Abstract
The current study examined the effect of age on both glutamatergic and GABAergic signaling in the rodent medial prefrontal cortex (mPFC), with an emphasis on revealing novel changes contributing to increased inhibition in age. Whole-cell patch clamp recordings were obtained from layer 2/3 mPFC pyramidal neurons in acute cortical slices prepared from either young (4 month) or aged (20–24 month) male F344 rats. Results indicated that GABAB receptors on GABAergic, but not on glutamatergic, inputs to layer 2/3 pyramidal cells are tonically activated by ambient GABA in young animals, and further demonstrated that this form of tonic inhibition is significantly attenuated in aged mPFC. Moreover, concurrent with loss of tonic presynaptic GABAB autoreceptor activation, layer 2/3 pyramidal cells in aged mPFC are subjected to increased tonic activation of extrasynaptic GABAA and GABAB receptors. These data demonstrate a shift in the site of GABABR mediated inhibitory tone in the aged mPFC that clearly promotes increased inhibition of pyramidal cells in aged animals, and that may plausibly contribute to impaired executive function.
Keywords: GABAB, GABAA, prefrontal cortex, working memory, L2/3 pyramidal neurons, age-related cognitive decline
1. Introduction
Global life expectancy has increased by more than 6 years since 1990, and has nearly doubled in the last century. This has led to a substantially increased prevalence of individuals who experience age-related declines in cognitive performance during their lifetime. Among the most functionally debilitating of age related cognitive declines are those that impair working memory. Working memory is defined narrowly as the ability to act on recently available information that is no longer present in the environment (Goldman-Rakic, 1995). It is supported by the dorsolateral prefrontal cortex in primates and by the medial prefrontal cortex (mPFC) in rodents (Dalley, et al., 2004, Hoover and Vertes, 2007, Ongur and Price, 2000, Owen, et al., 1999, Uylings, et al., 2003, Verwer, et al., 1997). Working memory performance declines with advancing age across species, contributing to impaired decision making and a decreased ability for abstract thought and complex problem solving (Alexander, et al., 2012, Banuelos, et al., 2014, Beas, et al., 2013, Bizon, et al., 2012, Glisky, 2007, Morrison and Baxter, 2012, Park, 2000).
From a mechanistic standpoint, working memory is likely to depend on persistent firing of distinct functional subsets of cortical pyramidal neurons that remain active during the delay phase of a specific task (e.g. Arnsten, et al., 2012, Funahashi, et al., 1989, Goldman-Rakic, 1996, Wang, et al., 2011). However, recent experimental and computational studies have highlighted the fact that the ability to fire persistently is generally not a property intrinsic to the pyramidal cells themselves, but rather one that depends on precise regulation of synaptic excitation and inhibition in complex cortical networks. Indeed, it now seems likely that persistent firing, and by extension working memory performance itself, can be disrupted by shifts in prefrontal cortical excitatory-inhibitory dynamics (Konstantoudaki, et al., 2013, Murray, et al., 2014, Wang, et al., 2013).
A primary goal of the current study was to use whole cell patch clamp recordings in layer 2/3 pyramidal cells of the rodent mPFC to provide a more detailed mechanistic understanding of how aging alters synaptic excitation and inhibition in cortical networks that subserve working memory. A core working hypothesis was that age-related changes would ultimately favor increased basal inhibition of cortical pyramidal cells over increased basal activity. This hypothesis was partially motivated by the recent observation that GABAB receptors in the prefrontal cortex regulate tonic inhibition of pyramidal cells in young animals (Wang, et al., 2010), and by studies of both excitatory and inhibitory spontaneous synaptic transmission in primates (Luebke, et al., 2004) and rodents (Bories, et al., 2013) which have suggested an overall net increase in synaptic inhibition accompanies advanced aging. It was further supported by our recent observation that expression of GAD67, an enzyme that promotes synthesis of GABA, is elevated in the aged mPFC, while expression of the neuronal GABA transporter, GAT-1 concurrently declines (Banuelos, et al., 2014). Collectively these findings support that the hypothesis that tonic GABAergic inhibition may be increased in the aged mPFC.
Intriguingly, data obtained during the current study did not indicate a simple increase in tonic inhibition in aged mPFC, but rather revealed an unexpected shift in the location and nature of tonic GABAB receptor activation that is consistent with decreased pyramidal cell excitability in aged cortex. These data should help elucidate recently observed effects of mPFC GABAB receptor modulation in young and aged animals, and may also ultimately provide additional insight on appropriate molecular targets and time points for therapeutic intervention in the treatment of age-related cognitive decline.
2. Materials and Methods
2.1. Slice preparation
Young adult (4–6 month) and aged (20–24 month) male F344 rats obtained from the National Institute of Aging (NIA) were anesthetized with an intraperitoneal injection containing 75–100 mg/kg ketamine and 5–10 mg/kg xylazine. Once a surgical plane of anesthesia was achieved, animals were transcardially perfused with ice-cold oxygenated artificial cerebrospinal fluid (ACSF) which contained in mM: 206 sucrose, 2 KCl, 25 NaHCO3, 1.25 NaH2PO4, 1 CaCl2, 1 MgSO4, 0.01 Glycine, 10 D-glucose, saturated with 95% O2 and 5% CO2. Immediately after perfusion, animals were decapitated with a small animal guillotine, the brain was rapidly removed, and 300 μM thick coronal slices through the medial prefrontal cortex (mPFC) were made in the same ice-cold ACSF using a Leica VT 1000s vibratome. Slices were then transferred to an incubator containing ACSF that contained in mM: 124 NaCl, 2.5 KCl, 25 NaHCO3, 1.23 NaH2PO4, 1 CaCl2, 3 MgSO4, 10 D-glucose. This ACSF was preheated to 30–35 °C, and was also saturated with 95% O2 and 5% CO2. Slices were allowed to equilibrate to room temperature for a minimum of 60 minutes before experimental use. All animal procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Florida and conform to the National Institutes of Health (NIH) animal welfare guidelines.
2.2 Whole cell recording
After incubation, slices were transferred to a recording chamber where they were perfused at 2 ml/min with ACSF that contained in mM: 124 NaCl, 5 KCl, 25 NaHCO3, 1.25 NaH2PO4, 2.5 CaCl2, 1.2 MgSO4, 25 D-glucose. This ACSF was also saturated with 95% O2 and 5% CO2. Temperature in the recording chamber was maintained at 30 ± 2 °C. For experiments that recorded spontaneous inhibitory postsynaptic currents (spontaneous IPSCs), evoked inhibitory postsynaptic currents (evoked IPSCs), or tonic GABAA receptor mediated current, the patch electrode was filled with an internal solution which contained in mM: 95 CsMeSO3, 55 CsCl, 1 MgCl2, 0.2 EGTA, 10 HEPES, 2 Na2-ATP, 0.3 Na-GTP, 5 QX-314. For experiments that recorded spontaneous excitatory postsynaptic currents (spontaneous EPSCs) or evoked excitatory postsynaptic currents (evoked EPSCs), the patch electrode was filled with an internal solution which contained in mM: 140 CsMeSO3, 3 NaCl, 1 MgCl2, 0.2 Cs-EGTA, 4 Na2-ATP, 0.3 Na-GTP, 10 HEPES, 5 QX-314. For experiments that measured the tonic activation of postsynaptic GABAB receptors, the patch electrode was filled with an internal solution that contained in mM: 130 K-Glu, 10 KCl, 10 NaCl, 2 MgCl2,1 EGTA, 2 Na2-ATP, 0.3 Na-GTP, 10 HEPES. All internal solutions were pH adjusted to 7.3 with CsOH or KOH, and were volume adjusted to 285–300 mOSm. Liquid junction potentials were estimated at 8.4 mV (95 CsMeSO3), 11.6 mV (140 CsMeSO3), and 13.1 mV (K-Glu) and were uncorrected. Slices were visualized with infrared differential interference contrast microscopy (IR DIC) using an Olympus BX51WI microscope. Whole cell voltage-clamp recordings were performed using micropipettes pulled from borosilicate glass using a Flaming/Brown electrode puller (Sutter P-97, Sutter Instruments, Novato, CA, USA). Electrode tip resistance was 3–5 MΩ when filled with cesium-based internals described above, and 5–7 MΩ using the K-gluconate based internal. Access resistance, membrane resistance, and whole cell capacitance were measured in voltage clamp mode in response to a 10 mV hyperpolarizing step delivered every 10 seconds. Cells were discarded if access resistance increased by ~30% or more during the course of an experiment, or if changes in access resistance were temporally related to changes in parameters of experimental interest (e.g. evoked or spontaneous synaptic currents or holding current). To generate evoked postsynaptic currents a small tip (~1 μm inner diameter) glass monopolar electrode filled with extracellular solution was connected to a constant current stimulus isolator (World Precision Instruments, Sarasota, FL) and placed in Layer 2/3. Stimuli lasting 0.1 msec were delivered at a frequency of 0.1 Hz. Voltage clamp recordings were performed using an Axon Multiclamp 700A or 700B amplifier (Molecular Devices, Sunnyvale, CA). Data were sampled at 20 kHz, filtered at 2 kHz, and digitally recorded by a Digidata 1322A or 1440 A/D converter using Clampex v. 9 or v. 10 (Molecular Devices, Sunnyvale, CA). Chemicals were obtained from Tocris Cookson, Sigma-Aldrich, or Fisher Scientific.
