elovl2 genotype affects DHA accretion in mothers in a systemic manner during pregnancy and lactation. Instead, the proportion of DHA in mammary gland of control females seemed to have a higher correlation coefficient with WAT (Fig. 4D). In contrast, a low correlation was found between WAT and liver or serum in any of the females (Fig. 4E, F).
Fig. 4.
The correlation of DHA level across different tissues changes with pregnancy and lactation. Scatter plots across different tissues of mothers and control females representing correlation for DHA level between serum and liver (A), liver and mammary gland (MG) (B), serum and mammary gland (C), mammary gland and WAT (D), liver and WAT (E), and serum and WAT (F).
Effects of Elovl2 genotype on fatty acid composition in mothers and nonpregnant females
The complete analysis of fatty acid composition of liver, serum, mammary glands, and WAT is presented in supplemental Tables S16–S19, respectively. However, because we expressed the DHA proportion as a percentage of the total fatty acids for a given tissue, we did not analyze differences within/between other fatty acids because these might be considered as potential confounders in regard to total fatty acid composition. Although, when we performed PCA, loading plots indicated that the main fatty acids contributing to PC1 were 18:1n-9, 22:6n-3, 20:4n-6, and 18:2n-6 (supplemental Fig. S3A) and for PC2, it was mainly 16:0, 18:2n-6, 20:4n-6, and 14:0 (supplemental Fig. S3B).
PCA of all the tissue samples showed a distinct fatty acid composition within each tissue [female WAT, serum, mammary glands, and liver, and offspring liver (supplemental Fig. S3C)], as well as in total fatty acid composition between mothers and control females (supplemental Fig. S3D).
Samples from mammary glands (green) were split into two subgroups along PC2 (supplemental Fig. S3C), where one subgroup was different from the other tissues (PCA scores exclusively from the mothers dataset), while the other subgroup exclusively overlapped with WAT (PCA scores for both mothers and nonpregnant females dataset) supporting the correlation data in Fig. 4 that the fatty acid content of the maternal mammary glands resembles both WAT and liver signatures; whereas in control females, it resembles only the WAT signature.
DHA levels in offspring liver and serum in regard to maternal and offspring Elovl2 genotype
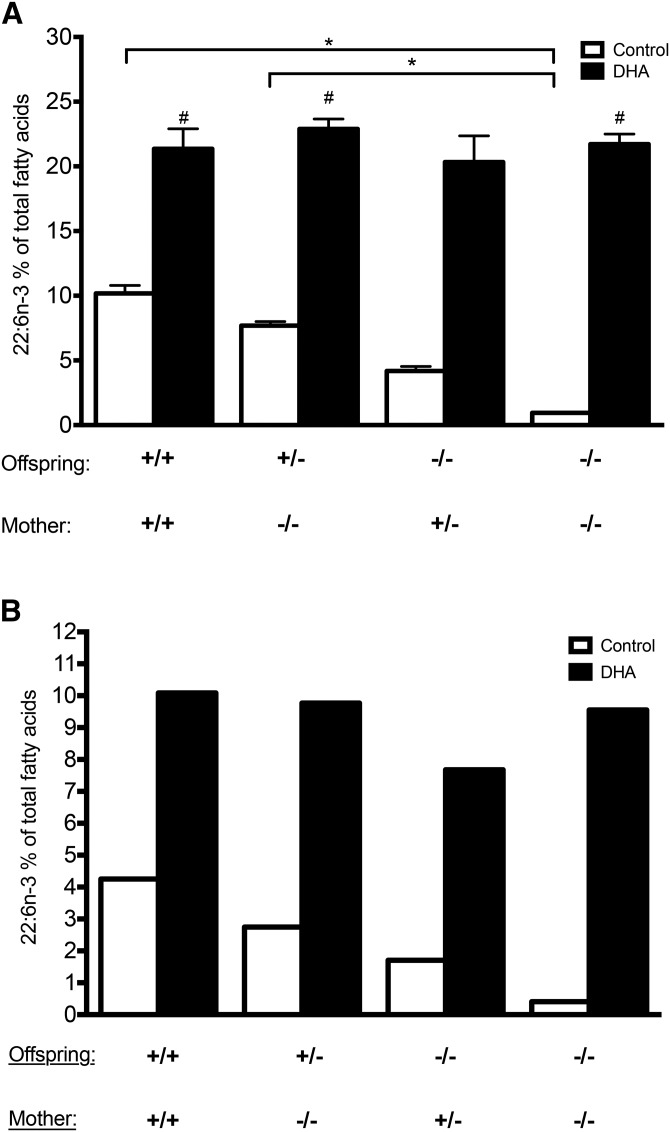
Regarding the contribution of maternal and offspring Elovl2 genotypes on perinatal DHA accretion during pregnancy and lactation, we analyzed eight distinct groups of pups of particular genotype (Elovl2+/+, Elovl2+/−, and Elovl2−/−) delivered by mothers of certain genotype (Elovl2+/+, Elovl2+/−, and Elovl2−/−) fed control or DHA-enriched diet. The complete analysis of the fatty acid composition of liver and serum is presented in supplemental Tables S20 and S21, respectively. We could observe that the proportion of DHA in the liver of wild-type offspring delivered by wild-type mothers accounted for 10% of total fatty acids, which was significantly higher than in the Elovl2−/− neonates coming from Elovl2+/− mothers (4%) and Elovl2−/− mothers (1%) (Fig. 5A). Heterozygote +/− pups from DHA-deficient −/− mothers showed slightly reduced DHA levels (7%) compared with +/+ pups delivered by +/+ mothers, but the level was significantly higher than in −/− pups without any maternal DHA input (Fig. 5A). Analysis of pooled serum samples from the same groups of offspring showed the same relationship of DHA deposition as in the liver (Fig. 5B) implying that offspring liver, like in adult mice, is the major contributor of systemic DHA under standard dietary conditions and that, at least before weaning, endogenous DHA synthesis in the pups can almost fully compensate for maternal DHA deficiency.
