Abstract
apoA-I, apoA-I mimetic peptides, and their lipid complexes or reconstituted high-density lipoprotein (HDL) have been studied as treatments for various pathologies. However, consensus is lacking about the best method for administration, by intravenous (IV) or intraperitoneal (IP) routes, and formulation, as an HDL particle or in a lipid-free form. The objective of this study was to systematically examine peptide plasma levels, cholesterol mobilization, and lipoprotein remodeling in vivo following administration of lipid-free apoA-I peptide (22A) or phospholipid reconstituted 22A-sHDL by IV and IP routes. The mean circulation half-life was longer for 22A-sHDL (T1/2 = 6.27 h) than for free 22A (T1/2 = 3.81 h). The percentage of 22A absorbed by the vascular compartment after the IP dosing was ∼50% for both 22A and 22A-sHDL. The strongest pharmacologic response came from IV injection of 22A-sHDL, specifically a 5.3-fold transient increase in plasma-free cholesterol (FC) level compared with 1.3- and 1.8-fold FC increases for 22A-IV and 22A-sHDL-IP groups. Addition of either 22A or 22A-sHDL to rat plasma caused lipoprotein remodeling and appearance of a lipid-poor apoA-I. Hence, both the route of administration and the formulation of apoA-I peptide significantly affect its pharmacokinetics and pharmacodynamics.
Keywords: HDL, lipoproteins/metabolism, apolipoprotein A-I mimetic peptides, lipoprotein remodeling, PK-PD modeling
The therapeutic use of apoA-I, its mutants, and peptide mimetics for the treatment of atherosclerosis has been studied in a variety of animal models and clinical trials (1, 2). However, there is a lack of consensus regarding whether the apoA-I protein or peptide should be administered in a lipid-free form or bound to phospholipids as a reconstituted HDL particle. Early clinical trials showed that infusion of lipid-free apoA-I failed to increase circulation level of HDL cholesterol (HDL-C) and resulted in shorter circulation time than that for endogenous apoA-I (3). Consequently, the majority of clinically developed apoA-I products have been administered as reconstituted HDL particles: apoA-I/soybean phosphatidylcholine (CSL-111 and CSL-112), apoA-I-Milano/palmitoyl-oleoyl-phosphotidylcholine (ETC-216), and apoA-I/sphingomyelin/dipalmitoyl-phosphorylglycerol (CER-001) (2, 4, 5). In contrast, many preclinical studies have been performed using infusions of lipid-free proteins, and apoA-I peptides have been optimized for their pharmacological activity in lipid-free form (6).
There is also an uncertainty concerning the mechanism (or mechanisms) by which reconstituted HDL infusions elicit pharmacological effects and whether such mechanisms differ from that used by lipid-free apoA-I. The ability of lipid-free apoA-I to efflux lipids through interaction with ABCA1 and its capacity to form de novo functional preβ HDL particles are considered to be critical for cholesterol efflux in vivo (7, 8). However, infused apoA-I peptides or proteins do not remain in the lipid-free form. Rather, they bind and remodel endogenous HDL, improving its functionality and thereby eliciting a pharmacological effect (9, 10). In contrast, when apoA-I is dosed in the form of an HDL particle, the magnitude of its pharmacological effect, measured by the degree of cholesterol mobilization from tissues to plasma, depends on the HDL’s phospholipid composition (11, 12).
In a similar fashion, there is a lack of consensus surrounding the optimal route of administration for apoA-I and its mimetic peptides. For long-term dosing in rodents, intraperitoneal (IP) administration is preferred for a technical reason, namely, the difficulty of repeated dosing in the tail vein (13). However, in many acute studies, apoA-I and HDL are administered intravenously (IV), specifically because these compounds act in the vascular compartment through either remodeling endogenous lipoproteins or direct efflux of the excess of cholesterol from foam cells in atheroma (14, 15). The effective dose of apoA-I or reconstituted HDL that reaches circulation is likely lower following IP administration in comparison to IV administration because of loss occurring during absorption from tissue to the vascular compartment. Yet the fraction of apoA-I or HDL that is actually capable of reaching the vasculature following IP dose has not been experimentally determined.
With growing interest around the potential therapeutic roles of administered reconstituted HDL and apoA-I in the treatment of sepsis, diabetes, rheumatoid arthritis, lupus, and other diseases (11, 16, 17), it is important to develop a basic understanding of HDL/apoA-I pharmacokinetics in order to select the proper dose, dosing intervals, and route of administration. In addition, the ability to measure basic HDL-related biomarkers of pharmacological effect to understand how these biomarkers relate to the dose, dosing interval, and administration route is important. Having this information at hand will allow investigators to select pharmacologically relevant doses and avoid obtaining false negative results. Pharmacokinetic-pharmacodynamic (PK/PD) modeling is a scientific tool to relate PK models (describing the relationship among dose, systemic drug exposure, and time) to PD models (describing the mathematical relationship between exposure level and the pharmacological effect) (18). By establishing PK/PD modeling, the relationship between the PK and PD profile can be quantified, providing an assessment of effect onset/duration in relation to the plasma PK profile (19).
In this study, we selected a “two by two” experimental design to compare the administration of apoA-I peptide and apoA-I peptide reconstituted HDL following IV and IP administrations in normal adult male rats. We selected the synthetic peptide 22A, or ESP24218, as a model apoA-I mimetic peptide. This peptide was the first apoA-I mimetic peptide to reach clinical development, which has been administered clinically in both single- and multiple-dose trials, and its human pharmacokinetic data are available (20, 21). We determined peptide and phospholipid pharmacokinetics and measured cholesterol mobilization in plasma, distribution of mobilized cholesterol among HDL, LDL, and VLDL particles, plasma efflux capacity, and lipoprotein remodeling following free 22A and 22A reconstituted HDL dosing.
MATERIALS AND METHODS
Materials
ApoA-I mimetic peptides 22A, PVLDLFRELLNELLEALKQKLK, and 5A, DWLKAFYDKVAEKLKEAFPDWAKAAYDKAAEKAKEAA, were synthesized by Genscript Inc. (Piscataway, NJ). The purities of peptides were determined to be over 95% by reserve phase HPLC. Phospholipids 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) and 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) were generously donated by Nippon Oils and Fats (Osaka, Japan). All other materials were obtained from commercial sources.
Preparation and characterization of 22A-sHDL particles
Synthetic HDL particles were prepared by the thin film-hydration method. Briefly, DPPC and POPC were dissolved in chloroform at 20 mg/ml. The 22A peptide was dissolved in methanol:water (1:1 vol ratio) at 10 mg/ml. DPPC, POPC, and 22A were mixed in a 4 ml glass vial at different weight ratios and vortexed for 5 s. The mixture was dried by nitrogen gas flow and then placed in the vacuum oven overnight to remove residual solvent. The resulting lipid film was hydrated with PBS (pH 7.4) (final concentration of 22A =15 mg/ml) and vortexed. The suspension was homogenized in a bath sonicator for 5 min and then with a probe sonicator intermittently (50 W×10 S×12 cycles) to form a clear or translucent 22A-sHDL solution.
Analysis of 22A-sHDL particles
The purity of 22A-sHDL was analyzed by gel permeation chromatography (GPC) with UV detection at 220 nm using Tosoh TSK gel G3000SWx 7.8 mm × 30 cm column (Tosoh Bioscience, King of Prussia, PA) on a Waters Breeze Dual Pump system. The HDL samples were diluted to 1 mg/ml peptide concentration, and an injection volume of 10 μl was used. The samples were eluted with PBS (pH 7.4) at a flow rate of 1 ml/min. The sHDL hydrodynamic diameters were determined by dynamic light scattering, using a Zetasizer Nano ZSP (Malvern Instruments, Westborough, MA). Samples were diluted to 1.5 mg/ml peptide concentration. The volume intensity average values were reported.
Rat pharmacokinetics and cholesterol mobilization
Male Sprague-Dawley rats (300∼350 mg) were purchased from Charles River Laboratories (Wilmington, MA) and acclimated for 1 week. The animals were maintained on a chow diet. Prior to dosing, the animals were fasted overnight and received either 22A peptide or 22A-sHDL particles at 75 mg/kg by either intraperitoneal (IP) or intravenous (IV) injection. Four groups of rats (n = 4 per group) were dosed as follows: IV dose of 22A solution group; IP dose of 22A solution group; IV dose of 22A-sHDL group; and IP dose of 22A-sHDL group. Blood (300–500 μl) was drawn from the jugular vein into BD microtainer (TM) tubes (BD, Franklin Lakes, NJ) for capillary blood collection at predose and 0.25, 0.5, 1, 2, 4, 8, and 24 h after dosing. The animals were fed following the 8 h time point and fasted again prior to the 24 h bleed. Serum was isolated immediately from the whole blood by centrifugation at 14,000 rpm for 10 min at 4°C. Serum was aliquoted into multiple tubes and stored at −80°C prior to analysis.
