Abstract
Gamma globin induction remains a promising pharmacological therapeutic treatment mode for sickle cell anemia and beta thalassemia, however Hydroxyurea remains the only FDA approved drug which works via this mechanism. In this regard, we assayed the γ-globin inducing capacity of Cis-vaccenic acid (CVA). CVA induced differentiation of K562, JK1 and transgenic mice primary bone marrow hematopoietic progenitor stem cells. CVA also significantly up-regulated γ-globin gene expression in JK-1 and transgenic mice bone marrow erythroid progenitor stem cells (TMbmEPSCs) but not K562 cells without altering cell viability. Increased γ-globin expression was accompanied by KLF1 suppression in CVA induced JK-1 cells. Erythropoietin induced differentiation of JK-1 cells 24 h before CVA induction did not significantly alter CVA induced differentiation and γ-globin expression in JK-1 cells. Inhibition of JK-1 and Transgenic mice bone marrow erythroid progenitor stem cells Fatty acid elongase 5 (Elovl5) and Δ9 desaturase suppressed the γ-globin inductive effects of CVA. CVA treatment failed to rescue γ-globin expression in Elovl5 and Δ9-desaturase inhibited cells 48 h post inhibition in JK-1 cells. The data suggests that CVA directly modulates differentiation of JK-1 and TMbmEPSCs, and indirectly modulates γ-globin gene expression in these cells. Our findings provide important clues for further evaluations of CVA as a potential fetal hemoglobin therapeutic inducer
Keywords: Cis-vaccenic acid, Fetal hemoglobin, Sickle cell anemia, Erythroid progenitor stem cells, Mono-unsaturated fatty acid, Gamma globin
1. Introduction
The hallmark of current molecular strategies in the therapy of sickle cell anemia (SCA) and beta thalassemia is centered on the pharmacological reactivation of fetal hemoglobin in these patients (Musallam et al., 2013). The central feature of the human β-globin gene expression is the existence of a two stage-specific switch that regulates the expression of hemoglobin from embryonic hemoglobin to fetal hemoglobin to adult hemoglobin (Bieker, 2010; Perrine, 2011; Sankaran et al., 2010). Several studies have documented the clinical benefits of increased fetal hemoglobin synthesis in the hemoglobinopathies (Fibach et al., 1993; Platt et al., 1984). However, Hydroxyurea remains the only FDA approved sickle cell treatment drug that functions by inducing HbF. But its widespread use is limited due to concerns over its long term side effects, and moreover, a significant number of patients do not respond to hydroxyurea therapy (Kinney et al., 1999; Platt et al., 1984; Steinberg et al., 2003). Studies by Perrine and colleagues revealed that butyrate, a short chain fatty acid, induced significant γ-globin gene expression and fetal hemoglobin synthesis in SCA and β-thalassemia patients (Perrine et al., 1987). Butyrate was shown to increase γ-globin gene expression through mechanisms dependent on histone deacetylase inhibition (Perrine et al., 1993).
Bone marrow stem cell transplant (which is unaffordable to a significant population of people living with SCA) remains the only cure for SCA (Walters, et al., 2000), it involves the complete replacement of the individuals’ bone marrow using stem cells from a normal compatible donor to replace that of the affected individual. The heightened risks associated with the procedure also contribute to limiting its applications. Gene therapy is also another SCA therapeutic procedure that attempts to overcome the limitations of bone marrow transplant using stem cells from patient’s own blood transduced with a lentiviral vector containing an anti-sickling gene leading to improved production of healthy RBCs (Pawliuk et al., 2001). However the clinical success of gene therapy has been greatly limited due to the low titers observed as a result of regulatory elements of the β-globin gene locus used for the improvement of the transgene’s expression and the eventual silencing of the transgene (Papanikolaou and Anagnou, 2010). More recently, some progress have also been made using zinc finger nucleases or transcription factor activator-like nucleases targeted editing of the abnormal β-globin gene leading to mutations such as frame-shift or deletions or to stimulate homologous recombination thus activating fetal hemoglobin production in these cells (Bauer et al., 2012). This approach however is not advanced enough for human therapeutic purposes and would require precise specificity to prevent off target mutagenic effects which could be deleterious. This underscores the requirement for a pharmacologic intervention which can up-regulate fetal hemoglobin with minimal toxicity.
Primary erythroid progenitor stem cell cultures from bone marrow or peripheral blood remain the best in vitro models for determining potential pharmacologically active agents, although cell lines have been used widely as good in vitro models for drug screening and have been widely used to screen and identify novel gamma globin inducers (Bianchi et al., 2001; Cioe et al., 1981; Gambari, 2003; Zein et al., 2010). Human K562 cells established by Lozzio and Lozzio (1975), co-express ε and γ but not β-globin genes; however, transgenic mouse bone marrow erythroid progenitor stem cells (TMbmEPSCs) and human JK-1 cells express β in addition to γ-globin, similar to the in vivo scenario of humans (Blau et al., 2005; Okunno et al., 1990).
Globin synthesis is developmentally regulated by a host of factors, key amongst which is the Kruppel like factor 1 (KLF1) (Zhou et al., 2010). Knockdown of KLF 1 an erythroid specific transcription factor (Bieker, 2010), in human and mouse adult erythroid progenitors leads to reduced expression of B cell lymphoma 11a (BCL11a) and consequently induced γ-globin levels. Studies have also demonstrated that happloinsufficiency of KLF 1 leads to hereditary persistence of fetal hemoglobin (Zhou et al., 2010) thus illuminating KLF1 as a molecular target for the reactivation of fetal hemoglobin synthesis in humans.
In vivo inhibition of the mechanistic target of Rapamycin (mTOR) synthesis has been shown to remarkably improve erythroid cell maturation and anemia in a model of β-thalassemia (Zhang et al., 2014). (Z) 11 octadecenoic acid also called Cis-vaccenic acid (CVA) an 18 carbon n-7 mono-unsaturated fatty acid is biosynthesized in humans by hepatic fatty acid elongase 5 (Elovl5). CVA has also been shown to be the fatty acid precursor of 9-cis 11-trans octadecenoic acid an isomer of conjugated linolenic acid (CLA), a reaction also catalyzed by Elovl5 (Tripathy and Jump, 2013). Elovl5 expression studies have shown that it is down regulated during post natal development and its activity shown to be linked to the control of the mTORC2-Akt-FOXO1 pathway (Tripathy et al., 2010; Wang et al., 2008). The significance of this down-regulation was previously demonstrated and shown to be diet linked (Wang et al., 2008). CLA and its derivatives have been shown to induce differentiation and inhibit proliferation of HT-29 cells in a dose and time dependent fashion (Palombo et al., 2002). Studies have also showed that Vaccenic acid in the form of either Cis or Trans, significantly reduced growth of HT-29 human colon cancer cells by 23% when compared with control cells (Awad et al., 1995; Banni et al., 2001). Several other studies have demonstrated the anti-inflammatory effects of mono-unsaturated fatty acids (MUFA). Increase in RBC membrane CVA content has been shown to protect humans against coronary heart disease (Djoussé et al., 2012), However, very little is known about the link between CVA metabolism and hemoglobin expression. We have previously reported the fetal hemoglobin inducing activity of a water purified fraction of Terminalia catappa leaf extract on primary hematopoietic progenitor cells (Aimola et al., 2014). Further chromatographic studies on this fraction revealed that this fraction contained CVA (un-published data).
Herein we report the findings of the differentiation inducing effects and γ-globin inducing activity of CVA and the possible mechanisms up-stream and downstream of CVA metabolism on its gamma globin inducing activity.
2. Materials and methods
2.1. Compound
CVA was obtained from Sigma. Stock solution of CVA was prepared in ethanol (molecular grade). CVA was further diluted to desired concentrations using culture media consisting of RPMI 1640 supplemented with 20% FBS in the presence of penicillin streptomycin mix (1%).