2.3 Data analysis
All electrophysiological data was analyzed using custom software written in OriginC (OriginLab, Northampton, MA) by CJF. For experiments that involved spontaneous synaptic currents, events were detected using a parameter based event detection algorithm. Data for event detection was filtered off-line using exponential convolution (kernel time constant = 1 msec). RMS noise of the filtered data was generally < 3 pA. Event detection parameters were chosen conservatively to eliminate false positives (e.g. typical peak threshold = 9 pA, typical area threshold = 70 pA * msec). In all cases an algorithm for detecting complex peaks (peaks that occur during the decay period of a previous event) was employed. This algorithm calculates event amplitude for the second (and subsequent) peaks within the decay period based on an extrapolated monoexponential decay from the peak of the initial event. To rule out significant or variable contributions of dendritic filtering we carefully evaluated the relationship between rise time and amplitude of detected events for all cells presented in Table 2. Slope of the best fit linear regression line was typically near 0, and adjusted R-squared values indicated that rise time consistently accounted for less than 3% of the observed variance in amplitude. To evaluate basal frequency of spontaneous EPSCs or spontaneous IPSCs, data was collected for a five-minute period under control conditions, and binned frequency was calculated for each cell using either 4.5 or 8.5 second bins (See results and Table 2). It should be noted that miniature IPSCs and miniature EPSCs were not evaluated in these experiments because addition of TTX would have prevented simultaneous collection of evoked responses. A standard unpaired Student’s two-sample t-test for means was used to compare basal intrinsic and synaptic properties of Layer 2/3 pyramidal neurons in young vs. aged animals. An unpaired t-test with Welch’s correction (for unequal variance) was used to compare shifts in holding current produced by GABA receptor antagonists in young vs. aged neurons. For experiments that involved more complex manipulation of evoked or spontaneous synaptic responses with multiple bath-applied drugs, age comparisons were made using two-factor repeated measures ANOVAs (age X drug condition). When the data revealed a significant interaction between age and drug condition, post-hoc analyses were used to further evaluate the effects of drug condition within each age group and/or across age. These analyses were conducted using two tailed one-sample t-tests for data normalized to the baseline mean (null hypotheses: mean = 1.0), or on paired two-sample t-tests for raw data (null hypothesis: mean for condition 1 = mean for condition 2). Error bars in all figures represent the standard error of the mean.
Table 2. Aging does not alter frequency or amplitude of spontaneous glutamatergic or GABAergic inputs to L2/3 pyramidal neurons.
Values presented in this table were obtained from L2/3 pyramidal cells voltage clamped at −70 mV. Spontaneous IPSCs were isolated using a high chloride CsMeSO3 based internal solution and bath antagonists for ionotropic glutamate receptors (see Methods, Results). Spontaneous EPSCs were isolated using a lower chloride CsMeSO3 based internal solution and a bath antagonist for GABAA receptors. See Methods, Results for more details. In all experiments, basal spontaneous event frequency and amplitude were quantified over a 5 min period that began approximately 15 minutes after initiation of whole cell recording. Cells without stable event frequency during that time, or with unstable access resistance or holding current, were discarded from the dataset. N values represent neurons and animals, respectively.
| Frequency (Hz) | Amplitude (pA) | N values | ||||||
|---|---|---|---|---|---|---|---|---|
| Young | Aged | p | Young | Aged | p | Young | Aged | |
| Isolated sIPSCs | 2.0 ± 0.26 | 1.7 ± 0.43 | 0.53 | 17.5 ± 0.92 | 16.2 ± 0.76 | 0.29 | 37, 15 | 31, 13 |
| Isolated sEPSCs | 7.1 ± 0.96 | 5.1 ± 0.95 | 0.17 | 12.9 ± 1.11 | 12.3 ± 0.75 | 0.68 | 13, 5 | 11, 4 |
3. Results
3.1 Whole cell patch clamp recording from layer 2/3 pyramidal neurons in young and aged rodent medial prefrontal cortex
Historically, obtaining high quality whole-cell patch clamp recordings in tissue from aged animals has presented technical challenges. To facilitate such experiments, we anesthetized animals with a ketamine-xylazine cocktail, performed an intracardial perfusion with ice-cold, oxygenated, sucrose-laden aCSF prior to slice collection, and incubated the slices in a modified aCSF medium containing elevated magnesium and decreased calcium concentrations (see also Moyer and Brown, 1998, Ting, et al., 2014). Collectively, these changes were implemented in an effort to minimize osmotic stress and excitotoxity during the slice making procedure, and to reduce metabolic demands on the tissue during both dissection and incubation. These methods reliably produced viable slices and robust whole cell recordings in both young and aged cortical brain slices. Specifically, in the current studies, whole-cell patch clamp recordings were performed in Layer 2/3 pyramidal cells localized to the infralimbic or prelimbic medial prefrontal cortex (mPFC) is slices generated from young (4–6 month) and aged (20–24 month) Fisher 344 rats. When L2/3 neurons were patched with a K-gluconate based internal solution (see Methods), no differences were found between neurons from young and aged animals in resting membrane potential, input resistance, or whole cell capacitance (Table 1).
Table 1. Aging does not alter basic intrinsic properties of Layer 2/3 pyramidal neurons.
Values presented in this table were obtained from L2/3 pyramidal cells using whole-cell recording pipettes filled with a K-gluconate based internal solution (see Methods). The resting membrane potential was measured shortly after patching in current clamp mode with holding current set to 0 pA. Membrane resistance and whole cell capacitance were calculated from whole-cell capacitive transients observed in response to a voltage step (−10 mV in amplitude and 50 msec in duration) delivered to neurons voltage clamped at −70 mV. For these experiments, fast capacitance compensation was adjusted to minimize impact of the electrode capacitance on instantaneous current amplitude. N values represent neurons and animals, respectively.
| Property | Young (n=17,4) | Aged (n=17,5) | p value |
|---|---|---|---|
| Resting Membrane Potential (mV) | −66.9 ± 0.87 | −65.7 ± 0.90 | 0.33 |
| Membrane Resistance (MΩ) | 55.2 ± 5.12 | 52.5 ± 6.12 | 0.73 |
| Capacitance (pF) | 83.0 ± 8.96 | 87.0 ± 8.79 | 0.75 |
3.2 Neither inhibitory nor excitatory spontaneous synaptic inputs to layer 2/3 pyramidal cells are significantly altered by age
Significant prior work suggests that aging may alter the balance between excitation and inhibition in cortex. Therefore, as an initial step, we sought to evaluate basic properties of spontaneous inhibitory postsynaptic currents (spontaneous IPSCs) and spontaneous excitatory postsynaptic currents (spontaneous EPSCs) impinging on L2/3 pyramidal cells in young and aged mPFC.
