Abstract
Several members of the SLC9A family of Na+/H+ exchangers are expressed in the gut, with varying expression patterns and cellular localization. Not only do they participate in the regulation of basic epithelial cell functions, including control of transepithelial Na+ absorption, intracellular pH (pHi), cell volume, and nutrient absorption, but also in cellular proliferation, migration, and apoptosis. In addition, they modulate the extracellular milieu to facilitate other nutrient absorption and to regulate the intestinal microbial microenvironment. Na+/H+ exchangers are frequent targets of inhibition in gastrointestinal pathologies, either by intrinsic factors (eg, bile acids, inflammatory mediators) or infectious agents and associated microbial toxins. Based on emerging evidence, disruption of Na+/H+ exchange activity via impaired expression or function of respective isoforms may contribute not only to local and systemic electrolyte imbalance, but also to the disease severity via multiple mechanisms. Here, we review the current state of knowledge about the roles Na+/H+ exchangers play in the pathogenesis of disorders of diverse origin and affecting a range of gastrointestinal tissues.
Keywords: Barrett’s Esophagus, Esophageal Adenocarcinoma, Epithelial Injury, Epithelial Restitution, Diarrhea, Infection, Inflammation, Microbiota, Inflammatory Bowel Disease, Hypertension
Abbreviations used in this paper: cAMP, cyclic adenosine monophosphate; CD, Crohn’s disease; CFTR, cystic fibrosis transmembrane conductance regulator; cGMP, cyclic guanosine monophosphate; CPA, cation/proton antiporter; CSD, congenital sodium diarrhea; EGF, epidermal growth factor; EPEC, enteropathogenic Escherichia coli; IBD, inflammatory bowel disease; IL, interleukin; IRCX, inhibitor regulatory complex; mRNA, messenger RNA; mTOR, mechanistic or mammalian target of rapamycin; mTORC, mTOR complex; Muc2, mucin 2; NHERF, sodium hydrogen exchange regulatory factor; NHE, Na+/H+ exchange; ORS, oral rehydration solution; PDZ structural domain, postsynaptic density protein, Drosophila disc large tumor suppressor, and zona occludens-1 protein; pHi, intracellular pH; PKC, protein kinase C; SAR, SAR218034; SCFA, short-chain fatty acids; UC, ulcerative colitis
Summary.
Epithelial Na+/H+ exchange is a pleiotropic membrane transport mechanism that participates in intestinal NaCl transport, in the regulation of basic cellular functions and the extracellular milieu to facilitate other nutrient absorption, and to regulate the gut microbial microenvironment. In this review, we summarize the findings describing the basic roles of Na+/H+ exchange and the consequences of its dysregulation in the pathogenesis of gastrointestinal disorders.
Na+/H+ exchange (NHE) is an evolutionarily conserved membrane transport mechanism attributed to members of the cation/proton antiporters (CPA) superfamily.1, 2 Among the 4 inclusive families: CPA1, CPA2, Pho1 phosphate permease, and Na+-transporting carboxylic acid decarboxylase, the CPA1 (SLC9A) family contains the best-characterized plasmalemmal and intracellular isoforms (SLC9A1-9). In addition, the SLC9B subgroup consists of 2 Na+/H+ antiporter isoforms, Na+/H+ antiporter 1 and Na+/H+ antiporter 2 (SLC9B1 and SLC9B2). In the gut, expression of all NHE isoforms except NHE5 have been described. NHE1 (SLC9A1), NHE2 (SLC9A2), NHE3 (SLC9A3), and NHE8 (SLC9A8) not only have been implicated in regulating the basic functions of the epithelial cells, including control of the intracellular pH (pHi), acidic mucosal microclimate, cell volume, and nutrient absorption, but also in cell proliferation,3 cell migration,3, 4 and apoptosis.5 Intestinal expression of NHE4 (SLC9A4) in the small and large intestine remains controversial. Although gastric expression and function have been confirmed,6, 7 its presence in the intestine, at least in rodents, was contested.7 In light of these observations, the interpretation of the more recent studies on the functional contribution of NHE4 to pH regulation in the human colonic crypts8, 9 has to be performed with care, although species differences may account for the reported discrepancies.
As a principle, Na+/H+ exchangers use energy stored in the form of the electrochemical Na+ concentration gradient developed across the plasma membrane (secondary active transport) by the basolateral Na+/K+-adenosine triphosphatase. NHE is a critical mechanism for transepithelial movement of Na+ and HCO3- (and thus for luminal and systemic salt, volume, and acid-base homeostasis) (Figure 1). It also serves as a supporting role for other nutrient transporters by providing the proton gradient for the proton-coupled absorption of amino acids, peptides, organic anions, short-chain fatty acids, folate, and iron.10, 11 Only mutations in the NHE6 or NHE9 genes have been linked to human disease (X-linked mental retardation and familial autism, respectively).12, 13 Although long assumed, only recently has Janecke et al14 showed novel recessive missense, splicing, and truncation mutations in the NHE3-encoding SLC9A3 gene as being linked to the pathogenesis of congenital sodium diarrhea. Non–genetically based functional alterations of intestinal NHEs also have been linked with epithelial dysfunction. Details on the molecular mechanisms of regulation of expression and function of NHEs are beyond the scope and limit of this article, but have been covered comprehensively in other reviews.15, 16, 17, 18 In this review, we summarize the current state of knowledge regarding the physiological roles of NHE isoforms in the gut, and roles that aberrant epithelial Na+/H+ exchange plays in the context of gastrointestinal disorder pathogenesis.
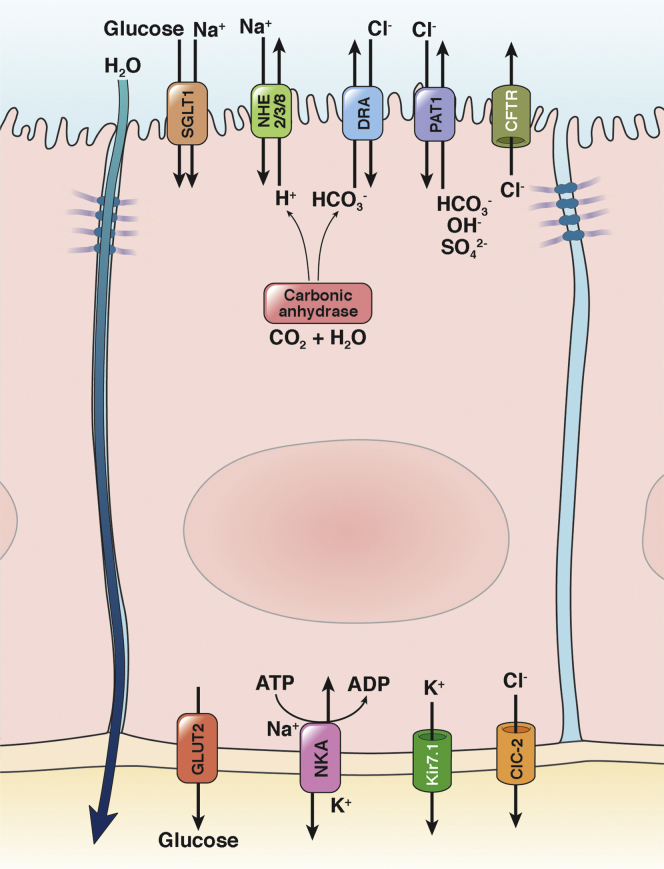
Figure 1.
Simplified depiction of the apical Na+/H+ exchange in transepithelial sodium, glucose, and water absorption. The inward Na+ gradient (low intracellular [Na+]) is maintained by the basolateral Na+/K+-adenosine triphosphatase (NKA), which provides the driving force for several secondary active transporters membrane transport proteins that import glucose, amino acids (not shown), and other nutrients into the cell by use of the sodium gradient. The intracellular concentration of potassium is coordinated by basolateral efflux via Kir7.1 (potassium inwardly rectifying channel, subfamily J, member 13, KCNJ13). Electroneutral NaCl absorption is driven by parallel Na+/H+ and Cl−/HCO3- exchange, the latter mediated by down-regulated in adenoma (DRA; SLC26A3), and putative anion transporter 1 (PAT1; SLC26A6). This coupled activity is made possible by carbonic anhydrase, which provides intracellular HCO3-. NHEs are cross-regulated with SLC26 family members and the Cl- and HCO3- transporting CFTR. In addition to Na+/D-glucose apical transport, SGLT1 activity stimulates NHE3 via an increased amount of apical NHE3 (see section Contribution of Na+/H+ Exchange to the Efficacy of Oral Rehydration Therapy). GLUT2 (glucose transporter 2, SLC2A2) provides a basolateral exit route for glucose. ClC-2 (chloride channel protein 2, CLCN2) is capable of basolateral Cl- secretion, although its key role appears to be the regulation of intestinal barrier function by altering tight junction composition and recovery from injury. An additional route of electrogenic Na+ transport is provided by apical Na+ channels (not shown).
Na+/H+ Exchange in Esophageal and Gastric Pathology
The Role of NHE1 in the Esophageal Epithelium in Barrett’s Esophagus and in the Progression to Esophageal Adenocarcinoma
Barrett’s esophagus is a precancerous condition characterized by the metaplasia of columnar epithelium with goblet cells that replace the normal squamous stratified epithelium in response to chronic reflux of gastric acid and bile acids. Although it is thought to represent a protective adaptive response to noxious components of the gastric chyme, metaplasia also is associated with an increased risk of esophageal adenocarcinoma. When esophageal luminal acidity reaches a pH of 2.0, the microenvironment adjacent to the surface cells of the stratified squamous epithelium is reduced to a pH of 2.0–3.0.19 On exposure to acid, esophageal epithelial cells regulate their pH primarily via the combined actions of Na+/H+ antiport and Na+-dependent Cl-/HCO3- exchange.20 NHE1 is the sole plasmalemmal Na+/H+ exchanger isoform expressed in the rabbit and rat esophagus,21 and is regulated allosterically (activated) by reduced pHi in a protein kinase C (PKC)-dependent mechanism.22 In addition, epidermal growth factor (EGF), abundant in the saliva, exerts a cytoprotective effect in acid-exposed cells via Ca2+/calmodulin- and PKC-dependent activation of NHE1.23 Consistent with that, patients with low salivary EGF levels were found to be susceptible to severe esophageal damage as a potential consequence of gastroesophageal reflux and to form a high-risk group for the development of Barrett's esophagus.24, 25 NHE1 expression is increased in patients with gastroesophageal reflux disease26 and in Barrett’s esophagus,27 where it likely represents a cellular defensive mechanism to manage the acute and chronic acid overload. Bile acids present in reflux chyme reduce the ability of the cells to control their pHi by nitric oxide–mediated NHE1 inhibition, thus leading to increased DNA damage and potentially to mutations and cancer progression.27
However, NHE1 has diverse physiological roles extending well beyond pHi and cell volume control, including cell proliferation, growth, migration, and apoptosis, and contributes to pathologic processes such as cancer cell invasion and heart failure.28, 29 In a Barrett's adenocarcinoma cell line, acid pulse-induced NHE1 activity correlated with increased proliferation, which could be reduced by inhibition of NHE1 or PKC.30 This finding led to a somewhat paradoxic proposal that NHE1 inhibition may be of therapeutic value in Barrett’s esophagus and prevention of its progression to cancer.