2.2. Cell culture
K562 and JK-1 cell lines were maintained in RPMI 1640 medium supplemented with 20% FBS (Sigma) in the presence of penicillin streptomycin mix (100 U/ml penicillin and 200 μg/ml streptomycin) (Zhang and Bieker, 1998). JK-1 erythroleukemic cells were established from a patient with chronic myelogenous leukemia in erythroid crisis (Okunno et al., 1990) and their differentiation potential has been shown to be enhanced by differentiation inducers. Cells were seeded at a density of 1.5×104 cells/ml. Cells were cultured in a humidified environment at 37 °C in 5% CO2 and passaged every 48 h (Kourembanas et al., 1991). Induction was carried out by adding CVA to the cell culture at specified concentrations for varying time lengths. Viable cell count was done using Trypan blue staining as previously described (Lee et al., 2006). Accumulation of hemoglobinized cells was assayed using Benzidine staining. Cell morphology was determined using cytospin preparations stained with Benzidine-Giemsa staining and May Grumwald-Giemsa staining (Ji et al., 2008).
2.3. Isolation of bone marrow cells
Mice bone marrow was flushed from the femurs of sickle cell transgenic mice using 1× PBS (Tanimoto et al., 1999). Bone marrow cells were washed twice with 1× PBS. Hematopoietic progenitor stem cells were enriched by plastic adherence as previously described (Sieff et al., 1986). Hematopoietic progenitor stem cells were subsequently cultured at a density of 2×106 cells/ml in IMDM supplemented with 20% FBS 250 units/ml penicillin and 200 μg/ml streptomycin. Culture was carried out in a humidified environment at 37 °C in 5% CO2.
2.4. Clonogenic assay
TMbmEPSCs were cultured at a density of 5×103 cells in 35 mm petri dish containing semi-solid media containing Iscove Modified Dulbecco Medium supplemented with 20% FBS, 200U/ml penicillin, 250 μg/ml streptomycin, 2 mM L-glutamine and 0.9% methylcellulose at 37 °C in 5% CO2 in a humidified environment. To quantify the number of BFUe colonies, plates were estimated daily for hemoglobinized colonies with large aggregates of 65 or more hemoglobinized cells.
2.5. Fetal hemoglobin (HbF) assay
fetal hemoglobin was assayed using Cell lysates according to the method of Jonxis and Huisman (1956). Briefly, cells were harvested at the end of culture by centrifuging at 450g. Cells were then washed twice with phosphate-buffered saline (PBS) pH7.2. Washed cells were lysed using 10% saponin (Sigma), in PBS and the mixture was centrifuged at 300g for 10 min. Adult hemoglobin in the supernatant was denatured using Drabkin’s reagent for 30 min and then precipitated out of the solution using saturated ammonium sulfate. Absorbance of HbF was then assayed at 415 nm using a Nanodrop spectrophotometer.
2.6. Quantitative real time PCR
RNA was isolated from cells using TRIZOL as previously described (Valadi et al., 2007), RNA was treated with DNAse I and subsequently cleaned using RNAeasy minikit (Qiagen) according to manufacturers protocol. Complementary DNA was synthesized from equal amounts of total RNA with Promega Reverse Transcription System (A3500) with a combination of random primers and oligo dT. γ-globin, β-globin and KLF1/EKLF gene expression was quantified using a Applied Biosystems 7900HT Fast Real-Time PCR. The level of each gene was normalized with that of GAPDH expression in the respective cells. The PCR conditions used were as follows: denaturation at 95 °C for 15 s, annealing and extension at 60 °C and 72 °C for 1 min respectively for up to 50 cycles.
All the primers for real time and regular PCR are listed in Supplementary Table 1.
3. Results
3.1. CVA induces differentiation of K562, JK-1 and transgenic mice bone marrow erythroid progenitor cells (TMbmEPSCs)
To identify novel compounds capable of differentiation induction, we employed K562 and JK-1 erythroleukemic cell lines and TMbmEPSCs. K562 and JK-1 cells have been shown to differentiate to the erythroid lineage and express γ-globin gene in response to differentiation inducers (Lozzio and Lozzio, 1975; Okuno et al., 1990). To ascertain the differentiation effect of CVA, we added CVA exogenously to the cell cultures on Day 1 of the culture and cells were assessed for differentiation using benzidine and May Grumwald Giemsa staining at specified time points. CVA increased the percentage of benzidine positive cells in K562 cell cultures comparable to Hemin (40 μM), a known inducer of fetal Hb (Cioe et al., 1981) (Fig. 1A). CVA induced differentiation in K562 cells was assayed with increasing concentrations of CVA at sub-toxic concentrations of CVA (50 μM, 75 μM and 100 μM) as assessed from previous studies (Briggs and Lefkowitz, 1980) and compared with vehicle alone (Fig. 1A). CVA induced differentiation appeared to be concentration dependent in K562 cells with 50 μM CVA being the most effective concentration with more than 20% of the K562 cells showing positive for benzidine stain after 48 h of incubation with CVA (Fig. 1B). Additional tests were carried out on TMbmEPSCs and JK-1 cells using CVA at 50 μM (the concentration at which it was most effective in K562 differentiation induction). CVA induction of TMbmEPSCs led to a significantly higher percentage of reticulocytes (which was evident 24 h post induction) as assessed with benzidine-giemsa staining compared to control TMbmEPSCs cultures (Fig. 1C). Erythroid lineage Cell differentiation usually proceeds from the stem cells to erythroid burst forming units (BFUe) colonies. We observed that CVA increased the percentage of TMbmEPSCs BFUe colonies (Fig. 1D). CVA at 50 μM also increased the percentage of benzidine positive JK-1 cell (Fig. 1E), however JK-1 cell growth and viability was not significantly altered by CVA at this concentration (Fig. 2A and B). Trypan blue assays of cell suspensions of JK-1 cell in RPMI 1640% and 20% FBS revealed that cell growth rate of CVA induced cells and control cells did not differ significantly. Although JK-1 cells induced with CVA differentiated faster than control cells, we observed that the overall growth rate of CVA induced cells was comparable with the un-induced cells. Higher concentrations of CVA affected JK-1 cell viability significantly (P < 0.05).
Fig. 1.
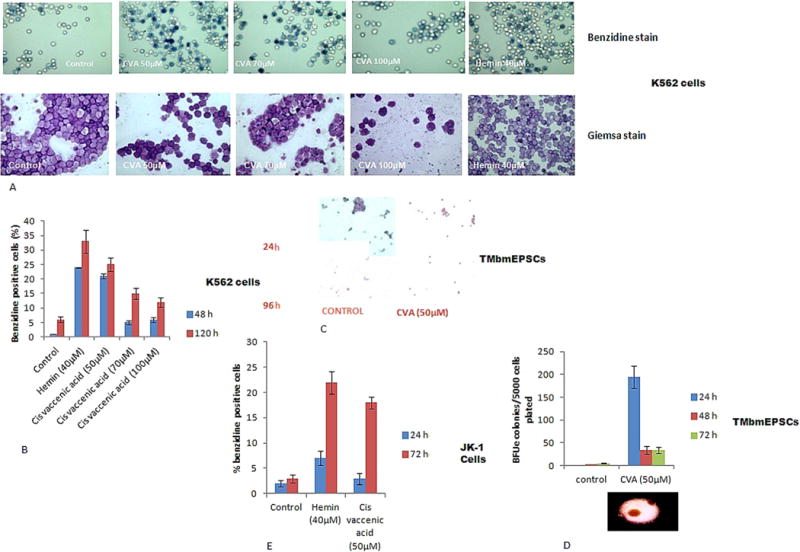
CVA induces differentiation to the erythroid lineage. Differentiation was assayed as a measure of the emergence of hemoglobinized cells positive for benzidine. Analysis was carried out with K562, transgenic mice bone marrow progenitor stem cells and JK-1 cells. A. K562 cells induced with CVA for 72 h and stained with Benzidine and Giemsa stains. B. K562 cells were induced with varying concentrations of CVA and monitored for percentage of benzidine positive cells at 48 and 120 h post induction. C. Transgenic mice bone marrow progenitor stem cells depleted of plastic adherent cells in IMDM supplemented with 20% FBS, 100 U/ml penicillin, 200 μg/ml streptomycin and 2 U/ml EPO. Cells were induced with CVA for 96 h, and cytospin preparations of the cells were stained using benzidine-giemsa stain. D. Percentage of BFUe colony formation from TMbmEPSCs induced by CVA. TMbmEPSCs depleted of plastic adherent cells were seeded in plates containing semisolid IMDM supplemented with 20% FBS, 100 U/ml penicillin and 200 μg/ml streptomycin. E. JK-1 cells induced with 50 μM CVA and monitored for percentage of benzidine positive cells at 24 and 72 h post induction.