Fig. 5.
DHA accretion in the liver and serum of offspring depends on both maternal and offspring genotype and the maternal dietary DHA. Proportion of DHA (22:6n-3) in the liver (A) and serum (B) of offspring with Elovl2+/+ (+/+), Elovl2+/− (+/−), and Elovl2−/− (−/−) genotype delivered by Elovl2+/+ (+/+), Elovl2+/− (+/−), and Elovl2−/− (−/−) mothers fed either control diet (Control) or DHA-enriched diet (DHA) expressed as a percent of total fatty acids. For the percent of remaining fatty acids, see respectively, supplemental Tables S20, S21. Results are presented as means ± SEM of two to five mice (A) or as pooled samples from three to five individuals (B). *Represents statistical significance (P < 0.05) between genotypes; #Represents statistical significance (P < 0.05) between control (Control) and maternal DHA-enriched diet (DHA).
Interestingly, independently of maternal or offspring Elovl2 genotype, maternal dietary DHA supplementation during the gestation and lactation periods resulted in an increased proportion of DHA up to 20% of total fatty acids in the liver (Fig. 5A) and 10% in serum (Fig. 5B) of all offspring groups. When we performed PCA score plots on total fatty acid composition, offspring liver samples showed clear differences between samples coming from pups supplied by mothers fed a DHA-enriched diet and those that were delivered by mothers kept on a control diet (supplemental Fig. S4), suggesting that maternal DHA supplementation has noticeable effect on offspring total fatty acid composition.
Effect of maternal and offspring genotype and maternal diet on offspring hepatic gene expression
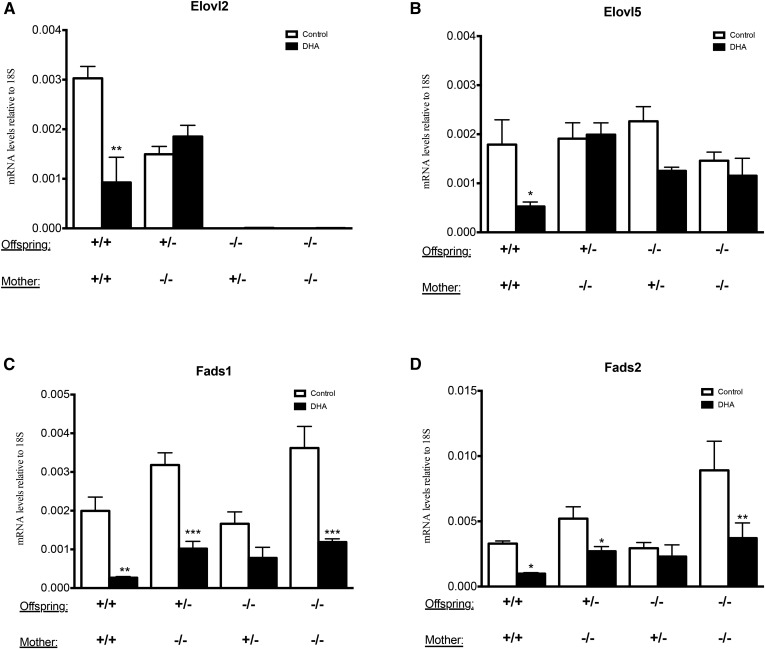
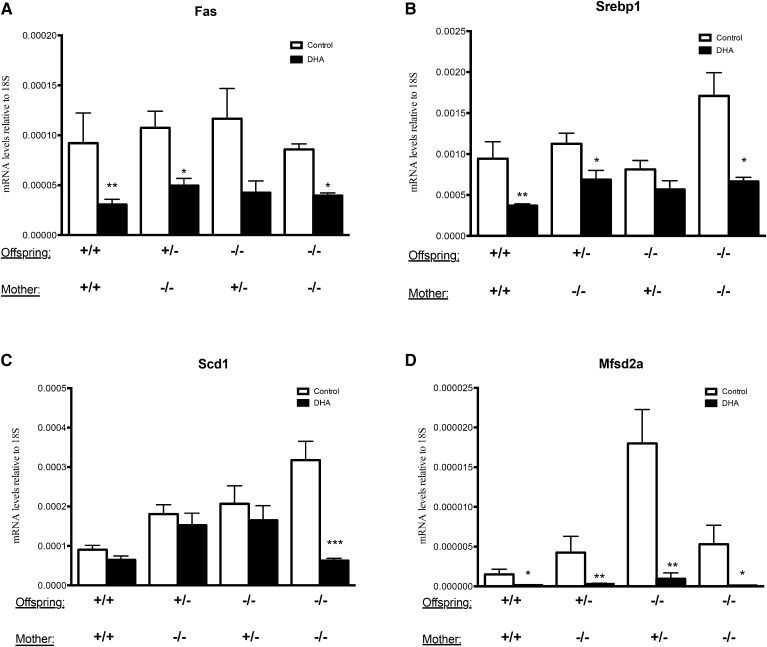
In order to see whether the DHA levels in the offspring groups described in Fig. 5 correlated with hepatic gene expression, we analyzed mRNA levels of genes regulating PUFA synthesis in the liver of the offspring. The levels of Elovl2 expression confirmed the genotypes of the offspring and indicated, as previously reported (24), a haploinsufficiency by 50% in heterozygote (Elovl2+/−) offspring (Fig. 6A). Interestingly, we did not observe any significant upregulation of Elovl5 as a compensatory mechanism for the loss of Elovl2 (Fig. 6B). The expression of Fads1 and Fads2 showed a tendency to be higher especially in the Elovl2−/− pups fed by Elovl2−/− mothers maintained on control diet (Fig. 6C, D). Maternal DHA supplementation led to a downregulation of Fads1 and Fads2 in all genotypes (Fig. 6C, D); whereas, Elovl2 and Elovl5 were only downregulated in +/+ neonates fed by +/+ mothers and Elovl5 also when fed by +/− mothers. Conclusively, the hepatic expression of the PUFA enzymes in the offspring is regulated in a noncoherent manner and affected not only by the Elovl2 genotype but also by the amount of maternal DHA supply. Moreover, the offspring of mothers maintained on a DHA-enriched diet showed a significant downregulation of the classical lipogenic transcription factor, Srebp-1c, and its target genes, Fas and Scd1 (Fig. 7A–C), which supports the concept of DHA as a suppressor of lipogenesis described previously in adult animals (25–27).
