Abstract
Initially, lipoprotein (a) [Lp(a)] was believed to be a genetic variant of lipoprotein (Lp)-B. Because its lipid moiety is almost identical to LDL, Lp(a) has been deliberately considered to be highly atherogenic. Lp(a) was detected in 1963 by Kare Berg, and individuals who were positive for this factor were called Lpa+. Lpa+ individuals were found more frequently in patients with coronary heart disease than in controls. After the introduction of quantitative methods for monitoring of Lp(a), it became apparent that Lp(a), in fact, is present in all individuals, yet to a greatly variable extent. The genetics of Lp(a) had been a mystery for a long time until Gerd Utermann discovered that apo(a) is expressed by a variety of alleles, giving rise to a unique size heterogeneity. This size heterogeneity, as well as countless mutations, is responsible for the great variability in plasma Lp(a) concentrations. Initially, we proposed to evaluate the risk of myocardial infarction at a cut-off for Lp(a) of 30–50 mg/dl, a value that still is adopted in numerous epidemiological studies. Due to new therapies that lower Lp(a) levels, there is renewed interest and still rising research activity in Lp(a). Despite all these activities, numerous gaps exist in our knowledge, especially as far as the function and metabolism of this fascinating Lp are concerned.
Keywords: review, atherosclerosis, myocardial infarction, metabolism
The number of scientific works dealing with lipoprotein (a) [Lp(a)] is reaching the ten thousands; wherefore it is not our intention here to give credit to all, or even a fraction of them, we would like to list some key works that may help the reader working in a different field, or those newly working in the field, to get to know the historical development of this topic. In addition to the articles of this Thematic Review Series on Lp(a) in the Journal of Lipid Research, two books that focus solely on Lp(a) are noteworthy: 1) the monograph published by Angelo Scanu in 1990 (1) that provides a very comprehensive overview of most work conducted until that year; and 2) the Lp(a) handbook published by a consensus panel of the European Society of Atherosclerosis (2). Current Opinion in Lipidology has also published close to 60 reviews, so far, dealing with all aspects of Lp(a) research. Worth mentioning here are also the article by Gerd Utermann, “The mysteries of lipoprotein (a)” (3), the editorial by Barnathan, “Has lipoprotein ‘little’ (a) shrunk?” (4), and the review published by us “Lipoprotein(a): still an enigma?” (5). In the following paragraphs, we intend to provide an insight into the historical developments pertinent to the particular field, giving credit to the pioneers of Lp(a) research. At the end, we summarize some more recent aspects of Lp(a) metabolism that may help to design new therapies for elevated plasma Lp(a) concentrations.
PIONEERS IN Lp DIVERSITY RESEARCH
In the “Roaring Sixties” of Lp research, three views concerning plasma Lp diversity existed: 1) electrophoretic entities comprising β-, preβ-, and α-Lp; 2) Lp density classes with the main fractions VLDL, LDL, and HDL; and 3) Lp families with the main fractions Lp-A, Lp-B, and Lp-C [reviewed in (6)]. The nomenclature relating to electrophoretic separation was used by the “East-Coast Lipid Laboratories” represented by Don Fredrickson and his collaborators (7), the density fractions were introduced by John Gofman and Frank Lindgren from the “West-Coast group from Donner Laboratories” (8), and the ABC concept was pioneered by Petar Alaupovic from Oklahoma City (9). apoB, the main component of VLDL and LDL, was recognized as a rather polymorphic apo-Lp with numerous allotypes that were highlighted by Allison and Blumberg (10). These authors tested sera from multi-transfused patients by immune-diffusion (Ouchterlony test) for the presence of iso-antibodies against LDL. Many of these antibodies were unique, as they showed no cross-reactivity in Ouchterlony tests and were comprised in the “Ag” system. In order not to depend on iso-precipitins that were not easily accessible and, in addition, exhibited a nonreproducible heterogeneity, Kare Berg set out to hyper-immunize rabbits with β- Lps isolated from 20 healthy arbitrarily chosen donors (11). Although these antisera could not distinguish between the β-Lp from the 20 individuals, Berg succeeded in preparing an antibody after cross-absorption that recognized a “Lp antigen” called Lpa, and was present in some, but not in all, sera. Sera that were positive for this factor were called Lpa+ and those negative were called Lpa−. Studying a panel of sera from 314 healthy adult donors, 34% were Lpa+. Notably, in our own studies, we quantified Lp(a) in a group of 107 healthy and 76 myocardial infarction (MI) patients from Venice and found that 35% of them had Lp(a) levels of >30 mg/dl, the value that had been adopted as the cut-off for coronary heart disease (CHD) and MI in numerous subsequent studies (12). In early days, Lp(a) was considered to be a qualitative genetic trait and a gene frequency of 0.1881 was calculated in the Norwegian population by K. Berg (11).
The Ag system mentioned above obviously reflects some sequence variations in the APOB gene or possibly variations in the sugar moiety, with approximately 14 different alleles (reviewed in Ref. 13). Additional independent polymorphisms of apoB that might not be related to the Ag system, called the Tl system (from “trypsin-treated Lps”) and the El system (from “electrophoresis”), have been described (reviewed in Ref. 8). Other suggested polymorphisms, the Ld system and the Lt system, turned out to be, in fact, Ag alleles (13).
For completeness, it is noteworthy to mention “Lp(x)” that was described by Bundschuh and Vogt (14) as a factor distinct from Lp(a) that was recognized with xeno-antibodies from horse, but not from rabbits, and was believed to be a heterogeneous form of Lp(a). All these polymorphisms have not been followed in detail in later studies, as they apparently had no relevance for either atherosclerosis or for cardiovascular diseases.
PIONEERS IN Lp(a) RESEARCH
The first pioneers of Lp(a) research came from Scandinavian countries with Kare Berg as the most prominent person, but there were others working in his group, such as Gösta Dahlen and Martin Frick (15). This group was the first to suggest that Lp(a) might be associated with CHD. Pioneering work in this field was also published by Angelo Scanu and his coworkers, Gunther Fless and Celinda Edelstein. At the beginning, Scanu’s group was mostly interested in Lp(a) structure and composition. They described an original method for isolating apo(a) from Lp(a) by rate zonal centrifugation (16). In this study, they also pointed out that apo(a) associates with apoB-100 not only by disulfide linking, but also by noncovalent interaction. When Scanu’s group first realized that the amino acid sequence of apo(a) was partially homologous to plasminogen, it opened up the avenues for apo(a) cloning (17) and further investigations in genetics. The results of apo(a) cloning provoked a wealth of studies addressing the significance of Lp(a) for blood clotting and fibrinolysis and identified the bridging between atherosclerosis and thrombosis. Celinda Edelstein, on the other hand, published a series of works on purification and stabilization of Lp(a) that provided the basis for the investigation of Lp(a) metabolism in cultured cells (18). The role of Lp(a) in hemostasis and fibrinolysis was also addressed by Angles-Cano working in the laboratory of John Chapman (19). It was suggested that Lp(a) inhibits the activation of plasminogen by t-PA during fibrin binding. There are numerous other names belonging to this chapter that contributed greatly to the interference of apo(a) with hemostasis, the most prominent being Joseph Loscalzo, Edward Plow, Robert Hegele, Joel Morrisett, and John Gaubatz. The research of White, Rainwater, and Lanford (20) provided ample insight into the abundance of Lp(a) in primates, as well as into the cellular metabolism and assembly of Lp(a).
Richard Lawn, James Tomlinson, John McLean, and Dan Eaton (21) were, beyond any doubt, pioneers in cloning and molecular biology of apo(a). These investigators not only cloned apo(a) from humans, but also from a variety of animal species, such as baboon, rhesus monkey, and hedgehog. Lawn’s research group demonstrated that apo(a) is confined to primates, yet hedgehogs express an apo(a)-like gene that is evolutionarily distinct from human apo(a), as both genes evolved are phylogenetically different. In contrast to humans, the apo(a)-like protein from hedgehog derives from plasminogen kringle-3 and lacks the kringle-V as well as the protease domain. This is considered the only example of an independent parallel evolution of an allogenic protein (22).
Marlys Koschinsky and Sally McCormick, two more pioneers in Lp(a) research, need to be mentioned here: Koschinsky was mostly involved in elucidating the apo(a) gene and protein structure and the structural features responsible for its interaction with clotting factors and macrophages (23). McCormick also contributed to the elucidation of apo(a) structure by cloning and expressing mutated apo(a) and apoB and by studying their impact on apoB metabolism and on apo(a) assembly (24).
With regard to methodology of Lp(a) measurements in the clinical laboratory, there are two eminent people who set the field: John Albers and Santica Marcovina. Albers was among the first who quantified Lp(a) by ELISA and radio-immunoassays (25). Marcovina worked on enzymatic methods for protein quantification. She produced a whole panel of monoclonal antibodies that recognized numerous different epitopes on apo(a), and most importantly, some of them reacting only with nonrepetitive structures in apo(a). This led her to standardize Lp(a) measurements in human plasma irrespective of the size polymorphism (25).
Last but not least, one of the most eminent people in illuminating the genetics of Lp(a) is Gerd Utermann. Together with his collaborators Hans Dieplinger and Hans-Jörg Kraft, Utermann not only realized that there is an extraordinary size heterogeneity of apo(a) expressed by numerous different alleles, but also that this size polymorphism is responsible for the large variation of plasma Lp(a) concentrations among individuals (26). Utermann’s group also identified numerous mutations and polymorphisms in the promoter and in the coding region of the apo(a) gene, among them the so called “null-allele” that causes the expression of a truncated form of apo(a), which does not assemble readily with LDL and is responsible for the extremely low plasma Lp(a) concentration in affected homozygous individuals (27). They made the important observations that these individuals lacking functional apo(a) are fully healthy and do not suffer from any deficiency. This raises the question that has not been answered until today, whether apo(a) might have any important physiological function.
Lp(a): FROM “PLATELET DUST” TO ONE OF THE MOST SIGNIFICANT RISK FACTORS FOR CORONARY ARTERY DISEASE
Does Lp(a) exist at all? As mentioned in the Introduction, Lp researchers in former days preferred it simple. There were lipid-stainable electrophoretic bands called α-, β-, or preβ-Lp that corresponded to HDL, LDL, and VLDL in the ultracentrifuge and, according to their major protein moiety, were more or less synonymous to LpA, LpB, and LpB:LpC, respectively. The genes of these proteins have been cloned and their inheritance and genetic variants have been established in the last century. Other apo-Lps beyond apoC have been recognized only in later days. When Berg (11) suddenly came up with a Lp that migrated like preβ, floated like HDL, and contained a protein like LpB, it was rather suspicious.
While one of us spent his postdoctoral education in the laboratory of Pierre Alaupovic in the early 1970s, Ken Walton, a recognized pathologist in atherosclerosis, was invited for a local meeting and we discussed the significance of Lps for atherosclerosis and MI and asked him what he thought about Lp(a). Ken said, “Lp(a), I never have seen such a Lp, probably it is just platelet dust”. But ultimately, he became aware of the importance of Lp(a) and a few years later Walton published that Lp(a) is likely atherogenic because he detected it in atherosclerotic lesions as well (28).
