Abstract
Oxidized HDL (ox-HDL), unlike native HDL that exerts antiatherogenic effects, plays a proatherogenic role. However, the underlying mechanisms are not completely understood. This study was designed to explore the inductive effect of ox-HDL on endoplasmic reticulum (ER) stress-CCAAT-enhancer-binding protein homologous protein (CHOP)-mediated macrophage apoptosis and its upstream mechanisms. Our results showed that ox-HDL could be ingested by macrophages, causing intracellular lipid accumulation. As with tunicamycin (an ER stress inducer), ox-HDL induced macrophage apoptosis with concomitant activation of ER stress pathway, including nuclear translocation of activating transcription factor 6, phosphorylation of protein kinase-like ER kinase and eukaryotic translation initiation factor 2α, and upregulation of glucose-regulated protein 78 and CHOP, which were inhibited by 4-phenylbutyric acid (PBA, an ER stress inhibitor) and CHOP gene silencing. Additionally, diphenyleneiodonium (DPI, an oxidative stress inhibitor), probucol (a reactive oxygen species scavenger), and toll-like receptor 4 (TLR4) silencing reduced ox-HDL-induced macrophage apoptosis, oxidative stress, and CHOP upregulation. Moreover, HDL isolated from patients with metabolic syndrome induced macrophage apoptosis, oxidative stress, and CHOP upregulation, which were blocked by PBA and DPI. These data indicate that ox-HDL may activate ER stress-CHOP-induced apoptotic pathway in macrophages via enhanced oxidative stress and that this pathway may be mediated by TLR4.
Keywords: endoplasmic reticulum stress, CCAAT-enhancer-binding protein homologous protein, atherosclerosis, oxidative stress
Atherosclerosis, one of the prime causes of heart attack and stroke, has been well known as a lipid-driven inflammatory disease characterized by macrophage accumulation in subendothelial space. Apoptosis of macrophage-derived foam cells, especially in advanced atherosclerotic lesions where phagocytic clearance of dead and dying cells is defective, results in acellular necrotic lesions that are prone to plaque rupture and thrombosis formation, leading to the majority of acute vascular events (1). Thus, deeper understanding of the mechanisms of macrophage apoptosis may pave the way for developing novel therapeutic strategies to effectively prevent atherosclerotic plaque rupture and subsequent clinical complications (2). Oxidized LDL (ox-LDL) has been recognized as a major inducer, promoting foam cell formation and apoptosis in macrophages (3). In contrast, HDL exerts antiatherogenic functions such as promoting reverse cholesterol transport, suppressing inflammation, inhibiting LDL oxidation, and increasing endothelial nitric oxide production (4). However, accumulating evidence suggests that HDL, like LDL, is also susceptible to oxidative modification. Oxidized HDL (ox-HDL) loses the antiatherogenic functions of native HDL (5–7) and exerts effects instead by inducing proliferation and migration of vascular smooth muscle cells (8), promoting platelet activation (9), and causing apoptosis in endothelial cells (10). Ox-HDL has been detected in atherosclerotic plaques (11). It has been reported that ox-HDL exerts a cytotoxic effect on macrophages and accelerates atherosclerosis progression (12, 13). However, the precise underlying mechanisms are poorly understood.
Prolonged or severe endoplasmic reticulum (ER) stress is one major cause of apoptosis, in which CCAAT-enhancer-binding protein homologous protein (CHOP) is a specific proapoptotic molecule (14). CHOP can mediate macrophage apoptosis and contribute to the instability of atherosclerotic plaques (15–17). We have previously shown that ox-LDL can induce macrophage apoptosis by upregulating CHOP expression (18, 19). Here, we investigated the potential role of the ER stress-CHOP pathway in ox-HDL-induced macrophage apoptosis. We found that ox-HDL prepared in vitro and HDL isolated from patients with metabolic syndrome (MS) activated the ER stress-CHOP-mediated apoptotic pathway in macrophages, which could be blocked by oxidative stress inhibitors, toll-like receptor 4 (TLR4)-specific small interfering RNA (siRNA), and TLR4 antibody.
MATERIALS AND METHODS
Reagents
Tunicamycin (TM), 4-phenylbutyric acid (PBA), diphenyleneiodonium (DPI), probucol, Oil Red O, antibody against β-actin, siRNA against TLR4, and CHOP were purchased from Sigma (St. Louis, MO). DMEM and FBS were obtained from Gibco (Rockville, MD). DiI-ox-HDL and DiI-ox-LDL were from Xiesheng Biotech (Beijing, China). RIPA lysis buffer and 3-(4,5-dimethylthiazol-2- y-l)-2,5-diphenyl-2H-tetrazolium bromide (MTT) were purchased from Solarbio (Beijing, China) and Genview (Houston, TX), respectively. Rabbit antibody against phospho-double-stranded RNA-activated protein kinase-like ER kinase (p-PERK) and rat anti-mouse TLR4 antibody were from Abcam (Cambridge, MA) and eBioscience (San Diego, CA), respectively. Rabbit polyclonal antibodies against activating transcription factor 6 (ATF6), phospho-eukaryotic translation initiation factor 2α (p-eIF2α) and glucose- regulated proteins 78 (GRP78) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse monoclonal CHOP antibody was from Cell Signaling Technology, Inc. (Danvers, MA). SABC-Cy3 immunohistochemistry kits and 2’,7’-dichlorofluorescin diacetate (DCHF-DA), Nile red, were from Boshide (Wuhan, China) and Molecular Probes (Eugene, OR), respectively. Annexin V-FITC apoptosis detection kits were obtained from KeyGEN Biotech (Nanjing, China). Polyvinylidene difluoride (PVDF) membranes and enhanced chemiluminescence kits were obtained from Millipore (Bedford, MA) and Thermo Scientific Pierce (Rockford, IL), respectively. Terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL) assay kit (In Situ Cell Death Detection kit, TMR red) and NADPH oxidase assay kits were from Roche (Mannheim, Germany) and Genmed Scientifics (Shanghai, China), respectively. Assay kits of lactate dehydrogenase (LDH), malondialdehyde (MDA), and superoxide dismutase (SOD) were obtained from Jiancheng Biotech (Nanjing, China).
Subjects
Twenty-six MS patients (male/female: 12/14; age: 49.5 ± 9.8 years), diagnosed according to the criteria for clinical diagnosis of MS (20), were enrolled from the Department of Endocrinology, Central Hospital of Taian, Taian, China. Subjects were excluded when they presented diabetes, unstable coronary artery disease, myocardial infarction or stroke within 6 months preceding the study, impaired hepatic or renal function, acute and chronic inflammatory diseases, autoimmune disorders, thyroid diseases, or other endocrine diseases; those who consumed more than 20 g alcohol/day were also excluded. In parallel, 34 healthy volunteers (male/female: 15/19; age: 43.8 ± 8.6 years) were recruited as controls. The study protocol was approved by the ethics committee of Taishan Medical University, and all the participants provided written informed consent before enrollment in the study.
Lipoprotein preparation and oxidation
The fresh fasting plasma of every 4–5 subjects from the MS group or the healthy control group were pooled, and then LDL (1.019–1.063 g/ml) and HDL (1.063–1.210 g/ml) were separated by density-gradient ultracentrifugation in a Beckman Optima XPN-100 ultracentrifuge with a fixed angle rotor, as has been previously described (18). The isolated LDL and HDL were dialyzed extensively against endotoxin-free PBS (pH = 7.4) containing 0.1 mmol/L EDTA at 4°C and sterilized with a 0.22 µm filter. Ox-LDL and ox-HDL were prepared by incubating native LDL (n-LDL; 1 mg/ml) and native HDL (n-HDL; 1 mg/ml) under sterile conditions with 10 µmol/L CuSO4 in EDTA-free PBS at 37°C for 24 h, as has been described previously (18, 21). Oxidative modification was prevented by addition of EDTA, and then the oxidized lipoproteins were stored at 4°C and used within 2 weeks. The oxidative extent of lipoproteins was determined by a thiobarbituric acid-reactive substances (TBARS) assay. The value for TBARS was <1.5 nmol MDA/mg protein in n-LDL and in n-HDL, whereas it was 30.9 ± 6.1 and 6.3 ± 1.2 nmol MDA/mg protein in ox-LDL and ox-HDL, respectively. Additionally, the oxidized phosphatidylcholines in ox-HDL and HDL from MS patients increased significantly in comparison with n-HDL (supplemental Fig. S1). The Limulus Amoebocyte Lysate kit (Bio Whittaker, Walkersville, MD) was used to tested for possible endotoxin contamination, and lipoproteins with lipopolysaccharide (LPS) content of >50 pg/mg protein were discarded (22).
Cell culture and SiRNA transfection
RAW264.7 macrophages were procured from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China) and cultured in DMEM supplemented with 10% FBS and 100 U/ml penicillin/streptomycin in a humidified incubator containing 5% CO2 at 37°C.
