Abstract
A rather new approach in the treatment of long-chain fatty acid oxidation disorders is represented by triheptanoin, a triglyceride with three medium-odd-chain heptanoic acids (C7), due to its anaplerotic potential. We here investigate the effects of a 1-year triheptanoin-based diet on the clinical phenotype of very long-chain-acyl-CoA-dehydrogenase-deficient (VLCAD−/−) mice. The cardiac function was assessed in VLCAD−/− mice by in vivo MRI. Metabolic adaptations were identified by the expression of genes regulating energy metabolism and anaplerotic processes using real-time PCR, and the results were correlated with the measurement of the glycolytic enzymes pyruvate dehydrogenase and pyruvate kinase. Finally, the intrahepatic lipid accumulation and oxidative stress in response to the long-term triheptanoin diet were assessed. Triheptanoin was not able to prevent the development of systolic dysfunction in VLCAD−/− mice despite an upregulation of cardiac glucose oxidation. Strikingly, the anaplerotic effects of triheptanoin were restricted to the liver. Despite this, the hepatic lipic content was increased upon triheptanoin supplementation. Our data demonstrate that the concept of anaplerosis does not apply to all tissues equally.
Keywords: anaplerosis, VLCAD deficiency, cardiac function
Mitochondrial β-oxidation is essential for energy production from fat. Deficiency of one of the enzymes involved is associated with life-threatening events and death. Very-long chain acyl-CoA dehydrogenase (VLCAD) deficiency (OMIM 609575) is the second most common disorder of fatty acid oxidation in Europe and the USA, with an incidence of 1:25,000–1:100,000 newborns (1–4). Pathophysiological mechanisms responsible for the development of symptoms include i) severe energy deficiency due to a deficient fatty acid oxidation and subsequent impairment of ketone body biosynthesis and ii) accumulation of toxic long-chain acylcarnitines. Therefore, catabolic situations in which the organism mainly relies on fatty acid oxidation induce symptoms and severe metabolic derangement. The clinical phenotype is very heterogeneous and presents with different severity and age of onset (3), involving organs and tissues that mostly rely on fatty acid β-oxidation for energy production. To date, treatment recommendations (5) include a long-chain fat-restricted and fat-modified diet in which long-chain fatty acids are fully or in part replaced by medium-chain triglycerides (MCTs) (1, 5) and the avoidance of prolonged fasting. In contrast to long-chain fatty acids, medium-chain fatty acids (MCFAs) are oxidized by medium-chain acyl-CoA dehydrogenase, bypassing VLCAD; therefore, MCTs may be fully metabolized, supplying the organism with the required energy. Many reports confirm the effectiveness of MCT application in the treatment of cardiomyopathy in long-chain fatty acid oxidation disorders (FAODs) (6–9). In patients with exercise-induced muscle pain, the application of an MCT bolus immediately prior to physical exercise has been proven effective (1, 5). Our own studies on the VLCAD-deficient (VLCAD−/−) mouse showed the beneficial effects of MCTs when applied during increased demand (10). Although an MCT diet is considered to be a safe dietary intervention and is applied in different FAODs for longer periods of time, recent reports highlight the adverse effects of an MCT diet in the murine model of VLCAD deficiency (11–14). A rather new therapeutic approach for the treatment of FAODs is represented by the application of MCTs in the form of triheptanoin, a triglyceride with three medium odd-chain heptanoic acids (C7). The rationale behind the application of triheptanoin is that it has a potential anaplerotic effect, supplying the citric acid cycle (CAC) with the required substrates (15). Our study on the long-term effects of triheptanoin supplementation in VLCAD−/− mice showed that, similarly to MCTs, this diet strongly stimulates lipogenesis, resulting in a disturbed fatty acid composition of plasma membranes in liver, heart, and skeletal muscle (16). Whether triheptanoin is able to better supply all tissues with the required energy by additionally replenishing the CAC with substrates is unknown. An FDA phase 2 clinical trial with double blind comparison of physiologic effects of MCT oil versus triheptanoin in patients with long-chain FAOD is ongoing. However, in patients it is not possible to evaluate metabolic adaptations in tissues in response to dietary modifications over a long period of time. Here we explored how a triheptanoin-based diet, applied as a regular diet long term and not to correct acute metabolic decompensation, affects tissue metabolism in mice. We measured biochemical parameters such as blood lipids, glucose, insulin, and FFAs in serum and assessed cardiac morphology and function by in vivo MRI. The data were complemented by the evaluation of the cardiac metabolic adaptation in response to triheptanoin. Furthermore, lipid accumulation and markers of oxidative stress were examined in the liver in addition to anaplerotic effects in heart and liver.
MATERIALS AND METHODS
Animals
Experiments were performed on intercrosses of C57BL6+129sv VLCAD genotypes. Littermates served as controls, and genotyping of mice was performed as described previously by Exil et al. (17). Serum parameters were determined under standard conditions. Blood from mice at the age of 3 and 12 months was drawn after 5 h of food withdrawal. Mice were euthanized by CO2 asphyxiation.
Blood samples were collected by heart puncture. Serum was obtained by centrifugation at 16,000 g for 10 min and stored at −80°C for further analysis. Tissues were rapidly removed and immediately frozen in liquid nitrogen.
All animal studies were performed with the approval of the University’s Institutional Animal Care and Use Committee and in accordance with the Committees’ guidelines (approval number: 35-9185.81/G-14/20).
Diet composition and supplementation
At 5 weeks of age, mice of each genotype were divided in two groups and fed with different diets for 1 year. The first group received a normal purified mouse diet containing 5% crude fat in the form of long-chain TGs, corresponding to 12% of metabolizable energy as calculated with Atwater factors (ssniff® EF R/M Control; ssniff Spezialdiäten GmbH, Soest, Germany). The treatment recommendation for long-chain fatty acid oxidation diseases includes a strict fat-modified diet in which the normal long-chain fatty acids are completely replaced by even- and medium-chain MCTs (C8 and C10 chains) (1, 18). To evaluate the effects of triheptanoin, which is an odd medium-chain triglyceride (C7 chain), we prepared a strict diet in which triheptanoin fully replaced the normal long-chain fatty acids, with the exception of the essential fatty acids. Therefore, the second group was fed a diet corresponding as well to 12% of total metabolizable energy. Here, 4.4% from a total of 5% fat was Triheptanoin (CREMER OLEO GmbH and Co. KG, basis GmbH, Hamburg, Germany), and the remaining 0.6% was derived from soybean oil, providing the required essential long-chain fatty acids. The necessary amount of essential long-chain fatty acids was calculated in accordance to the Nutrient Requirements of Laboratory Animals (Subcommittee on Laboratory Animal Nutrition, Committee on Animal Nutrition, Board on Agriculture, National Research Council). Both diets were based on purified feed ingredients and contained the same nutrient concentration as follows: 94.8% dry matter, 17.8% crude protein (N × 6.25), 5% crude fat, 5% crude fiber, 5.3% crude ash, 61.9% nitrogen-free extract, 36.8% starch, 14.8% dextrin, and 11% sugar. The detailed fatty acid composition of the diets is reported in supplementary Table S1. In both diets, the carbohydrate and protein contents corresponded to 69% and 19% of metabolizable energy, respectively. The mice were fed control diet or triheptanoin-based diet either over 5 weeks or over 12 months. All mouse groups received water ad libitum.
MRI
Data were recorded on an AvanceIII 9.4 Tesla Wide Bore (89 mm) nuclear magnetic resonance spectrometer (Bruker, Billerica, MA) operating at frequencies of 400.13 MHz for 1H as previously described (11, 19).
Protein homogenates
Tissues were homogenized in CelLytic MT Buffer (Sigma-Aldrich, Steinheim, Germany) in the presence of 1 mg/ml protease inhibitors and centrifuged at 4°C and 16,000 g for 10 min to pelletize any cell debris. The clear supernatant was immediately used for the enzyme assays or stored at –80°C.