The first reports pointing toward the atherogenicity of Lp(a) came from Kare Berg’s laboratory and are summarized in (29). It was reported that the preβ1 Lp, also called “sinking preβ1 Lp (SPB)” because of its behavior in the ultracentrifuge (considered at that time as a qualitative genetic trait), could be demonstrated 2.6 times more frequently in patients with CHD. In female patients with a positive history of CHD, SPB was found with a 2.8-fold higher frequency as compared with healthy controls. Dahlen also reported that in angiographically proven coronary artery disease (CAD) patients, Lp(a) was approximately 2-fold more abundant than in healthy controls (29). He finally studied the occurrence of the preβ1 band in patients suffering from MI and found a positive correlation. All the studies mentioned above were based on the diagnosis of Lp(a) by electrophoresis as a preβ1 band, or by ultracentrifugation as a SPB, and patients were called either Lpa+ or Lpa−.
The first quantitative measurements of Lp(a) were reported by Albers and Hazzard (30) using radial immune-diffusion. They reported that in a sample of 340 untreated fasting subjects, mean Lp(a) levels were 14 mg/dl and median values were 8 mg/dl. No significant correlation with age, sex, plasma cholesterol, or triglycerides was found. Albers, Wall, and Hazzard (31) also studied the genetics of Lp(a) in 300 mother-father-offspring triplets and suggested a polygenic model of inheritance. In 1977, the group of Albers measured Lp(a) in 1,000 subjects by a sensitive and specific double antibody radioimmunoassay (32). In a subgroup of 90 MI survivors, it was reported that Lp(a) levels were significantly higher and above the 50th percentile cut-point and it was suggested that Lp(a) may be associated with premature CAD.
In 1981, we published a report in which we studied the plasma concentration of Lp(a) in 76 male post-MI patients and in 107 control individuals matched for socio-economic status, age, body weight, and blood glucose from the area of Venice (12). Lp(a) was quantified by the use of “rocket electrophoresis”. The results of that study are summarized in Table 1: We actually found that the distribution of the serum concentration of Lp(a) was highly skewed toward lower concentrations. Patients and controls were divided into “normolipemics” (at this time <6.7 mmol/l of total cholesterol and <1.82 mmol/l of triglycerides) or patients with hyperlipoproteinemia according to Fredrickson (types IIA, IIB, and IV). Next, we calculated the number and percent of patients in each subgroup that suffered from MI. From the ratio of individuals above a given cut-off level in the MI group and the group without MI (control group), we calculated a virtual risk ratio (RR). As shown in Table 1, RRs for normolipemics and type IIA patients at a cut-off level >20 mg/dl were 1.53 and 1.21, respectively, and for all MI patients, the RR was 1.20. The corresponding RRs at a cut-off value of 50 mg/dl were 2.29, “infinite”, and 1.91. For reasons that have never been explored, RRs for type IV hyperlipemics were below zero (0.48).
TABLE 1.
Quantitation of Lp(a) by rocket electrophoresis in 76 post-MI patients and in 107 matched controls
| Lp(a) >20 mg/dl | Lp(a) >50 mg/dl | Lp(a) >30 mg/dl | ||||
| Percent | RR | Percent | RR | Percent | RR | |
| Normolipemics | ||||||
| Controls | 30.9 | — | 10.9 | — | n.d. | n.d. |
| MI | 47.3 | 1.53 | 25.0 | 2.29 | n.d. | n.d. |
| Type IIa | ||||||
| Controls | 46.7 | — | 0.0 | — | n.d. | n.d. |
| MI | 56.3 | 1.21 | 18.8 | Infinite | n.d. | n.d. |
| Type IIB | ||||||
| Controls | 50,0 | — | 8,3 | — | n.d. | n.d. |
| MI | 45.0 | 0.90 | 18.8 | 2.27 | n.d. | n.d. |
| Type IV | ||||||
| Controls | 44.0 | — | 16.0 | — | n.d. | n.d. |
| MI | 30.8 | 0.70 | 7.7 | 0.80 | n.d. | n.d. |
| All subjects | ||||||
| Controls | 38.3 | — | 10.3 | — | 25.5 | — |
| MI | 46.1 | 1.20 | 19.7 | 1.91 | 44.4 | 1.74 |
Patients and controls were divided into normolipemics and different types of hyperlipoproteinemia according to Fredrickson. The table lists the percent of patients and controls with Lp(a) above the three indicated cut-off values. The RRs were calculated from percent of MI patients divided by the percent of controls belonging to the corresponding groups. The table was adapted from (12). See main text for details. n.d., not determined.
Because of the low number of individuals in each subgroup, all the differences shown above were not significant, yet when we combined all controls and all MI patients and calculated the RR at a cut-off of 30 mg/dl, the difference was significant (P < 0.05). These results were taken as the reference in countless subsequent publications where cut-off values for Lp(a) were assumed to be 30 mg/dl. In the original publication, we also pointed out that an increased risk for MI extends to plasma Lp(a) levels of <<30 mg/dl depending on the constellation of other plasma Lps. This was particularly true for patients suffering from type IIA hyperlipoproteinemia, i.e., familial hypercholesterolemia (FH).
Given the observations of Kare Berg that Lp(a) levels are genetically determined (33), we conducted a further study with close to 1,500 young male probands subjected to medical examinations on the occasion of their military draft to the Austrian army (34). We assessed the family history for possible MI and, in addition to plasma Lp(a), we also measured several lipid parameters in the probands. Fifty-two of the interrogated participants reported an early MI in their parents (10 in mothers and 42 in fathers). Whereas there was no difference in plasma total cholesterol, HDL cholesterol (HDL-C), LDL cholesterol (LDL-C), and triglycerides in sons of parents with or without MI (case vs. control group), 32.7% of the cases, but only 13.3% of the control group had plasma Lp(a) levels >25 mg/dl (Table 2). Thus, there was a 2.46-fold higher incidence rate for MI in parents if their sons had elevated plasma Lp(a).
TABLE 2.
Offspring study
| Controls (Parents without MI) | Cases (Parents with MI) | Significance | |
| Number | 1,434 | 52 | — |
| Age (years) | 54.7 | 55.5 | n.s. |
| Lp(a) >25 mg/dl | 192 (13.3%) | 17 (32.7%) | P < 0.05 |
| LDL-C/HDL-C | 1.88 | 2.13 | P < 0.05 |
Plasma Lp(a) levels were quantified in 1,500 young soldiers on the occasion of their military draft to the Austrian army whose parents suffered from MI (Cases) or were free of MI (Controls). The table lists the number and percent of soldiers with >25 mg/dl of Lp(a) in the two categories. The values in the table are taken from (37). See main text for details. n.s., not significant.
Although our two studies cited above were not free of biases, they may have served as the basis for subsequent Mendelian randomization studies conducted by the Innsbruck and Copenhagen laboratories. At the time of publication, our results attracted little attention because they were not prospective studies that were in fashion at this time, such as the PROCAM and Framingham studies. Gerd Assmann and his associates succeeded almost 20 years later to confirm our results in the PROCAM collective (35). The geometric mean of plasma Lp(a) in MI patients aged <46 years was 2.4-fold higher than that of control individuals. Moreover, the Framingham data comparing 321 men aged 50 ± 7 years who suffered from CAD to 901 control subjects confirmed that Lp(a) distinguishes CAD patients from normal individuals irrespective of apo-AI, apoB, smoking, and hypertension (36). All that was in fact only the beginning of a large series of case-control and prospective studies aimed at identifying the role of Lp(a) in the development of CAD, MI, and stroke.
A damper for Lp(a) researchers was the publication in 1993 of a report by Ridker, Hennekens, and Stampfer (37) in the Journal of the American Medical Association that proclaimed that Lp(a) should not be considered for the evaluation of the risk for MI. In this prospective study, almost 15,000 physicians aged between 40 and 84 years with no prior MI or stroke were observed for 5 years. During this time period, close to 300 participants suffered from an MI. Plasma Lp(a) of this latter group was analyzed and compared with control individuals matched for age and smoking. Median Lp(a) levels from MI patients and controls were identical (103.0 mg/l vs. 102.5 mg/l) and the authors concluded:
“In this prospective study of predominantly middle-aged white men, we found no evidence of association between Lp(a) level and risk of future MI. These data do not support the use of Lp(a) level as a screening tool to define cardiovascular risk among this population.” (ref. 37; p. 2195)
This publication was probably one of the reasons why Lp(a) went out of focus for more than 15 years. In a later report published in 2004, this study was repeated by use of a highly standardized method that was independent of apo(a) size (38). In this study, high Lp(a) values predicted the risk of angina pectoris, and considering apo(a) size resulted in a greater predictive power than Lp(a) concentrations.
Although it was never fully elucidated why this study was so divergent from results obtained in previous and subsequent reports, it was speculated that it was biased by the methodology used; this is addressed in more detail in recent reviews (39, 40).
We cannot reiterate here the entire list of works supporting the atherogenicity of Lp(a), but we instead try to provide evidence in the following that a causal relationship is likely to exist between elevated plasma Lp(a) and atherosclerosis and its consequences. A final proof awaits results from intervention trials that are, as yet, due to be designed. An upward trend in the appraisal of Lp(a) as a risk factor occurred after the genome-wide haplotype association study by Tregouet et al. (41) was published in Nature Genetics. These authors identified the SLC22A3-LPAL2-LPA gene cluster as a significant risk locus for CAD. Because of the negative correlation of the apo(a) isoform size with the plasma concentration of Lp(a), it was of interest to know whether individuals with small apo(a) isoforms might be at an increased CHD risk. In that respect, Erqou et al. (42) performed a meta-analysis including 40 publications and comprising 58,000 phenotyped individuals comprising 11,396 CHD patients. The results revealed that individuals with smaller isoforms [<22 kringle-IV (K-IV) type 2] had a 2.08-fold higher relative risk for CHD than individuals with more than 22 K-IV type 2 repeats.
Two of the most convincing reports for the causal role of Lp(a) as a CHD risk factor were published from the Copenhagen laboratories (43, 44). First, it was shown that Lp(a) was an equally significant risk factor for MI in women and men. This was established in the report of Kamstrup (45) where individuals of both sexes from the Copenhagen City Heart Collective were investigated. During a 10 year follow-up, 498 out of 9,330 individuals suffered from MI. Multivariable-adjusted hazard ratios (HRs) for MI for increasing Lp(a) levels were 1.1–3.6 in women and 1.5–3.7 in men. In a further prospective study from this group that comprised 2,824 MI events from a collective study of almost 40,500 individuals from Denmark, it was demonstrated that the risk of MI increased with plasma Lp(a) concentrations: For individuals with Lp(a) levels between the 22nd and 66th percentiles, the HR was 1.2; between the 67th and 89th percentiles, it was 1.6; between the 90th and 95th percentiles, it was 1.9; and >95th percentile, it was 2.6. Individuals at the 67th to 89th percentiles had Lp(a) plasma concentrations between 30 and 76 mg/dl and it was reassuring to note how close the HRs obtained in this large collective were compared with our early study with only 76 MI patients and a completely different set of laboratory methods (12). The Copenhagen group also found a stepwise increase in MI risk with increasing plasma Lp(a) levels and no evidence for a threshold effect. The final line of evidence that strongly supported the causal relationship of elevated plasma Lp(a) levels with CHD and MI are the results from Mendelian randomization assays published for the first time by Utermann and colleagues (46). A similar approach was applied by the Copenhagen group on a much larger population (reviewed in Ref. 25). This led the European Atherosclerosis Society Consensus Panel to consider elevated Lp(a) as an independent actual cardiovascular risk factor and to recommend a “desirable level for Lp(a) <80th percentile, i.e., less than approximately 50 mg/dl” (47).