NHEs in Gastric Epithelial Injury and Repair
The integrity of the gastric epithelium is essential for protection from noxious luminal contents, which include gastric acid, proteases, and food-borne pathogens. As a consequence of damage to the surface epithelium, adjacent healthy cells rapidly migrate into the injured area to restore the mucosal barrier, and failure of this restitution process may lead to ulceration. Expression and activity of NHE1, and to a lesser extent NHE2 and NHE3, has been described in gastric myofibroblasts, cells that play a central role in wound healing, deposition of the extracellular matrix, and epithelial function.31 These functions are correlated tightly with the myofibroblasts’ ability to migrate and proliferate within the subepithelial compartment. NHE1 and its stimulation by insulin-like growth factor-II and carbachol has been postulated to contribute to human gastric myofibroblast migration and to be indispensable for insulin-like growth factor-II–induced human gastric myofibroblast proliferation.31 Gastric epithelium expresses 5 NHE isoforms: apical NHE2 (in surface/neck mucous cells), apical NHE3 (in rat but not rabbit parietal cells), apical NHE8 (mouse fundic and pyloric glands), basolateral NHE1 (all cells), and basolateral NHE4 (in parietal and chief cells).32, 33, 34 Initially, NHE1 was implicated in gastric repair in guinea pig mucosa in vitro.35, 36 Later studies with more definitive mouse knockout models, and the use of intravital 2-photon laser microscopy after injury, showed that it is NHE2 and not NHE1 that is required for the cytoprotective effects of trefoil factor 3 in the gastric epithelium.37 These findings were also in agreement with the in vitro studies by Furukawa et al,38 and in the description of progressive gastritis and gastric atrophy with loss of parietal cells in NHE2-/- mice.39, 40 Consistent with its expression pattern, loss of NHE4 in knockout mice resulted in reduced numbers of parietal cells, a loss of mature chief cells, increased numbers of mucous and undifferentiated cells, and an increase in the number of necrotic and apoptotic cells.6 More recent studies from our laboratory also showed that gastric expression of NHE8 was critical for the maintenance of bicarbonate secretion by the Cl-/HCO3- exchanger down-regulated-in-adenoma (DRA; SLC26A3) and the associated mucosal protection. Compared with their wild-type littermates, mice lacking NHE8 had a higher incidence of gastric ulcer formation.32 Although the mechanism responsible for DRA down-regulation is not yet clear, it appears to be a phenomenon consistent across organs because it was described also in the colon,41 conjunctiva, the cornea, and the lacrimal glands.42
Intestinal Na+/H+ Exchange in Diarrheal Diseases
Congenital Sodium Diarrhea
Congenital sodium diarrhea (CSD) is a rare autosomal-recessive diarrheal disorder originally described in 2 patients in 1984 and 1985.43, 44 To date, there have been fewer than 50 CSD cases reported in the literature.45 Before birth, polyhydroamnios (an excess of amniotic fluid in the amniotic sac) is observed, which is suggestive of an increase in colonic output by the fetus. After birth the disease is characterized by the secretion of a large volume of persistent high Na+ diarrhea, often mistaken for urine, which leads to irritability, dehydration, and metabolic acidosis.45 Although profuse diarrhea continues, electrolyte replacement therapy promotes normal growth and development.46
Two of the early reports of CSD linked diarrhea to defective NHE in the jejunum,44, 46 although the identity of the transporter remained unknown. In an attempt to identify the specific isoform, Muller et al47 studied a small CSD cohort of 5 infants from 2 inbred Austrian families and found no contribution from any of the 6 then known NHEs. Subsequent analyses showed additional intestinal and corneal pathophysiology (intestinal epithelia dysplasia and corneal epithelial erosions) among other symptoms accompanying high Na+ diarrhea. These affect approximately a third of CSD patients, are referred to as the syndromic CSD form, and have been linked to a mutation in SPINT2.48 The classic or nonsyndromic form of CSD is linked strongly to defective NHE activity, and, recently, using a cohort of 18 patients from 16 families, Janecke et al45 determined that a variety of mutations (point, missense, and truncation) in the NHE3-encoding SLC9A3 gene occurred in a subset (9 of 18) of the studied CSD cases. The identified SLC9A3 mutations included 1 whole-gene deletion, 1 splicing, and 2 frame-shift mutations, all of which were expected to abolish protein production. Four missense mutations/variants, p.Arg382Gln, p.Ala311Val, p.Ala269Thr, and p.Ala127Thr, were tested in vitro, and all but p.Ala127Thr (benign variant) conferred decreased basal Na+/H+ exchange activity.45
In addition, an activating mutation in the catalytic domain of the gene GUCY2C also was identified and may account for 20% of sporadic CSD cases.49, 50 This mutation likely is linked mechanistically to hyperactivation of the cystic fibrosis transmembrane conductance regulator (CFTR)49 and reduced NHE3 function via increased intracellular cyclic guanosine monophosphate (cGMP) and NHE3 inhibition via a cGKII kinase-dependent mechanism.51, 52 Consequently, NHE3 inhibition may contribute to at least 70% of CSD cases. Additional research is required to identify the remaining contributing factors, especially in the fetal and neonatal period when the contribution of NHE3 to total epithelial NHE is thought to be minimal. Although NHE8 has a reciprocal ontogenic pattern of expression, with higher levels in young than in older animals,53, 54 no mutations were found in the exonic regions of the SLC9A8 gene in a small cohort of 5 CSD patients.54
Inhibition of Na+/H+ Exchange During Infectious Diarrhea
Diarrhea caused by enteric infections is a major factor in morbidity and mortality worldwide. The mechanisms are multifactorial and include alterations in motility, changes in paracellular permeability, loss of absorptive surface, and changes in electrolyte fluxes. A significant component of altered electrolyte flux in infectious diarrhea is increased chloride secretion via activation of apical chloride channels, including the CFTR and Ca2+-activated Cl- channels.55 However, there is a significant component or subset of infectious diarrheal cases related to inhibition of Na+ and fluid absorption. The underlying mechanism for the latter is a reduction in Na+ absorption via impaired NHE activity, with the affected NHE isoforms and the mechanisms involved varying among pathogens. During infection with Vibrio cholerae, a prime example of secretory diarrhea, cholera toxin is capable of reducing epithelial Na+ absorption via cyclic adenosine monophosphate (cAMP)-dependent inhibition of NHE2 and NHE3.56 Extrapolating from the known mechanisms governing NHE regulation,57, 58 one could generalize that bacterial pathogens that increase intracellular Ca2+, cAMP, and/or cGMP concentration inhibit electroneutral NaCl absorption. Indeed, this was shown in mouse models of infection with Salmonella typhimurium, Shigella dysenteriae type 1 toxin, and Campylobacter jejuni.59, 60, 61
However, other mechanisms leading to NHE inhibition also have been described.
Clostridium difficile, the leading cause of nosocomial diarrhea and pseudomembranous colitis, also exerts inhibitory effects on epithelial NHE. C difficile toxin B inhibits Rho-family guanosine triphosphatases and alters the interaction between NHE3 and the microvillar actin cytoskeleton to facilitate its internalization and loss of function.62 C difficile toxin B also was shown to disrupt the stimulating effects of rho guanine nucleotide exchange factor 7, which, along with SH3 and multiple ankyrin repeat domains 2, maintains apical membrane NHE3 expression.63 More recently, Engevik et al64 postulated that NHE3 inhibition by C difficile toxin resulted in alteration of the intestinal environment and gut microbiota, which could facilitate colonization and expansion of the pathogen. They showed that when compared with healthy controls, decreased expression of NHE3 in infected patients correlated with increased Na+ and pH of the stools, increased Bacteroidetes and Proteobacteria, and decreased Firmicutes phyla. Interestingly, in vitro growth of C difficile was promoted by increased Na+ and an alkaline media pH; consequently, the investigators concluded that inhibition of NHE3 creates an altered environment favored by C difficile.64
Several classes of pathogenic E coli strains are responsible for diarrheal outbreaks. These include enteropathogenic E coli (EPEC), enterohemorrhagic E coli, enterotoxigenic E coli, enteroaggregative E coli, enteroinvasive E coli, and diffusely adherent E coli. A variety of NHE-independent mechanisms that contribute to diarrheagenic effects of EPEC are known (outside the scope of this review).65 Surprisingly, the effects of EPEC in vitro (Caco-2 BBE, HT-29 cells, and T84 cells) were isoform-specific, with significant increases in NHE1 (basolateral) and NHE2 (apical) activity and a concurrent 50% inhibition of NHE3.66 In a follow-up study, Hodges et al67 showed that the inhibitory effects of EPEC on NHE3 were dependent on E. coli secreted protein F-like protein from prophage U (EspF), a component of the EPEC type III secretion system. On the host side, Chen et al68 showed that E coli heat-stable enterotoxin, which binds to guanylate cyclase-C in the luminal enterocyte membrane and increases intracellular cGMP levels,69 requires the presence of the regulatory structural domain (post synaptic density protein, Drosophila disc large tumor suppressor, and zona occludens-1 protein) (PDZ) adaptor protein sodium hydrogen exchange regulatory factor (NHERF)2.68 The downstream effector of increased cGMP, cGKII, is a part of a NHE3 signaling complex,70 and inhibition of NHE3 activity by cGMP/cGKII requires NHE3 protein phosphorylation at Ser554 and Ser607,52 although the relationship of NHERF2/cGMP/cGKII in the context of exposure to E coli enterotoxin is not yet clear. Increased apical NHE2 activity may be compensatory to the loss of NHE3, although it remains unknown whether it outweighs NHE3 inhibition in vivo. Contrary to NHE3-/- mice,71 targeting of the SLC9A2 gene did not result in diarrhea,39, 72 suggesting that NHE2 is not a major absorptive Na+/H+ exchanger. Inhibition of NHE4 in T-84 cells by the heat-stable enterotoxin of enterotoxigenic E coli also has been reported, although the relevance of this finding is unclear considering the inconsistent data on intestinal expression of this isoform.7, 9
Less is known about the role of NHE in diarrhea during viral gastroenteritis. Astroviral infections have been associated with Na+ malabsorption, and 1 study suggested that infection leads to decreased levels of NHE3 in the insoluble protein fraction in the enterocytes, presumed to represent the apical membrane.73 Recently communicated data from rotavirus-infected patients showed that both NHE2 and NHE3 proteins are down-regulated, and the remaining NHE3 is mislocalized.74
Contribution of Na+/H+ Exchange to the Efficacy of Oral Rehydration Therapy
The glucose transporter SGLT1 uses a Na+ gradient to transport Na+ and glucose at a 2:1 stoichiometric ratio against a glucose gradient.75 In each cycle, a sugar molecule is co-transported with Na+ across the cell, which is accompanied by 260 water molecules.76 This mechanism was calculated to account for 5 L of water absorbed per day in the human intestine and formed the molecular basis of oral rehydration therapy aimed to control mortality associated with cholera and other infectious diarrheal diseases.77 Lin et al78 showed a key NHE3 contribution to the efficacy of oral rehydration solution. They showed that SGLT1-mediated Na-glucose co-transport stimulates NHE3 activity in vivo by a protein kinase B (Akt/PKB)- and NHERF2-dependent pathway. This increase was associated with increased brush-border NHE3 recruitment through its release from the endosomal storage pool. Activation of NHE3 by glucose reversed cholera toxin–induced NHE3 inhibition, a phenomenon that at least partially may explain the efficacy of oral rehydration solution (ORS).78 In 2004, both the World Health Organization and the United Nations Children’s Fund suggested the supplementation of ORS with zinc. This recommendation was based on observations linking a reduction in tissue and an increase in fecal zinc levels in infants and children with diarrhea,79 and that zinc deficiency is responsible for decreased net water and sodium transport from the small and large intestine.80 Clinical studies showed a decrease in diarrheic stool volume and episode duration when zinc was administered in conjunction with ORS.81 In addition to the antisecretory effects of zinc,82 Hoque et al83 showed that zinc not only stimulated basal NHE3 activity by 50%, but it also counteracted forskolin-induced cAMP-mediated NHE3 inhibition. Although conventional ORS targets primarily small intestinal Na+ and water absorption, an improved formulation has been proposed to target the colon as well.84 In this approach, D-glucose is replaced with a relatively amylase-resistant cornstarch. Although some of the starch is broken down enzymatically in the jejunum to stimulate Na+ absorption, most of it would enter the colon where it could be fermented to short-chain fatty acids such as propionate, butyrate, and acetate by the resident bacteria. Butyrate increases the expression and/or activity of apical NHEs85, 86, 87, 88 and aids in transepithelial Na+ absorption via a neutral linked Na+ absorptive process that exchanges short-chain fatty acids for OH- ions along with the apical Na+/H+ exchange. In a small randomized clinical trial, this ORS formulation performed better than standard ORS by reducing both the duration and volume loss of severe acute diarrhea.84 Two other randomized controlled trials with the addition of high-amylose maize starch to ORS performed in South India have shown a substantial decrease in diarrhea duration in both adults and children hospitalized for acute diarrhea.89, 90 One drawback of such modified ORS is the precipitation of the starch in the ORS formulation. New formulations containing an antisettling agent have been proposed and clinical trials were reported in 2014 by Binder et al91 as underway.