LC/MS analysis of peptide plasma levels
Immediately after serum separation, 10 μl of 5A peptide (3 mg/ml) was added to 10 μl of serum as an internal standard, and peptides were extracted with 100 μl of methanol containing 0.1% acetic acid. After vortexing for 30 s, the mixture was centrifuged at 14,000 rpm for 10 min at 4°C. The top layer was drawn off and used for quantifying peptide levels in the serum, using Agilent 6520 Accurate-Mass Q-TOF LC/MS equipped with a dual electrospray ionization source (Dual-ESI; Agilent Technologies, CA). The HPLC separation was performed using the Agilent 300SB-C18 column (2.1 mm × 50 mm, 3.5 μm). The mobile phase consisted of (A) water containing 0.1% (v:v) formic acid and (B) methanol (pH 2.2) containing 0.1% (v:v) formic acid using a gradient elution of 10% to 60% B at 0–3.5 min, and 60% to 95% B at 3.5–8 min. The flow rate was 0.4 ml/min with an injection volume of 10 μl. Mass spectra were acquired in negative ion mode with the mass range set at m/z 100–3,200. The conditions used for the ESI source included a capillary voltage of 3,500 V, a drying gas temperature of 332°C, a drying gas flow of 5 L/min, and a nebulizer pressure of 45 psi as well as a fragmentor voltage of 225 V. MassHunter Workstation software (Agilent Technologies, CA) was used for data acquisition and processing. The extracted ion chromatogram (EIC) of 22A was exported from the total ion chromatogram by monitoring the key fragment of 22A at m/z 656.6. Analogously, the EIC of 22A (-)Lys metabolite and IS 5A was extracted at m/z 832.5 and m/z 844.4, respectively. The total integral area of 22A peak and 22A (-)Lys metabolite peak was used to calculate concentration.
Measurement of plasma lipids
The levels of serum phospholipids (PL), total cholesterol (TC), and unesterified or free cholesterol (FC) were determined by enzymatic analysis using commercially available kits (Wako Chemicals, Richmond, VA). The cholesterol ester (CE) level was calculated as the difference between TC and FC levels at each time point. Briefly, serum samples were diluted with PBS (pH 7.4) 10-fold for TC detection and 3-fold for FC detection and with Milli Q water 10 times for PL detection. Defined amounts of standards or diluted samples were transferred into 96-well plate (50 μl, 60 μl, and 20 μl for TC, FC, and PL, respectively). Color reagents were added according to manufacturer instructions. The plates were gently shaken using an orbital shaker and incubated at 37°C for 5 min. The UV absorbance at 600 nm was measured by a Synergy NEO HTS Multi-Mode Microplate Reader (BioTek).
Pharmacokinetic parameters calculation and PK-PD modeling
Pharmacokinetic (PK) and pharmacodynamic (PD) analyses of the data were performed by least-squares regression analysis, weighted by the inverse of the fitted value, using Phoenix© WinNonlin® (Pharsight Corporation, Mountain View, CA). Serum 22A and PL PK parameters were estimated using a one-compartment disposition model for IV bolus administration, and a one-compartment disposition model with first-order absorption and no lag time for IP administration. The PK parameters included time to reach maximum serum concentration (Tmax), maximum serum concentration (Cmax), area under the serum concentration-time curve (AUC), first-order absorption rate constant after IP injection (k01), first-order elimination rate constant (k10), elimination half-life (T1/2), apparent total clearance (CL or CL/F, where F is bioavailability), and apparent volume of distribution (Vd or Vd/F). The goodness of fit was determined using Akaike Information Criterion (AIC) and by visual inspection of the fits and residuals. The coefficient of variation for each fitted parameter was also reported. The resulting pharmacokinetic parameters of 22A or phospholipids were then used as constants in the integrated PK-PD model.
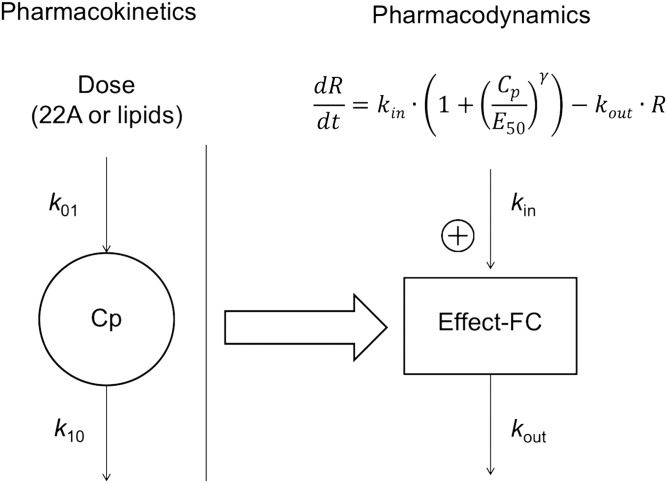
The PK-PD model was established by relating serum concentrations of either 22A or the phospholipid components of 22A-sHDL to FC levels as the PD endpoint using indirect pharmacodynamic response models (19). Figure 1 shows the indirect response model described by Jusko et al. for PK-PD modeling in this study (22). The pharmacologic response (free-cholesterol mobilization) is controlled by a zero-order rate constant for generation of response (kin) and a first-order rate constant for loss of response (kout). The response compartment can be modulated by stimulating kin using a sigmoidal infinite model. Steepness of the sigmoidal curve (γ) and the serum concentration needed to achieve a 50% maximum stimulation of response of a dosed agent (EC50) were calculated. After the PK parameters were obtained by fitting to compartmental models, they were regarded as fixed values and then used to estimate PD parameters (i.e., kin, kout, EC50, and γ). The final models were chosen on the basis of best fit in terms of sum-of-squared residuals, diagnosis plots, and log-likelihood value.
Fig. 1.
Scheme of the pharmacokinetic-pharmacodynamic model based on a one-compartment PK model. k01, the first-order absorption rate constant for IP groups only; k10: the first-order elimination rate constant; kin, the zero-order constant for production of response; kout: the first-order constant for loss of the response; EC50, the serum concentration needed to achieve a 50% of maximum stimulation achieved at the effect site of a dosed agent; γ, steepness of the sigmoidal curve.
Distribution of mobilized cholesterol in lipoproteins
The rat sera samples were analyzed to assess cholesterol distribution between VLDL, LDL, and HDL lipoprotein fractions. Separation of lipoproteins was performed on a Waters HPLC system equipped with Superose 6, 10/300 GL column (GE Healthcare, Piscataway, NJ) and on-line detection of cholesterol by postcolumn enzymatic reactions (23). Rat sera prior to dosing, and 0.25, 2, and 24 h postinjection were analyzed. Sera aliquots (50 μl) were injected and eluted with a 154 mM sodium chloride per 0.02% sodium azide solution at 0.8 ml/min. The postcolumn reaction was done using a 5 ml reaction coil at 37°C and detected by UV at 490 nm. The cholesterol detection enzymatic reagents were delivered at a 0.2 ml/min flow rate and mix-in with separated lipoprotein postcolumn. The enzymatic reagent solution comprised 100 mM phosphate buffer (pH 7.0), 4 M sodium chloride, 0.2% triton X-100, 10 mM sodium cholate, 2.5 mM 4-aminoantipyrine, 7.54 mM 2-hydroxy-3,5 dichlorobenzene, 0.0625 U/ml cholesterol oxidase, 1.25 U/ml peroxidase, 1.25 U/ml lipase, and 0.1 U/ml cholesterol ester hydrolase. All enzymatic reagents were purchased from Sigma.
Remodeling of endogenous plasma lipoprotein by lipid-free peptide and HDL particles
Remodeling of endogenous lipoproteins in serum was assessed after incubation of 22A peptide or 22A-sHDL with human sera. Solutions of 22A peptide or 22A-sHDL at 0.1, 0.5, 1.5, and 3 mg/ml peptide concentrations in sera were incubated at 37°C for 1 h with shaking at 300 rpm. The various subclasses of HDL were separated by size and charge by one-dimensional native polyacrylamide gel electrophoresis (PAGE) and visualized by Western blot. Samples were subjected to electrophoresis using 10-well Tris-Borate-EDTA gradient (3-25%) acrylamide native gels (Jule, Inc., Milford, CT) (24). For each well, 5 µl of human sera after incubation with PBS, 22A peptide or 22A-sHDL was mixed with 5 µl of 2× Tris-borate-EDTA sample buffer and 6 µl of the resulting mixtures were loaded per well. Gels were run at 200 V until the sample dye was 2.5 cm away from the bottom of the gel. Proteins were visualized by Western blot by transfer onto polyvinylidene difluoride membrane and incubation overnight with anti-human apoA-I-HRP conjugated antibody (Meridian Life Science, Memphis, TN). Bands were visualized using SuperSignalTM West Pico Chemiluminescence Substrate (Thermo Fisher), images were acquired on a FluorChem M Imager (Protein Simple, San Jose, CA), and Image J was used for spot densitometry.
Statistical analyses
Statistical analyses of the data were performed by Student’s t test for comparing two treatment groups or by one-way ANOVA/Dunnett’s test for comparing multiple treatment groups, with 22A-sHDL/IV serving as the control. Data are expressed as mean ± standard deviation of at least three independent experiments. P < 0.05 was considered to be statistically significant.
