Abstract
Objectives
A link between negative life stress and the onset of mood episodes in bipolar disorder (BD) has been established, but processes underlying such a link remain unclear. Growing evidence suggests that stress can negatively affect reward processing and related neurobiological substrates, indicating that a dysregulated reward system may provide a partial explanation. The aim of this study was to test the impact of stress on reward-related neural functioning in BD.
Methods
Thirteen euthymic or mildly depressed individuals with BD and 15 controls performed a Monetary Incentive Delay task while undergoing functional magnetic resonance imaging during no-stress and stress (negative psychosocial stressor involving poor performance feedback and threat of monetary deductions) conditions.
Results
In hypothesis-driven region-of- interest-based analyses, a significant group by condition interaction emerged in the amygdala during reward anticipation. Relative to controls, while anticipating a potential reward, subjects with BD were characterized by amygdalar hyperactivation in the no-stress condition but hypoactivation during stress. Moreover, relative to controls, subjects with BD had significantly larger amygdala volumes. After controlling for structural differences, the effects of stress on amygdalar function remained, whereas groups no longer differed during the no-stress condition. During reward consumption, a group by condition interaction emerged in the putamen due to increased putamen activation to rewards in participants with BD during stress, but an opposite pattern in controls.
Conclusions
Overall, findings highlight possible impairments in using reward-predicting cues to adaptively engage in goal-directed actions in BD, combined with stress-induced hypersensitivity to reward consumption. Potential clinical implications are discussed.
Keywords: amygdale, bipolar disorder, fMRI, MID, putamen, reward, stress
Bipolar disorder (BD) causes marked impairment across social, cognitive, and occupational domains of functioning (1, 2). Although BD is highly heritable, environmental effects such as negative life stress play a significant role in the development and maintenance of mood symptoms, including symptom onset (3), increased time to recovery (4), and higher likelihood of relapse (5). Despite clear links between life stress and BD, these relationships are not well understood. The reward system may be a key substrate in this regard. This system is particularly interesting because individuals with BD often experience anhedonia (e.g., reduced reactivity to pleasurable stimuli) during depressive episodes and hyperhedonia (e.g., increased pleasure-seeking behavior and reactivity to pleasurable stimuli) during manic episodes (6, 7). Various neuroimaging findings suggest that individuals with BD may have an underlying hypersensitivity to rewards [see (8) for review]: euthymic individuals with BD have been found to exhibit hyperactivation of the amygdala in response to rewards and reward reversal contingencies (9), and elevated ventral striatum and orbitofrontal cortex (OFC) activity during reward anticipation (10). In spite of these findings, inconsistencies exist [e.g., (11, 12)], including a recent report of hypoactivation of dorsal striatal regions in unmedicated sub-syndromal individuals with BD during reward anticipation (13), which points more towards blunted reward processing as a vulnerability factor. Mixed findings may partially stem from the fact that structural abnormalities in reward-related regions have also emerged in BD (e.g., 8,14,15), but volumetric findings are rarely controlled for in functional magnetic resonance imaging (fMRI) reports. Such inconsistencies highlight the need for additional research, including the importance of accounting for possible structural abnormalities while probing functional activation.
Convergent lines of evidence suggest that focusing on the reward system in BD in relation to negative stress might be particularly important, since stress often precedes depressive episodes in BD (3). Preclinical and clinical studies have shown that acute stress can reduce reward responsiveness and trigger anhedonic-like behaviors (16–18). Given the behavioral and neurobiological influence of stressors on reward processing in psychiatrically healthy individuals, in combination with the characteristic hyperhedonia and anhedonia seen in BD, it is imperative to investigate the ways in which environmental stressors may disrupt the reward system in BD. To this end, we investigated stress-induced reward dysfunction in euthymic patients with BD utilizing a monetary incentive delay task, an acute psychosocial stress manipulation, and fMRI.
Given previous fMRI studies with the Monetary Incentive Delay Task (MID) task (19, 20), we hypothesized that, relative to controls, euthymic individuals with BD would show reward-related heightened activation in the amygdala [e.g., (9)], but reduced activation in basal ganglia regions [e.g., (25)] during both anticipation and consumption of rewards. Regarding the impact of negative psychosocial stress, we previously found that controls exposed to acute psychosocial stress exhibited greater activation in the basal ganglia and amygdala during reward anticipation and blunted activation of striatal regions during reward consumption (22). Given the very limited neuroimaging studies reporting on stress-related reward processing in BD (23), we broadly anticipated that individuals with BD would also show dysfunctional reward processing under stress, but could not predict whether the patterns hypothesized above would be blunted or exaggerated by stress.
Methods
Fifteen volunteers with BD (13 bipolar I disorder and two bipolar II disorder) and 18 demographically matched psychiatrically healthy controls (HC) participated in this study. The Committee on the Use of Human Subjects in Research at Harvard University and the Partners Human Research Committee approved this study, and all participants provided written informed consent. Participants were screened using the Structured Clinical Interview for DSM-IV Disorders (SCID) (24) and the attention-deficit hyperactivity disorder (ADHD) section of the Mini-International Neuropsychiatric Interview (MINI) (25). In addition, all participants completed questionnaires probing depressive and anxiety symptoms: Beck Depression Inventory (BDI-II) (26), Mood and Anxiety Symptom Questionnaire (MASQ-short) (27); positive and negative affect: Positive and Negative Affective Schedule (PANAS–Trait) (28); anhedonia: Snaith–Hamilton Pleasure Scale (SHPS) (29); perceived stress levels: Perceived Stress Scale (PSS) (30); and nicotine use: Nicotine Craving Questionnaire (NCQ) (31). Participants with BD also completed a brief interview to assess current mood symptoms: Hamilton Rating Scale for Depression (HAM-D) (32)] and Young-Mania Rating Scale (YMRS) (33). Participants earned $55 for the study session and $10 to $60 in earnings from the MID task. Detailed inclusion and exclusion criteria, as well as medication information are provided in the Supplementary Data. Neuroimaging data from two patients with BD and three HC participants were unusable due to excessive head motion in the scanner (4 mm to 15 mm), leaving 13 BD and 15 HC included in the final analyses.