Fig. 2.
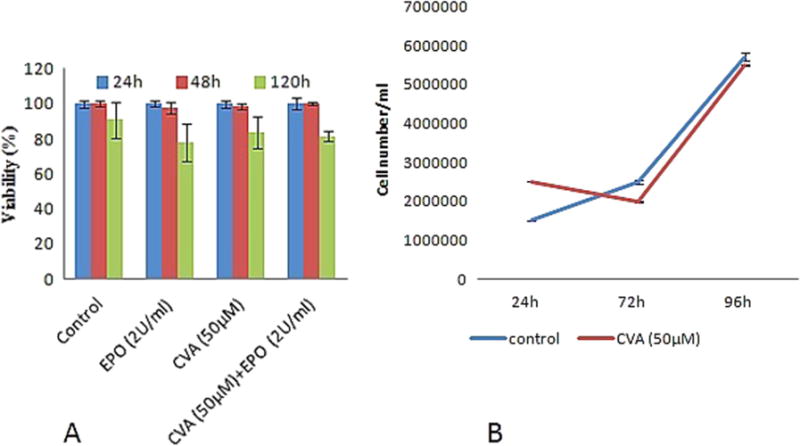
CVA does not alter JK-1 Cell survival or growth. A. Cell survival was assayed as a measure of percentage positive Trypan blue stained cells. Cell viability was monitored at 24, 48 and 120 h post CVA induction. B. JK-1 cell growth was monitored for 96 h. The data represents the mean and corresponding S.D. of three independent experiments.
3.2. CVA increases gamma globin mRNA levels and fetal HB in JK-1 and TMbmEPSCs
We then examined the effects of CVA on γ-globin gene expression in K562, JK-1 and TMbmEPSCs using either semi-quantitative PCR or real time PCR or both. K562 cells express primarily γ-globin and to a lesser extent embryonal hemoglobins (Portland, Gower 1 and Hb X) (Cioe et al., 1981). Semi-quantitative RT-PCR of isolated RNA from CVA treated K562 cells revealed no significant γ-globin induction beyond the control (data not shown). However we observed that CVA induced and increased significantly (P < 0.05) amounts of γ-globin mRNA in JK-1 cells treated with CVA (Fig. 3A; quantitative real time PCR data is presented as fold changes of mRNA amounts relative to vehicle treated cells in Fig. 3B). CVA induction of γ-globin gene expression was time dependent (Fig. 3A) and concentration dependent (Fig. 3C). Significant increase of JK-1 γ-globin mRNA was evident 48 h post induction with CVA and the γ-globin mRNA level peaked after 96 h of induction after which its level tapered off. CVA was most effective at inducing JK-1 γ-globin gene expression at 50 μM; at this concentration CVA was also able to up-regulate JK-1 β-globin gene expression, suggesting a threshold requirement of CVA for globin gene expression.
Fig. 3.
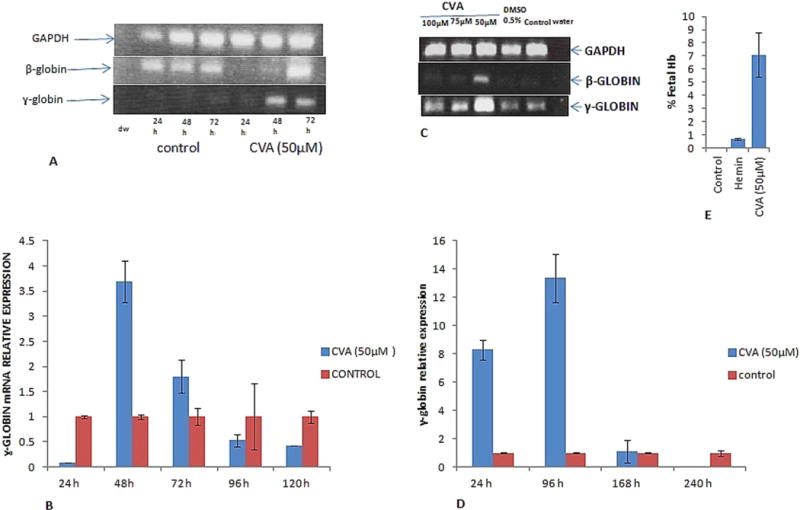
CVA up-regulates γ and β-globin gene expression and fetal hemoglobin synthesis. CVA induced γ-globin gene expression was monitored in A. JK-1 cells using RT-PCR at 24, 48 and 72 h post CVA induction. B. qRT-PCR was used to measure JK-1 cell relative γ-globin gene expression 24, 48, 72, 96 and 120 h post CVA induction. Expression data was normalized to JK-1 GAPDH expression. C. Concentration dependent effect of CVA on JK-1 cell γ-globin gene expression was assayed using semi-quantitative RT-PCR. D. Effect of CVA induction on transgenic mice bone marrow erythroid progenitor stem cells γ-globin gene expression. Relative γ-globin gene expression was assessed using qRT-PCR at 24, 96, 168 and 240 h post CVA induction. γ-globin gene expression was normalized to transgenic mice bone marrow progenitor stem cells GAPDH gene expression. E. effect of CVA on JK-1 cells fetal hemoglobin synthesis. Fetal hemoglobin was assayed in JK-1 cell lysates using alkaline denaturation assay as previously described (Fibach et al., 1993). Hemin was used as a positive control inducer. The experiment was carried out in duplicate, and although precise globin mRNA levels appeared to fluctuate from culture to culture, but relative γ-globin levels were always higher in cells induced in transgenic mice primary bone marrow progenitor stem cells induced with 50 μM CVA. The data represents the mean and corresponding S.D. of three independent experiments. ANOVA was used to analyse the differences between groups and differences were considered significant at P<0.05.
We then studied the effect of CVA on primary erythroid progenitor stem cells isolated from transgenic mice bone marrow. Cells were treated with 50 μM CVA on day one of culture and γ-globin was monitored at 24, 96, 168 and 240 h post incubation (Fig. 3D). The data revealed that CVA up-regulated γ-globin expression in TMbmEPSCs. CVA was able to increase γ-globin mRNA levels relative to the control within 24 h of incubation with 50 μM CVA (Fig. 3D). Increased γ-globin levels were still apparent in the progenitor cells after 96 h of induction.
HbF synthesis stems from increased transcription of gamma globin gene, so we assessed the effects of CVA treatment on HbF levels in JK1 cells. Cells were grown in RPMI medium and HbF level was assessed 72 h after incubation using the alkaline denaturation assay. HbF levels were significantly (P < 0.05) higher in CVA induced cells compared to the control (Fig. 3E). However Hemin, an established inducer of Fetal Hb inducer in K562, cells failed to induce significant Fetal Hb synthesis in JK-1 cells compared to CVA.
