Abstract
The PNPLA3 p.I148M, TM6SF2 p.E167K, and MBOAT7 rs641738 variants represent genetic risk factors for nonalcoholic fatty liver disease (NAFLD). Here we investigate if these polymorphisms modulate both steatosis and fibrosis in patients with NAFLD. We recruited 515 patients with NAFLD (age 16–88 years, 280 female patients). Liver biopsies were performed in 320 patients. PCR-based assays were used to genotype the PNPLA3, TM6SF2, and MBOAT7 variants. Carriers of the PNPLA3 and TM6SF2 risk alleles showed increased serum aspartate aminotransferase and alanine transaminase activities (P < 0.05). The PNPLA3 genotype was associated with steatosis grades S2–S3 (P < 0.001) and fibrosis stages F2–F4 (P < 0.001). The TM6SF2 genotype was associated with steatosis (P = 0.003) but not with fibrosis (P > 0.05). The MBOAT7 variant was solely associated with increased fibrosis (P = 0.046). In the multivariate model, variants PNPLA3 (P = 0.004) and TM6SF2 (P = 0.038) were associated with steatosis. Fibrosis stages were affected by the PNPLA3 (P = 0.042) and MBOAT7 (P = 0.021) but not by the TM6SF2 polymorphism (P > 0.05). The PNPLA3, TM6SF2, and MBOAT7 variants are associated with increased liver injury. The TM6SF2 variant seems to modulate predominantly hepatic fat accumulation, whereas the MBOAT7 polymorphism is linked to fibrosis. The PNPLA3 polymorphism confers risk of both increased steatosis and fibrosis.
Keywords: adiponutrin, fatty liver, fibrosis, steatosis
Nonalcoholic fatty liver disease (NAFLD) affects more than 30% of adults in developed countries. Given the increasing prevalence of environmental risk factors for this condition (e.g., hypercaloric diets and sedentary lifestyles) (1), the frequency of fatty liver is predicted to further increase in the coming years. In addition to environmental triggers, genetic predisposition is known to modulate the degree of steatosis and liver injury (2). Conceptually, the term “hepatic steatosis” refers to traits that are governed by multiple variants with modest effects. The major part of the genetic predisposition is, according to current knowledge, related to two common missense SNPs: PNPLA3 p.I148M and TM6SF2 p.E167K. These two polymorphisms, detected in genome-wide (3) and exome-wide (4) association studies in patients with fatty livers, seem to impose different risks on their carriers. The PNPLA3 (patatin-like phospholipase domain containing 3, also known as adiponutrin) p.I148M polymorphism is commonly regarded to be the risk factor for both increased fat accumulation and fibrosis (5, 6). The association with steatosis was demonstrated in several candidate studies, whereas the link between PNPLA3 and liver scarring was substantiated by meta-analyses in patients with chronic hepatitis C virus (HCV) infection (7) and in alcoholics (8). The data concerning the involvement of TM6SF2 (transmembrane 6 superfamily member 2) in liver injury are less definitive. So far, only a few studies investigating the TM6SF2 risk genotype in NAFLD (9) and in HCV (10) have been published. Liu et al. (11) reported that carriers of the minor allele are at risk of increased steatosis and fibrosis. Interestingly, both PNPLA3 and TM6SF2 variants have been associated with “metabolically silent” NAFLD; i.e., carriers of the risk genotypes seem to develop NAFLD and its severe forms even in the absence of characteristics commonly associated with fatty liver (5, 12). Indeed, numerous genetic studies failed to detect equivocal evidence for the association between TM6SF2 and PNPLA3 variants and traits such as obesity, insulin resistance, or hyperlipidemia. Most recently, the MBOAT7 polymorphism rs641738 was identified as the new risk factor for NAFLD (13), also associated with severity of fibrosis in alcoholic liver disease (14) and in HCV infection (15).
Liver biopsy represents the gold-standard method of quantifying the degree of NAFLD (16). Although several noninvasive methods have been developed, liver biopsy represents the only reliable tool to distinguish between nonalcoholic fatty liver and nonalcoholic steatohepatitis. Analysis of liver specimens also provides exact data concerning steatosis, fibrosis, and inflammation. Hence, it is a powerful tool for quantifying the role of inherited predisposition in liver injury.
To further elucidate the role of the genetic predisposition in modulation of NAFLD, we performed genetic analyses in a large cohort of patients with fatty liver to analyze the signs of liver injury in combination with the carriage of the PNPLA3 p.I148M, TM6SF2 p.E67K, and MBOAT7 rs641738 variants The frequencies of these variants were related to i) results of liver biopsy, ii) circulating levels of markers of liver injury, and iii) metabolic traits. Analysis of genotype-phenotype interactions performed in this group of patients demonstrated different effects of the PNPLA3, TM6SF2, and MBOAT7 variants on hepatic steatosis and fibrosis, underscoring the notion that they play distinct roles in NAFLD progression.
MATERIALS AND METHODS
Patients
Patients for the study were recruited in eight German university centers within the framework of the NAFLD Clinical Study Group (NAFLD CSG) project (17). In brief, the project was started in 2012 as a multicentric study in Germany and was intended to investigate triggers and modulators of NAFLD development, including common genetic variants. All patients gave written informed consent to participate in these studies. The ethical committees at participating centers approved the study protocol. Ethanol intake (>20 g per day for women and >30 g for men) was regarded as exclusion criterion. NAFLD was diagnosed either by imaging techniques (abdominal sonography, MRI, CT) or by liver biopsy. Liver biopsies were performed percutaneously under ultrasound guidance or intraoperatively. Acquired liver samples were evaluated by experienced local pathologists. The presence of acute and chronic liver diseases other than NAFLD was excluded in all patients. All study subjects underwent a standardized clinical examination. Fasted venous blood samples were drawn for routine biochemical analyses, including liver function tests and DNA genotyping. Liver function tests were determined by clinical-chemical assays in the central laboratories of participating centers. In a subgroup of 320 patients with NAFLD with available histology, hepatic steatosis (grades S0–S3) and fibrosis (grades F0–F4) were quantified according to the Kleiner score (18).
Genotyping of the PNPLA3 (rs738409), TM6SF2 (rs58542926), and MBOAT7 (rs641738) variants
Genotyping of the PNPLA3 (rs738409), TM6SF2 (rs58542926), and MBOAT7 (rs641738) variants was performed in a central laboratory (Homburg) by a technician blinded to the phenotype of patients. DNA was extracted from peripheral blood mononuclear cells using the DNeasy Blood and Tissue Kit (Qiagen). DNA concentrations were measured using a NanoDrop spectrophotometer. All variants were genotyped using TaqMan assays (19). The fluorescence data were analyzed with allelic discrimination 7500 Software v.2.0.2.
