Abstract
Recent advances in analytical and sweat collection techniques provide new opportunities to identify noninvasive biomarkers for the study of skin inflammation and repair. This study aims to characterize the lipid mediator profile including oxygenated lipids, endocannabinoids, and ceramides/sphingoid bases in sweat and identify differences in these profiles between sweat collected from nonlesional sites on the unflared volar forearm of subjects with and without atopic dermatitis (AD). Adapting routine procedures developed for plasma analysis, over 100 lipid mediators were profiled using LC-MS/MS and 58 lipid mediators were detected in sweat. Lipid mediator concentrations were not affected by sampling or storage conditions. Increases in concentrations of C30–C40 [NS] and [NdS] ceramides, and C18:1 sphingosine, were observed in the sweat of study participants with AD despite no differences being observed in transepidermal water loss between study groups, and this effect was strongest in men (P < 0.05, one-way ANOVA with Tukey’s post hoc HSD). No differences in oxylipins and endocannabinoids were observed between study groups. Sweat mediator profiling may therefore provide a noninvasive diagnostic for AD prior to the presentation of clinical signs.
Keywords: oxidized lipids, endocannabinoids, ceramides, sphingolipids, skin, metabolic profiling, non-invasive sampling, atopic dermatitis
Blood and urine have been extensively studied in the context of biomedical research and diagnostics. However, the collection of these biofluids can be physically and/or culturally invasive, affecting subject compliance. The use of noninvasive matrices, such as hair, oral fluid, sweat, and tears, has improved subject compliance in pharmacokinetic studies (1). Recently, metabolomic analyses of sweat have begun for similar reasons (2).
Sweat is a complex fluid excreted by apocrine and eccrine glands of the skin, reported to contain small molecules, including electrolytes, urea, lactate, amino acids, metals, and xenobiotics (2). Sweat can be used for diagnostics when disease alters its composition, as has been demonstrated in cystic fibrosis where sweat chloride levels are elevated (3). Sweat has also been used in forensic settings as a test matrix, as most illicit drugs are excreted in sweat following administration by a variety of routes (4).
Despite the potential diagnostic uses, there are few studies examining the metabolic profile of sweat due to a lack of uniform and reproducible sweat collection protocols that generate sweat in sufficient quantities for analytical sample processing (2, 5). Reported sweat collection devices include simple occlusive or nonocclusive absorbent bandages applied to a body surface, bags that can encase a limb, and the Macroduct® sweat collector (Wescor Inc., Logan, UT). In each case, sweat collection is preceded by stimulation of sweating by either physiological (exercise or thermal induction) or pharmacological (pilocarpine iontophoresis) means (2). Of these, the Macroduct® sweat collector, which uses a concave disk and plastic capillary tubing to immediately collect and isolate sweat excreted to the skin surface following stimulation by pilocarpine, is perhaps the most suitable for metabolomics analysis because sweat collection is relatively fast (∼30 min per sample) and the design of the device prevents hydromeiosis and/or encapsulation of the skin surface (2, 6).
While several proteomic analyses of sweat have been reported (7, 8), only five attempts at metabolomic analyses of sweat have been described. High-resolution nuclear magnetic resonance spectroscopy of sweat suggested the presence of several amino acids and lipid-associated groups (5, 9). More recent MS-based approaches confirmed the presence of amino acid- and lipid-derived molecules, while additionally showing the presence of carbohydrates and organic acids in sweat (10, 11). MS-based metabolomics of sweat was also used to develop a screening tool for lung cancer (12).
Despite the potential utility of sweat in diagnostic settings, it has rarely been used in the context of cutaneous research. To the best of our knowledge, only a single study exists that examines the composition of sweat in the context of cutaneous disease, a study that demonstrated no difference in the inflammation regulating lipid prostaglandin (PG)E2 levels in subjects with atopic dermatitis (AD), psoriasis, or hyperhidrosis, relative to healthy controls (13).
Bioactive lipids come in many forms and regulate a variety of processes, including inflammation, cell growth and differentiation, and vascular homeostasis (14). These mediators, which include oxygenated lipids (“oxylipins”), endocannabinoids, and ceramides, are generally thought to be produced locally via biosynthetic pathways in response to extracellular stimuli and function similarly to local hormones or autacoids (14). Additionally, ceramides play an important structural role in the epidermal barrier (15). While lipid mediators have been studied in cutaneous research, these studies have focused on a limited number of analytical targets and/or a single class of analytes (16), barring the identification of mediator pathway cross-talk and limiting the scope of discovery in these studies.
Understanding the actions and interactions of lipid mediators has proven useful in other contexts (14), and doing so in a noninvasive manner in the context of skin research could enhance our understanding of cutaneous biochemistry while ensuring minimal subject discomfort. The present study aims to demonstrate the potential for sweat analysis to reflect biochemical impacts of skin diseases by examining the impact of AD on the sweat profile of >100 lipid mediators from three chemical super-classes using targeted analyses.
MATERIALS AND METHODS
Chemicals and reagents
Ultra-performance LC (UPLC)-grade methanol, acetonitrile, 2-propanol, and water used during sample preparation and chromatographic analysis were purchased from Fisher Scientific (Waltham, MA). Glacial acetic acid, formic acid, and ammonium formate used during chromatographic analysis were purchased from either Fisher Scientific or Sigma-Aldrich (St. Louis, MO). Lipid mediator standards, analytical surrogates, and internal standards were synthesized or purchased from Cayman Chemicals (Ann Arbor, MI), Avanti Polar Lipids Inc. (Alabaster, AL), or Larodan (Malmö, Sweden).