RESULTS
Composition optimization, assembly, and characterization of 22A-sHDL particles
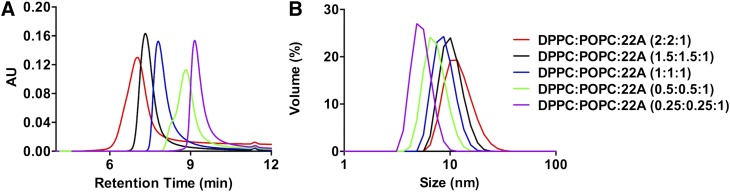
HDL composition was optimized using an apoA-I mimetic peptide, 22A, and phospholipids to match the size of endogenous preβ HDL particles. The 22A peptide was previously clinically tested in dyslipidemia patients as ETC-642 (25). The composition of ETC-642 is approximately 1:1:1 weight ratios of 22A peptide, DPPC, and sphingomyelin, combined to form homogeneous preβ HDL-like discs (25). In this study we replaced sphingomyelin with POPC in order to increase sHDL interaction with lecithin-cholesteryl acyltransferase (LCAT). Unsaturated phospholipids such as POPC are preferred substrates for LCAT, while sphingomyelin is not a substrate of the enzyme (26). In order to optimize 22A-sHDL particle size and purity, we varied the weight ratio of 22A to total phospholipids varied between 1:0.5 to 1:4 (Table 1). Gel permeation chromatography was used to examine the purity and the size distributions of newly generated 22A-sHDL particles. As is shown in Fig. 2A, the retention times of different 22A-sHDL particles were between 7 and 10 min, with the peak of unbound or lipid-free peptide appearing at around 11.5 min. The amount of lipid-free peptide was less than 0.48% for all formulations. The retention time of sHDL decreased with the increase of lipid-to-peptide ratio, indicating formation of larger sHDL particles.
TABLE 1.
The characterization summary of different 22A-sHDL particles
| sHDL Formulations (wt:wt:wt ratio) | Retention Time (min) | Particle Size (nm) | Polydispersity Index |
| DPPC:POPC:22A (2:2:1) | 7.02 | 12.5 ± 0.1 | 0.29 ± 0.07 |
| DPPC:POPC:22A (1.5:1.5:1) | 7.32 | 10.5 ± 0.1 | 0.17 ± 0.06 |
| DPPC:POPC:22A (1:1:1) | 7.80 | 9.0 ± 0.1 | 0.16 ± 0.03 |
| DPPC:POPC:22A (0.5: 0.5:1) | 8.83 | 7.4 ± 0.1 | 0.23 ± 0.04 |
| DPPC:POPC:22A (0.25:0.25:1) | 9.16 | 5.5 ± 0.1 | 0.56 ± 0.09 |
Fig. 2.
Characterization of 22A reconstituted sHDL particles. Gel permeation chromatography (A), and dynamic light scattering (B).
Dynamic light scattering analysis confirmed the increase of particle size from 5.5 nm to 12.5 nm with the increase of lipid:peptide ratio (Fig. 2B), which was consistent with the GPC results. The particle polydispersity index (PDI) for 0.5:1, 1:1, and 4:1 formulations were high, indicating heterogeneity of particle size (Table 1). Large PDI and small size for 0.5:1 and 1:1 formulations indicate insufficient amount of lipids for complete peptide binding and perhaps presence of peptide aggregates in the solution (27). Increasing the ratio to 4:1 resulted in larger PDI as well, indicating the presence of large lipid vehicles due to phospholipid excess. The optimal lipid-to-peptide weight ratio for 22A peptide appears to be between 2:1 and 3:1. The 1:1:1 weight ratio of 22A:DPPC:POPC was selected for future examination. These 22A-sHDL particles had almost no impurities and a homogeneous size of 9.0 ± 0.1 nm. Many other studies verified that the size of natural human HDL ranges from 8.5 to 12.0 nm (28). To further confirm similarity of size for selected 22A-sHDL with endogenous HDL, we analyzed purified human HDL by GPC (supplemental Fig. S1). The retention time for endogenous human HDL was 7.35 min, demonstrating a size similarity to that of the selected 22A-sHDL.
Validation of LC/MS method for peptide quantification in serum
A new LC/MS method capable of accurate and sensitive detection of 22A and its main metabolite was developed. For this, we used a different apoA-I-mimetic peptide, 5A, as an IS. We have compared solid-state extraction of peptide from serum using Oasis® HLB extraction cartridges (Waters, Milford, MA) and organic solvent precipitation methods for sample preparation prior to LC/MS analysis. Product recovery using the solid-state extraction method was less than 30% (data were not shown). The peptide recovery using methanol to precipitate proteins was greater than 90% (29). The LC/MS analysis indicated a rapid decrease in 22A peak area in serum and the appearance of a terminal lysine-truncated metabolite (22A(-)Lys). The 22A(-)Lys metabolite was stable in plasma for up to 48 h. For the pharmacokinetic evaluation, a sum of serum concentrations of 22A and 22A (-)Lys was plotted as a function of time.
A limited validation was performed for serum extraction of peptide and LC/MS analysis. Linearity of the LC/MS analysis was observed for the peptide concentration range of 5 to 200 μg/ml, with r2 = 0.995 (supplemental Fig. S2). The limit of quantification was determined to be 5 μg/ml. The extraction recovery of 22A ranged between 92% and 112%. The accuracy of concentration determination for serum samples spiked with 22A at 6, 50, and 160 μg/ml ranged from 7.8% to 12.0% of the target concentrations, with precision (n = 6) ranging between 2.3% and 1.5%. The interassay precision (n = 3) was between 3.8% and 4.3% (shown in supplemental Table S1). On the basis of the analysis of percentage of 22A Lys (-) peptide in serum for the first four time points, metabolism appears to occur in a similar rapid manner for all four groups (as is shown in supplemental Fig. S3).
Pharmacokinetic evaluation of apoA-I peptide
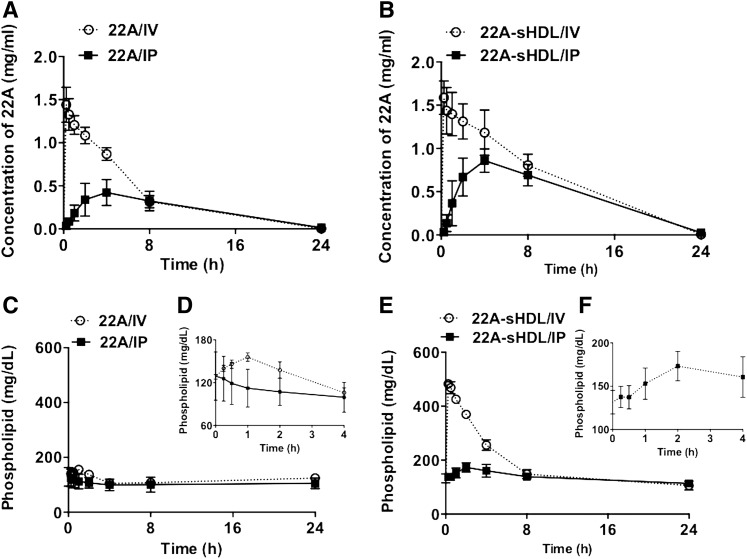
This experiment evaluated the dependence of apoA-I peptide’s pharmacokinetics on its formulation and administration route. We found that following IV administration of either 22A solution or 22A-sHDL, peptide elimination followed first-order kinetics (Fig. 3A, B). As is shown in Table 2, after IV dosing the clearance (CL) of 22A was more rapid when administered as free peptide than as 22A-sHDL particles (CL = 0.025 vs. 0.014 dl/h). Given the same volume of distribution between IV formulations (Vd = 0.13–14 dl), a similar increase was observed for the 22A elimination rate constant (K10 = 0.18 vs. 0.112 h−1), with a concomitant decrease in the elimination half-life of 22A (T1/2 = 3.81 vs. 6.27 h). As a result of the slower clearance, AUC was 1.79-fold higher for 22A-sHDL than for the 22A peptide. This PK difference is significant, and it is possibly due to peptide proteolysis in vivo or potentially different organs responsible for elimination of lipid-free peptide (kidney and sHDL particles) liver (30, 31). Following IP administration of 22A and 22A-sHDL, a first-order absorption was observed from the injection site into the systemic circulation. The plasma peptide levels following IP administration were lower than those following IV administration and peaked at 4 h. The AUCs of 22A, after IP dosing of 22A and 22A-sHDL, were only 52.0% and 54.1% of the IV administration, respectively. Because the vascular compartment is a target organ for most HDL therapeutics, dose adjustment appears to be critical because only about half of the dose reaches the systemic circulation following IP administration.
Fig. 3.
Pharmacokinetics of 22A peptide after administration of lipid-free 22A peptide (A) or 22A-sHDL (B). The kinetics of phospholipid mobilization and elimination following administration of lipid-free 22A peptide (C, insert D) or 22A-sHDL (E, insert F). Sprague-Dawley rats received 75 mg/kg of 22A or 22A-sHDL by either IV or IP injection. For 22A-sHDL a dose of 75 mg/kg of peptide corresponded to a 150 mg/kg dose of phospholipids. Serum peptide concentrations were determined by LC/MS, and total choline- containing phospholipids was measured by a commercial choline oxidase assay.
TABLE 2.