Fig. 6.
Hepatic gene expression of PUFA synthesizing enzymes. Expression of Elovl2 (A), Elovl5 (B), Fads1 (C), and Fads2 (D) in the liver of offspring with Elovl2+/+ (+/+), Elov2+/− (+/−), and Elovl2−/− (−/−) genotype delivered by Elovl2+/+ (+/+), Elovl2+/− (+/−), and Elovl2−/− (−/−) mothers fed either control diet (Control) or DHA-enriched diet (DHA) expressed as relative mRNA levels (target gene/18S). Results are presented as mean ± SEM of two to five mice. *P < 0.05, **P < 0.01, ***P < 0.001.
Fig. 7.
Analyses of lipogenic gene expression of Fas (A), Srebp-1c (B), Scd1 (C), and the DHA transporter Mfsd2a (D) in the liver of offspring with Elovl2+/+ (+/+), Elovl2+/− (+/−), and Elovl2−/− (−/−) genotype delivered by Elovl2+/+ (+/+), Elovl2+/− (+/−), and Elovl2−/− (−/−) mothers fed either control diet (Control) or DHA-enriched diet (DHA) expressed as relative mRNA levels (target gene/18S). Results are presented as mean ± SEM of two to five mice. *P < 0.05, **P < 0.01, ***P < 0.001.
Because our data suggests that the DHA level in the offspring is a result of a dynamic interplay between hepatic DHA synthesis and DHA uptake, we examined to determine whether the Elovl2 genotype and the level of maternal DHA supply during pregnancy and lactation may affect the hepatic expression of the DHA transporter, Mfsd2a. As seen in Fig. 7D, Mfsd2a was significantly expressed in all offspring groups and there was a tendency of increased expression, particularly in Elovl2−/− neonates fed by Elovl2+/− mothers kept on control diet, in both +/− and −/− offspring groups (Fig. 7D). Remarkably, maternal dietary DHA supplementation significantly downregulated the expression of the transporter in all groups, suggesting that Mfsd2a expression complements hepatic DHA synthesis to balance the level of DHA in the offspring.
DISCUSSION
To fulfill the physiological demands of the offspring, researchers have previously pointed out the importance of high systemic levels of the omega-3 fatty acid DHA in females during their reproduction cycle. In this study, we investigated the role of ELOVL2 and PUFA synthesis in establishing physiological levels of DHA in pregnant and lactating mice, as well as in their offspring. For this we used Elovl2-ablated (Elovl2−/−) mice, which previously have been shown to have severely reduced DHA levels to about 10% of normal levels in serum (21), and analyzed fatty acid levels in various tissues and serum in lactating mothers and their offspring, as well as in nonpregnant females. Our results show that both endogenous synthesis and maternal provision of DHA are required sources for obtaining normal systemic DHA levels in the offspring during pregnancy and lactation. However, in contrast to common beliefs, we can show that before weaning, endogenous DHA synthesis in the offspring can almost fully compensate for maternal DHA deficiency. Our data also imply that endogenous PUFA synthesis in mothers cannot fully compensate for deficient synthesis in mice during perinatal life.