Enzyme activities
Pyruvate kinase (PK) activity was performed in duplicate using the Pyruvate Kinase Assay Kit (BioVision, Mountain View, CA) as recommended by the manufacturer. Pyruvate dehydrogenase (PDH) assay was measured using the PDH Assay Kit (Abcam, Cambridge, UK) following the manufacturer’s protocol. α-Ketoglutarate-CoA dehydrogenase (αKGDH) activity was assayed by monitoring the rate of NAD+ reduction at 340 nm upon addition of 5.0 mM MgCl2, 40 μM rotenone, 2.5 mM α-ketoglutarate, 0.1 mM CoA, 0.2 mM thymine pyrophosphate, and 1.0 mM NAD to protein homogenate as previously described (20). The measurement of citrate synthase is performed by assessing the reduction of 5,5′-dithiobis-nitrobenzoic acid in the presence of acetyl-CoA and oxaloacetate (21) and after the reduction of this product at 412 nm at 30°C for 5 min. GSH was measured in liver homogenates by using an enzymatic kit (Glutathione Assay Kit; Bio Trend, Cologne, Germany). Measurement of protein carbonylation was performed with the Protein Carbonylation Assay Kit (Cayman Chemical, Ann Arbor, MI). Glutathione peroxidase activity was determined by calculating the oxidation rate of NADPH to NADP+ spectrophotometrically at 340 nm for 4 min as previously described (22, 23).
Analysis of serum variables and HOMA Index
FFA, triacylglycerides (TAGs), and lipoprotein concentrations were measured in duplicate in serum samples as described previously (13). Glucose and ketone bodies were determined with a Precision Xceed blood sugar meter (Abbott, Wiesbaden, Germany). The enzymatic method used for detection of C4 ketone bodies did not allow the detection of C5 species.
Insulin was measured in duplicate by using the Ultrasensitive Mouse Insulin ELISA Kit (Mercodia AB, Uppsala Sweden). Insulin resistance was calculated by the homeostasis model assessment (HOMA) formula (24). The HOMA of insulin resistance index, as described by Matthews et al. (24), is the most easily obtained measurement of insulin resistance and can be used as a reliable surrogate measure of in vivo insulin sensitivity because this method correctly differentiates between insulin sensitivity and insulin resistance (25). HOMA Index was calculated with glucose and insulin concentrations obtained after 5 h of fasting using the following formula: fasting blood glucose (md/dl) × fasting insulin (µU/ml)/22.5.
Lipoprotein concentrations were measured in duplicate in serum samples by using enzymatic kits (EnzyChrom HDL and VLDL/LDL Assay kit; BioTrend, Cologne, Germany) on an Infinite M200 Tecan (Crailsheim, Germany) plate reader.
Intrahepatic lipid content, fatty acid, and thiobarbituric acid reactive substances analysis
The intrahepatic lipid content was measured gravimetrically according to a method by Folch et al. (26) and was modified as previously reported (14). Fatty acid profiles have been analyzed as previously described (16). Thiobarbituric acid reactive substances have been analyzed as previously reported (11).
Analysis of acylcarnitines in dried blood spots
Analysis of acylcarnitines was performed as described previously (10, 27). Briefly, acylcarnitines were extracted from dried blood spots and tissues with acetonitrile/water (80/20% v/v) in the presence of [2H3]-free carnitine, [2H3] octanoyl-carnitine, and [2H3] palmitoyl-carnitine as internal standards. The extracted supernatant was dried, and the butylated acylcarnitines were analyzed by ESI-MS/MS. All even- and odd-chain C0–C19 acylcarnitines (saturated and unsaturated) were measured.
Western blot analysis
Protein expression in the different tissues was performed by Western blot analysis. Protein homogenate (20–40 µg) from tissue lysate was separated on a gradient (4–12%) SDS polyacrylamide gel and transferred to nitrocellulose. Detection was carried out with anti-propionyl-CoA carboxylase antibody (monoclonal mouse; Santa Cruz Biotechnology, Dallas, TX), anti-αKGDH antibody (polyclonal rabbit; Abcam), and anti-succinyl-CoA synthetase (SCoA) antibody (polyclonal rabbit; Cell Signaling, Danvers, MA) used at a dilution 1:1,000–1:2,000. Anti-GAPDH and anti-actin were used as a loading control at 1:4,000. HRP-conjugated secondary antibodies were used at 1:5,000. Samples have been analyzed as pool (n = 10–12). Signals were detected and quantified Fusion FX Analyzer (Peqlab, Erlangen, Germany).
RT-PCR analysis
Total RNA from heart was isolated with the RNeasy mini kit (Qiagen, Hilden, Germany). Forward and reverse primers were designed with the Primer Design Tool from NCBI (http://www.ncbi.nlm.nih.gov/tools). Gene function and primer sequences are reported in supplementary Table S2. RT-PCR was performed in a single-step procedure with the iTaq™ Universal SYBR® Green Supermix (Biorad, München, Germany) on a CFX96 Touch (Biorad). Gene coding for the 18S ribosomal subunit was used as reference.
Statistical analysis
MRI data are reported as means ± SD. All other reported data are presented as means ± SEM; n denotes the number of animals tested. Analysis for the significance of differences was performed using Student’s t-tests for paired and unpaired data. To test the effects of the variables, diet and genotype two-way ANOVA with Bonferroni posttest was performed (GraphPad Prism 5.0; GraphPad Software, San Diego, CA). Differences were considered significant if p < 0.05.
RESULTS
Clinical phenotype and blood lipids
We did not observe genotype- or diet-dependent effects on the body weight of mice after either short- or long-term supplementation (Table 1).
TABLE 1.
Clinical parameters in WT and VLCAD−/− mice either under control or triheptanoin diet at the age of 3 and 12 months
| Age | WT | VLCAD−/− | |||
| Control | C7 | Control | C7 | ||
| Clinical phenotype | 3 months | ||||
| Body weight (g) | 21.6 ± 0.4 | 23.1 ± 1.2 | 22.7 ± 0.8 | 23 ± 0.9 | |
| Serum lipids | |||||
| FFA (µM) | 317 ± 43 | 369 ± 47 | 382 ± 26 | 377 ± 35 | |
| TAG (mg/dl) | 26.3 ± 5.6 | 33.4 ± 4.5 | 52.2 ± 6.6a | 66.1 ± 16.1a | |
| Cholesterol total (mg/dl) | 58.2 ± 5.6 | 63.6 ± 6 | 78.7 ± 5.2a | 87 ± 4.5a | |
| HDL (mg/dl) | 35.7 ± 3 | 39.8 ± 3.7 | 55.2 ± 3.3a | 65.3 ± 4.6a | |
| VLDL/LDL (mg/dl) | 14.6 ± 1 | 17.1 ± 1.7 | 18.8 ± 0.9 | 21.7 ± 1.8 | |
| Serum variables | |||||
| Glucose (mg/dl) | 217 ± 19 | 218 ± 25 | 245 ± 11b | 301 ± 13a | |
| Insulin (pmol/L) | 107 ± 11 | 106 ± 16 | 72 ± 9 | 105 ± 18b | |
| HOMA Index | 8.2 ± 1.1 | 8.3 ± 1.9 | 6.2 ± 0.8 | 11.2 ± 2b | |
| Ketone bodies C4 (mmol/L) | 0.77 ± 0.1 | 1.07 ± 0.2 | 1.26 ± 0.1a | 0.95 ± 0.1 | |
| Clinical phenotype | 12 months | ||||
| Body weight (g) | 27.6 ± 2.5 | 28.6 ± 3.7 | 27.4 ± 2.9 | 30.1 ± 4.4 | |
| Serum lipids | |||||
| FFA (µM) | 277 ± 14 | 319 ± 36 | 308 ± 29 | 260 ± 22 | |
| TAG (mg/dl) | 19.6 ± 1.8 | 29.6 ± 4.2b | 37.2 ± 3.2a | 40.2 ± 3.2a | |
| Cholesterol total (mg/dl) | 69.3 ± 5.3 | 78.4 ± 4.3 | 84.5 ± 6.5a | 76.3 ± 8.9 | |
| HDL, mg/dl | 59.5 ± 5.6 | 62.6 ± 5.3 | 67.5 ± 4.5 | 63.4 ± 8.6 | |
| VLDL/LDL (mg/dl) | 27.4 ± 2.4 | 23.4 ± 1.5 | 25.7 ± 2.3 | 24.2 ± 2.3 | |
| Serum variables | |||||
| Glucose (mg/dl) | 196 ± 22 | 213 ± 22 | 256 ± 29 | 308 ± 16ab | |
| Insulin (pmol/L) | 104 ± 15 | 154 ± 19b | 184 ± 25a | 162 ± 16 | |
| HOMA Index | 7.5 ± 1.7 | 12.2 ± 2.6b | 9.8 ± 1.5 | 18 ± 1.8ab | |
| Ketone bodies (mmol/L) | 1.19 ± 0.1 | 0.63 ± 0.09b | 1.25 ± 0.10 | 0.78 ± 0.05b | |
Values are mean ± SEM (n = 10–12).