METABOLISM OF Lp(a) AND CHALLENGES FOR THE FUTURE
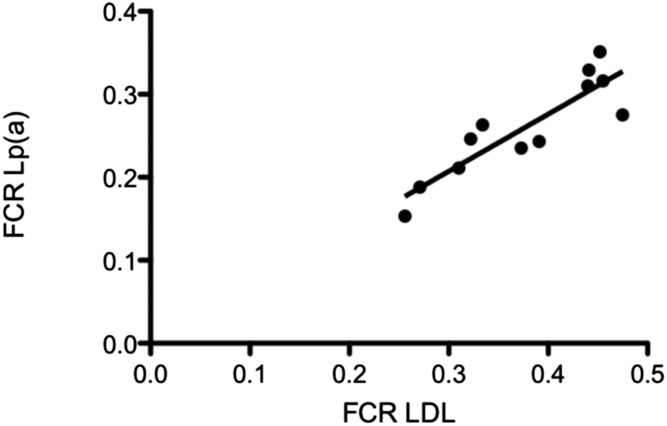
Because little reference is given to Lp(a) metabolism in this Thematic Review Series, we consider it important to devote a section to this topic. From the work of Lawn et al. (21), we know that apo(a) is almost exclusively expressed in the liver. Considering that apoB-100 is a main component of Lp(a), it was assumed in the early days of Lp(a) research that VLDL might be the metabolic precursor of Lp(a), or in other words, that Lp(a) might be secreted as triglyceride-rich particles from the liver and, after enzymatic modification of the lipid moiety, finally end up in the HDL-2 density fraction of plasma. In order to prove this, we isolated VLDL from several “Lp(a) positive” and “Lp(a) negative” donors and after labeling it, the 125I-VLDL was reinjected intravenously. The decay of radioactivity was followed for 5 days. No precursor-product relationship could be verified in these studies and we concluded that Lp(a) was synthesized independently as a separate Lp (48). In a consecutive study, we investigated the metabolism of Lp(a) with respect to biosynthesis and catabolism in nine apparently healthy volunteers. Interestingly, in contrast to the concentrations of the major apoB-containing Lp, LDL, the plasma levels of Lp(a) correlated highly significantly with the rate of biosynthesis, whereas there was no correlation with the fractional catabolic rate (FCR) (49). These results have been confirmed by several other groups using radioactive or stable isotope labeling techniques (50, 51).We have also been interested in the mechanism of Lp(a) catabolism via specific cell surface receptors. In order to study this, binding studies of LDL and Lp(a) to cultured human fibroblasts were performed. Comparable binding characteristics and inhibition of HMG-CoA reductase activity were observed with both LDL and Lp(a). Specific high-affinity binding to fibroblasts was abolished if Lp(a) or LDL was treated with cyclohexanedione, which blocks lysine (Lys) residues that are required for binding of apoB-100 to the LDL receptor (LDL-R). Fibroblasts from a homozygous FH patient did not show high affinity binding of LDL or Lp(a). In addition, turnover studies were conducted by injecting 125I-labeled Lp(a) plus 131I-labeled LDL simultaneously into 12 male volunteers and the decay of the radioactivity over time was followed (52). The metabolic parameters obtained in in vivo turnover studies are shown in Table 3. The FCR was significantly lower for Lp(a) as compared with LDL and, again, the rate of synthesis correlated significantly with the plasma Lp(a) concentration. What was interesting, however, was the fact that we observed a significant correlation between the FCR of Lp(a) and that of LDL (Fig. 1). These data were compatible with the assumption that Lp(a) might be cleared by the LDL-R. On the other hand, the FCR of Lp(a) in one homozygous FH patient was not different from that of normolipemic controls, suggesting that the LDL-R was not involved in Lp(a) clearance.
TABLE 3.
Metabolic parameters of Lp(a) in comparison to LDL
| Metabolic Parameters of Individuals Studies | Normolipemic Healthy Volunteers | FH Patient | ||||
| LDL | Lp(a) | LDL | Lp(a) | |||
| Number of volunteers | 12 | 1 | ||||
| Sex | Male | Male | ||||
| Age | 65 ± 13 | 16 | ||||
| Body weight (kg) | 66.3 ± 10.3 | 46 | ||||
| Plasma cholesterol (mg/dl) | 200.7 ± 31.7 | 521 | ||||
| Plasma triglycerides (mg/dl) | 120.8 ± 42.3 | 65 | ||||
| LDL-apoB (mg/dl) | 73.4 ± 11.4 | 253 | ||||
| Plasma Lp(a) (mg/dl) | 41.6 ± 32.7 | 17 | ||||
| Half-life (t/2) | 3.84 ± 0.62 | 3.93 ± 0.83 | 5.17 | 4.81 | ||
| FCR | 0.377 ± 0.077 | 0.260 ± 0.060 | 0.205 | 0.21 | ||
| Rate of LDL apoB synthesis (mg/kg/day) | 12.44 ± 2.09 | 23.44 | ||||
| Rate of Lp(a) synthesis (mg/kg/day)a | 4.60 ± 3.64 | 1.61 | ||||
| Percent intravascular pool | 64.2 ± 9.1 | 76.1 ± 7.7 | 69.4 | 71.6 | ||
125I-Lp(a) and 131I-LDL were injected simultaneously into 12 healthy individuals and the metabolic parameters were calculated from the die-away curves followed over 14 days. Values in the table are adapted from (59). See main text for details.
The units for Lp(a) synthesis refer to the entire Lp(a) Lp.
Fig. 1.
Correlation of the FCRs of Lp(a) to those of LDL. Twelve volunteers were injected simultaneously with 125I-labeled Lp(a) plus 131I-labeled LDL and the decay of radioactivity was measured at different time points up to 1 week. FCR is displayed in pools per day (r = 0.853; P < 0.01). See main text for details.
There are numerous subsequent reports that are relevant for the potential binding of Lp(a) to the LDL-R. We were actually the first to report that statins known to significantly increase the LDL-R activity do not reduce plasma Lp(a) in most cases, but in fact raise it in some individuals (53). This is reviewed in depth in a recent report (54). The increase of Lp(a) by statins argues against a role of the LDL-R in Lp(a) catabolism. Interestingly, the group of Koschinsky recently reported that PCSK9 modulates Lp(a) binding to Hep-G2 cells and that Lp(a) binds to the LDL-R on fibroblasts and liver cells (55). This was taken as an explanation of why new generation LDL-C lowering drugs, notably PCSK-9 inhibitors, reduce plasma Lp(a) by approximately 35%. These drugs are very effective in lowering LDL-C by inhibition of the lysosomal degradation of LDL-Rs and elevating the number of these receptors on cell surfaces. In view of these findings, the question on the role of LDL-R on Lp(a) catabolism has been warmed up again and is currently a hot topic in Lp(a) research.
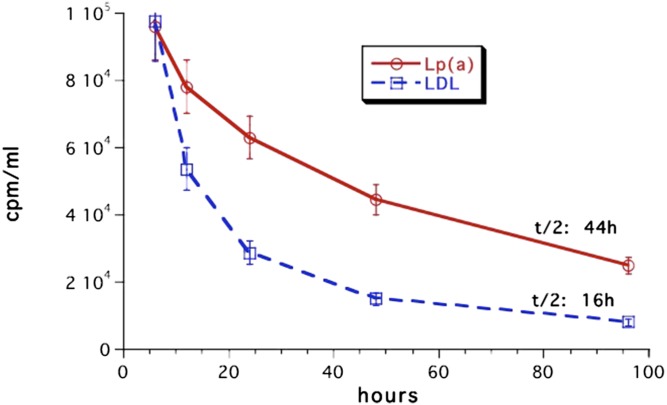
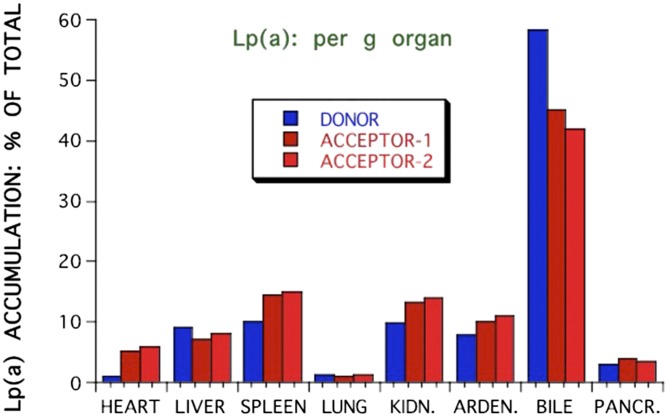
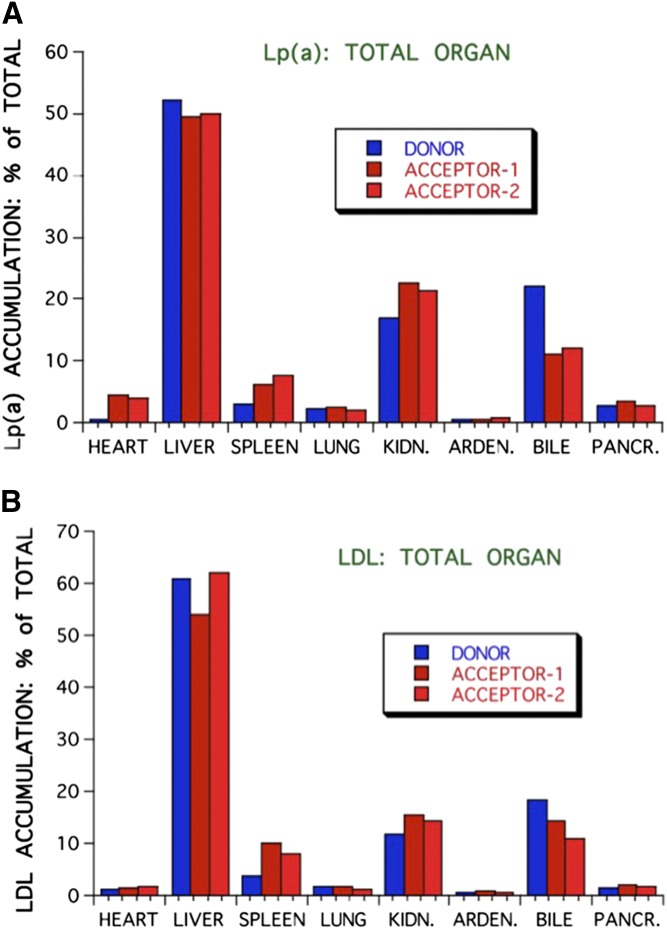
The most burning questions relating to Lp(a) catabolism today are: 1) What is the site of Lp(a) removal? 2) Is the LDL-R pathway involved in the Lp(a) metabolism and, if so, what is the role of PCSK9? 3) Are there other (Lp) receptors involved? 4) Is Lp(a) catabolized as intact Lp or are components of it catabolized separately? With respect to the site of removal in vivo, data only exist from experimental animals that, in their wild-type form, do not express human-like apo(a). In order to still get a hint on the site of Lp(a) removal, we investigated the Lp(a) catabolism in hedgehogs, who synthesize an Lp(a)-like Lp (56). Lps were labeled with the nondegradable label, 125I tyramine cellobiose, and the biological half-life and the accumulation in different organs of Lp(a) was studied in comparison to LDL. Unfortunately, we could not isolate sufficient amounts of hedgehog Lp(a), and therefore performed these studies with human Lps. As can be seen in Fig. 2, the in vivo half-life of Lp(a) was almost three times as long (44 h) as compared with LDL (16 h). Because we found a great deal of Lp(a) accumulation in spleen in preliminary experiments, radiolabeled Lp(a) or LDL were biologically screened in some cases by injecting them intravenously into a donor hedgehog, and after 3 h, blood was taken from the donor and injected into two different “acceptor”-hedgehogs. Figure 3 shows that the major amount of 125I label was found in bile in the form of nonprecipitable free iodine. On a gram basis, spleen and kidney accumulated the major amount of precipitable Lp(a), followed by adrenals, liver, heart, and pancreas. Considering the whole organs, liver was the most important organ, taking up approximately 50% of the injected Lp(a) 3 h after injection, followed by kidney and spleen (Fig. 4). Biologically screened Lp(a) was taken up by the spleen at an even higher rate than unscreened Lp(a), indicating that isolation and labeling of Lp(a) did not grossly alter the metabolic properties of the injected Lps. When compared with LDL, there was no great difference apparent concerning organ distribution. A major question that was not addressed in these studies is where the label was situated, on apoB, apo(a), or both. This would certainly add to the interpretation of these results.