Pharmacokinetic parameters (% CV) of 22A peptide after 75 mg/kg doses of 22A by four different treatments
| Group | ||||
| Parameter | 22A/IV | 22A/IP | 22A-sHDL/IV | 22A-sHDL/IP |
| Tmax (h) | — | 4.27 (15.3) | — | 4.04 (21.5) |
| Cmax (mg/dl) | 152.16 (5.0), ns | 34.83 (14.0)a | 165.23 (7.6) | 68.87 (19.9)a |
| AUC (mg·h/dl) | 836.3 (7.8)b | 434.5 (17.3)a | 1495.5(13.9) | 809.4 (24.6)b |
| k01 (h−1) | — | 0.33 (34.3) | — | 0.35 (47.7) |
| k10 (h−1) | 0.18 (9.6)c | 0.16 (10.9)d | 0.11 (16.6) | 0.17 (14.4)d |
| T1/2 (h) | 3.81 (9.6)b | 4.43 (10.9)c | 6.27 (16.6) | 4.14 (14.4)c |
| CL (dl/h) | 0.025 (7.8), ns | 0.048 (17.4)a | 0.014 (13.9) | 0.026 (24.6)d |
| Vd (dl) | 0.14 (5.0), ns | 0.30 (26.1)c | 0.13 (7.6) | 0.15 (36.16), ns |
| AIC | 15.48 | 12.35 | 23.36 | 7.00 |
Mean ± SD (n = 3). % CV, coefficient of variation; ns, no significant difference in comparison with 22A-sHDL/IV treatment.
P< 0.0001.
P< 0.001.
P< 0.01.
P< 0.05.
No difference was observed when comparing the volume of distribution (Vd) of 22A after IV and IP administrations of 22A (i.e., 0.14 for IV and 0.16 dl [Vd/F × F or 0.30 × 0.52] for IP). Likewise, the clearance (CL) of 22A did not change between these two dosing routes (i.e., 0.025 for IV and 0.025 dl [CL/F × F or 0.048 × 0.52] for IP). As a result, the elimination rate constants (K) and half-lives (T1/2) of 22A were very similar following IV and IP administrations. In contrast, the Vd of 22A was about 40% lower when administered as 22A-sHDL/IP (0.081 dl [Vd/F × F or 0.15 × 0.54]) in comparison with 22A-sHDL/IV (0.13 dl). The reason for this difference is unclear but may be related to partial dissociation of 22A and phospholipid during absorption into systemic circulation following IP administration and peptide degradation/tissue binding during absorption. Because there was no difference in the CL of 22A after IV and IP treatments of 22A-sHDL (i.e., 0.014 for IV and 0.014 dl/h [CL/F × F or 0.026 × 0.54] for IP), the 22A elimination rate constant (K) was higher (0.17 vs. 0.11 h−1) and the 22A half-life (T1/2) lower (4.14 vs. 6.27 h) following IP administration in comparison with IV dosing.
Phospholipid kinetics
Monitoring lipid plasma kinetics provides indirect information not only about the formation of HDL following administration of naked apoA-I peptide but also about the in vivo stability of administered sHDL and elimination of its lipid component. Administration of apoA-I peptide in sHDL formulation at a dose of 75 mg/kg peptide dose corresponds to administration of 150 mg/kg of phospholipids (PL). The plasma levels of both endogenous and 22A-sHDL administered lipids were measured by choline oxidase assay. The elimination kinetics of total PL following 22A-sHDL injection are shown in Fig. 3C–F. After subtracting the predose plasma PL levels, the pharmacokinetic parameters were determined, and these are summarized in Table 3. The maximum PL level after IV injection of sHDL reached 483.0 mg/dl and constituted a 2.7-fold increase over the baseline PL level of 132.2 mg/dl (Fig. 3E). The AUC of PL after IV dosing of 22A-sHDL was 1559.6 mg · hr/dl. The AUC after IP administration of 22A-sHDL was 416.2 mg · hr/dl, indicating that the bioavailability of lipids into the systemic circulation for IP injection was only 26.7%. Following IP administration of sHDL, the bioavailability of lipids is lower than that of 22A peptide (26.7% vs. 54.1%), indicating some degree of dissociation of peptide from sHDL lipids during absorption. Although no exogenous PL was given in the case of peptide injection, it is believed that apoA-I mimetic peptides administered in vivo are capable of forming new HDL particles by lipid and cholesterol efflux via ATP-binding cassette transporter ABCA1 or by mobilizing phospholipid directly from cellular membranes (8, 32). Hence, the slight increase in plasma lipid levels is suggestive of de novo HDL formation. As is shown in Fig. 3C, D, a small increase in circulating lipids was observed for IV administration of 22A. In contrast, for IP administration of 22A, there was no obvious increase in plasma PL, likely because of tissue binding of peptide and decreased bioavailability to systemic circulation in comparison with IV dosing of peptide.
TABLE 3.
Pharmacokinetic parameters (% CV) of phospholipids after 150 mg/kg doses of phospholipids by two different treatments
| Group | ||
| Parameter | 22A-sHDL/IV | 22A-sHDL/IP |
| Tmax (h) | — | 2.33 (14.6) |
| Cmax (mg/dl) | 420.9 (2.9) | 58.3 (10.1) |
| AUC (mg·h/dl) | 1,559.6 (3.9) | 416.2 (14.2)a |
| k01 (h−1) | — | 0.67 (46.9) |
| k10 (h−1) | 0.27 (4.9) | 0.25 (40.2) |
| T1/2 (h) | 2.57 (4.9) | 2.74 (40.2), ns |
| CL (dl/h) | 0.027 (3.9) | 0.10 (14.2)b |
| Vd (dl) | 0.10 (2.9) | 0.40 (35.1)c |
| AIC | 13.51 | 19.34 |
Mean ± SD (n = 3). % CV, coefficient of variation; ns, no significant difference compared with 22A-sHDL/IV treatment.
P< 0.0001.
P< 0.01.
P< 0.05.
Cholesterol mobilization and esterification
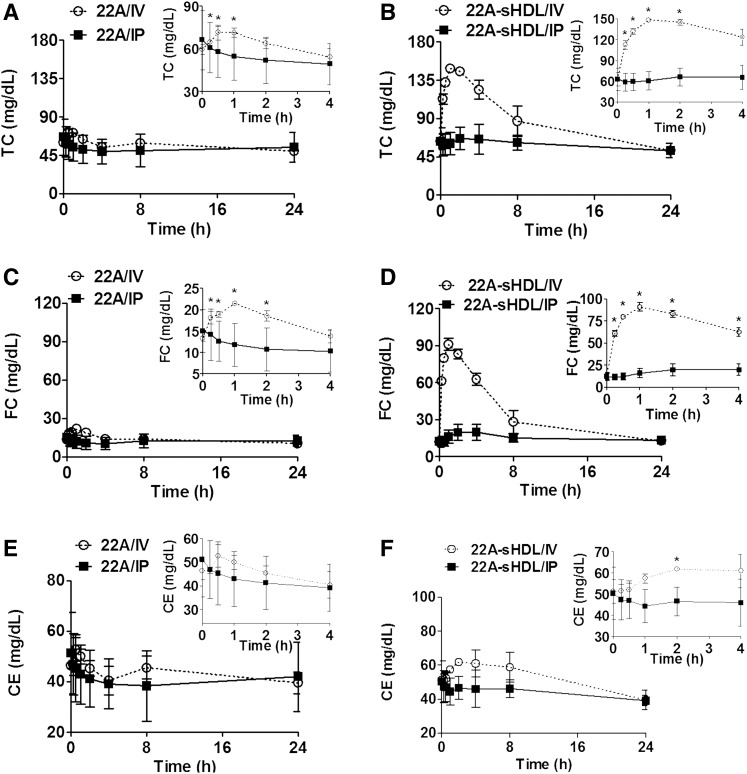
To investigate the impact of lipidation and route of administration on apoA-I peptide ability to elicit pharmacological response, we examined the kinetics of plasma cholesterol biomarkers. Both free apoA-I peptide and sHDL infusions are capable of facilitating reverse cholesterol transport (RCT) by enhancing cholesterol efflux. Therefore, transient increases in plasma levels of cholesterol following treatment are expected. The kinetic changes in plasma total and unesterified cholesterol levels were measured directly by plate assays, and the cholesterol ester levels were calculated (Fig. 4). Administration of 22A-sHDL by IV resulted in a rapid two-fold increase in total cholesterol (TC) within 0.5–1 h postdose (Fig. 4A, B). The TC levels also increased slightly following IV administration of lipid-free 22A peptide (P < 0.05, 0.25–2 h postdose). In contrast, no statistically significant increase in TC was observed for administration of both lipid-free 22A and 22A-sHDL by IP route (Fig. 4A, B).
Fig. 4.
Pharmacodynamic assessment of sHDL therapeutics after IV or IP administration of lipid-free 22A peptide or 22A-sHDL. Mobilization of total TC (A, B), FC (C, D), and CE (E, F) after injection of 75 mg/kg of 22A peptide solution (A, C, E) or 22A-sHDL (B, D, F). *Statistically significant differences of TC, FC, or EC changes in comparison with their predose levels with p values of at least <0.05.
The mobilized cholesterol for 22A-sHDL infusions was predominantly unesterified or free cholesterol (Fig. 4C, D). Normal predose levels of rat plasma free cholesterol (FC) were approximately 11.7 mg/dl, and these increased to 91.0 mg/dl within 1 h for IV dosing. The IP dosing of 22A-sHDL or IV dosing of lipid-free 22A also generated an increase in FC, but the effect was much smaller than that caused by IV injection of 22A-sHDL. There was no FC increase detected after IP peptide solution administration (Fig. 4C, D). For 22A-sHDL IV administration, limited conversion of mobilized free cholesterol into cholesterol ester (CE) was observed (Fig. 4E). It is possible that mobilized free cholesterol overwhelmed the esterification capacity of circulating LCAT or that 22A-sHDL was not a good activator of rat lecithin cholesterol acyltransferase. Cholesterol seemed to be predominantly eliminated from plasma in its unesterified form following mobilization and returned to the baseline levels 24 h postdose. Hence, the pharmacological effect of apoA-I peptide was remarkably affected by the formulation and administration route, in which the IV dose of 22A-sHDL generated the strongest cholesterol transfer and mobilization efficacy.