Significant difference (P < 0.05, two-way ANOVA and Student’s t-test) between WT and VLCAD−/− mice under the same dietary regimen.
Significant differences (P < 0.05, two-way ANOVA and Student’s t-test) between mice of the same genotype under different dietary regimen.
Blood lipid analysis revealed no alteration due to a triheptanoin diet, with the exception of the serum TAGs and total cholesterol. In line with previous reports (11, 14), in mice at the age of 3 months, serum TAGs (52.2 ± 6.6 vs. 26.3 ± 5.6 mg/dl) and total cholesterol content (78.7 ± 5.2 vs 58.2 ± 5.6 mg/dl) were significantly higher in mutants as compared with WT littermates. Long-term treatment did not substantially alter serum and cholesterol levels. However, in WT mice on the triheptanoin diet, the concentration of serum TAGs was significantly increased, as compared with WT under control diet (29.6 ± 4.21 vs. 19.6 ± 1.82 mg/dl).
Triheptanoin affects glucose concentration and HOMA Index
As reported previously (28), mice on the control diet VLCAD−/− did not display different glucose concentrations as compared with WT mice at the age of 3 or 12 months (Table 1). Three-month-old mutant mice on the triheptanoin diet showed significantly higher glucose values as compared with VLCAD−/− mice on the control diet (301 ± 23 vs. 218 ± 25 mg/dl). Regarding insulin levels, we observed significantly higher insulin concentrations in triheptanoin-treated mutants as compared with mutant mice on the control diet, as also reflected by the significantly higher HOMA Index (11.2 ± 2 vs. 8.3 ± 1.9 mg/dl). After 1 year of triheptanoin supplementation, WT mice displayed a marked increase of insulin concentration and a nearly a 2-fold higher HOMA Index (Table 1).
Analysis of C4 ketone bodies species showed a significantly higher content in VLCAD−/− mice under control diet as compared with mutants treated with triheptanoin. Interestingly, at the age of 1 year the concentration of these metabolites was significantly lower in both genotypes in triheptanoin-treated mice (Table 1).
Triheptanoin diet does not prevent the gradual development of cardiomyopathy in VLCAD−/− mice
Previous studies showed that VLCAD−/− mice at the age of 3 months present a normal cardiac function that progressively degenerates in an age-dependent manner due to chronic energy deficiency (29). In accordance with these findings, the analysis of cardiac function by cine 1H MRI showed the development of a systolic dysfunction in VLCAD−/− mice at the age of 12 months (Table 2). Indeed, VLCAD−/− mice displayed a significantly increased end-systolic volume (ESV), resulting in a pronounced reduction of ejection fraction as compared with WT mice (62.1 ± 7.8% vs. 73.3 ± 8.1%) (Table 2). Planimetry of the myocardial walls also revealed a significant reduction in systolic wall thickness in VLCAD−/− mice as compared with WT (1.52 ± 0.14 vs. 1.72 ± 0.28 mm), corroborating the finding of systolic dysfunction.
TABLE 2.
Characterization of cardiac function by MRI in WT and VLCAD−/− mice either under control or triheptanoin diet
| WT | VLCAD−/− | |||
| Control | C7 | Control | C7 | |
| Heart rate (bpm) | 579 ± 70 | 440 ± 31 | 569 ± 50 | 470 ± 32 |
| Cardiac output (ml/min) | 23.7 ± 3.5 | 17.7 ± 2.1 | 21.5 ± 3.7 | 19 ± 2.9 |
| End-diastolic volume (µl) | 57 ± 11.1 | 54 ± 10.2 | 61.8 ± 12 | 66.5 ± 19.3 |
| End-systolic volume (µl) | 15.8 ± 6.6 | 13.6 ± 4.3 | 24.1 ± 8.7a | 25.7 ± 12.33a |
| Stroke volume (µl) | 41.2 ± 5.7 | 40.4 ± 6 | 37.7 ± 5.1 | 40.8 ± 7 |
| Ejection fraction (%) | 73.3 ± 8.1 | 75.2 ± 3.1 | 62.1 ± 7.8a | 63.4 ± 7.7a |
| Left ventricular mass (mg) | 140.4 ± 11 | 109.7 ± 37.4b | 136.1 ± 10.9 | 146.8 ± 29.4a |
| Heart/body weight ratio | 4.45 ± 0.4 | 3.96 ± 1.3 | 4.67 ± 0.25 | 4.87 ± 0.6 |
| Wall thickness, diastole (mm) | 1.04 ± 0.11 | 0.94 ± 0.13 | 1.09 ± 0.08 | 1.08 ± 0.1 |
| Wall thickness, systole (mm) | 1.72 ± 0.28 | 1.53 ± 0.21 | 1.52 ± 0.14a | 1.53 ± 0.12 |
Values are mean ± SD (n = 10–12).
Significant differences (P < 0.05, two-way ANOVA and Student’s t-test) between WT and VLCAD−/− mice under the same dietary regimen.
Significant differences (P < 0.05, two-way ANOVA and Student’s t-test) between mice of the same genotype under different dietary regimen.
Triheptanoin provides substrates for the CAC and as consequence redox equivalents for energy production via oxidative phosphorylation. We investigated whether a long-term triheptanoin diet could prevent the development of the systolic dysfunction. Surprisingly, this diet neither improved nor aggravated the observed phenotype. Although the ESV in VLCAD−/− mice on the triheptanoin diet was significantly increased as compared with WT mice under the same dietary conditions, we could not observe a further increase or a decrease of the ESV as compared with mutants fed the control diet. The ejection fraction was also not affected by triheptanoin (63.4 ± 7.7% vs. 62.1 ± 7.8%) (Table 2).
Triheptanoin impairs cardiac energy metabolism and upregulates glucose oxidation
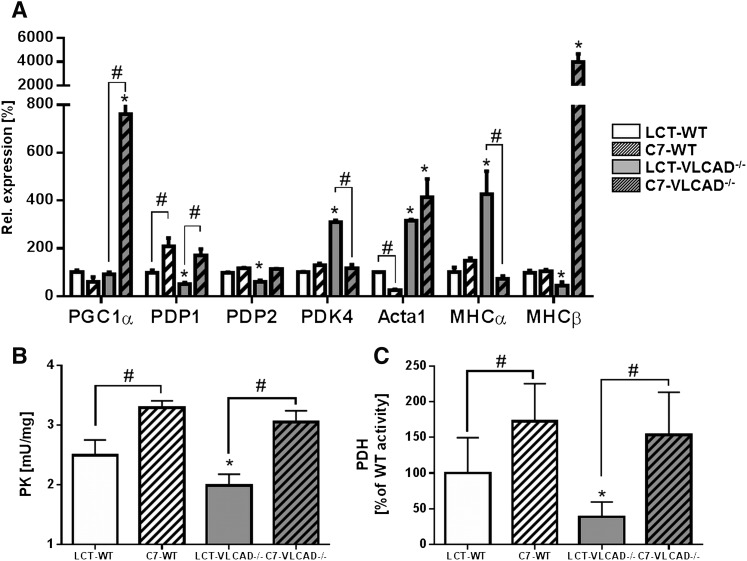
Because triheptanoin did not affect cardiac function, we measured the expression of genes involved in cardiac energy metabolism. VLCAD−/− mice fed the triheptanoin diet displayed a significant upregulation of PGC1α as reported in situations of increased energy demand (30) (Fig. 1A). This was accompanied by an upregulation of the pyruvate dehydrogenase phosphatase (PDP1), which activates PDH, thereby stimulating the glucose oxidation pathway, whereas the pyruvate kinase 4 was unaffected. Because this protein inhibits PDH by phosphorylation, we proposed stimulation of glycolysis and glucose oxidation to compensate for a defective fatty acid oxidation machinery. The specific activities of PK and PDH were significantly increased on triheptanoin in both genotypes, with a more prominent effect in the mutants (Fig. 1B, C). With triheptanoin, the activity of PK significantly increased from 1.98 ± 0.19 to 3.15 ± 0.17 mU/mg (Fig. 1B, C). Similarly, the turnover rate of PDH in supplemented VLCAD−/− mice was nearly four times higher as compared with mutants fed the control diet (153.7 ± 59.8% of WT vs. 38.8 ± 20.8% of WT). However, the expression of genes typically expressed in developing hearts and downregulated in adult organs known as genes of the fetal gene program (i.e., Acta1, MHCα, and MHCβ) showed a typical expression profile detected during cardiac metabolic derangement (Fig. 1A) (31).