DHA levels during pregnancy and lactation tend to show a quite dynamic pattern for both mothers and their offspring. During the first two thirds of pregnancy, there is an observed increase of DHA levels in human serum that is a result from increased endogenous synthesis as well as mobilization from internal stores (4, 28). A recent study shows that DHA levels can start to increase as early as 29 days post luteinizing hormone intervention (29). However, in late pregnancy and after parturition, a stage of DHA reduction has been shown to occur in human plasma (30). This period correlates with increased demands of DHA supply into the fetus or neonates for, e.g., optimal brain development (2). In breastfeeding mothers, up to a 30% depletion in the maternal circulation level of DHA has been observed (31), which may explain why we did not observe higher DHA levels in lactating mothers compared with the control females. The PCA scores revealed a clear distinction in fatty acid composition between mothers and control females, which implies maternal adipose depletion in mammary glands due to lactation. To fully understand the role of ELOVL2 and DHA synthesis during fetal and neonatal life it would therefore be valuable to monitor the DHA status in serum in our experimental groups during different time points throughout the pregnancy and lactation periods.
Several population studies have pointed out an association between human carriers of Fads1 and Fads2 haplotypes and the DHA level in pregnant and lactating mothers, as well as in their offspring (32–34). Although our data here imply that gene variants of Elovl2 can contribute to differences in the maternal DHA pool in mammals, a limited number of studies have pointed out a role for Elovl2 during pregnancy and lactation. Rodriguez-Cruz and coworkers have reported that Elovl2 upregulation in the maternal rat liver at 20 days of pregnancy can contribute to increased long-chain PUFA synthesis and DHA levels during late pregnancy and lactation (35). Moreover, the same group observed an increased Elovl2 expression in adipose tissue within the first half of the lactation period (36), suggesting improved mobilization of DHA from internal sources. There are also reports regarding low levels of Elovl2 mRNA and protein in placenta and in mammary glands during lactation (35–38).
Several studies have implied that maternal DHA supplementation may rescue DHA depletion during pregnancy and lactation and lead to a successful supply of DHA to the offspring (30, 39–42). In agreement with this, maternal DHA supplementation increased the level of DHA in all tissues of mothers and control females, as well as in the offspring. Interestingly, the DHA level in the pups reached a ceiling of about 20% of total fatty acids regardless of genotype, which implies the existence of a plausible set-point for how much DHA can be accreted. In addition, Elovl2−/− mothers fed a DHA-enriched diet exhibited accumulation of DHA, for all types of analyzed tissues, much over the level of mothers of other genotypes, as well as the control females of the same genotype. This may indicate the existence of a specific compensatory mechanism in response to DHA deficiency during pregnancy and lactation for Elovl2−/− mothers when DHA is available in the diet. Moreover, the relative amount of DHA in the offspring liver reached a relatively higher level than in the maternal liver, which is a good illustration of a bio-magnification phenomenon (43). Although there has been a growing interest in the field of diet-genotype interplay and body DHA levels (44), to our knowledge, this is the first report that focuses on the effect of maternal DHA supplementation in a condition of genetically acquired systemic DHA-deficiency and not by dietary manipulation such as depletion of DHA or its precursors, such as ALA.
Previous studies have revealed that FADS2-ablation or dietary DHA deficiency affects fertility of both genders, as well as breeding outcomes in general (45–48). In contrast to this, we observed that Elovl2−/− mothers fed a DHA-enriched diet had a lower level of successful pregnancies (Table 3). Regarding the Elovl2−/− genotype, which is a model system reflecting insufficient conversion of EPA into DHA, we observed relatively high maternal levels of EPA under standard conditions, which upon dietary supplementation, became even higher (supplemental Tables S16–S19) and may have a negative effect on pregnancy outcome (49, 50). Nevertheless, this hypothesis needs to be supported by further and more detailed studies.
Several studies have reported effects of DHA supplementation during pregnancy and lactation on the length of gestation and, consequently, the birth weight of the offspring (17, 51, 52). In the present study, DHA supplementation seemed to increase offspring body weight, which was linked to increased lean weight, but not fat mass. This suggests that, before weaning, DHA modulates growth and developmental features rather than the fat metabolism (53). However, we did not record the occurrence of vaginal plug and therefore the precise length of gestation was not monitored nor did we make any adjustments for litter size, which both may have a strong impact on body weight and composition.
In line with the previous reports on fetuses and neonates (11, 13), we detected relatively high expression of genes involved in PUFA synthesis (Elovl5, Fads1, and Fads2) in the liver of suckling mice. Although experimental data suggest that the activity of the PUFA enzymes is mainly controlled at the transcriptional level, Elovl2-ablation in the offspring did not lead to a general compensatory upregulation of any of the genes. Recently, Park et al. (54) reported that Fads2 transcript in human cells can catalyze Δ4-desaturation and be actively involved in direct synthesis of DHA from 22:5n-3. While we did not detect any effects on the DHA level in Elovl2−/− pups fed by Elovl2−/− mothers, the maternal genotype appeared to have a significant impact on the expression levels of the desaturases, but not on Elovl5.