Subjects
A total of 26 subjects (n = 13 in each group) were recruited to participate in a case-control study to examine the effects of AD on the sweat mediator lipidome between February 2015 and February 2016. Subjects were recruited from the University of California-Davis Dermatology Clinic and the Sacramento region. Inclusion criteria for the study included a diagnosis of AD by a dermatologist or the absence of any inflammatory skin conditions for control subjects. Subjects on systemic immunosuppressive medications were excluded, and all subjects with AD were sampled while they were in the unflared state. Written informed consent was obtained from all subjects prior to participation, and all study protocols were approved by the Institutional Review Board of the University of California-Davis (Protocol #605131). Of the 26 subjects recruited, three were excluded. Two subjects with AD did not produce sweat upon stimulation, and one subject without AD had flared acne vulgaris at the time of sampling. Therefore, the study proceeded with 11 subjects with and 12 subjects without AD. Group characteristics along with sampling and storage parameters are shown in Table 1.
TABLE 1.
Sampling and storage parameters of sweat collected from subjects with and without AD
| Parameter | AD (n = 11) | Control (n = 12) |
| Gender (male/female) (n) | 7/4 | 7/5 |
| Age (years)a | 33.8 ± 12.0b | 29.3 ± 3.8 |
| Sweat collected (μl)c | 31.2 [7.1–73]d | 61.3 [25–100] |
| Transepidermal water loss (g/h/m2)a | 8.4 ± 3.9 | 7.4 ± 3.9 |
| Sampling time (n) | ||
| 0900–1400 | 6 | 6 |
| 1400–1800 | 5 | 6 |
| Storage at −80°C (n) | ||
| 0–19 days | 7 | 5 |
| 20–30 days | 4 | 7 |
Data reported as mean ± SD.
These data include one female subject with AD was an outlier with respect to age (62.2 years vs. ADn=10 = 30.9 ± 7.7 years), but not with respect to observed lipid mediators.
Data reported as geometric mean [range].
P < 0.05 versus control group. Significance assessed by two-tailed heteroscedastic Student’s t-test.
Sweat collection and transepidermal water loss measurement
Subjects were asked to refrain from using any topical moisturizers or medications for at least 12 h prior to the study visit. Sweat was stimulated and collected from an approximately 7 cm2 area on the volar surfaces of the bilateral forearms at nonlesional sites present within 8 cm of the wrist using the Macroduct® sweat collector (generously donated by Wescor, Inc.) according to manufacturer’s instructions (http://wescor.com/translations/Translations/M2551-7A-EN.pdf, accessed July 11, 2016). Briefly, the forearm was prepared by wiping with a 70% isopropanol swab (Covidien, Minneapolis, MN) followed by distilled water-saturated sterile cotton gauze. Sweating was then stimulated sequentially on each forearm by pilocarpine iontophoresis using manufacturer-supplied pilocarpine gel disks attached to a power source containing two 9 V batteries. Pilocarpine iontophoresis was conducted for 5 min, after which the forearm was wiped with fresh distilled water-saturated sterile cotton gauze, and sweat was collected using the Macroduct® device. An image of the Macroduct® sweat collector and the typical sweat collection site used in this study can be found in supplemental Fig. S1. Collected sweat was exuded into methanol-rinsed 2 ml amber vials with Teflon lined closures (Waters, Milford, MA) by passing air through the collection tubing using a gastight syringe (Hamilton, Reno, NV) and samples were stored at −80°C until analysis.
Transepidermal water loss was measured at a nonlesional site immediately adjacent to the site of sweat collection using a Tewameter TM 300 (Courage and Khazaka Electronic GmbH, Cologne, Germany) in accordance with the manufacturer’s instructions. Briefly, the Tewameter probe was placed at the sampling site and triplicate measurements of transepidermal water loss were recorded in 30 s intervals. Data were reported in grams of water lost per hour per square meter of skin (g/h/m2).
Analysis of lipid mediators
Oxylipins, endocannabinoids, sphingoid bases, and ceramides were isolated from sweat by direct evaporation of the matrix. Prior to evaporation, sample volume was determined by a gastight syringe (Hamilton) and samples were enriched with 5 μl anti-oxidant solution (0.2 mg/ml solution butylated hydroxytoluene/EDTA in 1:1 methanol:water) and 5 μl of 1 μM deuterated or C17-analog analytical surrogates in methanol. A complete list of target analytes and their associated analytical surrogates are shown in supplemental Tables S1–S3. Samples were then diluted with 100 μl methanol and 10 μl of a 20% glycerol solution in methanol was added as a keeper to reduce analyte loss during evaporation. Samples were dried by vacuum evaporation and reconstituted in 35 μl of an internal standard solution containing 50 nM each of 1-cyclohexyl-3-ureido dodecanoic acid (Sigma-Aldrich) and 1-phenyl,3-ureido hexanoic acid (gift from B. D. Hammock, University of California-Davis) in 1:1 (v/v) methanol:acetonitrile prior to analysis.
UPLC-MS/MS analysis was conducted using modifications of previously published protocols (17, 18). Briefly, three 10 μl aliquots of the reconstituted sample were sequentially injected onto an Acquity UPLC system (Waters), with one injection per lipid mediator profile analysis. Oxylipins and endocannabinoids were separated on a 2.1 × 150 mm, 1.7 μm BEH C18 column (Waters), while ceramides/sphingoid bases were separated on a 2.1 × 100 mm, 1.7 μm BEH C8 column (Waters) using the solvent gradients described in supplemental Tables S1–S3. MS/MS was performed on an API 4000 QTrap (Sciex, Framingham, MA) with either negative mode (oxylipins) or positive mode (endocannabinoids and ceramides/sphingoid bases) electrospray ionization. Ionization voltages, MS/MS parameters, and chromatographic retention time are listed in supplemental Tables S1–S3. Analytes were quantified using internal standard methodology with five to seven point calibration curves (r ≥ 0.997). Data were processed using AB Sciex MultiQuant version 3.0.2 and all calculated lipid mediator concentrations were reported in picomoles per milliliter (i.e., nM) of collected sweat.