Fig. 1.
Impaired cardiac energy metabolism and upregulation of glucose oxidation upon triheptanoin. A: Expression of genes involved in cardiac energy metabolism and glucose oxidation in mice at the age of 12 months. B and C: Specific enzyme activities of PK and PDH, respectively. Values are means ± SEM (n = 10–12). *Significant differences (P < 0.05, two-way ANOVA and Student’s t-test) between WT and VLCAD−/− mice under the same dietary regimen. #Significant differences (P < 0.05, two-way ANOVA and Student’s t-test) between mice of the same genotype under different dietary regimen. LCT, long-chain triglyceride.
Effects of triheptanoin on intrahepatic lipid accumulation and oxidative stress
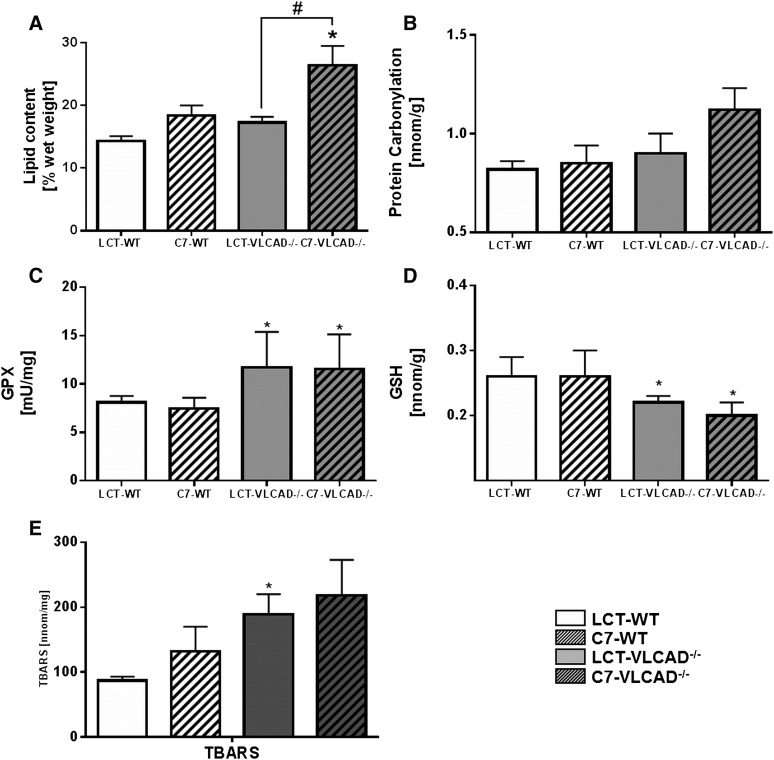
A previous study on the effects of even MCTs showed that they induce steatohepatitis when supplemented for 1 year (11). To test whether triheptanoin displays similar effects, we quantified gravimetrically the intrahepatic lipid content in mice at the age of 1 year. Triheptanoin resulted in a significant accumulation of lipids as compared with VLCAD−/− mice fed the control diet (26.4 ± 3.1% vs. 17.3 ± 0.9%) (Fig. 2A).
Fig. 2.
Intrahepatic lipid accumulation and oxidative stress in mice under either control or triheptanoin diet. A: Intrahepatic lipid accumulation. B: Protein carbonylation. C: GPX. D: GSH. E: TBARSs. Values are means ± SEM (n = 18–20). *Significant differences (P < 0.05, two-way ANOVA and Student’s t-test) between WT and VLCAD−/− mice under the same dietary regimen. #Significant differences (P < 0.05, two-way ANOVA and Student’s t-test) between mice of the same genotype under different dietary regimen. LCT, long-chain triglyceride.
Oxidative stress, which often accompanies intrahepatic lipid accumulation (32), was verified by the presence of established markers. We did not observe significant differences in protein carbonylation in VLCAD−/− mice fed the control diet as compared with WT under the same dietary regimen (Fig. 2B). However, mutants supplemented with triheptanoin showed a significant increase as compared with WT mice (1.12 ± 0.11 vs. 0.85 ± 0.09 nmol/g). Interestingly, we also observed a trend, although not significant (P= 0.051), toward higher protein carbonylation in mutants fed the triheptanoin diet. In contrast, glutathione peroxidase (GPX) activity was significantly increased in VLCAD−/− mice under control conditions as compared with WT mice (Fig. 2C). However, triheptanoin did not further affect GPX activity. In accordance with these findings, quantification of GSH as a substrate for GPX showed a direct correlation between increased GPX activity and reduced GSH content in mice fed the control diet (Fig. 2D): VLCAD−/− mice displayed a significant decrease in GSH concentration as compared with WT mice. Also in this case, triheptanoin had no effect on GSH concentration. In line with previous work (11), thiobarbituric acid reactive substances were significantly increased in VLCAD−/− mice at the age of 1 year under control diet; however, in contrast to MCT, triheptanoin did not aggravate hepatic oxidative stress (Fig. 2E).
Triheptanoin provides anaplerosis in the liver
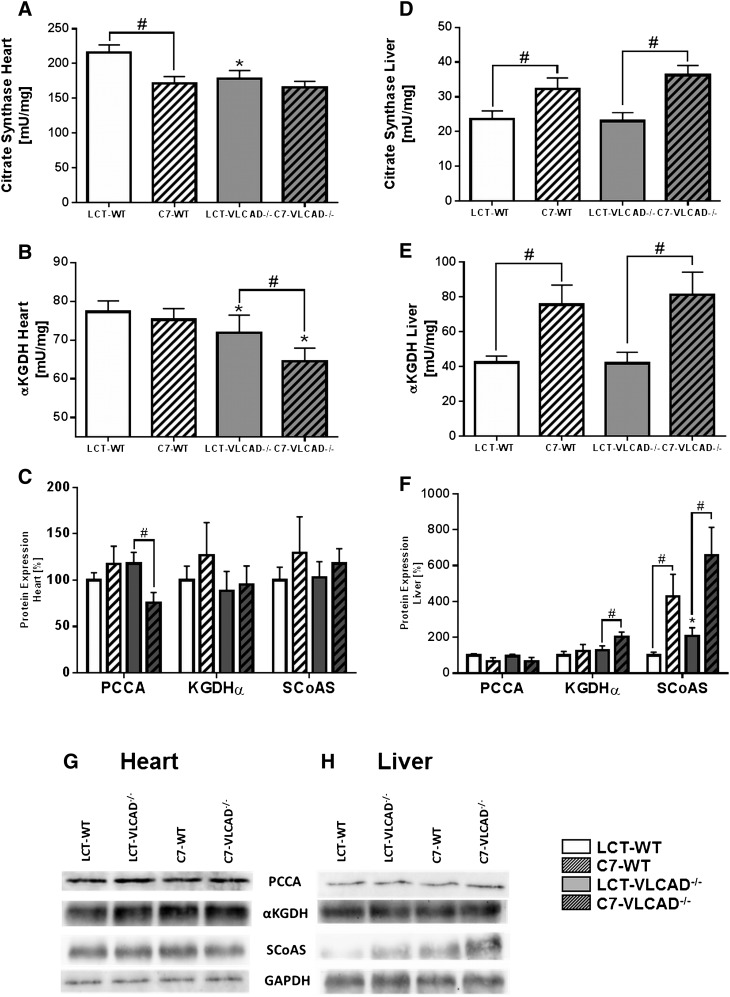
The supposed advantage in the application of triheptanoin is the potential additional anaplerotic effect, supplying the CAC with the required substrates (15). To characterize the anaplerotic efficacy of triheptanoin over a long period, we measured the specific activity of two enzymes of the CAC in heart and liver as well as the expression of proteins that should be upregulated in response to anaplerosis (i.e., propionyl-CoA carboxylase, αKGDH, and SCoAS) instead of the evaluation of CAC intermediates.