3.3. CVA induction suppresses KLF1 expression in JK-1 cells
We further assessed the effects of CVA on KLF1 (formerly called EKLF) expression. KLF1, an erythroid specific trans-acting factor, is known to be critical in erythroid cells gene expression and is known to indirectly modulate γ-globin expression (Yien and Bieker, 2013). Simvastatin and T. hydroquinoline have been shown to induce γ-globin expression via suppression of KLF1 expression (Macari et al., 2013). Our results show a slight but significant (P < 0.05) suppression of KLF1 expression in CVA treated JK-1 cells (Fig. 4).
Fig. 4.
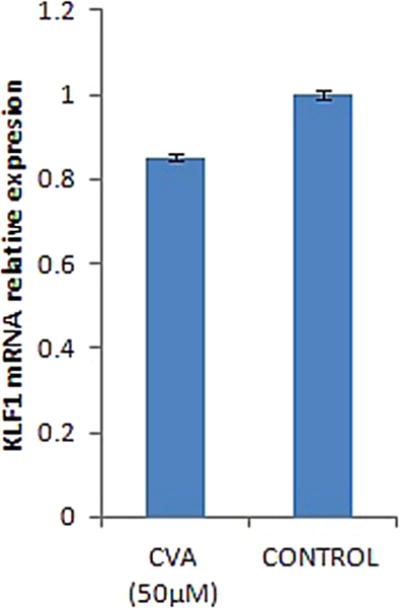
CVA lowers KLF1 gene expression in JK-1 cells. JK-1 cells were grown in RPMI 1640 medium supplemented with 20% FBS, 100U/ml penicillin and 200 μg/ml streptomycin, cells induced with CVA (50 μM). KLF1 gene expression was monitored quantitatively using qRT-PCR 24 h post induction. KLF1 gene expression was normalized to JK-1 GAPDH gene expression and compared to un-induced controls. The data represents the mean and corresponding S.D. of three independent experiments. ANOVA was used to analyse the differences between groups and differences were considered significant at P<0.05.
3.4. CVA gamma globin gene induction is enhanced by erythropoietin
Erythropoietin (EPO) has been shown to direct hematopoietic progenitor cell fate (Orkin and Zon, 2008), and is known to be required for normal erythropoiesis. To test the effects of EPO on CVA induced JK-1 cell γ-globin gene expression. We examined the potential of CVA to alter JK-1 cell differentiation potential and globin gene expression in the presence and absence of EPO (2 U/ml). The results suggest that CVA induced gamma globin expression is enhanced by EPO (Fig. 5A). However, pre-differentiation of JK-1 cells with EPO did not enhance γ-globin gene expression in these cells. The experiment was set-up with two groups of JK-1 cell cultures; the first group was pre-differentiated with 2 U/ml EPO for 48 h before induction with CVA, the control consisted of EPO pre-differentiated JK-1 cells un-induced with CVA, while the second group consisted of largely un-differentiated JK-1 cells induced with CVA (Fig. 5A). Cells from either groups were induced with either CVA (50 μM) or EPO (2 U/ml) alone or CVA (50 μM)+EPO (2 U/ml).
Fig. 5.
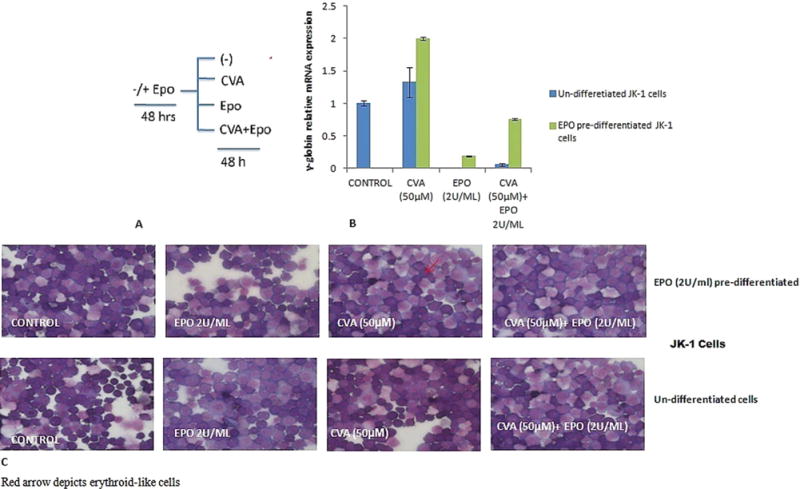
Effects of Erythropoietin on CVA induced JK-1 cell differentiation and γ-globin gene expression. EPO has been shown to direct erythroid cell development and maturation (Orkin and Zon, 2008). JK-1 cells were induced with EPO (2 U/ml) for 48 h prior to induction with CVA; cells were then induced using 50 μM CVA, EPO (2 U/ml) and ‘CVA (50 μM)+EPO (2 U/ml)’ for 48 h. Controls were set-up for each treatment using JK-1 cells which have not been induced with EPO. JK-1 cell differentiation and γ-globin gene expression were assayed 48 h post CVA induction. A. Experimental set-up B. JK-1 relative γ-globin gene expression normalized to GAPDH C. JK-1 cell differentiation assayed by benzidine-giemsa staining. The data represents the mean and corresponding S.D. of three independent experiments. ANOVA was used to analyse the differences between groups and differences were considered significant at P<0.05.
JK-1 cells treated with EPO 2U/ml and CVA (50 μM) simultaneously in both experiments reflected a suppressed γ-globin mRNA synthesis compared to cells induced with CVA alone. However CVA induced γ-globin gene expression was significantly (P < 0.05) higher in JK-1 cells pre-differentiated with EPO suggesting a requirement of EPO for CVA γ-globin induction in JK-1 cells (Fig. 5A).
JK-1 cells have been shown to have bi-potential differentiation capabilities (Tani et al., 1996), with a potential to commit to either the erythroid or megakaryocytic cell lineages depending on the differentiation inducer. CVA was able to induce differentiation of JK-1 cells to the erythroid lineage as assessed by benzidine-giemsa staining (Fig. 5B). However pre-differentiation of these cells with EPO 48 h before CVA induction did not significantly increase the percentage of benzidine positive JK-1 cells compared to the control, which consisted of JK-1 cells not treated with EPO before inducing differentiation with CVA (50 μM) (Fig. 5B).
3.5. CVA induces differentiation preferentially of immature and early erythroid progenitor stem cells
In order to assess the stage of erythroid progenitor cell differentiation most responsive to CVA induced differentiation and γ-globin induction, the cells were grown in either semisolid IMDM before induction or in a phase II liquid culture medium consisting of IMDM, 20% FBS and Erythropoietin as the only cytokine which favors erythroid differentiation (Nagata et al., 1998). This two phase liquid culture system of isolated bone marrow closely reflects the in vivo physiological relevant culture system for erythroid differentiation (Wada et al., 1990; Pope et al., 2000). The results revealed that CVA (50 μM) significantly increased γ-globin expression per microgram of RNA 8 fold at 48 h post induction (Fig. 6).
Fig. 6.