The abbreviations used to describe the oxylipins, endocannabinoids, sphingoid bases, and ceramides quantified in this study follow standard conventions in the field and are fully described in the supplemental data.
Statistical analysis
Statistical analysis generally followed previously published protocols (19). All lipid mediator data were assessed by partial least squares discriminant analysis (PLS-DA) using disease state and/or subject gender, hand sampled, time of collection, or storage time as the classifier. All statistical analyses were performed in the R statistical environment (R Foundation for Statistical Computing, Vienna, Austria) using imDEV v1.42, a Microsoft Excel add-in (Microsoft Corporation, Redmond, WA) (20).
Prior to PLS-DA, data were curated such that analytes with <70% completeness of data were removed from consideration. Curated data were screened for outliers using the Grubb’s test (21), and missing data were imputed by a two-component probabilistic principle components analysis with univariate scaling (22). Additionally, ceramide and sphingoid base data were not collected for four male subjects, two with and two without AD. To allow the unbiased use of these subjects’ oxylipin and endocannabinoid data in PLS-DA analyses, this missing data was interpolated as the gender-independent mean of the remaining subject’s data in each AD group. The interpolated ceramide results were not used for the mean comparisons described below. Following normalization of data according to the procedures of Box and Cox (23), PLS-DA was conducted using the orthogonal scores algorithm with univariate scaling and leave-one-out cross-validation. Variables were clustered by Spearman correlation coefficients using the Minkowski distance and Ward agglomeration.
All means comparisons were performed using MetaboAnalyst 3.0 (24). For subjects with and without AD classified by gender, mean differences were tested by false discovery rate-corrected one-way ANOVA with Tukey’s post hoc HSD. Mean differences in sampling or storage were tested for AD groups separately using Student’s t-test with false discovery rate correction. Additionally, three subjects with and three subjects without AD were randomly selected and mean sweat lipid mediator concentrations from the right and left volar forearms were compared by false discovery rate-corrected repeated measures ANOVA with Tukey’s post hoc HSD.
RESULTS
Lipid mediators in eccrine sweat
A total of 58 lipid mediators were quantified in the sweat of subjects with and without AD, including 33 oxylipins, 3 nitrolipids, 13 endocannabinoids, 7 [NS] or [NdS] ceramides, and 2 sphingoid bases. A complete list of lipid mediators screened for and detected along with their associated concentrations in eccrine sweat can be found in supplemental Table S4. Representative chromatograms of all three classes of lipid mediators can be found in supplemental Figs. S2–S4. As seen in supplemental Fig. S3, the monoacylglycerol derivatives of arachidonic, linoleic, and oleic acid (i.e., 2-AG/1-AG, 2-LG/1-LG, and 2-OG/1-OG, respectively) all demonstrated isomerization, with the 1-isomer present at higher concentrations than the 2-isomer. However, as the same degree of isomerization was noted in the analytical surrogate associated with these targets (d5-2-AG), this isomerization was considered acceptable, and quantitative data on each isomer could still be obtained.
AD alters sweat lipid mediator profile, but not barrier function, in unflared nonlesional skin
While one female subject with AD was substantially older than the other women in the study (62.2 years vs. ADn=10 = 30.9 ± 7.7 years or ADfemales = 34.1 ± 10.6 years), this extreme age did not influence the measured lipid mediators. As shown in Fig. 1, subjects with AD had increased (P < 0.05) concentrations of C14–C24 ceramides, i.e., C32–C42 [NS] ceramides; C18 and C24 dihydroceramides, i.e., C36 and C42 [NdS] ceramides; C18:1 sphingosine; 10-nitrooleate; and the arachidonate-derived alcohol 8-HETE. PLS-DA discriminated subjects with and without AD (Fig. 2A), and identified increases in ceramides, dihydroceramides, and C18:1 sphingosine as the dominant discriminating factors (Fig. 2B). The PLS-DA also highlighted a gender-dependent effect, with mediator profiles increasing more in men than in women, and where men with and without AD are better separated than women (Fig. 2A). Comparing the relative fold differences in ceramides and sphingosine between subjects with and without AD, when separated by gender, highlights this observation (Fig. 2C, D). Specifically, men with AD have ∼2- to 6-fold higher ceramides and sphingosine compared with men without AD, whereas women with AD only have ∼1- to 1.5-fold higher ceramides and sphingosine compared with women without AD. Transepidermal water loss, an indicator of skin barrier status, was not different between subjects with and without AD (Table 1).
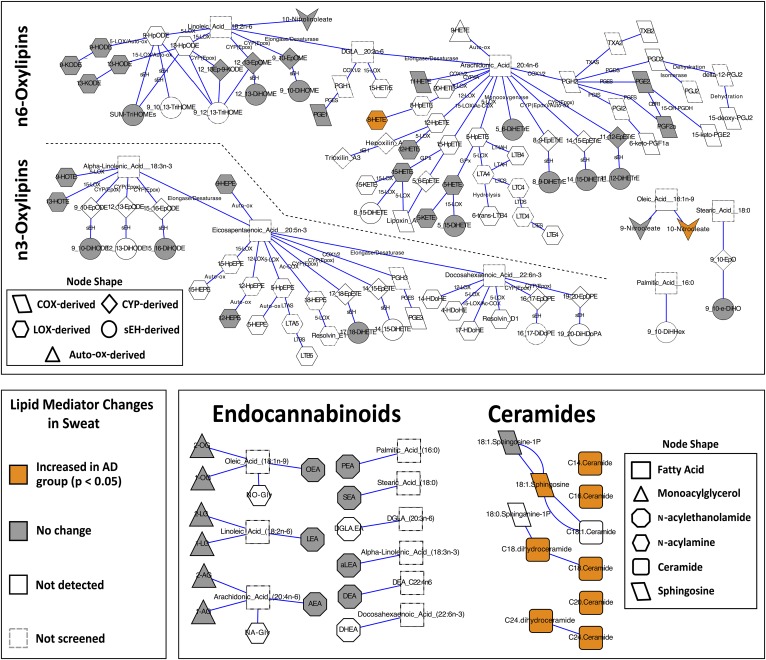
Fig. 1.