Lipoprotein distribution of mobilized cholesterol
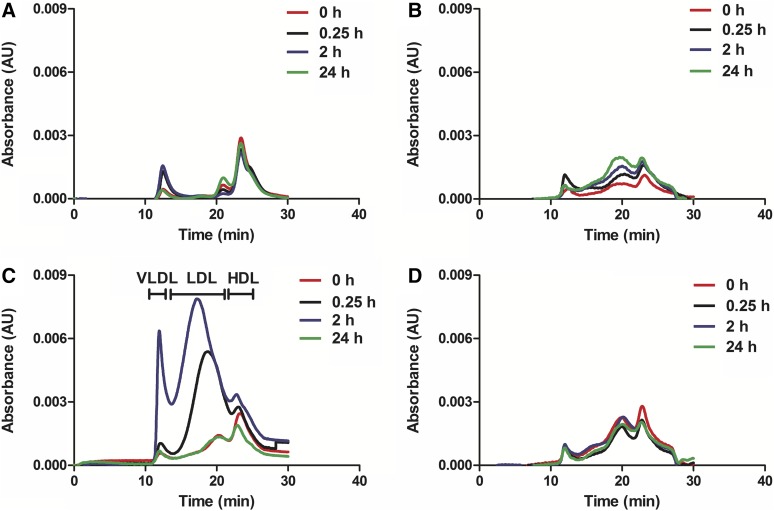
To investigate in greater detail the mechanism of cholesterol mobilization and elimination following apoA-I peptide or sHDL administrations, we determined the relative distribution of mobilized cholesterol in the HLD, LDL, and VLDL fractions. Serum lipoproteins were separated by gel permeation chromatography, and total cholesterol was detected after postcolumn enzymatic reaction (Fig. 5). Again, for the 22A-sHDL IV group, drastic but transient changes in lipoprotein profiles were observed over 24 h. Cholesterol was mobilized by injected HDL-sized particles, causing a rapid increase in particle size upon free-cholesterol uptake. Because sHDL are prepared with a short, single-helical peptide, the size of the nanoparticle is not constrained by the length and structure of lipid-bound full-length apoA-I, a major protein component of endogenous HDL. Therefore, we saw a rapid transition of cholesterol-carrying particles from HDL to LDL size (15 min postdose, with the maximum increase in cholesterol levels associated with LDL-sized particles detected by 2 h postdose). Although mobilization of FC was significant, the increase in levels of CE was disproportionally lower, indicating that cholesterol is likely to be taken up by the liver in its unesterified form (Fig. 4D, F). At 2 h postdose we also observed a significant increase in cholesterol levels in VLDL-sized lipoproteins. The VLDL-C increase could be due to saturation of liver receptors and enzymes that internalize, metabolize, and secrete large amounts of mobilized FC. However, the cholesterol changes were transient, and lipoprotein-cholesterol distribution returned to a predose profile 24 h postdose. In contrast to the 22A-sHDL IV group, only limited lipoprotein changes were observed for all other groups. A small transient increase in LDL-C level was observed for the 22A peptide group at 0.25 h and 2 h postdose, returning to baseline by 24 h postdose. Because of limited cholesterol mobilization for all groups except for 22A-sHDL, it was difficult to assess differences in in vivo mechanisms of cholesterol efflux, mobilization, lipoprotein transfer, and elimination for both lipid-fee peptide administrations and IP dosing of sHDL.
Fig. 5.
The cholesterol distribution among VLDL, LDL, and HDL lipoprotein fractions following IV (A) or IP (B) administration of 22A peptide solution or IV (C) or IP (D) administration of 22A-sHDL.
Plasma remodeling
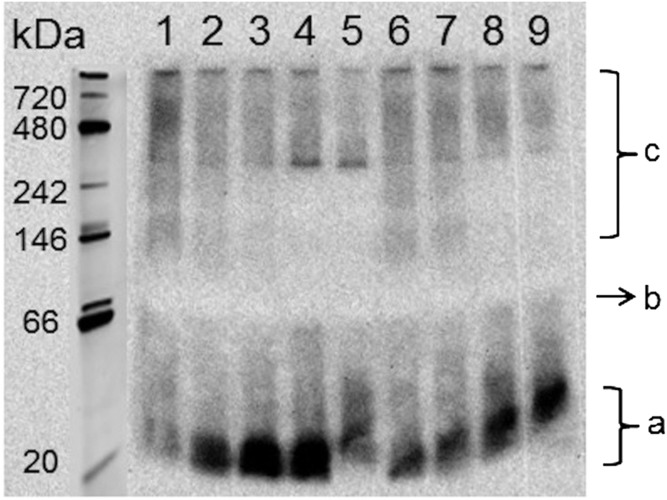
Potential differences in how apoA-I peptide or sHDL induce remodeling of endogenous lipoproteins were examined following in vitro incubation of both formulations with human serum. We added 22A and 22A-sHDL at 0, 0.15, 0.5, 1, and 3 mg/ml peptide concentrations, incubated them for 30 min, and separated them by 1D native PAGE electrophoresis (Fig. 6). The 1D gels were visualized by Western blot, staining for human apoA-I. The incubations were performed with a broad concentration range of 22A peptide, corresponding to in vivo concentration ranges of 0–1.5 mg/ml 22A, as measured by LC/MS. Compared with control serum (Fig. 6, lane 1), all incubations showed reduction in the apoA-I content of α-HDL, seen by decreased stain intensity between 720 and 200 kDa. Along with this decrease, a 22A-peptide concentration-dependent appearance of lipid-free or lipid-poor apoA-I protein was observed. The band of lipid-poor ApoA-I was ∼25–30 kDa, and it had slightly higher intensity for 22A-serum incubations but slightly larger size for 22A-sHDL-serum incubation. Hence, both naked and lipid-bound 22A were capable of associating with endogenous HDL and displacing endogenous apoA-I on HDL particles. Lipoprotein remodeling and release of lipid-free apoA-I could potentially be responsible for the therapeutic effect, independent of cholesterol mobilization and RCT.
Fig. 6.
Free apoA-I and various subclasses of HDL were separated by 1D native PAGE electrophoresis and visualized by Western blot using anti-apoA-I antibody. Lane 1 was the control serum; lanes 2, 3, 4, and 5 represented 0.15, 0.5, 1, and 3 mg/ml, respectively, of 22A, and lanes 6, 7, 8, and 9 represented 0.15, 0.5, 1, and 3 mg/ml, respectively, 22A-sHDL. Labels a, b, and c refer to the approximate positions of lipid-poor apoA-I, small preβ HDL particles, and large α-HDL particles.
Pharmacokinetics and pharmacodynamics correlation
Based on experimental pharmacokinetic (PK) and free-cholesterol mobilization pharmacodynamic (PD) data, we developed the indirect response PK-PD models for 22A and phospholipids (Fig. 1). The parameters obtained from PK-PD modeling allow for the prediction of timing and magnitude of FC increase, such that the dosing regimens can be further optimized. The best fit for the PK data was obtained using a one-compartment disposition model, with lowest AIC values for both IV and IP injections without lag time. PD parameters obtained from 22A-FC or phospholipids-FC PK-PD models are listed in Table 4. For IP injection of the 22A group, the model failed to fit the data because FC mobilization was limited for this group.
TABLE 4.
Estimated pharmacodynamic parameters (with % CV of the estimate) for 22A peptide
| Group | ||||
| Parameter | 22A/IV | 22A/IP | 22A-sHDL/IV | 22A-sHDL/IP |
| kin [mg/(dl·h)] | 30.38 (53.7) | — | 30.48 (18.3) | 30.60 (70.4) |
| kout (h−1) | 2.65 (61.6) | — | 2.61 (10.5) | 3.46 (60.1) |
| EC50 (mg/dl) | 142.81 (8.6) a | — | 53.77 (21.2) | 47.63 (247.8), ns |
| γ | 2.08 (71.9), ns | — | 1.92 (10.6) | 0.36 (90.6)a |
| LogLik | −9.96 | — | −15.97 | −11.94 |
Mean ± SD (n = 3). % CV, coefficient of variation; LogLik, log-likelihood of best-fitted model; ns, no significant difference versus 22A-sHDL/IV group.
P< 0.001.