RAW264.7 cells were cultured in 6-well plates using DMEM without antibiotics and allowed to reach 30%∼50% confluence. A mixture of siRNA oligomers specific for TLR4, CHOP (200 pmol), or control siRNA oligomers (200 pmol) with 250 µl of Opti-MEM I reduced serum medium (Invitrogen, CA) was prepared and incubated with 5 µl of Lipofectamine 2000 (Invitrogen) prediluted in 250 µl of Opti-MEM at room temperature for 20 min. The transfection complexes were then added to cells in a final volume of 2.5 ml of medium and maintained for 6 h, after which the medium was replaced with DMEM with 10% FBS but without antibiotics. After transfection for 48 h, the cells were treated with ox-HDL (100 mg/L) for 24 h. The silencing of TLR4 and CHOP were validated by Western blot. The TLR4 siRNAs are 5′-GCCUAAACCCAGUCUGUUUdTdT-3′ (sense) and 5′-AAACAGACUGGGUUUAGG CdTdT-3′ (antisense), and the CHOP siRNAs are 5′-GUCACACGCACAUCCCAAAdTdT-3′ (sense) and 5′-UUUGGGAUGUGCGUGUGACdTdT-3′ (antisense).
Cell viability and LDH assay
The viability of the treated cells was measured with a MTT assay, as has been previously described (18), and expressed as the percentage of the optical density value of the treated cells in relation to that of the untreated control cells.
To further evaluate the extent of cell injury, we determined LDH activity in media by using an assay kit, according to the manufacturer’s instructions.
Cell apoptosis measurement
Cell apoptosis was measured by annexin V-FITC/propidium iodide (PI) double-staining assay and TUNEL assay, as has been reported in our previous work (19). In brief, the treated cells were harvested, washed with PBS, and then incubated in 500 μl binding buffer containing 5 µl annexin V-FITC and 5 µl PI for 15 min in the dark at room temperature. The apoptosis percentage was analyzed on a FACScan flow cytometer (Becton Dickinson, San Jose, CA). On the other hand, the treated cells on glass coverslips were washed with PBS, fixed with 4% paraformaldehyde, and then permeabilized with 0.1% Triton X-100. Thereafter, cells were incubated with TUNEL reaction mixture in the dark at 37°C for 1 h and with 4’, 6-diamidino-2- phenylindole (DAPI) for 5 min, respectively, and then observed using fluorescence microscopy (Olympus BX51, Tokyo, Japan). The cell apoptosis was expressed as the percentage of the number of TUNEL-positive cells to total cells.
Uptake of Dil-ox-HDL and Dil-ox-LDL
Cells were treated with 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (Dil)-ox-HDL (12.5–200 mg/L) or Dil-ox-LDL (100 mg/L) for 6 h. Thereafter, the cells were washed with PBS, fixed with 4% paraformaldehyde, and counterstained with DAPI, and then the mean fluorescence intensity per cell was calculated using Image-Pro Plus software (version 6.0, Media Cybernetics, MD).
Intracellular lipid measurement
The accumulation of intracellular lipid was determined by using oil red O and Nile red staining. For oil red O staining, the lipoprotein-treated cells grown on coverslips were washed with PBS, fixed with 4% paraformaldehyde, stained with oil red O (0.5%) in isopropanol for 30 min, and then counterstained with hematoxylin. Stained cells were examined under an Olympus BX51 microscope (Tokyo, Japan) and analyzed with Image-Pro Plus software. The lipid droplet content was expressed as the average value of integrated optical density, as has been described previously (18).
For Nile red staining, after being washed with PBS, the treated cells were stained with 1 μg/ml Nile red in PBS for 30 min at room temperature. Next, cells were washed and resuspended in PBS and then analyzed with a FACScan flow cytometer (Becton Dickinson, San Jose, CA) between 568 and 590 nm. Unstained cells were used as a negative control. At least 10,000 cells were analyzed for each sample, and the results were reported as mean fluorescence intensity.
Immunofluorescence assay for ATF6 nuclear relocation
ATF6 nuclear relocation was tested by fluorescent immunocytochemistry, as was described in our previous work (18). Briefly, the treated cells were washed with PBS, fixed with 4% paraformaldehyde, permeabilized with 0.1% Triton-X 100, and blocked with 3% BSA. Next, the cell coverslips were incubated with ATF6 antibody (1:200) overnight at 4°C, exposed to a secondary antibody, treated with SABC-Cy3 complex and DAPI, and then photographed under a fluorescence microscope (Olympus BX51, Tokyo, Japan).
Western blot analysis
Western blotting was carried out as previously described (18, 23). In brief, the treated cells were lysed using a RIPA buffer supplemented with 1% phenylmethylsulphonyl fluoride (PMSF). An equal amount (40 µg) of protein sample was subjected to sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis and then transferred to PVDF membranes. After blocking with 5% nonfat dry milk, the membranes were incubated with primary antibodies overnight at 4°C. After incubation with anti-immunoglobulin G antibody conjugated to horseradish peroxidase, the immunoproteins were visualized using an enhanced chemiluminescence substrate system, and the protein expression levels were calculated with Image-Pro Plus software and normalized to β-actin levels.
Quantitative real-time PCR
RNA was isolated from the treated cells using the Trizol reagent from Invitrogen (Carlsbad, CA), and reverse transcription was performed with the QuantScript 1st strand complementary DNA synthesis kit (Tiangen Biological Chemistry, Beijing, China). Quantitative real-time PCR was carried out with SYBR-green PCR master mix kits (Tiangen Biological Chemistry) in a Rotor-Gene Q real-time PCR cycler (Qiagen, Shanghai, China), analyzed using the Rotor-Gene Q software (version 1.7; Qiagen), and then mRNA levels were calculated on the basis of the relative expression method with the formula 2–△△Ct, as has been described previously (18). Primers used in this study were obtained from Sangon Biotech (Shanghai, China), and the sequences were as follows: 5′-CCACCACACCTGAAAGCAGAA-3′(forward primer) and 5′-GGTGCCCCCAAT TTCATCT-3′(reverse primer) for CHOP; 5′-ACATGGACCTGTTCCGCTCTA-3′ (forward primer) and 5′-TGGCTCCTTGCCATTGAAGA-3′ (reverse primer) for GRP78; and 5′-CGGGGA CCTGACTGACTACC-3′ (forward primer) and 5′-AGGAAGGCTGGAAGAGTGC-3′ (reverse primer) for β-actin.
Measurement of NADPH oxidase activity
The activity of NADPH oxidase in the treated cells was measured with a cytochrome C-chromometry detection kit according to the manufacturer’s instructions and expressed as nmol per min per mg protein.
Reactive oxygen species (ROS) measurement
DCHF-DA, a redox-sensitive dye that could be oxidized into 2’,7’- dichlorofluorescein (DCF) by intracellular ROS, was used to detect intracellular ROS levels. The treated cells in 6-well plates were washed with PBS and then incubated with 10 μmol/L DCHF-DA in DMEM at 37°C for 20 min. After washing with PBS, cells were harvested and suspended in PBS, and the mean fluorescence intensity was analyzed on a FACScan flow cytometer (Becton-Dickinson, San Jose, CA).
Measurement of SOD activity and MDA content
The treated cells were harvested, washed with PBS, resuspended in 0.5 ml lysis buffer, and lysed with an electromotive cell crusher. The SOD activity and MDA content were determined by using commercial kits according to the manufacturers’ instructions and were expressed as U/mg protein and nmol/mg protein, respectively.
Statistical analysis
Data were expressed as the mean ± SD and analyzed by one-way ANOVA with the Student–Newmann–Keuls test for multiple comparisons and Student’s t test for comparison between two groups using the SPSS 13.0 software for Windows. P values less than 0.05 were considered significant.
RESULTS
Ox-HDL decreases cell viability and induces apoptosis in RAW264.7 cells
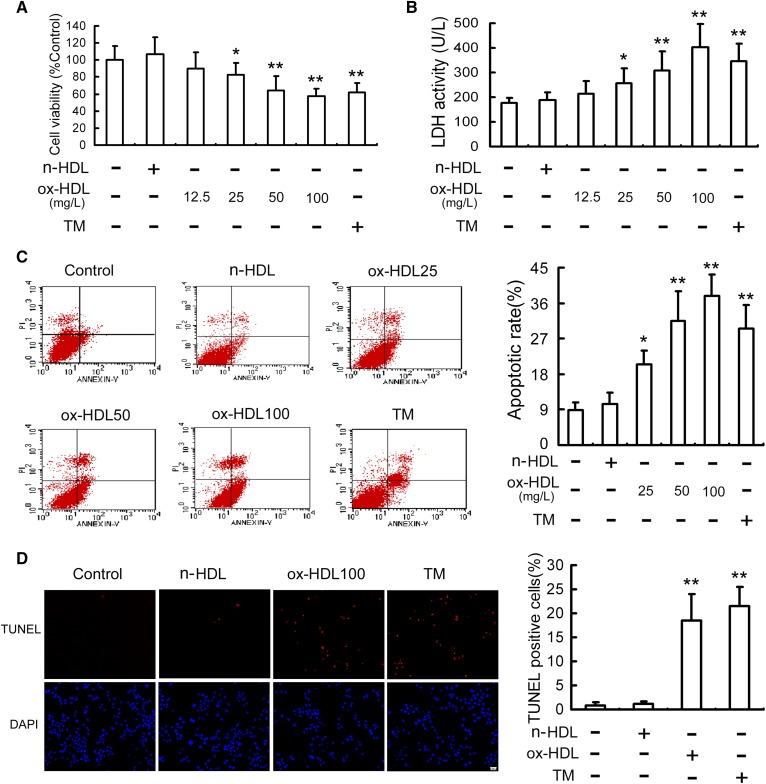
N-HDL did not exhibit significant cytotoxicity on RAW264.7 cells, whereas treatment with ox-HDL for 24 h markedly decreased cell viability and led to a dramatic elevation in LDH leakage in a dose-dependent manner (Fig. 1A, B). Both annexin V-FITC/PI double staining and TUNEL assay revealed that treatment with ox-HDL resulted in a significant increase in apoptosis with the peak at 100 mg/L concentration (Fig. 1C, D). There were similar data, including the decreased cell viability and elevated apoptosis in cells treated by TM, an ER stress inducer (Fig. 1A–D).