Statistical analysis
Unless stated otherwise, all statistical analyses were performed with SPSS 20.0 (SPSS, Munich, Germany) or GraphPad Prism 5.0 (GraphPad Software Inc., CA). Quantitative data were expressed as medians and ranges. The association between the PNPLA3, TM6SF2, and MBOAT7 variants and markers of liver injury was tested using ANOVA with post hoc tests. Exact tests were performed to check the consistency of genotyping results in with Hardy-Weinberg equilibrium (http://ihg.gsf.de/cgi-bin/hw/hwa1.pl). Genotype frequencies were compared in contingency tables. Power analysis was performed using PS: Power and Sample Size Calculation v.3.0 (http://biostat.mc.vanderbilt.edu/wiki/Main/PowerSampleSize). Differences in anthropometric and clinical traits between patients with PNPLA3 and MBOAT7 genotypes were compared using linear regression analysis under an additive genetic model. Comparisons between carriers of the TM6SF2 genotypes were performed under a dominant genetic model (due to the low number of homozygotes for the 167K mutant allele) using linear regression analysis. All models were adjusted for confounding factors (age, gender, BMI, diabetes mellitus, and statin use, as appropriate). The effects of the studied variants, as well as additional risk factors, on hepatic steatosis and fibrosis were analyzed in univariate and multivariate models using logistic regression analysis.
RESULTS
Characteristics of the study cohort
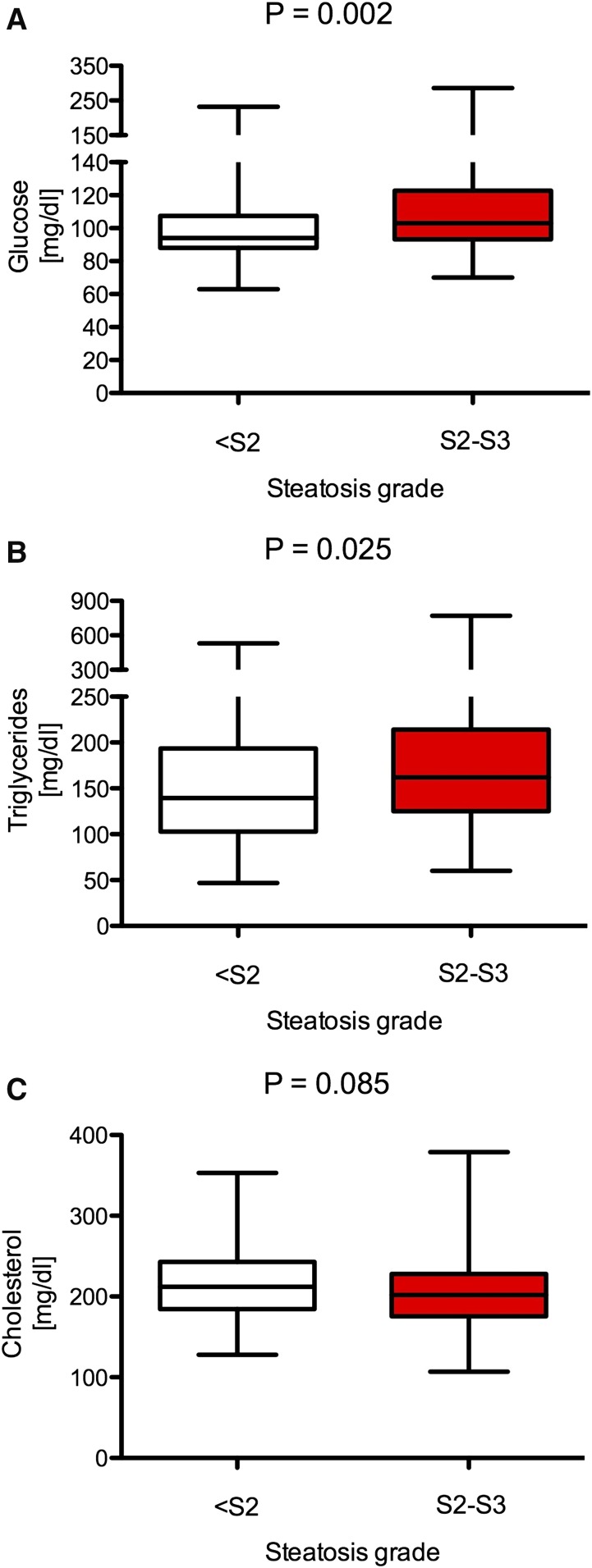
A total of 515 German patients with NAFLD (99.9% white) were recruited. Table 1 summarizes the baseline data of this study cohort, and Table 2 presents the results of liver biopsies in 320 biopsied patients. More women (54%) than men (46%) were included. The median age was 50 years. In 320 patients who underwent liver biopsy, 57% had steatosis grades 2 or 3 (Table 2). Fibrosis stage F2 or higher was present in 30% of patients. Patients undergoing liver biopsy had significantly higher alanine transaminase (ALT) and aspartate aminotransferase (AST) (both P < 0.001) but not γ−glutamyl transferase (GGT) activities (P = 0.26) (Table 1). We did not detect any differences in serum glucose, triglyceride, and cholesterol concentrations between biopsied and nonbiopsied patients (all P > 0.05). Individuals presenting with steatosis grade 2 or 3 had significantly higher serum glucose (P = 0.002) and triglyceride (P = 0.025) concentrations as compared with individuals with lower grades of steatosis (Fig. 1A, B). There were no differences in terms of serum cholesterol in relation to hepatic steatosis (P > 0.05) (Fig. 1C).
TABLE 1.