Biochemical network maps summarizing assayed and detected lipid mediators in the eccrine sweat of subjects with and without AD. Subjects with AD had increased concentrations of arachidonic acid-derived 8-HETE, oleic acid-derived 10-nitrooleate, C30–C40 [NS] and [NdS] ceramides, and C18:1 sphingosine. Lipid mediators were clustered based on known biochemical pathways and significance was assessed using a one-way ANOVA with Tukey’s post hoc HSD and false discovery rate correction (q = 0.05). Descriptions of lipid mediator abbreviations can be found in the supplemental data.
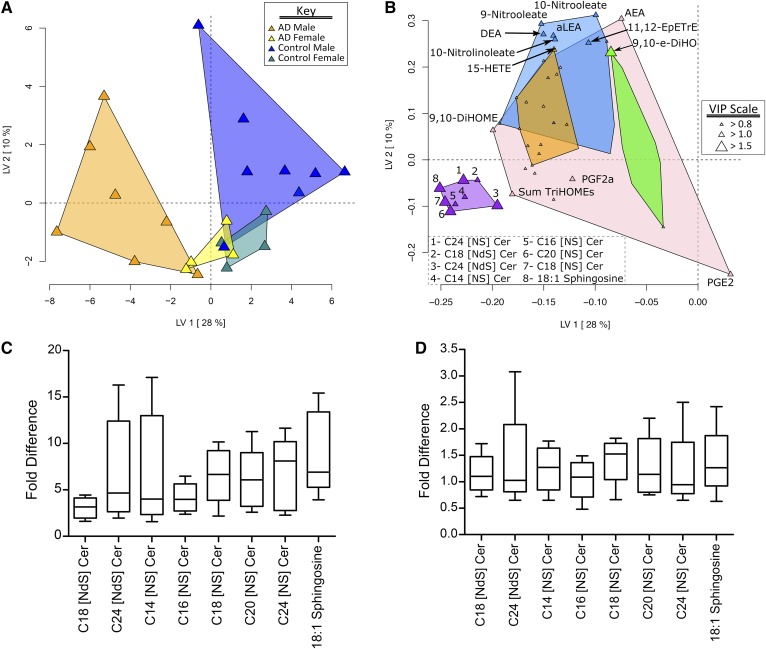
Fig. 2.
Eccrine sweat lipid mediator discrimination of men and women with and without AD. A: The PLS-DA scores plot showing AD group and gender discrimination. B: The PLS-DA loadings plot showing variable weight in discrimination. Point sizes are defined by the variable importance in projection (VIP) scores, with VIPs >1 considered significant factors in the discriminant model. Variables were grouped by their correlation (Spearman’s ρ) using a hierarchical cluster analysis with cluster identified by a unique color. Ceramides and sphingosine are elevated in subjects with AD. C: Fold-difference comparisons of lipid mediators increased in male subjects with AD relative to the average subject without AD. In men, AD caused a 2- to 6-fold elevation in ceramides and C18:1 sphingosine. D: Fold-difference comparisons of lipid mediators increased in female subjects with AD relative to the average subject without AD. In women, AD caused 1- to 1.5-fold higher concentrations of ceramides and C18:1 sphingosine. Descriptions of lipid mediator abbreviations can be found in the supplemental data.
Impact of sampling and storage on sweat lipid mediator profile
Lipid mediators discriminating between subjects with and without AD (i.e., ceramides and sphingoid bases) were unaffected by the sampling time, sampling site, or storage conditions used in this study. In particular, no differences were observed within each subject group (i.e., subjects with and without AD) when the data were grouped by time of collection (0900–1400 or 1400–1800), duration of storage at −80°C (0–19 days or 20–30 days), or arm sampled (right or left volar forearm) (P > 0.05) (supplemental Figs. S5–S7). Sampling and storage time impacted a few of the identified lipid mediators in subjects without AD (supplemental Table S5); however, these changes were excluded after false discovery rate correction for multiple comparisons (q = 0.05).
DISCUSSION
Sweat’s physiological role in heat dissipation is well-known, as is its basic chemical composition, i.e., water and salt. However, sweat also contains a wide array of other small molecules that can be influenced by disease states and environmental exposures, thus offering access to a potentially valuable and noninvasive biological sample. Lipid biochemistry produces a wide variety of bioactive molecules that have important regulatory roles in inflammation and cell growth, among other things, and many of these are produced by and found in the skin. This study represents an extensive characterization of bioactive lipid mediators in eccrine sweat. The only previous attempt was to quantify a single lipid mediator (PGE2) in sweat collected from the forearm following thermal stimulation of sweating (13). Interestingly, despite differences in sweat initiation, collection, and analysis between Forstrom, Goldyne, and Winkelman (13) and the present study, the concentrations of PGE2 in the sweat of healthy individuals (1.77 ± 1.22 nM and 2.19 ± 3.02 nM, respectively) were not different (P = 0.7) between the two studies. While it would be premature to suppose that sweat lipid mediator concentrations are unaffected by thermal versus pilocarpine iontophoretic sweat stimulation, these results are intriguing.