During the fMRI session, participants completed four runs of a revised version of the MID task (see below); two runs under no-stress and two runs under stress conditions, in the following order: Run #1: no-stress, Run #2: stress, Run #3: stress, and Run #4: no-stress. The stress manipulation involved negative feedback about task performance. More specifically, in order to induce mental stress during the stress runs (Runs #2 and #3), participants were given negative performance feedback immediately before the start of these runs. Participants were told that they were performing worse than prior participants and, as a result, there was a chance they would receive sudden $5 penalty deductions if they continued to perform poorly. In contrast, participants were given positive performance feedback immediately before the start of the two no-stress runs (Runs #1 and #4) with no possibility of receiving $5 penalties (for more details, see Supplementary Data). Immediately after each run, and prior to performance feedback, subjects completed brief computerized affective ratings that included rating the extent to which they experienced 12 different emotions (e.g., tense, anxious, relaxed, in control) on a scale from 1–5 (1 = not at all/very slightly, 5 = extremely).
MID task
The MID task was a variant of a monetarily-reinforced button-press task designed to elicit neural responses during reward anticipation and consumption [e.g., (28, 29)]. Briefly, at the beginning of each trial, participants were presented with a visual cue (1.5 sec) indicating the reinforcer associated with performance (‘+$’ for reward or ‘0$’ for no-incentive), followed by a visual cue (a red square, 0.2 sec) that indicated they should execute a button press as quickly as possible. Following response execution, participants received visual feedback about their performance (gain or no-gain on reward trials, and no-change on no-incentive trials). Successful performance during reward trials was associated with monetary gain, and occurred if subjects executed the button press within the 66th percentile of their individual reaction times (RT) obtained from the preceding run (for Run #1, the threshold was calculated using the practice block RT). Gains on successful reward trials varied between $0.95 and $1.15 (mean: $1.05); unsuccessful performance of reward trials was associated with no gain. For no-incentive trials, a ‘no-change’ feedback was presented regardless of the RT. The task was organized into four runs of 33 trials, with 22 reward and 11 no-incentive trials pseudo-randomized in each run. Subjects were instructed that the probability of success was contingent upon how fast they pressed the button after disappearance of the red square. A brief practice run (identical in design but without feedback) was completed immediately prior to the first run.
Imaging data acquisition
The MRI data were acquired on a 1.5-T Symphony/Sonata scanner (Siemens Medical Systems, Iselin, NJ, USA) using a 12-channel head coil. Structural data were collected using a T1-weighted magnetization-prepared rapid acquisition with gradient echo (MPRAGE) imaging sequence with the following parameters: repetition time (TR) = 2730 msec; echo time (TE) = 3.39 msec; field of view (FOV) = 256 mm; voxel dimensions = 1 × 1 × 1.33 mm; 128 slices. fMRI data were acquired using a gradient echo T2*-weighted echoplanar imaging sequence with an optimized pulse sequence from a previous study in our laboratory (34), including the following parameters: TR = 2500 msec; TE = 35 msec; FOV = 200 mm; voxel dimensions = 3.125 × 3.125 × 3 mm; 35 interleaved slices; tilted slice acquisition; and z-shimming to recover signal in regions affected by susceptibility artifacts.
Behavioral data analyses
Demographic and clinical variables
Independent samples t-tests were conducted to compare groups on their ratings (BDI-II, MASQ, SHPS, PSS, PANAS-Trait, NCQ).
In-scanner affective ratings
Positive and negative affect were calculated by averaging the scores obtained on five positive (in control, alert, energetic, relaxed, happy) and seven negative (tense, anxious, powerless, defeated, challenged, stressed, out of control) emotions, respectively, after every run. These ratings were analyzed using a 2 × 2 × 2 repeated measures ANOVA with Valence (positive, negative) × Stress (stress, no-stress) as within-subject factors, and Group (HC, BD) as a between-subject factor.
MID task
In line with our previous publication in healthy controls (22), analyses were restricted to Runs #1 and #2 to focus on the acute effects of the stress manipulation without potentially confounding carry-over effects. Both behavioral and fMRI analyses were, therefore, conducted on Run #1 (No-stress) and Run #2 (Stress) conditions. While positive feedback was given after Run #3 to mitigate potential carryover effects of the stress manipulation, our prior analyses in healthy controls tested with this paradigm indicated that differences between the first two runs more strongly reflected the effects of ‘acute’ stress (22).
With respect to behavioral performance, groups were compared using unpaired t-tests across different variables: % of reward trials in which subjects received successful reward feedback, number of errors and total amount of money won during no-stress and stress blocks. A Group (HC, BD) × Stress (stress, no-stress) ANOVA was run to assess task performance.
For RT data, outlier responses were removed before analyses. Outliers were defined as responses < 150 msec or > 1000 msec; responses exceeding three standard deviations above or below the individual's mean; and error trials (trials in which subjects pressed the button too soon before the cue or too late after the cue). Next, a 2 × 2 × 2 repeated measures ANOVA of RT with Incentive (reward, no-incentive) and Stress (stress, no-stress) as within-subject factors, and Group (HC, BD) as a between-subject factor was run.
Neuroimaging data analyses
fMRI data processing was completed using FEAT (FMRI Expert Analysis Tool) version 5.98, part of FSL version 4.1.5 (FMRIB's Software Library, www.fmrib.ox.ac.uk/fsl). After removal of non-brain structures using Brain Extraction Tool (BET) (35), the following pre-processing steps were performed: motion correction [using MCFLIRT (36)]; slice-timing correction using Fourier-space time-series phase-shifting; spatial smoothing using a Gaussian kernel with 6.0-mm full-width half-maximum; grand-mean intensity normalization by a single multiplicative factor; high pass temporal filtering (Gaussian-weighted least-squares straight line fitting, with sigma = 60 sec); in addition, we used the automatic outlier weighing option available in FSL. FLIRT was used to register functional data to the high-resolution structural images, and FSL's Non-linear Image Registration Tool (FNIRT) (36) was used to register structural images to 2-mm Montreal Neurological Institute (MNI) standard space.