In order to measure basal spontaneous IPSC frequency and amplitude, recordings of L2/3 pyramidal neurons were performed in the presence of ionotropic glutamate receptor antagonists DNQX (10 μM) and APV (40 μM). Cells were voltage clamped at −70 mV and filled with a CsMeSO3 based internal solution that contained a high concentration of chloride (see Methods). This internal solution created an outward driving force on chloride at −70 mV (estimated Cl− reversal potential −23 mV) allowing the detection of spontaneous IPSCs as inward currents. Results from these experiments indicated that aging had no significant effect on basal spontaneous IPSC frequency or amplitude (see Table 2 for specific values and statistical tests).
In order to measure basal spontaneous EPSC frequency and amplitude, the same experiments were performed in a separate group of cells in which the high chloride CsMeSO3 internal solution was replaced with a version that had a lower chloride concentration (see Methods), and in which the bath antagonists for ionotropic glutamate receptors were replaced with 50 μM picrotoxin (PTX, a GABAA receptor antagonist). The results of these experiments indicated that aging also had no significant effect on basal spontaneous EPSC frequency or amplitude (see Table 2 for specific values and statistical tests).
Next, in order to evaluate overall distribution of events collected from young and aged animals, cumulative probability histograms were constructed from the interevent intervals and amplitudes pooled across all cells, but within each age group. For this analysis, data were limited to events with interevent intervals of ≤ 2250 msec and amplitude of ≤ 150 pA. The maximum interevent interval was set at ½ the smallest bin size used to collect spontaneous data across all cells, while the maximum amplitude was set to eliminate a very small number of large outliers produced by compound events. For the sIPSC data, Kolmogorov-Smirnov tests conducted on the resulting CPH plots revealed that age had no effect on the distribution of interevent intervals (Z stat: 0.014, p=0.99, n=12,539 events young, 9,945 events aged) or amplitudes (Z stat: 0.03, p=0.27, n=12,671 events young, 10,228 events aged). Similarly, age also had no effect on the overall distribution of interevent intervals (Z stat: 0.06, p=0.08, n= 23,717 events young, 14,419 events aged) or amplitudes (Z stat: 0.03, p=0.27, n= 23,735 events young, 14,449 events aged) for the sEPSC data.
Finally, we also converted interevent intervals observed within each cell to a mean instantaneous frequency (as opposed to the original frequency in bin as reported above and in Table 2), and these values were also not significantly different with age (IPSCs Inst. Frequency: 2.62 ± 0.23 Hz in young, 2.34 ± 0.41 Hz in aged, p=0.54, n=37, 30 respectively, EPSCs Inst. Frequency: 7.23 ± 0.91 Hz in young, 5.3 ± 0.98 Hz in aged, p=0.16, n=13, 11 respectively).
3.3 Tonic inhibition mediated by GABAB autoreceptors on inhibitory inputs to layer 2/3 pyramidal cells is reduced in aged mPFC
A lack of change in basal frequency and amplitude of spontaneous synaptic events is not sufficient to conclude that there is no age related change in how excitatory and inhibitory synaptic transmission is regulated. Therefore, subsequent experiments were designed to more directly evaluate the role of presynaptic GABAB autoreceptors expressed on inhibitory inputs to L2/3 pyramidal cells in modulation of GABAergic transmission. In these experiments, L2/3 pyramidal cells were patch clamped at −70 mV using a high chloride CsMeSO3 based internal solution (see Methods), and a glass monopolar stimulator, also placed in layer 2/3, was used to generate evoked IPSCs every 10 seconds in the presence of DNQX (10 μM) and APV (40 μM). After 10 minutes of stable baseline recording, we bath applied the GABAB receptor agonist baclofen (10 μM) for 10 min, followed by the GABAB receptor antagonist CGP 55845 (CGP, 10 μM) for an additional 10 minutes, in the continued presence of baclofen (Fig. 1). A two factor repeated measures ANOVA (age X drug condition (baclofen, or baclofen + CGP)) was performed on data normalized to the baseline mean. This analysis revealed a main effect of drug condition (F(1,120)=44.64, p<0.0001), and a significant interaction between age and drug condition (F(1,120)=4.38, p=0.038).
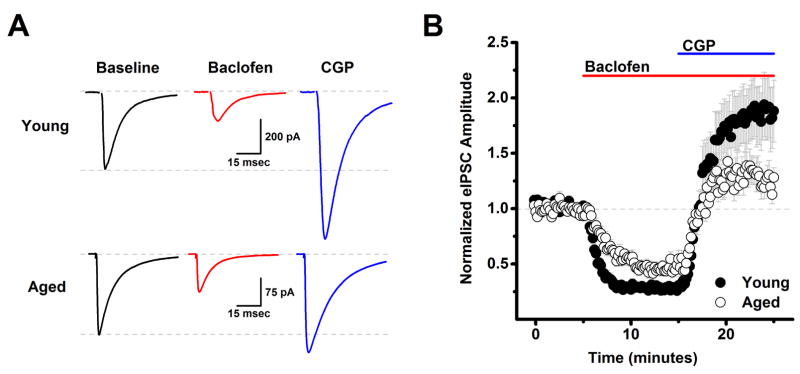
Figure 1. Aging reduces tonic activation of presynaptic GABAB autoreceptors on inhibitory inputs to layer 2/3 pyramidal neurons.
A) Raw data traces representative of the population mean are illustrated from a young neuron (top) and an aged neuron (bottom) in baseline conditions, after application of 10 μM baclofen, and in the presence of both baclofen and 10 μM CGP. Each trace is an average of 15–30 consecutive sweeps (generated at 0.1 Hz) in the stated conditions. Note that while baclofen reduced evoked IPSC amplitude in both cells, amplitude in the presence of baclofen and CGP exceeded baseline amplitude much more strongly in the neuron extracted from a young animal. The top dashed line in each set of traces illustrates a common baseline, while the bottom dashed line represents the evoked IPSC amplitude in baseline conditions. B) Population data illustrate that the same effects are apparent across cells when evoked IPSC amplitude is normalized to the baseline mean (young: n=39, 8, aged: n=23, 7, in neurons, animals respectively). See text for specific values. DNQX (10 μM) and APV (40 μM) were present in the bath solution throughout the experiment.
Post-hoc analyses were performed within each age group separately using two-tailed one-sample t tests (null hypothesis: mean=1), or across ages using unpaired two-tailed two sample t-tests (null hypothesis: mean 1 = mean 2). These analyses revealed that baclofen significantly reduced evoked IPSC amplitude in both young and aged neurons (to 28.2 ± 2.7% of baseline in young, n=39 neurons, 8 animals, p<0.001, and to 46.3 ± 4.0% of baseline in aged, n=23 neurons, 7 animals, p<0.001), and further indicated that the magnitude of this baclofen mediated inhibition was reduced in aged neurons (p<0.001). We also observed that evoked IPSC amplitude in the presence of both baclofen and CGP exceeded baseline levels in the young neurons (185.6 ± 26.6% of baseline, p=0.002). Intriguingly, the magnitude of this apparent tonic GABAB autoreceptor activation was significantly reduced in the aged neurons (to 128.6 ± 9.9% of baseline, p=0.050). Finally, the maximal amount of inhibition produced by activation of GABAB autoreceptors, measured as the difference between the eIPSC amplitude during the baclofen and baclofen+CGP time points, was also significantly reduced in the aged neurons (p=0.012, Fig. 1). Collectively, these results suggest that GABAergic inputs to layer 2/3 pyramidal cells in young animals are tonically inhibited by basal activation of GABAB autoreceptors, and further suggest that this form of tonic inhibition is significantly attenuated in aged animals.
An additional advantage of the high chloride cesium internal employed in this experiment is that the cesium blocks postsynaptic potassium conductances coupled to GABAB receptors expressed by L2/3 pyramidal cells, and thus effects of baclofen and CGP described above are very likely to be mediated by action on presynaptic GABAB autoreceptors. Consistent with that interpretation, baclofen had no significant effect on holding current or input resistance in young neurons (ΔiHold: −3.9 ± 5.0 pA, ΔRm: −12.3 ± 7.6 MΩ, n=25, 8 animals, p=0.38, 0.40 respectively), or in aged neurons (ΔiHold: −3.5 ± 3.7 pA, ΔRm: 1.7 ± 3.3 MΩ, n=19, 7 animals p=0.66, 0.64 respectively).