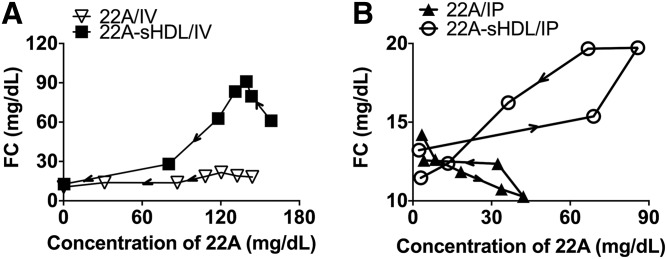
In all four groups, a plot of the relationship between 22A serum concentration and FC levels showed an anticlockwise hysteresis, indicating a significant delay in peak levels of free cholesterol in relation to the Cmax of 22A or phospholipids (Fig. 7). This relationship was described by an indirect response model in which serum concentrations of 22A or PL had a stimulatory effect on the production of FC. Under normal physiological conditions without HDL injection, the endogenous free-cholesterol level change can be described by a basic turnover model, which includes a zero-order turnover or synthesis rate constant (kin) and a first-order rate constant for cholesterol elimination (kout). The 22A or 22A-sHDL worked as a stimulatory factor, having an effect on production of the response (Fig. 1). Among all stimulation functions, the infinite stimulation model fits the data best, giving the lowest sum-of-squared residual value. The parameters kin and kout are independent of drug concentration; thus estimated values for all three groups were similar. The free-cholesterol baseline level R0, which is assumed to be constant, can be calculated by R0 = kin/kout for the three modeled groups. Similar baseline values were observed to be approximately 12 mg/dl, meeting the values detected for predose.
Fig. 7.
Plot of the relationship between 22A serum concentration and FC increase at individual time points following IV (A) or IP (B) administration of 22A peptide solution or 22A-sHDL at a dose of 75 mg/kg.
There was no significant difference between the sigmoidicity factor γ (γ), except in the case of the 22A-PD model for the 22A-sHDL IP group, whose value was much lower than for the IV group. The EC50 represents the plasma concentration needed to achieve a 50% of maximum stimulation achieved at the effect site of a dosed agent. From Table 4, 22A peptide had a significantly lower EC50 value after IV dosing of 22A-sHDL than after IV dosing of free peptide (53.8 mg/dl vs. 142.8 mg/dl), indicating that the sHDL formulation had a more potent effect on cholesterol efflux than did the free peptide. There was no significant difference between the 22A-sHDL IV and IP groups. By combining EC50 and γ values, lipidation of 22A increases the potency of peptide, whereas altering the administration route can increase the sensitivity of cholesterol efflux toward any 22A concentration change at the effect site. However, the smaller value of phospholipids EC50 value for phospholipids in the 22A-sHDL IV group compared with IP groups (27.1 mg/dl vs. 74.0 mg/dl) showed that the phospholipids in sHDL triggered higher cholesterol efflux after IV injection than it did with IP injection at the same dose, which may result from sHDL particle disassembly during the absorption process (Table 5).
TABLE 5.
Estimated pharmacodynamic parameters (with % CV of the estimate) for phospholipids
| Group | ||
| Parameter | 22A-sHDL/IV | 22A-sHDL/IP |
| kin [mg/(dl·hr)] | 30.48 (18.2) | 22.26 (27.2) |
| kout (hr−1) | 2.61 (10.5) | 1.85 (27.3) |
| EC50 (mg/dl) | 27.11 (51.6) | 74.02 (9.93)a |
| γ | 0.78 (10.6) | 1.25 (25.2), ns |
| LogLik | −15.97 | −7.34 |
Mean ± SD (n = 3). % CV, coefficient of variation; LogLik, log-likelihood of best-fitted model; ns, no significant difference versus 22A-sHDL/IV group.
P < 0.05.
The log-likelihood value reflects the quality of the fitted model. In the 22A-sHDL IP group, the phospholipid-FC PK/PD model appears to provide a better fit for the data than do the 22A-FC PK/PD models, as is highlighted by the larger log-likelihood of best-fitted model values (Tables 4 and 5). This better fit underscores the notion that FC mobilization is likely elicited by the presence of cholesterol-free lipid bilayers of sHDL in the plasma and to a lesser degree by peptide-mediated cholesterol efflux.
DISCUSSION
We found that both the physical state of the apoA-I peptide (i.e., naked or lipid-bound sHDL) and the route of administration (IV vs. IP) profoundly affected its pharmacokinetics and the mechanism of eliciting pharmacological response. Only lipidated 22A-sHDL administered by IV resulted in rapid and massive mobilization of free cholesterol. Additionally, only a partial conversion of FC to CE was observed, and all mobilized cholesterol was subsequently eliminated within 24 h. The difference in plasma-mobilized cholesterol levels did not directly correlate with differences in the pharmacokinetics of apoA-I peptide administered in a lipid-free form or in the bioavailability of apoA-I peptide to systemic circulation following IP dosing. The half-life of peptide administered in lipid-free form was only 0.61-fold shorter in relation to 22A-sHDL. However, 1- to 2-h postdose, the maximum levels of mobilized FC reached 22 mg/dl for 22A and 91 mg/dl for 22A-sHDL (Fig. 4 C, D, Table 2), constituting a 1.3- and 5.3-fold increase from predose level, respectively. This significant difference in the magnitude of plasma cholesterol mobilization indicated a potential mechanistic difference in how cholesterol efflux is elicited for lipid-free peptide and sHDL in vivo. The rapid cholesterol mobilization following IV administration of sHDL was likely caused in part by a physical partitioning of FC from cellular membranes to cholesterol-free lipid bilayers of sHDL. For administration of lipid-free 22A peptide, cholesterol mobilization possibly occurs in a receptor-mediated manner following interaction with peptide or de novo formed HDL following of plasma remodeling of lipoprotein by 22A peptide. Extensive mobilization of FC in serum led to rapid conversion of the injected sHDL particles to LDL-sized, cholesterol-loaded particles, causing a spike in LDL-C levels between 2 and 8 h postdose. These transient increases in LDL-C and triglyceride levels have been observed previously for both free ApoA-I and HDL infusions and has been attributed to saturating the liver’s capacity to excrete free cholesterol and inhibiting hepatic and lipoprotein lipases (33). Although the changes in lipoproteins appeared to be very dramatic following 22A-sHDL IV infusion, they were also transient, as all lipoprotein levels returned to baseline 24-h postdose. Similar transient changes in lipoproteins have been observed in clinical settings for infusions of other HDL products, such as CER-001 (34) and ETC-216 (35).
When either lipid-free or lipidated peptide was mixed with serum, each was capable of remodeling of endogenous lipoproteins, leading to the appearance of a lipid-poor apoA-I fraction. The appearance of lipid-poor apoA-I indicates that upon administration, 22A peptide binds to endogenous HDL particles and causes endogenous apoA-I displacement from HDL. It is unclear how this phenomenon impacts the antiatherosclerotic activity of apoA-I mimetic peptide as well as how the sequences of various peptides influence endogenous lipoprotein binding. The PK-PD modeling that correlated 22A with an increase in FC, a plasma biomarker of cholesterol efflux, revealed EC50 values to be much lower for 22A-sHDL-IV compared with lipid-free-22A-IV (Table 4).
The initial decision to use reconstituted HDL particles rather than lipid-free apoA-I in clinical development occurred in the 1990s following the first clinical evaluation by Miller et al (3, 15). In these studies, prolonged IV infusion and bolus administration of lipid-free apoA-I up to 50 mg/kg dose failed to increase plasma levels of HDL-C. Instead, transient increases in LDL particles were noted and attributed to inhibition of lipoprotein and hepatic lipases. In contrast, infusions of reconstituted HDL particles at 25 and 40 mg/kg of apoA-I resulted in transient increases in HDL unesterified cholesterol levels, followed by cholesterol esterification and elimination. Since these first clinical trials, the elevation of HDL-C levels has become a primary biomarker of sHDL’s pharmacological effect. The clinical dose selection and preclinical optimization for many follow-on HDL products (ETC-642, CER-001) were performed based on the HDL-C increase as a biomarker (2, 5). However, it is becoming clear that there are mechanistic differences in how sHDL and free apoA-I elicit cholesterol efflux. Lipid-free apoA-I interacts with ABCA-1 receptors to efflux, forms de novo HDL, and remodels existing lipoproteins. In contrast, the cholesterol-free lipid bilayers of sHDL particles are strong acceptors of cholesterol from ABCG1 and SR-BI and via passive efflux from cellular membranes. Indeed, recent studies have shown that SR-BI receptors are largely responsible for free-cholesterol elevation following rHDL infusion and that phospholipids, not ApoA-I, dictate FC efflux (36, 37). Because of these factors, CSL-112 pharmacological efficacy in early clinical trials was monitored by increases in ABCA1 cholesterol efflux capacity of patient plasma following drug administration (38).
Several studies have directly compared the anti-inflammatory effects of apoA-I and HDL administered by IV infusion in animal models of arthritis (11) and a carotid periarterial collar model (12). When lipid-free apoA-I and reconstituted HDL were administered at a low dose of 8 mg/kg, they exhibited similar measurable anti-inflammatory activity. This indicates that some of protective mechanisms of apoA-I and sHDL infusions could not be characterized simply by monitoring cholesterol mobilization and that additional biomarkers are needed to establish the PK-PD relationship.