At the individual level, statistical analyses of fMRI data were conducted using a general linear model (GLM) with separate regressors for each incentive cue (Reward, No-incentive) and the three types of feedback [successful reward feedback (gain), unsuccessful reward feedback, no-change (on no-incentive trials) feedback]. Each of these events was modeled using a gamma function and constructed as a hemodynamic response function, convolved with the event onset times. The following were included as covariates of no interest: the six rigid-body motion time courses from the motion correction, the target, errors (e.g., responding prior to the target or not responding at all), and penalties (when $5 penalties were presented during the stress runs). Contrast maps were constructed to identify brain regions involved in reward anticipation (reward versus no-incentive cue) and reward consumption (gain versus no-change feedback).
To test a priori hypotheses that bipolar patients would exhibit abnormal reward processing, anatomical masks were created using the Wake Forest University School of Medicine (WFU) PickAtlas for each of the following regions (for the left and right hemispheres separately): caudate, putamen, nucleus accumbens, and amygdala. Next, for each subject and condition (stress, no-stress), the parameter estimates were extracted using featquery from reward anticipation (reward versus no-incentive cue) and consumption (gain versus no-change feedback) contrast maps and entered into SPSS.
Exploratory analyses were conducted to test for the influence of trait affect (measured by the PANAS), depression (as measured by the BDI-II) and perceived stress (measured by the stress scale) on reward processing during the acute stressor, by correlating these scores with the parameter estimates from each significant region (see Supplementary Data).
A repeated measures ANOVA with Stress (stress, no-stress) and hemisphere (left, right) as within-subject factors, and Group as a between-subjects factor, was run for each region-of-interest (ROI) and Phase (anticipation, consumption) individually. A Bonferroni correction was used to correct for the number of ANOVAs performed (p = 0.05/8 = 0.006265). In light of the modest sample size, effect sizes (Cohen's d for paired and unpaired t-tests or η2p values for ANOVA effects) were computed to evaluate the robustness of putative findings. A commonly used interpretation is to refer to effect sizes for independent and dependent groups as small (d = 0.2), medium (d = 0.5), and large (d = 0.8). Similarly, for partial eta squared values, effect size are interpreted as small (η2p = 0.01), medium (η2p = 0.06) and large (η2p = 0.14) based on benchmarks suggested by Cohen (37).
Structural analyses
Volumetric segmentation was performed with the FreeSurfer image analysis suite [FreeSurfer Vol. 5.3, (38, 39)]. Freesurfer estimates cortical and subcortical volumes via a whole brain segmentation procedure (39). The brain parcellation and segmentation were run using the standard ‘recon-all’ script using default settings. The post-processing output for each subject was thoroughly inspected for segmentation errors and no manual edits were required. Intracranial volume (ICV) was also calculated to correct for inter-individual differences in total brain size. All volumes measured were exported to IBM, SPSS, Vol. 21 for statistical analyses. Age and gender were also controlled for, as both of these factors are known to influence structural morphology in humans (40).
Results
Participant characteristics
There were no significant differences between groups on any of the following demographic variables: gender, age, ethnicity, years of education, current/past smoker, tobacco dependency, or current caffeine consumption (p > 0.10) (Table 1). Relative to controls, participants with BD had a wider range of depressive symptoms as measured by the BDI-II (range: 0–16), but groups did not differ (p > 0.30). On the BDI-II, 12 of the 13 subjects with BD were in the ‘minimal’ range (0–13) and only one subject was in the ‘mild’ range (14–19, score: 16). Similarly, groups did not differ in their PANAS-Trait Positive Affect, MASQ-AD, or PSS [all p > 0.10]. Relative to controls, subjects with BD reported higher scores on the PANAS-Trait Negative Affect [t(26) = -2.41, p = 0.024] and the MASQ [general distress anxious subscale only; missing data on one control subject; HC: t(25) = -2.49, p = 0.020]. On the day of the scan, YMRS ratings indicated that all BD participants were below the cut-off for hypomania (< 12). HAM-D ratings on the day of the scan indicated that 8 BD participants were in the ‘normal range’ (0–7) and five participants were in the ‘mildly depressed’ (8–15) range.
Table 1. Characteristics of participants by groups.
| Controls (n = 15) | Bipolar disorder (n = 13) | p-value | |
|---|---|---|---|
| Gender, % female | 67% (n = 10) | 62% (n = 8) | 0.89 |
| Age, years | 31.73 (12.35) | 27.01 (6.25) | 0.21 |
| Ethnicity, % Caucasian | 64% (n = 9) | 77% (n = 10) | 0.92 |
| Education, years | 16.86 (2.03) | 15.73 (2.02) | 0.16 |
| BDI-II scorea | 3.21 (4.61) | 5.15 (5.01) | 0.31 |
| Anhedonia (MASQ)a | 52.64 (11.18) | 55.69 (11.18) | 0.48 |
| General distress anxious (MASQ)a | 14.17 (3.85) | 19.00 (6.25) | 0.02 |
| PANAS NA (trait) | 12.53 (2.50) | 15.38 (3.73) | 0.02 |
| PANAS PA (trait) | 35.20 (6.82) | 34.23 (8.01) | 0.73 |
| PSSa | 18.21 (7.94) | 20.77 (5.45) | 0.34 |
| HAM-D score | N/A | 5.62 (3.62) | N/A |
| YMRS score | N/A | 3.08 (2.84) | N/A |
Values are expressed as mean (standard deviation) unless indicated otherwise. BDI-II = Beck Depression Inventory; MASQ = Mood and Anxiety Symptom Questionnaire; PANAS = Positive (PA) and Negative Affect (NA) Schedule; PSS = Perceived Stress Scale; HAM-D = Hamilton Rating Scale for Depression; YMRS = Young Mania Rating Scale.
Data was missing for one healthy control.
Behavioral results
Affective ratings
As hypothesized, a significant Valence × Stress interaction emerged [F(1,26) = 55.85, p < 0.001] but this effect did not interact with group (p > 0.10). Post-hoc t-tests revealed that the stress manipulation significantly increased negative affect and decreased positive affect across all participants (p < 0.003) (Figs. 1A and 1B).