Baseline characteristics and genotype frequencies in the study cohort
| Variables | Entire cohort | Biopsied patients |
| N (female/male) | 515 (280/235) | 320 (186/134) |
| Age (years) | 50 (16–88) | 49 (16–88) |
| BMI (kg/m2) | 32 (17–70) | 33 (17–69) |
| ALT (U/l) | 52 (12–279) | 58 (13–279)a |
| AST (U/l) | 38 (5–397) | 42 (4–397)a |
| GGT (U/l) | 61 (4–1,658) | 67 (4–1,463) |
| Triglycerides (mg/dl) | 152 (45–770) | 154 (49–770) |
| Total cholesterol (mg/dl) | 204 (72–379) | 206 (107–379) |
| Glucose (mg/dl) | 98 (55–367) | 99 (63–286) |
| Incidence of diabetes type 2 (%) | 24.7 | 26.7 |
| Statin use (%) | 10.6 | 10.6 |
| TM6SF2 p.E167K genotypes (n) | ||
| [EE] | 409 | 253 |
| [EK] | 97 | 61 |
| [KK] | 9 | 6 |
| PNPLA3 p.I148M genotypes (n) | ||
| [II] | 215 | 126 |
| [IM] | 222 | 138 |
| [MM] | 78 | 56 |
| MBOAT7 rs641738 genotypes (n) | ||
| [CC] | 159 | 98 |
| [CT] | 242 | 157 |
| [TT] | 114 | 65 |
E, glutamic acid; I, isoleucine; K, lysine; M, methionine; MBOAT7, membrane bound O-acyltransferase domain containing 7; p, protein (amino acid number); PNPLA3, patatin-like phospholipase domain-containing protein 3; TM6SF2, transmembrane 6 superfamily member 2. Values are given as medians (ranges), unless stated otherwise.
P < 0.001 as compared with nonbiopsied individuals.
TABLE 2.
Distribution of steatosis and fibrosis in biopsied individuals with NAFLD
| Biopsy results | Distribution |
| Grade of steatosisa | |
| 0/1 | 48% |
| 2 | 27% |
| 3 | 25% |
| Grade of fibrosisb | |
| 0/1 | 70% |
| 2 | 16% |
| 3 | 7% |
| 4 | 7% |
Data available for 320 patients.
Data available for 295 patients.
Fig. 1.
Relation between steatosis grade at liver biopsy and metabolic traits. Increased steatosis was associated with higher serum glucose (A) and triglyceride (B) levels, but it did not affect total cholesterol (C).
PNPLA3 p.I148M and TM6SF2 p.E167K variants are associated with increased serum markers of liver injury
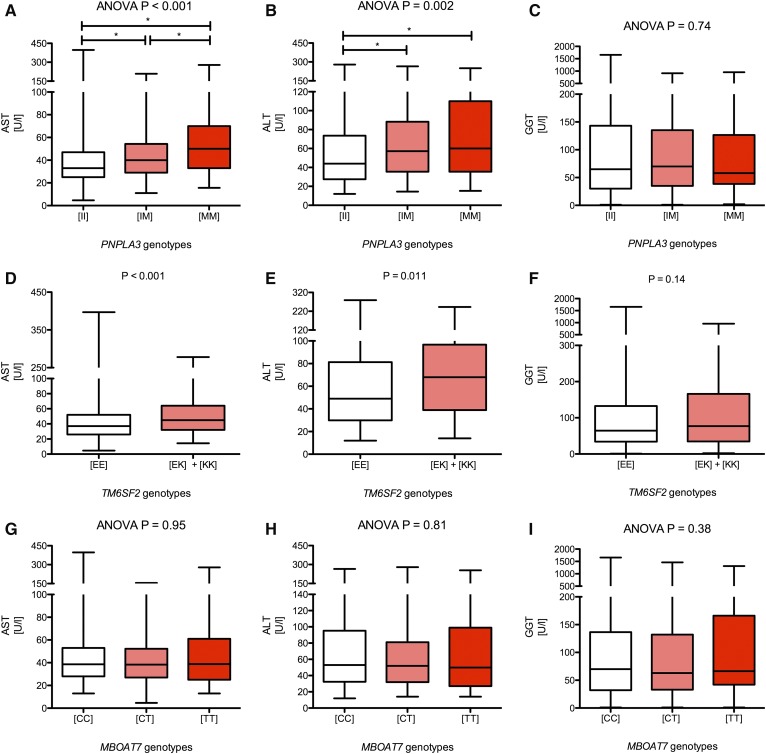
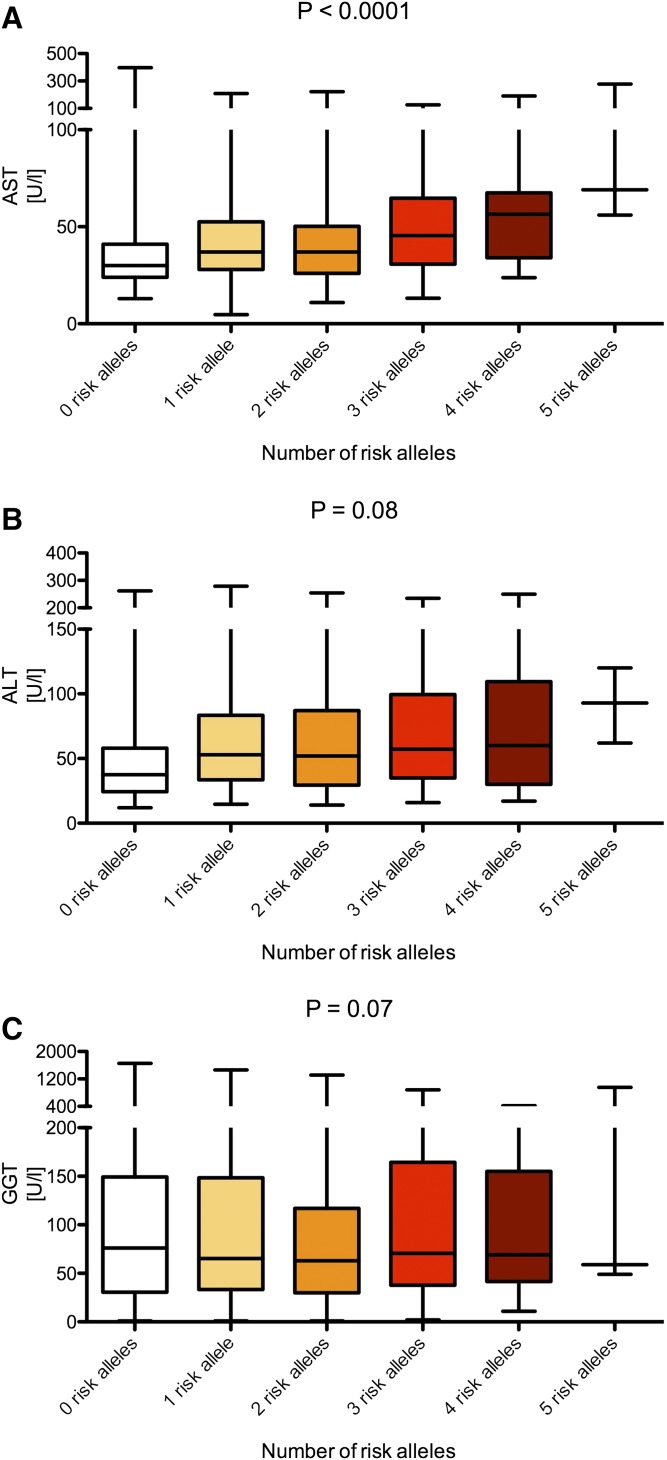
The PNPLA3 p.I148M, TM6SF2 p.E167K, and MBOAT7 rs641738 variants were successfully genotyped in all patients. The genotype frequencies (Table 1) do not differ from frequencies presented in previous publications and are localized on the Hardy-Weinberg equilibrium parabola (P > 0.05, exact test), which validates the genotyping quality. Relations of the studied variants to patient baseline characteristics are presented in supplemental Table S1 (for PNPLA3 p.I148M), supplemental Table S2 (for TM6SF2 p.E167K), and supplemental Table S3 (for the MBOAT7 rs641738). As presented in the supplemental materials, the PNPLA3 and TM6SF2 variants were significantly associated with BMI (both P = 0.01). We did not detect any significant association between clinical characteristics and the MBOAT7 polymorphism (supplemental Table S3). In the entire cohort (i.e., 515 patients with NAFLD), the PNPLA3 p.I148M polymorphism was associated with increased serum AST (ANOVA, P < 0.001) (Fig. 2A) and ALT (ANOVA, P = 0.002) (Fig. 2B) but not with GGT activities (ANOVA, P = 0.74) (Fig. 2C). Similarly, the TM6SF2 variant was associated with increased AST (P < 0.001) (Fig. 2D) and ALT (P = 0.011) (Fig. 2E) but not with GGT activities (P = 0.14) (Fig. 2F). We did not detect any significant association between the MBOAT7 polymorphism and liver function tests (all P > 0.05) (Fig. 2G–I). We detected a significant (P < 0.0001) increase of serum AST activities with the increment of risk alleles of either of the genotypes (Fig. 3A). We also detected trends for increased ALT (P = 0.08) and GGT (P = 0.07) levels with increasing risk allele number (Fig. 3B, C).
Fig. 2.
Box-and-whisker plots illustrating liver function tests in carriers of distinct PNPLA3, TM6SF2, and MBOAT7 variants. Carriers of either PNPLA3 or TM6SF2 risk alleles present with increased AST and ALT activities (A and B for PNPLA3; D and E for TM6SF2). We did not detect any major effects of these variants on the GGT activities (C and F). The MBOAT7 polymorphism did not affect liver function tests. All tests were performed using ANOVA with post hoc tests or with Mann-Whitney U as appropriate. *P < 0.05 in post hoc tests.