Fig. 2.
Half-life of Lp(a) in comparison to LDL in hedgehogs. Human Lps were labeled with the nondegradable label, 125I tyramine cellobiose, and injected individually into male or female hedgehogs (n = 20). Blood was taken at the indicated time intervals and the radioactivity was measured in a β-counter. See main text for details.
Fig. 3.
Accumulation of Lp(a) in various organs of hedgehogs. Human Lp(a), labeled with 125I tyramine cellobiose, was injected into hedgehogs. After 24 h animals were euthanized, the organs were perfused with ice-cold saline, and the radioactivity was counted. For biological screening purposes, some animals received the radiolabeled Lp and 2 h later, blood from donor animals was harvested and reinjected into two “acceptor animals”. After 24 h, the animals were euthanized and the radioactivity of various organs was counted. Values are expressed in percent radioactivity per gram of organ. See main text for details.
Fig. 4.
Accumulation of Lp(a) in various organs (A) in comparison to LDL (B). The experimental setup was the same as described in Fig. 3, but the values are expressed in percent radioactivity accumulated after 24 h in total organs. See main text for details.
Subsequent in vivo experiments were carried out in wild-type and in WHHL rabbits (57). In WHHL rabbits, the FCR of Lp(a) (1.355 ± 0.189 pools per day) and of LDL (1.278 ± 0.397 pools per day) were significantly lower than in wild-type animals (2.008 ± 0.083 pools per day and 2.855 ± 0.759 pools per day, respectively). In wild-type rabbits, Lp(a) was catabolized significantly more slowly than LDL, yet in WHHL rabbits the FCRs of both Lps were comparable. After 48 h, the ratios of Lp(a):LDL in liver, kidney, spleen, and bile were 1.525, 1.020, 1.819, and 1.967 in WHLL rabbits. The corresponding values in wild-type rabbits were 0.590, 0.677, 0.862, and 0.766, respectively. From these results, we concluded that there must be other mechanisms in addition to the LDL-R that might be responsible for Lp(a) clearance. The results also undermined the role of spleen in Lp(a) catabolism with the caveat that these experiments were carried out with human Lp(a) in animal models that did not have Lp(a).
Because apo(a) is a highly glycosylated protein containing a large amount of sialic acid, we considered the possibility that Lp(a) might be removed from circulation by the asialoglycoprotein receptor. Asialo-Lp(a) injected into hedgehogs exhibited an extremely short half-life (0.55 h) in comparison to native Lp(a) [13.8 h (58)]. Asialo-orosomucoid competitively inhibited not only the fast catabolism of asialo-Lp(a), but also of native Lp(a). These latter results suggest that a fraction of even native Lp(a) might be catabolized by the asialoglycoprotein receptor under physiological conditions. It is tempting to note that a strategy for lowering plasma Lp(a) by the asialoglycoprotein receptor was published by Theo van Berkel’s group in 1995 (59). They synthesized a tri-antennary hydrophobic galactoside that was capable of binding avidly to Lp(a) and, in turn, led to a 20-fold increase of its hepatic Lp(a) uptake.
On the whole, we found that, in various animal species, approximately 50% of the injected radioactively labeled Lp(a) is removed by the liver and degradation products of Lp(a) are found in bile. The major organs for removal were liver, spleen, and kidney. The mechanism for Lp(a) catabolism by the liver has not been resolved in detail, yet we believe that the asialoglycoprotein receptor may play a role in the removal of a minor Lp(a) fraction, at least as far as the desialylated form of apo(a) is concerned (58).
Role of the kidney in Lp(a) metabolism
There are several important aspects that have been published and it is impossible to reference even a fraction of them. One of the first observations pinpointing elevated plasma Lp(a) levels in kidney disease was published by the group of Fruchart in 1987 (60). In this short communication, it was reported that in patients with chronic renal failure treated by hemodialysis, plasma Lp(a) levels were approximately 3-fold higher as compared with controls. We published in1989 that patients with membranoproliferative glomerulonephritis had significantly higher plasma Lp(a) levels than controls, yet Lp(a) concentrations correlated negatively with daily urinary protein loss (61). In these early studies, we suggested that kidney may have a regulatory role in Lp(a) metabolism.
In 1996, two review articles were published that summarize the work on Lp(a) and kidney disease until then (62, 63). From there on, more than 500 related articles have been published that may deserve a separate review article. Here also, the work of Kronenberg et al. (64) needs mentioning, who measured the arteriovenous difference in plasma Lp(a) concentration and found some 13.5% lower values in the venous compartment. If one kidney would remove that fraction of Lp(a) during a single passage, the FCR of Lp(a) would have been extremely high, which in fact has not been observed.
Urinary excretion of apo(a) fragments
Mooser et al. (65) described for the first time that low molecular weight fragments of apo(a) are excreted into the urine. In subsequent experiments, we measured the amount of apo(a) fragments in humans secreted by the kidney over 2 week intervals and calculated the amount of Lp(a) cleared by this mechanism (66). From these studies, we concluded that the catabolism of Lp(a) by this mechnism accounts for less than 1% of the daily clearance. Also, there was a highly significant correlation between plasma and urine apo(a) concentrations and urinary apo(a) was at least equal, if not better, in distinguishing coronary patients from control individuals. In order to get some insight into the mechanism of this pathway, larger N-terminal fragments of apo(a) were expressed in mice and the excretion of their cleavage products into the urine was followed (67). These studies revealed that metalloproteinases located on skeletal muscle, kidney, and other organs cleave apo(a) into lower molecular fragments; fragments up to a size of six K-IV repeats are secreted into the urine of mice; yet in humans, fragments with a higher number of K-IV repeats are found. This is a very fast process, because if such fragments were injected from donor mice into acceptor mice they appeared within minutes in the urine. Whether or not these observations might be relevant for the metabolism of Lp(a) in humans remains to be resolved.
Lp(a) assembly
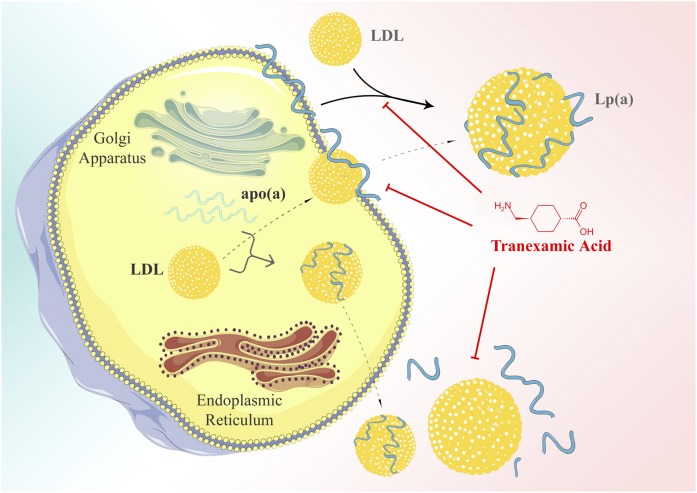
The assembly of Lp(a) appears to take place from LDL and apo(a) in two steps. This was shown by studies where we isolated recombinant apo(a) by chromatography by Lys-Sepharose followed by incubation in vitro with purified LDL. It was found that, in the first step, apo(a) binds to LDL through the interaction of Lys groups in apoB-100 and through the kringle structure in apo(a) (68). This step may be inhibited by Lys analogs with tranexamic acid being the most effective. In the second step, the mature Lp(a) is formed and stabilized by a disulfide bridge (69). A two-step model for Lp(a) formation has been subsequently confirmed by Trieu and McConnathy (70).
Kringles, in fact, are secondary protein structures responsible for the binding to Lys groups in proteins. For an efficient assembly of Lp(a), certain K-IV subtypes are essential. Gabel et al. (71) suggested that K-IV types 7 and 8 are required for a maximal efficient formation of Lp(a), whereas the Lys binding domain in K-IV type 10 was not of importance. Koschinsky et al. (72) identified Cys4057 in K-IV type 9 to be involved in the covalent linkage of apo(a) to apoB-100. Results from our own laboratory confirmed the importance of K-IV type 9 in the formation of a covalent bridge between apo(a) and apoB; in extension of this, we provided evidence that K-IV type 6 is essential for the first step of assembly (68).
Although any of the kringles in apo(a) potentially bind to Lys, the binding affinity to specific kringles varies quite substantially. Even more important is the observation by the group of Marlys Koschinsky that there are mutants of K-IV type 10 (Trp70→Arg or Phe; Arg35→Lys) with much lower or absent binding to Lys-Sepharose and it is suggested that this affects the atherogenicity of Lp(a) via interfering with fibrinolysis or the interaction with cell surface structures (73).
We also studied the binding of Lp(a) to Lys-Sepharose in view of plasminogen interaction and found that only part of the Lp(a) applied to the columns was retained, the rest of some 25–30% eluted without binding (74). If this latter fraction was rechromatographed over Lys-Sepharose, none of it was bound. The exact nature of this phenomenon has not been pursued further, and is an additional proof of the heterogeneity of Lp(a).
The interaction of Lys groups with kringle structures might be inhibited by Lys analogs. This was addressed in more detail using several natural and synthetic small compounds (75). Tranexamic acid, a compound that, in fact, is used as medication to interfere with fibrinolysis in vivo, exhibited the highest affinity (IC50 of 0.65) followed by several physiological compounds, with decreasing affinity (IC50 0.76–55): δ-aminovaleric acid, γ-aminobutyric acid, ε-aminohexanoic acid, spermine, spermidine ornithine, proline, Lys, and taurine (Table 3). Thus, all substances listed here ahead of Lys may, in fact, have an impact on Lp(a) metabolism in vivo and certainly should be studied in closer detail in view of the pathophysiology of Lp(a).
The morphology of LDL also appears to be important. This was delineated from the observation that LCAT-deficient patients had extremely low plasma Lp(a) levels (76). We isolated the LDL fraction of control individuals and homozygous and heterozygous patients suffering from LCAT deficiency. LDL from the latter group of patients was almost free of core lipids (cholesteryl esters) and consisted of large vesicles and small spheres with apoB as the main protein component. LDL from homozygous LCAT-deficient patients did not assemble with apo(a), whereas LDL from heterozygous patients that had a normal appearance and, in comparison to LDL from control individuals, yielded a normal assembly and formed a “native-like” Lp(a).