Fig. 1.
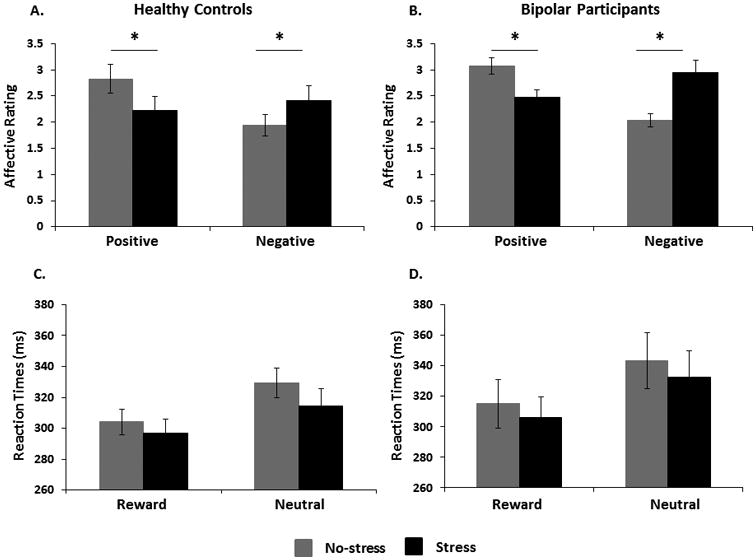
Affective ratings (A and B) and Reaction times (C and D) across no-stress (Run #1) and stress (Run #2) runs in healthy controls and participants with bipolar disorder. Error bars indicate standard errors.
MID performance
Overall, there were no behavioral differences between groups and stress runs in terms of the amount of reward feedback received, number of error trials, total number of errors, and total amount of money won during the task (all p > 0.50) (see Supplementary Data). On average, across all runs and participants, approximately 65% of reward trials (∼14 trials) were successful (i.e., participants were faster than the set threshold of 66%) and 35% (∼8 trials) were not successful (i.e., participants were slower than the 66% threshold).
RT
When examining RT to the target in Runs #1 and #2, the Cue (reward, no-incentive) × Stress (stress, no-stress) × Group (HC, BD) ANOVA yielded significant main effects of both Cue [F(1,26) = 28.12, p < 0.01] and Condition [F(1,26) = 5.82, p = 0.02]; all other p > 0.43. As evident from Figures 1C and 1D, RT was shorter for reward than no-incentive trials (confirming motivated responding) and shorter during the stress (Run #2) than no-stress (Run #1) block (in line with the stress manipulation).
Neuroimaging results
Parameter estimates from our ROIs were normal and satisfied the homogeneity of variance assumption.
Putamen
A Group × Stress × Hemisphere ANOVA on beta weights extracted for reward anticipation (Reward cue minus No-incentive cue) revealed no significant effects (Fig. 2A). An analogous ANOVA for the reward consumption phase (gain minus no-gain) highlighted a significant three-way interaction [F(1,26) = 4.80, p = 0.04, η2p = 0.16]. Separate Group × Stress ANOVAs run for each hemisphere individually clarified that this interaction was driven by the left putamen [F(1,26) = 4.83, p = 0.04, η2p = 0.16] (Fig. 2B]. Post-hoc t-tests revealed, however, no differences between HC and BD in Run #1 [t(26) = 1.58, p = 0.13, ds = 0.60] or Run #2 [t(26) = -1.38, p = 0.18, ds = 0.52], indicating that groups differed only in their relative activation in the no-stress versus stress condition.
Fig. 2.
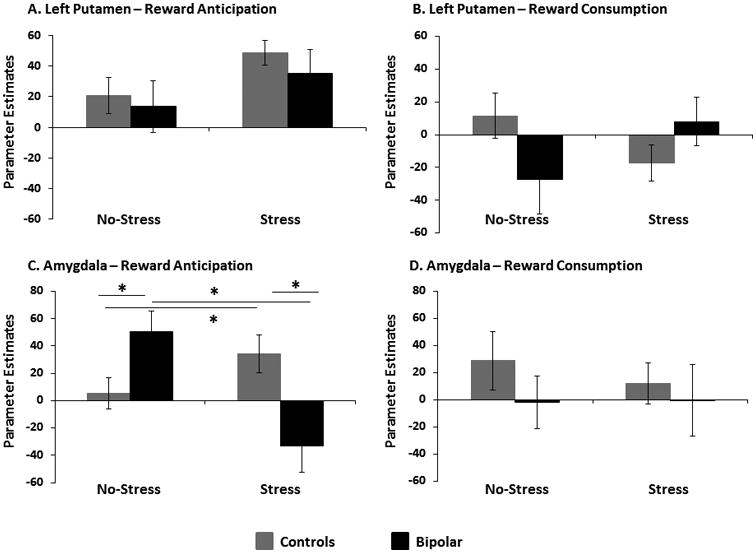
Parameter estimates extracted from functional region-of-interest (ROIs) during anticipation and consumption in the putamen (A and B) and amygdala (C and D) during stress (Run #1) and no-stress (Run #2) conditions in healthy controls and bipolar participants with bipolar disorder. Error bars indicate standard errors.
Caudate and nucleus accumbens
No effects involving Stress or Group were observed in the caudate or nucleus accumbens during reward anticipation or consumption.