To explore the potential impact of a common skin disorder on sweat lipid mediator composition, we enrolled and evaluated men and women with either unflared AD or healthy skin. Subjects with AD showed elevated concentrations of [NS] and [NdS] ceramides and C18:1 sphingosine in their sweat. Increases in short-chain (30–40 total carbons) [NS], [AS], [NH], and [AH] ceramides have been previously reported in the stratum corneum of subjects with AD and have been correlated with an impairment of the epidermal barrier (25, 26). More recently, increases in C18:1 sphingosine have also been shown to be associated with increases in membrane permeability in an AD mouse model (27).
The observed increases in C18:1 sphingosine in the sweat of subjects with AD appear contrary to the reported decreases in C18:1 sphingosine in the epidermis of subjects with AD (28). However, given the antimicrobial action of C18:1 sphingosine (29), these decreases in subjects with AD are consistent with their increased susceptibility to epidermal colonization by bacteria, such as Staphylococcus aureus (28). If linked, it is interesting to speculate that the elevated sweat C18:1 sphingosine levels may represent a compensatory mechanism to transport this antimicrobial molecule to the skin surface.
The increases in short-chain [NS] ceramides appear to be at odds with the generally reported decrease in total skin ceramides with AD (30). However, ceramides as a class are complex molecules, consisting of 12 reported species and present in different proportions in the skin, as described in supplemental Fig. S8. Of these 12 ceramide species, 8 species (including phytosphingosine- and hydroxysphingosine-containing ceramides and ω-esterified ceramides) are believed to form and maintain the epidermal barrier and collectively constitute ∼80% of total skin ceramides (15). Concentrations of all of these eight species decrease in the stratum corneum of subjects with AD, consistent with a damaged epidermal barrier (25, 26). Of the remaining four ceramide species, [NS] and [NdS] ceramides collectively constitute ∼15% of total skin ceramides and appear to have functions related to keratinocyte signaling (15, 31). As mentioned previously, concentrations of short-chain [NS] and [NdS] ceramides have been shown to increase in the stratum corneum of subjects with AD, while concentrations of long-chain (>50 total carbons) [NS] and [NdS] ceramides have been shown to decrease in the stratum corneum of subjects with AD (25). In addition, increases in short-chain [NS] and [NdS] ceramides have also been correlated with an impaired epidermal barrier, and altered lipid organization in the stratum corneum due to these ceramide increases has been proposed as the mechanism for epidermal barrier impairment (32).
It has recently been reported that subjects with AD have increased expression of ceramide synthase 4 (CerS4) (33). CerS4 is responsible for the linking of a fatty acid chain to a sphingosine backbone to form [NdS] or [AdS] ceramides that are eventually desaturated to [NS] or [AS] ceramides and its substrates are typically C14–C26 fatty acids (34). Other members of the ceramide synthase family of enzymes are responsible for the formation of other ceramide classes and their activity has been well reviewed elsewhere (35). Given that C18:1 sphingosine is the most abundant sphingoid base in human skin (36), the action of CerS4 on some of its typical substrates would form C32–C44 [NdS] ceramides, which could be further desaturated to C32–C44 [NS] ceramides. As we have reported increases in C32–C42 [NS] ceramides and C36 and C42 [NdS] ceramides in the sweat of subjects with AD, it would appear that our findings are consistent with the reported increases in CerS4 activity in subjects with AD. It remains unclear why CerS4, in particular, is increased in subjects with AD, whereas no reports exist of other ceramide synthase expression levels being affected by AD (30). One possibility is that increased short-chain [NS] ceramide levels would induce increased keratinocyte differentiation as a compensatory mechanism to repair a damaged epidermal barrier, but further research would be necessary to verify this or other explanations for the differential regulation of ceramide synthases in AD.
Our discovery of gender-specific AD impacts on sweat ceramide concentrations was unexpected. Previous studies examining [NS] ceramides in AD did not consider gender-specific differences in [NS] ceramide concentrations (25, 26). While gender differences in total skin ceramides, with men having increased skin ceramide concentrations, have been reported by some (37), others have found no differences in either total or individual ceramide species when comparing men and women (38, 39). However, while the magnitude of change was greater in men, the vector of change was consistent between genders, with AD increasing in the short-chain ceramide and sphingosine concentration in the sweat, suggesting that these changes in [NS] and [NdS] ceramides and C18:1 sphingosine may be linked to the pathogenesis of AD.
To the best of our knowledge, no previous associations have been established between levels of 10-nitrooleate or 8-HETE and AD. However, increases in cutaneous 8-HETE concentrations have been reported in psoriatic scales (40) and in response to sunburn (41), and 8-HETE synthesis during skin wound repair has been observed in our laboratory (unpublished observations). The role of 8-HETE in cutaneous disease remains unclear, but it is believed that as a potent neutrophil chemoattractant, 8-HETE may promote cutaneous inflammation (41). On the other hand, 10-nitrooleate has been demonstrated to be a signaling molecule in cultured murine keratinocytes (42), and to reduce the severity of murine allergic airway disease, an atopic disorder similar to asthma, by suppressing inflammation and hypersensitivity (43). Therefore, it is possible that 8-HETE and 10-nitrooleate influence cutaneous inflammation and systemic atopy, and further testing of lesional AD skin may be necessary to establish an association between these lipid mediators and AD.
Other lipid mediators reportedly associated with AD include PGE2, PGD2, and the endocannabinoid, arachidonoylethanolamide (AEA) or anandamide, all of which have been implicated in acute cutaneous inflammation and immunomodulation (44–46). While PGE2 and AEA were quantified in the sweat of subjects with and without AD, concentration differences were not observed between the groups (P > 0.05). PGD2 was not detected. Given that the study subjects with AD were sampled in an unflared state and samples were obtained from nonlesional skin, these findings support the pathognomonic association of PGE2 and AEA with the inflammatory sequela of AD, rather than its pathogenesis.