Fig. 1.
Ox-HDL decreases cell viability and induces apoptosis in RAW264.7 cells. RAW264.7 cells were treated with the indicated concentration of ox-HDL, n-HDL (100 mg/l), or TM (4 mg/l) for 24 h. Cell viability (A) and LDH activity in media (B) were determined by MTT assay and a kit, respectively. C: Cell apoptosis was analyzed by flow cytometry, and the total apoptotic cells (early- and late-stage apoptosis) were represented by the right side of the panel (annexin V staining alone or together with PI). D: Cell apoptosis was measured by TUNEL assay and expressed as the percentage of the number of TUNEL-positive cells to total cells. Scale bar = 20 µm. Data are expressed as the mean ± SD of at least four independent experiments. *P < 0.05; **P < 0.01 versus control group.
ER stress-CHOP pathway mediates macrophage apoptosis induced by ox-HDL
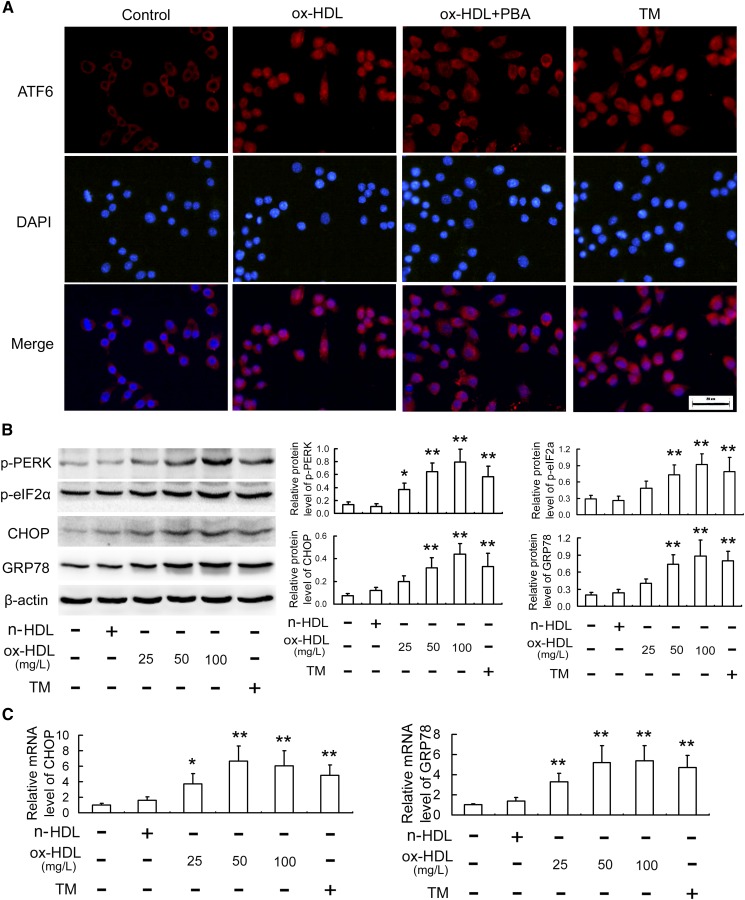
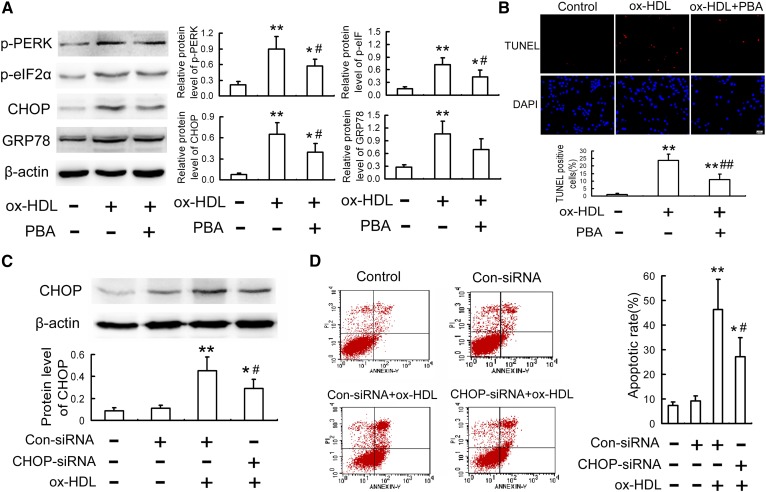
Because the ER stress-CHOP pathway plays an important role in macrophage apoptosis (15–17), we explored the effect of ox-HDL on CHOP and its two important upstream molecules, ATF6 and PERK. As is seen in Fig. 2A–C, as with TM, ox-HDL led to activation of an ER stresss-CHOP pathway, as assessed by nuclear translocation of ATF6, phosphorylation of PERK, and eIF2α, as well as upregulation of GRP78 and CHOP, both at the protein and mRNA levels. However, PBA, an ER stress inhibitor, significantly attenuated the ox-HDL-induced ER stress response and macrophage apoptosis (Fig. 2A, Fig. 3A, B).
Fig. 2.
Ox-HDL induces activation of ER stress-CHOP pathway in RAW264.7 cells. A: Cells were pretreated with or without PBA (5 mmol/l) for 1 h, followed by incubation with ox-HDL (100 mg/l) or TM (4 mg/l) for 24 h, and then immunofluorescence experiments showed ATF6 stained with Cy3 (red) and nuclei visualized by DAPI (blue). Representative fluorescent images photographed by a fluorescence microscopy are shown. Scale bar = 20 μm. B, C: Cells were treated as described in Fig. 1, and then the protein and mRNA levels of ER stress markers were determined with Western blot and quantitative real-time PCR, respectively. Data are expressed as the mean ± SD of at least three independent experiments. *P < 0.05; **P < 0.01 versus control group.
Fig. 3.
Alleviation of ER stress-CHOP pathway mitigates ox-HDL-induced macrophage apoptosis. A, B: RAW264.7 cells were treated with 100 mg/l ox-HDL in the presence or absence of PBA (5 mmol/l) for 24 h, and then the protein levels of ER stress markers and apoptosis were detected by Western blot and TUNEL assay, respectively. Scale bar = 20 µm. C, D: RAW264.7 cells were transfected with siRNA specific for CHOP, followed by treatment with 100 mgl ox-HDL for 24 h, and then CHOP level and apoptosis were determined by Western blot and flow cytometry, respectively. Data are expressed as the mean ± SD of at least three independent experiments. Con, control. *P < 0.05; **P < 0.01 versus control group; #P < 0.05; ##P < 0.01 versus ox-HDL group.
To further confirm the contribution of CHOP to ox-HDL-induced macrophage apoptosis, we investigated whether silencing of CHOP could mitigate ox-HDL-induced apoptosis. As is indicated in Fig. 3C, D, siRNA specific for CHOP showed efficient inhibition of CHOP expression and reduced ox-HDL-induced apoptosis by 41.2%. In addition, PERK is one of the key upstream factors that positively regulate CHOP expression during ER stress (18), so the effect of PERK inhibitor GSK2606414 on ox-HDL-induced macrophage apoptosis was also explored. As can be seen in supplemental Fig. S2, GSK2606414 not only attenuated the upregulation of p-PERK, p-eIF2α, and CHOP but also inhibited apoptosis in RAW264.7 cells induced by ox-HDL. These results suggest that the ER stress-CHOP pathway plays a crucial role in ox-HDL-induced macrophage apoptosis.