Amygdala
Two significant outliers (1 BD, 1 HC), as listed by SPSS, were identified in the left amygdala during reward anticipation, so these participants were removed from analyses. For reward anticipation, the Group × Stress × Hemisphere ANOVA revealed a significant main effect of Stress [F(1,24) = 6.49, p = 0.018, η2p = 0.21], which significantly interacted with Group [F(1,24) = 27.52, p < 0.001, η2p = 0.53]. Irrespective of the hemisphere, stress increased amygdalar activation in controls, whereas subjects with BD had a stress-induced reduction in amygdala activation. Given that Hemisphere did not interact with Group × Stress, activation from the left and right amygdala were averaged in subsequent analyses. Within Run #1 (no-stress), control subjects demonstrated lower activation than subjects with BD during reward anticipation [t(24) = -2.75, p = 0.01, ds = 1.08]; conversely, HCs demonstrated higher activation than subjects with BD [t(24) = 3.51, p = 0.002, ds = 1.38] during reward anticipation in Run #2 (stress) (Fig. 2C). In addition, within the BD group, there was a significant reduction in the amygdalar activation with stress [t(11) = 4.35, p = 0.001, ds = 1.25], whereas HCs had a significant stress-induced increase in amygdalar activation during reward anticipation [t(13) = -2.59, p = 0.02, ds = 0.69]. No significant findings emerged in the amygdala during reward consumption in the stress condition (Fig. 2D).
Structural Freesurfer analyses
While controlling for age, gender, and ICV, no significant group differences emerged in the basal ganglia. However, the left and right amygdala were both found to be structurally larger in bipolar subjects as compared to controls [left: t(26) = -2.83, p = 0.009, ds = 1.07; right: t(26) = -2.65, p = 0.020, ds = 1.00] (Fig. 3A). No other significant structural group differences emerged. Unstandardized residuals of averaged left and right amygdala controlling for age, gender, and ICV, were calculated and the aforementioned functional analyses for the amygdala were repeated controlling for these residuals. Results did not change in the basal ganglia after controlling for structural volume. Specifically, Group × Stress interaction in the left putamen remained after controlling for volume [F(1,25) = 4.75, p = 0.039, ηp = 0.16]. Similar to above results, post-hoc t-tests revealed no group differences in Run #1 or Run #2 (p > 0.10), indicating that groups differed only in their relative activation in the no-stress versus stress condition with no influence from structural variability. Similarly, significant Group × Stress interaction for the amygdala was confirmed when taking into account the structural differences [F(1,23) = 15.17, p = 0.001, η2p = 0.39]. More specifically, under stress (Run #2), HC subjects continued to demonstrate higher amygdalar activation during reward anticipation than subjects with BD (p = 0.003, η2p = 0.32) (Fig. 3C). When considering the no-stress condition (Run #1), the group difference in amygdalar activation during reward anticipation was no longer significant (p = 0.21)1. Finally, across the entire sample, amygdala volume correlated positively with amygdalar activation while anticipating potential rewards during Run #1 (r = 0.59, p = 0.001) (Fig. 3B); this effect was mainly driven by the BD group (BD: r = 0.58, p = 0.046; HC: r = 0.21, p = 0.47), but the groups did not differ in their correlations (z = 1.04, p > 0.05).
Fig. 3.
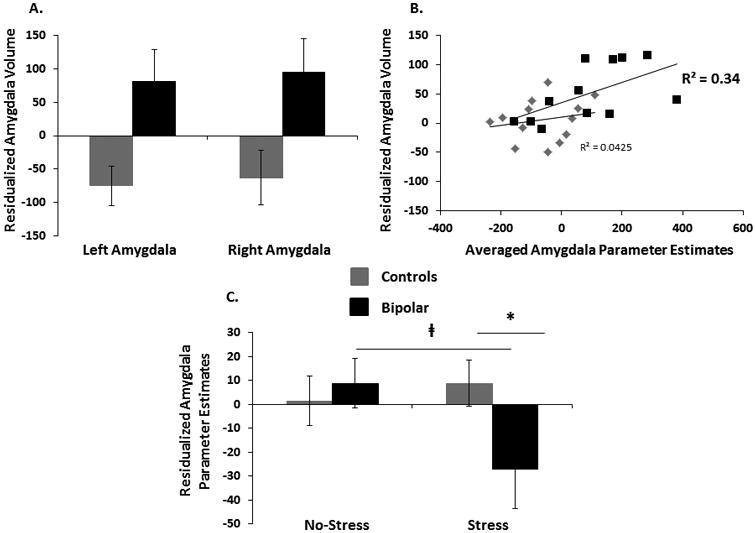
Gray matter (corrected for age, gender, and intracranial volume) differences between healthy controls and participants with bipolar disorder (A). Association between structural volume and function activation to reward anticipation in the amygdala under no-stress in both groups (B). Parameter estimates from the amygdala during reward anticipation after controlling for structural volume in healthy controls and participants with bipolar disorder (C). Error bars indicate standard errors.
Discussion
Using fMRI in conjunction with the MID task and a negative psychosocial stress manipulation, the current study examined the impact of an acute stressor on reward processing in individuals with BD and HCs. The main finding emerging from the fMRI analyses was a stress-dependent effect in the amygdala: relative to controls, individuals with BD showed significantly higher amygdalar activation during reward anticipation under no-stress, whereas the opposite pattern was seen under stress conditions (BD < HC). Notably, groups did not differ in terms of behavioral performance or any of the pre-scan baseline ‘in-the-moment’ affective questionnaires, suggesting that fMRI findings were not confounded by group differences in task difficulty or mood state on the day of the scan.
Interestingly, structural analyses revealed that both the left and right amygdala were larger in subjects with BD than HC. These findings are in line with some prior studies that have reported similar structural abnormalities in amygdala volume in BD [e.g., (40, 14, 25, 43)], although opposite patterns have also been described (8). A recent meta-analysis reported overall reduced amygdala volumes in BD (43), although these conclusions were driven by studies of children and adolescents with BD, who may show meaningful structural brain differences from adults with BD (44). Medication history may contribute to inconsistencies in amygdala volume differences, since certain BD medications may increase amygdala volumes (14, 45) making it more likely to find larger amygdala volumes in adults with BD as compared to controls and youth with BD. Of note, in the current study, amygdala volume correlated positively with amygdala activation suggesting that prior reports of amygdalar hyperactivation in response to reward anticipation under no-stress [e.g., (9, 44)] might be partially confounded by structural abnormalities in this region.