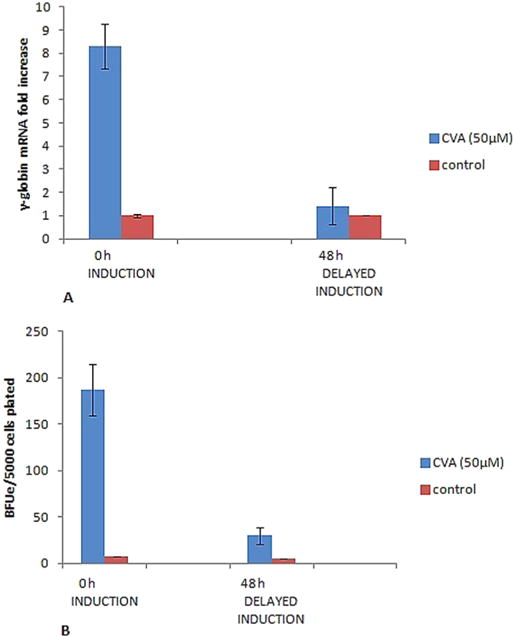
Effects of CVA on Transgenic Mice Bone Marrow Erythroid Progenitor Stem Cell Differentiation. Cells were induced with 50 μM CVA at the start of culture or 48 h after the commencement of culture. A. TMbmEPSCs were grown in IMDM supplemented with 20% FBS, 100U/ml penicillin, 200 μg/ml streptomycin and 2 U/ml EPO. γ-globin gene expression was assayed 48 h post CVA induction. B. TMbmEPSCs were grown in semisolid IMDM supplemented with 20% FBS, 100U/ml penicillin, 200 μg/ml streptomycin and 0.9% methylcellulose. BFUe colonies were enumerated 24 h post CVA induction. The data represents the mean and corresponding S.D. of three independent experiments. ANOVA was used to analyse the differences between groups and differences were considered significant at P<0.05.
Similarly, CVA significantly (P < 0.05) increased γ-globin gene expression in TMbmEPSCs when CVA was used to induce TMbmEPSCs at the start of culture and γ-globin gene expression assayed 48 h post CVA induction. However, pre-differentiation of TMbmEPSCs 48 h before CVA-induction with erythropoietin reduced the CVA γ-globin induction potential in these cells (Fig. 6A).
Likewise, differentiation of TMbmEPSCs 48 h before CVA induction reduced the percentage of CVA induced TMbmEPSCs BFUe forming potential (Fig. 6B).
3.6. Inhibition of fatty acid elongase 5 (Elvol5) and Δ9-desaturase activity with Isoxyl and Cycloate respectively alters CVA induced JK-1 γ-globin gene expression inducing activity and TMbmEPSCs differentiation and γ-globin gene expression
We further assessed the role of CVA metabolism in modulating γ-globin expression in JK-1 cells. We inhibited Elovl5 and Δ9-desaturase enzymes involved in CVA turnover in vivo. Elovl5 is vital in modulating hepatic fatty acid and carbohydrate metabolism. It elongates unsaturated fatty acids like Palmitoleic acid (16:1, n-7) to form CVA (18:1, n-7). Elovl5 has also recently been identified as a key mediator of the mammalian target of rapamycin Akt linked FOXO1 (mTORC2-Akt–FOXO1) pathway through the control of CVA synthesis (Tripathy and Jump, 2013). CVA induced JK-1 and TMbmEPSCs γ-globin gene expression potential was greatly diminished by inhibition of either JK-1 Elovl5 (with Cycloate) or Δ9-desaturase (with Isoxyl) (Fig. 7A and B). To further understand how CVA metabolism influences its effects on γ-globin expression, we tried to rescue γ-globin expression in JK-1 cells treated with Isoxyl and Cycloate by exogenous addition of CVA 24 h post inhibition. γ-globin expression was assayed and presented as fold increase over JK-1 cells not inhibited nor induced. CVA (50 μM) failed to rescue γ-globin expression in these pre-treated JK-1 cells (Fig. 7). Elovl5 and Δ9-desaturase inhibition in TMbmEPSCs also diminished CVA induced γ-globin gene expression in these cells (Fig. 7C). The data suggests that CVA indirectly modulates γ-globin gene expression in JK-1 cells. Inhibition of TMbmEPSCs Δ9-desa-turase appeared to enhance CVA induced TMbmEPSCs BFUe forming capacity (Fig. 8), however inhibition of TMbmEPSCs Elovl5 diminished CVA induced TMbmEPSCs BFUe forming capacity.
Fig. 7.
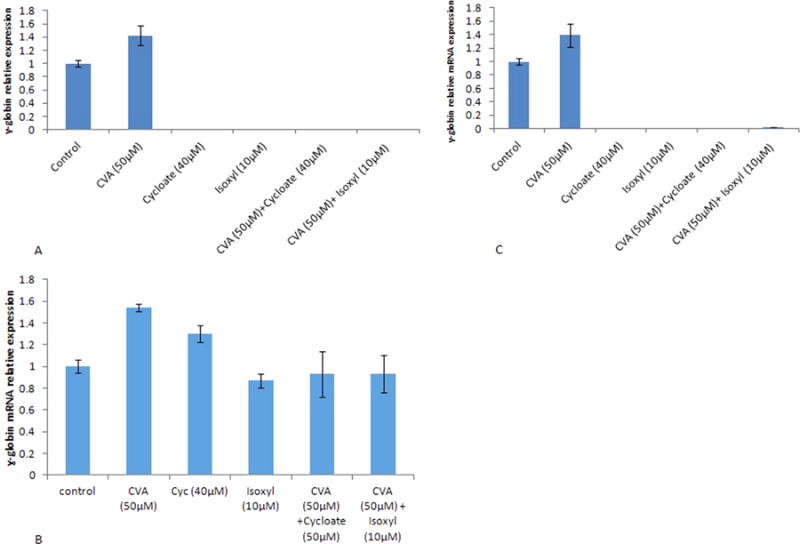
Inhibition of Eukaryotic Fatty acid Elongase V and Δ9-desaturase Modulates CVA induced differentiation and γ-globin induction capacity. JK-1 cells were grown in RPMI 1640 medium supplemented with 20% FBS, 100U/ml penicillin and 200 μg/ml streptomycin. Fatty acid elongase V (Elovl5) and Δ9-desaturase were inhibited at the start of culture in separate experiments using Cycloate (40 μM) or Isoxyl (10 μM) respectively or jointly. CVA was used to induce JK-1 cell γ-globin gene expression in both inhibited culture systems. A. CVA was used to induce Elovl5 and Δ9-desaturase inhibited JK-1 cells at the start of cultures. B. CVA (50 μg) was used to induce Elovl5 and Δ9-desaturase inhibited TMbmEPSCs cells at the start of cultures. C. CVA (50 μM) was added to Elovl5 and Δ9-desaturase inhibited JK-1 cells 48 h post inhibition, γ-globin gene expression was assayed 48 h post induction. The data represents the mean and corresponding S.D. of three independent experiments. ANOVA was used to analyse the differences between groups and differences were considered significant at P<0.05.
Fig. 8.
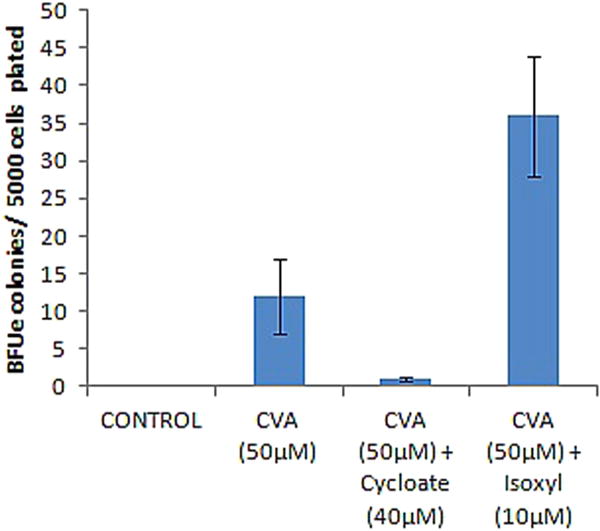
Effect of CVA Rescue on TMbmEPSCs differentiation. CVA (50 μM) was added to Elovl5 and Δ9-desaturase inhibited TMbmEPSCs 48 h post inhibition on semisolid IMDM supplemented with 20% FBS, 100 U/ml penicillin and 200 μg/ml streptomycin, BFUe colonies were enumerated 24 h post induction. The data represents the mean and corresponding S.D. of three independent experiments. ANOVA was used to analyse the differences between groups and differences were considered significant at P<0.05.