Ox-HDL induces intracellular lipid accumulation in RAW264.7 cells
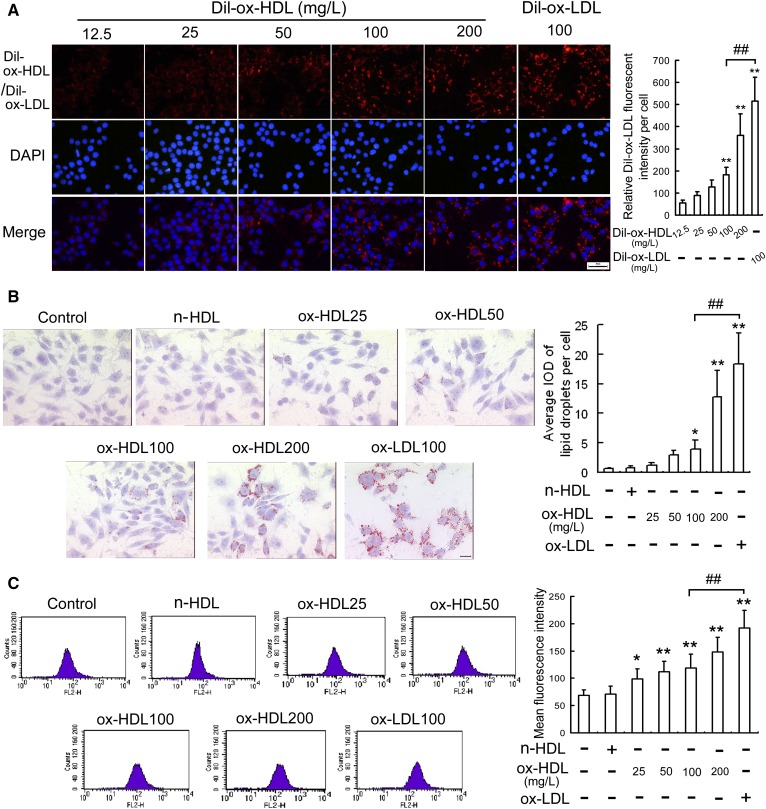
Because the accumulation of intracellular lipid induced by ox-LDL is an important inducer of ER stress-CHOP-mediated macrophage apotosis (18, 19, 24), we examined whether ox-HDL could be taken up by macrophages and induce foam cell formation. As is seen in Fig. 4A, Dil-ox-HDL was ingested by macrophages in a concentration-dependent manner but was less significant than was the uptake of Dil-ox-LDL. The Oil Red O staining (Fig. 4B) and Nile red staining (Fig. 4C) also indicated that lipid content in RAW264.7 cells was increased by ox-HDL in a dose-dependent manner, but was less significant than ox-LDL, suggesting that ox-HDL may induce the formation of macrophage-derived foam cells, but the effect is less potent than that of ox-LDL.
Fig. 4.
Ox-HDL induces intracellular lipid accumulation in RAW264.7 cells. A: Dil-ox-HDL and Dil-ox-LDL fluorescence intensity in cells incubated with Dil-ox-HDL and Dil-ox-LDL at the indicated concentrations for 6 h. Scale bar = 20 μm. B, C: Cells were treated with the indicated concentration of ox-HDL, n-HDL (100 mg/l), or ox-LDL (100 mg/l) for 24 h, and the levels of intracellular lipid were determined using oil red O staining and Nile red staining, respectively. Representative lipid droplet staining images are shown. Scale bar = 20 μm. Data are expressed as the mean ± SD of at least four independent experiments. *P < 0.05; **P < 0.01 versus control group; ##P < 0.01.
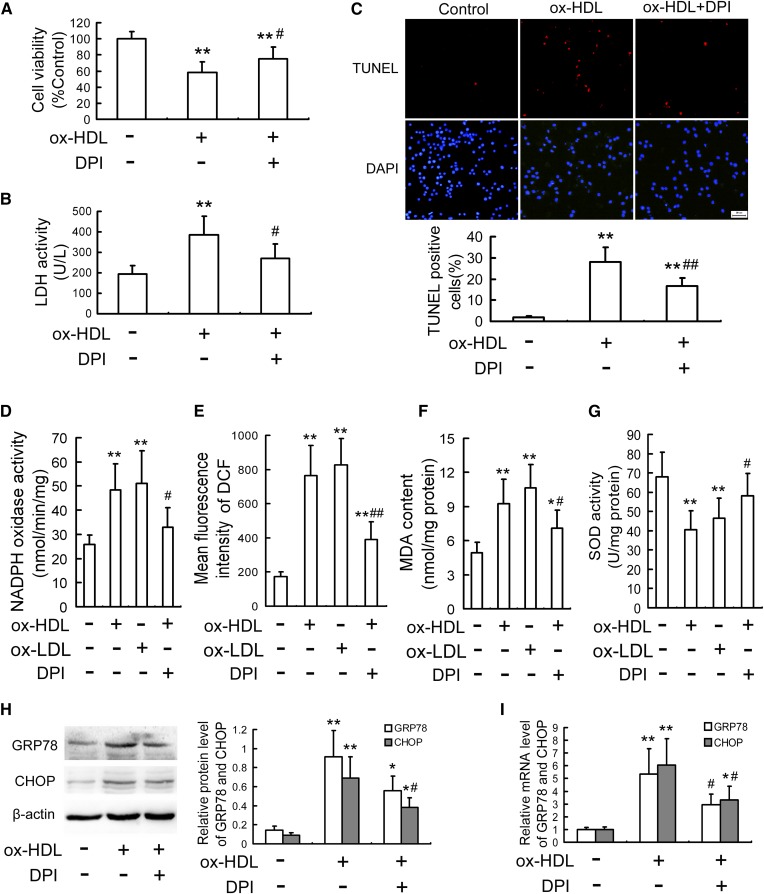
Alleviation of oxidative stress mitigates CHOP-mediated macrophage apoptosis induced by ox-HDL
Because the enhancement of oxidative stress induced by various stimuli acts as an inducer leading to ER stress and macrophage apoptosis (25), the alterations of oxidative stress in RAW264.7 cells treated with ox-HDL were investigated to identify causal factors for CHOP-mediated macrophage apoptosis. As is seen in Fig. 5A-C, preincubation with DPI (a selective inhibitor of NADPH oxidase) attenuated ox-HDL-induced macrophage injury as assessed by the elevated cell viability and the reduced LDH release and cell apoptosis. As with ox-LDL, ox-HDL significantly stimulated the oxidative stress response as determined by the increased NADPH oxidase activity, ROS production, and MDA content, as well as the decreased SOD activity, which were inhibited by DPI (Fig. 5D-G). In addition, pretreatment with DPI markedly inhibited ox-HDL-upregulated GRP78 and CHOP expression both at the protein and mRNA levels (Fig. 5H, I). Similar results were obtained from ROS scavenger probucol-pretreated cells as assessed by the decreased apoptosis, generation of ROS and MDA, and the protein and mRNA expression of CHOP (supplemental Fig. S3), indicating that oxidative stress plays a key role in the ox-HDL-induced CHOP upregulation and macrophage apoptosis.
Fig. 5.
DPI attenuates CHOP-mediated macrophage apoptosis induced by ox-HDL. RAW264.7 cells were pretreated with or without 5 µmol/l DPI (NADPH oxidase inhibitor) for 1 h, and then stimulated with ox-HDL (100 mg/l) for 24 h. Cell viability (A) and LDH activity in media (B) were determined by MTT assay and a kit, respectively. C: Cell apoptosis was measured by TUNEL assay. Scale bar = 20 µm. Cells were pretreated with or without DPI (5 µmol/l) for 1 h, followed by stimulation with ox-HDL (100 mg/l) or ox-LDL (100 mg/l) for 24 h. D: NADPH oxidase activity was determined by cytochrome C chromometry. E: Intracellular ROS levels were measured by DCF analysis using a flow cytometer. F, G: MDA content and SOD activity were determined using commercial kits. H, I: Cells were treated as described in A, and then the protein and mRNA levels of GRP78 and CHOP were determined by Western blot and quantitative real-time PCR, respectively. Data are expressed as the mean ± SD of at least three independent experiments. *P < 0.05; **P < 0.01 versus control group; #P < 0.05; ##P < 0.01 versus ox-HDL treatment.
To further confirm the role of oxidative stress and CHOP pathway in ox-HDL-induced macrophage apoptosis, we also used mouse peritoneal macrophages in this study. As is seen in supplemental Fig. S4A-C, ox-HDL caused injury of mouse peritoneal macrophages, as assessed by the decreased cell viability and increased LDH leakage and apoptosis, which were inhibited by PBA and DPI. Additionally, ox-HDL induced the oxidative stress response, including the elevated NADPH oxidase activity, ROS production, and MDA content, as well as the decreased SOD activity, which were attenuated by DPI (supplemental Fig. S4D–G). Furthermore, ox-HDL upregulated CHOP expression, which was inhibited by PBA and DPI (supplemental Fig. S4H).