Direct comparisons of the antiatherosclerotic potency of IP injections of 30 mg/kg of either ATI-5261 peptide or reconstituted HDL were performed in a high-fat-diet-fed ApoE−/− mouse model of atherosclerosis (39). Although no increase in plasma cholesterol levels was detected, sHDL injections showed slightly better atheroma reduction; however, the difference was not statistically significant. In our study, IP administrations resulted in only limited plasma cholesterol mobilization, with slightly higher mobilization for sHDL infusions than for those using lipid-free peptide. After IP dosing 52% of lipid-free apoA-I peptide, 54% of lipidated apoA-I peptide and 27% of sHDL phospholipids reached the systemic circulation. In addition, the values for absorption (K01) and elimination (K10) constants differed for PL and 22A following IP administration of 22A-sHDL (Tables 2 and 3), indicating that some of 22A-sHDL was dissociated prior to absorption. Peptide tissue-binding, proteolysis, and disassembly of 22A-sHDL particles are potential reasons for the reduced and different bioavailabilities. Peptide tissue-binding and proteolysis depend on the primary sequence of peptide and, thus, differ for various peptides and full-length apoA-I. Stability of sHDL in vivo and extent of endogenous HDL remodeling depend on the lipid-binding affinity of amphipathic helixes toward lipids used in sHDL formulation, endogenous lipoproteins, and plasma lipid membrane, as well as the peptide’s tendency to self-associate (4, 40). Furthermore, lipid formulation of sHDL (peptide-lipid ratio, lipid type, and sHDL particle size) affects particle stability, cholesterol affinity, and interaction with LCAT (41). Thus, in vivo behavior of apoA-I peptide and sHDL formulation could be distinctly different for various peptide sequences and lipid formulations.
The magnitude of cholesterol mobilization and pharmacokinetic parameters depend on the dose of administered apoA-I peptide. The dose used in this study, 75 mg/kg, was selected to assure the detection of peptide in plasma following IP administration. The selected dose is slightly higher than doses used in animal pharmacology studies for apoA-I and HDL, which range between 1 and 50 mg/kg (42, 43). However, in phase I clinical dosing the infusions were given up to 45 mg/kg for CER-001, 135 mg/kg for CSL-112, and 50 mg/kg for lipid-free apoA-I (2–4). Hence, 75 mg/kg was a reasonable dose for the current study. In addition, our study was conducted in healthy rats to allow for the multiple blood draws required to determine the PK and PD parameters. However, the cholesterol pool mobilized in healthy rats is likely to be different from that present in human atheromas, and thus it would be beneficial to evaluate how apoA-I lipidation affects cholesterol mobilization in hyperlipidemic disease models.
The results of this study emphasize the criticality of considerations for formulation, route of administration, and dose used in pharmacological studies of apoA-I peptide and sHDL particles. Historic drug development considerations for the selection of HDL dose and formulation were based on measurements of plasma cholesterol level increase, which were primarily observed upon IV administration of sHDL particles. Yet the increase in plasma cholesterol primarily depends on sHDL lipid composition and particle stability in vivo and is seen upon administration of relatively high doses of sHDL. Thus, endogenous lipoporotein remodeling, anti-inflammatory effects, and apoA-I protein-mediated ABCA-1 efflux that are exhibited at lower doses are often underemphasized. Consequently, a mechanistic understanding of which therapeutic effects of sHDL are mediated by lipoprotein particles and which are mediated by their apoA-I component, as well as correlating elicited pharmacologic effect in vivo with the actual systemic concentrations, AUC, and pharmacokinetic parameters for administered apoA-I peptide, will significantly improve clarity around the development of HDL mimetics. Identification of in vivo biomarkers indicative of HDL mimetic potency in addition to HDL-C plasma increase and rapid assessment of in vivo PK and biomarker response for novel apoA-I mimetic peptides could improve their development outcomes, because extensive in vitro optimization does not account for peptide proteolysis and excessive tissue binding in vivo.
Supplementary Material
Acknowledgments
The authors acknowledge Mass Spectrometry Core, Department of Chemistry, University of Michigan.
Footnotes
Abbreviations:
- CE
- cholesterol ester
- DPPC
- 1,2-dipalmitoyl-sn-glycero-3-phosphocholine
- FC
- unesterified or free cholesterol
- GPC
- gel permeation chromatography
- HDL-C
- HDL cholesterol
- IP
- intra-peritoneal
- IV
- intravenous
- PD
- pharmacodynamic
- PK
- Pharmacokinetic
- PL
- phospholipids
- POPC
- 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine
- TC
- total cholesterol
- 5A
- apoA-I mimetic peptide consisting of 37 amino acids: DWLKAFYDKVAEKLKEAFPDWAKAAYDKAAEKAKEAA
- 22A
- apoA-I mimetic peptide consisting of 22 amino acids: PVLDLFRELLNELLEALKQKLK
- 22A-sHDL
- phospholipid reconstituted HDL based on 22A
This research was funded in part by the American Heart Association (AHA 13SDG17230049 to A.S. and AHA 16POST27760002 to W.Y.), the Michigan College of Pharmacy Upjohn Award, and the National Institutes of Health R01 GM113832 and R21 NS091555. E.E.M. was partially supported by a fellowship from the Cellular Biotechnology Training Program (T32 GM008353) and Translational Cardiovascular Research and Entrepreneurship Training Grant (T32 HL125242). L.D. was partially supported by a fellowship from the Pharmacological Sciences Training Program (T32 GM07767). S.D. was supported by a fellowship from the Interdisciplinary Research Experiences for Undergraduates Program in the Structure and Function of Proteins (National Science Foundation Division of Biological Infrastructure #1263079). The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Smith J. D. 2010. Apolipoprotein AI and its mimetics for the treatment of atherosclerosis. Curr. Opin. Investig. Drugs. 11: 989–996. [PMC free article] [PubMed] [Google Scholar]
- 2.van Capelleveen J. C., Brewer H. B., Kastelein J. J., and Hovingh G. K.. 2014. Novel therapies focused on the high-density lipoprotein particle. Circ. Res. 114: 193–204. [DOI] [PubMed] [Google Scholar]
- 3.Nanjee M. N., Crouse J., King J., Hovorka R., Rees S., Carson E., Morgenthaler J-J., Lerch P., and Miller N.. 1996. Effects of intravenous infusion of lipid-free apo AI in humans. Arterioscler. Thromb. Vasc. Biol. 16: 1203–1214. [DOI] [PubMed] [Google Scholar]
- 4.Kuai R., Li D., Chen Y. E., Moon J. J., and Schwendeman A.. 2016. High-density lipoproteins: nature’s multifunctional nanoparticles. ACS Nano. 10: 3015–3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nissen S. E., Tsunoda T., Tuzcu E. M., Schoenhagen P., Cooper C. J., Yasin M., Eaton G. M., Lauer M. A., Sheldon W. S., and Grines C. L.. 2003. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. J. Am. Med. Assoc. 290: 2292–2300. [DOI] [PubMed] [Google Scholar]
- 6.Osei-Hwedieh D. O., Amar M., Sviridov D., and Remaley A. T.. 2011. Apolipoprotein mimetic peptides: mechanisms of action as anti-atherogenic agents. Pharmacol. Ther. 130: 83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Favari E., Calabresi L., Adorni M. P., Jessup W., Simonelli S., Franceschini G., and Bernini F.. 2009. Small discoidal pre-β1 HDL particles are efficient acceptors of cell cholesterol via ABCA1 and ABCG1. Biochemistry. 48: 11067–11074. [DOI] [PubMed] [Google Scholar]
- 8.Duong P. T., Weibel G. L., Lund-Katz S., Rothblat G. H., and Phillips M. C.. 2008. Characterization and properties of preβ-HDL particles formed by ABCA1-mediated cellular lipid efflux to apoA-I. J. Lipid Res. 49: 1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kee P., Rye K-A., Taylor J. L., Barrett P. H. R., and Barter P. J.. 2002. Metabolism of apoA-I as lipid-free protein or as component of discoidal and spherical reconstituted HDLs: studies in wild-type and hepatic lipase transgenic rabbits. Arterioscler. Thromb. Vasc. Biol. 22: 1912–1917. [DOI] [PubMed] [Google Scholar]
- 10.Patel S., Di Bartolo B. A., Nakhla S., Heather A. K., Mitchell T. W., Jessup W., Celermajer D. S., Barter P. J., and Rye K-A.. 2010. Anti-inflammatory effects of apolipoprotein AI in the rabbit. Atherosclerosis. 212: 392–397. [DOI] [PubMed] [Google Scholar]
- 11.Wu B. J., Ong K. L., Shrestha S., Chen K., Tabet F., Barter P. J., and Rye K-A.. 2014. Inhibition of arthritis in the Lewis rat by apolipoprotein AI and reconstituted high-density lipoproteins. Arterioscler. Thromb. Vasc. Biol. 34: 543–551. [DOI] [PubMed] [Google Scholar]