Although the determination of CAC is a robust method to follow the acute response to triheptanoin, such as after the administration of a bolus, this method is unsuitable to measure chronic effects developed over a long period of time. Indeed, mice at the age of 3 months fed over 5 weeks with triheptanoin did not show any differences in citrate synthase (CS) and αKGDH activities as compared with mice fed the control diet (supplementary Fig. S1). In contrast, the applied diet modulated, in the long-term, the enzyme activities, as measured in mice at the age of 1 year (Fig. 3).
Fig. 3.
Cardiac and hepatic anaplerotic effects of triheptanoin in mice at the age of 1 year. A–D: Citrate synthase enzyme activity. B–E: αKetoglutarate dehydrogenase enzyme activity. C, F, G, H: Immunodetection and quantification of protein expression using Western blot. GAPDH was used as loading control. Values are means ± SEM (n = 10–12). *Significant differences (P < 0.05, two-way ANOVA and Student’s t-test) between WT and VLCAD−/− mice under the same dietary regimen. #Significant differences (P < 0.05, two-way ANOVA and Student’s t-test) between mice of the same genotype under different dietary regimen. LCT, long-chain triglyceride; PCCA, propionyl-CoA carboxylase.
In mice fed the control diet, we observed a significantly reduced specific activity of the enzymes CS and αKGDH in the heart of VLCAD−/− mice as compared with WT mice (Fig. 3A, B), which were not stimulated by triheptanoin. Under this diet the specific activity of CS was significantly reduced also in WT mice, with a value of 171.8 ± 9.5 mU/mg versus 216 ± 11 mU/mg of WT under control diet. Similarly, the αKGDH-specific activity in VLCAD−/− mice was lower with triheptanoin (71.8 ± 4.6 mU/mg with a normal diet and 64.5 ± 3.5 mU/mg on the triheptanoin diet) (Fig. 3A, B). These data were supported by protein expression analysis, which showed no increase in propionyl-CoA carboxylase, αKGDH, and SCoAS, suggesting that long-term supplementation with triheptanoin may not stimulate anaplerosis in the heart (Fig. 3G).
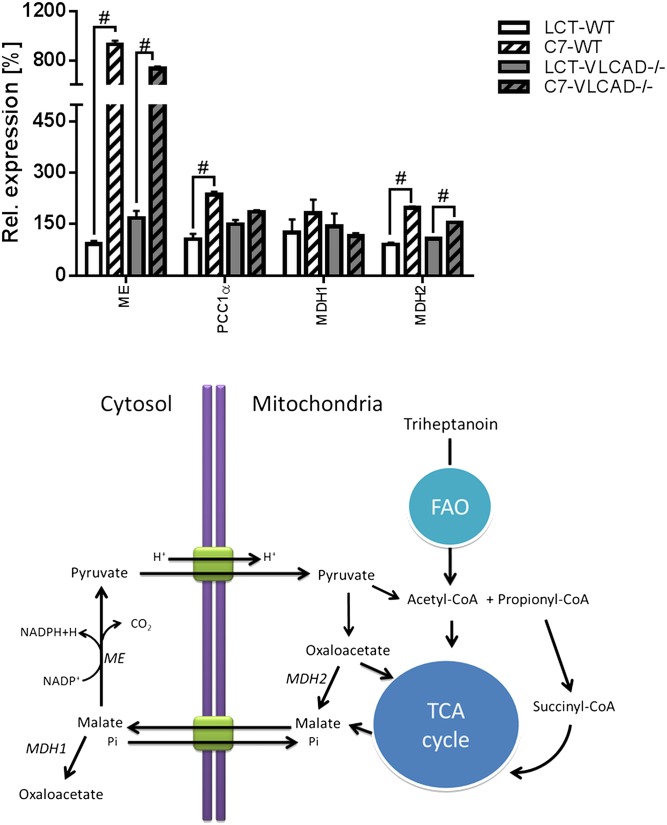
We observed a different results in the liver (Fig. 3D, E). The specific activity of CS and αKGDH was strongly upregulated. This was a clear diet-dependent effect because it was evident in both genotypes upon triheptanoin. Protein expression analysis also showed an increased expression of SCoAS (Fig. 3H) that, together with the upregulated expression of malic enzyme and the mitochondrial malate dehydrogenase (Fig. 4), is suggestive of an adaptation mechanism to higher substrates in response to the triheptanoin diet.
Fig. 4.
Upregulation of anaplerotic genes in the liver on triheptanoin (A) and schematic representation of anaplerotic metabolism of triheptanoin via cytosolic malic enzyme. #Significant differences (P < 0.05, two-way ANOVA and Student’s t-test) between mice of the same genotype under different dietary regimens. LCT, long-chain triglyceride; MDH1, cytosolic malate dehydrogenase; MDH2, mitochondrial malate dehydrogenase; ME, malic enzyme.
Increment of long-chain acylcarnitines in blood despite long-term supplementation with triheptanoin
Because of the supposed anaplerotic effect of triheptanoin, we analyzed in dried blood spots the concentration of long-chain acylcarnitines, which are a reliable parameter for β-oxidation performance. We observed higher concentrations of long-chain acylcarnitines in 1-year-old mice treated with triheptanoin as compared with mice fed the control diet but observed no accumulation of odd-chain species (Fig. 5). In particular, the content of the monounsaturated long-chain acylcarnitines C16:1 and C18:1 increased significantly in VLCAD−/− mice as compared with mutants on the control diet. Moreover, the concentration of free carnitine and acetyl-carnitine (C0 and C2) was significantly reduced in both genotypes (Fig. 5A).
Fig. 5.
Acylcarnitine profiles from dried blood spots of WT and VLCAD−/− mice fed with either long-chain triglycerides (LCTs) or triheptanoin diet. Values are means ± SEM (n = 18–20). *Significant differences (P < 0.05, two-way ANOVA and Student’s t-test) between WT and VLCAD−/− mice under the same dietary regimen. #Significant differences (P < 0.05, two-way ANOVA and Student’s t-test) between mice of the same genotype under different dietary regimens. FAO, fatty acid oxidation; ME, malic enzyme; TCA, tricarboxylic acid.
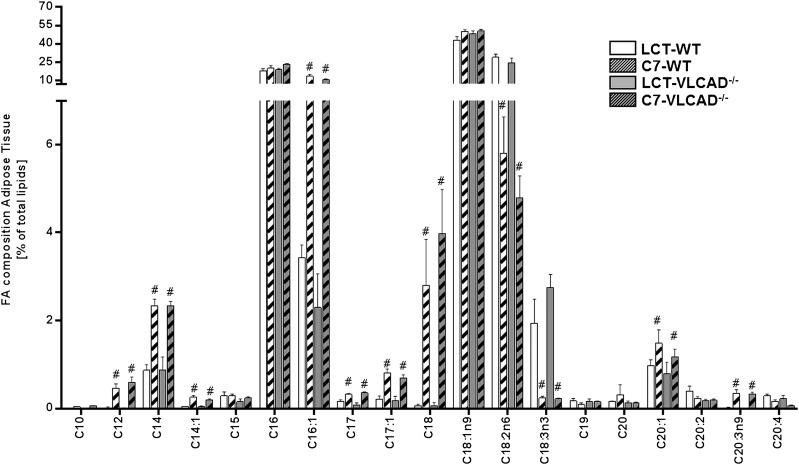
Triheptanoin does not accumulate in adipose tissue
Because mice on triheptanoin did not show increased odd-chain acylcarnitines, we analyzed the total fatty acid profile in white adipose tissue. Fatty acids with a chain length ≤C10 presented only in trace and have not been included in the histogram. As shown in Fig. 6, we observed diet-dependent effects, but no genotype-dependent effects, on the fatty acid profile in white adipose issue. In particular, we observed a marked increment in the content of saturated fatty acids such as C12, C14, C17, and C18. In parallel, the monounsaturated fatty acids C14:1, C16:1, and C17:1 were strongly increased, supporting previous data on the stimulation of de novo fatty acid biosynthesis and elongation (16). With the exception of C17 and C17:1, no other odd-chain species accumulated in the white adipose tissue with the long-term application, suggesting a low incorporation and processing of C7 introduced by the diet.