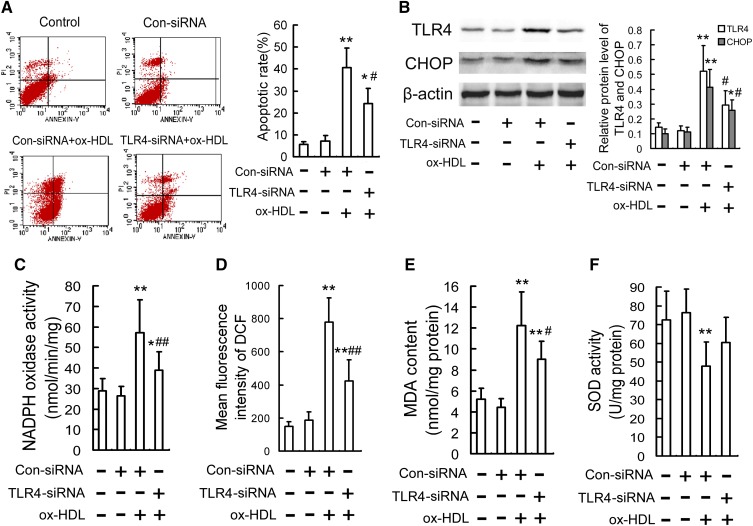
TLR4 is involved in ox-HDL-induced oxidative stress and CHOP-mediated macrophage apoptosis
TLR4 is one of the major transmembrane receptors for signaling transduction with the ability to modulate inflammation and immune-related genes and mediate the activation of macrophages and the formation of foam cells. Our previous work has proved that TLR4 mediates minimally modified low-density lipoprotein (mm-LDL)-induced ER stress in macrophages (26). So we investigated whether TLR4 could mediate ox-HDL-induced oxidative stress and CHOP-mediated macrophage apoptosis. As is shown in Fig. 6A, B, treatment with ox-HDL significantly upregulated TLR4 protein expression, whereas TLR4 silencing by TLR4 siRNA reduced ox-HDL-induced macrophage apoptosis and CHOP protein by 40.3% and 37.4%, respectively. In addition, TLR4 silencing also inhibited ox-HDL-induced oxidative stress response, as determined by the decreased NADPH oxidase activity, ROS production, and MDA content, as well as the increased SOD activity (Fig. 6C–F). To further confirm the role of TLR4 in ox-HDL-induced oxidative stress and CHOP-mediated macrophage apoptosis, we performed antibody neutralization tests using anti-TLR4 antibody, and similar results were obtained as assessed by the inhibitory effect of anti-TLR4 antibody (TLR4 Ab) on macrophage apoptosis, oxidative stress, and CHOP upregulation induced by ox-HDL (supplemental Fig. S5).
Fig. 6.
TLR4 siRNA attenuates ox-HDL-induced oxidative stress and CHOP-mediated macrophage apoptosis. RAW264.7 cells were transfected with siRNA directed against TLR4 and then stimulated with ox-HDL (100 mg/l) for 24 h. A: Cell apoptosis was measured by flow cytometry. B: The protein levels of TLR4 and CHOP were determined by Western blot. C: NADPH oxidase activity was determined by cytochrome C chromometry. D: Intracellular ROS levels were measured by DCF analysis using a flow cytometer. E, F: MDA content and SOD activity were determined using commercial kits. Data are expressed as the mean ± SD of at least three independent experiments. Con, control. *P < 0.05; **P < 0.01 versus control group; #P < 0.05; ##P < 0.01 versus ox-HDL treatment.
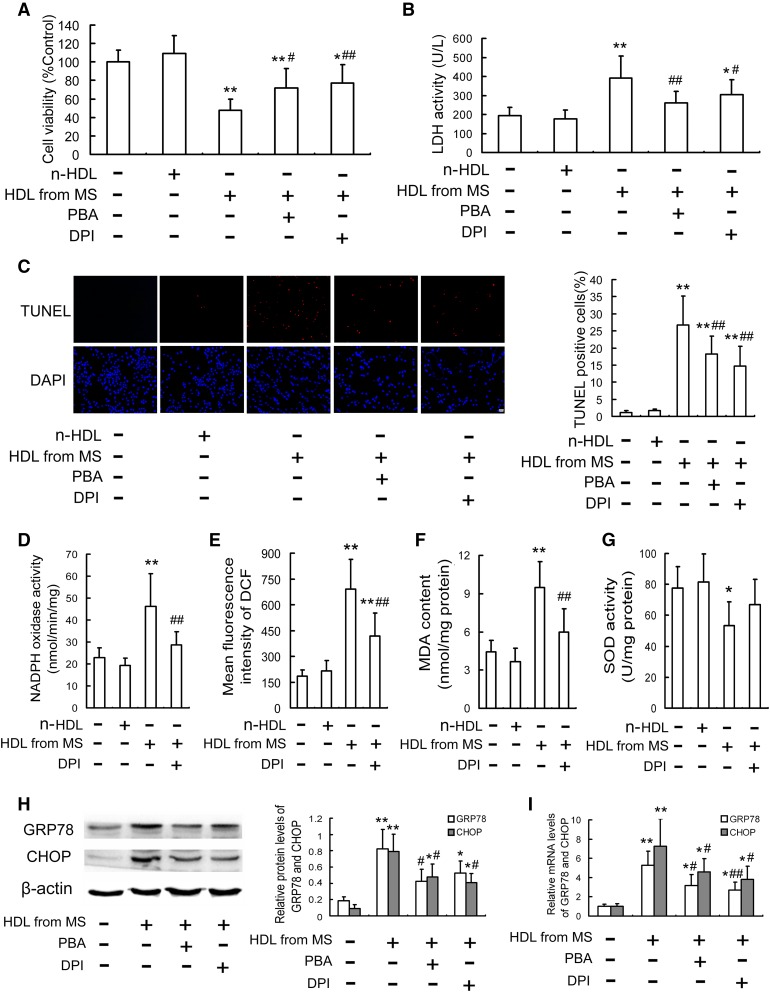
HDL from MS patients induces oxidative stress and CHOP-mediated macrophage apoptosis
HDL is susceptible to oxidative modification under certain circumstances, such as in diabetes (27), metabolic syndrome, and cardiovascular diseases (28, 29). To further clarify the pathophysiologic significance of oxidative modification of HDL in clinical diseases, we isolated HDL from MS patients and investigated its effects on oxidative stress and CHOP-mediated apoptosis in RAW264.7 macrophages. The subject characteristics are presented in supplemental Table S1, and the content of oxidized phosphatidylcholines in HDL from MS patients was much higher than that in n-HDL, which was similar to ox-HDL prepared in vitro (supplemental Fig. S1). As is seen in Fig. 7A–C, treatment with HDL isolated from MS patients led to macrophage injury as assessed by the decreased cell viability and the elevated LDH leakage and apoptosis, which were blocked by PBA and DPI. In addition, HDL from MS patients induced oxidative stress response, including the increased NADPH oxidase activity, ROS production, and MDA content, as well as the decreased SOD activity (Fig. 7D–G), which were inhibited by DPI. Furthermore, HDL from MS patients upregulated expression of GRP78 and CHOP both at the protein and mRNA levels, which were attenuated by PBA and DPI (Fig. 7H, I). Additionally, GSK2606414 not only attenuated the upregulation of p-PERK, p-eIF2α, and CHOP, but also inhibited apoptosis in RAW264.7 cells induced by HDL from MS patients (supplemental Fig. S6). To further elevate the value of the data from pooled samples of MS patients, we isolated HDL samples from individual plasma of MS patients and explored their effects on macrophage apoptosis and CHOP expression. As is shown in supplemental Fig. S7, similar results were obtained showing that treatment with HDL from MS patients led to macrophage apoptosis and CHOP upregulation, which were inhibited by PBA.
Fig. 7.
HDL from MS patients induces oxidative stress and CHOP-mediated macrophage apoptosis. RAW264.7 cells were pretreated with or without PBA (5 mmol/l) or DPI (5 µmol/l) for 1 h and then stimulated with HDL from MS patients (100 mg/l) or n-HDL (100 mg/l) for 24 h. Cell viability (A) and LDH activity in media (B) were determined by MTT assay and a kit, respectively. C: Cell apoptosis was measured by TUNEL assay. Scale bar = 20 µm. D: NADPH oxidase activity was determined by cytochrome C chromometry. E: Intracellular ROS levels were measured by DCF analysis using a flow cytometer. F, G: MDA content and SOD activity were determined using commercial kits. H, I: The protein and mRNA levels of GRP78 and CHOP were analyzed by Western blot and quantitative real-time PCR, respectively. Data are expressed as the mean ± SD of at least three independent experiments. *P < 0.05; **P < 0.01 versus control group; #P < 0.05; ##P < 0.01 versus HDL from MS patients’ treatment.