An interesting observation was that the expression levels of Mfsd2a, which is identified as a transporter of DHA as lysophosphatidylcholine-DHA (55) and is significantly expressed in liver, presented a mirror image of the DHA levels found in offspring liver and serum and was almost undetectable in the groups fed by mothers on a DHA-enriched diet, implying a negative feed-back mechanism when the DHA levels have reached a ceiling in the offspring. This is in line with previous results indicating that Mfsd2a is a nutritionally regulated gene that plays numerous roles in body growth and development, motor function, and lipid metabolism (56, 57). Its expression in liver is found to be dependent on PPARα and glucagon signaling and is increased in liver upon food deprivation. Accordingly, Mfsd2a has therefore been suggested to play a role in adaptation to fasting. However, to our knowledge, this is the first in vivo study on the regulation of Mfsd2a regarding DHA deficiency and DHA supplementation.
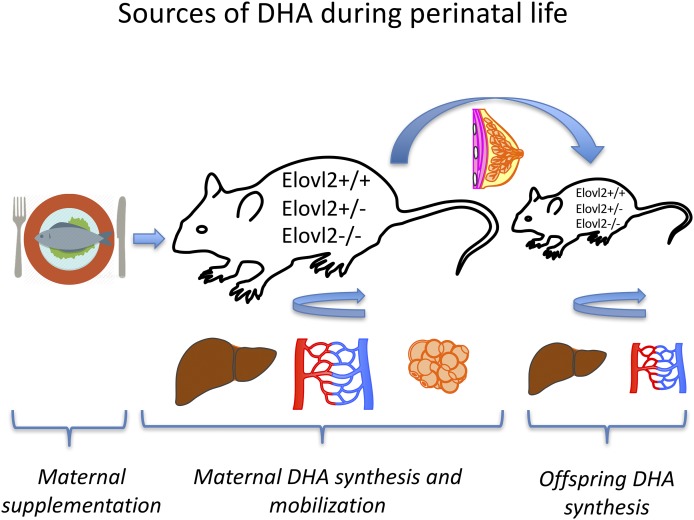
In conclusion, analysis of DHA levels in the diverse Elovl2 genotypes of mothers and their offspring, gave us a possibility to address questions concerning the source of DHA during perinatal life. Combining our findings, we can conclude that both maternal and offspring Elovl2 genotypes, as well as maternal dietary supplementation, contribute to DHA accretion during perinatal life and are controlled by a dynamic interplay between hepatic DHA synthesis and DHA uptake in the offspring (Fig. 8). Undoubtedly, additional precise investigations are required regarding DHA sources and offspring fatty acid deposition during the whole pregnancy and lactation period.
Fig. 8.
Maternal and offspring Elovl2 genotype and maternal diet contribute to DHA accretion through perinatal life.
Supplementary Material
Acknowledgments
The authors thank S. Wagenius, E. Ljunglöf, N. Petrescu, and L. Oellig for technical assistance.
Footnotes
Abbreviations:
- ALA
- α-linolenic acid
- ELOVL
- elongation of very long-chain fatty acid
- FADS
- fatty acid desaturase
- Mfsd2a
- major facilitator superfamily domain containing 2a
- PC
- principal component
- PCA
- principal component analysis
- Scd1
- steroyl-CoA desaturase 1
- Srebp-1c
- sterol regulatory element-binding protein 1c
- WAT
- white adipose tissue
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Innis S. M. 2008. Dietary omega 3 fatty acids and the developing brain. Brain Res. 1237: 35–43. [DOI] [PubMed] [Google Scholar]
- 2.Lauritzen L., Brambilla P., Mazzocchi A., Harslof L. B., Ciappolino V., and Agostoni C.. 2016. DHA effects in brain development and function. Nutrients. 8: E6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haggarty P. 2010. Fatty acid supply to the human fetus. Annu. Rev. Nutr. 30: 237–255. [DOI] [PubMed] [Google Scholar]
- 4.Lauritzen L., and Carlson S. E.. 2011. Maternal fatty acid status during pregnancy and lactation and relation to newborn and infant status. Matern. Child Nutr. 7(Suppl 2): 41–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guillou H., Zadravec D., Martin P. G., and Jacobsson A.. 2010. The key roles of elongases and desaturases in mammalian fatty acid metabolism: insights from transgenic mice. Prog. Lipid Res. 49: 186–199. [DOI] [PubMed] [Google Scholar]
- 6.Giltay E. J., Gooren L. J., Toorians A. W., Katan M. B., and Zock P. L.. 2004. Docosahexaenoic acid concentrations are higher in women than in men because of estrogenic effects. Am. J. Clin. Nutr. 80: 1167–1174. [DOI] [PubMed] [Google Scholar]
- 7.Extier A., Langelier B., Perruchot M. H., Guesnet P., Van Veldhoven P. P., Lavialle M., and Alessandri J. M.. 2010. Gender affects liver desaturase expression in a rat model of n-3 fatty acid repletion. J. Nutr. Biochem. 21: 180–187. [DOI] [PubMed] [Google Scholar]
- 8.Stark K. D., Beblo S., Murthy M., Buda-Abela M., Janisse J., Rockett H., Whitty J. E., Martier S. S., Sokol R. J., Hannigan J. H., et al. . 2005. Comparison of bloodstream fatty acid composition from African-American women at gestation, delivery, and postpartum. J. Lipid Res. 46: 516–525. [DOI] [PubMed] [Google Scholar]
- 9.Stewart F., Rodie V. A., Ramsay J. E., Greer I. A., Freeman D. J., and Meyer B. J.. 2007. Longitudinal assessment of erythrocyte fatty acid composition throughout pregnancy and post partum. Lipids. 42: 335–344. [DOI] [PubMed] [Google Scholar]