3.4 Spontaneous ISPCs are less reliable reporters of presynaptic GABAB autoreceptor activation than evoked IPSCs
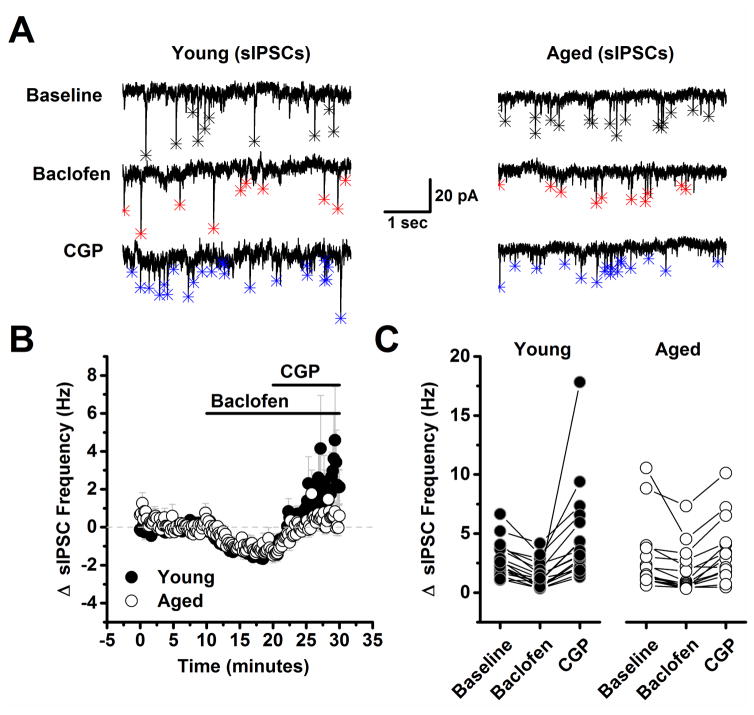
While reduced tonic activation of GABAB autoreceptors might be expected to produce increased basal spontaneous IPSC frequency in the aged animals, as reported above, this was not the case. In order to more carefully evaluate the potential role of presynaptic GABAB autoreceptors in regulating frequency of spontaneous IPSCs, we recorded spontaneous IPSC frequency for 10 minutes in cells voltage clamped at −70 mV with the same high CsMeSO3 based internal used to study evoked IPSCs, and then bath applied baclofen (10 μM) for 10 minutes, followed by CPG (10 μM) for an additional 10 minutes in the continued presence of baclofen (Fig. 2). A two factor repeated measures ANOVA (age X drug condition (baseline, baclofen, or baclofen + CGP) was performed on data representing the raw spontaneous IPSC frequency. This analysis revealed a main effect of drug condition (F(2,60)=14.84, p<0.0001), but there was no effect of age nor a significant interaction between age and drug condition (F(2,60)=1.77, P=0.179). To further explore the effects of drug condition, post-hoc analyses were performed on data collapsed by age. These analyses indicated that baclofen significantly reduced spontaneous IPSC frequency from baseline (from 2.90 ± 0.398 Hz to 1.63 ± 0.276 Hz, n=32, p<0.001), and interestingly, revealed that frequency was significantly increased from baseline in the presence of both baclofen and CGP (2.90 ± 0.398 Hz vs. 4.1 ± 0.61 Hz, n=32, p=0.04).
Figure 2. Spontaneous inhibitory postsynaptic currents are less reliable reporters of presynaptic GABAB autoreceptor activation than are evoked inhibitory postsynaptic currents.
A) Representative traces from a young neuron (left) and aged neuron (right) in baseline conditions, after application of 10 μM baclofen, and in the presence of both baclofen and 10 μM CGP. Asterisks in each trace indicate the peak of detected events. Baclofen clearly reduced spontaneous IPSC frequency in both neurons (middle traces, red asterisks). Notably, event frequency also exceeded baseline after application of 10 μM CGP in young, but not in the aged neuron (bottom traces, blue asterisks). B) Illustrates the average change in spontaneous IPSC frequency across all cells tested as in panel A. Although the mean spontaneous IPSC frequency in the presence of both baclofen and CGP more strongly trended above baseline in neurons from young animals, overall the effect was not statistically significant (young: n=19, 9, aged: n=15, 8, in neurons, animals respectively, p=0.09, see text of the results section for more details). C) Raw spontaneous IPSC frequency is plotted in all three conditions for every neuron tested in both the young and aged population. DNQX (10 μM) and APV (40 μM) were present in the bath solution throughout the experiment.
3.5 Presynaptic GABAB receptors also exist on glutamatergic inputs to L2/3 pyramidal neurons in mPFC, but are not subject to tonic activation in young or in aged tissue
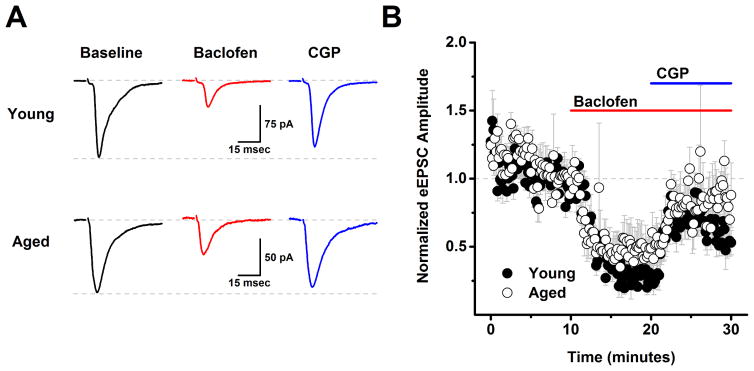
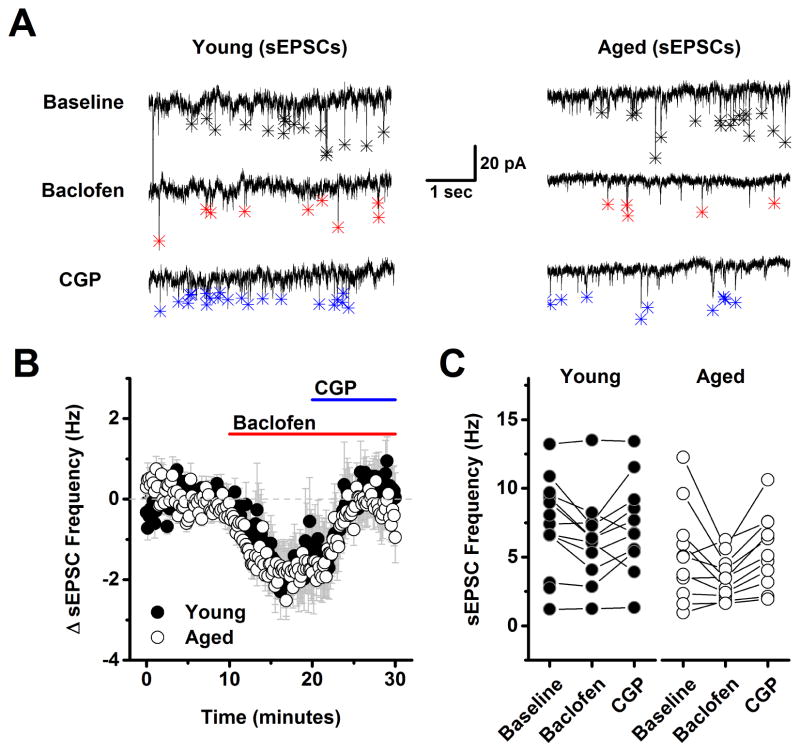
In order to evaluate the effects of aging on GABAB receptor dependent modulation of glutamatergic inputs to L2/3 pyramidal cells the experiments presented in Figs. 1–2 were repeated using a low chloride CsMeSO3 based internal solution instead of the high chloride version (see Methods), and with 50 μM PTX (a GABAA receptor antagonist) in the bath, instead of DNQX and APV. These conditions effectively isolated evoked and spontaneous EPSCs (see Methods). With respect to both evoked EPSC amplitude and spontaneous EPSC frequency a two factor repeated measures ANOVA (age X drug condition (baseline, baclofen and baclofen + CGP) revealed a main effect of drug condition (F(1,8)=21.15, p=0.002 for evoked EPSC amplitude, F(2,46)=11.56, p<0.0001 for sEPSC frequency) but no main effect of age nor an interaction between age and drug condition (F(1,8)=0.24, p=0.63 for evoked EPSC amplitude, F(2,46)=0.20, p=0.82 for spontaneous EPSC frequency). To further explore the effects of drug condition, post-hoc analyses were performed on data collapsed by age. A one factor ANOVA on these data revealed a significant effect of drug condition on both evoked EPSC amplitude (F(2,9)=33.0, p<0.0001) and spontaneous EPSC frequency (F(2,24)=11.9, p<0.0001) such that baclofen significantly reduced both evoked EPSC amplitude (to 38.5 ± 8.1% of baseline, n=10, p<0.0005, one sample t-test on data normalized to the baseline mean) and spontaneous EPSC frequency (from 6.3 ± 0.7 Hz to 4.7 ± 0.6 Hz, n=25, P=0.0005, paired t-test on raw sIPSC frequency). Nevertheless, further analysis revealed that neither of these parameters significantly exceeded baseline values in the presence of both baclofen and CGP (evoked EPSC amplitude in baclofen + CGP = 72.5% of baseline, n=10, p=0.005, spontaneous EPSC frequency in baclofen + CGP: 6.4 ± 0.6 Hz vs. 6.3 ± 0.7 Hz in baseline, n=25, p=0.75). Collectively, these results indicate that presynaptic GABAB receptors exist on the axon terminals of glutamatergic inputs to layer 2/3 pyramidal cells, and that activation of these receptors can inhibit both evoked EPSC amplitude and spontaneous EPSC frequency. However, these data provided no evidence of tonic basal activation of these GABAB heteroreceptors in either young or aged neurons. Finally, it should be emphasized that the primary effects of baclofen in the experiments with evoked and spontaneous EPSCs are very likely to be mediated by activation of presynaptic GABAB receptors on excitatory inputs to layer 2/3 pyramidal cells, again because the CsMeSO3 based internal solutions used in these experiments is expected to effectively block primary postsynaptic potassium conductances coupled to GABAB receptors. Consistent with that interpretation we found no significant effect of baclofen on holding current or input resistance across all cells presented in Fig 3 (ΔiHold: −6.2 ± 19.5 pA, ΔRm: −11.0 ± 7.4 MΩ, n=10 neurons, 4 animals, p=0.76, 0.17 respectively).