4. Discussion
γ-globin induction is key to sustainable management of SCA. The management of this disease using the only FDA approved drug (hydroxyurea) is largely limited due to its attendant side effects (Macari et al., 2013; Sankaran et al., 2008). Although several inducers of HbF have been identified, most are still inadequate for SCA and β-thalassemia therapeutic uses; hence a critical need remains to identify effective γ-globin pharmacological inducers with much less side effects.
Ineffective erythropoiesis has been implicated in erythroid disorders which have been shown to be orchestrated by dysregulation of the mTOR pathway which has been shown to be controlled by Elovl5 responsible for CVA synthesis (Tripathy and Jump, 2013; Zhang et al., 2014). Addition of exogenous CVA increased fetal hemoglobin (HbF) synthesis in K562, JK-1, and TMbmEPSCs. To determine the concentration of CVA that did not significantly alter cell viability and proliferation, we assayed the effects of CVA on K562 cells at varying sub-toxic concentrations (Takahashi et al., 1993). CVA induced differentiation of K562, JK-1 and TMbmEPSCs and significantly increased γ-globin mRNA levels in JK-1 and TMbmEPS cells but not K562 cells. These findings differ from previous reports that induction of fetal hemoglobin is a property exhibited by short chain fatty acids (Liakopoulou et al., 2002). CVA an 18 carbon n-7 mono-unsaturated fatty acid showed significant and potent γ-globin inducing potentials in JK-1 and TMbmEPSCs but surprisingly not K562 cells. The reason for this cell selective γ-globin induction is unclear but could be related to the fact that these cells express little KLF1 which is required for normal globin expression (Donze et al., 1995). JK-1 cells were identified by Okuno et al. (1990) as a hemopoietic cell line from a patient with chronic myelogenous leukemia in erythroid crisis. JK-1 cells consist of largely immature immortal cells capable of differentiating to the erythroid lineage and expressing fetal hemoglobin at very low levels when treated with appropriate inducers. CVA induced differentiation and increased γ-globin gene expression were shown to be concentration and time dependent, with a concentration of 50 μM CVA being most effective. The earliest detection of γ-globin mRNA increase in JK-1 cells was at 48 h. We observed that CVA did not significantly alter cell proliferation in these cells. Similarly CVA (50 μM) induced TMbmEPSCs differentiation as assessed by CVA ability to drive BFUe formation of TMbmEPSCs.
γ-globin transcription is developmentally regulated (Donze et al., 1995; Mabaera et al., 2007; Sankaran et al., 2008), such that γ-globin gene expression is down-regulated in adult erythroid cells. Studies have revealed that a subset of erythroid cells (F-cells) retain the ability to express γ-globin; however, findings in neonatal BFUe cultures suggest that hemoglobin switching does occur in the cells of the same stem cell lineages (Tani et al., 1996). Our data suggest that CVA acts preferentially on relatively early erythroid precursors probably altering progenitor stem cell globin transcription. This may be significant as it appears to elicit its effects by altering γ-globin gene expression within erythroid stem cells of the same lineage.
KLF1 is an erythroid specific developmental stage-enriched zinc finger protein that preferentially activates human β-globin gene expression (Donze et al., 2005). KLF1 is an important trans-acting factor that is known to modulate γ-globin to β-globin gene switching. The transcriptional up-regulation of γ-globin gene induction is controlled by KLF1 both directly and indirectly (Siatecka and Bieker, 2011) and haploinsufficiency of KLF1 expression has been shown to be responsible for hereditary persistence of fetal hemoglobin (HPFH) (Borg et al., 2011). CVA lowered JK-1 cell KLF1 gene expression compared to un-induced cells, in a similar fashion to the Statins which were previously reported to induce γ-globin gene expression in erythroid cells by lowering KLF1 gene expression (Macari et al., 2013).
CVA is a monounsaturated n-7 fatty acid that is a product of Elovl5 activity and has been previously identified as a mediator of mTORC2-Akt-FoxO1 pathway (Tripathy and Jump, 2013), a pathway which has been implicated in the differentiation of B cells (Limon and Fruman, 2012). Δ9 desaturase converts vaccenic acid to CLA in vivo in humans, a pathway which has been shown to be dysregulated in sickle cell anemia individuals (Enomoto et al., 1998). CLA (9-cis 11-trans octadecenoic acid) is one of a group of conjugated C18: Δ9,11 fatty acids that are known to mediate various beneficial health effects in humans (Turpeinen et al., 2002). Inhibition of Δ9 desaturase using 10 μM Isoxyl significantly suppressed γ-globin gene expression in JK-1 and TMbmEPSCs suggesting that CVA is not directly responsible for the observed increase in γ-globin expression in these cells and may be linked to induced rictor proteins (Tripathy and Jump, 2013). Similarly, Cyloate inhibition of in vivo biosynthesis of CVA from palmitoleic acid the precursor of CVA completely suppressed γ-globin expression in JK-1 and TMbmEPSCs. Addition of CVA (50 μM) to the culture medium 48 h after inhibition failed to restore γ-globin gene synthesis in both cell types suggesting that CVA induced γ-globin expression is mediated primarily by a downstream biosynthetic product of CVA. Inhibition of Δ9 desaturase and Elovl5 also completely suppressed the CVA-induced BFUe forming capacity of TMbmEPSCs on semisolid IMDM; however addition of CVA (50 μM) to the semisolid culture medium significantly restored CVA induced BFUe activity in cells treated with Isoxyl but not cells treated with Cycloate, suggesting that CVA directly modulates TMbmEPSCs differentiation and commitment to the erythroid lineage.
The results described above strongly suggest that CVA induces erythroid differentiation and up-regulates human γ-globin gene expression, however γ-globin gene expression appeared to be indirectly modulated by CVA. Site directed mutagenesis of erythroid Elovl5 and Δ9 desaturase in progenitor stem cells in vitro or in vivo could provide additional information on the endogenous role of this fatty acid on fetal hemoglobin gene switching.
Supplementary Material
Acknowledgments
This work was partially supported by grants from the Fulbright foundation 15130884 (to IA) and the NIH, United States RC1 DK86200 (to JJB). We acknowledge Li Xue for technical advice.
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.ejphar.2016.02.041.
