Abstract
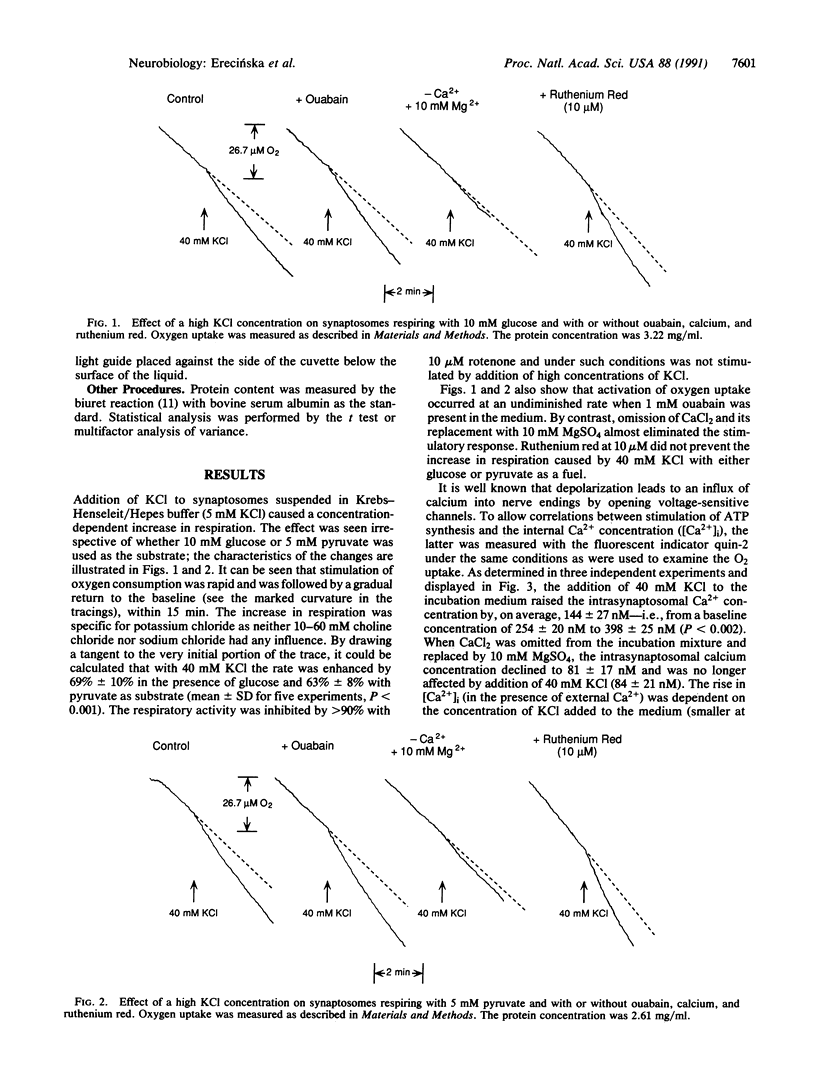
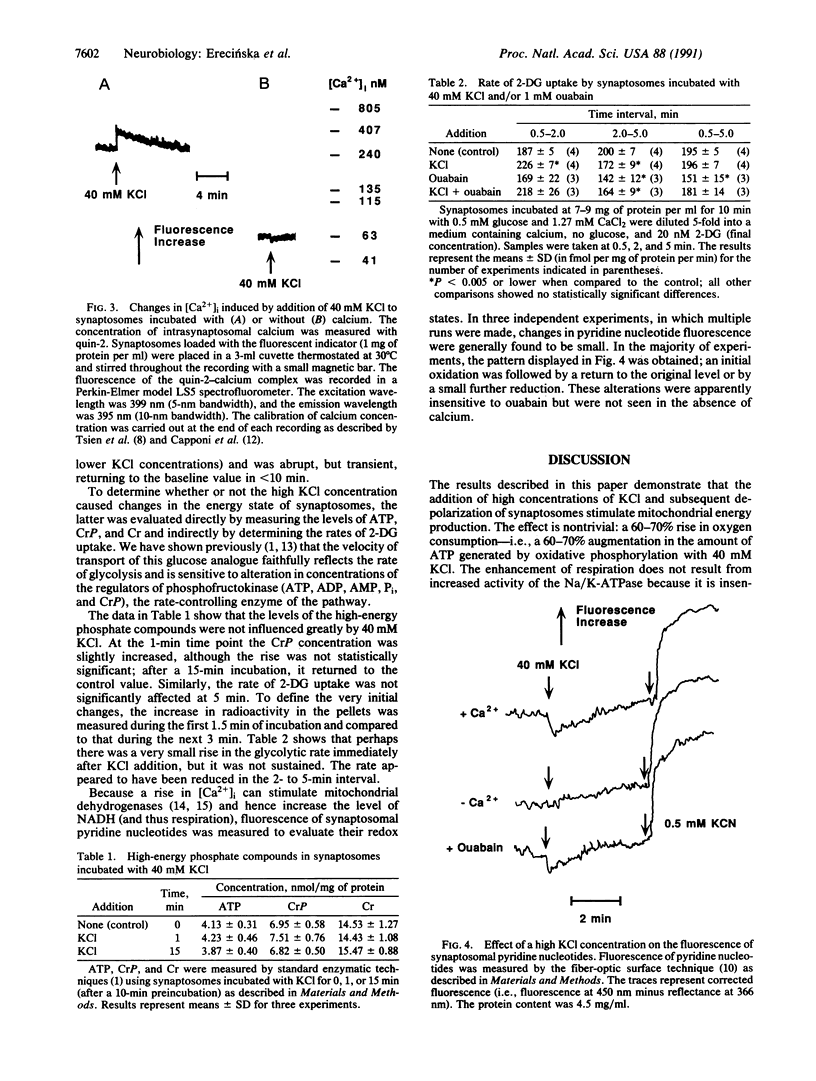
Addition of high concentrations of KC1 to preparations of rat brain synaptosomes incubated with either glucose or pyruvate caused a transient stimulation of oxygen uptake. This increased respiration was insensitive to 1 mM ouabain and 10 microM ruthenium red but was dependent upon the presence of calcium. With 40 mM KCl in the incubation medium, the levels of high-energy phosphate compounds in the synaptosomes were unaltered, whereas pyridine nucleotides underwent a rapid, albeit small and temporary, oxidation. It is postulated that there is a calcium-dependent mechanism in synaptosomes through which the function of the mitochondrial respiratory chain or of oxidative phosphorylation is stimulated directly without the involvement of either adenine nucleotides or mitochondrial dehydrogenases.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Astrup J., Sørensen P. M., Sørensen H. R. Oxygen and glucose consumption related to Na+-K+ transport in canine brain. Stroke. 1981 Nov-Dec;12(6):726–730. doi: 10.1161/01.str.12.6.726. [DOI] [PubMed] [Google Scholar]
- Booth R. F., Clark J. B. A rapid method for the preparation of relatively pure metabolically competent synaptosomes from rat brain. Biochem J. 1978 Nov 15;176(2):365–370. doi: 10.1042/bj1760365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capponi A. M., Lew P. D., Schlegel W., Pozzan T. Use of intracellular calcium and membrane potential fluorescent indicators in neuroendocrine cells. Methods Enzymol. 1986;124:116–135. doi: 10.1016/0076-6879(86)24012-4. [DOI] [PubMed] [Google Scholar]
- Collins R. C., Posner J. B., Plum F. Cerebral energy metabolism during electroshock seizures in mice. Am J Physiol. 1970 Apr;218(4):943–950. doi: 10.1152/ajplegacy.1970.218.4.943. [DOI] [PubMed] [Google Scholar]
- Denton R. M., McCormack J. G. Ca2+ transport by mammalian mitochondria and its role in hormone action. Am J Physiol. 1985 Dec;249(6 Pt 1):E543–E554. doi: 10.1152/ajpendo.1985.249.6.E543. [DOI] [PubMed] [Google Scholar]
- Erecińska M., Dagani F. Relationships between the neuronal sodium/potassium pump and energy metabolism. Effects of K+, Na+, and adenosine triphosphate in isolated brain synaptosomes. J Gen Physiol. 1990 Apr;95(4):591–616. doi: 10.1085/jgp.95.4.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erecińska M. Stimulation of the Na+/K+ pump activity during electrogenic uptake of acidic amino acid transmitters by rat brain synaptosomes. J Neurochem. 1989 Jan;52(1):135–139. doi: 10.1111/j.1471-4159.1989.tb10907.x. [DOI] [PubMed] [Google Scholar]