Modulation of Intestinal Na+/H+ Exchange in Noninfectious Diarrhea Associated With Immunosuppression (Rapamycin)
Rapamycin or sirolimus is a drug isolated from the actinomycete Streptomyces hygroscopius that shows immunosuppressive, antiproliferative, and antifungal properties. Rapamycin and its analogs (Rapalogs) have been shown to inhibit the activity of mammalian or mechanistic target of rapamycin (mTOR).92 mTOR is a serine-threonine kinase that is known to form 2 different catalytic complexes, the mTOR complex 1 or 2 (mTORC1 or mTORC2), and to induce autophagy.92 Rapamycin administration inhibits mTORC1, while long-term use partially inhibits mTORC2. Its use to prevent organ transplant rejection and to treat lymphangioleiomyomatosis is associated with several side effects, which include diarrhea. Among patients receiving postrenal transplant rapamycin treatment and in those hospitalized for noninfectious diarrhea, ileal NHE3 expression was reduced at the apical membrane.93 Of 367 records of renal transplant cases at the Albany Medical College, 20 patients had 39 events of acute, severe diarrhea requiring hospitalization. In all events, rapamycin levels at the time of diarrhea were increased significantly (2–7 times higher than the baseline levels), and in all cases diarrhea resolved within 3–5 days after the serum rapamycin levels returned to baseline.93 This finding was reproduced in rapamycin-treated wild-type, but not in autophagy-resistant, Atg7-/- mice. Deletion of mTOR in mice also led to reduced NHE activity, luminal accumulation of fluid, and reduced levels of NHE3 and NHERF1.93
Intestinal Na+/H+ Exchange in Clinical and Experimental Inflammatory Bowel Diseases
Inflammatory bowel disease (IBD) is a group of chronic inflammatory diseases that includes Crohn’s disease (CD) and ulcerative colitis (UC). Both conditions are characterized by immune system activation, microbial dysbiosis, and disruption of the epithelial barrier, which may lead to bacterial translocation, the latter being a more prominent feature of CD.94 Alterations in the function of epithelial cells in IBD extend into transcellular nutrient transport.95 In some instances, those alterations of transport protein complexes at the apical or basolateral membranes have consequences transcending their basal functions as nutrient or electrolyte transporters to modulate epithelial barrier function and gut microbiota, potentially shaping the immune response in the course of IBD.
Inhibition of Intestinal Na+/H+ Exchange as a Contributor to Inflammation-Associated Diarrhea
Diarrhea is one of the common symptoms in patients with IBD, occurring in approximately 50% of acute flare-ups in CD and in nearly all UC patients.96 The pathophysiology of diarrhea in IBD is complex and multifactorial. It involves altered intestinal motility as well as abnormal epithelial ion transport and defective Na+ absorption in the colonic mucosa in particular.97, 98 Contrary to infectious diarrhea in which active and excessive Cl- secretion is predominant, in IBD, both electrogenic Na+ transport mediated by sodium channels99, 100, 101 as well as electroneutral NHE and coupled Na-Cl absorption are reduced.102 At least for UC, a model was proposed whereby the associated diarrhea is the result of a combination of factors: increased paracellular permeability to monovalent ions, reduction of electrogenic Na+ absorption (secondary to decreased apical Na+ channel and basolateral Na+/K+-adenosine triphosphatase expression), electroneutral Na+ absorption and coupled Na+-H+/Cl--HCO3- exchange, and as a result compromised active Cl- absorption.102
The mechanisms involved in the inhibition of epithelial NHEs in IBD are not well defined and conflicting results have been published. Interferon γ inhibits the expression and function of NHE3 and NHE2 both in vitro and in vivo.103 Inhibition of NHE3 expression and activity also has been described in several experimental models of colitis, including interleukin (IL)2-/- mice,104 and in dextran sulfate sodium– and trinitrobenzene sulfonic acid–induced colitis.105 The latter study described decreased NHE3 protein expression in sigmoid mucosal biopsy specimens from most of the cases of active UC and/or CD, in ileal mucosal biopsy specimens of active CD, as well as in approximately 50% of sigmoid biopsy specimens from inactive UC or CD. Siddique et al106 showed that in both the untreated and treated patients with CD and UC, NHE3 protein and activity also were reduced. Furthermore, NHE3 messenger RNA (mRNA) was reduced only in CD, but not in patients with UC. However, in IL10-/- mice, NHE3 activity, measured in the apical enterocytes within isolated colonic crypts, was decreased significantly without altered NHE3 expression and localization.107 The investigators speculated that decreased expression of 2 key NHE3-regulatory proteins, SLC9A3 regulator 2 (SLC9A3R2/NHERF-2) and PDZ domain containing 1 (PDZK1), may be responsible for decreased NHE3 activity.107 Additional studies with UC patients by Yeruva et al108 and Farkas et al109 showed a significant reduction of NHE3 activity despite the preserved protein and mRNA expression, thus arguing for post-translational regulation of the antiporter activity. In an in vivo model of diarrhea mediated by anti-CD3 monoclonal antibody–induced T-cell activation, Clayburgh et al110 showed that in the jejunum, tumor necrosis factor induces NHE3 internalization. PKCα activation triggered by tumor necrosis factor was responsible for NHE3 internalization and the resulting Na+ malabsorption.110 Although it is clear that the NHE3-mediated apical Na+/H+ exchange and epithelial Na+ absorption are inhibited in IBD, the exact mechanism remains unclear and may depend on the specific disease, segment involved, and/or the severity of inflammation.
NHE8 has a broader expression pattern than other apical NHEs.53, 111, 112 Moreover, its expression is not limited to the apical membrane; in vitro studies with ectopically expressed NHE8 have shown that it is both present and functional in the trans-Golgi network and multivesicular bodies.113 It is broadly expressed in the colon, including the goblet cells, where it participates in the regulation of mucin 2 (Muc2) expression and mucous secretion.41, 114, 115 Although we observed a dramatic decrease in the expression of NHE8 in UC patients (Li et al, unpublished data), loss of NHE8 expression and activity may not contribute to inflammation-associated diarrhea directly because NHE8-/- mice do not show symptoms of impaired Na+ absorption or diarrhea.41 The consequences of NHE8 deficiency in IBD may be more related to microbial–host interactions, as discussed in the next section.
Both the role of the basolateral NHE1 and the potential changes in its expression and/or activity in the colonic epithelial cells in clinical and experimental IBD remain unclear, and the available data are inconsistent. Expression of NHE1 mRNA and protein was increased in both acetic acid and trinitrobenzene sulfonic acid–induced colitis in rats,116 and nonspecific pharmacologic NHE inhibition with amiloride was deemed beneficial in rodent models of IBD.117, 118 However, in human patients, opposing data have been published. Contrary to the same group’s earlier rat data, Khan et al119 and Siddique and Khan120 showed decreased NHE1 mRNA in the biopsy specimens of UC and CD patients, whereas Farkas et al109 showed consistently increased NHE1 activity and expression in the inflamed mucosa of UC patients. The importance of these controversial findings remains unclear. Although it may be consequential to some aspects of epithelial cell function, the notion that “NHE1 suppression may reduce an uptake of sodium chloride and water from the inflamed colonic lumen and thus contribute to diarrhea”119 currently lacks experimental support.