- 10.Childs C. E., Hoile S. P., Burdge G. C., and Calder P. C.. 2012. Changes in rat n-3 and n-6 fatty acid composition during pregnancy are associated with progesterone concentrations and hepatic FADS2 expression. Prostaglandins Leukot. Essent. Fatty Acids. 86: 141–147. [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez A., Sarda P., Nessmann C., Boulot P., Poisson J. P., Leger C. L., and Descomps B.. 1998. Fatty acid desaturase activities and polyunsaturated fatty acid composition in human liver between the seventeenth and thirty-sixth gestational weeks. Am. J. Obstet. Gynecol. 179: 1063–1070. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y., Botolin D., Christian B., Busik J., Xu J., and Jump D. B.. 2005. Tissue-specific, nutritional, and developmental regulation of rat fatty acid elongases. J. Lipid Res. 46: 706–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carnielli V. P., Simonato M., Verlato G., Luijendijk I., De Curtis M., Sauer P. J., and Cogo P. E.. 2007. Synthesis of long-chain polyunsaturated fatty acids in preterm newborns fed formula with long-chain polyunsaturated fatty acids. Am. J. Clin. Nutr. 86: 1323–1330. [DOI] [PubMed] [Google Scholar]
- 14.Salem N. Jr., Wegher B., Mena P., and Uauy R.. 1996. Arachidonic and docosahexaenoic acids are biosynthesized from their 18-carbon precursors in human infants. Proc. Natl. Acad. Sci. USA. 93: 49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sauerwald T. U., Hachey D. L., Jensen C. L., Chen H., Anderson R. E., and Heird W. C.. 1997. Intermediates in endogenous synthesis of C22:6 omega 3 and C20:4 omega 6 by term and preterm infants. Pediatr. Res. 41: 183–187. [DOI] [PubMed] [Google Scholar]
- 16.Szitanyi P., Koletzko B., Mydlilova A., and Demmelmair H.. 1999. Metabolism of 13C-labeled linoleic acid in newborn infants during the first week of life. Pediatr. Res. 45: 669–673. [DOI] [PubMed] [Google Scholar]
- 17.Larqué E., Gil-Sánchez A., Prieto-Sánchez M. T., and Koletzko B.. 2012. Omega 3 fatty acids, gestation and pregnancy outcomes. Br. J. Nutr. 107(Suppl 2): S77–S84. [DOI] [PubMed] [Google Scholar]
- 18.Harauma A., Salem N. Jr., and Moriguchi T.. 2010. Repletion of n-3 fatty acid deficient dams with alpha-linolenic acid: effects on fetal brain and liver fatty acid composition. Lipids. 45: 659–668. [DOI] [PubMed] [Google Scholar]
- 19.Niculescu M. D., Lupu D. S., and Craciunescu C. N.. 2011. Maternal alpha-linolenic acid availability during gestation and lactation alters the postnatal hippocampal development in the mouse offspring. Int. J. Dev. Neurosci. 29: 795–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niculescu M. D., Lupu D. S., and Craciunescu C. N.. 2013. Perinatal manipulation of alpha-linolenic acid intake induces epigenetic changes in maternal and offspring livers. FASEB J. 27: 350–358. [DOI] [PubMed] [Google Scholar]
- 21.Pauter A. M., Olsson P., Asadi A., Herslof B., Csikasz R. I., Zadravec D., and Jacobsson A.. 2014. Elovl2 ablation demonstrates that systemic DHA is endogenously produced and is essential for lipid homeostasis in mice. J. Lipid Res. 55: 718–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hara A., and Radin N. S.. 1978. Lipid extraction of tissues with a low-toxicity solvent. Anal. Biochem. 90: 420–426. [DOI] [PubMed] [Google Scholar]
- 23.Appelqvist L. 1968. Rapid methods of lipid extraction and fatty acid methyl ester preparation for seed and leaf tissue with special remarks on preventing the accumulation of lipid contaminants. Arkiv för Kemi, Royal Swedish Academy of Science (Kungliga Svenska Vetenskapsakademien) 36. 28: 551–570. [Google Scholar]
- 24.Zadravec D., Tvrdik P., Guillou H., Haslam R., Kobayashi T., Napier J. A., Capecchi M. R., and Jacobsson A.. 2011. ELOVL2 controls the level of n-6 28:5 and 30:5 fatty acids in testis, a prerequisite for male fertility and sperm maturation in mice. J. Lipid Res. 52: 245–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu J., Borthwick F., Hassanali Z., Wang Y., Mangat R., Ruth M., Shi D., Jaeschke A., Russell J. C., Field C. J., et al. . 2011. Chronic dietary n-3 PUFA intervention improves dyslipidaemia and subsequent cardiovascular complications in the JCR:LA- cp rat model of the metabolic syndrome. Br. J. Nutr. 105: 1572–1582. [DOI] [PubMed] [Google Scholar]