DISCUSSION
As a main feature of advanced atherosclerotic plaques, macrophage apoptosis promotes enlargement of the necrotic cores and plaque rupture and then leads to cardiovascular complications (1, 2). Ox-LDL plays a crucial role in macrophage-derived foam cell formation and apoptosis, but the role of ox-HDL in this process is not fully understood. In this study, our results showed for the first time that ox-HDL induced macrophage apoptosis by triggering TLR4-mediated oxidative stress and subsequently activating an ER stress-CHOP pathway, which was supported by the following observations. First, as with TM (an ER stress inducer), ox-HDL induced macrophage injury and apoptosis with concomitant activation of the ER stress pathway, including nuclear translocation of ATF6, phosphorylation of PERK and eIF2α, and upregulation of GRP78 and CHOP. Second, ox-HDL-induced apoptosis and activation of ER stress were inhibited by PBA (an ER stress inhibitor), CHOP gene silencing, and GSK2606414 (PERK inhibitor). Third, ox-HDL could be taken up by macrophages and cause lipid accumulation, but less effectively than could ox-LDL. Fourth, DPI (an oxidative stress inhibitor) and probucol (a ROS scavenger) reduced ox-HDL-induced macrophage apoptosis, oxidative stress, and upregulation of GRP78 and CHOP. Fifth, ox-HDL-induced macrophage apoptosis, oxidative stress, and CHOP upregulation were blocked by TLR4 siRNA and antibody. Sixth, HDL isolated from metabolic syndrome patients induced macrophage injury and apoptosis, oxidative stress, and upregulation of GRP78 and CHOP, which were blocked by PBA and DPI.
It is well known that HDL provides a protective role in cardiovascular diseases. The atheroprotective effects of HDL are mainly attributed to its ability to promote cholesterol efflux from lipid-laden extrahepatic cells, inhibit LDL oxidation and adhesion molecule expression, and protect endothelial cells (4). So elevating HDL level was recognized as a promising strategy to suppress atherosclerosis progression (30, 31). Cholesteryl ester transfer protein (CETP) inhibitors were considered to be promising and applicable for markedly raising HDL levels in animal models (32). However, the excess mortality and lack of significant effects to reduce carotid intima-media thickness and attenuate the risk of recurrent cardiovascular events using CETP inhibitors such as torcetrapib and dalcetrapib (33–35) result in a reconsideration, which is more important for the antiatherosclerotic functions of HDL, quantity or quality (36, 37). Thus, the effects of modification of HDL and changes in HDL composition on HDL properties and pathological significance drew great attention from researchers. As the most common type of modification under certain circumstances, such as in diabetes (27), metabolic syndrome, and cardiovascular diseases (28, 29, 38), oxidation was shown to significantly affect the biological properties of HDL from many aspects, including altering paraoxonase structure and decreasing lactonase activity (39), which might cause HDL dysfunction, as assessed by decreased antiatherogenic abilities or even becoming proatherogenic (6). For example, ox-HDL has been shown to halt cholesterol efflux from foam cells in comparison with native HDL (5). Moreover, ox-HDL induces ROS production and NF-κB activation and increases the expression of several proinflammatory genes, including tumor necrosis factor-α and cyclooxygenase-2 in endothelial cells and macrophages, which accelerate atherosclerosis development (10, 40, 41). Furthermore, ox-HDL can induce the proliferation and migration of vascular smooth muscle cells by promoting ROS production (8). In this study, we found that ox-HDL resulted in macrophage injury in a dose-dependent manner, as assessed by decreased cell viability and elevated LDH leakage and apoptosis.
Accumulating evidence indicates that activated ER stress in macrophages promotes the progression of early and advanced atherosclerotic lesions (42). Previous studies, including our own, have demonstrated that ER stress mediates the upregulation of scavenger receptors on macrophages, such as lectin-like oxidized LDL receptor-1 and CD36, and promotes foam cell formation (23, 43). On the other hand, prolonged and severe ER stress triggers proapoptotic signals in macrophages, which in turn result in the instability of advanced atherosclerotic plaques (14, 44). CHOP, a specific proapoptotic molecule regulated by ATF6 and PERK under ER stress, has been demonstrated to play a crucial role in macrophage apoptosis and the destabilization of advanced atherosclerotic lesions, whereas CHOP deficiency inhibits macrophage apoptosis and reduces plaque necrosis in advanced atherosclerotic plaques (15–17). Our previous studies have shown that ox-LDL induces CHOP-mediated macrophage apoptosis by activating ATF6 and PERK pathways, whereas D4F, an apolipoprotein A-I mimetic peptide, alleviates macrophage-derived foam cell apoptosis by inhibiting the ER stress-CHOP pathway (18, 19). In this study, we found that as with TM, ox-HDL not only induced macrophage injury and apoptosis but also activated an ER stress pathway, as assessed by the increased nuclear translocation of ATF6, phosphorylation of PERK, and eIF2α, as well as upregulation of GRP78 and CHOP. However, ox-HDL-induced apoptosis and activation of an ER stress-CHOP pathway were inhibited by PBA (an ER stress inhibitor), CHOP gene silencing, and GSK2606414 (PERK inhibitor). All these data suggest that activation of an ER stress-CHOP pathway may be involved in the inductive effect of ox-HDL on macrophage apoptosis.
The accumulation of intracellular lipid and oxidative stress are the important inducers of ER stress and macrophage apoptosis in vivo and in vitro (24, 25, 45). Our previous studies have proven that minimally modified LDL (mm-LDL) and ox-LDL can induce cholesterol accumulation in macrophages and subsequently stimulate ER stress (18, 19, 23, 26). In this work, we showed that ox-HDL could be taken up by macrophages and cause a significant increase in lipid accumulation but was less potent than was ox-LDL in promoting the macrophage-derived foam cell formation, suggesting that the effect of ox-HDL to induce macrophage-derived foam cell formation is much less than that of ox-LDL. ROS are recognized to be closely responsible for activation of ER stress and apoptosis. NADPH oxidases are the primary enzymes responsible for inducing ROS production in the vascular system. Studies of endothelial cells and aortic smooth muscle cells treated with ox-LDL and 7-ketocholesterol, respectively, have shown that NADPH oxidase mediates ER stress and apoptosis (46, 47), whereas probucol, a ROS scavenger, can protect human umbilical vein endothelial cells from injury induced by hypoxia/reoxygenation by inhibiting CHOP upregulation (48). In addition, oxidative stress also resulted from excessive decrease in activities of antioxidative enzymes, such as SOD (49). In the present study, ox-HDL led to a significant increase in NADPH oxidase activity and a decrease in the SOD activity, with concomitant increased production of ROS and MDA, which were mimetic to ox-LDL treatment. However, DPI, a selective inhibitor of NADPH oxidase, and probucol significantly inhibited oxidative stress, macrophage injury, and apoptosis, as well as upregulation of GRP78 and CHOP induced by ox-HDL, indicating that oxidative stress may mediate ox-HDL-induced activation of the ER stress-CHOP-associated apoptotic pathway in macrophages.
TLR4 has been implicated in oxidative stress and inflammation and related to cardiovascular diseases including atherosclerosis, hypertension, and diabetes (50). Inhibition of TLR4 attenuates vascular dysfunction and oxidative stress in diabetic rats (51). In addition to microbial pathogens, TLR4 recognizes modified lipoprotein, such as mm-LDL and ox-LDL, and promotes lipid accumulation in macrophages (52). However, TLR4 deficiency reduces foam cell accumulation and inhibits expression of interleukin-1α, vascular cell adhesion molecule 1, and monocyte chemoattractant protein-1 in apolipoprotein E−/− mice (53). Our previous work has shown that TLR4 mediates cholesterol accumulation and subsequent activation of ER stress in macrophages induced by mm-LDL (26). The results in the present study showed that ox-HDL upregulated markedly TLR4 expression, whereas TLR4 silencing significantly alleviated macrophage apoptosis, oxidative stress, and CHOP upregulation induced by ox-HDL. Similar results were obtained in the TLR4 antibody-pretreated macrophages, further suggesting that TLR4 may mediate ox-HDL-induced oxidative stress and subsequent activation of a CHOP-induced apoptotic pathway in macrophages.
HDL is susceptible to structural modification, including oxidation and glycation under certain circumstances such as diabetes, MS, and atherosclerosis, and loses its antiatherogenic abilities or even becomes proatherogenic (27–29, 38). To further confirm the clinical significance of HDL modification, we explored the inductive effect of HDL isolated from MS patients on oxidative stress and the CHOP-mediated apoptotic pathway in macrophages. We showed that the content of oxidized phosphatidylcholines in HDL from MS patients was much higher than that in HDL from healthy subjects, which were similar to that of ox-HDL prepared in vitro. In addition, treatment with HDL from MS patients led to macrophage injury, as assessed by the decreased cell viability and elevated LDH leakage and apoptosis, induced oxidative stress response, and upregulated expression of GRP78 and CHOP, which were blocked by PBA and DPI. Furthermore, macrophage apoptosis and CHOP upregulation induced by HDL from MS patients were inhibited by PERK inhibitor GSK2606414.
In conclusion, the major findings of this study are that ox-HDL induced lipid accumulation and activated an ER stress-CHOP-induced apoptotic pathway in macrophages via an enhanced oxidative stress and that this pathway may be triggered by the binding of ox-HDL to TLR4, indicating that ox-HDL may also play a crucial, direct role in atherosclerotic pathogenesis. Therefore, we speculate that it may be possible to apply effective antioxidants to suppress HDL oxidation and improve HDL quality in patients with atherosclerotic risks, which may form an attractive therapeutic approach for combating atherosclerosis-related diseases in the near future.