The Lp(a) assembly, whether intra- or extracellular, appears to be a determinant for its plasma concentration. There are currently two controversial views on the site of Lp(a) assembly that are highlighted in a review by Dieplinger and Utermann (77). White and Lanford (78) concluded from their studies in cultured primary hepatocytes from baboon that, after intracellular maturation and secretion, apo(a) partly or as a whole binds to the surface of hepatocytes and as LDL passes by, the final apo(a):LDL complex is formed. This model of assembly is compatible with the observations that: 1) transgenic apo(a) mice secrete free apo(a) that is not bound to LDL; and 2) by mixing of recombinant apo(a) with LDL in vitro, an Lp(a)-like particle is formed that is indistinguishable from native Lp(a) (69). On the other hand, there are conflicting results published on the intracellular or extracellular occurrence of apo(a):apoB complexes: Lobentanz et al. (79) found no evidence of intracellular apo(a):apoB complexes in HepG2 cells even if cultivated at 15°C, a temperature where secretory proteins are captured in the endoplasmic reticulum. The apo(a):apoB complexes, on the other hand, were demonstrated by others in lysates of thoroughly washed primary human hepatocytes (80). The strongest support, finally, for an intracellular assembly is obtained from in vivo stable isotope turnover experiments of Lp(a) in humans (51). The authors followed the incorporation of the tracer, d3-leucine, into apo(a) and into apoB in Lp(a) or in LDL over time in nine healthy individuals with plasma Lp(a) levels >15 mg/dl. Whereas the production rate of apo(a) and apoB in Lp(a) was comparable, apoB in LDL exhibited an almost 30-fold higher production rate. Although other interpretations are feasible, the authors concluded that the assembly of Lp(a) most probably takes place intracellularly. Finally, an interesting finding must be mentioned here that was published by the group of Davidson in 1998 (81). The authors incubated HepG2 cells expressing a 17 IV apo(a) protein with oleate and demonstrated a 2- to 6-fold increase of apo(a) secretion that was apparently caused by protection of proteasomal apo(a) degradation. The inhibition of the microsomal triglyceride transfer protein, on the other hand, caused a dose-dependent decrease of apo(a) secretion. These studies suggest that apo(a) secretion might be coupled, to a certain extent, to the synthesis of triglyceride-rich Lp in the liver.
An important question that needs to be resolved is as to what the assembly might bear some impact on plasma Lp(a) concentrations. In order to study this, transgenic mice expressing only apo(a) or apo(a) plus human apoB-100 were treated with the Lys analogs, tranexamic acid or δ-amino valeric acid, and their influence on plasma-free and apoB-bound apo(a) concentrations was followed over 1–2 weeks (82). The results of this study may be summarized as follows: 1) Treatment of transgenic apo(a) or apo(a)-apoB mice with Lys analogs caused a doubling of the plasma concentration of apo(a). 2) The ratio of apo(a):apoB in the double-transgenic mice did not change during Lys-analog treatment. Tranexamic acid significantly increased the half-life of apo(a) from 6 to 8 h. 3) When McA-RH 7777 cells stably transfected with apo(a) were incubated with Lys analogs, we noticed a 1.4-fold increase of apo(a) in the incubation medium. We interpreted these results such that Lys analogs increase plasma Lp(a) concentrations by: 1) increasing the dissociation of cell surface bound apo(a) that otherwise might have been internalized and degraded; and 2) reducing the catabolism of Lp(a) (schematically displayed in Fig. 5). In a similar fashion, Wang and White (83) suggested that the Lys analog, 6-aminohexoic acid, that increased the secretion of a 17 KIV containing apo(a) protein transfected into HepG2 cells 8- to 14-fold might act as a chemical chaperone for apo(a). We believe that all of these findings might be relevant for the metabolism or function of Lp(a) in view of the occurrence of natural Lys analogs in plasma that are listed in Table 4.
Fig. 5.
Models of the assembly of Lp(a) from LDL and apo(a). Today, essentially two alternative possibilities are discussed: an intracellular and an extracellular assembly. Lys-analogs, such as tranexamic acid, inhibit the assembly in vitro and probably also in vivo. ©Medical University of Graz.
TABLE 4.
Lys and Lys-analogs that inhibit the assembly of Lp(a)
| Substance | IC50 (mmol/l) |
| Benzamidine | >100 |
| Serotonine | >100 |
| Glutamic acid | >100 |
| Taurine | 55 |
| N-ε-acetyl-Lys | 52 |
| Lys | 38 |
| p-Aminomethylbenzene-sulfonamide | 33 |
| Hydroxyproline | 25 |
| Proline | 21 |
| N-α-acetyl-Lys | 9.7 |
| Ornithine | 8.2 |
| Spermidine | 6.5 |
| Spermine | 5.4 |
| ε-Aminohexoic acid | 4.8 |
| γ-Aminobutyric acid | 2.1 |
| δ-Aminovaleric acid | 0.76 |
| Tranexamic acid | 0.65 |
Purified LDL and recombinant apo(a) were mixed and free apo(a) was separated from Lp(a) through ultracentrifugation. IC50 relates to the concentration of the substance that inhibits 50% of the assembly. Values are from (75).
TRANSCRIPTIONAL REGULATION OF apo(a) EXPRESSION
After cloning of apo(a) in primates and hedgehog (21, 22), it became apparent that its metabolism differs from that of all other plasma Lps. The apo(a) is almost exclusively expressed in the liver and, thus, most of the relevant work has been performed in cultured primary hepatocytes. The apo(a) gene cluster comprises some six homologous genes and pseudogenes related to plasminogen. Despite the fact that these genes contain extensive regions of great homology characterized by the presence of numerous cis-elements, the regulation and expression of these genes is distinct (84). In 1994, Wade, Lindahl, and Lawn (85) characterized numerous positive and negative regulatory elements in the promoter region of apo(a) with HNF-1a as an important transcription factor for the tissue-specific expression of apo(a). In early studies, relatively short promoter fragments up to 500 bp had been cloned. In subsequent experiments from the group of Taramelli, it was demonstrated that fragments up to 3 kb upstream of the transcriptional start site are required to account for the liver-specific expressional activity of apo(a) (86).
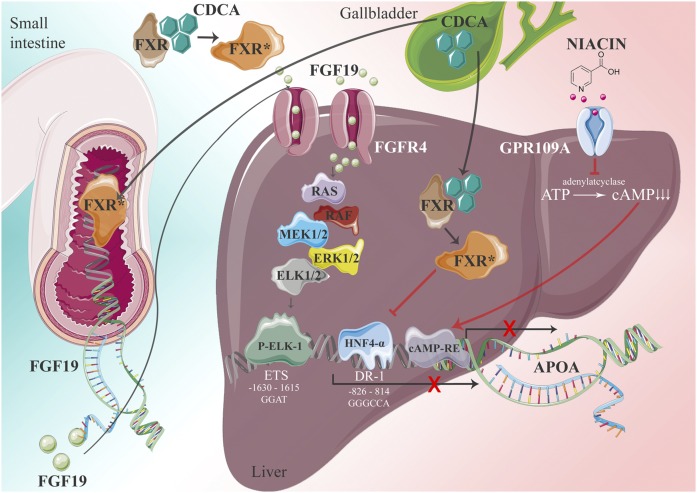
There are several secondary factors that have a great impact on plasma Lp(a) levels. Among them are sex hormones, thyroid hormones and insulin, kidney disease (59–63), and liver disease, which will not be reviewed here. Interestingly, heavy alcohol consumers, even if they showed no symptoms for liver disease, were found to have extremely low plasma Lp(a) levels (87). What mechanism, in fact, causes this reduction of Lp(a) has never been clarified. More importantly, we found that patients suffering from cholestasis also had extremely low plasma Lp(a) and when patients were successfully treated for this disease, Lp(a) levels rose to “normal”. In a series of experiments conducted in transgenic mice and humans, we elucidated the mechanism of low Lp(a) in cholestasis. The culprit factor was bile acid that serves as a ligand for FXR, an important transcription factor for liver proteins (88). We found that apo(a) expression is driven to a significant extent by a HNF-4α-responsive element in the apo(a) promoter. Bile acid binding to FXR causes the translocation of this nuclear receptor to the nucleus and the competitive displacement of HNF-4α from the promoter. This pathway accounts for approximately 60% of apo(a) expression. On the other hand, bile acids in the gut promote the expression of FGF-15/19 that, in turn, binds on the liver to its cognate receptor and by signaling via a cascade of signal factors, where at the end stands Elk-1, that interact with an ETS-1 binding element in the −1,630/−1,615 bp region of the human APOA promoter. This element functions as an Elk-1 binding site that mediates repression of APOA transcription by FGF19.This pathway accounts for approximately 40% of the FXR effect (89), as shown in the cartoon of Fig. 6.
Fig. 6.
Cartoon showing the transcriptional regulation of APOA. Influence of bile acids [chenodeoxycholic acid (CDCA) and nicotinic acid]. See main text for details. ©Medical University of Graz.
The transcriptional regulation of apo(a), in fact, appears to be much more complex than outlined above. In silico search of the promoter region of APOA revealed response elements for more than 70 transcription factors and nuclear receptors, among them HNFs, FXR, PPARs, RXR, SREBPs, CCAAT-enhancer, cAMP, IL-6, and others. With respect to IL-6, it has been suggested that apo(a) is an acute phase protein, yet the exact nature of this is controversial. Whether or not all the observed cis-elements in the promoter region might be operational needs further investigation. Of note is the cAMP-response element that appears to be important for the action of nicotinic acid (niacin) to reduce plasma Lp(a) concentrations. Niacin is one of the few older generation lipid-lowering drugs that, at high doses, significantly lowers Lp(a) up to 35%. We addressed the question of the molecular mechanism of niacin in transgenic mice and found that, on liver cells, niacin binds to its receptor, GPR109A, thereby inhibiting the activation of adenylate cyclase. This leads to the downregulation of the cAMP level in the liver and reduced binding to the cAMP response element in the APOA promoter that apparently is a positive regulatory element for APOA transcription (90).
The inhibition of apo(a) gene expression is currently the most promising strategy for an effective treatment of patients with grossly elevated Lp(a). In early experiments along this line, we transduced transgenic mice expressing a fragment of apo(a) with an adenovirus that led to the synthesis of a hammer-head mRNA directed against specific sequences in the apo(a) mRNA (91). This led to an almost complete disappearance of the corresponding mRNA and, in turn, of the expressed apo(a) fragment from plasma.
Post-transcriptional regulation
The plasma concentration of Lp(a), on the other hand, in addition is regulated by several posttranscriptional events. White, Rainwater, and Lanford (20) pioneered this work in the early 1990s; they studied the metabolism of Lp(a) in primary hepatocytes from baboons and found that the maturation process of apo(a) differs between small and large isoforms of apo(a) and has an impact on the apo(a)/Lp(a) abundance in plasma: The apo(a) is biosynthesized as a lower molecular weight precursor with a prolonged residence time in the endoplasmic reticulum and early Golgi compartment. After maturation and probably glycosylation, a larger mature apo(a) is secreted. Micro-RNAs appear to have additional effects: There are a few thousands of micro-RNAs that operate by fine-tuning the translation of almost all known genes. A preliminary in silico search performed in our laboratory by Manjula Vinod revealed several micro-RNAs with putative binding sites to APOA mRNA (M. Vinod and G. Kostner, unpublished observations).
A promising strategy to reduce apo(a) biosynthesis is currently being pursued by the company, IONIS®, who generated single stranded chimeric oligonucleotides directed against APOA mRNA [ISIS-APO(a)Rx]. They were tested at doses between 50 and 400 mg in patients with different plasma Lp(a) concentrations. At the most effective treatment regime, ISIS-APO(a)Rx lowered plasma Lp(a) by 77.8%. This treatment also led to a significant reduction of oxidized phospholipids (oxPLs) in plasma (92).
A new form of this antisense, which has agalactose moiety attached to target it to the liver, can reduce Lp(a) up to 99% and at doses that are 1/10 to 1/30 of the dose of the ASO used in the first generation of antisense oligonucleotides. A detailed review on this topic is found in (93).