Fig. 3.
Combined analysis of the PNPLA3 p.I148M, TM6SF2 p.E167K, and MBOAT7 rs641738 risk alleles on liver function tests. The graphs demonstrate median AST (A), ALT (B), and GGT (C) by the number of risk alleles in either of the tested genes. Analyses were performed using trend test. The following frequencies of carriers of risk alleles were detected: zero risk alleles, n = 56; one risk allele, n = 142; two risk alleles, n = 170; three risk alleles, n = 117; four risk alleles, n = 27; five risk alleles, n = 3.
PNPLA3 p.I148M and TM6SF2 p.E167K have different effects on hepatic steatosis and fibrosis
We performed separate analysis of the variants’ effects on the risk of developing hepatic steatosis and fibrosis in specimens acquired by liver biopsy. Overall, carriers of the PNPLA3 risk allele (P = 0.043), but not TM6SF2 or MBOAT7 variants (both P > 0.05), were more frequently scheduled for liver biopsy. The PNPLA3 polymorphism was significantly associated with the risk of developing steatosis grades S2 and S3 [common odds ratio (OR) = 1.896; P < 0.001] and fibrosis stages F2–F4 (common OR, 2.348; P < 0.001) (Tables 3 and 4). Analysis of TM6SF2 genotype frequencies (Tables 5 and 6) reveals that this variant was associated with steatosis (common OR, 1.539; P = 0.003) but had no major effects on fibrosis (P > 0.05). Based on the frequency of the minor allele among individuals with fibrosis grade <F2 (Table 6), this analysis had a power of 0.81 to detect genetic effects with OR of at least 2.0. Although the MBOAT7 polymorphism was not associated with hepatic steatosis (all P > 0.05), it was significantly associated with the development of liver fibrosis (common OR, 1.446; P = 0.046) (Table 7). We also detected an increase in the number of risk PNPLA3, TM6SF2, and MBOAT7 alleles with increasing hepatic fibrosis (supplemental Fig. S1) and most of all steatosis (supplemental Fig. S2). In the univariate model, PNPLA3 and TM6SF2 polymorphisms, but not MBOAT7, were associated with increased steatosis (Table 8). The association remained significant for these two genotypes in the multivariate analysis (Table 8). In the analyses of liver function tests restricted to biopsied patients, the PNPLA3 polymorphism was associated with significantly increased AST (P = 0.013) (supplemental Fig. S3A) but not ALT (P = 0.17) (supplemental Fig. S3B) or GGT (P = 0.13) (supplemental Fig. S3C). Notably, among individuals scheduled for the liver biopsy, the TM6SF2 polymorphism was associated with increased AST (P = 0.005) (supplemental Fig. S3D), ALT (P = 0.025) (supplemental Fig. S3E), and GGT (P = 0.025) (supplemental Fig. S3F). We did not detect any significant association between liver function test and the MBOAT7 polymorphism in biopsied patients (supplemental Fig. S3G-I). Table 9 summarizes the results of regression analyses for factors associated with liver fibrosis in biopsied patients. Of note, in the multivariate model we detect a significant association for PNPLA3 and MBOAT7 genotypes (both P < 0.05) but not for the TM6SF2 polymorphism (P > 0.05).
TABLE 3.
Distribution of alleles and genotypes for PNPLA3 p.I148M and association tests in respect to steatosis grade
| PNPLA3 p.I148M allele/genotype | Count of alleles/genotypes | |
| Steatosis grade <S2 (2N = 306) | Steatosis S2–S3 (2N = 334) | |
| [I] | 211 (0.69) | 197 (0.54) |
| [M] | 95 (0.31) | 155 (0.46) |
| [II] | 73 (0.48) | 53 (0.31) |
| [IM] | 65 (0.42) | 73 (0.44) |
| [MM] | 15 (0.10) | 41 (0.25) |
| Association test | OR | P value |
| Armitage’s trend test | 1.896 | <0.001 |
| OR statistics | OR (95% CI) | P value |
| [M] ↔ [I] | 1.923 (1.391–2.659) | <0.001 |
| [MM] ↔ [II] | 3.765 (1.890–7.499) | <0.001 |
| [MM] ↔ [IM + II] | 2.994 (1.580–5.671) | <0.001 |
| [MM + IM] ↔ [II] | 1.936 (1.246–3.091) | 0.003 |
I, isoleucine; M, methionine; p, protein (amino acid number); PNPLA3, adiponutrin. [M] represents the steatosis risk allele. Allele and genotype frequency differences were assessed by chi2 test or by Armitage’s trend test as appropriate (https://ihg.gsf.de/cgi-bin/hw/hwa1.pl).