References
- Aimola IA, Inuwa HM, Nok AJ, Mamman AI. Induction of foetal haemoglobin synthesis in erythroid progenitor stem cells: mediated by water-soluble components of Terminalia catappa. Cell Biochem and Funct. 2014;32(4):361–367. doi: 10.1002/cbf.3024. [DOI] [PubMed] [Google Scholar]
- Awad A, Hermann T, Finsk CS, Horvath PJ. 18:1 n7 fatty acids inhibit growth and decrease inositol phosphatase release in HT-29 cells compared to n9 fatty acids. Cancer Lett. 1995;91:55–61. doi: 10.1016/0304-3835(95)03725-c. [DOI] [PubMed] [Google Scholar]
- Banni S, Angioni E, Murru E, Carta G, Paola M, Bauman MD, Dong Y, Ip C. Vaccenic acid feeding increases tissue levels of conjugated linoleic acid and suppresses development of premalignant lesions in rat mammary gland. Nutr Cancer. 2001;41(1–2):91–97. doi: 10.1080/01635581.2001.9680617. [DOI] [PubMed] [Google Scholar]
- Bauer DE, Kamran SC, Orkin SH. Reawakening fetal hemoglobin: prospects for new therapies for the β-globin disorders. Blood. 2012;120(15):2945–2953. doi: 10.1182/blood-2012-06-292078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi N, Chiarabelli C, Borgatti M, Mischiati C, Fibach E, Gambari R. Accumulation of γ-globin mRNA and induction of erythroid differentiation after treatment of human leukaemic K562 cells with tallimustine. Br J Haematol. 2001;113(4):951–961. doi: 10.1046/j.1365-2141.2001.02843.x. [DOI] [PubMed] [Google Scholar]
- Bieker JJ. Putting a finger on the switch. Nat Genet. 2010;42:733–734. doi: 10.1038/ng0910-733. http://dx.doi.org/10.1038/ng0910-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blau CA, Barbas CF, Bomhoff AL, Neades R, Yan J, Navas PA, Peterson KR. γ-Globin gene expression in chemical inducer of dimerization (CID)-dependent multipotential cells established from human β-globin locus yeast artificial chromosome (β-YAC) transgenic mice. J Biol Chem. 2005;280(44):36642–36647. doi: 10.1074/jbc.M504402200. [DOI] [PubMed] [Google Scholar]
- Borg J, Patrinos GP, Felice AE, Philipsen S. Erythroid phenotypes associated with KLF1 mutations. Haematologica. 2011;96(5):635–638. doi: 10.3324/haematol.2011.043265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs MM, Lefkowitz RJ. Parallel modulation of catecholamine activation of adenylate cyclase and formation of the high-affinity agonist.cntdot receptor complex in Turkey erythrocyte membranes by temperature and cis-vaccenic acid. Biochemistry. 1980;19(19):4461–4466. doi: 10.1021/bi00560a012. [DOI] [PubMed] [Google Scholar]
- Cioe L, McNab A, Hubbell HR, Meo P, Curtis P, Rovera G. Differential expression of the globin genes in human leukemia K562 (S) cells induced to differentiate by hemin OR butyric acid. Cancer Res. 1981;41(1):237–243. [PubMed] [Google Scholar]
- Djousse L, Matthan NR, Lichtenstein AH, Gaziano JM. Red Blood cell membrane concentration of cis-palmitoleic and cis-vaccenic acids and risk of coronary heart disease. Am J Cardiol. 2012;110(4):539–544. doi: 10.1016/j.amjcard.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donze D, Townes TM, Bieker JJ. Role of erythroid Kruppel like factor in human γ- to β-globin gene switching. J Biol Chem. 1995;270(4):1955–1959. doi: 10.1074/jbc.270.4.1955. [DOI] [PubMed] [Google Scholar]
- Enomoto TM, Isichei C, VanderJagt DJ, Fry DE, Glew RH. Decreased polyunsaturated fatty acids in sickle cell anaemia. J Trop Paed. 1998;44(1):28–34. doi: 10.1093/tropej/44.1.28. [DOI] [PubMed] [Google Scholar]
- Fibach E, Burke LP, Schechter AN, Noguchi CT, Rodgers GP. Hydroxyurea increases fetal hemoglobin in cultured erythroid cells derived from normal individuals and patients with sickle cell anemia or beta-thalassemia. Blood. 1993;81:1630–1635. [PubMed] [Google Scholar]
- Gambari R. The human erythroleukemia K562 cell culture system for identification of inducers of fetal hemoglobin. Minerva Biotecnol. 2003;15(2):123–128. [Google Scholar]
- Ji P, Jayapal SR, Lodish HF. Enucleation of cultured mouse fetal erythro-blasts requires Rac GTPases and mDia2. Nat Cell Biol. 2008;10(3):314–321. doi: 10.1038/ncb1693. [DOI] [PubMed] [Google Scholar]
- Jonxis JHP, Huisman THJ. The detection and estimation of fetal hemoglobin by means of the alkali denaturation test. Blood. 1956;11(11):1009–1018. [PubMed] [Google Scholar]
- Kinney TR, Helms RW, Branski EEO, Ohene-frempong K, Wang W, Daeschner C, Vichinsky E, Redding-Lallinger R, Gee B, Platt OS, Ware RE. Safety of Hydroxyurea in children with sickle cell anemia: results of the HUG-KIDS study, a phase I/II trial. Blood. 1999;94:1550–1554. [PubMed] [Google Scholar]
- Kourembanas S, Marsden PA, McQuillan LP, Faller DV. Hypoxia induces endothelin gene expression and secretion in cultured human endothelium. J Clin Invest. 1991;88(3):1054. doi: 10.1172/JCI115367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Zhou HJ, Wu XH. Dihydroartemisinin downregulates vascular endothelial growth factor expression and induces apoptosis in chronic myeloid leukemia K562 cells. Cancer Chemother Pharmacol. 2006;57(2):213–220. doi: 10.1007/s00280-005-0002-y. [DOI] [PubMed] [Google Scholar]
- Liakopoulou E, Li Q, Stamatoyannopoulos G. Induction of fetal hemoglobin by propionic and butyric acid derivatives: correlations between chemical structure and potency of HB F induction. Blood Cells Mol Dis. 2002;29(1):48–56. doi: 10.1006/bcmd.2002.0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limon JJ, Fruman DA. Akt and mTOR in B cell activation and differentiation. Front Immunol. 2012;3:2012. doi: 10.3389/fimmu.2012.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozzio CB, Lozzio BB. Human chronic myelogenous leukemia cell-line with positive Philadelphia chromosome. Blood. 1975;45(3):321–334. [PubMed] [Google Scholar]
- Mabaera R, Richardson CA, Johnson K, Hsu M, Fiering S, Lowrey CH. Developmental and differentiation specific patterns of human γ and β-globin promoter DNA methylation. Blood. 2007;110:1343–1352. doi: 10.1182/blood-2007-01-068635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macari ER, Schaeffer EK, West RJ, Lowrey CH. Simvastatin and t- [butylhydroquinone suppress KLF1] and BCL11A gene expression and additively increase fetal hemoglobin in primary human erythroid cells. Blood. 2013;121(5):830–839. doi: 10.1182/blood-2012-07-443986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musallam KM, Taher AT, Cappellini MD, Sankaran VG. Clinical experience with fetal hemoglobin induction therapy in patients with β-thalassemia. Blood. 2013;121(12) doi: 10.1182/blood-2012-10-408021. http://dx.doi.org/10.1182/blood-2012-10-408021. [DOI] [PubMed] [Google Scholar]
- Nagata Y, Takahashi N, Davis RJ, Todokoro K. Activation of p38 MAP kinase and JNK but not ERK IS required for erythropoietin-induced erythroid differentiation. Blood. 1998;92(6):1859–1869. [PubMed] [Google Scholar]
- Okuno Y, Suzuki A, Ichiba S, Takahashi T, Nakamura K, Hitomi K, Sasaki R, Tada K, Imura H. Establishment of An erythroid cell line (JK-1) that spontaneously differentiates to red cells. Cancer. 1990;66(7):1544–1551. doi: 10.1002/1097-0142(19901001)66:7<1544::aid-cncr2820660719>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Orkin SH, Zon LI. Hematopoiesis: an evolving paradigm for stem cell biology. Cell. 2008;132(4):631–644. doi: 10.1016/j.cell.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palombo JD, Ganguly A, Bistrian BR, Menard MP. The antiproliferative effects of biologically active isomers of conjugated linoleic acid on human colorectal and prostatic Cancer cells. Cancer Lett. 2002;177(2):163–172. doi: 10.1016/s0304-3835(01)00796-0. [DOI] [PubMed] [Google Scholar]