Supplementary Material
Footnotes
Abbreviations:
- ATF6
- activating transcription factor 6
- CETP
- cholesteryl ester transfer protein
- CHOP
- CCAAT-enhancer-binding protein homologous protein
- DAPI
- 4’, 6-diamidino-2-phenylindole
- DCF
- 2’,7’- dichlorofluorescein
- DCHF-DA
- 2’,7’-dichlorofluorescin diacetate
- DPI
- diphenyleneiodonium
- ER
- endoplasmic reticulum
- GRP78
- glucose regulated proteins 78
- LDH
- lactate dehydrogenase
- MDA
- malondialdehyde
- MS
- metabolic syndrome
- MTT
- 3-(4,5- dimethylthiazol-2-y-l)-2,5-diphenyl-2H-tetrazolium bromide
- ox-HDL
- oxidized HDL
- ox-LDL
- oxidized LDL
- PBA
- 4-phenylbutyric acid
- p-eIF2α
- phospho-eukaryotic translation initiation factor 2α p-PERK, phospho-double-stranded RNA-activated protein kinase-like ER kinase
- ROS
- reactive oxygen species
- siRNA
- small interfering RNA
- SOD
- superoxide dismutase
- TLR4
- toll-like receptor 4
- TM
- tunicamycin
- TUNEL
- terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Moore K. J., and Tabas I.. 2011. Macrophages in the pathogenesis of atherosclerosis. Cell. 145: 341–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tiwari R. L., Singh V., and Barthwal M. K.. 2008. Macrophages: an elusive yet emerging therapeutic target of atherosclerosis. Med. Res. Rev. 28: 483–544. [DOI] [PubMed] [Google Scholar]
- 3.Maiolino G., Rossitto G., Caielli P., Bisogni V., Rossi G. P., and Calò L. A.. 2013. The role of oxidized low-density lipoproteins in atherosclerosis: the myths and the facts. Mediators Inflamm. 2013: 714653–714665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rader D. J., and Hovingh G. K.. 2014. HDL and cardiovascular disease. Lancet. 384: 618–625. [DOI] [PubMed] [Google Scholar]
- 5.Hewing B., Parathath S., Barrett T., Chung W. K., Astudillo Y. M., Hamada T., Ramkhelawon B., Tallant T. C., Yusufishaq M. S., Didonato J. A., et al. 2014. Effects of native and myeloperoxidase-modified apolipoprotein a-I on reverse cholesterol transport and atherosclerosis in mice. Arterioscler. Thromb. Vasc. Biol. 34: 779–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Otocka-Kmiecik A., Mikhailidis D. P., Nicholls S. J., Davidson M., Rysz J., and Banach M.. 2012. Dysfunctional HDL: a novel important diagnostic and therapeutic target in cardiovascular disease? Prog. Lipid Res. 51: 314–324. [DOI] [PubMed] [Google Scholar]
- 7.Ferretti G., Bacchetti T., Nègre-Salvayre A., Salvayre R., Dousset N., and Curatola G.. 2006. Structural modifications of HDL and functional consequences. Atherosclerosis. 184: 1–7. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y., Ji L., Jiang R., Zheng L., and Liu D.. 2014. Oxidized high-density lipoprotein induces the proliferation and migration of vascular smooth muscle cells by promoting the production of ROS. J. Atheroscler. Thromb. 21: 204–216. [DOI] [PubMed] [Google Scholar]
- 9.Assinger A., Koller F., Schmid W., Zellner M., Babeluk R., Koller E., and Volf I.. 2010. Specific binding of hypochlorite-oxidized HDL to platelet CD36 triggers proinflammatory and procoagulant effects. Atherosclerosis. 212: 153–160. [DOI] [PubMed] [Google Scholar]
- 10.Matsunaga T., Hokari S., Koyama I., Harada T., and Komoda T.. 2003. NF-kappa B activation in endothelial cells treated with oxidized high-density lipoprotein. Biochem. Biophys. Res. Commun. 303: 313–319. [DOI] [PubMed] [Google Scholar]
- 11.Nakajima T., Origuchi N., Matsunaga T., Kawai S., Hokari S., Nakamura H., Inoue I., Katayama S., Nagata A., and Komoda T.. 2000. Localization of oxidized HDL in atheromatous plaques and oxidized HDL binding sites on human aortic endothelial cells. Ann. Clin. Biochem. 37: 179–186. [DOI] [PubMed] [Google Scholar]
- 12.Soumyarani V. S., and Jayakumari N.. 2014. Oxidized HDL induces cytotoxic effects: implications for atherogenic mechanism. J. Biochem. Mol. Toxicol. 28: 481–489. [DOI] [PubMed] [Google Scholar]
- 13.Ru D., Zhiqing H., Lin Z., Feng W., Feng Z., Jiayou Z., Yusheng R., Min F., Chun L., and Zonggui W.. 2015. Oxidized high-density lipoprotein accelerates atherosclerosis progression by inducing the imbalance between treg and teff in LDLR knockout mice. APMIS. 123: 410–421. [DOI] [PubMed] [Google Scholar]
- 14.Scull C. M., and Tabas I.. 2011. Mechanisms of ER stress-induced apoptosis in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 31: 2792–2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu X., Wang Y., Zhao W., Zhou H., Yang W., and Guan X.. 2014. Toll-like receptor 7 promotes the apoptosis of THP-1-derived macrophages through the CHOP-dependent pathway. Int. J. Mol. Med. 34: 886–893. [DOI] [PubMed] [Google Scholar]
- 16.Tsukano H., Gotoh T., Endo M., Miyata K., Tazume H., Kadomatsu T., Yano M., Iwawaki T., Kohno K., Araki K., et al. 2010. The endoplasmic reticulum stress-C/EBP homologous protein pathway-mediated apoptosis in macrophages contributes to the instability of atherosclerotic plaques. Arterioscler. Thromb. Vasc. Biol. 30: 1925–1932. [DOI] [PubMed] [Google Scholar]
- 17.Thorp E., Li G., Seimon T. A., Kuriakose G., Ron D., and Tabas I.. 2009. Reduced apoptosis and plaque necrosis in advanced atherosclerotic lesions of Apoe−/− and Ldlr−/− mice lacking CHOP. Cell Metab. 9: 474–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yao S., Zong C., Zhang Y., Sang H., Yang M., Jiao P., Fang Y., Yang N., Song G., and Qin S.. 2013. Activating transcription factor 6 mediates oxidized LDL-induced cholesterol accumulation and apoptosis in macrophages by up-regulating CHOP expression. J. Atheroscler. Thromb. 20: 94–107. [DOI] [PubMed] [Google Scholar]
- 19.Yao S., Tian H., Miao C., Zhang D. W., Zhao L., Li Y., Yang N., Jiao P., Sang H., Guo S., et al. 2015. D4F alleviates macrophage-derived foam cell apoptosis by inhibiting CD36 expression and ER stress-CHOP pathway. J. Lipid Res. 56: 836–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alberti K. G., Eckel R. H., Grundy S. M., Zimmet P. Z., Cleeman J. I., Donato K. A., Fruchart J. C., James W. P., Loria C. M., and Smith S. C. Jr.. 2009. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 120: 1640–1645. [DOI] [PubMed] [Google Scholar]
- 21.Girona J., LaVille A. E., Solà R., Motta C., and Masana L.. 2003. HDL derived from the different phases of conjugated diene formation reduces membrane fluidity and contributes to a decrease in free cholesterol efflux from human THP-1 macrophages. Biochim. Biophys. Acta. 1633: 143–148. [DOI] [PubMed] [Google Scholar]
- 22.Boullier A., Li Y., Quehenberger O., Palinski W., Tabas I., Witztum J. L., and Miller Y. I.. 2006. Minimally oxidized LDL offsets the apoptotic effects of extensively oxidized LDL and free cholesterol in macrophages. Arterioscler. Thromb. Vasc. Biol. 26: 1169–1176. [DOI] [PubMed] [Google Scholar]
- 23.Yao S., Miao C., Tian H., Sang H., Yang N., Jiao P., Han J., Zong C., and Qin S.. 2014. Endoplasmic reticulum stress promotes macrophage-derived foam cell formation by up-regulating cluster of differentiation 36 (CD36) expression. J. Biol. Chem. 289: 4032–4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Devries-Seimon T., Li Y., Yao P. M., Stone E., Wang Y., Davis R. J., Flavell R., and Tabas I.. 2005. Cholesterol-induced macrophage apoptosis requires ER stress pathways and engagement of the type A scavenger receptor. J. Cell Biol. 171: 61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown D. I., and Griendling K. K.. 2015. Regulation of signal transduction by reactive oxygen species in the cardiovascular system. Circ. Res. 116: 531–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yao S., Yang N., Song G., Sang H., Tian H., Miao C., Zhang Y., and Qin S.. 2012. Minimally modified low-density lipoprotein induces macrophage endoplasmic reticulum stress via toll-like receptor 4. Biochim. Biophys. Acta. 1821: 954–963. [DOI] [PubMed] [Google Scholar]