TABLE 5.
Distribution of alleles and genotypes for TM6SF2 p.E167K and association tests with respect to steatosis grade
| TM6SF2 p.E167K allele/ genotype | Count of alleles/genotypes | |
| Steatosis grade <S2 (2N = 306) | Steatosis S2–S3 (2N = 334) | |
| [E] | 280 (0.92) | 287 (0.86) |
| [K] | 26 (0.08) | 47 (0.14) |
| [EE] | 130 (0.85) | 123 (0.74) |
| [EK] | 20 (0.13) | 41 (0.25) |
| [KK] | 3 (0.02) | 3 (0.01) |
| Association test | OR | P value |
| Armitage’s trend test | 1.539 | 0.003 |
| OR statistics | OR (95% CI) | P value |
| [K] ↔ [E] | 1.764 (1.063–2.927) | 0.026 |
| [KK] ↔ [EE] | 1.057 (0.209–5.336) | 0.946 |
| [KK] ↔ [EK + EE] | 0.915 (0.182–4.601) | 0.913 |
| [KK + EK] ↔ [EE] | 2.022 (1.153–3.544) | 0.001 |
CI, confidence interval; E, glutamic acid; K, lysine; p, protein (amino acid number); TM6SF2, transmembrane 6 superfamily member 2. [K] represents the steatosis risk allele. Allele and genotype frequency differences were assessed by chi2 test or by Armitage’s trend test as appropriate (https://ihg.gsf.de/cgi-bin/hw/hwa1.pl).
TABLE 6.
Distribution of alleles and genotypes for TM6SF2 p.E167K and association tests with respect to fibrosis grade
| TM6SF2 p.E167K allele/ genotype | Count of alleles/genotypes | |
| Fibrosis grade <F2 (2N = 410) | Fibrosis grade F2–F4 (2N = 180) | |
| [E] | 366 (0.89) | 156 (0.87) |
| [K] | 44 (0.11) | 24 (0.13) |
| [EE] | 164 (0.80) | 68 (0.76) |
| [EK] | 38 (0.19) | 20 (0.22) |
| [KK] | 3 (0.01) | 2 (0.02) |
| Association test | OR | P value |
| Armitage’s trend test | 1.269 | 0.370 |
| OR statistics | OR (95% CI) | P value |
| [K] ↔ [E] | 1.280 (0.752–2.177) | 0.362 |
| [KK] ↔ [EE] | 1.608 (0.269–9.838) | 0.608 |
| [KK] ↔ [EK + EE] | 1.530 (0.251–9.319) | 0.642 |
| [KK + EK] ↔ [EE] | 1.294 (0.717–2.335) | 0.391 |
CI, confidence interval; E, glutamic acid; K, lysine; p, protein (amino acid number); TM6SF2, transmembrane 6 superfamily member 2. [K] represents the steatosis risk allele. Allele and genotype frequency differences were assessed by chi2 test or by Armitage’s trend test as appropriate (https://ihg.gsf.de/cgi-bin/hw/hwa1.pl).
TABLE 7.
Distribution of alleles and genotypes for MBOAT7 rs641738 and association tests in respect to fibrosis grade
| MBOAT7 rs641738 allele/genotype | Count of alleles/genotypes | |
| Fibrosis ade F0 (2N = 206) | Fibrosis grade F1–F4 (2N = 384) | |
| [C] | 122 (0.59) | 194 (0.51) |
| [T] | 44 (0.41) | 190 (0.49) |
| [CC] | 34 (0.33) | 53 (0.28) |
| [CT] | 54 (0.52) | 88 (0.46) |
| [TT] | 15 (0.15) | 51 (0.26) |
| Association test | OR | P value |
| Armitage’s trend test | 1.446 | 0.046 |
| OR statistics | OR (95% CI) | P value |
| [T] ↔ [C] | 1.422 (1.010–2.003) | 0.043 |
| [TT] ↔ [CC] | 2.181 (1.063–4.476) | 0.031 |
| [TT] ↔ [CT + CC] | 2.122 (0.251–9.319) | 0.012 |
| [TT + CT] ↔ [CC] | 1.292 (0.770–2.170) | 0.350 |
CI, confidence interval; p, protein (amino acid number); MBOAT7, membrane bound O-acyltransferase domain containing 7. [T] represents the risk allele. Allele and genotype frequency differences were assessed by chi2 test or by Armitage’s trend test as appropriate (https://ihg.gsf.de/cgi-bin/hw/hwa1.pl).
TABLE 8.
Risk factors for developing hepatic steatosis
| Factor | OR | 95% CI | P value |
| Univariate analysis | |||
| PNPLA3 p.I148M | 2.418 | 1.323–4.419 | 0.004 |
| TM6SF2 p.E167K | 4.622 | 1.077–19.831 | 0.039 |
| MBOAT7 rs641738 | 1.260 | 0.749–2.119 | 0.384 |
| Glucose | 1.015 | 0.994–1.037 | 0.168 |
| BMI | 0.966 | 0.933–1.001 | 0.055 |
| Age (years) | 1.005 | 0.979–1.033 | 0.692 |
| Sex | 2.080 | 0.933–4.634 | 0.073 |
| Presence of diabetes | 1.224 | 0.504–2.973 | 0.656 |
| Triglycerides | 1.002 | 0.996–1.007 | 0.594 |
| Cholesterol | 0.997 | 0.988–1.007 | 0.539 |
| Multivariate analysis | |||
| PNPLA3 p.I148M | 2.424 | 1.326–4.419 | 0.004 |
| TM6SF2 p.E167K | 4.725 | 1.093–20.429 | 0.038 |
CI, confidence interval; E, glutamic acid; I, isoleucine; K, lysine; M, methionine; MBOAT7, membrane bound O-acyltransferase domain containing 7; p, protein (amino acid number); PNPLA3, adiponutrin; TM6SF2, transmembrane 6 superfamily member 2. The relationships between steatosis PNPLA3, TM6SF2, and MBOAT7 variants as well as other potentially prosteatotic factors were assessed by univariate and multivariate logistic regression analysis. Genetic analyses were calculated by using either additive (for PNPLA3 and MBOAT7) or dominant (for TM6SF2) models.