Figure 3. Analysis of evoked EPSCs indicates that GABAB heteroreceptors are expressed on excitatory inputs to layer 2/3 pyramidal neurons, but are not subject to tonic activation in either young or aged neurons.
A) Raw data traces representative of the population mean are illustrated from a young neuron (top) and an aged neuron (bottom) in baseline conditions, after application of 10 μM baclofen, and in the presence of both baclofen and 10 μM CGP. Each trace is an average of 15–30 consecutive sweeps (generated at 0.1 Hz) in the stated conditions. Baclofen reduced evoked IPSC amplitude in both cells, but amplitude in the presence of baclofen and CGP did not exceed baseline values in either cell. The top dashed line in each set of traces illustrates a common baseline, while the bottom dashed line represents the evoked IPSC amplitude in baseline conditions. B) Population data illustrate that the same effects are apparent across cells when evoked EPSC amplitude is normalized to the baseline mean (young: n=5, 2, aged: n= 5, 2, in neurons, animals respectively). See text for specific values. PTX (50 μM) was present in the bath solution throughout the experiment.
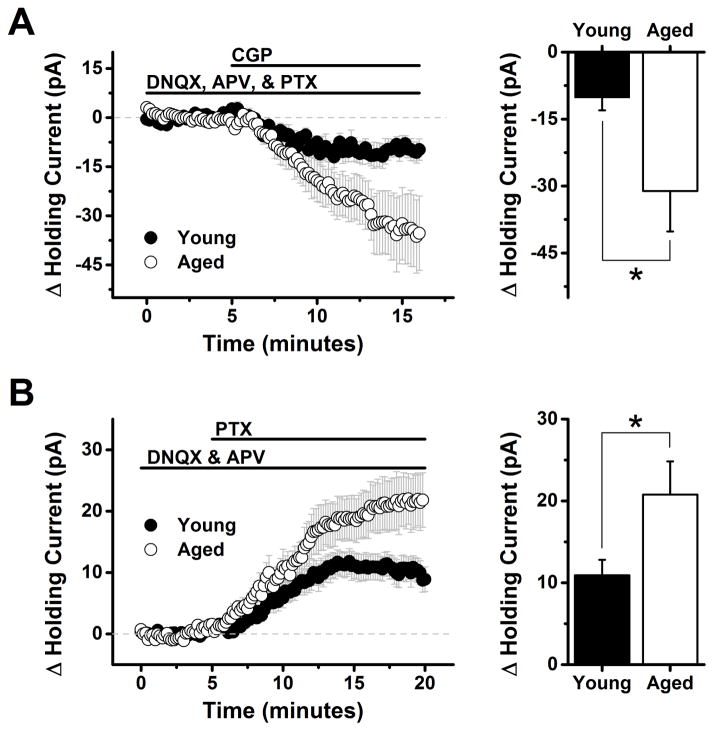
3.6 Extrasynaptic GABAA and GABAB receptors are expressed by L2/3 pyramidal neurons, and are subject to greater tonic activation in aged animals
The results presented above suggest that aging produces disinhibition of inhibitory inputs to L2/3 pyramidal cells, but further indicate that this phenomenon is not directly associated with increased spontaneous IPSC frequency. Therefore, we next sought to test the hypothesis that age-related changes in GABAergic signaling could otherwise lead to increased basal activation of extrasynaptic high affinity GABAB and/or GABAA receptors on postsynaptic pyramidal neurons. To test the hypothesis with respect to GABAB receptors, we evaluated the effect of CGP on the holding current required to voltage clamp L2/3 pyramidal cells at −50 mV using a K-gluconate based internal solution (see Methods). Antagonists for ionotropic glutamate receptors (DNQX, 10 μM, APV, 40 μM) and GABAA receptors (PTX, 100 μM) were present in the bath solution throughout the experiment. Under these conditions, bath application of 10 μM CGP caused a negative shift in holding current in both age groups, consistent with removal of a GABAB receptor dependent tonic inhibitory current. The CGP induced change in holding current was −10.2 ± 2.9 pA in young neurons (n=8 neurons, 5 animals, p=0.009) and −31.1 ± 9.06 pA in aged neurons (n=15 neurons, 7 animals, p=0.004). Because variance was significantly greater in the aged population (F(7,14)=18.93, p=0.0007) we used an unpaired t-test with Welch’s correction to compare across groups. This analysis revealed that GABAB receptor mediated tonic inhibitory current was significantly larger in the aged population (p=0.04).
In order to isolate tonic inhibitory current mediated by extrasynaptic GABAA receptors, we voltage clamped L2/3 pyramidal neurons at −70 mV in the presence of DNQX (10 μM) and APV (40 μM) using a high chloride CsMeSO3 based internal solution (see Methods). Under these conditions bath application of PTX (100 μM) caused a positive shift in holding current in both age groups, consistent with removal of a tonic outward flow of chloride through GABAA receptors. The PTX induced change in holding current was 10.9 ± 1.91 pA in young neurons (n=18 neurons, 7 animals, P<0.001) and 20.8 ± 4.07 pA in aged neurons (n=17 neurons, 7 animals, p<0.001). Although variance was again greater in the aged population (F(17,16)=4.3, p=0.005), an unpaired t-test with Welch’s correction indicated that aged neurons were also subject to a significantly greater tonic activation of extrasynaptic GABAA receptors (p<0.001).
4. Discussion
In this study we used an in vitro electrophysiological approach to examine the effect of GABAB receptor activation and antagonism on spontaneous and evoked glutamatergic and GABAergic synaptic inputs to Layer 2/3 pyramidal cells in the mPFC of both young and aged rodents. We also examined the extent to which age effects tonic activation of extrasynaptic GABA receptors expressed by these neurons. The work was most directly motivated by the recent observation that intra-PFC administration of a GABAB receptor antagonist significantly improved working memory performance in aged rodents, and yet failed to do so in young animals (Banuelos, et al., 2014). One goal of the current study was to identify a specific population of GABAB receptors in the cortical circuitry of mPFC that is tonically activated only in aged neurons, as such receptors would be considered a likely site of action for the positive behavioral effects of CGP. However, in a broader context, we also sought to provide a more complete description than previously available of when, where, and to what end both presynaptic and extrasynaptic GABAB receptors are tonically active in the mPFC of both young and aged animals. Our experiments provided several results which can be considered in context with recent findings about other types of age related changes in cortical GABAergic systems.