Apical Na+/H+ Exchange as a Modulator of the Gut Microbiome and Inflammatory Response During Colitis
The lumen of the gastrointestinal tract is a habitat for 100 trillion bacterial cells, which form a virtual organ.121 This gastrointestinal bacterial consortium, collectively referred to as the gut microbiome, is a highly functional, complex, and dynamic entity. The composition of this virtual organ, known as the gut microbiota or microbiome, generally is conserved at the phylum level and is unique for each individual at lower taxonomic (genus and species) levels. The adult colon is colonized predominantly by 2 phyla, Bacteroidetes and Firmicutes, followed by Proteobacteria and Actinobacteria.122 Cells of both the innate immune system (including epithelial cells) as well as of the adaptive immune system can sense microorganisms or their metabolic products and translate the signals into host physiological responses, which in turn can regulate the gut microbial ecology.123, 124 This cross-talk is essential for normal gut development, maturation of the immune system, optimal nutrient bioavailability, participation in xenobiotic and hormone metabolism, and the maintenance of the epithelial and immune cell microenvironment. Any perturbations in microbial–host homeostasis results in dysbiosis, a detrimental shift in the composition or microbial abundance, with consequences ranging from enteric infections,125, 126 colorectal cancer,127 and autoimmune diseases,128 through metabolic syndrome (type 2 diabetes and obesity),129 to neuropsychiatric diseases and autism spectrum disorders.130, 131, 132, 133
Dysbiosis in both IBD patients and animal models is characterized by reduced general biodiversity,134 expanded relative abundance of the Bacteroidetes, Proteobacteria, and Actinobacteria phyla, decreased abundance of Firmicutes,135 and, importantly, the presence of Clostridia species belonging to the clostridia IV and XIVa clusters, the major butyrate-producing bacteria and inducers of colonic CD4+FoxP3+ T-regulatory cells.136 Similar changes have been observed in mouse models of IBD.137, 138, 139 The mechanisms by which the gut microbial community is affected by the inflammatory process, or whether dysbiosis is sufficient to induce and/or modulate mucosal immune responses, remain a subject of debate. Although some studies have indicated that dysbiotic communities can be colitogenic upon transmission to wild-type mice,140 others have shown that mucosal bacteria from UC patients only confer susceptibility and are not sufficient to induce disease in untreated or otherwise nongenetically manipulated hosts.141
We hypothesized that inhibition of epithelial NHE, common in IBD patients, may lead to altered epithelial cell function and mucosal milieu that could lead or contribute to the observed shifts in microbial gut ecology and, as a consequence, influence mucosal inflammation (Figure 2). Indeed, mice lacking the dominant epithelial apical NHE isoform NHE3, when raised in a conventional facility, spontaneously develop distal colitis with mild diarrhea.142 Colitis symptoms were alleviated by broad-spectrum antibiotics and reduced by rederivation into a barrier facility.142, 143 NHE3-/- mice develop microbial dysbiosis similar to IBD patients.143 Although they appear to have normal mucous thickness, structure, and Muc2 mRNA expression, higher bacterial penetrance with increased bacterial adhesion to the mucosa and bacterial translocation was described, similar to IL10-/- mice.142, 144 These findings suggested that mucus thickness alone is not a major determinant of bacterial penetration, and that other factors may contribute to mucosal bacterial penetration in IBD.144 It is plausible, however, that the molecular structure of mucin 2 (MUC2) polymers within the inner layer may change with impaired apical Na+/H+ exchange. Because MUC2 is packed into secretory granules in the goblet cells, the organellar pH changes from 7.2 in the endoplasmic reticulum to 6.0 in the trans-Golgi network, and to 5.2 in the secretory granulae, along with increasing Ca2+ concentration.145 Upon secretion, and before the 1000-fold expansion, it remains densely packed to form the impenetrable for bacteria inner stratified layer, which is best preserved at a pH of 6.2 in the form of sheets of N-terminal trimers.145 Disruption of the regulatory mechanisms responsible for maintenance of the acidic microclimate could make the MUC2 network structure more penetrable for bacteria. However, this would require experimental confirmation.
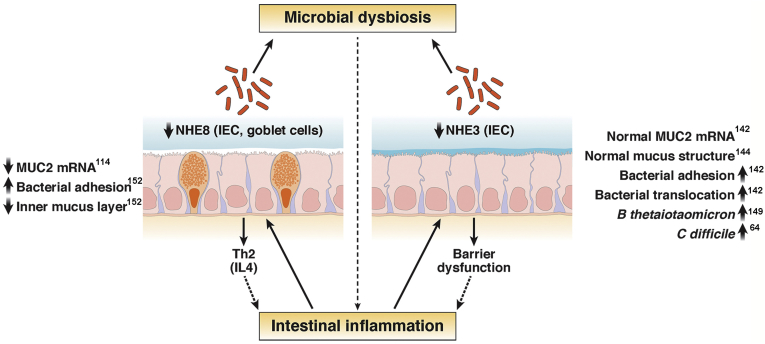
Figure 2.
Summary graph illustrating the roles of NHE3 and NHE8 and the consequences of their inhibition during mucosal inflammation. IEC, intestinal epithelial cell; Th, T-helper.
Importantly, NHE3-/- mice are highly susceptible to experimental colitis,146, 147 although the relative contributions of epithelial dysfunction and microbial dysbiosis still are unclear. Most recently, our group showed that in adoptive T-cell transfer colitis, NHE3 deficiency and the associated dysbiosis dramatically accelerated and exacerbated the disease, increased mucosal CD4+ T-cell and neutrophil homing, and increased intestinal permeability, all of which was attenuated with broad-spectrum antibiotics.148 Strikingly, a dysbiotic microbiome was associated more strongly with NHE3 deficiency than with T-cell–mediated colitis per se.148 We concluded that Na+/H+ exchange is, therefore, a crucial process for maintaining microbial homeostasis within the gut and that its disruption in IBD is likely a strong contributor to dysbiosis, which in turn affects the progression and severity of disease. Another group also showed that higher ileal luminal Na+ concentration, and not changes in pH, is responsible for the relative expansion of Bacteroides thetaiotaomicron and expression and activity of fucosylase FUT2.149 B thetaiotaomicron has been shown to be colitigenic,150 and an aberrantly high IL8 response to B thetaiotaomicron has been shown in CD patients,149 findings that may provide a link between inhibition of NHE3 and mucosal neutrophil influx in IBD.
As suggested earlier in this review, NHE8 also may contribute to mucosal homeostasis through mechanisms distinct from that of NHE3, possibly through its as-yet-undefined roles in the goblet cell function. Indeed, it is down-regulated and confers higher susceptibility during experimental colitis with a T-helper 2–like response.151, 152 Reduced expression or absence of NHE8 also is related to decreased Muc2 mRNA expression in goblets cells, and decreased mucosal expression of antimicrobial peptides.114 NHE8-/- mice have reduced Muc2 expression, which also could be reproduced in colonic organoids from this strain (Xu et al, unpublished data), and show a reduced inner mucous layer and closer proximity of luminal bacteria to the brush-border membrane.152 Consequently, loss of NHE8 in vivo led to higher adhesion of S typhimurium.152 In vitro, NHE8 small interfering RNA knockdown showed a similar increase in S typhimurium adhesion, which appeared to be selective and did not affect adhesion of a probiotic strain of Lactobacillus plantarum JDM1.152 In summary, data from NHE3 and NHE8 knockout mice suggest that their inhibition during inflammation or infection, albeit acting via different mechanisms, may be critical in shaping the host-microbial homeostasis in the gut and affect mucosal immune responses during IBD. The development of new pharmacologic approaches to either prevention of inhibition of the 2 apical NHEs, or restoration of their activity, may be of significant benefit to IBD patients not only as means of reducing episodes of diarrhea, but possibly as means of modulating the gut microbiome and host’s inflammatory response.
Therapeutic Targeting of Intestinal Na+/H+ Exchange
Potential for Modulation of Intestinal Fluid and Na+ Absorption in Diarrheal and Inflammatory Conditions by Targeting NHE3 Activity
As described earlier in this review, part of the success of oral rehydration therapy and its modifications relies on the modulation of electroneutral epithelial Na+/H+ exchange. Considering the role of the C-terminus of the NHE3 protein in forming regulatory complexes responsible for NHE3 endocytic retrieval,18 Zachos et al153 postulated that a peptide designed to mimic the region of the NHE3 protein involved in the formation of the inhibitor regulatory complex (IRCX; NHERFs 1–4, phospholipase C-γ, casein kinase 2, and Ca2+/calmodulin-dependent protein kinase II) may increase the apical pool of NHE3 and improve its activity. This 38–amino acid decoy peptide dubbed IRCX complex formation stimulated basal NHE3 activity by 40% and prevented inhibition of NHE3 activity by both forskolin and carbachol, without altering the stimulatory effect of EGF on NHE3 activity.153 In addition, in vivo, IRCX complex formation prevented the cholera toxin–induced increase in luminal fluid accumulation. It remains to be seen whether these promising data translate into a viable treatment. However, it clearly shows that the formation of the regulatory complexes at the C-terminus of NHE3 represents an attractive target for compound screening and development of drugs to stimulate NHE3 as potentially useful in the treatment of infectious and noninfectious diarrhea, and perhaps in modulating microbial and inflammatory responses in IBD.
Inhibition of Intestinal NHE3 Activity as a Treatment Modality for Hypertension, Constipation-Predominant Irritable Bowel Syndrome, and Hyperphosphatemia for End-Stage Renal Disease Patients on Dialysis
On the opposite spectrum, excessive sodium intake and/or reduced excretion in the intestine leads to sodium–fluid imbalances that result in hypertension, which then can lead to vision impairment in addition to a variety of cardiovascular and renal diseases such as heart failure, chronic kidney disease, stroke, and coronary heart disease. Typically, hypertension is considered to be one of the most common modifiable risk factors for cardiovascular disease and death154 because it can be controlled by reducing dietary sodium intake.155 Unfortunately, compliance with a low-salt diet is low.156 Drugs that limit intestinal sodium absorption, given alone or in conjunction with other antihypertensive medications, have been considered. To this end, 2 oral nonabsorbable NHE3 inhibitors have been developed, SAR218034 (SAR) and tenapanor. Pharmacokinetic analyses in rats, dogs, and human beings have shown that neither of these compounds crossed the intestinal barrier in biologically active doses,157, 158 and tenapanor was well tolerated in phase I clinical study.159 Both drugs were found to increase fecal and reduce urinary Na+ concentrations in rats (SAR and tenapanor) and human beings (tenapanor). Not surprisingly, both drugs caused an increase in luminal fluid resulting from increased Na+, leading to loose stools (a laxative effect).157, 158 In a spontaneously hypertensive rat model, SAR administration in conjunction with sodium chloride–laden drinking water markedly reduced systolic blood pressure.158 Furthermore, in a salt-fed 5/6 nephrectomized rat model (a chronic kidney disease model associated with hypertension, hypervolemia, cardiac hypertrophy, and arterial stiffening), tenapanor reduced extracellular volume expansion, albuminuria, and blood pressure, in addition to promoting protective cardiorenal effects such as reducing left ventricular hypertrophy.157 Both drugs show enhanced effects if administered in conjunction with an angiotensin-converting enzyme inhibitor, which was deemed important in cases in which hypertension could not be controlled by the administration of a single medication.157, 158 However, more recently, the development of tenapanor and SAR, and the general concept of inhibition of intestinal Na+/H+ exchange as an antihypertensive strategy, appears to have been abandoned by Ardelyx (Fremont, CA) and Sanofi (Paris, France), respectively. Ardelyx continues the investigation into the use of tenapanor and its close analogs in patients with constipation-predominant irritable bowel syndrome and for the treatment of hyperphosphatemia in end-stage renal disease patients on dialysis.160, 161 The potential of NHE3 inhibitors as stool softeners in constipation-predominant irritable bowel syndrome is obvious, although it remains to be seen if they can be applied without significant adverse effects. A somewhat similar strategy to modulate ion transport in constipation has been applied recently to develop CFTR activator to increase the net chloride output in the gut.162 The concept behind the use of NHE3 inhibition in hyperphosphatemia is based on the described increased fecal Pi excretion and reduced urinary Pi excretion in NHE3 inhibitor-treated rats with chronic kidney disease with vascular calcification, in which tenapanor markedly reduced ectopic calcification and protected renal function.161 The mechanism of this phenomenon remains unclear. It also is not yet evident whether targeting NHE3 would be more efficacious and have less adverse effects that the Pi binders currently used clinically.