TABLE 9.
Risk factors for developing hepatic fibrosis
| Factor | OR | 95% CI | P value |
| Univariate analysis | |||
| PNPLA3 p.I148M | 1.679 | 1.192–2.367 | 0.003 |
| TM6SF2 p.E167K | 1.060 | 0.587–1.914 | 0.846 |
| MBOAT7 rs641738 | 1.410 | 1.003–1.982 | 0.048 |
| Glucose | 1.020 | 1.008–1.033 | 0.002 |
| BMI | 0.989 | 0.965–1.015 | 0.413 |
| Age (years) | 1.020 | 1.002–1.039 | 0.027 |
| Sex | 1.088 | 0.671–1.763 | 0.732 |
| Presence of diabetes | 2.092 | 1.136–3.852 | 0.018 |
| Triglycerides | 1.003 | 1.000–1.007 | 0.083 |
| Cholesterol | 0.997 | 0.991–1.003 | 0.314 |
| Multivariate analysis | |||
| PNPLA3 p.I148M | 1.676 | 1.019–2.757 | 0.042 |
| MBOAT7 rs641738 | 1.766 | 1.089–2.864 | 0.021 |
CI, confidence interval; E, glutamic acid; I, isoleucine; K, lysine; M, methionine; MBOAT7, membrane bound O-acyltransferase domain containing 7; p, protein (amino acid number); PNPLA3, adiponutrin; TM6SF2, transmembrane 6 superfamily member 2 The relationships between steatosis PNPLA3, TM6SF2, and MBOAT7 variants as well as other potentially profibrotic factors were assessed by univariate and multivariate logistic regression analysis. Genetic analyses were calculated using either additive (for PNPLA3 and MBOAT7) and dominant (for TM6SF2) models.
TABLE 4.
Distribution of alleles and genotypes for PNPLA3 p.I148M and association tests in respect to fibrosis grade
| PNPLA3 p.I148M allele/genotype | Count of alleles/genotypes | |
| Fibrosis grade <F2 (2N = 410) | Fibrosis grade F2–F4 (2N = 180) | |
| [I] | 211 (0.69) | 197 (0.54) |
| [M] | 95 (0.31) | 155 (0.46) |
| [II] | 95 (0.46) | 18 (0.20) |
| [IM] | 83 (0.41) | 44 (0.49) |
| [MM] | 27 (0.13) | 28 (0.31) |
| Association test | OR | P value |
| Armitage’s trend test | 2.348 | <0.001 |
| OR statistics | OR (95% CI) | P value |
| [M] ↔ [I] | 2.491 (1.740–3.565) | <0.001 |
| [MM] ↔ [II] | 5.473 (2.637–11.361) | <0.001 |
| [MM] ↔ [IM + II] | 2.977 (1.630–5.439) | <0.001 |
| [MM + IM] ↔ [II] | 3.455 (1.925–6.200) | <0.001 |
CI, confidence interval; I, isoleucine; M, methionine; p, protein (amino acid number); PNPLA3, adiponutrin. [M] represents the steatosis risk allele. Allele and genotype frequency differences were assessed by chi2 test or by Armitage’s trend test as appropriate (https://ihg.gsf.de/cgi-bin/hw/hwa1.pl).
DISCUSSION
In the current study we analyzed a thoroughly phenotyped cohort of patients with NAFLD. According to current knowledge (2, 5), the three variants that we chose to genotype might play major roles in the development of hepatic steatosis. We demonstrate that both PNPLA3 and TM6SF2 polymorphisms are associated with increased aminotransferase activities, which might mirror enhanced liver injury in NAFLD. However, the analysis of biopsy samples underscored that the deleterious effects conferred by the tested variants are apparently related to distinct mechanisms: whereas the PNPLA3 genotype modulates the progression of both fibrosis and steatosis, the TM6SF2 variant seems to be predominantly associated with steatosis. The MBOAT7 polymorphism is likely to be, in turn, associated with the risk of liver scarring.
Our observations with respect to the PNPLA3 variant are in line with the majority of previous studies in patients with NAFLD (6). Patients with PNPLA3-associated steatohepatitis are known to be at risk of progressive liver fibrosis, cirrhosis, and eventually hepatocellular carcinoma (20). Previously published results concerning the TM6SF2 variant are less consistent. The association between this variant and fibrosis postulated by Liu et al. (11) was not replicated a biopsy-based study from Argentina (21). In this study, the authors analyzed a total of 361 patients, among them 226 with biopsy-proven mostly mild NAFLD, and found a genetic association with steatosis but not with fibrosis. These results are in line with our study. In contrast, Sookoian et al. (21) did not detect any major effects of the TM6SF2 polymorphism on liver function tests. Interestingly, the analysis of patients with chronic HCV infection (10) provided hints that the presence of variant TM6SF2 enhances liver fibrogenesis in this setting. Also, alcoholics carrying the susceptible TM6SF2 genotype seem to be at risk of liver cirrhosis (14). Recently, Eslam et al. (22) analyzed the effects of this variant on metabolic traits and liver status in a cohort of 3,260 individuals, among which a total of 502 presented with NAFLD. In this study, variant TM6SF2 was overrepresented in patients with NAFLD, among whom presence of the minor TM6SF2 allele was associated with increased fibrosis and lower serum triglycerides. It did not affect other metabolic traits, NAFLD activity score (NAS) or transaminase activities. Overall, these data might suggest that the TM6SF2 polymorphism is associated with advanced liver fibrosis in the presence of additional nongenetic factors (e.g., alcohol or viral hepatitis). However, these additional factors that might promote fibrogenesis in patients with NAFLD carrying the TM6SF2 risk genotype are yet to be defined. The MBOAT7 polymorphism has lately emerged as a new risk factor for severe liver diseases. First detected by Buch et al. (14) as a genetic determinant of an increased cirrhosis risk in alcoholics, it was subsequently associated with severe NAFLD by Mancina et al. (13). Most recently, it was demonstrated that its presence is associated with an increased fibrosis risk in patients with HCV (15). The association between fibrosis and MBOAT7 in our cohort is, hence, in line with the previous studies.