There would therefore appear to be agreement between published cutaneous lipid mediator changes and those observed in sweat in the context of AD, particularly with respect to ceramides and sphingoid bases. It is interesting that these differences in ceramides and sphingoid bases usually correlated with epidermal barrier dysregulation were observed in nonlesional skin from subjects with unflared AD, given that an impaired epidermal barrier in AD is usually associated with active lesions or nonlesional skin adjacent to active lesions (47). Therefore, the sweat mediator lipidome appears to indicate a sustained biochemical dysregulation in subjects with AD and may provide an opportunity for preclinical diagnostics. This potential utility warrants further studies to assess the specificity and sensitivity of the observed changes by assessing the impact of other inflammatory skin conditions, such as psoriasis or acne vulgaris, on the sweat mediator lipidome. It may also be of interest to collect sweat from lesional and nonlesional skin of subjects with AD in the flared state in order to evaluate the impact of epidermal barrier dysregulation and/or inflammation on the sweat mediator lipidome.
While the source(s) of lipid mediators in sweat remain unidentified, both tissue production and plasma transdermal diffusion are feasible. Skin is a rich source of eicosanoids, and the epithelial cells of the sweat gland themselves are capable of synthesizing the endocannabinoids, AEA, and 2-AG (48). Moreover, small xenobiotics are believed to emerge in sweat by either transdermal migration or passive diffusion from blood (49), and the same may be true for lipid mediators. Ultimately, understanding the source of mediators found in the sweat will be important for the interpretation of the resulting data, and this will require additional focused studies in the future.
Overall, this study represents a broad characterization of the sweat mediator lipidome, demonstrating the presence of endocannabinoids, oxylipins, nitrolipids, [NS] and [NdS] ceramides, and sphingoid bases in eccrine sweat collected from the volar forearm. Additionally, this study demonstrates an apparently robust and novel noninvasive tool that could be used to support cutaneous research. Future studies will investigate the ability of sweat to report on the biochemical milieu of skin inflammation, directly compare the impact of physiologic versus pharmacologic sweat stimulation, and assess the variance in sweat composition associated with collection from other anatomical regions as we begin to scratch the surface of this novel and exciting addition to cutaneous research.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the assistance and advice provided by M. R. LaFrano, I. J. Gray, W. R. Keyes, and B. Rust during sample preparation and data analysis and the assistance provided by M. Notay during subject recruitment. The authors also gratefully acknowledge the donation of the Macroduct® sweat collection system by Wescor, Inc., an Elitech Company.
Footnotes
Abbreviations:
- AD
- atopic dermatitis
- AEA
- arachidonoyl ethanolamide (anandamide)
- AG
- arachidonoylglycerol
- CerS4
- ceramide synthase 4
- LG
- linoleoylglycerol
- OG
- oleoylglycerol
- PG
- prostaglandin
- PLS-DA
- partial least squares discriminant analysis
- UPLC
- ultra-performance LC
This study was supported by National Institute of General Medical Sciences Grant T32-GM008799 (K.A.), the University of California-Davis Medical Student Research Fellowship from School of Medicine, University of California, Davis (L.A.H.), US Department of Agriculture Intramural Project 2032-51530-022-00D, and National Institute of Diabetes and Digestive and Kidney Diseases Grant U24DK097154-01 (J.W.N.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the US Department of Agriculture. The US Department of Agriculture is an equal opportunity provider and employer. The authors declare that they have no financial conflicts of interest.
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Raju K. S., Taneja I., Singh S. P., and Wahajuddin. 2013. Utility of noninvasive biomatrices in pharmacokinetic studies. Biomed. Chromatogr. 27: 1354–1366. [DOI] [PubMed] [Google Scholar]
- 2.Mena-Bravo A., and Luque de Castro M. D.. 2014. Sweat: a sample with limited present applications and promising future in metabolomics. J. Pharm. Biomed. Anal. 90: 139–147. [DOI] [PubMed] [Google Scholar]
- 3.Gibson L. E., and Cooke R. E.. 1959. A test for concentration of electrolytes in sweat in cystic fibrosis of the pancreas utilizing pilocarpine by iontophoresis. Pediatrics. 23: 545–549. [PubMed] [Google Scholar]
- 4.De Giovanni N., and Fucci N.. 2013. The current status of sweat testing for drugs of abuse: a review. Curr. Med. Chem. 20: 545–561. [DOI] [PubMed] [Google Scholar]
- 5.Kutyshenko V. P., Molchanov M., Beskaravayny P., Uversky V. N., and Timchenko M. A.. 2011. Analyzing and mapping sweat metabolomics by high-resolution NMR spectroscopy. PLoS One. 6: e28824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ely M. R., Ely B. R., Chinevere T. D., Lacher C. P., Lukaski H. C., and Cheuvront S. N.. 2012. Evaluation of the megaduct sweat collector for mineral analysis. Physiol. Meas. 33: 385–394. [DOI] [PubMed] [Google Scholar]