- Papanikolaou E, Anagnou P. Major challenges for gene therapy of tha-lassemia and sickle cell disease. Curr Gene Ther. 2010;10(5):404–412. doi: 10.2174/156652310793180724. [DOI] [PubMed] [Google Scholar]
- Pawliuk R, Westerman KA, Fabry ME, Payen E, Tighe R, Bouhassira EE, Acharya SA, Ellis J, London IM, Eaves CJ, Humphries RK, Beuzard Y, Nagel RL, Leboulch P. Correction of sickle cell disease in transgenic mouse models by gene therapy. Science. 2001;294(5550):2368–2371. doi: 10.1126/science.1065806. [DOI] [PubMed] [Google Scholar]
- Perrine SP. Switching Globin, raising red cells. Blood. 2011;118:834–836. doi: 10.1182/blood-2011-06-354373. [DOI] [PubMed] [Google Scholar]
- Perrine SP, Ginder GD, Faller DV, Dover GH, Ikuta T, Witkowska HE, Shiping CMS, Vichinsky EP, Olivieri NF. A short-term trial of butyrate to stimulate fetal-globin-gene expression in the β-globin disorders. New Engl J Med. 1993;328(2):81–86. doi: 10.1056/NEJM199301143280202. [DOI] [PubMed] [Google Scholar]
- Perrine SP, Miller BA, Greene MF, Cohen RA, Cook N, Shackleton C, Faller DV. Butryic acid analogues augment γ globin gene expression in neonatal erythroid progenitors. Biochem Biophys Res Commun. 1987;148(2):694–700. doi: 10.1016/0006-291x(87)90932-6. [DOI] [PubMed] [Google Scholar]
- Platt OS, Orkin SH, Dover G, Beardsley GP, Miller B, Nathan DG. Hydroxyurea enhances fetal hemoglobin production in sickle cell anemia. J Clin Invest. 1984;74:652–656. doi: 10.1172/JCI111464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope SH, Fibach E, Sun J, Chin K, Rodgers GP. Two-phase liquid culture system models normal human adult erythropoiesis at the molecular level. Eur J Haematol. 2000;64(5):292–303. doi: 10.1034/j.1600-0609.2000.90032.x. [DOI] [PubMed] [Google Scholar]
- Sankaran VG, Xu J, Orkin SH. Advances in the understanding of haemoglobin switching. Br J Haematol. 2010;149(2):181–194. doi: 10.1111/j.1365-2141.2010.08105.x. http://dx.doi.org/10.1111/$10.1365-2141.2010.08105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaran VG, Menne TF, Xu J, Akie TE, Lettre G, Van Handel B, Mikkola HKA, Hirschhorn JN, Cantor AB, Orkin SH. Human fetal hemoglobin expression IS regulated by the developmental stage-specific repressor BCL11A. Sci (NY, NY) 2008;322(5909):1839–1842. doi: 10.1126/science.1165409. http://dx.doi.org/10.1126/scienc.1165409. [DOI] [PubMed] [Google Scholar]
- Siatecka M, Bieker JJ. The multifunctional role of EKLF/KLF1 during erythropoiesis. Blood. 2011;118(8):2044–2054. doi: 10.1182/blood-2011-03-331371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieff CA, Emerson SG, Mufson A, Gesner TG, Nathan DG. Dependence of highly enriched human bone marrow progenitors on hemopoietic growth factors and their response to recombinant erythropoietin. J Clin Invest. 1986;77(1):74. doi: 10.1172/JCI112305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg MH, Barton F, Castro O, Pegelow CH, Ballas SK, Kutlar A, Orringer E, Bellevue R, Olivieri N, Eckman J, Varma M, Ramirez G, Adler B, Smith W, Carlos T, Ataga K, DeCastro L, Bigelow C, Saunthararajah Y, Telfer M, Vichinsky E, Claster S, Shurin S, Bridges K, Waclawiw M, Bonds D, Terrin M. Effect of Hydroxyurea on mortality and morbidity in adult sickle cell anemia risks and benefits up to 9 years of treatment. J Am Med Assoc. 2003;289(13):1645–1652. doi: 10.1001/jama.289.13.1645. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Ozawa K, Takahashi K, Okuno Y, Muto Y, Takaku F, Asano S. DNA replication of parvovirus B 19 in a human erythroid leukemia cell line (JK-1) in vitro. Arch Virol. 1993;131(1–2):201–208. doi: 10.1007/BF01379092. [DOI] [PubMed] [Google Scholar]
- Tani T, Ylänne J, Virtanen I. Expression of megakaryocytic and Erythroi properties in human leukemic cells. Exp Hematol. 1996;24(2):158–168. [PubMed] [Google Scholar]
- Tanimoto K, Liu Q, Bungert J, Engel JD. Effects of altered gene order OR orientation of the locus control region on human beta-globin gene expression in mice. Nature. 1999;398:344–348. doi: 10.1038/18698. [DOI] [PubMed] [Google Scholar]
- Tripathy S, Jump DB. Elovl5 regulates the mTORC2-Akt–FOXO1 pathway by controling hepatic cis-vaccenic acid synthesis in diet=induced obese mice. J Lipid Res. 2013;54:71–84. doi: 10.1194/jlr.M028787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathy S, Torres-Gonzalez M, Jump DB. Elevated hepatic fatty acid elongase-5 activity corrects dietary fat-induced hyperglycemia in obese BL/6J mice. J Lipid Res. 2010;51(9):2642–2654. doi: 10.1194/jlr.M006080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turpeinen AM, Mutanen M, Aro A, Salminen I, Basu S, Palmquist DL, Griinari JM. Bioconversion of vaccenic acid to conjugated linoleic acid in humans. Am J Clin Nutr. 2002;76(3):504–510. doi: 10.1093/ajcn/76.3.504. [DOI] [PubMed] [Google Scholar]
- Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs IS a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- Wada H, Suda T, Miura Y, Kajii E, Ikemoto S, Yawata Y. Expression of major Blood group antigens on human erythroid cells in a two phase liquid culture system. Blood. 1990;75(2):505–511. [PubMed] [Google Scholar]
- Walters MC, Storb R, Patience M, Leisenring W, Taylor T, Sanders JE, Buchannan GE, Rogers ZR, Dinndorf P, Roberts Irene AG, Dickerhoff R, Yeager Andrew M, Hsu Lewis, Kurtzberg J, Ohene-Frempong K, Bunin N, Bernaudin F, Wing-Yen W, Scott JP, Margolis D, Vichinsky E, Wall Donna A, Wayne Allen S, Pegelow C, Redding-Lallinger R, Dinndorf J, Klemperer M, Mentzer William C, Smith Franklin O, Sullivan Keith M. Impact of bone marrow transplantation for symptomatic sickle cell disease: an interim report. Blood. 2000;95(6):1918–1924. [PubMed] [Google Scholar]
- Wang Y, Torres-Gonzalez M, Tripathy S, Botolin D, Christian B, Jump DB. Elevated hepatic fatty acid elongase-5 activity affects multiple pathways controlling hepatic lipid and carbohydrate composition. J Lipid Res. 2008;49(7):1538–1552. doi: 10.1194/jlr.M800123-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yien YY, Bieker JJ. EKLF/KLF1, a tissue-restricted integrator of transcriptional control, chromatin remodeling, and lineage determination. Mol Cell Biol. 2013;33(1):4–13. doi: 10.1128/MCB.01058-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zein S, Li W, Ramakrishnan V, Lou TF, Sivanand S, Mackie A, Pace B. Identification of fetal hemoglobin-inducing agents using the human leukemia KU812 cell line. Exp Biol Med. 2010;235(11):1385–1394. doi: 10.1258/ebm.2010.010129. [DOI] [PubMed] [Google Scholar]
- Zhang X, Campreciós G, Rimmele P, Liang R, Yalcin S, Mungamuri SK, Ghaffari S. FOXO3-mTOR metabolic cooperation in the regulation of erythroid cell maturation and homeostasis. Am J Hematol. 2014;89(10):954–963. doi: 10.1002/ajh.23786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Bieker JJ. Acetylation and modulation of erythroid Krüppel-like factor (EKLF) activity by interaction with histone acetyltransferases. Proc Natl Acad Sci. 1998;95(17):9855–9860. doi: 10.1073/pnas.95.17.9855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Liu K, Sun CW, Pawlik KM, Townes TM. KLF1 regulates BCL11A expression and [gamma]-to [beta]-globin gene switching. Nat Genet. 2010;42(9):742–744. doi: 10.1038/ng.637. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