- Fein A., Tsacopoulos M. Activation of mitochondrial oxidative metabolism by calcium ions in Limulus ventral photoreceptor. Nature. 1988 Feb 4;331(6155):437–440. doi: 10.1038/331437a0. [DOI] [PubMed] [Google Scholar]
- Fein A., Tsacopoulos M. Light-induced oxygen consumption in Limulus ventral photoreceptors does not result from a rise in the intracellular sodium concentration. J Gen Physiol. 1988 Apr;91(4):515–527. doi: 10.1085/jgp.91.4.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn I. M., Karlish S. J. The sodium pump. Annu Rev Physiol. 1975;37:13–55. doi: 10.1146/annurev.ph.37.030175.000305. [DOI] [PubMed] [Google Scholar]
- Halestrap A. P. The regulation of the matrix volume of mammalian mitochondria in vivo and in vitro and its role in the control of mitochondrial metabolism. Biochim Biophys Acta. 1989 Mar 23;973(3):355–382. doi: 10.1016/s0005-2728(89)80378-0. [DOI] [PubMed] [Google Scholar]
- Hansford R. G., Castro F. Role of Ca2+ in pyruvate dehydrogenase interconversion in brain mitochondria and synaptosomes. Biochem J. 1985 Apr 1;227(1):129–136. doi: 10.1042/bj2270129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansford R. G., Chappell J. B. The effect of Ca2+ on the oxidation of glycerol phosphate by blowfly flight-muscle mitochondria. Biochem Biophys Res Commun. 1967 Jun 23;27(6):686–692. doi: 10.1016/s0006-291x(67)80090-1. [DOI] [PubMed] [Google Scholar]
- Hansford R. G. Relation between mitochondrial calcium transport and control of energy metabolism. Rev Physiol Biochem Pharmacol. 1985;102:1–72. doi: 10.1007/BFb0034084. [DOI] [PubMed] [Google Scholar]
- Jöbsis F. F., O'Connor M., Vitale A., Vreman H. Intracellular redox changes in functioning cerebral cortex. I. Metabolic effects of epileptiform activity. J Neurophysiol. 1971 Sep;34(5):735–749. doi: 10.1152/jn.1971.34.5.735. [DOI] [PubMed] [Google Scholar]
- Klingenberg M. Localization of the glycerol-phosphate dehydrogenase in the outer phase of the mitochondrial inner membrane. Eur J Biochem. 1970 Apr;13(2):247–252. doi: 10.1111/j.1432-1033.1970.tb00924.x. [DOI] [PubMed] [Google Scholar]
- Landowne D., Ritchie J. M. On the control of glycogenolysis in mammalian nervous tissue by calcium. J Physiol. 1971 Jan;212(2):503–517. doi: 10.1113/jphysiol.1971.sp009338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landowne D., Ritchie J. M. Optical studies on the kinetics of the sodium pump in mammalian non-myelinated nerve fibres. J Physiol. 1971 Jan;212(2):483–502. doi: 10.1113/jphysiol.1971.sp009337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis D. V., Schuette W. H. NADH fluorescence, [K+]0 and oxygen consumption in cat cerebral cortex during direct cortical stimulation. Brain Res. 1976 Jul 16;110(3):523–535. doi: 10.1016/0006-8993(76)90863-5. [DOI] [PubMed] [Google Scholar]