- 7.Raiszadeh M. M., Ross M. M., Russo P. S., Schaepper M. A., Zhou W., Deng J., Ng D., Dickson A., Dickson C., Strom M., et al. 2012. Proteomic analysis of eccrine sweat: implications for the discovery of schizophrenia biomarker proteins. J. Proteome Res. 11: 2127–2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Csősz É., Emri G., Kalló G., Tsaprailis G., and Tőzsér J.. 2015. Highly abundant defense proteins in human sweat as revealed by targeted proteomics and label-free quantification mass spectrometry. J. Eur. Acad. Dermatol. Venereol. 29: 2024–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harker M., Coulson H., Fairweather I., Taylor D., and Daykin C. A.. 2006. Study of metabolite composition of eccrine sweat from healthy male and female human subjects by 1H NMR spectroscopy. Metabolomics. 2: 105–112. [Google Scholar]
- 10.Calderón-Santiago M., Priego-Capote F., Jurado-Gámez B., and Luque de Castro M. D.. 2014. Optimization study for metabolomics analysis of human sweat by liquid chromatography-tandem mass spectrometry in high resolution mode. J. Chromatogr. A. 1333: 70–78. [DOI] [PubMed] [Google Scholar]
- 11.Delgado-Povedano M. M., Calderón-Santiago M., Priego-Capote F., and Luque de Castro M. D.. 2016. Development of a method for enhancing metabolomics coverage of human sweat by gas chromatography-mass spectrometry in high resolution mode. Anal. Chim. Acta. 905: 115–125. [DOI] [PubMed] [Google Scholar]
- 12.Calderón-Santiago M., Priego-Capote F., Turck N., Robin X., Jurado-Gámez B., Sanchez J. C., and Luque de Castro M. D.. 2015. Human sweat metabolomics for lung cancer screening. Anal. Bioanal. Chem. 407: 5381–5392. [DOI] [PubMed] [Google Scholar]
- 13.Förström L., Goldyne M. E., and Winkelmann R. K.. 1974. Prostaglandin activity in human eccrine sweat. Prostaglandins. 7: 459–464. [DOI] [PubMed] [Google Scholar]
- 14.Murakami M. 2011. Lipid mediators in life science. Exp. Anim. 60: 7–20. [DOI] [PubMed] [Google Scholar]
- 15.van Smeden J., Janssens M., Gooris G. S., and Bouwstra J. A.. 2014. The important role of stratum corneum lipids for the cutaneous barrier function. Biochim. Biophys. Acta. 1841: 295–313. [DOI] [PubMed] [Google Scholar]
- 16.Kendall A. C., and Nicolaou A.. 2013. Bioactive lipid mediators in skin inflammation and immunity. Prog. Lipid Res. 52: 141–164. [DOI] [PubMed] [Google Scholar]
- 17.Bielawski J., Pierce J. S., Snider J., Rembiesa B., Szulc Z. M., and Bielawska A.. 2009. Comprehensive quantitative analysis of bioactive sphingolipids by high-performance liquid chromatography-tandem mass spectrometry. Methods Mol. Biol. 579: 443–467. [DOI] [PubMed] [Google Scholar]
- 18.Grapov D., Adams S. H., Pedersen T. L., Garvey W. T., and Newman J. W.. 2012. Type 2 diabetes associated changes in the plasma non-esterified fatty acids, oxylipins and endocannabinoids. PLoS One. 7: e48852 10.1371/journal.pone.0048852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dunn T. N., Keenan A. H., Thomas A. P., Newman J. W., and Adams S. H.. 2014. A diet containing a nonfat dry milk matrix significantly alters systemic oxylipins and the endocannabinoid 2-arachidonoylglycerol (2-AG) in diet-induced obese mice. Nutr. Metab. (Lond.). 11: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grapov D., and Newman J. W.. 2012. imDEV: a graphical user interface to R multivariate analysis tools in Microsoft Excel. Bioinformatics. 28: 2288–2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grubbs F. E. 1950. Sample criteria for testing outlying observations. Ann. Math. Statist. 21: 27–58. [Google Scholar]
- 22.Wang C., and Wang W.. 2006. Links between PPCA and subspace methods for complete Gaussian density estimation. IEEE Trans. Neural Netw. 17: 789–792. [DOI] [PubMed] [Google Scholar]
- 23.Box G. E. P., and Cox D. R.. 1964. An analysis of transformations (with discussion). J. Roy. Statist. Soc. Ser.B. 26: 211–252. [Google Scholar]
- 24.Xia J., Sinelnikov I. V., Han B., and Wishart D. S.. 2015. MetaboAnalyst 3.0–making metabolomics more meaningful. Nucleic Acids Res. 43: W251–W257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ishikawa J., Narita H., Kondo N., Hotta M., Takagi Y., Masukawa Y., Kitahara T., Takema Y., Koyano S., Yamazaki S., et al. 2010. Changes in the ceramide profile of atopic dermatitis patients. J. Invest. Dermatol. 130: 2511–2514. [DOI] [PubMed] [Google Scholar]
- 26.Janssens M., van Smeden J., Gooris G. S., Bras W., Portale G., Caspers P. J., Vreeken R. J., Hankemeier T., Kezic S., Wolterbeek R., et al. 2012. Increase in short-chain ceramides correlates with an altered lipid organization and decreased barrier function in atopic eczema patients. J. Lipid Res. 53: 2755–2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loiseau N., Obata Y., Moradian S., Sano H., Yoshino S., Aburai K., Takayama K., Sakamoto K., Holleran W. M., Elias P. M., et al. 2013. Altered sphingoid base profiles predict compromised membrane structure and permeability in atopic dermatitis. J. Dermatol. Sci. 72: 296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arikawa J., Ishibashi M., Kawashima M., Takagi Y., Ichikawa Y., and Imokawa G.. 2002. Decreased levels of sphingosine, a natural antimicrobial agent, may be associated with vulnerability of the stratum corneum from patients with atopic dermatitis to colonization by Staphylococcus aureus. J. Invest. Dermatol. 119: 433–439. [DOI] [PubMed] [Google Scholar]
