Abstract
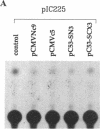
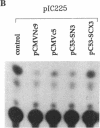
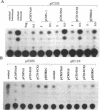
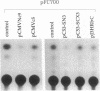
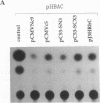
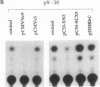
The aberrant overexpression of interleukin 6 (IL-6) is implicated as an autocrine mechanism in the enhanced proliferation of the neoplastic cell elements in various B- and T-cell malignancies and in some carcinomas and sarcomas; many of these neoplasms have been shown to be associated with a mutated p53 gene. The possibility that wild-type (wt) p53, a nuclear tumor-suppressor protein, but not its transforming mutants might serve to repress IL-6 gene expression was investigated in HeLa cells. We transiently cotransfected these cells with constitutive cytomegalovirus (CMV) enhancer/promoter expression plasmids overproducing wt or mutant human or murine p53 and with appropriate chloramphenicol acetyltransferase (CAT) reporter plasmids containing the promoter elements of human IL-6, c-fos, or beta-actin genes or of porcine major histocompatibility complex (MHC) class I gene in pN-38 to evaluate the effect of the various p53 species on these promoters. Murine and human wt p53 derived from pCMVNc9 and pC53-SN3, respectively, strongly repressed the IL-6 (promoter position -225 to +13), c-fos (-711 to +42), beta-actin (-3400 to +912), and MHC (-528 to -38) promoters in serum-induced HeLa cells; additionally, IL-6 promoter/CAT transcription unit constructs induced by IL-1, phorbol ester, or pseudorabies virus were also repressed by wt human and murine p53. The murine transforming mutant p53 (pCMVc5) was less active in repressing the IL-6, c-fos, beta-actin, and MHC promoter constructs. The human p53 mutant derived from pC53-SCX3 was also less active than the wt protein in repressing the IL-6, c-fos, beta-actin, and MHC promoters, except that serum-induced IL-6/CAT expression was equally repressed by both human wt and mutant p53. In similar transient transfection experiments in HeLa cells, overexpression of the wt human retinoblastoma susceptibility gene product, RB, was found to repress the serum-induced IL-6 (-225 to +13), c-fos (-711 to +42), and beta-actin (-3400 to +912) promoters but not the PRV-induced IL-6 (-110 to +13) or the serum-induced MHC (-528 to -38) promoters. These observations identify transcriptional repression as a property of p53 and suggest that p53 and RB may be involved as transcriptional repressors in modulating IL-6 gene expression during cellular differentiation and oncogenesis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akira S., Isshiki H., Sugita T., Tanabe O., Kinoshita S., Nishio Y., Nakajima T., Hirano T., Kishimoto T. A nuclear factor for IL-6 expression (NF-IL6) is a member of a C/EBP family. EMBO J. 1990 Jun;9(6):1897–1906. doi: 10.1002/j.1460-2075.1990.tb08316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker S. J., Markowitz S., Fearon E. R., Willson J. K., Vogelstein B. Suppression of human colorectal carcinoma cell growth by wild-type p53. Science. 1990 Aug 24;249(4971):912–915. doi: 10.1126/science.2144057. [DOI] [PubMed] [Google Scholar]
- Banks L., Matlashewski G., Crawford L. Isolation of human-p53-specific monoclonal antibodies and their use in the studies of human p53 expression. Eur J Biochem. 1986 Sep 15;159(3):529–534. doi: 10.1111/j.1432-1033.1986.tb09919.x. [DOI] [PubMed] [Google Scholar]
- Ehrlich R., Maguire J. E., Singer D. S. Identification of negative and positive regulatory elements associated with a class I major histocompatibility complex gene. Mol Cell Biol. 1988 Feb;8(2):695–703. doi: 10.1128/mcb.8.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliyahu D., Goldfinger N., Pinhasi-Kimhi O., Shaulsky G., Skurnik Y., Arai N., Rotter V., Oren M. Meth A fibrosarcoma cells express two transforming mutant p53 species. Oncogene. 1988 Sep;3(3):313–321. [PubMed] [Google Scholar]
- Eliyahu D., Michalovitz D., Eliyahu S., Pinhasi-Kimhi O., Oren M. Wild-type p53 can inhibit oncogene-mediated focus formation. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8763–8767. doi: 10.1073/pnas.86.22.8763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields S., Jang S. K. Presence of a potent transcription activating sequence in the p53 protein. Science. 1990 Aug 31;249(4972):1046–1049. doi: 10.1126/science.2144363. [DOI] [PubMed] [Google Scholar]
- Gannon J. V., Greaves R., Iggo R., Lane D. P. Activating mutations in p53 produce a common conformational effect. A monoclonal antibody specific for the mutant form. EMBO J. 1990 May;9(5):1595–1602. doi: 10.1002/j.1460-2075.1990.tb08279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman R. M., Krueger J., Yourish D., Granelli-Piperno A., Murphy D. P., May L. T., Kupper T. S., Sehgal P. B., Gottlieb A. B. Interleukin 6 is expressed in high levels in psoriatic skin and stimulates proliferation of cultured human keratinocytes. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6367–6371. doi: 10.1073/pnas.86.16.6367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halevy O., Michalovitz D., Oren M. Different tumor-derived p53 mutants exhibit distinct biological activities. Science. 1990 Oct 5;250(4977):113–116. doi: 10.1126/science.2218501. [DOI] [PubMed] [Google Scholar]