GAPS IN OUR KNOWLEDGE ABOUT Lp(a) AND CHALLENGES FOR THE FUTURE
Angelo M. Scanu edited a compiled series of reports on Lp(a) that summarized all the accumulated knowledge about this Lp up to 1990 (1). The topics of this book comprised the structure, molecular biology, genetics, metabolism, methodology, epidemiology, and the relationship of Lp(a) to hemostasis and fibrinolysis. All well-established groups working in Lp(a) research displayed their results in the 15 chapters of this book, which provided a comprehensive overview of knowledge in the field up to 1990. There is no point or even space to reiterate all the information detailed in Scanu’s book, but we intend to pinpoint open questions that need to be resolved.
There are important issues that need clarification regarding Lp(a) structure and composition: Kare Berg described Lp(a) from the beginning as a rather homogenous preβ1 band that in later work turned out to float in the density region of HDL-2 (1, 15). The presence of apo(a) in other density fractions was not really pursued. Yet, in earlier studies, we detected apo(a) in almost any density fraction that we investigated (94, 95). Even in the triglyceride-rich fraction of d < 1.006 g/l, we found approximately 5% of the immunochemical reactivity of apo(a). The apo(a) is also found, to a variable extent, in traditional LDL fractions, particularly in donors with high plasma Lp(a) concentrations. There is also a rather large amount of apo(a) (up to 8%) that does not precipitate with heparin, dextran sulfate, polyethylene glycol, or phosphotungstate. Furthermore, up to 3% of the total plasma immune reactivity can be detected in the bottom fraction at d > 1.21 g/l after ultracentrifugation (66, 96). This latter fraction represents mostly apo(a) fragments that are not associated to apoB-100 and are free of lipids. The fragments represent a proteolytic product of Lp(a) degradation and are secreted into urine (see below). Even in the HDL-2 fraction, Lp(a) is heterogenous due to the size polymorphism of apo(a). The theoretical molecular mass of various apo(a) isoforms together with their carbohydrate content ranges from approximately 400 kDa to 800 kDa, causing an overspill of Lp(a) by ultracentrifugation into density ranges of LDL, on one hand, and to HDL-3, on the other hand. The significance of apo(a) in VLDL is far from being clear: does it represent a direct biosynthetic product or a precursor of Lp(a) with HDL density characteristics, or is it just an artifact caused by the isolation procedure? Another question that needs further clarification is the role of different kringles in binding to Lys groups of different proteins. Do they have an additional role above the interaction with apoB-100 or cell surface components? Are there specific receptors, e.g., the plasminogen receptor family, that specifically recognize these structures?
PATHOPHYSIOLOGY OF Lp(a)
As mentioned above, there are numerous gaps in our knowledge concerning the physiology of Lp(a); yet it appears that our knowledge relating to Lp(a) pathophysiology is more advanced. In early days, we studied the interaction of Lp(a) with cell surface components, notably with glycosaminoglycans (GAGs) and proteoglycans (97). GAGs isolated from human aorta had a 4-fold higher affinity to Lp(a) than to LDL. When GAG-Lp(a) complexes were incubated with mouse peritoneal macrophages, we observed a 1.3-fold higher stimulation of cholesterol esterification than with LDL-GAG complexes. Thus, it appears that Lp(a) preferentially binds to the vessel wall via GAGs and, in turn, migrates to the intimal space where it is avidly taken up by macrophages, a process that is of key importance for atherogenesis. There are nice studies by Nordestgaard and Nielsen (98) that show this in vivo in animal models.
In addition, there are numerous pathophysiological mechanisms put forward pointing toward the mechanism of the atherogenicity of Lp(a) that cannot be reviewed here. We would, however, like to mention a very appealing pathway elucidated in recent years. Oxidized lipids, in general, and oxidized LDL, in particular, are widely accepted as playing a key role in atherogenesis (99, 100). Phospholipids, particularly lecithin (the major component of cell membranes), are prone to oxidation because of their unsaturated fatty acid content. oxPLs bind with high affinity to Lp(a). Such oxPL-Lp(a) complexes promote the biosynthesis of pro-inflammatory cytokines, notably IL-1 that is an earmark for the promotion of atherosclerosis (101).These findings may also explain why Lp(a) is very atherogenic in some individuals, and in others probably much less so because the genetic control of IL-1 responses is rather complex, and the responses of IL-1 may stratify the population risk for cardiovascular diseases in combination with oxPL-Lp(a) (102). The affinity of oxPLs to Lp(a) may also give a hint on a possible physiological role of Lp(a): It has recently been shown by the La Jolla research group in collaboration with the laboratory of J. Handa that Lp(a) may protect mice from macular degradation mediated by oxPLs (103).
Acknowledgments
The authors acknowledge the countless researchers that could not be mentioned here, without whom our knowledge on Lp(a) would be far less advanced. The authors thank Emrah Eroglu for drawing the cartoons in Figs. 5 and 6.
Footnotes
Abbreviations:
- APOA
- gene or mRNA of apo(a)
- apo(a)
- characteristic glycoprotein component of Lp(a)
- CAD
- coronary artery disease
- CHD
- coronary heart disease
- FCR
- fractional catabolic rate
- FH
- familial hypercholesterolemia
- GAG
- glycosaminoglycan
- HDL-C
- HDL cholesterol
- HR
- hazard ratio
- K-IV
- kringle-IV
- LDL-C
- LDL cholesterol
- LDL-R
- LDL receptor
- Lp
- lipoprotein
- Lp(a)
- lipoprotein (a)
- Lys
- lysine
- MI
- myocardial infarction
- oxPL
- oxidized phospholipid
- RR
- risk ratio
- SPB
- sinking pre-β1 lipoprotein [synonymous to lipoprotein (a)]
REFERENCES
- 1.Scanu A. M. 1990. Lipoprotein (a). Academic Press, San Diego. [Google Scholar]
- 2.Nordestgaard B. G., Chapman M. J., and Ginsberg H. N.. 2013. Lipoprotein(a): A Handbook for Clinicians. Available at: https://itunes.apple.com/gb/app/lipoprotein-atherosclerosis/id648424814?mt=8. [Google Scholar]
- 3.Utermann G. 1989. The mysteries of lipoprotein (a). Science. 246: 904–910. [DOI] [PubMed] [Google Scholar]
- 4.Barnathan E. S. 1993. Has lipoprotein ‘little’ (a) shrunk? JAMA. 270: 2224–2225. [PubMed] [Google Scholar]
- 5.Kostner K. M., and Kostner G. M.. 2002. Lipoprotein(a): still an enigma? Curr. Opin. Lipidol. 13: 391–396. [DOI] [PubMed] [Google Scholar]
- 6.Kostner G. M. 1983. Apolipoproteins and lipoproteins of human plasma: significance in health and disease. Adv. Lipid Res. 20: 1–43. [PubMed] [Google Scholar]
- 7.Fredrickson D. S., Levy R. I., and Lees R. S.. 1967. Fat transport in lipoproteins–an integrated approach to mechanisms and disorders. N. Engl. J. Med. 276: 148–156. [DOI] [PubMed] [Google Scholar]
- 8.Gofman J. W., Lindgren F. T., and Elliott H.. 1949. Ultracentrifugal studies of lipoproteins of human serum. J. Biol. Chem. 179: 973–979. [PubMed] [Google Scholar]
- 9.Alaupovic P., Kostner G., Lee D. M., McConnathy W. J., and Magnani H. N.. 1972. Peptide composition of human plasma lipoproteins A, B and C. Expos. Annu. Biochim. Med. 31: 145–160. [PubMed] [Google Scholar]
- 10.Allison A. C., and Blumberg B. S.. 1961. An isoprecipitation reaction distinguishing human serum protein types. Lancet. 1: 634–637. [DOI] [PubMed] [Google Scholar]
- 11.Berg K. 1963. A new serum type system in man–the Lp system. Acta Pathol. Microbiol. Scand. 59: 369–382. [DOI] [PubMed] [Google Scholar]
- 12.Kostner G. M., Avogaro P., Cazzolato G., Marth E., Bittolo-Bon G., and Quinci G. B.. 1981. Lipoptotein Lp(a) and the risk for myocardial infarction. Atherosclerosis. 38: 51–61. [DOI] [PubMed] [Google Scholar]
- 13.Kostner G. M. 1976. Lp(a) lipoproteins and the genetic polymorphisms of lipoprotein B. In Low Density Lipoproteins. C. E. Day and R. S. Levy, editors. Plenum Press, New York. 229–269. [Google Scholar]
- 14.Bundschuh G., and Vogt A.. 1965. Die Häufigkeit des Merkmals Lp(a) in der Berliner Bevölkerung. Humangenetik. 1: 379–382. [DOI] [PubMed] [Google Scholar]
- 15.Berg K., Dahlen G., and Frick M. H.. 1974. Lp(a) lipoprotein and pre-β1 lipoprotein in patients with coronary heart disease. Clin. Genet. 6: 230–235. [DOI] [PubMed] [Google Scholar]
- 16.Fless G. M., ZumMallen M. E., and Scanu A. M.. 1985. Isolation of apolipoprotein(a) from lipoprotein(a). J. Lipid Res. 26: 1224–1229. [PubMed] [Google Scholar]
- 17.McLean J. W., Tomlinson J. E., Kuang W. J., Eaton D. L., Chen E. Y., Fless G. M., Scanu A. M., and Lawn R. M.. 1987. cDNA sequence of human apolipoprotein(a) is homologous to plasminogen. Nature. 330: 132–137. [DOI] [PubMed] [Google Scholar]
- 18.Scanu A. M., Himman J., Pfaffinger D., and Edelstein C.. 2004. Sucessful utilization of lyophilized lipoprotein(a) as a biological reagent. Lipids. 39: 589–593. [DOI] [PubMed] [Google Scholar]
- 19.Rouy D., Grailhe P., Nigon F., Chapman J., and Angles-Cano E.. 1991. Lipoprotein(a) impairs generation of plasmin by fibrin-bound tissue-type plasminogen activator. In vitro studies in a plasma millieu. Arterioscler. Thromb. 11: 629–638. [DOI] [PubMed] [Google Scholar]
- 20.White A. L., Rainwater D. L., and Lanford R. E.. 1993. Intracellular maturation of apolipoprotein(a) and assembly of lipoprotein(a) in primary baboon hepatocytes. J. Lipid Res. 34: 509–517. [PubMed] [Google Scholar]
- 21.Lawn R. M., Tomlinson J. E., McLean W. J., and Eaton D. L.. 1990. Molecular biology of apolipoprotein(a). In Lipoprotein (a). A. M. Scanu, editor. Academic Press, San Diego. 25–40. [Google Scholar]