Conclusions
NHEs are important regulators of a wide array of processes on a cellular, tissue, and systemic level. The majority of current knowledge is limited to the function of plasmalemmal isoforms, which play protective roles during disease pathogenesis in a segment- and isoform-specific fashion and are frequent targets of inhibition by microbial or inflammatory stimuli. As such, future studies may bring new approaches that selectively target NHEs in the gut to enhance or inhibit their function for the benefit of the patients. Importantly, a large body of knowledge relies on studies focused on individual isoforms, frequently with knockout mice, while their true function always occurs in the larger context of frequently interdependent transporters, also subject to regulation during physiological and pathophysiological conditions. Thus, more integrative approaches to function, regulation, and targeting of NHEs in gut-associated pathologies may be needed. Although the usefulness of orally available inhibitors remains to be established, the emerging focus on the development of agonists of intestinal apical Na+/H+ exchange may provide significant benefits to the future treatment of infectious and inflammatory disorders of the gut.
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases grant 2R01 DK-041274 (F.K.G. and P.R.K.). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Chen J.S., Reddy V., Chen J.H. Phylogenetic characterization of transport protein superfamilies: superiority of SuperfamilyTree programs over those based on multiple alignments. J Mol Microbiol Biotechnol. 2011;21:83–96. doi: 10.1159/000334611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brett C.L., Donowitz M., Rao R. Evolutionary origins of eukaryotic sodium/proton exchangers. Am J Physiol Cell Physiol. 2005;288:C223–C239. doi: 10.1152/ajpcell.00360.2004. [DOI] [PubMed] [Google Scholar]
- 3.Walker J., Undem C., Yun X. Role of Rho kinase and Na+/H+ exchange in hypoxia-induced pulmonary arterial smooth muscle cell proliferation and migration. Physiol Rep. 2016;4:e12702. doi: 10.14814/phy2.12702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rigiracciolo D.C., Scarpelli A., Lappano R. GPER is involved in the stimulatory effects of aldosterone in breast cancer cells and breast tumor-derived endothelial cells. Oncotarget. 2016;7:94–111. doi: 10.18632/oncotarget.6475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao Y., Cui G., Zhang N. Lipopolysaccharide induces endothelial cell apoptosis via activation of Na(+)/H(+) exchanger 1 and calpain-dependent degradation of Bcl-2. Biochem Biophys Res Commun. 2012;427:125–132. doi: 10.1016/j.bbrc.2012.09.023. [DOI] [PubMed] [Google Scholar]
- 6.Gawenis L.R., Greeb J.M., Prasad V. Impaired gastric acid secretion in mice with a targeted disruption of the NHE4 Na+/H+ exchanger. J Biol Chem. 2005;280:12781–12789. doi: 10.1074/jbc.M414118200. [DOI] [PubMed] [Google Scholar]
- 7.Bookstein C., Xie Y., Rabenau K. Tissue distribution of Na+/H+ exchanger isoforms NHE2 and NHE4 in rat intestine and kidney. Am J Physiol. 1997;273:C1496–C1505. doi: 10.1152/ajpcell.1997.273.5.C1496. [DOI] [PubMed] [Google Scholar]
- 8.Beltran A.R., Ramirez M.A., Carraro-Lacroix L.R. NHE1, NHE2, and NHE4 contribute to regulation of cell pH in T84 colon cancer cells. Pflugers Arch. 2008;455:799–810. doi: 10.1007/s00424-007-0333-0. [DOI] [PubMed] [Google Scholar]
- 9.Arena E.A., Longo W.E., Roberts K.E. Functional role of NHE4 as a pH regulator in rat and human colonic crypts. Am J Physiol Cell Physiol. 2012;302:C412–C418. doi: 10.1152/ajpcell.00163.2011. [DOI] [PubMed] [Google Scholar]
- 10.Thwaites D.T., Anderson C.M. H+-coupled nutrient, micronutrient and drug transporters in the mammalian small intestine. Exp Physiol. 2007;92:603–619. doi: 10.1113/expphysiol.2005.029959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shawki A., Engevik M.A., Kim R.S. Intestinal brush-border Na+/H+ exchanger-3 drives H+-coupled iron absorption in the mouse. Am J Physiol Gastrointest Liver Physiol. 2016;311:G423–G430. doi: 10.1152/ajpgi.00167.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takahashi Y., Hosoki K., Matsushita M. A loss-of-function mutation in the SLC9A6 gene causes X-linked mental retardation resembling Angelman syndrome. Am J Med Genet B Neuropsychiatr Genet. 2011;156B:799–807. doi: 10.1002/ajmg.b.31221. [DOI] [PubMed] [Google Scholar]
- 13.Kondapalli K.C., Hack A., Schushan M. Functional evaluation of autism-associated mutations in NHE9. Nat Commun. 2013;4:2510. doi: 10.1038/ncomms3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janecke A.R., Heinz-Erian P., Yin J. Reduced sodium/proton exchanger NHE3 activity causes congenital sodium diarrhea. Hum Mol Genet. 2015;24:6614–6623. doi: 10.1093/hmg/ddv367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kiela P.R., Ghishan F.K. Na+-H+ exchange in mammalian digestive tract. In: Johnson L.R., editor. Physiology of the gastrointestinal tract. Vol 2. 4th ed. Academic Press; London, UK: 2006. pp. 1847–1879. [Google Scholar]
- 16.Orlowski J., Grinstein S. Na+/H+ exchangers. Compr Physiol. 2011;1:2083–2100. doi: 10.1002/cphy.c110020. [DOI] [PubMed] [Google Scholar]
- 17.Donowitz M., Ming Tse C., Fuster D. SLC9/NHE gene family, a plasma membrane and organellar family of Na(+)/H(+) exchangers. Mol Aspects Med. 2013;34:236–251. doi: 10.1016/j.mam.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donowitz M., Mohan S., Zhu C.X. NHE3 regulatory complexes. J Exp Biol. 2009;212:1638–1646. doi: 10.1242/jeb.028605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orlando R.C. Esophageal epithelial defense against acid injury. J Clin Gastroenterol. 1991;13(Suppl 2):S1–S5. [PubMed] [Google Scholar]
- 20.Layden T.J., Schmidt L., Agnone L. Rabbit esophageal cell cytoplasmic pH regulation: role of Na(+)-H+ antiport and Na(+)-dependent HCO3- transport systems. Am J Physiol. 1992;263:G407–G413. doi: 10.1152/ajpgi.1992.263.3.G407. [DOI] [PubMed] [Google Scholar]
- 21.Shallat S., Schmidt L., Reaka A. NHE-1 isoform of the Na+/H+ antiport is expressed in the rat and rabbit esophagus. Gastroenterology. 1995;109:1421–1428. doi: 10.1016/0016-5085(95)90626-6. [DOI] [PubMed] [Google Scholar]
- 22.Malo M.E., Fliegel L. Physiological role and regulation of the Na+/H+ exchanger. Can J Physiol Pharmacol. 2006;84:1081–1095. doi: 10.1139/y06-065. [DOI] [PubMed] [Google Scholar]
- 23.Fujiwara Y., Higuchi K., Takashima T. Roles of epidermal growth factor and Na+/H+ exchanger-1 in esophageal epithelial defense against acid-induced injury. Am J Physiol Gastrointest Liver Physiol. 2006;290:G665–G673. doi: 10.1152/ajpgi.00238.2005. [DOI] [PubMed] [Google Scholar]
- 24.Gray M.R., Donnelly R.J., Kingsnorth A.N. Role of salivary epidermal growth factor in the pathogenesis of Barrett's columnar lined oesophagus. Br J Surg. 1991;78:1461–1466. doi: 10.1002/bjs.1800781218. [DOI] [PubMed] [Google Scholar]
- 25.Marcinkiewicz M., Grabowska S.Z., Czyzewska E. Role of epidermal growth factor (EGF) in oesophageal mucosal integrity. Curr Med Res Opin. 1998;14:145–153. doi: 10.1185/03007999809113354. [DOI] [PubMed] [Google Scholar]
- 26.Siddique I., Khan I. Regulation of Na/H exchanger-1 in gastroesophageal reflux disease: possible interaction of histamine receptor. Dig Dis Sci. 2003;48:1832–1838. doi: 10.1023/a:1025503318409. [DOI] [PubMed] [Google Scholar]
- 27.Goldman A., Shahidullah M., Goldman D. A novel mechanism of acid and bile acid-induced DNA damage involving Na+/H+ exchanger: implication for Barrett's oesophagus. Gut. 2010;59:1606–1616. doi: 10.1136/gut.2010.213686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harguindey S., Orive G., Luis Pedraz J. The role of pH dynamics and the Na+/H+ antiporter in the etiopathogenesis and treatment of cancer. Two faces of the same coin–one single nature. Biochim Biophys Acta. 2005;1756:1–24. doi: 10.1016/j.bbcan.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 29.Karmazyn M., Sawyer M., Fliegel L. The Na(+)/H(+) exchanger: a target for cardiac therapeutic intervention. Curr Drug Targets Cardiovasc Haematol Disord. 2005;5:323–335. doi: 10.2174/1568006054553417. [DOI] [PubMed] [Google Scholar]
- 30.Fitzgerald R.C., Omary M.B., Triadafilopoulos G. Altered sodium-hydrogen exchange activity is a mechanism for acid-induced hyperproliferation in Barrett's esophagus. Am J Physiol. 1998;275:G47–G55. doi: 10.1152/ajpgi.1998.275.1.G47. [DOI] [PubMed] [Google Scholar]
- 31.Czepan M., Rakonczay Z., Jr., Varro A. NHE1 activity contributes to migration and is necessary for proliferation of human gastric myofibroblasts. Pflugers Arch. 2012;463:459–475. doi: 10.1007/s00424-011-1059-6. [DOI] [PubMed] [Google Scholar]
- 32.Xu H., Li J., Chen H. NHE8 plays important roles in gastric mucosal protection. Am J Physiol Gastrointest Liver Physiol. 2013;304:G257–G261. doi: 10.1152/ajpgi.00433.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pizzonia J.H., Biemesderfer D., Abu-Alfa A.K. Immunochemical characterization of Na+/H+ exchanger isoform NHE4. Am J Physiol. 1998;275:F510–F517. doi: 10.1152/ajprenal.1998.275.4.F510. [DOI] [PubMed] [Google Scholar]
- 34.Rossmann H., Sonnentag T., Heinzmann A. Differential expression and regulation of Na(+)/H(+) exchanger isoforms in rabbit parietal and mucous cells. Am J Physiol Gastrointest Liver Physiol. 2001;281:G447–G458. doi: 10.1152/ajpgi.2001.281.2.G447. [DOI] [PubMed] [Google Scholar]
- 35.Yanaka A., Suzuki H., Shibahara T. EGF promotes gastric mucosal restitution by activating Na(+)/H(+) exchange of epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2002;282:G866–G876. doi: 10.1152/ajpgi.00150.2001. [DOI] [PubMed] [Google Scholar]
- 36.Joutsi T., Paimela H., Bhowmik A. Role of Na(+)-H(+)-antiport in restitution of isolated guinea pig gastric epithelium after superficial injury. Dig Dis Sci. 1996;41:2187–2194. doi: 10.1007/BF02071399. [DOI] [PubMed] [Google Scholar]