One key finding of the current study was that GABAB autoreceptors present on inhibitory inputs to Layer 2/3 pyramidal neurons contribute to tonic inhibition of action potential dependent release of GABA in young animals, and that this effect is markedly reduced in aged animals. In general, this observation is consistent with the recent finding that aging reduces expression of a GABAB receptor isoform (GABABR1a) that is believed to be localized to presynaptic terminals (Banuelos, et al., 2014, McQuail, et al., 2012, Vigot, et al., 2006). While it might be expected that disinhibition of GABAergic afferents in aged mPFC would lead to measurable increases in spontaneous IPSC frequency, this was not the case in our experiments. Indeed, although there was a slight trend towards decreased frequency of pharmacologically isolated spontaneous EPSCs, overall our data revealed no significant effect of age on spontaneous synaptic transmission from either GABAergic or glutamatergic terminals. Some prior studies of spontaneous synaptic transmission in the prefrontal cortex have shown modest changes favoring inhibition, however on balance none of the prior publications are directly incompatible with our observations. For example, Bories et al (2013) reported increased miniature, rather than spontaneous, ISPC frequency in aged Fischer 344 rats that were impaired on a corner exploration task; however, similar to our results, they noted no significant effect on either excitatory or inhibitory events across the general aged population. By contrast, Luebke et al. (2004), working with tissue from young or aged rhesus monkeys, noted no change in miniature glutamatergic or GABAergic events, but did report decreased frequency of spontaneous EPSCs and increased frequency of spontaneous IPSCs in Layer 2/3 pyramidal cells. These data raise the possibility that age related changes in spontaneous synaptic events in mPFC are more pronounced in primates, a possibility that could plausibly be related to their longer lifespan.
A second key finding was that, although presynaptic GABAB receptors were clearly expressed on axon terminals of glutamatergic inputs to Layer 2/3 pyramidal cells, these receptors did not appear to be subject to tonic activation by ambient GABA in either young or aged rodents. This finding is interesting because it indicates that there are likely microdomains of ambient GABA in cortical tissue and that, at least in young animals, concentrations tend to be higher near GABA terminals than near glutamate terminals. This might be a simple reflection of the fact that GABAB autoreceptors are closer to the release site than GABAB heteroreceptors, however as a significant percentage of ambient GABA in most cortical networks studied to date is believed to be activity independent (Roth and Draguhn, 2012), it may also reflect structural architecture that reduces access of ambient GABA to heteroreceptors under basal conditions, and/or molecular systems that promote increased uptake of GABA in the vicinity of glutamatergic terminals.
A third key finding was that extrasynaptic GABA receptors expressed by pyramidal cells themselves are clearly subject to increased tonic activation in aged mPFC. From a mechanistic standpoint, this is another effect that may result from the observed disinhibition of GABAergic inputs. However, as spontaneous IPSC frequency was not significantly increased, it seems likely that there would be other underlying causes for increased extrasynaptic GABA receptor activation. Indeed, recent work has indicated that aging in the mPFC is associated with increased local expression of GAD67 and decreased expression of the neuronal GABA transporter, GAT-1 (Banuelos, et al., 2014), both of which are changes that might promote increases in ambient GABA. Interestingly, however, other recent work indicates that expression of a postsynaptic isoform of GABAB receptors (GABABR1b) is reduced in aged mPFC, and that this reduction is inversely correlated with working memory performance (Banuelos, et al., 2014, McQuail, et al., 2012, Vigot, et al., 2006). These observations, when considered alongside data from the current study, suggest the possibility that reduced expression of GABABR1b in aged animals is driven by large increases in ambient GABA, and yet is ultimately insufficient to return tonic inhibitory currents to levels observed in young animals. In that event, GABABR1b expression in mPFC would be inversely correlated with working memory performance for the same reason that application of an exogenous GABAB receptor antagonist to the mPFC improves it: because reduced extrasynaptic inhibition of Layer 2/3 pyramidal cells promotes improved working memory performance in aged rodents. Interestingly, the idea that a net shift towards inhibition may be associated with impaired cognitive performance may not hold true for all brain areas. Consider that GABABR expression during aging is more preserved in hippocampus than in PFC (Banuelos, et al., 2014, Beas, et al., 2016, McQuail, et al., 2012, McQuail, et al., 2015), that aged hippocampal pyramidal cells are likely subject to reduced dendritic inhibition associated with reduced numbers of inhibitory interneurons (Bakker, et al., 2012, Spiegel, et al., 2013, Stanley, et al., 2012, Thome, et al., 2015), and that some mouse models of Alzheimer’s are associated with increased hippocampal excitability (Davis, et al., 2014, Hall, et al., 2015, Palop, et al., 2011).
Finally, it is worth considering effects of CGP on young animals more carefully. Based on current data, intra-PFC administration of CGP in young animals might be expected to have a disinhibitory effect on GABAergic inputs to Layer 2/3 pyramidal cells that would be absent in aged animals. Further, any direct ability of CGP to remove tonic inhibition of Layer 2/3 pyramidal cells by antagonism of extrasynaptic receptors appears to be significantly reduced in the young population. Indeed, in this situation the overall effect of CGP on working memory performance might be expected to depend on whether the presynaptic disinhibitory mechanism or the reduced direct antagonism of extrasynaptic receptors had a bigger overall impact on cortical function. If the disinhibitory mechanism were predominant, CGP might even be expected to impair performance in the younger population by increasing synaptic release of GABA. Indeed, Banuelos et al. (2014) did note a trend toward impaired performance in young animals that received intra-PFC administration of CGP at longer delay times, and they also reported a statistically significant impairment in performance across all delay times in young animals that received systemic injection of CGP. By contrast, the powerful positive effect of CGP on working memory performance in aged animals likely reflects a situation where intra-PFC administration of CGP is much more heavily skewed towards reducing inhibition of pyramidal cells produced by tonic activation of extrasynaptic GABAB receptors (a phenomenon revealed in the current study).
Based on results presented here, we believe there are several interesting and important avenues for future investigation. For example, it is important to highlight that aging did not alter intrinsic properties of mPFC pyramidal cells as observed at −70 mV in the current study, and also did not affect basal spontaneous event frequency. Therefore, additional work should focus on further revealing the functional impact of tonic activation of GABAB receptors on pyramidal cell physiology. Similarly, based on the broad diversity of interneurons in prefrontal cortical circuits (Zaitsev, et al., 2009), it will be important to more precisely determine which subclasses of interneurons are subject to tonic GABAB autoreceptor activation in young animals that is lost with aging, when affected interneurons are most active during normal cortical processing, and whether they are lost or modified in other disease states. Further, given that our results revealed a net age related increase in activation of extrasynaptic GABAA and GABAB receptors, there are a number of challenging but fascinating questions that remain about the relative mechanistic contribution of these two types of inhibitory systems. In that regard it is interesting to note that in other systems tonic activation of GABAA receptors is consistently implicated in control of neuronal offset or gain (Pavlov, et al., 2009, Semyanov, et al., 2004), and in generation of patterned activity and neuronal synchrony (Pavlov, et al., 2014, Traub, et al., 1996). By contrast, GABAB receptors expressed by cortical pyramidal cells have been implicated in modulation of NMDA mediated calcium influx (Chalifoux and Carter, 2010), and voltage gated calcium channels (Chalifoux and Carter, 2011), and even in theoretical generation and direct experimental termination of an upstate that is likely to support persistent firing (Craig, et al., 2013, Mann, et al., 2009, Sanders, et al., 2013).
Figure 4. Frequency of spontaneous excitatory postsynaptic currents is also sensitive to baclofen but not subject to tonic GABAB receptor mediated inhibition.