We did not detect a link between the MBAOT7 genotype and increased steatosis. This lack of association is in line with our recent results in patients undergoing bariatric surgery (23) but might be also related to an insufficient power of our cohort, which included fewer subjects than the above-mentioned studies (13–15). Furthermore, the currently studied cohort encompassed well-characterized patients from eight centers, but each biopsy was evaluated only by local pathologists, so interobserver discrepancies in defining fibrosis and steatosis and their effects of the association tests could not be excluded.
Metabolic syndrome is believed to be the major trigger of hepatic steatosis. The presence of steatosis was associated with increased serum glucose and triglyceride concentrations in our cohort as well (Fig. 1). No major association between the risk of PNPLA3, TM6SF2, or MABOT7 genotypes and distorted metabolic status has been described. Because PNPLA3- and TM6SF2-driven steatosis might even be “metabolically silent” (12), the inclusion of these two genotypes in the diagnostic work-up of patients with NAFLD could help, together with a detailed analysis of environmental determinants of fatty liver, to identify individuals at increased risk of liver injury even in the absence of the full ensemble of metabolic traits commonly associated with fatty liver disease. By adding the MBOAT7 polymorphism as the third genetic factor to the clinical work-up of the patients with NAFLD, one could further improve the chance of detecting patients who are at risk of liver fibrosis. According to a recent study (24), fibrosis represents the most important factor affecting the long-term survival in patients with NAFLD. Currently, liver biopsy is mostly recommended in patients with signs of severe steatohepatitis or fibrosis, which might lead to a selection bias in genetic studies. Hence, combined analyses of invasive and noninvasive markers of liver injury might be required in the future to elucidate the risks conferred by the PNPLA3, TM6SF2, and MBOAT7 variants.
Recently, it has been suggested that increased serum aminotransferase activities in patients with NAFLD might indicate metabolic adaptation of the liver to the fat overload rather than hepatic injury (25). Hence, it is not surprising that although both PNPLA3 and TM6SF2 polymorphisms were associated in our patients with NAFLD with increased liver functions tests, analyses of liver biopsy results demonstrate their different involvement in steatosis and fibrosis. Although the PNPLA3 and MBOAT7 risk variants display a clear association with NAFLD-driven liver fibrosis, in our cohort the TM6SF2 polymorphism was linked solely to the grade of steatosis. Importantly, the presence of variant TM6SF2 might even represent a protective factor against metabolic challenges (9, 12, 26). Based on our current results, one can still argue that, in comparison to the TM6SF2 and MBOAT7 genotypes, the PNPLA3 p.I148M variant plays a more important role as the determinant of severe hepatic phenotypes ranging from steatosis to fibrosis and cirrhosis. This is in line with our latest controlled-attenuation, parameter-based study in patients with chronic liver diseases (19). In this analysis, we did not identify any major effects of the TM6SF2 variant on liver injury, which, however, were detected in carriers of the PNPLA3 minor allele. These discrepancies might be related to substantially higher frequencies of the PNPLA3 risk allele in the general population as compared with the TM6SF2 minor allele. Indeed, the first one is carried by ∼10% of Europeans in the homozygous form, whereas <1% of individuals are homozygous carriers of the TM6SF2 p.167K allele. Hence, as in the case of MBOAT7, larger cohorts of patients might be required to fully elucidate the involvement of the TM6SF2 polymorphism in hepatic injury. Indeed, as described by Mancina et al. (13), the PNPLA3 variant has larger impact on the whole spectrum of liver disease than TM6SF2. Therefore, we could not exclude the possibility that the lack of association may be related to insufficient power in our study.
In conclusion, PNPLA3, TM6SF2, and MBOAT7 variants might be associated with liver injury in patients with NAFLD. Carriers of variant PNPLA3 present with progressive disease, but the TM6SF2 and MBAOT7 polymorphisms might also have deleterious effects on liver health. Future longitudinal studies are warranted to fully elucidate the involvement of these variants in the modulation of NAFLD.
Supplementary Material
Acknowledgments
The authors thank Annika Bohner (Homburg) for technical assistance.
Footnotes
Abbreviations:
- ALT
- alanine transaminase
- AST
- aspartate aminotransferase
- GGT
- gamma glutamyl transferase
- HCV
- hepatitis C virus
- NAFLD
- nonalcoholic fatty liver disease
- OR
- odds ratio
This work was supported, in part, by Interdisciplinary Center for Clinical Research (IZKF) Würzburg and by Grant SFB 841 from the Deutsche Forschungsgemeinschaft (J.K.). The authors declare no conflicts of interest.
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Marchesini G., Petta S., and Dalle Grave R.. 2016. Diet, weight loss, and liver health in nonalcoholic fatty liver disease: pathophysiology, evidence, and practice. Hepatology. 63: 2032–2043. [DOI] [PubMed] [Google Scholar]
- 2.Anstee Q. M., and Day C. P.. 2015. The genetics of nonalcoholic fatty liver disease: spotlight on PNPLA3 and TM6SF2. Semin. Liver Dis. 35: 270–290. [DOI] [PubMed] [Google Scholar]
- 3.Romeo S., Kozlitina J., Xing C., Pertsemlidis A., Cox D., Pennacchio L. A., Boerwinkle E., Cohen J. C., and Hobbs H. H.. 2008. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat. Genet. 40: 1461–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kozlitina J., Smagris E., Stender S., Nordestgaard B. G., Zhou H. H., Tybjaerg-Hansen A., Vogt T. F., Hobbs H. H., and Cohen J. C.. 2014. Exome-wide association study identifies a TM6SF2 variant that confers susceptibility to nonalcoholic fatty liver disease. Nat. Genet. 46: 352–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krawczyk M., Portincasa P., and Lammert F.. 2013. PNPLA3-associated steatohepatitis: toward a gene-based classification of fatty liver disease. Semin. Liver Dis. 33: 369–379. [DOI] [PubMed] [Google Scholar]