The amygdala is involved in appetitive motivated learning (47) and plays an important role in reward-related DA release in order to generate ‘approach’ behaviors (48). Thus, in the face of stress, increased amygdalar activation during reward anticipation may reflect that controls have increased appetitive motivation to engage in actions to cope with the stressor, gain rewards, and avoid punishments. In contrast, among individuals with BD, blunted amygdalar activation while anticipating a potential reward may indicate an impaired ability to use reward-predicting cues to appropriately engage in goal-directed actions when under stress.
During reward consumption, ROI analyses revealed a significant Group × Stress interaction in the left putamen. Although post-hoc tests did not reveal significant group differences within any run, the overall significant interaction showed that bipolar subjects had a stress-induced increase in putamen activation in response to reward feedback, whereas controls showed the opposite pattern. Decreased striatal activation in controls fits prior reports that acute stressors blunt reward responsiveness or ‘liking’ of positive stimuli (17, 18, 22, 49), and lead to reduced striatal activation to rewards (50, 51). Conversely, the pattern of increased striatal activation in BD suggests this group may experience stress-induced heightened reward responsiveness, which is consistent with models of hypersensitivity to rewards in BD (52), and findings of reward-related dorsal striatal hyperactivation in BD [e.g., (50)]. Interestingly, participants with BD baseline (under no-stress) putamen activation during reward consumption correlated with their trait positive affect (see Supplementary Data).
Limitations and future directions
Several limitations should be acknowledged. First, the sample size was small and the BD group was heterogeneous with regard to BD subtype. Second, individuals in the BD group were taking psychotropic medications, as maintenance drugs are often necessary to control symptoms, but sample size was too small to allow sub-analyses evaluating the potential effects of different classes of medication. Third, although affective self-report ratings indicated that the psychosocial stress manipulation was successful, there were no physiological data to confirm the effectiveness of the stress manipulation (or to parse those participants with strong vs. weak physiological stress responses during the experiment). When a Bonferroni correction for multiple comparisons was applied, the Group × Stress interaction in the amygdala survived (p = 0.001) correction, whereas the Group × Stress interaction in the putamen (p = 0.04) becomes insignificant. However, our effect sizes show a large effect (0.16–0.39).
Conclusions
Despite these limitations, findings from the present study extend previous lines of research by highlighting potential atypical patterns of neural functioning—e.g., dysregulated stress-related activation of the amygdala and putamen—that may underlie the relationship between a dysfunctional reward-processing system and BD, at least among predominantly euthymic (and medicated) individuals. Given the euthymic status of the BD group, atypical functioning of these neural regions may represent trait markers of the illness. However, future research is necessary to determine if these neural findings are more appropriately conceptualized as vulnerability factors to BD or effects of the illness.
Supplementary Material
Acknowledgments
This project was supported by R01 MH068376 from the National Institute of Mental Health (DAP), A NARSAD Independent Investigator Award from the National Alliance for Research on Schizophrenia and Depression (DAP), UL1 RR025758-04 from the National Center for Research Resources, and M01-RR-01066 from the National Center for Research Resources. PK was supported by The John and Charlene Madison Cassidy Fellowship in Translational Neuroscience through McLean Hospital, a Livingston and a NARSAD Young Investigator awards. LHB was supported in part by the Sackler Fellowship in Psychobiology and an NRSA Predoctoral Training Grant in Advanced Multimodal Neuroimaging.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The authors would like to acknowledge Dr. Jill Hooley, Ms. Nancy Hall Brooks, Ms. Alex Rodman, and Mr. Andrew Cohen for their help with this project.
Disclosures: TD's research has been funded by National Institute of Health (NIH), National Institute of Mental Health (NIMH), NARSAD, TSA, IOCDF, Tufts University, DBDAT, Otsuka Pharmaceuticals and Cogito, Inc.; he has received honoraria, consultation fees and/or royalties from the MGH Psychiatry Academy, BrainCells Inc., Clintara, LLC., Systems Research and Applications Corporation, Boston University, the Catalan Agency for Health Technology Assessment and Research, the National Association of Social Workers Massachusetts, the Massachusetts Medical Society, Tufts University, NIDA, NIMH, and Oxford University Press; and he has also participated in research funded by DARPA, NIH, NIMH, NIA, AHRQ, PCORI, Janssen Pharmaceuticals, The Forest Research Institute, Shire Development, Inc., Medtronic, Cyberonics, Northstar, and Takeda. DO has received research support from Roche Genentech. Over the past three years, DAP has received consulting fees from Akili Interactive Labs, BlackThorn Therapeutics, Otsuka America Pharmaceutical, and Pfizer for activities unrelated to the current research.
Footnotes
Even though there were no group differences in depressive symptoms (as measured by BDI-II score), there was a greater variability of depressive symptomology in the BD group. Accordingly, we repeated all neuroimaging analyses controlling for BDI-II score and found that stress-induced reward dysfunction in participants with BD remained significant.
LHB, PK, DNG, and SD report no biomedical financial interests.