Fig. 6.
Fatty acid profiles from adipose tissue of mice under either a control or a triheptanoin diet. Values are means ± SEM (n = 10–12). #Significant differences (P < 0.05, two-way ANOVA and Student’s t-test) between mice of the same genotype under different dietary regimen. DBS, dried blood spot; LCT, long-chain triglyceride.
DISCUSSION
In this work, we show that the long-term application of dietary triheptanoin induces enzymatic changes resulting in upregulated cardiac glucose oxidation. However, the observed substrate switch does not prevent the gradual development of systolic dysfunction in VLCAD−/− mice. More importantly, triheptanoin does not have an anaplerotic potential in the heart but only in the liver, despite the intrahepatic lipid accumulation. However, this was not associated with increased oxidative stress.
In the last 10 years there has been accumulating evidence describing the positive effects of triheptanoin, especially in the treatment of diseases associated with derangement of brain cellular metabolism. Triheptanoin has been successfully applied as a ketogenic diet in epilepsy (33, 34) and reduces the severity of ischemic strokes (35). A recent study of patients with Huntington disease supplemented with triheptanoin at the early stage of the disease showed an improvement in the metabolic profile of the brain after 1 month of supplementation (36, 37). In a similar manner, in patients with glucose transporter type I deficiency the cerebral metabolic rate, measured as the cerebral metabolic rate of oxygen consumption by MRI, was enhanced (38). In patients with these diseases, triheptanoin is attributed a crucial role in the supply of citric acid intermediates via anaplerosis. Application of anaplerotic therapy in FAOD has also been extensively discussed (15, 38–40). The first studies in patients with FAOD display a positive trend in terms of the number of catabolic events and hospitalizations (41). Because previous studies in rats showed that intravenous administration of triheptanoin induces lipolysis (42), which is not desirable during metabolic decompensation in FAOD, we tested the effect of long-term dietary supplementation with triheptanoin in VLCAD−/− mice. Our findings show that long-term triheptanoin supplementation in VLCAD−/− mice resulted in higher circulating glucose concentration but not in higher insulin concentration, in line with previous reports (43). In addition, this diet did not result in a metabolic syndrome as reported in mice on even MCTs (28), although the HOMA Index was progressively increased in groups of triheptanoin-treated mice.
Hypertrophic cardiomyopathy and arrhythmias are typical clinical manifestations of the severe early-onset phenotype of VLCAD deficiency. In a previous work we showed that long-term supplementation with dietary MCTs aggravated the cardiac dysfunction of VLCAD−/− mice to a dilated cardiomyopathy (29). Because triheptanoin supplies the CAC with anaplerotic substrates, we hypothesized that the long-term supplementation would inhibit the development of the progressive cardiac dysfunction. Surprisingly, we found that the cardiac function in triheptanoin-treated VLCAD−/− mice did not differ in any parameter compared with the cardiac function of VLCAD−/− mice on control diet. The analysis of genes typically expressed in adult hearts during functional impairment and metabolic derangement (44), and responsible for the substrate switch toward enhanced glucose oxidation, showed a marked upregulation that correlated with the increased specific activity of PDH and PK. A similar effect on glucose metabolism has been described in rats with pressure overload treated over 6 weeks with a triheptanoin-based diet (45). The treatment with a triheptanoin-enriched diet appreciably improved diastolic function and reduced ventricular hypertrophy in rats subjected to pressure overload (45), indicating that anaplerosis by heptanoate was sufficient to maintain the contractile function supplying the CAC with the required substrates. These data were in line with the findings reported by Russel and Taegtmeyer (46) showing that depletion of CAC intermediates induces contractile dysfunction in isolated perfused rat hearts (46). However, VLCAD−/− mice did not display improvement in the systolic function when they were treated long term with triheptanoin, despite a marked upregulation of enzymes involved in glucose oxidation. We postulated that the long-term dietary supplementation of triheptanoin may not be effective to avoid the chronic energy deficiency as suggested by our results. Although the anaplerotic supply of intermediates by triheptanoin has been demonstrated in isolated perfused heart (45), in our model organism we could show that by oral administration of triheptanoin the availability of triheptanoin in peripheral organs (e.g., heart or the skeletal muscles) is not sufficient to provide these organs with the required substrates in order to prevent chronic energy deficiency. Indeed, MCTs are quickly hydrolyzed to FFAs and as such reach the portal circulation because only a minor amount of MCFAs are incorporated into chylomicrons (47). Therefore, most of the circulating MCFAs are retained by the liver, and only a small amount appears in the peripheral blood for a short period of time (48–51). This would explain why triheptanoin-fed mice did not display an upregulation of the genes and enzymes involved in the anaplerotic pathway in the heart. In the liver the anaplerotic energy supply was indeed stimulated in mice at the age of 1 year, whereas short-term supplementation had no effect on the enzymes of the CAC. In accordance with these findings, we found that the higher intrahepatic lipid accumulation due to the stimulated lipogenesis (16) was not associated with higher oxidative stress as compared with mutants on the control diet, suggesting that anaplerotic processes protect the liver from oxidative stress. Indeed, the intrahepatic lipid accumulation in VLCAD−/− mice fed triheptanoin over 1 year was in the same range of that observed in VLCAD−/− mice supplemented only over 5 weeks with MCTs (14). The accumulation of long-chain acylcarnitines but not of odd-chain species indicates that the elongated fatty acids (16) are released due to energy deficiency. Indeed, MCFAs do not accumulate in white adipose tissue and are not available as energy source during higher energy demand. This is in contrast to the study of Zurier et al. (52). Overall, compared with intravenous injection, oral administration of triheptanoin results in a different availability of this macronutrient because it undergoes a whole body metabolism. Even after 1 year of supplementation, there was no trace of MCFAs in white adipose tissue. The hypothesis that triheptanoin is stored after supplementation and can be released during fasting is not supported by our findings. Quantification of anaplerotic triheptanoin effects right after ingestion would have given additional information. However, our aim was to study the long-term effects and long-term metabolic adaptations because patients may undergo life-long treatment, and the consequences of life-long treatment have not been unexplored. Despite the positive acute effects observed with trihepatnoin treatment in symptomatic patients (53), our study suggests that this diet may not be as effective as prophylactic application to prevent the development of symptoms.
CONCLUSIONS
We here show that the long-term application of a triheptanoin diet had no positive effect on the cardiac phenotype of VLCAD−/− mice. In particular, triheptanoin did not prevent the development of systolic dysfunction. This may be explained by the lack of anplerotic effects of triheptanoin in the heart in contrast to liver. In the liver, the medium odd-chain fatty acids (triheptanoin) are also elongated and accumulate, as occurs for MCTs; however, they result in less severe oxidative stress. The availability of MCFAs, either odd-chain or even-chain, in the various organs is very different, and further studies are required to develop targeted delivery to the tissue of interest.
Supplementary Material
Footnotes
Abbreviations:
- αKGDH
- α-ketoglutarate dehydrogenase
- CAC
- citric acid cycle
- CS
- citrate synthase
- ESV
- end-systolic volume
- FAOD
- fatty acid oxidation disorder
- HOMA
- homeostasis model assessment
- MCFA
- medium-chain fatty acid
- MCT
- medium-chain triglyceride
- PDH
- pyruvate dehydrogenase
- PK
- pyruvate kinase
- SCoA
- succinyl-CoA synthetase
- TAG
- triacylglyceride
- VLCAD
- very long-chain-acyl-CoA-dehydrogenase
- VLCAD−/−
- very long-chain-acyl-CoA-dehydrogenase deficient
The work was supported by grants from Deutsche Forschungsgemeinschaft (SFB612 A09) and from the Eva-Uth Foundation (1027055102) and theMüller-Fahnenberg Foundation (1027062101S4). The authors have no conflicts of interest to disclose.