- 37.Xue L., Aihara E., Wang T.C. Trefoil factor 2 requires Na/H exchanger 2 activity to enhance mouse gastric epithelial repair. J Biol Chem. 2011;286:38375–38382. doi: 10.1074/jbc.M111.268219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Furukawa O., Matsui H., Suzuki N. Epidermal growth factor protects rat epithelial cells against acid-induced damage through the activation of Na+/H+ exchangers. J Pharmacol Exp Ther. 1999;288:620–626. [PubMed] [Google Scholar]
- 39.Schultheis P.J., Clarke L.L., Meneton P. Targeted disruption of the murine Na+/H+ exchanger isoform 2 gene causes reduced viability of gastric parietal cells and loss of net acid secretion. J Clin Invest. 1998;101:1243–1253. doi: 10.1172/JCI1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boivin G.P., Schultheis P.J., Shull G.E. Variant form of diffuse corporal gastritis in NHE2 knockout mice. Comp Med. 2000;50:511–515. [PubMed] [Google Scholar]
- 41.Xu H., Zhang B., Li J. Impaired mucin synthesis and bicarbonate secretion in the colon of NHE8 knockout mice. Am J Physiol Gastrointest Liver Physiol. 2012;303:G335–G343. doi: 10.1152/ajpgi.00146.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu H., Zhao Y., Li J. Loss of NHE8 expression impairs ocular surface function in mice. Am J Physiol Cell Physiol. 2015;308:C79–C87. doi: 10.1152/ajpcell.00296.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Holmberg C., Perheentupa J. Congenital Na+ diarrhea: a new type of secretory diarrhea. J Pediatr. 1985;106:56–61. doi: 10.1016/s0022-3476(85)80465-0. [DOI] [PubMed] [Google Scholar]
- 44.Booth I.W., Stange G., Murer H. Defective jejunal brush-border Na+/H+ exchange: a cause of congenital secretory diarrhoea. Lancet. 1985;1:1066–1069. doi: 10.1016/s0140-6736(85)92369-4. [DOI] [PubMed] [Google Scholar]
- 45.Janecke A.R., Heinz-Erian P., Muller T. Congenital sodium diarrhea: a form of intractable diarrhea, with a link to inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2016;63:170–176. doi: 10.1097/MPG.0000000000001139. [DOI] [PubMed] [Google Scholar]
- 46.Fell J.M., Miller M.P., Finkel Y. Congenital sodium diarrhea with a partial defect in jejunal brush border membrane sodium transport, normal rectal transport, and resolving diarrhea. J Pediatr Gastroenterol Nutr. 1992;15:112–116. doi: 10.1097/00005176-199208000-00002. [DOI] [PubMed] [Google Scholar]
- 47.Muller T., Wijmenga C., Phillips A.D. Congenital sodium diarrhea is an autosomal recessive disorder of sodium/proton exchange but unrelated to known candidate genes. Gastroenterology. 2000;119:1506–1513. doi: 10.1053/gast.2000.20514. [DOI] [PubMed] [Google Scholar]
- 48.Heinz-Erian P., Muller T., Krabichler B. Mutations in SPINT2 cause a syndromic form of congenital sodium diarrhea. Am J Hum Genet. 2009;84:188–196. doi: 10.1016/j.ajhg.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fiskerstrand T., Arshad N., Haukanes B.I. Familial diarrhea syndrome caused by an activating GUCY2C mutation. N Engl J Med. 2012;366:1586–1595. doi: 10.1056/NEJMoa1110132. [DOI] [PubMed] [Google Scholar]
- 50.Muller T., Rasool I., Heinz-Erian P. Congenital secretory diarrhoea caused by activating germline mutations in GUCY2C. Gut. 2016;65:1306–1313. doi: 10.1136/gutjnl-2015-309441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arshad N., Visweswariah S.S. The multiple and enigmatic roles of guanylyl cyclase C in intestinal homeostasis. FEBS Lett. 2012;586:2835–2840. doi: 10.1016/j.febslet.2012.07.028. [DOI] [PubMed] [Google Scholar]
- 52.Chen T., Kocinsky H.S., Cha B. Cyclic GMP kinase II (cGKII) inhibits NHE3 by altering its trafficking and phosphorylating NHE3 at three required sites: identification of a multifunctional phosphorylation site. J Biol Chem. 2015;290:1952–1965. doi: 10.1074/jbc.M114.590174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu H., Chen R., Ghishan F.K. Subcloning, localization, and expression of the rat intestinal sodium-hydrogen exchanger isoform 8. Am J Physiol Gastrointest Liver Physiol. 2005;289:G36–G41. doi: 10.1152/ajpgi.00552.2004. [DOI] [PubMed] [Google Scholar]
- 54.Baum M., Martin M.G., Booth I.W. Nucleotide sequence of the Na+/H+ exchanger-8 in patients with congenital sodium diarrhea. J Pediatr Gastroenterol Nutr. 2011;53:474–477. doi: 10.1097/MPG.0b013e318227ad6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thiagarajah J.R., Donowitz M., Verkman A.S. Secretory diarrhoea: mechanisms and emerging therapies. Nat Rev Gastroenterol Hepatol. 2015;12:446–457. doi: 10.1038/nrgastro.2015.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Subramanya S.B., Rajendran V.M., Srinivasan P. Differential regulation of cholera toxin-inhibited Na-H exchange isoforms by butyrate in rat ileum. Am J Physiol Gastrointest Liver Physiol. 2007;293:G857–G863. doi: 10.1152/ajpgi.00462.2006. [DOI] [PubMed] [Google Scholar]
- 57.Zachos N.C., Tse M., Donowitz M. Molecular physiology of intestinal Na+/H+ exchange. Annu Rev Physiol. 2005;67:411–443. doi: 10.1146/annurev.physiol.67.031103.153004. [DOI] [PubMed] [Google Scholar]
- 58.Cha B., Donowitz M. The epithelial brush border Na+/H+ exchanger NHE3 associates with the actin cytoskeleton by binding to ezrin directly and via PDZ domain-containing Na+/H+ exchanger regulatory factor (NHERF) proteins. Clin Exp Pharmacol Physiol. 2008;35:863–871. doi: 10.1111/j.1440-1681.2008.04931.x. [DOI] [PubMed] [Google Scholar]
- 59.Khurana S., Ganguly N.K., Khullar M. Studies on the mechanism of Salmonella typhimurium enterotoxin-induced diarrhoea. Biochim Biophys Acta. 1991;1097:171–176. doi: 10.1016/0925-4439(91)90031-4. [DOI] [PubMed] [Google Scholar]
- 60.Kaur T., Singh S., Gorowara S. Role of enteric nervous system in Shigella dysenteriae type 1 toxin-induced fluid secretion in rabbit ileum. J Diarrhoeal Dis Res. 1995;13:159–165. [PubMed] [Google Scholar]
- 61.Kanwar R.K., Ganguly N.K., Kanwar J.R. Impairment of Na+,K(+)-ATPase activity following enterotoxigenic Campylobacter jejuni infection: changes in Na+, Cl- and 3-O-methyl-D-glucose transport in vitro, in rat ileum. FEMS Microbiol Lett. 1994;124:381–385. doi: 10.1111/j.1574-6968.1994.tb07312.x. [DOI] [PubMed] [Google Scholar]
- 62.Hayashi H., Szaszi K., Coady-Osberg N. Inhibition and redistribution of NHE3, the apical Na+/H+ exchanger, by Clostridium difficile toxin B. J Gen Physiol. 2004;123:491–504. doi: 10.1085/jgp.200308979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee J.S., Lee Y.M., Kim J.Y. BetaPix up-regulates Na+/H+ exchanger 3 through a Shank2-mediated protein-protein interaction. J Biol Chem. 2010;285:8104–8113. doi: 10.1074/jbc.M109.055079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Engevik M.A., Engevik K.A., Yacyshyn M.B. Human Clostridium difficile infection: inhibition of NHE3 and microbiota profile. Am J Physiol Gastrointest Liver Physiol. 2015;308:G497–G509. doi: 10.1152/ajpgi.00090.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lapointe T.K., O'Connor P.M., Buret A.G. The role of epithelial malfunction in the pathogenesis of enteropathogenic E. coli-induced diarrhea. Lab Invest. 2009;89:964–970. doi: 10.1038/labinvest.2009.69. [DOI] [PubMed] [Google Scholar]
- 66.Hecht G., Hodges K., Gill R.K. Differential regulation of Na+/H+ exchange isoform activities by enteropathogenic E. coli in human intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2004;287:G370–G378. doi: 10.1152/ajpgi.00432.2003. [DOI] [PubMed] [Google Scholar]
- 67.Hodges K., Alto N.M., Ramaswamy K. The enteropathogenic Escherichia coli effector protein EspF decreases sodium hydrogen exchanger 3 activity. Cell Microbiol. 2008;10:1735–1745. doi: 10.1111/j.1462-5822.2008.01163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen M., Sultan A., Cinar A. Loss of PDZ-adaptor protein NHERF2 affects membrane localization and cGMP- and [Ca2+]- but not cAMP-dependent regulation of Na+/H+ exchanger 3 in murine intestine. J Physiol. 2010;588:5049–5063. doi: 10.1113/jphysiol.2010.198721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vaandrager A.B., Schulz S., De Jonge H.R. Guanylyl cyclase C is an N-linked glycoprotein receptor that accounts for multiple heat-stable enterotoxin-binding proteins in the intestine. J Biol Chem. 1993;268:2174–2179. [PubMed] [Google Scholar]
- 70.Cha B., Kim J.H., Hut H. cGMP inhibition of Na+/H+ antiporter 3 (NHE3) requires PDZ domain adapter NHERF2, a broad specificity protein kinase G-anchoring protein. J Biol Chem. 2005;280:16642–16650. doi: 10.1074/jbc.M500505200. [DOI] [PubMed] [Google Scholar]
- 71.Schultheis P.J., Clarke L.L., Meneton P. Renal and intestinal absorptive defects in mice lacking the NHE3 Na+/H+ exchanger. Nat Genet. 1998;19:282–285. doi: 10.1038/969. [DOI] [PubMed] [Google Scholar]
- 72.Ledoussal C., Woo A.L., Miller M.L. Loss of the NHE2 Na(+)/H(+) exchanger has no apparent effect on diarrheal state of NHE3-deficient mice. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1385–G1396. doi: 10.1152/ajpgi.2001.281.6.G1385. [DOI] [PubMed] [Google Scholar]
- 73.Nighot P.K., Moeser A., Ali R.A. Astrovirus infection induces sodium malabsorption and redistributes sodium hydrogen exchanger expression. Virology. 2010;401:146–154. doi: 10.1016/j.virol.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Baetz N.W., Gupta A., Kapoor A. Rotavirus infection in patients is associated with altered trafficking of apical membrane transport proteins. Gastroenterology. 2016;150:S113–S114. [Google Scholar]
- 75.Chen X.Z., Coady M.J., Jackson F. Thermodynamic determination of the Na+: glucose coupling ratio for the human SGLT1 cotransporter. Biophys J. 1995;69:2405–2414. doi: 10.1016/S0006-3495(95)80110-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Loo D.D., Zeuthen T., Chandy G. Cotransport of water by the Na+/glucose cotransporter. Proc Natl Acad Sci U S A. 1996;93:13367–13370. doi: 10.1073/pnas.93.23.13367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hirschhorn N., Kinzie J.L., Sachar D.B. Decrease in net stool output in cholera during intestinal perfusion with glucose-containing solutions. N Engl J Med. 1968;279:176–181. doi: 10.1056/NEJM196807252790402. [DOI] [PubMed] [Google Scholar]