- 29.Bibel D. J., Aly R., and Shinefield H. R.. 1992. Antimicrobial activity of sphingosines. J. Invest. Dermatol. 98: 269–273. [DOI] [PubMed] [Google Scholar]
- 30.Imokawa G., and Ishida K.. 2014. Role of ceramide in the barrier function of the stratum corneum, implications for the pathogenesis of atopic dermatitis. J. Clin. Exp. Dermatol. Res. 5: 1000206. [Google Scholar]
- 31.Geilen C. C., Wieder T., and Orfanos C. E.. 1997. Ceramide signalling: regulatory role in cell proliferation, differentiation and apoptosis in human epidermis. Arch. Dermatol. Res. 289: 559–566. [DOI] [PubMed] [Google Scholar]
- 32.Mojumdar E. H., Kariman Z., van Kerckhove L., Gooris G. S., and Bouwstra J. A.. 2014. The role of ceramide chain length distribution on the barrier properties of the skin lipid membranes. Biochim. Biophys. Acta. 1838: 2473–2483. [DOI] [PubMed] [Google Scholar]
- 33.Ito S., Ishikawa J., Naoe A., Yoshida H., Hachiya A., Fujimura T., Kitahara T., and Takema Y.. Ceramide synthase 4 is highly expressed in involved skin of patients with atopic dermatitis. J. Eur. Acad. Dermatol. Venereol. Epub ahead of print. June 29, 2016; doi:10.1111/jdv.13777. [DOI] [PubMed] [Google Scholar]
- 34.Mizutani Y., Kihara A., and Igarashi Y.. 2005. Mammalian LASS6 and its related family members regulate synthesis of specific ceramides. Biochem. J. 390: 263–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rabionet M., Gorgas K., and Sandhoff R.. 2014. Ceramide synthesis in the epidermis. Biochim. Biophys. Acta. 1841: 422–434. [DOI] [PubMed] [Google Scholar]
- 36.Stewart M. E., and Downing D. T.. 1995. Free sphingosines of human skin include 6-hydroxysphingosine and unusually long-chain dihydrosphingosines. J. Invest. Dermatol. 105: 613–618. [DOI] [PubMed] [Google Scholar]
- 37.De Paepe K., Weerheim A., Houben E., Roseeuw D., Ponec M., and Rogiers V.. 2004. Analysis of epidermal lipids of the healthy human skin: factors affecting the design of a control population. Skin Pharmacol. Physiol. 17: 23–30. [DOI] [PubMed] [Google Scholar]
- 38.Denda M., Koyama J., Hori J., Horii I., Takahashi M., Hara M., and Tagami H.. 1993. Age- and sex-dependent change in stratum corneum sphingolipids. Arch. Dermatol. Res. 285: 415–417. [DOI] [PubMed] [Google Scholar]
- 39.Mutanu Jungersted J., Hellgren L. I., Hogh J. K., Drachmann T., Jemec G. B., and Agner T.. 2010. Ceramides and barrier function in healthy skin. Acta Derm. Venereol. 90: 350–353. [DOI] [PubMed] [Google Scholar]
- 40.Camp R. D. R., Mallet A. I., Woollard P. M., Brain S. D., Kobza Black A., and Greaves M. W.. 1983. The identification of hydroxy fatty acids in psoriatic skin. Prostaglandins. 26: 431–447. [DOI] [PubMed] [Google Scholar]
- 41.Rhodes L. E., Gledhill K., Masoodi M., Haylett A. K., Brownrigg M., Thody A. J., Tobin D. J., and Nicolaou A.. 2009. The sunburn response in human skin is characterized by sequential eicosanoid profiles that may mediate its early and late phases. FASEB J. 23: 3947–3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zheng R., Heck D. E., Black A. T., Gow A., Laskin D. L., and Laskin J. D.. 2014. Regulation of keratinocyte expression of stress proteins and antioxidants by the electrophilic nitrofatty acids 9- and 10-nitrooleic acid. Free Radic. Biol. Med. 67: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reddy A. T., Lakshmi S. P., Dornadula S., Pinni S., Rampa D. R., and Reddy R. C.. 2013. The nitrated fatty acid 10-nitro-oleate attenuates allergic airway disease. J. Immunol. 191: 2053–2063. [DOI] [PubMed] [Google Scholar]
- 44.De Filippis D., D’Amico A., and Iuvone T.. 2008. Cannabinomimetic control of mast cell mediator release: new perspective in chronic inflammation. J. Neuroendocrinol. 20: 20–25. [DOI] [PubMed] [Google Scholar]
- 45.Boehme S. A., Chen E. P., Franz-Bacon K., Šášik R., Sprague L. J., Ly T. W., Hardiman G., and Bacon K. B.. 2009. Antagonism of CRTH2 ameliorates chronic epicutaneous sensitization-induced inflammation by multiple mechanisms. Int. Immunol. 21: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McIlroy A., Caron G., Blanchard S., Frémaux I., Duluc D., Delneste Y., Chevailler A., and Jeannin P.. 2006. Histamine and prostaglandin E2 up-regulate the production of Th2-attracting chemokines (CCL17 and CCL22) and down-regulate IFN-γ-induced CXCL10 production by immature human dendritic cells. Immunology. 117: 507–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hata M., Tokura Y., Takigawa M., Sato M., Shioya Y., Fujikura Y., and Imokawa G.. 2002. Assessment of epidermal barrier function by photoacoustic spectrometry in relation to its importance in the pathogenesis of atopic dermatitis. Lab. Invest. 82: 1451–1461. [DOI] [PubMed] [Google Scholar]
- 48.Czifra G., Szöllősi A. G., Tóth B. I., Demaude J., Bouez C., Breton L., and Bíró T.. 2012. Endocannabinoids regulate growth and survival of human eccrine sweat gland-derived epithelial cells. J. Invest. Dermatol. 132: 1967–1976. [DOI] [PubMed] [Google Scholar]
- 49.Cone E. J., Hillsgrove M. J., Jenkins A. J., Keenan R. M., and Darwin W. D.. 1994. Sweat testing for heroin, cocaine, and metabolites. J. Anal. Toxicol. 18: 298–305. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.