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Arnold G. L., Van Hove J., Freedenberg D., Strauss A., Longo N., Burton B., Garganta C., Ficicioglu C., Cederbaum S., Harding C., et al. . 2009. A Delphi clinical practice protocol for the management of very long chain acyl-CoA dehydrogenase deficiency. Mol. Genet. Metab. 96: 85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lindner M., Hoffmann G. F., and Matern D.. 2010. Newborn screening for disorders of fatty-acid oxidation: experience and recommendations from an expert meeting. J. Inherit. Metab. Dis. 33: 521–526. [DOI] [PubMed] [Google Scholar]
- 3.Spiekerkoetter U., Sun B., Zytkovicz T., Wanders R., Strauss A. W., and Wendel U.. 2003. MS/MS-based newborn and family screening detects asymptomatic patients with very-long-chain acyl-CoA dehydrogenase deficiency. J. Pediatr. 143: 335–342. [DOI] [PubMed] [Google Scholar]
- 4.Therrell B. L. Jr., Lloyd-Puryear M. A., Camp K. M., and Mann M. Y.. 2014. Inborn errors of metabolism identified via newborn screening: ten-year incidence data and costs of nutritional interventions for research agenda planning. Mol. Genet. Metab. 113: 14–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spiekerkoetter U., Bastin J., Gillingham M., Morris A., Wijburg F., and Wilcken B.. 2010. Current issues regarding treatment of mitochondrial fatty acid oxidation disorders. J. Inherit. Metab. Dis. 33: 555–561. [DOI] [PubMed] [Google Scholar]
- 6.Brown-Harrison M. C., Nada M. A., Sprecher H., Vianey-Saban C., Farquhar J. Jr., Gilladoga A. C., and Roe C. R.. 1996. Very long chain acyl-CoA dehydrogenase deficiency: successful treatment of acute cardiomyopathy. Biochem. Mol. Med. 58: 59–65. [DOI] [PubMed] [Google Scholar]
- 7.Lund A. M., Dixon M. A., Vreken P., Leonard J. V., and Morris A. A.. 2003. What is the role of medium-chain triglycerides in the management of long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency? J. Inherit. Metab. Dis. 26: 353–360. [DOI] [PubMed] [Google Scholar]
- 8.Pervaiz M. A., Kendal F., Hegde M., and Singh R. H.. 2011. MCT oil-based diet reverses hypertrophic cardiomyopathy in a patient with very long chain acyl-coA dehydrogenase deficiency. Indian J. Hum. Genet. 17: 29–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharef S. W., Al-Senaidi K., and Joshi S. N.. 2013. Successful treatment of cardiomyopathy due to very long-chain acyl-CoA dehydrogenase deficiency: first case report from Oman with literature review. Oman Med. J. 28: 354–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Primassin S., Tucci S., Herebian D., Seibt A., Hoffmann L., ter Veld F., and Spiekerkoetter U.. 2010. Pre-exercise medium-chain triglyceride application prevents acylcarnitine accumulation in skeletal muscle from very-long-chain acyl-CoA-dehydrogenase-deficient mice. J. Inherit. Metab. Dis. 33: 237–246. [DOI] [PubMed] [Google Scholar]
- 11.Tucci S., Flogel U., Sturm M., Borsch E., and Spiekerkoetter U.. 2011. Disrupted fat distribution and composition due to medium-chain triglycerides in mice with a beta-oxidation defect. Am. J. Clin. Nutr. 94: 439–449. [DOI] [PubMed] [Google Scholar]
- 12.Tucci S., Herebian D., Sturm M., Seibt A., and Spiekerkoetter U.. 2012. Tissue-specific strategies of the very-long chain acyl-CoA dehydrogenase-deficient (VLCAD−/−) mouse to compensate a defective fatty acid beta-oxidation. PLoS One. 7: e45429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tucci S., Primassin S., and Spiekerkoetter U.. 2010. Fasting-induced oxidative stress in very long chain acyl-CoA dehydrogenase-deficient mice. FEBS J. 277: 4699–4708. [DOI] [PubMed] [Google Scholar]
- 14.Tucci S., Primassin S., Ter Veld F., and Spiekerkoetter U.. 2010. Medium-chain triglycerides impair lipid metabolism and induce hepatic steatosis in very long-chain acyl-CoA dehydrogenase (VLCAD)-deficient mice. Mol. Genet. Metab. 101: 40–47. [DOI] [PubMed] [Google Scholar]
- 15.Roe C. R., and Mochel F.. 2006. Anaplerotic diet therapy in inherited metabolic disease: therapeutic potential. J. Inherit. Metab. Dis. 29: 332–340. [DOI] [PubMed] [Google Scholar]
- 16.Tucci S., Behringer S., and Spiekerkoetter U.. 2015. De novo fatty acid biosynthesis and elongation in very long-chain acyl-CoA dehydrogenase- (VLCAD) deficient mice supplemented with odd or even medium-chain fatty acids. FEBS J. 282: 4242–4253. [DOI] [PubMed] [Google Scholar]
- 17.Exil V. J., Roberts R. L., Sims H., McLaughlin J. E., Malkin R. A., Gardner C. D., Ni G., Rottman J. N., and Strauss A. W.. 2003. Very-long-chain acyl-coenzyme a dehydrogenase deficiency in mice. Circ. Res. 93: 448–455. [DOI] [PubMed] [Google Scholar]
- 18.Spiekerkoetter U., Lindner M., Santer R., Grotzke M., Baumgartner M. R., Boehles H., Das A., Haase C., Hennermann J. B., Karall D., et al. . 2009. Treatment recommendations in long-chain fatty acid oxidation defects: consensus from a workshop. J. Inherit. Metab. Dis. 32: 498–505. [DOI] [PubMed] [Google Scholar]
- 19.Flogel U., Jacoby C., Godecke A., and Schrader J.. 2007. In vivo 2D mapping of impaired murine cardiac energetics in NO-induced heart failure. Magn. Reson. Med. 57: 50–58. [DOI] [PubMed] [Google Scholar]
- 20.Nulton-Persson A. C., and Szweda L. I.. 2001. Modulation of mitochondrial function by hydrogen peroxide. J. Biol. Chem. 276: 23357–23361. [DOI] [PubMed] [Google Scholar]
- 21.Trounce I. A., Kim Y. L., Jun A. S., and Wallace D. C.. 1996. Assessment of mitochondrial oxidative phosphorylation in patient muscle biopsies, lymphoblasts, and transmitochondrial cell lines. Methods Enzymol. 264: 484–509. [DOI] [PubMed] [Google Scholar]
- 22.Lawrence R. A., and Burk R. F.. 2012. Glutathione peroxidase activity in selenium-deficient rat liver. 1976. Biochem. Biophys. Res. Commun. 425: 503–509. [DOI] [PubMed] [Google Scholar]
- 23.Mantha S. V., Prasad M., Kalra J., and Prasad K.. 1993. Antioxidant enzymes in hypercholesterolemia and effects of vitamin E in rabbits. Atherosclerosis. 101: 135–144. [DOI] [PubMed] [Google Scholar]
- 24.Matthews D. R., Hosker J. P., Rudenski A. S., Naylor B. A., Treacher D. F., and Turner R. C.. 1985. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 28: 412–419. [DOI] [PubMed] [Google Scholar]
- 25.Lee S., Muniyappa R., Yan X., Chen H., Yue L. Q., Hong E. G., Kim J. K., and Quon M. J.. 2008. Comparison between surrogate indexes of insulin sensitivity and resistance and hyperinsulinemic euglycemic clamp estimates in mice. Am. J. Physiol. Endocrinol. Metab. 294: E261–E270. [DOI] [PubMed] [Google Scholar]
- 26.Folch J., Lees M., and Sloane Stanley G. H.. 1957. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226: 497–509. [PubMed] [Google Scholar]
- 27.Vreken P., van Lint A. E., Bootsma A. H., Overmars H., Wanders R. J., and van Gennip A. H.. 1999. Rapid diagnosis of organic acidemias and fatty-acid oxidation defects by quantitative electrospray tandem-MS acyl-carnitine analysis in plasma. Adv. Exp. Med. Biol. 466: 327–337. [DOI] [PubMed] [Google Scholar]