- 78.Lin R., Murtazina R., Cha B. D-glucose acts via sodium/glucose cotransporter 1 to increase NHE3 in mouse jejunal brush border by a Na+/H+ exchange regulatory factor 2-dependent process. Gastroenterology. 2011;140:560–571. doi: 10.1053/j.gastro.2010.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Naveh Y., Lightman A., Zinder O. Effect of diarrhea on serum zinc concentrations in infants and children. J Pediatr. 1982;101:730–732. doi: 10.1016/s0022-3476(82)80303-x. [DOI] [PubMed] [Google Scholar]
- 80.Ghishan F.K. Transport of electrolytes, water, and glucose in zinc deficiency. J Pediatr Gastroenterol Nutr. 1984;3:608–612. doi: 10.1097/00005176-198409000-00022. [DOI] [PubMed] [Google Scholar]
- 81.Bhatnagar S., Bahl R., Sharma P.K. Zinc with oral rehydration therapy reduces stool output and duration of diarrhea in hospitalized children: a randomized controlled trial. J Pediatr Gastroenterol Nutr. 2004;38:34–40. doi: 10.1097/00005176-200401000-00010. [DOI] [PubMed] [Google Scholar]
- 82.Hoque K.M., Rajendran V.M., Binder H.J. Zinc inhibits cAMP-stimulated Cl secretion via basolateral K-channel blockade in rat ileum. Am J Physiol Gastrointest Liver Physiol. 2005;288:G956–G963. doi: 10.1152/ajpgi.00441.2004. [DOI] [PubMed] [Google Scholar]
- 83.Hoque K.M., Sarker R., Guggino S.E. A new insight into pathophysiological mechanisms of zinc in diarrhea. Ann N Y Acad Sci. 2009;1165:279–284. doi: 10.1111/j.1749-6632.2009.04442.x. [DOI] [PubMed] [Google Scholar]
- 84.Ramakrishna B.S., Subramanian V., Mohan V. A randomized controlled trial of glucose versus amylase resistant starch hypo-osmolar oral rehydration solution for adult acute dehydrating diarrhea. PLoS One. 2008;3:e1587. doi: 10.1371/journal.pone.0001587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Krishnan S., Rajendran V.M., Binder H.J. Apical NHE isoforms differentially regulate butyrate-stimulated Na absorption in rat distal colon. Am J Physiol Cell Physiol. 2003;285:C1246–C1254. doi: 10.1152/ajpcell.00598.2002. [DOI] [PubMed] [Google Scholar]
- 86.Kiela P.R., Kuscuoglu N., Midura A.J. Molecular mechanism of rat NHE3 gene promoter regulation by sodium butyrate. Am J Physiol Cell Physiol. 2007;293:C64–C74. doi: 10.1152/ajpcell.00277.2006. [DOI] [PubMed] [Google Scholar]
- 87.Amin M.R., Dudeja P.K., Ramaswamy K. Involvement of Sp1 and Sp3 in differential regulation of human NHE3 promoter activity by sodium butyrate and IFN-gamma/TNF-alpha. Am J Physiol Gastrointest Liver Physiol. 2007;293:G374–G382. doi: 10.1152/ajpgi.00128.2007. [DOI] [PubMed] [Google Scholar]
- 88.Xu H., McCoy A., Li J. Sodium butyrate stimulates NHE8 expression via its role on activating NHE8 basal promoter activity. Am J Physiol Gastrointest Liver Physiol. 2015;309:G500–G505. doi: 10.1152/ajpgi.00194.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ramakrishna B.S., Venkataraman S., Srinivasan P. Amylase-resistant starch plus oral rehydration solution for cholera. N Engl J Med. 2000;342:308–313. doi: 10.1056/NEJM200002033420502. [DOI] [PubMed] [Google Scholar]
- 90.Raghupathy P., Ramakrishna B.S., Oommen S.P. Amylase-resistant starch as adjunct to oral rehydration therapy in children with diarrhea. J Pediatr Gastroenterol Nutr. 2006;42:362–368. doi: 10.1097/01.mpg.0000214163.83316.41. [DOI] [PubMed] [Google Scholar]
- 91.Binder H.J., Brown I., Ramakrishna B.S. Oral rehydration therapy in the second decade of the twenty-first century. Curr Gastroenterol Rep. 2014;16:376. doi: 10.1007/s11894-014-0376-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Laplante M., Sabatini D.M. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yang J., Zhao X., Patel A. Rapamycin inhibition of mTOR reduces levels of the Na+/H+ exchanger 3 in intestines of mice and humans, leading to diarrhea. Gastroenterology. 2015;149:151–162. doi: 10.1053/j.gastro.2015.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gutierrez A., Scharl M., Sempere L. Genetic susceptibility to increased bacterial translocation influences the response to biological therapy in patients with Crohn's disease. Gut. 2014;63:272–280. doi: 10.1136/gutjnl-2012-303557. [DOI] [PubMed] [Google Scholar]
- 95.Ghishan F.K., Kiela P.R. Epithelial transport in inflammatory bowel diseases. Inflamm Bowel Dis. 2014;20:1099–1109. doi: 10.1097/MIB.0000000000000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Seidler U., Lenzen H., Cinar A. Molecular mechanisms of disturbed electrolyte transport in intestinal inflammation. Ann N Y Acad Sci. 2006;1072:262–275. doi: 10.1196/annals.1326.024. [DOI] [PubMed] [Google Scholar]
- 97.Harris J., Shields R. Absorption and secretion of water and electrolytes by the intact human colon in diffuse untreated proctocolitis. Gut. 1970;11:27–33. doi: 10.1136/gut.11.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rask-Madsen J., Hammersgaard E.A., Knudsen E. Rectal electrolyte transport and mucosal permeability in ulcerative colitis and Crohn's disease. J Lab Clin Med. 1973;81:342–353. [PubMed] [Google Scholar]
- 99.Sandle G.I., Higgs N., Crowe P. Cellular basis for defective electrolyte transport in inflamed human colon. Gastroenterology. 1990;99:97–105. doi: 10.1016/0016-5085(90)91235-x. [DOI] [PubMed] [Google Scholar]
- 100.Greig E.R., Boot-Handford R.P., Mani V. Decreased expression of apical Na+ channels and basolateral Na+, K+-ATPase in ulcerative colitis. J Pathol. 2004;204:84–92. doi: 10.1002/path.1613. [DOI] [PubMed] [Google Scholar]
- 101.Amasheh S., Barmeyer C., Koch C.S. Cytokine-dependent transcriptional down-regulation of epithelial sodium channel in ulcerative colitis. Gastroenterology. 2004;126:1711–1720. doi: 10.1053/j.gastro.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 102.Sandle G.I. Pathogenesis of diarrhea in ulcerative colitis: new views on an old problem. J Clin Gastroenterol. 2005;39:S49–S52. doi: 10.1097/01.mcg.0000155520.04253.37. [DOI] [PubMed] [Google Scholar]
- 103.Rocha F., Musch M.W., Lishanskiy L. IFN-gamma downregulates expression of Na(+)/H(+) exchangers NHE2 and NHE3 in rat intestine and human Caco-2/bbe cells. Am J Physiol Cell Physiol. 2001;280:C1224–C1232. doi: 10.1152/ajpcell.2001.280.5.C1224. [DOI] [PubMed] [Google Scholar]
- 104.Barmeyer C., Harren M., Schmitz H. Mechanisms of diarrhea in the interleukin-2-deficient mouse model of colonic inflammation. Am J Physiol Gastrointest Liver Physiol. 2004;286:G244–G252. doi: 10.1152/ajpgi.00141.2003. [DOI] [PubMed] [Google Scholar]
- 105.Sullivan S., Alex P., Dassopoulos T. Downregulation of sodium transporters and NHERF proteins in IBD patients and mouse colitis models: potential contributors to IBD-associated diarrhea. Inflamm Bowel Dis. 2009;15:261–274. doi: 10.1002/ibd.20743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Siddique I., Hasan F., Khan I. Suppression of Na+/H+ exchanger isoform-3 in human inflammatory bowel disease: lack of reversal by 5'-aminosalicylate treatment. Scand J Gastroenterol. 2009;44:56–64. doi: 10.1080/00365520802321253. [DOI] [PubMed] [Google Scholar]
- 107.Lenzen H., Lunnemann M., Bleich A. Downregulation of the NHE3-binding PDZ-adaptor protein PDZK1 expression during cytokine-induced inflammation in interleukin-10-deficient mice. PLoS One. 2012;7:e40657. doi: 10.1371/journal.pone.0040657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yeruva S., Farkas K., Hubricht J. Preserved Na(+)/H(+) exchanger isoform 3 expression and localization, but decreased NHE3 function indicate regulatory sodium transport defect in ulcerative colitis. Inflamm Bowel Dis. 2010;16:1149–1161. doi: 10.1002/ibd.21183. [DOI] [PubMed] [Google Scholar]
- 109.Farkas K., Yeruva S., Rakonczay Z., Jr. New therapeutic targets in ulcerative colitis: the importance of ion transporters in the human colon. Inflamm Bowel Dis. 2011;17:884–898. doi: 10.1002/ibd.21432. [DOI] [PubMed] [Google Scholar]
- 110.Clayburgh D.R., Musch M.W., Leitges M. Coordinated epithelial NHE3 inhibition and barrier dysfunction are required for TNF-mediated diarrhea in vivo. J Clin Invest. 2006;116:2682–2694. doi: 10.1172/JCI29218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Xu H., Chen H., Dong J. Gastrointestinal distribution and kinetic characterization of the sodium-hydrogen exchanger isoform 8 (NHE8) Cell Physiol Biochem. 2008;21:109–116. doi: 10.1159/000113752. [DOI] [PubMed] [Google Scholar]
- 112.Goyal S., Mentone S., Aronson P.S. Immunolocalization of NHE8 in rat kidney. Am J Physiol Renal Physiol. 2005;288:F530–F538. doi: 10.1152/ajprenal.00229.2004. [DOI] [PubMed] [Google Scholar]
- 113.Lawrence S.P., Bright N.A., Luzio J.P. The sodium/proton exchanger NHE8 regulates late endosomal morphology and function. Mol Biol Cell. 2010;21:3540–3551. doi: 10.1091/mbc.E09-12-1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang A., Li J., Zhao Y. Loss of NHE8 expression impairs intestinal mucosal integrity. Am J Physiol Gastrointest Liver Physiol. 2015;309:G855–G864. doi: 10.1152/ajpgi.00278.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Xu H., Li Q., Zhao Y. Intestinal NHE8 is highly expressed in goblet cells and its expression is subject to TNF-alpha regulation. Am J Physiol Gastrointest Liver Physiol. 2016;310:G64–G69. doi: 10.1152/ajpgi.00367.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Khan I., al-Awadi F.M., Abul H. Colitis-induced changes in the expression of the Na+/H+ exchanger isoform NHE-1. J Pharmacol Exp Ther. 1998;285:869–875. [PubMed] [Google Scholar]
- 117.Nemeth Z.H., Deitch E.A., Szabo C. Na+/H+ exchanger blockade inhibits enterocyte inflammatory response and protects against colitis. Am J Physiol Gastrointest Liver Physiol. 2002;283:G122–G132. doi: 10.1152/ajpgi.00015.2002. [DOI] [PubMed] [Google Scholar]
- 118.Khan I., Oriowo M.A., Anim J.T. Amelioration of experimental colitis by Na-H exchanger-1 inhibitor amiloride is associated with reversal of IL-1ss and ERK mitogen-activated protein kinase. Scand J Gastroenterol. 2005;40:578–585. doi: 10.1080/00365520510012352. [DOI] [PubMed] [Google Scholar]
- 119.Khan I., Siddique I., Al-Awadi F.M. Role of Na+/H+ exchanger isoform-1 in human inflammatory bowel disease. Can J Gastroenterol. 2003;17:31–36. doi: 10.1155/2003/673819. [DOI] [PubMed] [Google Scholar]
- 120.Siddique I., Khan I. Mechanism of regulation of Na-H exchanger in inflammatory bowel disease: role of TLR-4 signaling mechanism. Dig Dis Sci. 2011;56:1656–1662. doi: 10.1007/s10620-010-1524-7. [DOI] [PubMed] [Google Scholar]