References
- 1.Goldberg JF, Chengappa KNR. Identifying and treating cognitive impairment in bipolar disorder. Bipolar Disord. 2009;11(Suppl. 2):123–137. doi: 10.1111/j.1399-5618.2009.00716.x. [DOI] [PubMed] [Google Scholar]
- 2.Dean BB, Gerner D, Gerner RH. A systematic review evaluating health-related quality of life, work impairment, and healthcare costs and utilization in bipolar disorder. Curr Med Res Opin. 2004;20(2):139–54. doi: 10.1185/030079903125002801. [DOI] [PubMed] [Google Scholar]
- 3.Hillegers MHJ, Burger H, Wals M, Reichart CG, Verhulst FC, Nolen WA, et al. Impact of stressful life events, familial loading and their interaction on the onset of mood disorders: Study in a high-risk cohort of adolescent offspring of parents with bipolar disorder. Br J Psychiatry. 2004;185(2):97–101. doi: 10.1192/bjp.185.2.97. [DOI] [PubMed] [Google Scholar]
- 4.Johnson SL, Miller I. Negative life events and time to recovery from episodes of bipolar disorder. J Abnorm Psychol. 1997;106(3):449–57. doi: 10.1037//0021-843x.106.3.449. [DOI] [PubMed] [Google Scholar]
- 5.Ellicott A, Hammen C, Gitlin M, Brown G, Jamison K. Life events and the course of bipolar disorder. Am J Psychiatry. 1990;147(9):1194–8. doi: 10.1176/ajp.147.9.1194. [DOI] [PubMed] [Google Scholar]
- 6.Johnson SL, Ruggero CJ, Carver CS. Cognitive, Behavioral, and Affective Responses to Reward: Links With Hypomanic Symptoms. J Soc Clin Psychol. 2005;24(6):894–906. [Google Scholar]
- 7.Mansell W, Colom F, Scott J. The nature and treatment of depression in bipolar disorder: A review and implications for future psychological investigation. Clin Psychol Rev. 2005;25:1076–100. doi: 10.1016/j.cpr.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 8.Phillips ML, Swartz H. a. A critical appraisal of neuroimaging studies of bipolar disorder: Toward a new conceptualization of underlying neural circuitry and a road map for future research. Am J Psychiatry. 2014;171(8):829–43. doi: 10.1176/appi.ajp.2014.13081008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Linke J, King AV, Rietschel M, Strohmaier J, Hennerici M, Gass A, et al. Increased medial orbitofrontal and amygdala activation: Eidence for a systems-level endophenotype of bipolar I disorder. Am J Psychiatry. 2012;169(3):316–25. doi: 10.1176/appi.ajp.2011.11050711. [DOI] [PubMed] [Google Scholar]
- 10.Nusslock R, Almeida JR, Forbes EE, et al. Waiting to win: elevated striatal and orbitofrontal cortical activity during reward anticipation in euthymic bipolar disorder adults. Bipolar Disord. 2012;14:249–260. doi: 10.1111/j.1399-5618.2012.01012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dickstein DP, Finger EC, Skup M, Pine DS, Blair JR, Leibenluft E. Altered neural function in pediatric bipolar disorder during reversal learning. Bipolar Disord. 2010;12:707–719. doi: 10.1111/j.1399-5618.2010.00863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jogia J, Dima D, Kumari V, Frangou S. Frontopolar cortical inefficiency may underpin reward and working memory dysfunction in bipolar disorder. World J Biol Psychiatry. 2011;13(8):605–15. doi: 10.3109/15622975.2011.585662. [DOI] [PubMed] [Google Scholar]
- 13.Yip SW, Worhunsky PD, Rogers RD, Goodwin GM. Hypoactivation of the Ventral and Dorsal Striatum During Reward and Loss Anticipation in Antipsychotic and Mood Stabilizer-Naive Bipolar Disorder. Neuropsychopharmacology. 2015;40(3):658–66. doi: 10.1038/npp.2014.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foland LC, Altshuler LL, Sugar Ca, Lee AD, Leow AD, Townsend J, et al. Increased volume of the amygdala and hippocampus in bipolar patients treated with lithium. Neuroreport. 2008;19(2):221–4. doi: 10.1097/WNR.0b013e3282f48108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hallahan B, Newell J, Soares JC, Brambilla P, Strakowski SM, Fleck DE, et al. Structural magnetic resonance imaging in bipolar disorder: An international collaborative mega-analysis of individual adult patient data. Biol Psychiatry. 2011;69(4):326–35. doi: 10.1016/j.biopsych.2010.08.029. [DOI] [PubMed] [Google Scholar]
- 16.Berenbaum H, Connelly J. The effect of stress on hedonic capacity. J Abnor psychology. 1993;102:474–81. doi: 10.1037//0021-843x.102.3.474. [DOI] [PubMed] [Google Scholar]
- 17.Berghorst LH, Bogdan R, Frank MJ, Pizzagalli DA. Acute stress selectively reduces reward sensitivity. Front Hum Neurosci. 2013;7(April):133. doi: 10.3389/fnhum.2013.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bogdan R, Pizzagalli DA. Acute stress reduces reward responsiveness: implications for depression. Biol Psychiatry. 2006;60(10):1147–54. doi: 10.1016/j.biopsych.2006.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knutson B, Westdorp A, Kaiser E, Hommer D. FMRI visualization of brain activity during a monetary incentive delay task. Neuroimage. 2000;12(1):20–7. doi: 10.1006/nimg.2000.0593. [DOI] [PubMed] [Google Scholar]
- 20.Santesso DL, Dillon DG, Birk JL, Holmes AJ, Goetz E, Bogdan R, et al. Individual differences in reinforcement learning: behavioral, electrophysiological, and neuroimaging correlates. Neuroimage. 2008;42(2):807–16. doi: 10.1016/j.neuroimage.2008.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strakowski SM, Delbello MP, Adler CM. The functional neuroanatomy of bipolar disorder: a review of neuroimaging findings. Mol Psychiatry. 2005;10(1):105–16. doi: 10.1038/sj.mp.4001585. [DOI] [PubMed] [Google Scholar]
- 22.Kumar P, Berghorst LH, Nickerson LD, Dutra SJ, Goer FK, Greve DN, et al. Differential Effects of Acute Stress on Anticipatory and Consummatory Phases of Reward Processing. Neuroscience. 2014;266:1–12. doi: 10.1016/j.neuroscience.2014.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harmon-Jones E, Abramson LY, Sigelman J, Bohlig A, Hogan ME, Harmon-Jones C. Proneness to hypomania/mania symptoms or depression symptoms and asymmetrical frontal cortical responses to an anger-evoking event. J Pers Soc Psychol. 2002;82(4):610–8. [PubMed] [Google Scholar]
- 24.First MB, Spitzer RL, Miriam G, Williams JB. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition (SCID-I/NP) Non-patien. New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 25.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry. 1998;58:22–33. [PubMed] [Google Scholar]