- 26.Pachikian B. D., Essaghir A., Demoulin J. B., Neyrinck A. M., Catry E., De Backer F. C., Dejeans N., Dewulf E. M., Sohet F. M., Portois L., et al. . 2011. Hepatic n-3 polyunsaturated fatty acid depletion promotes steatosis and insulin resistance in mice: genomic analysis of cellular targets. PLoS One. 6: e23365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rossmeisl M., Medrikova D., van Schothorst E. M., Pavlisova J., Kuda O., Hensler M., Bardova K., Flachs P., Stankova B., Vecka M., et al. . 2014. Omega-3 phospholipids from fish suppress hepatic steatosis by integrated inhibition of biosynthetic pathways in dietary obese mice. Biochim. Biophys. Acta. 1841: 267–278. [DOI] [PubMed] [Google Scholar]
- 28.Herrera E. 2002. Lipid metabolism in pregnancy and its consequences in the fetus and newborn. Endocrine. 19: 43–55. [DOI] [PubMed] [Google Scholar]
- 29.Meyer B. J., Onyiaodike C. C., Brown E. A., Jordan F., Murray H., Nibbs R. J., Sattar N., Lyall H., Nelson S. M., and Freeman D. J.. 2016. Maternal plasma DHA levels increase prior to 29 days post-LH surge in women undergoing frozen embryo transfer: a prospective, observational study of human pregnancy. J. Clin. Endocrinol. Metab. 101: 1745–1753. [DOI] [PubMed] [Google Scholar]
- 30.Al M. D., van Houwelingen A. C., and Hornstra G.. 2000. Long-chain polyunsaturated fatty acids, pregnancy, and pregnancy outcome. Am. J. Clin. Nutr. 71: 285S–291S. [DOI] [PubMed] [Google Scholar]
- 31.Makrides M., and Gibson R. A.. 2000. Long-chain polyunsaturated fatty acid requirements during pregnancy and lactation. Am. J. Clin. Nutr. 71: 307S–311S. [DOI] [PubMed] [Google Scholar]
- 32.Xie L., and Innis S. M.. 2008. Genetic variants of the FADS1 FADS2 gene cluster are associated with altered (n-6) and (n-3) essential fatty acids in plasma and erythrocyte phospholipids in women during pregnancy and in breast milk during lactation. J. Nutr. 138: 2222–2228. [DOI] [PubMed] [Google Scholar]
- 33.Lattka E., Koletzko B., Zeilinger S., Hibbeln J. R., Klopp N., Ring S. M., and Steer C. D.. 2013. Umbilical cord PUFA are determined by maternal and child fatty acid desaturase (FADS) genetic variants in the Avon Longitudinal Study of Parents and Children (ALSPAC). Br. J. Nutr. 109: 1196–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gonzalez-Casanova I., Rzehak P., Stein A. D., Garcia Feregrino R., Dommarco J. A., Barraza-Villarreal A., Demmelmair H., Romieu I., Villalpando S., Martorell R., et al. . 2016. Maternal single nucleotide polymorphisms in the fatty acid desaturase 1 and 2 coding regions modify the impact of prenatal supplementation with DHA on birth weight. Am. J. Clin. Nutr. 103: 1171–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rodriguez-Cruz M., Sanchez R., Sanchez A. M., Kelleher S. L., Sanchez-Munoz F., Maldonado J., and Lopez-Alarcon M.. 2011. Participation of mammary gland in long-chain polyunsaturated fatty acid synthesis during pregnancy and lactation in rats. Biochim. Biophys. Acta. 1811: 284–293. [DOI] [PubMed] [Google Scholar]
- 36.González R. S., Rodriguez-Cruz M., Maldonado J., and Saavedra F. J.. 2014. Role of maternal tissue in the synthesis of polyunsaturated fatty acids in response to a lipid-deficient diet during pregnancy and lactation in rats. Gene. 549: 7–23. [DOI] [PubMed] [Google Scholar]
- 37.Bautista C. J., Montano S., Ramirez V., Morales A., Nathanielsz P. W., Bobadilla N. A., and Zambrano E.. 2016. Changes in milk composition in obese rats consuming a high-fat diet. Br. J. Nutr. 115: 538–546. [DOI] [PubMed] [Google Scholar]
- 38.Rodríguez-Cruz M., González R. S., Maldonado J., López-Alarcón M., and Bernabe-García M.. 2016. The effect of gestational age on expression of genes involved in uptake, trafficking and synthesis of fatty acids in the rat placenta. Gene. 591: 403–410. [DOI] [PubMed] [Google Scholar]
- 39.Hornstra G. 2000. Essential fatty acids in mothers and their neonates. Am. J. Clin. Nutr. 71: 1262S–1269S. [DOI] [PubMed] [Google Scholar]
- 40.Dunstan J. A., Mori T. A., Barden A., Beilin L. J., Holt P. G., Calder P. C., Taylor A. L., and Prescott S. L.. 2004. Effects of n-3 polyunsaturated fatty acid supplementation in pregnancy on maternal and fetal erythrocyte fatty acid composition. Eur. J. Clin. Nutr. 58: 429–437. [DOI] [PubMed] [Google Scholar]