- 12.Nicholls S. J., Dusting G. J., Cutri B., Bao S., Drummond G. R., Rye K-A., and Barter P. J.. 2005. Reconstituted high-density lipoproteins inhibit the acute pro-oxidant and proinflammatory vascular changes induced by a periarterial collar in normocholesterolemic rabbits. Circulation. 111: 1543–1550. [DOI] [PubMed] [Google Scholar]
- 13.Peterson S. J., Husney D., Kruger A. L., Olszanecki R., Ricci F., Rodella L. F., Stacchiotti A., Rezzani R., McClung J. A., and Aronow W. S.. 2007. Long-term treatment with the apolipoprotein A1 mimetic peptide increases antioxidants and vascular repair in type I diabetic rats. J. Pharmacol. Exp. Ther. 322: 514–520. [DOI] [PubMed] [Google Scholar]
- 14.Shah P. K., Yano J., Reyes O., Chyu K-Y., Kaul S., Bisgaier C. L., Drake S., and Cercek B.. 2001. High-dose recombinant apolipoprotein a-imilano mobilizes tissue cholesterol and rapidly reduces plaque lipid and macrophage content in apolipoprotein e–deficient mice potential implications for acute plaque stabilization. Circulation. 103: 3047–3050. [DOI] [PubMed] [Google Scholar]
- 15.Nanjee M. N., Doran J., Lerch P., and Miller N.. 1999. Acute effects of intravenous infusion of ApoA1/phosphatidylcholine discs on plasma lipoproteins in humans. Arterioscler. Thromb. Vasc. Biol. 19: 979–989. [DOI] [PubMed] [Google Scholar]
- 16.Remaley A. T., Amar M., and Sviridov D.. 2008. HDL-replacement therapy: mechanism of action, types of agents and potential clinical indications. Expert Rev. Cardiovasc. Ther. 6: 1203–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharifov O. F., Xu X., Gaggar A., Grizzle W. E., Mishra V. K., Honavar J., Litovsky S. H., Palgunachari M. N., White C. R., and Anantharamaiah G.. 2013. Anti-inflammatory mechanisms of apolipoprotein AI mimetic peptide in acute respiratory distress syndrome secondary to sepsis. PLoS One. 8: e64486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meibohm B., and Derendorf H.. 2002. Pharmacokinetic/pharmacodynamic studies in drug product development. J. Pharm. Sci. 91: 18–31. [DOI] [PubMed] [Google Scholar]
- 19.Olsen C. K., Brennum L. T., and Kreilgaard M.. 2008. Using pharmacokinetic-pharmacodynamic modelling as a tool for prediction of therapeutic effective plasma levels of antipsychotics. Eur. J. Pharmacol. 584: 318–327. [DOI] [PubMed] [Google Scholar]
- 20.Li D., Gordon S., Schwendeman A., and Remaley A. T.. 2015. Apolipoprotein mimetic peptides for stimulating cholesterol efflux. In Apolipoprotein mimetics in the management of human disease. G. M. Anantharamaiah and D. Goldberg, editors. Springer, New York. 29–42. [Google Scholar]
- 21.Miles J., Khan M., Painchaud C., Lalwani N., Drake S., and Dasseux J. L.. 2004. Single-dose tolerability, pharmacokinetics, and cholesterol mobilization in HDL-C fraction following intravenous administration of ETC-642, a 22-mer ApoA-I analogue and phospholipids complex, in atherosclerosis patients. Proc. Arterioscler. Thromb. Vasc. Biol. 24: E19. [Google Scholar]
- 22.Dayneka N. L., Garg V., and Jusko W. J.. 1993. Comparison of four basic models of indirect pharmacodynamic responses. J. Pharmacokinet. Biopharm. 21: 457–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garber D. W., Kulkarni K. R., and Anantharamaiah G. M.. 2000. A sensitive and convenient method for lipoprotein profile analysis of individual mouse plasma samples. J. Lipid Res. 41: 1020–1026. [PubMed] [Google Scholar]
- 24.Freeman L. A. 2013. Native–native 2D gel electrophoresis for HDL subpopulation analysis. In Lipoproteins and Cardiovascular Disease: Methods and Protocols. Humana Press, New York. 353–367. [DOI] [PubMed] [Google Scholar]
- 25.Stoekenbroek R., Stroes E., and Hovingh G.. 2015. ApoA-I mimetics. In High Density Lipoproteins. E. J. Schaefer, editor. Springer, New York. 631–648. [DOI] [PubMed] [Google Scholar]
- 26.Subbaiah P. V., and Liu M.. 1993. Role of sphingomyelin in the regulation of cholesterol esterification in the plasma lipoproteins. Inhibition of lecithin-cholesterol acyltransferase reaction. J. Biol. Chem. 268: 20156–20163. [PubMed] [Google Scholar]
- 27.Dasseux J-L., Schwendeman A. S., and Zhu L.. 2010. Apolipoprotein ai mimics. Google Patents WO 2010093918 A1. Accessed at https://www.google.com/patents/WO2010093918A1?cl=en22 [Google Scholar]
- 28.Anderson D. W., Nichols A. V., Forte T. M., and Lindgren F. T.. 1977. Particle distribution of human serum high density lipoproteins. Biochim. Biophys. Acta. 493: 55–68. [DOI] [PubMed] [Google Scholar]
- 29.Tamvakopoulos C. 2007. Mass spectrometry for the quantification of bioactive peptides in biological fluids. Mass Spectrom. Rev. 26: 389–402. [DOI] [PubMed] [Google Scholar]
- 30.Spady D. K., Woollett L. A., Meidell R. S., and Hobbs H. H.. 1998. Kinetic characteristics and regulation of HDL cholesteryl ester and apolipoprotein transport in the apoA-I−/−mouse. J. Lipid Res. 39: 1483–1492. [PubMed] [Google Scholar]
- 31.Horowitz B. S., Goldberg I., Merab J., Vanni T., Ramakrishnan R., and Ginsberg H.. 1993. Increased plasma and renal clearance of an exchangeable pool of apolipoprotein AI in subjects with low levels of high density lipoprotein cholesterol. J. Clin. Invest. 91: 1743–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu S-C., Atangan L., Kim K. W., Chen M. M., Komorowski R., Chu C., Han J., Hu S., Gu W., and Véniant M.. 2012. An apoA-I mimetic peptibody generates HDL-like particles and increases alpha-1 HDL subfraction in mice. J. Lipid Res. 53: 643–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dasseux J-L., Oniciu D. C., and Ackermann R.. 2012. Lipoprotein complexes and manufacturing and uses thereof. Google Patents US 20120232005 A1. Publication no. US20120232005 A1. [Google Scholar]
- 34.Kootte R. S., Smits L. P., van der Valk F. M., Dasseux J-L., Keyserling C. H., Barbaras R., Paolini J. F., Santos R. D., van Dijk T. H., and Dallinga-van Thie G. M.. 2015. Effect of open-label infusion of an apoA-I-containing particle (CER-001) on RCT and artery wall thickness in patients with FHA. J. Lipid Res. 56: 703–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bisgaier C. L., Ackermann R., Rea T., Rodrigueza W. V., and Hartman D.. 2016. ApoA-IMilano phospholipid complex (ETC-216) infusion in human volunteers. Insights into the phenotypic characteristics of ApoA-IMilano carriers. Pharmacol. Res. 111: 86–99. [DOI] [PubMed] [Google Scholar]
- 36.Cuchel M., Lund-Katz S., de la Llera-Moya M., Millar J. S., Chang D., Fuki I., Rothblat G. H., Phillips M. C., and Rader D. J.. 2010. Pathways by which reconstituted high-density lipoprotein mobilizes free cholesterol from whole body and from macrophages. Arterioscler. Thromb. Vasc. Biol. 30: 526–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Herzog E., Pragst I., Waelchli M., Gille A., Schenk S., Mueller-Cohrs J., Diditchenko S., Zanoni P., Cuchel M., and Seubert A.. 2016. Reconstituted high-density lipoprotein can elevate plasma alanine aminotransferase by transient depletion of hepatic cholesterol: role of the phospholipid component. J. Appl. Toxicol. 36: 1038–1047. [DOI] [PubMed] [Google Scholar]
- 38.Gille A., Easton R., D’Andrea D., Wright S. D., and Shear C. L.. 2014. CSL112 enhances biomarkers of reverse cholesterol transport after single and multiple infusions in healthy subjects. Arterioscler. Thromb. Vasc. Biol. 34: 2106–2114. [DOI] [PubMed] [Google Scholar]
- 39.Bielicki J. K., Zhang H., Cortez Y., Zheng Y., Narayanaswami V., Patel A., Johansson J., and Azhar S.. 2010. A new HDL mimetic peptide that stimulates cellular cholesterol efflux with high efficiency greatly reduces atherosclerosis in mice. J. Lipid Res. 51: 1496–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yancey P. G., Bielicki J. K., Johnson W. J., Lund-Katz S., Palgunachari M. N., Anantharamaiah G., Segrest J. P., Phillips M. C., and Rothblat G. H.. 1995. Efflux of cellular cholesterol and phospholipid to lipid-free apolipoproteins and class A amphipathic peptides. Biochemistry. 34: 7955–7965. [DOI] [PubMed] [Google Scholar]
- 41.Schwendeman A., Sviridov D. O., Yuan W. M., Guo Y. H., Morin E. E., Yuan Y., Stonik J., Freeman L., Ossoli A., Thacker S., et al. 2015. The effect of phospholipid composition of reconstituted HDL on its cholesterol efflux and anti-inflammatory properties. J. Lipid Res. 56: 1727–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Di Bartolo B. A., Nicholls S. J., Bao S., Rye K-A., Heather A. K., Barter P. J., and Bursill C.. 2011. The apolipoprotein AI mimetic peptide ETC-642 exhibits anti-inflammatory properties that are comparable to high density lipoproteins. Atherosclerosis. 217: 395–400. [DOI] [PubMed] [Google Scholar]
- 43.Iwata A., Miura S-i., Zhang B., Imaizumi S., Uehara Y., Shiomi M., and Saku K.. 2011. Antiatherogenic effects of newly developed apolipoprotein AI mimetic peptide/phospholipid complexes against aortic plaque burden in Watanabe-heritable hyperlipidemic rabbits. Atherosclerosis. 218: 300–307. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.