- 26.Beck AT, Steer RA, Brown GK. Manual for the Beck depression inventory-II. San Antonio, TX: Psychol Corp; 1996. pp. 1–82. [Google Scholar]
- 27.Watson D, Weber K, Assenheimer JS, Clark LA, Strauss ME, McCormick RA. Testing a tripartite model: I. Evaluating the convergent and discriminant validity of anxiety and depression symptom scales. J Abnorm Psychol. 1995;104(1):3–14. doi: 10.1037//0021-843x.104.1.3. [DOI] [PubMed] [Google Scholar]
- 28.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54(6):1063–70. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 29.Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwell P. A scale for the assessment of hedonic tone. The Snaith-Hamilton Pleasure Scale. Br J Psychiatry. 1995;167:99–103. doi: 10.1192/bjp.167.1.99. [DOI] [PubMed] [Google Scholar]
- 30.Cohen S, Kamarck T, Mermelstein R. A Global measure of Perceived Stress. J Health Soc Behav. 1983;24(4):385–96. [PubMed] [Google Scholar]
- 31.Shiffman SM, Jarvik ME. Smoking withdrawal symptoms in two weeks of abstinence. Psychopharmacology (Berl) 1976;50(1):35–9. doi: 10.1007/BF00634151. [DOI] [PubMed] [Google Scholar]
- 32.Hamilton M. Rating depressive patients. J Clin Psychiatry. 1980;41(12 Pt 2):21–4. [PubMed] [Google Scholar]
- 33.Young RC, Biggs JT, Ziegler VE, Meyer Da. A rating scale for mania: Reliability, validity and sensitivity. Br J Psychiatry. 1978;133(11):429–35. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 34.Dillon DG, Holmes AJ, Jahn AL, Bogdan R, Wald LL, Pizzagalli DA. Dissociation of neural regions associated with anticipatory versus consummatory phases of incentive processing. Psychophysiology. 2009;45(1):36–49. doi: 10.1111/j.1469-8986.2007.00594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–55. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–41. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 37.Cohen J. Statistical power analysis for the behavioral sciences. Statistical Power Analysis for the Behavioral Sciences. 1988:567. [Google Scholar]
- 38.Dale AM, Fischl B, Sereno MI. Cortical Surface-Based Analysis I. Segmentation and Surface Reconstruction. Neuroimage. 1999;9:179–94. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 39.Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole Brain Segmentation. Neuron. 2002;33(3):341–55. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 40.Taki Y, Thyreau B, Kinomura S, Sato K, Goto R, Kawashima R, et al. Correlations among brain gray matter volumes, age, gender, and hemisphere in healthy individuals. PLoS One. 2011;6(7):e22734. doi: 10.1371/journal.pone.0022734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Altshuler LL, Bartzokis G, Grieder T, Curran J, Jimenez T, Leight K, et al. An MRI study of temporal lobe structures in men with bipolar disorder or schizophrenia. Biol Psychiatry. 2000;48(2):147–62. doi: 10.1016/s0006-3223(00)00836-2. [DOI] [PubMed] [Google Scholar]
- 42.Altshuler L, Bookheimer S, Townsend J, et al. Regional brain changes in bipolar I depression: a functional magnetic resonance imaging study. Bipolar Disord. 2008;10:708–717. doi: 10.1111/j.1399-5618.2008.00617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hibar DP, Westlye LT, van Erp TGM, Rasmussen J, Leonardo CD, Faskowitz J, et al. Subcortical volumetric abnormalities in bipolar disorder. Mol Psychiatry. 2016 doi: 10.1038/mp.2015.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pfeifer JC, Welge J, Strakowski SM, Adler C, Delbello MP. Meta-Analysis of Amygdala Volumes in Children and Adolescents With Bipolar Disorder. J Am Acad Child Adolesc Psychiatry. 2008;47(11):1289–98. doi: 10.1097/CHI.0b013e318185d299. [DOI] [PubMed] [Google Scholar]
- 45.Chang K, Karchemskiy A, Barnea-Goraly N, Garrett A, Simeonova DI, Reiss A. Reduced amygdalar gray matter volume in familial pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2005;44(6):565–73. doi: 10.1097/01.chi.0000159948.75136.0d. [DOI] [PubMed] [Google Scholar]
- 46.Blumberg HP, Fredericks C, Wang F, et al. Preliminary evidence for persistent abnormalities in amygdala volumes in adolescents and young adults with bipolar disorder. Bipolar Disord. 2005;7:570–576. doi: 10.1111/j.1399-5618.2005.00264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Knapska E, Walasek G, Nikolaev E, Neuhausser-Wespy F, Lipp HP, Kaczmarek L, et al. Differential involvement of the central amygdala in appetitive versus aversive learning. Learn Mem. 2006;13(2):192–200. doi: 10.1101/lm.54706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mahler SV, Berridge KC. What and when to “want”? Amygdala-based focusing of incentive salience upon sugar and sex. Psychopharmacology (Berl) 2012;221:407–26. doi: 10.1007/s00213-011-2588-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bogdan R, Santesso DL, Fagerness J, Perlis RH, Pizzagalli DA. Corticotropin-releasing hormone receptor type 1 (CRHR1) genetic variation and stress interact to influence reward learning. J Neurosci. 2011;31(37):13246–54. doi: 10.1523/JNEUROSCI.2661-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Born JM, Lemmens SGT, Rutters F, Nieuwenhuizen AG, Formisano E, Goebel R, et al. Acute stress and food-related reward activation in the brain during food choice during eating in the absence of hunger. Int J Obes Nature Publishing Group. 2009;34(1):172–81. doi: 10.1038/ijo.2009.221. [DOI] [PubMed] [Google Scholar]
- 51.Porcelli AJ, Lewis AH, Delgado MR. Acute stress influences neural circuits of reward processing. Front Neurosci. 2012;6(November):157. doi: 10.3389/fnins.2012.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Whitton AE, Treadway MT, Pizzagalli DA. Reward processing dysfunction in major depression, bipolar disorder and schizophrenia. Curr Opin Psychiatry. 2015;28(1):7–12. doi: 10.1097/YCO.0000000000000122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.O'Sullivan N, Szczepanowski R, El-Deredy W, Mason L, Bentall RP. fMRI evidence of a relationship between hypomania and both increased goal-sensitivity and positive outcome-expectancy bias. Neuropsychologia. 2011;49(10):2825–35. doi: 10.1016/j.neuropsychologia.2011.06.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