- 22.Lawn R. M., Boonmark W. N., Schwartz K., Lindahl G. E., Wade D. P., Byrne C. D., Fong K. J., Meer K., and Patthy L.. 1995. The recurring evolution of lipoprotein(a). Insights from cloning of hedgehog apolipoprotein(a). J. Biol. Chem. 270: 24004–24009. [DOI] [PubMed] [Google Scholar]
- 23.Anglés-Cano E., Hervio L., Rouy D., Fournier C., Chapman J. M., Laplaud M., and Koschinsky M. L.. 1994. Effects of lipoprotein(a) on the binding of plasminogen to fibrin and its activation by fibrin-bound tissue-type plasminogen activator. Chem. Phys. Lipids. 67–68: 369–380. [DOI] [PubMed] [Google Scholar]
- 24.McCormick S. P., and Nielsen L. B.. 1998. Expression of large genomic clones in transgenic mice: new insights into apolipoprotein B structure, function and regulation. Curr. Opin. Lipidol. 9: 103–111. [DOI] [PubMed] [Google Scholar]
- 25.Albers J. J., and Marcovina S. M.. 1994. Standardization of Lp(a) measurements. Chem. Phys. Lipids. 67–68: 257–263. [DOI] [PubMed] [Google Scholar]
- 26.Utermann G., Menzel H. J., Kraft H. G., Duba H. C., Kemmler H. G., and Seitz C.. 1987. Lp(a) glycoprotein phenotypes. Inheritance and relation to Lp(a)-lipoprotein concentrations in plasma. J. Clin. Invest. 80: 458–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parson W., Kraft H. G., Niederstatter H., Lingenhel A. W., Kochl S., Fresser F., and Utermann G.. 2004. A common nonsense mutation in the repetitive kringle IV-2 domain of human apolipoprotein(a) results in a truncated protein and low plasma Lp(a). Hum. Mutat. 24: 474–480. [DOI] [PubMed] [Google Scholar]
- 28.Walton K. W., Hitchens J., Magnani H. N., and Khan M.. 1974. A study of methods of identification and estimation of Lp(a) lipoprotein and of ist significance in health, hyperlipidemia and atherosclerosis. Atherosclerosis. 20: 323–346. [DOI] [PubMed] [Google Scholar]
- 29.Dahlén G. 1974. The pre-beta1 lipoprotein phenomenon in relation to serum cholesterol and triglyceride levels, the Lp(a) lipoprotein and coronary heart disease. Acta Med. Scand. Suppl. 570: 1–45. [PubMed] [Google Scholar]
- 30.Albers J. J., and Hazzard W. R.. 1974. Immunochemical quantification of human plasma Lp(a) lipoprotein. Lipids. 9: 15–26. [DOI] [PubMed] [Google Scholar]
- 31.Albers J. J., Wahl P., and Hazzard W. R.. 1974. Quantitative genetic studies of the human plasma Lp(a) lipoprotein. Biochem. Genet. 11: 475–486. [DOI] [PubMed] [Google Scholar]
- 32.Albers J. J., Adolphson J. L., and Hazzard W. R.. 1977. Radioimmunoassay of human plasma Lp(a) lipoprotein. J. Lipid Res. 18: 331–338. [PubMed] [Google Scholar]
- 33.Berg K. 1990. Lp(a) lipoprotein: an overview. In Lipoprotein (a). A. M. Scanu, editor. Academic Press, San Diego. 1–23. [Google Scholar]
- 34.Hoefler G., Harnoncourt F., Paschke E., Mirtl W., Pfeiffer K. P., and Kostner G. M.. 1988. Lipoprotein Lp(a). A risk factor for myocardial infarction. Arteriosclerosis. 8: 398–401. [DOI] [PubMed] [Google Scholar]
- 35.Sandkamp M., Funke H., Schulte H., Köhler E., and Assmann G.. 1990. Lipoprotein(a) is an independent risk factor for myocardial infarction at a young age. Clin. Chem. 36: 20–23. [PubMed] [Google Scholar]
- 36.Genest J. Jr., McNamara J. R., Ordovas J. M., Jenner J. L., Silberman S. R., Anderson K., Wilson P. W., Salem D. N., and Schaefer E. J.. 1992. Lipoprotein cholesterol, apolipoprotein A-I and B and lipoprotein (a) abnormalities in men with premature coronary artery disease. J. Am. Coll. Cardiol. 19: 792–802. [DOI] [PubMed] [Google Scholar]
- 37.Ridker P. M., Hennekens C. H., and Stampfer M. J.. 1993. A prospective study of lipoprotein(a) and the risk of myocardial infarction. JAMA. 270: 2195–2199. [PubMed] [Google Scholar]
- 38.Rifai N., Ma J., Sacks F. M., Ridker P. M., Hernandez W. J., Stampfer M. J., and Marcovina S. M.. 2004. Apolipoprotein(a) size and lipoprotein(a) concentration and future risk of angina pectoris with evidence of severe coronary atherosclerosis in men: the Physicians’ Health Study. Clin. Chem. 50: 1364–1371. [DOI] [PubMed] [Google Scholar]
- 39.Marcovina S. M., and Albers J. J.. 2016. Lipoprotein (a) measurements for clinical application. J. Lipid Res. 57: 526–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kostner K. M., März W., and Kostner G. M.. 2013. When should we measure lipoprotein(a). Eur. Heart J. 34: 3268–3276. [DOI] [PubMed] [Google Scholar]
- 41.Trégouët D. A., König I. R., Erdmann J., Munteanu A., Braund P. S., Hall A. S., Grosshennig A., Linsel-Nitschke P., Perret C., DeSuremain M., et al. . 2009. Genome-wide haplotype association study identifies the SLC22A3-LPAL2-LPA gene cluster as a risk locus for coronary artery disease. Nat. Genet. 41: 283–285. [DOI] [PubMed] [Google Scholar]
- 42.Erqou S., Thompson A., Di Angelantonio E., Saleheen D., Kaptoge S., Marcovina S., and Danesh J.. 2010. Apolipoprotein(a) isoforms and the risk of vascular disease: systematic review of 40 studies involving 58 000 participants. J. Am. Coll. Cardiol. 55: 2160–2167. [DOI] [PubMed] [Google Scholar]
- 43.Kamstrup P. R., Benn M., Tybjaerg-Hansen A., and Nordestgaard B. G.. 2008. Extreme lipoprotein(a) levels and risk of myocardial infarction in the general population: the Copenhagen City Heart Study. Circulation. 117: 176–184. [DOI] [PubMed] [Google Scholar]
- 44.Kamstrup P. R., Tybjærg-Hansen A., Steffensen R., and Nordestgaard B. G.. 2009. Genetically Elevated lipoprotein(a) and increased risk of myocardial infarction. JAMA. 301: 2331–2339. [DOI] [PubMed] [Google Scholar]
- 45.Kamstrup P. R. 2010. Lipoprotein(a) and ischemic heart disease–a causal association? A review. Atherosclerosis. 211: 15–23. [DOI] [PubMed] [Google Scholar]
- 46.Sandholzer C., Boerwinkle E., Saha N., Tong M. C., and Utermann G.. 1992. Apolipoprotein(a) phenotypes, Lp(a) concentration and plasma lipid levels in relation to coronary heart disease. J. Clin. Invest. 89: 1040–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nordestgaard B. G., Chapman J. M., Ray K., Boren J., Andreotti F., Watts G. F., Ginsberg H., Amarenco P., Catapano A., Descamps O. S., et al. . 2010. Lipoprotein(a) as a cardiovascular risk factor: current status. Eur. Heart J. 31: 2844–2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Krempler F., Kostner G., Bolzano K., and Sandhofer F.. 1979. Lipoprotein(a) is not a metabolic product of other lipoproteins containing apolipoproteinB. Biochim. Biophys. Acta. 575: 63–70. [DOI] [PubMed] [Google Scholar]
- 49.Krempler F., Kostne G. M., and Bolzano K.. 1980. Turnover of lipoprotein(a) in man. J. Clin. Invest. 65: 1483–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rader D. J., Cain W., Ikewaki K., Talley G., Zech L. A., Usher D., and Brewer H. B. Jr. 1994. The inverse association of plasma lipoprotein(a) concentrations with apolipoprotein(a) isoform size is not due to differences in Lp(a) catabolism but to differences in production rate. J. Clin. Invest. 93: 2758–2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Frischmann M. E., Ikewaki K., Trenkwalder E., Lamina C., Dieplinger B., Soufi M., Schweer H., Schaefer J. R., Konig P., Kronenberg F., et al. . 2012. In vivo stable-isotope kinetic study suggests intracellular assembly of lipoprotein(a). Atherosclerosis. 225: 322–327. [DOI] [PubMed] [Google Scholar]
- 52.Krempler F., Kostner G. M., Roscher A., Haslauer K., Bolzano K., and Sandhofer F.. 1983. Studies on the role of specific cell surface receptors in the removal of lipoprotein(a) in man. J. Clin. Invest. 71: 1431–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kostner G. M., Gavish D., Leopold B., Bolzano K., Weintraub M. S., and Breslow J. L.. 1989. HMG-CoA reductase inhibitors lower LDL cholesterol without reducing Lp(a) levels. Circulation. 80: 1313–1319. [DOI] [PubMed] [Google Scholar]
- 54.Yeang C., Hung M. Y., Byun Y. S., Clopton P., Yang X., Witztum J. L., and Tsimikas S. J.. 2016. Effect of therapeutic interventions on oxidized phospholipids on apolipoprotein B100 and lipoprotein(a). J. Clin. Lipidol. 10: 594–603. [DOI] [PubMed] [Google Scholar]
- 55.Romagnuolo R., Scipione C., Boffa M. B., Marcovina S., Seidah N. G., and Koschinsky M. L.. 2015. Lipoprotein(a) catabolism is regulated by proprotein convertase subtilisin/kexin type 9 trough the low density lipoprotein receptor. J. Biol. Chem. 290: 11649–11662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kostner G. M., Wo X., Frank S., Zimmermann R., and Steyrer E.. 1997. Metabolism of Lp(a): assembly and excretion. Clin. Genet. 52: 347–354. [DOI] [PubMed] [Google Scholar]
- 57.Liu R., Saku K., Kostner G. M., Hitata K., Zhang B., Shimori M., and Arakawa K.. 1993. In vivo kinetics of lipoprotein(a) in homozygous Watanabe heritable hyperlipidaemic rabbits. Eur. J. Clin. Invest. 23: 561–565. [DOI] [PubMed] [Google Scholar]
- 58.Hrzenjak A., Frank S., Wo X., Zhou Y., Van Berkel T., and Kostner G. M.. 2003. Galactose specific asialoglycoprotein receptor is involved in lipoprotein(a) catabolism. Biochem. J. 376: 765–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Biessen E. A., Vietsch H., and van Berkel T. J.. 1996. Induction of hepatic uptake of lipoprotein(a) by cholesterol-derivatized cluster galactosides. Arterioscler. Thromb. Vasc. Biol. 16: 1552–1558. [DOI] [PubMed] [Google Scholar]
- 60.Parra H. J., Mezdour H., Cachera C., Dracon M., Tacquet A., and Fruchart J. C.. 1987. Lp(a) lipoprotein in patients with chronic renal failure treated by hemodialysis. Clin. Chem. 33: 721. [PubMed] [Google Scholar]
- 61.Karádi I., Romics L., Pálos G., Domán J., Kaszás I., Hesz A., and Kostner G. M.. 1989. Lp(a) lipoprotein concentrations in serum of patients with heavy proteinuria of different origin. Clin. Chem. 35: 2121–2123. [PubMed] [Google Scholar]
- 62.Kronenberg F., Utermann G., and Dieplinger H.. 1996. Lipoprotein(a) in renal disease. Am. J. Kidney Dis. 27: 1–25. [DOI] [PubMed] [Google Scholar]
- 63.Stenvinkel P., and Berglund L.. 1996. Lipoprotein(a) in chronic renal disease. Miner. Electrolyte Metab. 22: 16–21. [PubMed] [Google Scholar]