- 6.Sookoian S., and Pirola C. J.. 2011. Meta-analysis of the influence of I148M variant of patatin-like phospholipase domain containing 3 gene (PNPLA3) on the susceptibility and histological severity of nonalcoholic fatty liver disease. Hepatology. 53: 1883–1894. [DOI] [PubMed] [Google Scholar]
- 7.Fan J. H., Xiang M. Q., Li Q. L., Shi H. T., and Guo J. J.. 2016. PNPLA3 rs738409 polymorphism associated with hepatic steatosis and advanced fibrosis in patients with chronic hepatitis C virus: a meta-analysis. Gut Liver. 10: 456–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salameh H., Raff E., Erwin A., Seth D., Nischalke H. D., Falleti E., Burza M. A., Leathert J., Romeo S., Molinaro A., et al. 2015. PNPLA3 gene polymorphism is associated with predisposition to and severity of alcoholic liver disease. Am. J. Gastroenterol. 110: 846–856. [DOI] [PubMed] [Google Scholar]
- 9.Pirola C. J., and Sookoian S.. 2015. The dual and opposite role of the TM6SF2-rs58542926 variant in protecting against cardiovascular disease and conferring risk for nonalcoholic fatty liver: a meta-analysis. Hepatology. 62: 1742–1756. [DOI] [PubMed] [Google Scholar]
- 10.Milano M., Aghemo A., Mancina R. M., Fischer J., Dongiovanni P., De Nicola S., Fracanzani A. L., D’Ambrosio R., Maggioni M., De Francesco R., et al. 2015. Transmembrane 6 superfamily member 2 gene E167K variant impacts on steatosis and liver damage in chronic hepatitis C patients. Hepatology. 62: 111–117. [DOI] [PubMed] [Google Scholar]
- 11.Liu Y. L., Reeves H. L., Burt A. D., Tiniakos D., McPherson S., Leathart J. B., Allison M. E., Alexander G. J., Piguet A. C., Anty R., et al. 2014. TM6SF2 rs58542926 influences hepatic fibrosis progression in patients with non-alcoholic fatty liver disease. Nat. Commun. 5: 4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou Y., Llaurado G., Oresic M., Hyotylainen T., Orho-Melander M., and Yki-Jarvinen H.. 2015. Circulating triacylglycerol signatures and insulin sensitivity in NAFLD associated with the E167K variant in TM6SF2. J. Hepatol. 62: 657–663. [DOI] [PubMed] [Google Scholar]
- 13.Mancina R. M., Dongiovanni P., Petta S., Pingitore P., Meroni M., Rametta R., Boren J., Montalcini T., Pujia A., Wiklund O., et al. 2016. The MBOAT7–TMC4 variant rs641738 increases risk of nonalcoholic fatty liver disease in individuals of European descent. Gastroenterology 150: 1219–1230 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buch S., Stickel F., Trepo E., Way M., Herrmann A., Nischalke H. D., Brosch M., Rosendahl J., Berg T., Ridinger M., et al. 2015. A genome-wide association study confirms PNPLA3 and identifies TM6SF2 and MBOAT7 as risk loci for alcohol-related cirrhosis. Nat. Genet. 47: 1443–1448. [DOI] [PubMed] [Google Scholar]
- 15.Thabet K., Asimakopoulos A., Shojaei M., Romero-Gomez M., Mangia A., Irving W. L., Berg T., Dore G. J., Gronbaek H., Sheridan D., International Liver Disease Genetics Consortium. 2016. MBOAT7 rs641738 increases risk of liver inflammation and transition to fibrosis in chronic hepatitis C. Nat. Commun. 7: 12757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chalasani N., Younossi Z., Lavine J. E., Diehl A. M., Brunt E. M., Cusi K., Charlton M., Sanyal A. J.. 2012. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 142: 1592–1609. [DOI] [PubMed] [Google Scholar]
- 17.Weiss J., Rau M., Bantel H., Bock H., Demir M., Kluwe J., Krawczyk M., Pathil-Warth A., Schattenberg J. M., Tacke F., et al. 2015. First data concerning the medical supply of patients with non-alcoholic fatty liver disease in Germany: a survey in university hospital centers of hepatology. [Article in German] Z. Gastroenterol. 53: 562–567. [DOI] [PubMed] [Google Scholar]
- 18.Kleiner D.E., Brunt E. M., Van Natta M., Behling C., Contos M. J., Cummings O. W., Ferrell L. D., Liu Y. C., Torbenson M. S., Unalp-Arida A., Yeh M., McCullough A. J., and Sanyal A. J.. 2005. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 41: 1313–1321. [DOI] [PubMed] [Google Scholar]
- 19.Arslanow A., Stokes C. S., Weber S. N., Grünhage F., Lammert F., and Krawczyk M.. 2016. The common PNPLA3 variant p.I148M is associated with liver fat contents as quantified by controlled attenuation parameter (CAP). Liver Int. 36: 418–426. [DOI] [PubMed] [Google Scholar]
- 20.Dongiovanni P., Romeo S., and Valenti L.. 2014. Hepatocellular carcinoma in nonalcoholic fatty liver: role of environmental and genetic factors. World J. Gastroenterol. 20: 12945–12955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sookoian S., Castano G. O., Scian R., Mallardi P., Fernandez Gianotti T., Burgueno A. L., San Martino J., and Pirola C. J.. 2015. Genetic variation in transmembrane 6 superfamily member 2 and the risk of nonalcoholic fatty liver disease and histological disease severity. Hepatology. 61: 515–525. [DOI] [PubMed] [Google Scholar]
- 22.Eslam M., Mangia A., Berg T., Chan H. L., Irving W. L., Dore G. J., Abate M. L., Bugianesi E., Adams L. A., Najim M. A. , et al., International Liver Disease Genetics Consortium. 2016. Diverse impacts of the rs58542926 E167K variant in TM6SF2 on viral and metabolic liver disease phenotypes. Hepatology. 64: 34–46. [DOI] [PubMed] [Google Scholar]
- 23.Krawczyk M., Jimenez-Aguero R., Alustiza J. M., Emparanza J. I., Perugorria M. J., Bujanda L., Lammert F., and Banales J. M.. 2016. PNPLA3 p.I148M variant is associated with greater reduction of liver fat content after bariatric surgery. Surg. Obes. Relat. Dis. In press. [DOI] [PubMed] [Google Scholar]
- 24.Angulo P., Kleiner D. E., Dam-Larsen S., Adams L. A., Bjornsson E. S., Charatcharoenwitthaya P., Mills P. R., Keach J. C., Lafferty H. D., Stahler A.. 2015. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 149: 389–397.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sookoian S., Castano G. O., Scian R., Fernandez Gianotti T., Dopazo H., Rohr C., Gaj G., San Martino J., Sevic I., Flichman D., et al. 2016. Serum aminotransferases in nonalcoholic fatty liver disease are a signature of liver metabolic perturbations at the amino acid and Krebs cycle level. Am. J. Clin. Nutr. 103: 422–434. [DOI] [PubMed] [Google Scholar]
- 26.Musso G., Cassader M., Paschetta E., and Gambino R.. 2016. TM6SF2 may drive postprandial lipoprotein cholesterol toxicity away from the vessel walls to the liver in NAFLD. J. Hepatol. 64: 979–981. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.