- 28.Tucci S., Flogel U., and Spiekerkoetter U.. 2015. Sexual dimorphism of lipid metabolism in very long-chain acyl-CoA dehydrogenase deficient (VLCAD(−/−)) mice in response to medium-chain triglycerides (MCT). Biochim. Biophys. Acta. 1852: 1442–1450. [DOI] [PubMed] [Google Scholar]
- 29.Tucci S., Flogel U., Hermann S., Sturm M., Schafers M., and Spiekerkoetter U.. 2014. Development and pathomechanisms of cardiomyopathy in very long-chain acyl-CoA dehydrogenase deficient (VLCAD(−/−)) mice. Biochim. Biophys. Acta. 1842: 677–685. [DOI] [PubMed] [Google Scholar]
- 30.Lin J., Wu H., Tarr P. T., Zhang C. Y., Wu Z., Boss O., Michael L. F., Puigserver P., Isotani E., Olson E. N., et al. . 2002. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature. 418: 797–801. [DOI] [PubMed] [Google Scholar]
- 31.Kuwahara K., Nishikimi T., and Nakao K.. 2012. Transcriptional regulation of the fetal cardiac gene program. J. Pharmacol. Sci. 119: 198–203. [DOI] [PubMed] [Google Scholar]
- 32.Jaeschke H., Gores G. J., Cederbaum A. I., Hinson J. A., Pessayre D., and Lemasters J. J.. 2002. Mechanisms of hepatotoxicity. Toxicol. Sci. 65: 166–176. [DOI] [PubMed] [Google Scholar]
- 33.Hadera M. G., McDonald T., Smeland O. B., Meisingset T. W., Eloqayli H., Jaradat S., Borges K., and Sonnewald U.. 2015. Modification of astrocyte metabolism as an approach to the treatment of epilepsy: triheptanoin and acetyl-L-carnitine. Neurochem. Res. 41(1-2): 86–95. [DOI] [PubMed] [Google Scholar]
- 34.Thomas N. K., Willis S., Sweetman L., and Borges K.. 2012. Triheptanoin in acute mouse seizure models. Epilepsy Res. 99: 312–317. [DOI] [PubMed] [Google Scholar]
- 35.Schwarzkopf T. M., Koch K., and Klein J.. 2015. Reduced severity of ischemic stroke and improvement of mitochondrial function after dietary treatment with the anaplerotic substance triheptanoin. Neuroscience. 300: 201–209. [DOI] [PubMed] [Google Scholar]
- 36.Adanyeguh I. M., Rinaldi D., Henry P. G., Caillet S., Valabregue R., Durr A., and Mochel F.. 2015. Triheptanoin improves brain energy metabolism in patients with Huntington disease. Neurology. 84: 490–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mochel F., Duteil S., Marelli C., Jauffret C., Barles A., Holm J., Sweetman L., Benoist J. F., Rabier D., Carlier P. G., et al. . 2010. Dietary anaplerotic therapy improves peripheral tissue energy metabolism in patients with Huntington’s disease. Eur. J. Hum. Genet. 18: 1057–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pascual J. M., Liu P., Mao D., Kelly D. I., Hernandez A., Sheng M., Good L. B., Ma Q., Marin-Valencia I., Zhang X., et al. . 2014. Triheptanoin for glucose transporter type I deficiency (G1D): modulation of human ictogenesis, cerebral metabolic rate, and cognitive indices by a food supplement. JAMA Neurol. 71: 1255–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roe C. R., Sweetman L., Roe D. S., David F., and Brunengraber H.. 2002. Treatment of cardiomyopathy and rhabdomyolysis in long-chain fat oxidation disorders using an anaplerotic odd-chain triglyceride. J. Clin. Invest. 110: 259–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roe C. R., Yang B. Z., Brunengraber H., Roe D. S., Wallace M., and Garritson B. K.. 2008. Carnitine palmitoyltransferase II deficiency: successful anaplerotic diet therapy. Neurology. 71: 260–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vockley J., Marsden D., McCracken E., DeWard S., Barone A., Hsu K., and Kakkis E.. 2015. Long-term major clinical outcomes in patients with long chain fatty acid oxidation disorders before and after transition to triheptanoin treatment: a retrospective chart review. Mol. Genet. Metab. 116: 53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gu L., Zhang G. F., Kombu R. S., Allen F., Kutz G., Brewer W. U., Roe C. R., and Brunengraber H.. 2010. Parenteral and enteral metabolism of anaplerotic triheptanoin in normal rats: II. Effects on lipolysis, glucose production, and liver acyl-CoA profile. Am. J. Physiol. Endocrinol. Metab. 298: E362–E371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guo W., Xie W., and Han J.. 2006. Modulation of adipocyte lipogenesis by octanoate: involvement of reactive oxygen species. Nutr. Metab. (Lond). 3: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nascimben L., Ingwall J. S., Lorell B. H., Pinz I., Schultz V., Tornheim K., and Tian R.. 2004. Mechanisms for increased glycolysis in the hypertrophied rat heart. Hypertension. 44: 662–667. [DOI] [PubMed] [Google Scholar]
- 45.Nguyen T. D., Shingu Y., Amorim P. A., Schwarzer M., and Doenst T.. 2015. Triheptanoin alleviates ventricular hypertrophy and improves myocardial glucose oxidation in rats with pressure overload. J. Card. Fail. 21: 906–915. [DOI] [PubMed] [Google Scholar]
- 46.Russell R. R. 3rd, and Taegtmeyer H.. 1991. Changes in citric acid cycle flux and anaplerosis antedate the functional decline in isolated rat hearts utilizing acetoacetate. J. Clin. Invest. 87: 384–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Swift L. L., Hill J. O., Peters J. C., and Greene H. L.. 1990. Medium-chain fatty acids: evidence for incorporation into chylomicron triglycerides in humans. Am. J. Clin. Nutr. 52: 834–836. [DOI] [PubMed] [Google Scholar]
- 48.Bach A. C., and Babayan V. K.. 1982. Medium-chain triglycerides: an update. Am. J. Clin. Nutr. 36: 950–962. [DOI] [PubMed] [Google Scholar]
- 49.Guillot E., Vaugelade P., Lemarchal P., and Rerat A.. 1993. Intestinal absorption and liver uptake of medium-chain fatty acids in non-anaesthetized pigs. Br. J. Nutr. 69: 431–442. [DOI] [PubMed] [Google Scholar]
- 50.Fernando-Warnakulasuriya G. J., Staggers J. E., Frost S. C., and Wells M. A.. 1981. Studies on fat digestion, absorption, and transport in the suckling rat: I. Fatty acid composition and concentrations of major lipid components. J. Lipid Res. 22: 668–674. [PubMed] [Google Scholar]
- 51.Mascioli E. A., Lopes S., Randall S., Porter K. A., Kater G., Hirschberg Y., Babayan V. K., Bistrian B. R., and Blackburn G. L.. 1989. Serum fatty acid profiles after intravenous medium chain triglyceride administration. Lipids. 24: 793–798. [DOI] [PubMed] [Google Scholar]
- 52.Zurier R. B., Campbell R. G., Hashim S. A., and Van Itallie T. B.. 1967. Enrichment of depot fat with odd and even numbered medium chain fatty acids. Am. J. Physiol. 212: 291–294. [DOI] [PubMed] [Google Scholar]
- 53.Vockley J., Charrow J., Ganesh J., Eswara M., Diaz G. A., McCracken E., Conway R., Enns G. M., Starr J., Wang R., et al. . 2016. Triheptanoin treatment in patients with pediatric cardiomyopathy associated with long chain-fatty acid oxidation disorders. Mol. Genet. Metab. 119: 223–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.