- 64.Kronenberg F., Trenkwalder E., Lingenhel A., Friedrich G., Lhotta K., Schober M., Moes N., König P., Utermann G., and Dieplinger H.. 1997. Renovascular arteriovenous differences in Lp(a) plasma concentrations suggest removal of Lp(a) from the renal circulation. J. Lipid Res. 38: 1755–1763. [PubMed] [Google Scholar]
- 65.Mooser V., Marcovina S. M., White A. L., and Hobbs H. H.. 1996. Kringle-containing fragments of apolipoprotein(a) circulate in human plasma and are excreted into the urine. J. Clin. Invest. 98: 2414–2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kostner K. M., Maurer G., Huber K., Stefenelli T., Dieplinger H., Steyrer E., and Kostner G. M.. 1996. Urinary excretion of apo(a) fragments. Role in apo(a) catabolism. Arterioscler. Thromb. Vasc. Biol. 16: 905–911. [DOI] [PubMed] [Google Scholar]
- 67.Frank S., Hrzenjak A., Blaschitz A., Dohr G., and Kostner G. M.. 2001. Role of various tissues in apo(a) fragmentation and excretion of fragments by the kidney. Eur. J. Clin. Invest. 31: 504–512. [DOI] [PubMed] [Google Scholar]
- 68.Frank S., Durovic S., and Kostner G. M.. 1994. Structural requirements of apo-a for the lipoprotein-a assembly. Biochem. J. 304: 27–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Frank S., Krasznai K., Durovic S., Lobentanz E. M., Dieplinger H., Wagner E., Zatloukal K., Cotten M., Utermann G., Kostner G. M., et al. . 1994. High-level expression of various apolipoprotein(a) isoforms by transferrinfection: the role of kringle IV sequences in the extracellular association with low-density lipoprotein. Biochemistry. 33: 12329–12339. [DOI] [PubMed] [Google Scholar]
- 70.Trieu V. N., and MacConnarthy W. J.. 1995. A two-step model for lipoprotein(a) formation. J. Biol. Chem. 270: 15471–15474. [DOI] [PubMed] [Google Scholar]
- 71.Gabel B. R., Ma L. F., Marcovina S. M., and Koschinsky M. L.. 1996. Lipoprotein(a) assembly. Quantitative assessment of the role of apo(a) kringle IV types 2-10 in particle formation. Arterioscler. Thromb. Vasc. Biol. 16: 1559–1567. [DOI] [PubMed] [Google Scholar]
- 72.Koschinsky M. L., Cote G. P., Gabel B., and van der Hoek Y. Y.. 1993. Identification of the cysteine residue in apolipoprotein(a) that mediates extracellular coupling with apolipoprotein B-100. J. Biol. Chem. 268: 19819–19825. [PubMed] [Google Scholar]
- 73.Rahman M. N., Petrounevitch V., Jia Z., and Koschinsky M. L.. 2001. Antifibrinolytic effect of single apo(a) kringle domains: relationship to fibrinogen binding. Protein Eng. 14: 427–438. [DOI] [PubMed] [Google Scholar]
- 74.Karàdi I., Kostner G. M., Gries A., Nimpf J., Romics L., and Malle E.. 1988. Lipoprotein (a) and plasminogen are immunochemically related. Biochim. Biophys. Acta. 960: 91–97. [DOI] [PubMed] [Google Scholar]
- 75.Frank S., Durovic S., Kostner K., and Kostner G. M.. 1995. Inhibitors of the in vitro assembly of Lp(a). Arterioscler. Thromb. Vasc. Biol. 15: 1774–1780. [DOI] [PubMed] [Google Scholar]
- 76.Steyrer E., Durovic S., Frank S., Gießauf W., Burger A., Dieplinge H., Zechner R., and Kostner G. M.. 1994. The role of lecithin:cholesterol acyltransferase for lipoprotein(a) assembly. Structural integrity of low density lipoproteins is a prerequisite for Lp(a) formation in human plasma. J. Clin. Invest. 94: 2330–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dieplinger H., and Utermann G.. 1999. The seventh myth of lipoprotein(a): where and how is it assembled. Curr. Opin. Lipidol. 10: 275–283. [DOI] [PubMed] [Google Scholar]
- 78.White A. L., and Lanford R. E.. 1995. Biosynthesis and metabolism of lipoprotein(a). Curr. Opin. Lipidol. 6: 75–80. [DOI] [PubMed] [Google Scholar]
- 79.Lobentanz E. M., Krasznai K., Gruber A., Brunner C., Muller H. J., Sattler J., Kraft H. G., Utermann G., and Dieplinger H.. 1998. Intracellular metabolism of human apolipoprotein(a) in stably transfected Hep G2 cells. Biochemistry. 37: 5417–5425. [DOI] [PubMed] [Google Scholar]
- 80.Edelstein C., Davidson N. O., and Scanu A. M.. 1994. Oleate stimulates the formation of triglyceride-rich particles containing apoB100-apo(a) in long-term primary cultures of human hepatocytes. Chem. Phys. Lipids. 67–68: 135–143. [DOI] [PubMed] [Google Scholar]
- 81.Nassir F., Bonen D. K., and Davidson N. O.. 1998. Apolipoprotein(a) synthesis and secretion from hepatoma cells is coupled to triglyceride synthesis and secretion. J. Biol. Chem. 273: 17793–17800. [DOI] [PubMed] [Google Scholar]
- 82.Frank S., Hrzenjak A., Kostner K., Sattler W., and Kostner G. M.. 1999. Effect of tranexamic acid and delta-aminovaleric acid on Lopoprotein(a) metabolism in transgenic mice. Biochim. Biophys. Acta. 1438: 99–110. [DOI] [PubMed] [Google Scholar]
- 83.Wang J., and White A. L.. 1999. 6-Aminohexanoic acid as a chemical chaperone for apolipoprotein(a). J. Biol. Chem. 274: 12883–12889. [DOI] [PubMed] [Google Scholar]
- 84.Wade D. P., Clarke J. G., Lindahl G. E., Liu A. C., Zysow B. R., Meer K., Schwartz K., and Lawn R. M.. 1993. 5′ control regions of the apolipoprotein(a) gene and members of the related plasminogen gene family. Proc. Natl. Acad. Sci. USA. 90: 1369–1373. [DOI] [PMC free article] [PubMed]
- 85.Wade D. P., Lindahl C. E., and Lawn R. M.. 1994. Apoliporotein(a) gene transcription is regulated by liver-enriched trans-actin factor hepatic nuclear factor-1a. J. Biol. Chem. 269: 19757–19765. [PubMed] [Google Scholar]
- 86.Bopp S., Kochl S., Acquati F., Magnaghi P., Petho-Schramm A., Kraft H. G., Utermann G., Muller H. J., and Taramelli R.. 1995. Ten allelic apolipoprotein[a] 5′ flanking fragments exhibit comparable promoter activities in HepG2 cells. J. Lipid Res. 36: 1721–1728. [PubMed] [Google Scholar]
- 87.Marth E., Cazzolato G., Bittolo Bon G., Avogaro P., and Kostner G. M.. 1982. Serum concentrations of Lp(a) and other lipoprotein parameters in heavy alcohol consumers. Ann. Nutr. Metab. 26: 56–62. [DOI] [PubMed] [Google Scholar]
- 88.Chennamsetty I., Claudel T., Kostner K. M., Baghdasaryan A., Kratky D., Levak-Frank S., Frank S., Gonzalez F. J., Trauner M., and Kostner G. M.. 2011. Farnesoid X receptor represses hepatic human APOA gene expression. J. Clin. Invest. 121: 3724–3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chennamsetty I., Claudel T., Kostner K. M., Trauner M., and Kostner G. M.. 2012. FGF19 signaling cascade suppresses APOA gene expression. Arterioscler. Thromb. Vasc. Biol. 32: 1220–1227. [DOI] [PubMed] [Google Scholar]
- 90.Chennamsetty I., Kostner K. M., Claudel T., Vinod M., Frank S., Weiss T. S., Trauner M., and Kostner G. M.. 2012. Nicotinic acid inhibits hepatic APOA gene expression: studies in humans and in transgenic mice. J. Lipid Res. 53: 2405–2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Frank S., Gauster M., Strauss J., Hrzenjak A., and Kostner G. M.. 2001. Adenovirus-mediated apo(a)-antisense-RNA expression efficiently inhibits apo(a) synthesis in vitro and in vivo. Gene Ther. 8: 425–430. [DOI] [PubMed] [Google Scholar]
- 92.Tsimikas S., Viney N. J., Hughes S. G., Singleton W., Graham M. J., Baker B. F., Burkey J. L., Yang Q., Marcovina S. M., Geary R. S., et al. . 2015. Antisense therapy targeting apolipoprotein(a): a randomised, double-blind, placebo controlled phase 1 study. Lancet. 386: 1472–1483. [DOI] [PubMed] [Google Scholar]
- 93.Graham M. J., Viney N., Crooke R. M., and Tsimikas S.. 2016. Antisense inhibition of apolipoprotein(a) to lower plasma lipoprotein (a) levels in humans. J. Lipid Res. 57: 340–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kronenberg F., Steinmetz A., Kostner G. M., and Dieplinger H.. 1996. Lipoprotein(a) in health and disease. Crit. Rev. Clin. Lab. Sci. 33: 495–543. [DOI] [PubMed] [Google Scholar]
- 95.Kostner G. M. 1990. The physiological role of Lp(a). In Lipoprotein (a). A. M. Scanu, editor. Academic Press, San Diego 183–204. [Google Scholar]
- 96.Gries A., Nimpf J., Nimpf M., Wurm H., and Kostner G. M.. 1987. Free and apoB-associated Lpa-specific protein in human serum. Clin. Chim. Acta. 164: 93–100. [DOI] [PubMed] [Google Scholar]
- 97.Bihari-Varga M., Gruber E., Rotheneder M., Zechner R., and Kostner G. M.. 1988. Interaction of lipoprotein Lp(a) and low density lipoprotein with glycosaminoglycans from human aorta. Arteriosclerosis. 8: 851–857. [DOI] [PubMed] [Google Scholar]
- 98.Nordestgaard B. G., and Nielsen L. B.. 1994. Atherosclerosis and arterial influx of lipoproteins. Curr. Opin. Lipidol. 5: 252–257. [DOI] [PubMed] [Google Scholar]
- 99.Klatt P., and Esterbauer H.. 1996. Oxidative hypothesis of atherogenesis. J. Cardiovasc. Risk. 3: 346–351. [DOI] [PubMed] [Google Scholar]
- 100.Steinberg D., and Witztum J. L.. 2010. Oxidized low-density lipoprotein and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 30: 2311–2316. [DOI] [PubMed] [Google Scholar]
- 101.Bergmark C., Dewan A., Orsoni A., Merki E., Miller E. R., Shin M. J., Binder C. J., Hörkkö S., Krauss R. M., Chapman M. J., et al. . 2008. A novel function of lipoprotein [a] as a preferential carrier of oxidized phospholipids in human plasma. J. Lipid Res. 49: 2230–2239. [DOI] [PubMed] [Google Scholar]
- 102.Tsimikas S., Gordon W., Duff M. D., Berger P. B., Rogus J., Huttner K., Clopton P., Birlaki E., Kornmann K. S., and Witztum J. L.. 2014. Pro-inflammatory interleukin-1 genotypes potentiate the risk of coronary artery disease and cardiovascular events mediated by oxidized phospholipids and lipoprotein(a). J. Am. Coll. Cardiol. 63: 1724–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Handa J. T., Tagami M., Katayoon E., Leibundgut G., Janiak A., Witztum J. L., and Tsimikas S.. 2015. Liporotein(a) with an intact lysine binding site protects the retina from an age-related macular degradation phenotype in mice. Trans. Am. Ophthalmol. Soc. 113: T51–T522. [PMC free article] [PubMed] [Google Scholar]