- Lothman E., Lamanna J., Cordingley G., Rosenthal M., Somjen G. Responses of electrical potential, potassium levels, and oxidative metabolic activity of the cerebral neocortex of cats. Brain Res. 1975 Apr 25;88(1):15–36. doi: 10.1016/0006-8993(75)90943-9. [DOI] [PubMed] [Google Scholar]
- Mata M., Fink D. J., Gainer H., Smith C. B., Davidsen L., Savaki H., Schwartz W. J., Sokoloff L. Activity-dependent energy metabolism in rat posterior pituitary primarily reflects sodium pump activity. J Neurochem. 1980 Jan;34(1):213–215. doi: 10.1111/j.1471-4159.1980.tb04643.x. [DOI] [PubMed] [Google Scholar]
- Mayevsky A., Chance B. Intracellular oxidation-reduction state measured in situ by a multichannel fiber-optic surface fluorometer. Science. 1982 Aug 6;217(4559):537–540. doi: 10.1126/science.7201167. [DOI] [PubMed] [Google Scholar]
- Robinson J. D. Substrate sites for the (Na+ + K+)-dependent ATPase. Biochim Biophys Acta. 1976 May 13;429(3):1006–1019. doi: 10.1016/0005-2744(76)90345-4. [DOI] [PubMed] [Google Scholar]
- Rosenthal M., Somjen G. Spreading depression, sustained potential shifts, and metabolic activity of cerebral cortex of cats. J Neurophysiol. 1973 Jul;36(4):739–749. doi: 10.1152/jn.1973.36.4.739. [DOI] [PubMed] [Google Scholar]
- Shibuki K. Calcium-dependent and ouabain-resistant oxygen consumption in the rat neurohypophysis. Brain Res. 1989 May 15;487(1):96–104. doi: 10.1016/0006-8993(89)90944-x. [DOI] [PubMed] [Google Scholar]
- Somjen G. G. Extracellular potassium in the mammalian central nervous system. Annu Rev Physiol. 1979;41:159–177. doi: 10.1146/annurev.ph.41.030179.001111. [DOI] [PubMed] [Google Scholar]
- Sánchez-Prieto J., González P. Occurrence of a large Ca2+-independent release of glutamate during anoxia in isolated nerve terminals (synaptosomes). J Neurochem. 1988 Apr;50(4):1322–1324. doi: 10.1111/j.1471-4159.1988.tb10611.x. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y., Pozzan T., Rink T. J. Calcium homeostasis in intact lymphocytes: cytoplasmic free calcium monitored with a new, intracellularly trapped fluorescent indicator. J Cell Biol. 1982 Aug;94(2):325–334. doi: 10.1083/jcb.94.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]