- 121.O'Hara A.M., Shanahan F. The gut flora as a forgotten organ. EMBO Rep. 2006;7:688–693. doi: 10.1038/sj.embor.7400731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Arumugam M., Raes J., Pelletier E. Enterotypes of the human gut microbiome. Nature. 2011;473:174–180. doi: 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Thaiss C.A., Zmora N., Levy M. The microbiome and innate immunity. Nature. 2016;535:65–74. doi: 10.1038/nature18847. [DOI] [PubMed] [Google Scholar]
- 124.Honda K., Littman D.R. The microbiota in adaptive immune homeostasis and disease. Nature. 2016;535:75–84. doi: 10.1038/nature18848. [DOI] [PubMed] [Google Scholar]
- 125.Engevik M.A., Yacyshyn M.B., Engevik K.A. Human Clostridium difficile infection: altered mucus production and composition. Am J Physiol Gastrointest Liver Physiol. 2015;308:G510–G524. doi: 10.1152/ajpgi.00091.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Milani C., Ticinesi A., Gerritsen J. Gut microbiota composition and Clostridium difficile infection in hospitalized elderly individuals: a metagenomic study. Sci Rep. 2016;6:25945. doi: 10.1038/srep25945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yamamoto M., Matsumoto S. Gut microbiota and colorectal cancer. Genes Environ. 2016;38:11. doi: 10.1186/s41021-016-0038-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Forbes J.D., Van Domselaar G., Bernstein C.N. The gut microbiota in immune-mediated inflammatory diseases. Front Microbiol. 2016;7:1081. doi: 10.3389/fmicb.2016.01081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sonnenburg J.L., Backhed F. Diet-microbiota interactions as moderators of human metabolism. Nature. 2016;535:56–64. doi: 10.1038/nature18846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Li Q., Zhou J.M. The microbiota-gut-brain axis and its potential therapeutic role in autism spectrum disorder. Neuroscience. 2016;324:131–139. doi: 10.1016/j.neuroscience.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 131.Gacias M., Gaspari S., Santos P.M. Microbiota-driven transcriptional changes in prefrontal cortex override genetic differences in social behavior. Elife. 2016;5:e13442. doi: 10.7554/eLife.13442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Luna R.A., Savidge T.C., Williams K.C. The brain-gut-microbiome axis: what role does it play in autism spectrum disorder? Curr Dev Disord Rep. 2016;3:75–81. doi: 10.1007/s40474-016-0077-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yarandi S.S., Peterson D.A., Treisman G.J. Modulatory effects of gut microbiota on the central nervous system: how gut could play a role in neuropsychiatric health and diseases. J Neurogastroenterol Motil. 2016;22:201–212. doi: 10.5056/jnm15146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ott S.J., Musfeldt M., Wenderoth D.F. Reduction in diversity of the colonic mucosa associated bacterial microflora in patients with active inflammatory bowel disease. Gut. 2004;53:685–693. doi: 10.1136/gut.2003.025403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Miyoshi J., Chang E.B. The gut microbiota and inflammatory bowel diseases. Transl Res. 2016 doi: 10.1016/j.trsl.2016.06.002. S1931-5244(16)30095-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Furusawa Y., Obata Y., Fukuda S. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446–450. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 137.Garrett W.S., Gallini C.A., Yatsunenko T. Enterobacteriaceae act in concert with the gut microbiota to induce spontaneous and maternally transmitted colitis. Cell Host Microbe. 2010;8:292–300. doi: 10.1016/j.chom.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Morampudi V., Dalwadi U., Bhinder G. The goblet cell-derived mediator RELM-beta drives spontaneous colitis in Muc2-deficient mice by promoting commensal microbial dysbiosis. Mucosal Immunol. 2016;9:1218–1233. doi: 10.1038/mi.2015.140. [DOI] [PubMed] [Google Scholar]
- 139.Devkota S., Wang Y., Musch M.W. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10-/- mice. Nature. 2012;487:104–108. doi: 10.1038/nature11225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Garrett W.S., Lord G.M., Punit S. Communicable ulcerative colitis induced by T-bet deficiency in the innate immune system. Cell. 2007;131:33–45. doi: 10.1016/j.cell.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Du Z., Hudcovic T., Mrazek J. Development of gut inflammation in mice colonized with mucosa-associated bacteria from patients with ulcerative colitis. Gut Pathog. 2015;7:32. doi: 10.1186/s13099-015-0080-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Laubitz D., Larmonier C.B., Bai A. Colonic gene expression profile in NHE3-deficient mice: evidence for spontaneous distal colitis. Am J Physiol Gastrointest Liver Physiol. 2008;295:G63–G77. doi: 10.1152/ajpgi.90207.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Larmonier C.B., Laubitz D., Hill F.M. Reduced colonic microbial diversity is associated with colitis in NHE3-deficient mice. Am J Physiol Gastrointest Liver Physiol. 2013;305:G667–G677. doi: 10.1152/ajpgi.00189.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Johansson M.E., Gustafsson J.K., Holmen-Larsson J. Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and patients with ulcerative colitis. Gut. 2014;63:281–291. doi: 10.1136/gutjnl-2012-303207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ambort D., Johansson M.E., Gustafsson J.K. Calcium and pH-dependent packing and release of the gel-forming MUC2 mucin. Proc Natl Acad Sci U S A. 2012;109:5645–5650. doi: 10.1073/pnas.1120269109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kiela P.R., Laubitz D., Larmonier C.B. Changes in mucosal homeostasis predispose NHE3 knockout mice to increased susceptibility to DSS-induced epithelial injury. Gastroenterology. 2009;137 doi: 10.1053/j.gastro.2009.05.043. 965–975 e1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Larmonier C.B., Laubitz D., Thurston R.D. NHE3 modulates the severity of colitis in IL-10-deficient mice. Am J Physiol Gastrointest Liver Physiol. 2011;300:G998–G1009. doi: 10.1152/ajpgi.00073.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Laubitz D., Harrison C.A., Midura-Kiela M.T. Reduced epithelial Na+/H+ exchange drives gut microbial dysbiosis and promotes inflammatory response in T cell-mediated murine colitis. PLoS One. 2016;11:e0152044. doi: 10.1371/journal.pone.0152044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Engevik M.A., Aihara E., Montrose M.H. Loss of NHE3 alters gut microbiota composition and influences Bacteroides thetaiotaomicron growth. Am J Physiol Gastrointest Liver Physiol. 2013;305:G697–G711. doi: 10.1152/ajpgi.00184.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hickey C.A., Kuhn K.A., Donermeyer D.L. Colitogenic bacteroides thetaiotaomicron antigens access host immune cells in a sulfatase-dependent manner via outer membrane vesicles. Cell Host Microbe. 2015;17:672–680. doi: 10.1016/j.chom.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Xu H., Chen H., Dong J. Tumor necrosis factor-{alpha} downregulates intestinal NHE8 expression by reducing basal promoter activity. Am J Physiol Cell Physiol. 2009;296:C489–C497. doi: 10.1152/ajpcell.00482.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Liu C., Xu H., Zhang B. NHE8 plays an important role in mucosal protection via its effect on bacterial adhesion. Am J Physiol Cell Physiol. 2013;305:C121–C128. doi: 10.1152/ajpcell.00101.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zachos N.C., van Rossum D.B., Murtazina R. A peptide mimicking the NHE3 C-terminus stimulates basal and blocks CA2+ and cAMP inhibition of NHE3 activity, and prevents cholera toxin-induced mouse jejunal fluid accumulation: potential role as drug therapy for diarrhea. Gastroenterology. 2012;142:S-1. [Google Scholar]
- 154.Oparil S., Schmieder R.E. New approaches in the treatment of hypertension. Circ Res. 2015;116:1074–1095. doi: 10.1161/CIRCRESAHA.116.303603. [DOI] [PubMed] [Google Scholar]
- 155.Feyh A., Bracero L., Lakhani H.V. Role of dietary components in modulating hypertension. J Clin Exp Cardiolog. 2016;7(4) doi: 10.4172/2155-9880.1000433. pii: 433. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kwan M.W., Wong M.C., Wang H.H. Compliance with the Dietary Approaches to Stop Hypertension (DASH) diet: a systematic review. PLoS One. 2013;8:e78412. doi: 10.1371/journal.pone.0078412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Spencer A.G., Labonte E.D., Rosenbaum D.P. Intestinal inhibition of the Na+/H+ exchanger 3 prevents cardiorenal damage in rats and inhibits Na+ uptake in humans. Sci Transl Med. 2014;6:227ra36. doi: 10.1126/scitranslmed.3007790. [DOI] [PubMed] [Google Scholar]
- 158.Linz D., Wirth K., Linz W. Antihypertensive and laxative effects by pharmacological inhibition of sodium-proton-exchanger subtype 3-mediated sodium absorption in the gut. Hypertension. 2012;60:1560–1567. doi: 10.1161/HYPERTENSIONAHA.112.201590. [DOI] [PubMed] [Google Scholar]
- 159.Johansson S., Rosenbaum D.P., Knutsson M. A phase 1 study of the safety, tolerability, pharmacodynamics, and pharmacokinetics of tenapanor in healthy Japanese volunteers. Clin Exp Nephrol. 2016 doi: 10.1007/s10157-016-1302-8. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zielinska M., Wasilewski A., Fichna J. Tenapanor hydrochloride for the treatment of constipation-predominant irritable bowel syndrome. Expert Opin Investig Drugs. 2015;24:1093–1099. doi: 10.1517/13543784.2015.1054480. [DOI] [PubMed] [Google Scholar]
- 161.Labonte E.D., Carreras C.W., Leadbetter M.R. Gastrointestinal inhibition of sodium-hydrogen exchanger 3 reduces phosphorus absorption and protects against vascular calcification in CKD. J Am Soc Nephrol. 2015;26:1138–1149. doi: 10.1681/ASN.2014030317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Cil O., Phuan P.W., Lee S. CFTR activator increases intestinal fluid secretion and normalizes stool output in a mouse model of constipation. Cell Mol Gastroenterol Hepatol. 2016;2:317–327. doi: 10.1016/j.jcmgh.2015.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]