A) Representative traces from a young neuron (left) and aged neuron (right) in baseline conditions, after application of 10 μM baclofen, and in the presence of both baclofen and 10 μM CGP. Asterisks in each trace indicate the peak of detected events. Baclofen clearly reduced spontaneous EPSC frequency in both neurons (middle traces, red asterisks). Event frequency returned to, but did not notably exceed baseline, after application of 10 μM CGP in both the young and the aged neuron (bottom traces, blue asterisks). B) Population data illustrating the average change in spontaneous EPSC frequency across all cells tested as in panel A. As per the representative cells, baclofen clearly inhabited spontaneous EPSC frequency, and subsequent application of CGP reversed this effect, however no difference was noted between ages, and no evidence of tonic GABAB heteroreceptors activation was apparent in either population (young: n=11, 4, aged: n=11, 5, in neurons, animals respectively). See text of the results section for more details. C) Raw spontaneous EPSC frequency is plotted in all three conditions for every neuron tested in both the young and aged population. PTX (100 μM) was present in the bath solution throughout the experiment.
Figure 5. Extrasynaptic high affinity GABAA and GABAB receptors are expressed by L2/3 pyramidal neurons, and are subject to greater tonic activation in aged animals.
A) Left panel: Bath application of 10 μM CGP (a GABAB receptor antagonist) causes a clear shift in holding current in L2/3 pyramidal cells voltage clamped at −70 mV with a K-gluconate based internal solution (see Methods). Antagonists for ionotropic glutamate (DNQX, 10 μM, APV 40 μM) and GABAA receptors (PTX, 100 μM) were present throughout the experiment. The polarity of the holding current shift is consistent with the hypothesis that CGP is blocking an inhibitory current mediated by tonic activation of extrasynaptic GABAB receptors. Right panel: When quantified between 12 and 16 minutes into the experiment (7 to 11 minutes after application of CGP) this effect was significantly greater in neurons from aged animals than in neurons from young animals (−10.2 ± 2.9 pA in young neurons vs. −31.1 ± 9.06 pA in aged neurons, n=8, 15 respectively, p=0.04, see test of results section for further details). Note: although we refer to GABAB receptors on layer 2/3 pyramidal cells that are tonically activated by ambient GABA as ‘extrasynaptic’ here, and throughout the manuscript, it is plausible that synaptically located GABAB receptors also have sufficiently high affinity for GABA to contribute to these tonic currents, particularly if GABA uptake near synapses is not prohibitive. B) Left panel: illustrates a similar experiment conducted in conditions designed to isolate and amplify current through extrasynaptic GABAA receptors. Specifically, L2/3 pyramidal neurons were voltage clamped at −70 mV in the presence of ionotropic glutamate receptor antagonists (DNQX, 10 μM, APV 40 μM) using a high chloride CsMeSO3 based internal solution. Application of the GABAA receptor antagonist PTX (100 μM) revealed a clear shift in holding current in both young and aged neurons. The polarity of the holding current shift is consistent with the hypothesis that PTX is blocking an excitatory current mediated by tonic activation of extrasynaptic GABAA receptors. The current is excitatory due to the strong outward driving force on chloride at −70 mV that is created when using an internal solution with a high chloride concentration. Right panel: When quantified between 15 and 20 minutes into the experiment (4 to 10 minutes after application of PTX) this effect was significantly greater in neurons from aged animals than in neurons from young animals (10.9 ± 1.91 pA in young neurons vs. 20.8 ± 4.07 pA in aged neurons, n=18 cells (7 animals), 17 cells (7 animals) respectively, p<0.001, see test of results section for further details).
Highlights.
We examined the effects of age synaptic transmission in rodent prefrontal cortex.
Aging reduces tonic activation of presynaptic GABAB autoreceptors.
Presynaptic GABAB heteroreceptors are not subject to tonic inhibition in young or aged animals.
Aging increases tonic activation of high affinity GABAA and GABAB receptors expressed on layer II/III pyramidal cells.
Acknowledgments
This work was supported by the McKnight Brain Research Foundation (CJF) and by R01-AG029421 (JLB).
Footnotes
Disclosure statement
The authors have no conflicts of interest, and all animal procedures were performed in compliance with the Institutional Animal Care and Use Committee (IACUC) of the University of Florida.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander GE, Ryan L, Bowers D, Foster TC, Bizon JL, Geldmacher DS, Glisky EL. Characterizing cognitive aging in humans with links to animal models. Front Aging Neurosci. 2012;4:21. doi: 10.3389/fnagi.2012.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF, Wang MJ, Paspalas CD. Neuromodulation of thought: flexibilities and vulnerabilities in prefrontal cortical network synapses. Neuron. 2012;76(1):223–39. doi: 10.1016/j.neuron.2012.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker A, Krauss GL, Albert MS, Speck CL, Jones LR, Stark CE, Yassa MA, Bassett SS, Shelton AL, Gallagher M. Reduction of hippocampal hyperactivity improves cognition in amnestic mild cognitive impairment. Neuron. 2012;74(3):467–74. doi: 10.1016/j.neuron.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banuelos C, Beas BS, McQuail JA, Gilbert RJ, Frazier CJ, Setlow B, Bizon JL. Prefrontal cortical GABAergic dysfunction contributes to age-related working memory impairment. J Neurosci. 2014;34(10):3457–66. doi: 10.1523/JNEUROSCI.5192-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beas BS, McQuail JA, Banuelos C, Setlow B, Bizon JL. Prefrontal cortical GABAergic signaling and impaired behavioral flexibility in aged F344 rats. Neuroscience. 2016 doi: 10.1016/j.neuroscience.2016.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beas BS, Setlow B, Bizon JL. Distinct manifestations of executive dysfunction in aged rats. Neurobiol Aging. 2013;34(9):2164–74. doi: 10.1016/j.neurobiolaging.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizon JL, Foster TC, Alexander GE, Glisky EL. Characterizing cognitive aging of working memory and executive function in animal models. Front Aging Neurosci. 2012;4:19. doi: 10.3389/fnagi.2012.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bories C, Husson Z, Guitton MJ, De Koninck Y. Differential balance of prefrontal synaptic activity in successful versus unsuccessful cognitive aging. J Neurosci. 2013;33(4):1344–56. doi: 10.1523/JNEUROSCI.3258-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalifoux JR, Carter AG. GABAB receptors modulate NMDA receptor calcium signals in dendritic spines. Neuron. 2010;66(1):101–13. doi: 10.1016/j.neuron.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalifoux JR, Carter AG. GABAB receptor modulation of voltage-sensitive calcium channels in spines and dendrites. J Neurosci. 2011;31(11):4221–32. doi: 10.1523/JNEUROSCI.4561-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig MT, Mayne EW, Bettler B, Paulsen O, McBain CJ. Distinct roles of GABAB1a- and GABAB1b-containing GABAB receptors in spontaneous and evoked termination of persistent cortical activity. J Physiol. 2013;591(Pt 4):835–43. doi: 10.1113/jphysiol.2012.248088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Cardinal RN, Robbins TW. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev. 2004;28(7):771–84. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Davis KE, Fox S, Gigg J. Increased hippocampal excitability in the 3xTgAD mouse model for Alzheimer’s disease in vivo. PLoS One. 2014;9(3):e91203. doi: 10.1371/journal.pone.0091203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funahashi S, Bruce CJ, Goldman-Rakic PS. Mnemonic coding of visual space in the monkey’s dorsolateral prefrontal cortex. J Neurophysiol. 1989;61(2):331–49. doi: 10.1152/jn.1989.61.2.331. [DOI] [PubMed] [Google Scholar]
- Glisky EL. Changes in Cognitive Function in Human Aging. In: Riddle DR, editor. Brain Aging: Models, Methods, and Mechanisms. Boca Raton (FL): 2007. [Google Scholar]
- Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14(3):477–85. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Regional and cellular fractionation of working memory. Proc Natl Acad Sci U S A. 1996;93(24):13473–80. doi: 10.1073/pnas.93.24.13473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall AM, Throesch BT, Buckingham SC, Markwardt SJ, Peng Y, Wang Q, Hoffman DA, Roberson ED. Tau-dependent Kv4.2 depletion and dendritic hyperexcitability in a mouse model of Alzheimer’s disease. J Neurosci. 2015;35(15):6221–30. doi: 10.1523/jneurosci.2552-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover WB, Vertes RP. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct. 2007;212(2):149–79. doi: 10.1007/s00429-007-0150-4. [DOI] [PubMed] [Google Scholar]
- Konstantoudaki X, Papoutsi A, Chalkiadaki K, Poirazi P, Sidiropoulou K. Modulatory effects of inhibition on persistent activity in a cortical microcircuit model. Frontiers in neural circuits. 2013;8:7. doi: 10.3389/fncir.2014.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luebke JI, Chang YM, Moore TL, Rosene DL. Normal aging results in decreased synaptic excitation and increased synaptic inhibition of layer 2/3 pyramidal cells in the monkey prefrontal cortex. Neuroscience. 2004;125(1):277–88. doi: 10.1016/j.neuroscience.2004.01.035. [DOI] [PubMed] [Google Scholar]
- Mann EO, Kohl MM, Paulsen O. Distinct roles of GABA(A) and GABA(B) receptors in balancing and terminating persistent cortical activity. J Neurosci. 2009;29(23):7513–8. doi: 10.1523/jneurosci.6162-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuail JA, Banuelos C, LaSarge CL, Nicolle MM, Bizon JL. GABA(B) receptor GTP-binding is decreased in the prefrontal cortex but not the hippocampus of aged rats. Neurobiol Aging. 2012;33(6):1124, e1–12. doi: 10.1016/j.neurobiolaging.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuail JA, Frazier CJ, Bizon JL. Molecular aspects of age-related cognitive decline: the role of GABA signaling. Trends in molecular medicine. 2015;21(7):450–60. doi: 10.1016/j.molmed.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison JH, Baxter MG. The ageing cortical synapse: hallmarks and implications for cognitive decline. Nat Rev Neurosci. 2012;13(4):240–50. doi: 10.1038/nrn3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer JR, Jr, Brown TH. Methods for whole-cell recording from visually preselected neurons of perirhinal cortex in brain slices from young and aging rats. J Neurosci Methods. 1998;86(1):35–54. doi: 10.1016/s0165-0270(98)00143-5. [DOI] [PubMed] [Google Scholar]
- Murray JD, Anticevic A, Gancsos M, Ichinose M, Corlett PR, Krystal JH, Wang XJJ. Linking microcircuit dysfunction to cognitive impairment: effects of disinhibition associated with schizophrenia in a cortical working memory model. Cerebral cortex (New York, NY : 1991) 2014;24(4):859–72. doi: 10.1093/cercor/bhs370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10(3):206–19. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Owen AM, Herrod NJ, Menon DK, Clark JC, Downey SP, Carpenter TA, Minhas PS, Turkheimer FE, Williams EJ, Robbins TW, Sahakian BJ, Petrides M, Pickard JD. Redefining the functional organization of working memory processes within human lateral prefrontal cortex. Eur J Neurosci. 1999;11(2):567–74. doi: 10.1046/j.1460-9568.1999.00449.x. [DOI] [PubMed] [Google Scholar]
- Palop JJ, Mucke L, Roberson ED. Quantifying biomarkers of cognitive dysfunction and neuronal network hyperexcitability in mouse models of Alzheimer’s disease: depletion of calcium-dependent proteins and inhibitory hippocampal remodeling. Methods in molecular biology (Clifton, NJ) 2011;670:245–62. doi: 10.1007/978-1-60761-744-0_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC. The basic mechanisms accounting for age-related decline in cognitive function. In: Park DC, Schwartz N, editors. Cognitive Aging: A Primer. Psychology Press; Philadelphia: 2000. pp. 3–18. [Google Scholar]
- Pavlov I, Savtchenko LP, Kullmann DM, Semyanov A, Walker MC. Outwardly rectifying tonically active GABAA receptors in pyramidal cells modulate neuronal offset, not gain. J Neurosci. 2009;29(48):15341–50. doi: 10.1523/JNEUROSCI.2747-09.2009. 29/48/15341 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov I, Savtchenko LP, Song I, Koo J, Pimashkin A, Rusakov DA, Semyanov A. Tonic GABAA conductance bidirectionally controls interneuron firing pattern and synchronization in the CA3 hippocampal network. Proc Natl Acad Sci U S A. 2014;111(1):504–9. doi: 10.1073/pnas.1308388110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth FC, Draguhn A. GABA metabolism and transport: effects on synaptic efficacy. Neural Plast. 2012;2012:805830. doi: 10.1155/2012/805830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders H, Berends M, Major G, Goldman MS, Lisman JE. NMDA and GABAB (KIR) conductances: the “perfect couple” for bistability. J Neurosci. 2013;33(2):424–9. doi: 10.1523/jneurosci.1854-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM, Silver RA. Tonically active GABA(A) receptors: modulating gain and maintaining the tone. Trends in Neurosciences. 2004;27(5):262–9. doi: 10.1016/j.tins.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Spiegel AM, Koh MT, Vogt NM, Rapp PR, Gallagher M. Hilar interneuron vulnerability distinguishes aged rats with memory impairment. J Comp Neurol. 2013;521(15):3508–23. doi: 10.1002/cne.23367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley EM, Fadel JR, Mott DD. Interneuron loss reduces dendritic inhibition and GABA release in hippocampus of aged rats. Neurobiol Aging. 2012;33(2):431, e1–13. doi: 10.1016/j.neurobiolaging.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thome A, Gray DT, Erickson CA, Lipa P, Barnes CA. Memory impairment in aged primates is associated with region-specific network dysfunction. Molecular psychiatry. 2015 doi: 10.1038/mp.2015.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting JT, Daigle TL, Chen Q, Feng G. Acute brain slice methods for adult and aging animals: application of targeted patch clamp analysis and optogenetics. Methods in molecular biology (Clifton, NJ) 2014;1183:221–42. doi: 10.1007/978-1-4939-1096-0_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub RD, Whittington MA, Colling SB, Buzsaki G, Jefferys JG. Analysis of gamma rhythms in the rat hippocampus in vitro and in vivo. JPhysiol. 1996;493( Pt 2):471–84. doi: 10.1113/jphysiol.1996.sp021397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uylings HB, Groenewegen HJ, Kolb B. Do rats have a prefrontal cortex? Behav Brain Res. 2003;146(1–2):3–17. doi: 10.1016/j.bbr.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Verwer RW, Meijer RJ, Van Uum HF, Witter MP. Collateral projections from the rat hippocampal formation to the lateral and medial prefrontal cortex. Hippocampus. 1997;7(4):397–402. doi: 10.1002/(SICI)1098-1063(1997)7:4<397::AID-HIPO5>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Vigot R, Barbieri S, Brauner-Osborne H, Turecek R, Shigemoto R, Zhang YP, Lujan R, Jacobson LH, Biermann B, Fritschy JM, Vacher CM, Muller M, Sansig G, Guetg N, Cryan JF, Kaupmann K, Gassmann M, Oertner TG, Bettler B. Differential compartmentalization and distinct functions of GABAB receptor variants. Neuron. 2006;50(4):589–601. doi: 10.1016/j.neuron.2006.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Gamo NJ, Yang Y, Jin LE, Wang XJ, Laubach M, Mazer JA, Lee D, Arnsten AF. Neuronal basis of age-related working memory decline. Nature. 2011;476(7359):210–3. doi: 10.1038/nature10243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Yang Y, Wang CJ, Gamo NJ, Jin LE, Mazer JA, Morrison JH, Wang XJ, Arnsten AF. NMDA receptors subserve persistent neuronal firing during working memory in dorsolateral prefrontal cortex. Neuron. 2013;77(4):736–49. doi: 10.1016/j.neuron.2012.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Neubauer F, Lüscher HR, Thurley K. GABAB receptor-dependent modulation of network activity in the rat prefrontal cortex in vitro. The European journal of neuroscience. 2010;31(9):1582–94. doi: 10.1111/j.1460-9568.2010.07191.x. [DOI] [PubMed] [Google Scholar]
- Zaitsev AV, Povysheva NV, Gonzalez-Burgos G, Rotaru D, Fish KN, Krimer LS, Lewis DA. Interneuron diversity in layers 2–3 of monkey prefrontal cortex. Cereb Cortex. 2009;19(7):1597–615. doi: 10.1093/cercor/bhn198. [DOI] [PMC free article] [PubMed] [Google Scholar]