- 41.Krauss-Etschmann S., Shadid R., Campoy C., Hoster E., Demmelmair H., Jimenez M., Gil A., Rivero M., Veszpremi B., Decsi T., et al. ; Nutrition and Health Lifestyle (NUHEAL) Study Group. 2007. Effects of fish-oil and folate supplementation of pregnant women on maternal and fetal plasma concentrations of docosahexaenoic acid and eicosapentaenoic acid: a European randomized multicenter trial. Am. J. Clin. Nutr. 85: 1392–1400. [DOI] [PubMed] [Google Scholar]
- 42.Bergmann R. L., Haschke-Becher E., Klassen-Wigger P., Bergmann K. E., Richter R., Dudenhausen J. W., Grathwohl D., and Haschke F.. 2008. Supplementation with 200 mg/day docosahexaenoic acid from mid-pregnancy through lactation improves the docosahexaenoic acid status of mothers with a habitually low fish intake and of their infants. Ann. Nutr. Metab. 52: 157–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Crawford M. A., Hassam A. G., and Williams G.. 1976. Essential fatty acids and fetal brain growth. Lancet. 1: 452–453. [DOI] [PubMed] [Google Scholar]
- 44.Minihane A. M. 2016. Impact of genotype on EPA and DHA status and responsiveness to increased intakes. Nutrients. 8: 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wathes D. C., Abayasekara D. R., and Aitken R. J.. 2007. Polyunsaturated fatty acids in male and female reproduction. Biol. Reprod. 77: 190–201. [DOI] [PubMed] [Google Scholar]
- 46.Stoffel W., Holz B., Jenke B., Binczek E., Gunter R. H., Kiss C., Karakesisoglou I., Thevis M., Weber A. A., Arnhold S., et al. . 2008. Delta6-desaturase (FADS2) deficiency unveils the role of omega3- and omega6-polyunsaturated fatty acids. EMBO J. 27: 2281–2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stroud C. K., Nara T. Y., Roqueta-Rivera M., Radlowski E. C., Lawrence P., Zhang Y., Cho B. H., Segre M., Hess R. A., Brenna J. T., et al. . 2009. Disruption of FADS2 gene in mice impairs male reproduction and causes dermal and intestinal ulceration. J. Lipid Res. 50: 1870–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roqueta-Rivera M., Abbott T. L., Sivaguru M., Hess R. A., and Nakamura M. T.. 2011. Deficiency in the omega-3 fatty acid pathway results in failure of acrosome biogenesis in mice. Biol. Reprod. 85: 721–732. [DOI] [PubMed] [Google Scholar]
- 49.Carlson S. E., Cooke R. J., Werkman S. H., and Tolley E. A.. 1992. First year growth of preterm infants fed standard compared to marine oil n-3 supplemented formula. Lipids. 27: 901–907. [DOI] [PubMed] [Google Scholar]
- 50.Hadley K. B., Ryan A. S., Forsyth S., Gautier S., and Salem N.. 2016. The essentiality of arachidonic acid in infant development. Nutrients. 8: 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carlson S. E., Colombo J., Gajewski B. J., Gustafson K. M., Mundy D., Yeast J., Georgieff M. K., Markley L. A., Kerling E. H., and Shaddy D. J.. 2013. DHA supplementation and pregnancy outcomes. Am. J. Clin. Nutr. 97: 808–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meher A., Randhir K., Mehendale S., Wagh G., and Joshi S.. 2016. Maternal fatty acids and their association with birth outcome: a prospective study. PLoS One. 11: e0147359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Muhlhausler B. S., Yelland L. N., McDermott R., Tapsell L., McPhee A., Gibson R. A., and Makrides M.. 2016. DHA supplementation during pregnancy does not reduce BMI or body fat mass in children: follow-up of the DHA to Optimize Mother Infant Outcome randomized controlled trial. Am. J. Clin. Nutr. 103: 1489–1496. [DOI] [PubMed] [Google Scholar]
- 54.Park H. G., Park W. J., Kothapalli K. S., and Brenna J. T.. 2015. The fatty acid desaturase 2 (FADS2) gene product catalyzes Delta4 desaturation to yield n-3 docosahexaenoic acid and n-6 docosapentaenoic acid in human cells. FASEB J. 29: 3911–3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nguyen L. N., Ma D., Shui G., Wong P., Cazenave-Gassiot A., Zhang X., Wenk M. R., Goh E. L., and Silver D. L.. 2014. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature. 509: 503–506. [DOI] [PubMed] [Google Scholar]
- 56.Angers M., Uldry M., Kong D., Gimble J. M., and Jetten A. M.. 2008. Mfsd2a encodes a novel major facilitator superfamily domain-containing protein highly induced in brown adipose tissue during fasting and adaptive thermogenesis. Biochem. J. 416: 347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Berger J. H., Charron M. J., and Silver D. L.. 2012. Major facilitator superfamily domain-containing protein 2a (MFSD2A) has roles in body growth, motor function, and lipid metabolism. PLoS One. 7: e50629. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.