- Hirano T., Akira S., Taga T., Kishimoto T. Biological and clinical aspects of interleukin 6. Immunol Today. 1990 Dec;11(12):443–449. doi: 10.1016/0167-5699(90)90173-7. [DOI] [PubMed] [Google Scholar]
- Kern S. E., Kinzler K. W., Baker S. J., Nigro J. M., Rotter V., Levine A. J., Friedman P., Prives C., Vogelstein B. Mutant p53 proteins bind DNA abnormally in vitro. Oncogene. 1991 Jan;6(1):131–136. [PubMed] [Google Scholar]
- Kishimoto T. The biology of interleukin-6. Blood. 1989 Jul;74(1):1–10. [PubMed] [Google Scholar]
- Krueger J., Ray A., Tamm I., Sehgal P. B. Expression and function of interleukin-6 in epithelial cells. J Cell Biochem. 1991 Apr;45(4):327–334. doi: 10.1002/jcb.240450404. [DOI] [PubMed] [Google Scholar]
- Lane D. P., Benchimol S. p53: oncogene or anti-oncogene? Genes Dev. 1990 Jan;4(1):1–8. doi: 10.1101/gad.4.1.1. [DOI] [PubMed] [Google Scholar]
- Lane D. P., Crawford L. V. T antigen is bound to a host protein in SV40-transformed cells. Nature. 1979 Mar 15;278(5701):261–263. doi: 10.1038/278261a0. [DOI] [PubMed] [Google Scholar]
- Leavitt J., Gunning P., Porreca P., Ng S. Y., Lin C. S., Kedes L. Molecular cloning and characterization of mutant and wild-type human beta-actin genes. Mol Cell Biol. 1984 Oct;4(10):1961–1969. doi: 10.1128/mcb.4.10.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalovitz D., Halevy O., Oren M. p53 mutations: gains or losses? J Cell Biochem. 1991 Jan;45(1):22–29. doi: 10.1002/jcb.240450108. [DOI] [PubMed] [Google Scholar]
- Nigro J. M., Baker S. J., Preisinger A. C., Jessup J. M., Hostetter R., Cleary K., Bigner S. H., Davidson N., Baylin S., Devilee P. Mutations in the p53 gene occur in diverse human tumour types. Nature. 1989 Dec 7;342(6250):705–708. doi: 10.1038/342705a0. [DOI] [PubMed] [Google Scholar]
- Ray A., LaForge K. S., Sehgal P. B. On the mechanism for efficient repression of the interleukin-6 promoter by glucocorticoids: enhancer, TATA box, and RNA start site (Inr motif) occlusion. Mol Cell Biol. 1990 Nov;10(11):5736–5746. doi: 10.1128/mcb.10.11.5736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray A., Sassone-Corsi P., Sehgal P. B. A multiple cytokine- and second messenger-responsive element in the enhancer of the human interleukin-6 gene: similarities with c-fos gene regulation. Mol Cell Biol. 1989 Dec;9(12):5537–5547. doi: 10.1128/mcb.9.12.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray A., Tatter S. B., May L. T., Sehgal P. B. Activation of the human "beta 2-interferon/hepatocyte-stimulating factor/interleukin 6" promoter by cytokines, viruses, and second messenger agonists. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6701–6705. doi: 10.1073/pnas.85.18.6701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray A., Tatter S. B., Santhanam U., Helfgott D. C., May L. T., Sehgal P. B. Regulation of expression of interleukin-6. Molecular and clinical studies. Ann N Y Acad Sci. 1989;557:353–362. [PubMed] [Google Scholar]
- Raycroft L., Wu H. Y., Lozano G. Transcriptional activation by wild-type but not transforming mutants of the p53 anti-oncogene. Science. 1990 Aug 31;249(4972):1049–1051. doi: 10.1126/science.2144364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rideout W. M., 3rd, Coetzee G. A., Olumi A. F., Jones P. A. 5-Methylcytosine as an endogenous mutagen in the human LDL receptor and p53 genes. Science. 1990 Sep 14;249(4974):1288–1290. doi: 10.1126/science.1697983. [DOI] [PubMed] [Google Scholar]
- Robbins P. D., Horowitz J. M., Mulligan R. C. Negative regulation of human c-fos expression by the retinoblastoma gene product. Nature. 1990 Aug 16;346(6285):668–671. doi: 10.1038/346668a0. [DOI] [PubMed] [Google Scholar]
- Rogel A., Popliker M., Webb C. G., Oren M. p53 cellular tumor antigen: analysis of mRNA levels in normal adult tissues, embryos, and tumors. Mol Cell Biol. 1985 Oct;5(10):2851–2855. doi: 10.1128/mcb.5.10.2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samad A., Carroll R. B. The tumor suppressor p53 is bound to RNA by a stable covalent linkage. Mol Cell Biol. 1991 Mar;11(3):1598–1606. doi: 10.1128/mcb.11.3.1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal P. B. Interleukin 6 in infection and cancer. Proc Soc Exp Biol Med. 1990 Nov;195(2):183–191. doi: 10.3181/00379727-195-43129d. [DOI] [PubMed] [Google Scholar]
- Tabibzadeh S. S., Poubouridis D., May L. T., Sehgal P. B. Interleukin-6 immunoreactivity in human tumors. Am J Pathol. 1989 Sep;135(3):427–433. [PMC free article] [PubMed] [Google Scholar]
- Tosato G., Seamon K. B., Goldman N. D., Sehgal P. B., May L. T., Washington G. C., Jones K. D., Pike S. E. Monocyte-derived human B-cell growth factor identified as interferon-beta 2 (BSF-2, IL-6). Science. 1988 Jan 29;239(4839):502–504. doi: 10.1126/science.2829354. [DOI] [PubMed] [Google Scholar]