- 27.Kontush A., and Chapman M. J.. 2008. Why is HDL functionally deficient in type 2 diabetes? Curr. Diab. Rep. 8: 51–59. [DOI] [PubMed] [Google Scholar]
- 28.Kotani K., Sakane N., Ueda M., Mashiba S., Hayase Y., Tsuzaki K., Yamada T., and Remaley A. T.. 2012. Oxidized high-density lipoprotein is associated with increased plasma glucose in non-diabetic dyslipidemic subjects. Clin. Chim. Acta. 414: 125–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peterson S. J., Vanella L., Gotlinger K., Jiang H., Singh S. P., Sodhi K., Maher E., O’Hanlon K., Shapiro J. I., and Abraham N. G.. 2016. Oxidized HDL is a potent inducer of adipogenesis and causes activation of the Ang-II and 20-HETE systems in human obese females. Prostaglandins Other Lipid Mediat. 123: 68–77. [DOI] [PubMed] [Google Scholar]
- 30.Parolini C., Marchesi M., Lorenzon P., Castano M., Balconi E., Miragoli L., Chaabane L., Morisetti A., Lorusso V., Martin B. J., et al. 2008. Dose-related effects of repeated ETC-216 (recombinant apolipoprotein A-I Milano/1-palmitoyl-2-oleoyl phosphatidylcholine complexes) administrations on rabbit lipid-rich soft plaques: in vivo assessment by intravascular ultrasound and magnetic resonance imaging. J. Am. Coll. Cardiol. 51: 1098–1103. [DOI] [PubMed] [Google Scholar]
- 31.Taylor A. J. 2008. Evidence to support aggressive management of high-density lipoprotein cholesterol: implications of recent imaging trials. Am. J. Cardiol. 101: 36B–43B. [DOI] [PubMed] [Google Scholar]
- 32.Hansen M. K., McVey M. J., White R. F., Legos J. J., Brusq J. M., Grillot D. A., Issandou M., and Barone F. C.. 2010. Selective CETP inhibition and PPARalpha agonism increase HDL cholesterol and reduce LDL cholesterol in human ApoB100/human CETP transgenic mice. J. Cardiovasc. Pharmacol. Ther. 15: 196–202. [DOI] [PubMed] [Google Scholar]
- 33.Bots M. L., Visseren F. L., Evans G. W., Riley W. A., Revkin J. H., Tegeler C. H., Shear C. L., Duggan W. T., Vicari R. M., Grobbee D. E., et al. 2007. Torcetrapib and carotid intima-media thickness in mixed dyslipidaemia (RADIANCE 2 study): a randomised, double-blind trial. Lancet. 370: 153–160. [DOI] [PubMed] [Google Scholar]
- 34.Nissen S. E., Tardif J. C., Nicholls S. J., Revkin J. H., Shear C. L., Duggan W. T., Ruzyllo W., Bachinsky W. B., Lasala G. P., and Tuzcu E. M.. 2007. Effect of torcetrapib on the progression of coronary atherosclerosis. N. Engl. J. Med. 356: 1304–1316. [DOI] [PubMed] [Google Scholar]
- 35.Schwartz G. G., Olsson A. G., Abt M., Ballantyne C. M., Barter P. J., Brumm J., Chaitman B. R., Holme I. M., Kallend D., Leiter L. A., et al. 2012. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N. Engl. J. Med. 367: 2089–2099. [DOI] [PubMed] [Google Scholar]
- 36.Gadi R., Amanullah A., and Figueredo V. M.. 2013. HDL-C: does it matter? An update on novel HDL-directed pharmacotherapeutic strategies. Int. J. Cardiol. 167: 646–655. [DOI] [PubMed] [Google Scholar]
- 37.Pirillo A., Norata G. D., and Catapano A. L.. 2013. Treating high density lipoprotein cholesterol (HDL-C): quantity versus quality. Curr. Pharm. Des. 19: 3841–3857. [DOI] [PubMed] [Google Scholar]
- 38.Shao B., Tang C., Sinha A., Mayer P. S., Davenport G. D., Brot N., Oda M. N., Zhao X. Q., and Heinecke J. W.. 2014. Humans with atherosclerosis have impaired ABCA1 cholesterol efflux and enhanced high-density lipoprotein oxidation by myeloperoxidase. Circ. Res. 114: 1733–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kar S., Patel M. A., Tripathy R. K., Bajaj P., and Pande A. H.. 2013. Oxidized-phospholipids in reconstituted high density lipoprotein particles affect structure and function of recombinant paraoxonase 1. Biochim. Biophys. Acta. 1831: 1714–1720. [DOI] [PubMed] [Google Scholar]
- 40.Soumyarani V. S., and Jayakumari N.. 2012. Oxidatively modified high density lipoprotein promotes inflammatory response in human monocytes-macrophages by enhanced production of ROS, TNF-α, MMP-9, and MMP-2. Mol. Cell. Biochem. 366: 277–285. [DOI] [PubMed] [Google Scholar]
- 41.Callegari E., Norata G. D., Inoue H., and Catapano A. L.. 2006. Oxidized-HDL3 modulates the expression of Cox-2 in human endothelial cells. Int. J. Mol. Med. 18: 209–213. [PubMed] [Google Scholar]
- 42.Ivanova E. A., and Orekhov A. N.. 2016. The role of endoplasmic reticulum stress and unfolded protein response in atherosclerosis. Int. J. Mol. Sci. 17: E193–E203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ishiyama J., Taguchi R., Akasaka Y., Shibata S., Ito M., Nagasawa M., and Murakami K.. 2011. Unsaturated FAs prevent palmitate-induced LOX-1 induction via inhibition of ER stress in macrophages. J. Lipid Res. 52: 299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Erbay E., Babaev V. R., Mayers J. R., Makowski L., Charles K. N., Snitow M. E., Fazio S., Wiest M. M., Watkins S. M., Linton M. F., et al. 2009. Reducing endoplasmic reticulum stress through a macrophage lipid chaperone alleviates atherosclerosis. Nat. Med. 15: 1383–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feng B., Yao P. M., Li Y., Devlin C. M., Zhang D., Harding H. P., Sweeney M., Rong J. X., Kuriakose G., Fisher E. A., et al. 2003. The endoplasmic reticulum is the site of cholesterol-induced cytotoxicity in macrophages. Nat. Cell Biol. 5: 781–792. [DOI] [PubMed] [Google Scholar]
- 46.Hong D., Bai Y. P., Gao H. C., Wang X., Li L. F., Zhang G. G., and Hu C. P.. 2014. Ox-LDL induces endothelial cell apoptosis via the LOX-1-dependent endoplasmic reticulum stress pathway. Atherosclerosis. 235: 310–317. [DOI] [PubMed] [Google Scholar]
- 47.Pedruzzi E., Guichard C., Ollivier V., Driss F., Fay M., Prunet C., Marie J. C., Pouzet C., Samadi M., Elbim C., et al. 2004. NAD(P)H oxidase Nox-4 mediates 7-ketocholesterol-induced endoplasmic reticulum stress and apoptosis in human aortic smooth muscle cells. Mol. Cell. Biol. 24: 10703–10717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chai Y. L., Xu J. Z., Zhang Y. L., and Sheng G. T.. 2016. Effects of probucol on cultured human umbilical vein endothelial cells injured by hypoxia/reoxygenation. Genet. Mol. Res. 15: 15016752–15016760. [DOI] [PubMed] [Google Scholar]
- 49.Chen X. P., Xun K. L., Wu Q., Zhang T. T., Shi J. S., and Du G. H.. 2007. Oxidized low density lipoprotein receptor-1 mediates oxidized low density lipoprotein-induced apoptosis in human umbilical vein endothelial cells: role of reactive oxygen species. Vascul. Pharmacol. 47: 1–9. [DOI] [PubMed] [Google Scholar]
- 50.den Dekker W. K., Cheng C., Pasterkamp G., and Duckers H. J.. 2010. Toll like receptor 4 in atherosclerosis and plaque destabilization. Atherosclerosis. 209: 314–320. [DOI] [PubMed] [Google Scholar]
- 51.Carrillo-Sepulveda M. A., Spitler K., Pandey D., Berkowitz D. E., and Matsumoto T.. 2015. Inhibition of TLR4 attenuates vascular dysfunction and oxidative stress in diabetic rats. J. Mol. Med. (Berl.). 93: 1341–1354. [DOI] [PubMed] [Google Scholar]
- 52.Choi S. H., Harkewicz R., Lee J. H., Boullier A., Almazan F., Li A. C., Witztum J. L., Bae Y. S., Miller Y. I.. 2009. Lipoprotein accumulation in macrophages via toll-like receptor-4-dependent fluid phase uptake. Circ. Res. 104: 1355–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Higashimori M., Tatro J. B., Moore K. J., Mendelsohn M. E., Galper J. B., and Beasley D.. 2011. Role of toll-like receptor 4 in intimal foam cell accumulation in apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 31: 50–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.